Abstract
Background
The OPTN has implemented medical criteria to determine which candidates are most appropriate for simultaneous liver-kidney transplantation (SLK) in comparison to liver-alone transplantation. We investigated prepolicy center-level variation among SLK-listing practice, in light of such criteria.
Methods
We identified 4,736 SLK-eligible candidates after Share-35 in the U.S. We calculated the proportion of candidates at each center who were listed for SLK within 6 months of eligibility. Multi-level logistic regression and parametric survival model was used to estimate the center-specific probability of SLK-listing, adjusting for patient and center-level characteristics.
Results
Among 4,736 SLK-eligible candidates, 64.8% were listed for SLK within 6-months of eligibility. However, the percentage of SLK-listing ranged from 0% to 100% across centers. African American race, male gender, prior transplant history, diabetes, and hypertension were associated with a higher likelihood of SLK-listing. Conversely, older age, was associated with a lower likelihood of SLK-listing. After adjusting for candidate characteristics, the percentage of SLK-listing still ranged from 3.8% to 80.2% across centers; this wide variation persisted even after further adjusting for center-level characteristics.
Conclusions
There was significant prepolicy center-level variation in SLK-listing for SLK-eligible candidates. Implementation of standardized SLK listing practices may reduce center-level variation and equalize access for SLK candidates across the US.
INTRODUCTION
Simultaneous liver-kidney transplantation (SLK) is preferred to liver-alone transplantation (LAT) for candidates with renal dysfunction that will not recover after LAT1,2. Prior to August 2017, it was the discretion of individual transplant centers to determine candidacy for SLK and to list appropriately. At that time, if a liver was allocated to a SLK candidate, the candidate received priority for a kidney from the same donor (if the donor and candidate are within the same DSA)3. This allocation paradigm prioritized SLK candidates to receive kidney allografts over kidney-alone transplant (KAT) candidates, regardless of their time on the waiting list or comorbidity. However, in an attempt to standardize allocation equity between SLK and LAT candidates, the Organ Procurement and Transplantation Network (OPTN) recently accepted medical eligibility criteria to help determine which transplant candidates are most appropriate for SLK3. This proposal only prioritizes SLK candidates who meet at least 1 of several key criteria: chronic kidney disease (CKD), sustained acute kidney injury, or certain types of metabolic diseases (Table 1)3.
Table 1.
OPTN proposed SLK medical eligibility criteria.3
| For adult SLK candidates, they must meet 1 of the 3 criteria listed below: | |
|---|---|
| If the candidate’s transplant nephrologist confirms a diagnosis of: | Then the transplant program must report in the UNOS computer system and document in the candidate’s medical record: |
| 1) Chronic kidney disease (CKD) with a measured or calculated glomerular filtration rate (GFR) less than or equal to 60 mL/min for greater than 90 consecutive days | At least 1 of the following:
|
| 2) Sustained acute kidney injury | At least 1 of the following, or a combination of both of the following, for the last 6 weeks:
|
| 3) Metabolic disease | A diagnosis of at least 1 of the following:
|
The proposed medical criteria were driven by a rise in SLK transplantation (from 135 in 2000 to 557 in 2014)4 in the absence SLK candidacy requirements. Medical criteria for SLK have been discussed in great detail4–7 over the past decade, but until recently there was not a consensus. Some observational studies have identified subgroups of liver transplant candidates who may benefit from SLK4,6–13. Specifically, Sharma et al reported higher survival with SLK vs LAT in nondialysis patients who receive high quality donor kidneys, and similar survival between SLK and LAT in dialysis patients12. However, previous studies have shown minimal short-term survival benefit for SLK recipients compared to LAT14 and high rates of renal recovery in acute dialysis LAT recipients14,15. Thus, clearly defining the appropriate medical criteria for SLK is imperative to creating an allocation system that maximizes organ usage and allocation equity.
Prior to the new OPTN SLK policy, a recent survey of 57 US transplant centers demonstrated a wide array of medical criteria for SLK-listing16. However, this center level variation has never been quantified. It is unclear how the likelihood of SLK-listing varies across transplant centers for a given transplant candidate. Additionally, it is unclear how well SLK-listing practices correlate with the new OPTN medical eligibility criteria. Characterization of prior center level SLK-listing practice is necessary to estimate changes in practice due to the recent policy implementation. Therefore, the goal of this study was to explore and quantify center variation of SLK-listing practices, in light of the new SLK-eligible criteria.
METHODS
Data source
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donors, waitlisted candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN) as per reported17. The Health Resources and Services Administration (HRSA), US Department of Health and Human Services, provides oversight to the activities of the OPTN and SRTR contractors.
Study population
We studied 55,396 adult active liver transplant candidates between June 18, 2013 (post-Share-35) and February 28, 2017 in the United States. Among all liver transplant candidates, 4,736 were SLK-eligible and met the new OPTN SLK medical criteria, i.e. having 1 of the following 3 conditions:
Chronic kidney disease - eGFR ≤ 60mL/min for greater than 90 consecutive days, and subsequent 1 time eGFR ≤ 30mL/min
Sustained acute kidney injury - Dialysis or eGFR ≤ 25ml/min for at least 6 weeks
Metabolic disease - Hyperoxaluria, atypical HUS from mutations in factor H/I, familial nonneuropathic systemic amyloidosis, or methylmalonic aciduria.
We calculated eGFR by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation18. Clinical information was only available in SRTR after candidates were wait-listed; as such, to determine eligibility resulting from 90 days eGFR ≤ 60mL/min, we excluded the first 90 days of person time to allow 90 days of data prior to determination.
Center level variation of SLK-listing
The primary outcome of this study was SLK-listing. We determined the overall and center-level percentage of SLK-eligible candidates who were listed within 6 months of eligibility. Six months was chosen because the majority of SLK-eligible candidates that were listed for SLK (96.2%) did so within this time frame. SLK-listing is reported as incidence rate of SLK-listing per 6 months at each center.
Patient-Level Adjustment
Based on observed SLK-listing among SLK-eligible candidates, we identified 2 distinct patient populations: candidates that were listed for SLK immediately upon eligibility, and candidates who were listed later in the study period, after becoming eligible (Figure 1). Therefore, we explored SLK-listing using 2 unique models based on the distribution of our observed data: (1) multilevel logistic regression for immediate listing and (2) multilevel mixed-effects parametric survival regression for listing after eligibility. We adjusted both models for age (per 10 years), gender, race (African-American vs non-African-American), biologic MELD score (per 5 points), eGFR (per 5 ml/min/1.73), previous transplant, hypertension, and diabetes. For the logistic regression model that described immediate SLK-listing among SLK-eligible candidates, we reported an odds ratio for listing. For this population, an odds ratio (OR) of greater than one indicates a higher chance of SLK-listing. For the parametric survival regression model that described SLK-listing after becoming eligible, we reported a time ratio (TR) to listing. A time ratio of greater than one indicates a longer time to SLK-listing and thus, a lower likelihood of listing.
Figure 1.
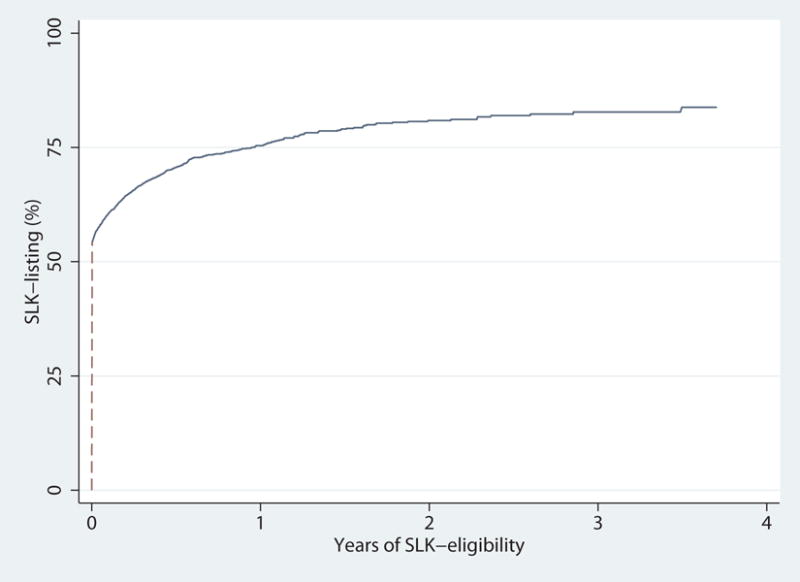
Percentage of SLK-listing among SLK-eligible candidates. More than 50% of SLK-eligible candidates were listed immediately when eligible (dash line). Among SLK-eligible candidates listed for SLK, 96.2% were listed within 6 months after becoming eligible. We explored SLK-listing using 2 unique models based on the distribution of our observed data: (1) multilevel logistic regression for immediate listing (dash line) and (2) multilevel mixed-effects parametric survival regression for listing after eligibility (solid line).
We further used empirical Bayes estimates of center-level random intercepts to calculate an estimated percentage of 6-month SLK-listing at each center for a reference candidate. The reference candidate was defined using the median value for specific covariates: a 60 year old, non-African American male with a MELD of 25, a GFR of 30, and no history of hypertension, diabetes or previous solid organ transplant.
Center-level adjustment
After patient-level adjustment, we explored the association of SLK-listing with center-level characteristics. Center-level characteristics were modeled and reported as continuous variables: per 5% increase in percentage of AA liver candidates, per 10 liver transplants for liver transplant volume, per 10 kidney transplants for kidney transplant volume, and per 10 SLKs for SLK volume. Transplant volume was recorded as a cumulative volume over the entire study period. We used empirical Bayes estimates to calculate the center-specific percentage of 6-month SLK-listing for the reference candidate, adjusting for candidate and center level characteristics.
Posttransplant mortality
Among SLK-eligible candidates during our study period, there were 1,132 SLK recipients and 613 LAT recipients. Kaplan-Meier methods and Log-Rank test were used to compare posttransplant mortality between SLK and LAT recipients. Cox regression was used to adjust for recipient gender, race, age, biologic MELD, eGFR, previous transplant, hypertension, diabetes, and the Donor Risk Index (DRI).
Sensitivity analysis
Based on the 90 day history of eGFR≤60 criterion for CKD in SLK criteria, we excluded 16,903 candidates with less than 90 days of follow-up on the waitlist. This exclusion criterion may exclude some SLK-eligible candidates. Therefore, as a sensitivity analysis, we included candidates with less than 90 days of follow-up on the waitlist and redefined SLK eligibility of CKD as 1-time eGFR ≤ 30mL/min. We repeated the analysis previously outlined to determine the stability of our inferences, and our inferences remained the same under these conditions.
Statistical analysis
Likelihood ratio tests were used to test the statistical significance of multi-level random effects by transplant centers. Confidence intervals and interquartile range are reported as per the method of Louis and Zeger19. All analyses were performed using Stata 14.0/MP for Linux (College Station, Texas).
RESULTS
Study population
Among adult active liver transplant candidates, 4,736 (8.5%) were SLK-eligible. Among SLK-eligible candidates, 1,525 (32.2%) had CKD, 3,173 (67.0%) had sustained acute kidney injury, and 38 (0.8%) had metabolic disease (Table 2). SLK-listing rates were 30.5%, 84.7%, and 97.4% for eligible candidates with CKD, sustained acute kidney injury, and metabolic diseases, respectively. Overall, 67.4% of SLK-eligible candidates (n=3,191) were listed (Table 2), while 1,545 SLK-eligible candidates were not listed.
Table 2. SLK-eligible and listed candidates by individual medical criteria.
The majority of the SLK-eligible candidates with sustained acute kidney injury or certain metabolic diseases were listed for SLK. However, the majority of the eligible candidates with chronic kidney disease were not listed for SLK.
| Diagnosis | Criteria | SLK-eligible N |
SLK-listing N (%) |
Eligible Nonlisting N (%) |
|---|---|---|---|---|
| Chronic kidney disease | eGFR ≤ 60mL/min ≥ 90 consecutive days and subsequent 1 time eGFR ≤ 30mL/min | 1,525 | 465 (30.5) | 1060 (69.5) |
| Sustained acute kidney injury | dialysis or eGFR ≤ 25ml/min ≥ 6 weeks | 3,173 | 2,689 (84.7) | 484 (15.3) |
| Metabolic disease | hyperoxaluria, atypical HUS, systemic amyloidosis, or methylmalonic aciduria | 38 | 37 (97.4) | 1 (2.6) |
| Total | 4,736 | 3,191 (67.4) | 1,545 (32.6) | |
Among SLK-eligible candidates, SLK-listed candidates and unlisted candidates had clinically similar MELD (202225 vs 202328, p<0.001), although there were statistically significantly differences (Table 3). SLK-listed candidates were less likely to have MELD exception points (10.8% vs 14.8%, p<0.001). SLK-listed candidates also had lower eGFR (9.616.624.9 vs 20.625.628.4, p<0.001). However, SLK-listed candidates were more likely to be younger (535963 vs 576266, p<0.001), African-American (15.1% vs 4.5%, p<0.001), male (61.4% vs 49.7%, p<0.001), to have had a prior transplant (11.3% vs 6.1%, p<0.001), diabetes (50.6% vs 43.0%, p<0.001), or hypertension (41.2% vs 23.4%, p<0.001).
Table 3. SLK-eligible candidate demographics and characteristics.
Among 4,736 adult SLK-eligible candidates between June 18, 2013 and February 28, 2017, 67.4% were listed for SLK.
| Listed SLK-eligible candidates (N=3,191) |
Unlisted SLK-eligible candidates (N=1,545) |
p value | |
|---|---|---|---|
| Age, median (IQR) | 59 (53–63) | 62 (57–66) | <0.001 |
| Male (%) | 61.4 | 49.7 | <0.001 |
| African American (%) | 15.1 | 4.5 | <0.001 |
| MELD, median (IQR)* | 22 (20–25) | 23 (20–28) | <0.001 |
| Exception points (%)* | 10.8 | 14.8 | <0.001 |
| eGFR, median (IQR)* | 16.6 (9.6–24.9) | 25.6 (20.6–28.4) | <0.001 |
| Prior transplant (%) | 11.3 | 6.1 | <0.001 |
| Diabetes (%) | 50.6 | 43.0 | <0.001 |
| Hypertension (%) | 41.2 | 23.4 | <0.001 |
These time-varying characteristics were obtained at eligibility.
At the 120 transplant centers with SLK-eligible liver candidates, the median percentage of African-American liver candidates was 6.6% (IQR: 3.9%–13.2%), median liver transplant volume was 156 (IQR: 79–280), median kidney transplant volume was 238 (IQR: 152–409), median SLK transplant volume was 14 (IQR: 7–27), and median MELD at transplant was 29 (IQR: 25–33) (Table 4).
Table 4.
Center level characteristics of the 120 transplant centers with SLK-eligible candidates.
| Minimum | 25th percentile | Median | 75th percentile | Maximum | |
|---|---|---|---|---|---|
| AA liver candidates, % | 0.0% | 3.9% | 6.6% | 13.2% | 24.8% |
| Liver transplant volume* | 0 | 79 | 156 | 280 | 765 |
| Kidney transplant volume* | 0 | 152 | 238 | 409 | 871 |
| SLK transplant volume* | 0 | 7 | 14 | 27 | 120 |
| Median MELD at transplant | 22 | 25 | 29 | 33 | 40 |
Liver, kidney, and SLK transplant volumes were cumulative transplant volumes over the study period. AA, African American.
Center level variation of SLK-listing
Over the study period, the total number of SLK-eligible candidates ranged from 1 to 185 across 120 transplant centers (Figure 2). Centers listed between 0% and 100% of their SLK-eligible candidates for SLK within 6 months of eligibility. Even among larger-volume centers with more than 50 total SLK-eligible candidates, the percentage of SLK-listing varied widely from 1.6% to 89.0%.
Figure 2.
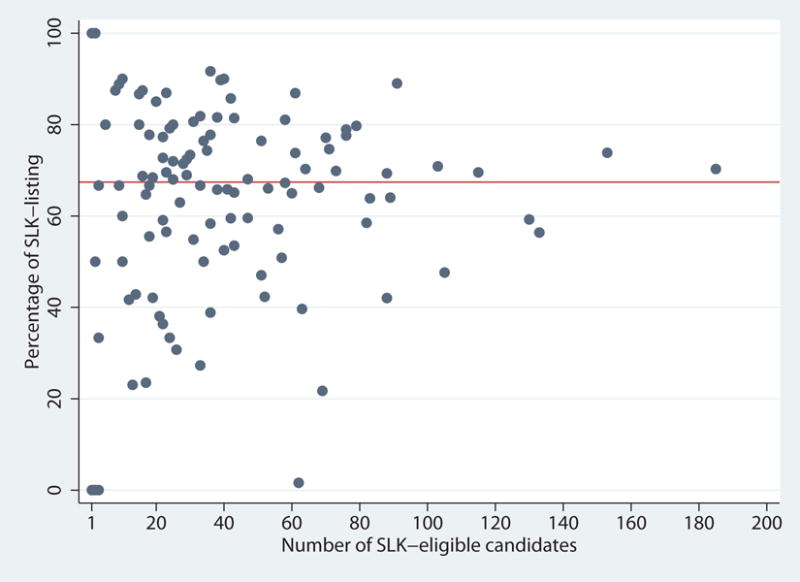
Percentage of SLK-listing (within 6 months of eligibility) by number of SLK-eligible liver candidates. The number of SLK-eligible candidates over the study period ranged from 1 to 185 across centers. The percentage of SLK-listing ranged from 0% to 100%. Even among centers with > 50 total SLK-eligible candidates, the percentage listed for SLK ranged from 1.6% to 89.0%.
Among SLK-eligible candidates (Table 5), African-American race (OR=1.752.232.84, p<0.001), male gender (OR=1.221.411.62, p<0.001), prior transplant (OR=1.301.682.17, p<0.001), diabetes (OR=1.181.351.56, p<0.001), and hypertension (OR=1.832.132.48, p<0.001) were associated with increased immediate SLK-listing. However, SLK-eligible candidates with higher MELD were less likely to be immediately listed for SLK (OR=0.770.820.88, p<0.001).
Table 5. Characteristics associated with SLK-listing among SLK-eligible liver candidates.
Odds ratios (ORs) represent likelihood of immediate SLK-listing; OR>1 indicates higher likelihood of immediate SLK listing. Time ratios (TRs) represent ratio of time to SLK-listing if candidates were not listed for SLK immediately; TR>1 indicates longer time and lower likelihood to SLK-listing.
| OR of immediate SLK-listing | P value | TR of later SLK-listing | P value | |
|---|---|---|---|---|
| Patient-level characteristics | ||||
| Age (per 10 year increments) | 0.50 0.55 0.60 | <0.001 | 1.25 1.39 1.56 | <0.001 |
| Male | 1.22 1.41 1.62 | <0.001 | 0.66 0.79 0.94 | 0.007 |
| African American | 1.75 2.23 2.84 | <0.001 | 0.40 0.55 0.75 | <0.001 |
| MELD (per 5 point increments) | 0.77 0.82 0.88 | <0.001 | 0.44 0.47 0.51 | <0.001 |
| eGFR (per 5 ml/min/1.73 increments) | 0.67 0.70 0.73 | <0.001 | 1.21 1.25 1.30 | <0.001 |
| Prior transplant | 1.30 1.68 2.17 | <0.001 | 0.81 1.15 1.63 | 0.4 |
| Diabetes | 1.18 1.35 1.56 | <0.001 | 0.62 0.74 0.89 | 0.001 |
| Hypertension | 1.83 2.13 2.48 | <0.001 | 0.68 0.82 1.01 | 0.06 |
|
| ||||
| Center-level characteristics | ||||
| Percentage of AA liver candidates | 0.90 1.01 1.14 | 0.8 | 0.99 1.13 1.28 | 0.07 |
| Liver transplant volume* | 0.94 0.96 0.97 | <0.001 | 1.02 1.03 1.06 | <0.001 |
| Kidney transplant volume* | 0.99 1.00 1.01 | 0.3 | 0.98 0.99 1.00 | 0.02 |
| SLK transplant volume* | 1.32 1.50 1.70 | <0.001 | 0.63 0.72 0.82 | <0.001 |
| Median MELD at transplant | 0.93 0.97 1.00 | 0.054 | 1.00 1.04 1.09 | 0.04 |
For liver, kidney, and SLK transplant volume, the adjusted OR is per 10 transplants. Liver, kidney, and SLK transplant volumes are cumulative transplant volumes over the study period.
We explored SLK-listing using 2 unique models based on the distribution of our observed data: (1) multilevel logistic regression for immediate listing and (2) multilevel mixed-effects parametric survival regression for listing after eligibility. For the logistic regression model that described immediate SLK-listing among SLK-eligible candidates, we reported an odds ratio for listing. For this population, an odds ratio (OR) of greater than one indicates a higher chance of SLK-listing. For the parametric survival regression model that described SLK-listing after becoming eligible, we reported a time ratio (TR) to listing. A time ratio of greater than one indicates a longer time to SLK-listing and thus, a lower likelihood of listing.
Among SLK-eligible candidates not immediately listed for SLK (Table 5), African-American race (TR=0.400.550.75, p<0.001), male gender (TR=0.660.790.94, p=0.007), higher MELD (TR=0.440.470.51, p<0.001), and diabetes (TR=0.620.740.89; p=0.001) were associated with shorter time to listing for SLK.
After adjustment, on average, across all centers, 55.5% of SLK-eligible candidates were listed for SLK. However, the center specific percentage ranged from 3.8% to 80.2% (Figure 3). Out of 120 centers, 38 (31.7%) listed less than 50% of candidates within 6 months of eligibility.
Figure 3.
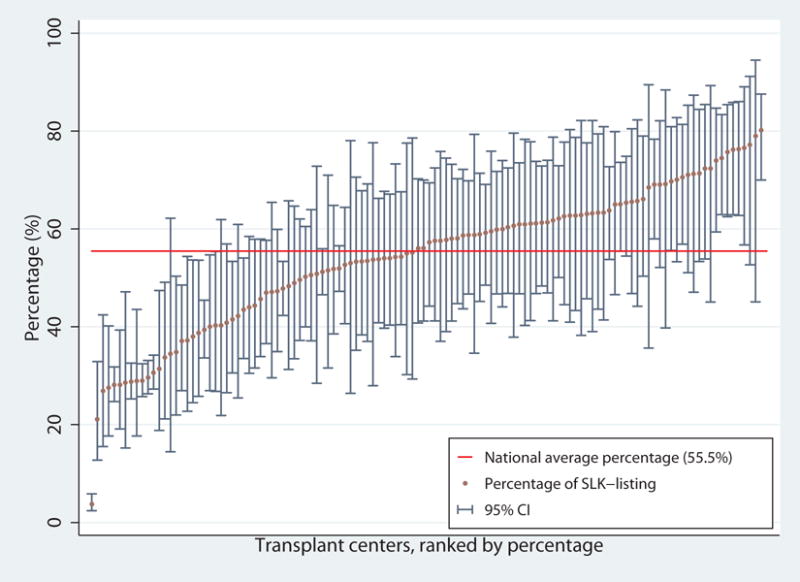
Casemix-adjusted SLK-listing percentage (within 6 months of eligibility) among SLK-eligible liver candidates by center. The percentage was calculated for a 60 year old, non-African American male with a MELD of 25, a GFR of 30, with no history of hypertension, diabetes or any previous transplant. The national average percentage for SLK-listing was 55.5% (red horizontal line), and ranged from 3.8% to 80.2%. There were 38 (31.7%) centers that had SLK-listing percentage less than 50%.
SLK-listing among SLK-eligible candidates was also independently associated with some center level characteristics (Table 5). SLK-listing decreased as liver transplant volume increased (OR=0.940.960.97, p<0.001; TR=1.021.031.06, p<0.001) and median MELD at transplant increased (TR=1.001.041.09, p=0.04). However, SLK-listing increased with increased SLK volume (OR=1.321.501.70, p<0.001; TR=0.630.720.82, p<0.001). After adjusting for patient and center level characteristics, SLK-listing (for a reference candidate) still varied significantly by center, from 9.3% to 70.7% (likelihood ratio test: p<0.001).
Posttransplant mortality
During our study period among SLK-eligible candidates, 1,132 underwent SLK (68.9% CKD, 78.1% acute kidney injury, 2.3% metabolic disease) with median (IQR) MELD of 25 (21–32), and 613 underwent LAT (49.8% CKD, 15.3% acute kidney injury, 0.2% metabolic disease) with median (IQR) MELD of 28 (22–35). SLK recipients had lower mortality than LAT recipients after transplant (Figure 4). One-year mortality was 9.1% for SLK recipients and 14.2% for LAT recipients (Log-Rank, p=0.003). After adjustment, SLK was associated with 39% lower mortality than LAT (HR=0.430.610.87, p=0.006).
Figure 4.
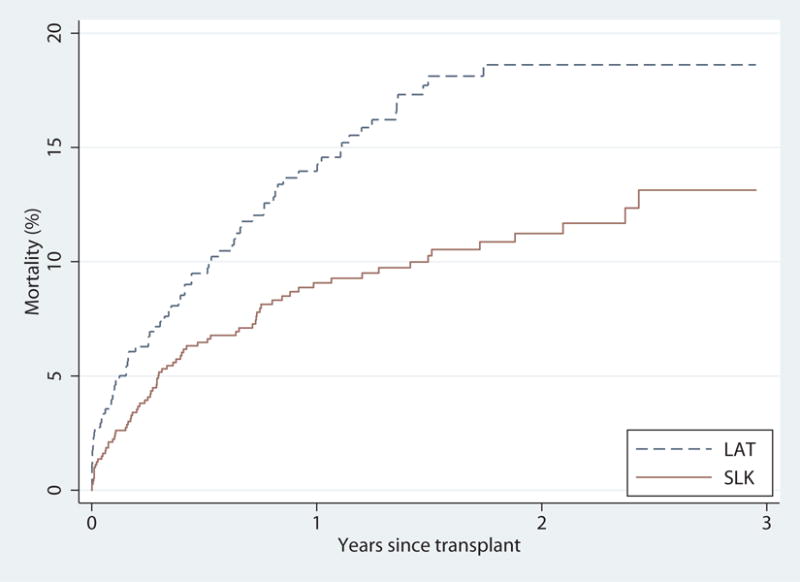
Posttransplant mortality of SLK and LAT (liver-alone transplantation) among SLK-eligible candidates. SLK recipients had higher posttransplant survival than LAT recipients (p<0.001). After adjusting for recipient gender, race, age, biologic MELD, eGFR, previous transplant, hypertension, diabetes, and the Donor Risk Index (DRI), posttransplant survival was still higher among SLK recipients (hazard ratio=0.430.610.87, p=0.006).
DISCUSSION
In this national study of SLK-listing practices, we found that 67.4% of SLK-eligible candidates were listed for SLK, with wide variation across the US centers (0%–100% listed). African American race, male gender, prior transplant history, diabetes, and hypertension were independently associated with a higher likelihood of SLK-listing. Conversely, older age, and higher eGFR were independently associated with a lower likelihood of SLK-listing. The adjusted percentage of SLK-listing varied substantially from 3.8% to 80.2% across centers for a reference candidate, even after adjusting for multiple patient and center level characteristics. Additionally, mortality risk after transplantation was 39% lower for SLK recipients compared to LAT recipients.
Qualitative proof of center variation in SLK-listing practices has been reported16. Our study is the first to quantify the center-level variation in SLK-listing. After adjustment, patient case mixture and center-level characteristics were unable to fully explain the variation in listing practices. This quantified variation has led to disparity in access to SLK transplantation throughout the US. In centers with lower listing rates, SLK-eligible candidates who might benefit from SLK are potentially being disadvantaged. The SLK allocation policy may help to address some of the disparities currently seen in SLK-listing and decrease listing variation throughout the US.
In addition to center level variation, there is variation in SLK transplantation for specific candidate populations. Sharma et al12 and Mindikoglu et al10 previously demonstrated that male gender and African-American race were associated with higher rates of SLK in comparison to LAT. Our study shows that these differences not only exist in transplantation, but exist in SLK-listing among SLK-eligible candidates. The disparities in SLK-listing might be biased by concern that male gender and African-American race were associated with faster decline of GFR and progression in CKD20–22. Particularly, African-American race has been shown to be associated with ESRD after LAT2. Therefore, lack of standard medical criteria for SLK might lead to the disparities in listing practice and access to transplantation. Implementation of SLK medical criteria may reduce the disparities in SLK-listing.
Our findings showed that increased MELD was associated with lower likelihood of immediate SLK-listing, but higher likelihood of SLK-listing over time, after becoming SLK-eligible. These 2 temporal SLK-listing processes may represent different SLK-eligible populations. For those that were immediately listed, the negative association of higher MELD and listing may reflect the theoretical concern of performing an SLK in a patient with high medical acuity. This issue was addressed by Lunsford et al as they showed that SLK recipients with higher MELD had greater mortality or need for renal replacement therapy at 3 months after SLK, making kidney transplant during that high medical acuity more futile23. The latter population likely represents SLK-eligible candidates with long-term progressive renal dysfunction. In this population, the association with listing and MELD is not surprising, as the MELD is likely driven by progression renal decline, with normal kidney function.
Based on the recently accepted medical criteria for SLK eligibility, 32.6% of eligible candidates were not listed for SLK. Wadei and colleagues, in an editorial to the American Journal of Transplantation, expressed concern that such medical criteria would lead to an inappropriate increase in SLK, specifically for individuals who may not benefit from SLK24. However, Formica et al argued that the SLK medical criteria can help clinicians make more appropriate decisions regarding SLK candidacy and uphold the OPTN’s Final Rule25,26. It is unclear how the policy implementation will affect listing practices given the substantial variation in documentation prior to the allocation change, notably the definition of CKD. Our results demonstrate only 30.5% of CKD SLK-eligible patients are listed for SLK prior to policy implementation. This CKD group will likely be affected after the new policy with additional data collection, but we will need to follow SLK-listing practices after implementation to know the true effect. Our study provides a quantified report of listing practices prior to the SLK allocation change, and we will be well equipped to assess changes in listing practices with the policy change.
Mortality and survival benefit after SLK versus LAT have been previously studied. Sharma et al reported lower mortality with SLK vs LAT in nondialysis patients with high quality donor kidneys and similar mortality between SLK and LAT in dialysis patients12. However, Brennan et al demonstrated lower 1-year mortality for SLK versus LAT, but minimal short-term survival benefit for SLK recipients due to inherent differences in the patient populations that led to the choice of transplantation14. Our results show lower mortality for SLK vs LAT recipients in the population of SLK-eligible patients. This may predict the mortality we will see with new policy implementation, but patients selected for SLK and SLK-listing were not based on standardized criteria.
Given that this is a retrospective analysis of registry data, our study is subject to the known limitations associated with secondary database analysis including missing data, misclassification and data-entry error. For example, certain metabolic diseases in the SLK criteria (atypical HUS, familial nonneuropathic systemic amyloidosis, or methylmalonic aciduria) are not coded as primary diagnosis in the registry data. However, it should also be noted that these diagnosis are rare diseases in adults in the U.S. and therefore inaccuracies in coding are unlikely to dramatically affect our inferences27,28. Furthermore, registry data lack serum creatinine and GFR information prior to listing. We excluded candidates who were listed for less than 90 days and without AKI and certain metabolic diseases since their SLK-eligible status was unknown. However, after inclusion of these candidates in a sensitivity analysis, our conclusions remained the same. Finally, GFR information was not available in the registry data and was calculated from serum creatinine and other candidate characteristics using the CKD-EPI equation. While this only provided estimated GFR, the CKD-EPI equation we used is a validated tool and has been cited as one of the most accurate GFR estimation methods29.
Our study is the first to quantify center variation in listing practices for SLK-eligible candidates accounting for the patient case mixture and possible future changes in allocation policy. Overall, more than half of candidates who met OPTN eligibility criteria were listed, and substantial center level variation in listing practices remained after adjusting for patient and center-level factors. The SLK-listing policy will possibly address some of the disparity in listing practices, but postimplementation practices will need to be followed closely to determine the ultimate downstream effects on both liver and kidney allocation.
Acknowledgments
Dr. Segev is supported by grant numbers K24DK101828 and 1R01DK111233-01 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Dr. Haugen is supported by grant number F32AG053025 from the National Institute on Aging (NIA). Ashton Shaffer is supported by grant number F30DK116658-01 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Funding: This work is supported by grants: K24DK101828 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), 1R01DK111233-01 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and F32AG053025 from the National Institute on Aging (NIA).
Abbreviations
- OPTN
organ procurement and transplantation network
- CKD
chronic kidney disease
- SLK
simultaneous liver-kidney transplantation
- MELD
model for end-stage liver disease
- OR
odds ratio
- TR
time ratio
- LAT
liver-alone transplantation
- DSA
donor service area
- KAT
kidney-alone transplantation
- SRTR
scientific registry of transplant recipients
- HRSA
health resources and services administration
- eGFR
estimated glomerular filtration rate
- HUS
hemolytic-uremic syndrome
- IQR
interquartile range
Footnotes
Author Contributions:
Xun Luo, Allan B. Massie, Dorry L. Segev, and Jacqueline M. Garonzik-Wang participated in research design. Xun Luo, Allan B. Massie, Dorry L. Segev, and Jacqueline M. Garonzik-Wang contributed to data analysis. Xun Luo, Allan B. Massie, Christine E. Haugen, Rashikh Choudhury, Jessica M. Ruck, Ashton A. Shaffer, Sheng Zhou, Dorry L. Segev, and Jacqueline M. Garonzik-Wang wrote the paper.
Conflict of Interest Disclosures: The authors declare no conflicts of interest.
References
- 1.O’Leary JG, Levitsky J, Wong F, Nadim MK, Charlton M, Kim WR. Protecting the Kidney in Liver Transplant Candidates: Practice-Based Recommendations From the American Society of Transplantation Liver and Intestine Community of Practice. Am J Transplant. 2016;16(9):2516–2531. doi: 10.1111/ajt.13790. [DOI] [PubMed] [Google Scholar]
- 2.Ruebner R, Goldberg D, Abt PL, et al. Risk of end-stage renal disease among liver transplant recipients with pretransplant renal dysfunction. Am J Transplant. 2012;12(11):2958–2965. doi: 10.1111/j.1600-6143.2012.04177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.OPTN/UNOS Policy Notice. Simultaneous Liver Kidney (SLK) Allocation. https://optn.transplant.hrsa.gov/media/1888/kidney_policynotice_slk_201606.pdf Published 2016. Accessed 2017.
- 4.Formica RN, Aeder M, Boyle G, et al. Simultaneous Liver-Kidney Allocation Policy: A Proposal to Optimize Appropriate Utilization of Scarce Resources. Am J Transplant. 2016;16(3):758–766. doi: 10.1111/ajt.13631. [DOI] [PubMed] [Google Scholar]
- 5.Nadim MK, Sung RS, Davis CL, et al. Simultaneous liver-kidney transplantation summit: current state and future directions. Am J Transplant. 2012;12(11):2901–2908. doi: 10.1111/j.1600-6143.2012.04190.x. [DOI] [PubMed] [Google Scholar]
- 6.Davis CL, Feng S, Sung R, et al. Simultaneous liver-kidney transplantation: evaluation to decision making. Am J Transplant. 2007;7(7):1702–1709. doi: 10.1111/j.1600-6143.2007.01856.x. [DOI] [PubMed] [Google Scholar]
- 7.Eason JD, Gonwa TA, Davis CL, Sung RS, Gerber D, Bloom RD. Proceedings of Consensus Conference on Simultaneous Liver Kidney Transplantation (SLK) Am J Transplant. 2008;8(11):2243–2251. doi: 10.1111/j.1600-6143.2008.02416.x. [DOI] [PubMed] [Google Scholar]
- 8.Fong TL, Khemichian S, Shah T, Hutchinson IV, Cho YW. Combined liver-kidney transplantation is preferable to liver transplant alone for cirrhotic patients with renal failure. Transplantation. 2012;94(4):411–416. doi: 10.1097/TP.0b013e3182590d6b. [DOI] [PubMed] [Google Scholar]
- 9.Locke JE, Warren DS, Singer AL, et al. Declining outcomes in simultaneous liver-kidney transplantation in the MELD era: ineffective usage of renal allografts. Transplantation. 2008;85(7):935–942. doi: 10.1097/TP.0b013e318168476d. [DOI] [PubMed] [Google Scholar]
- 10.Mindikoglu AL, Raufman JP, Seliger SL, Howell CD, Magder LS. Simultaneous liver-kidney versus liver transplantation alone in patients with end-stage liver disease and kidney dysfunction not on dialysis. Transplant Proc. 2011;43(7):2669–2677. doi: 10.1016/j.transproceed.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitt TM, Kumer SC, Al-Osaimi A, et al. Combined liver-kidney and liver transplantation in patients with renal failure outcomes in the MELD era. Transpl Int. 2009;22(9):876–883. doi: 10.1111/j.1432-2277.2009.00887.x. [DOI] [PubMed] [Google Scholar]
- 12.Sharma P, Shu X, Schaubel DE, Sung RS, Magee JC. Propensity Score-Based Survival Benefit of Simultaneous Liver-Kidney Transplant over Liver Transplant Alone for Recipients with Pre-Transplant Renal Dysfunction. Liver Transpl. 2016;22(1):71–9. doi: 10.1002/lt.24189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hmoud B, Kuo YF, Wiesner RH, Singal AK. Outcomes of liver transplantation alone after listing for simultaneous kidney: comparison to simultaneous liver kidney transplantation. Transplantation. 2015;99(4):823–828. doi: 10.1097/TP.0000000000000438. [DOI] [PubMed] [Google Scholar]
- 14.Brennan TV, Lunsford KE, Vagefi PA, Bostrom A, Ma M, Feng S. Renal outcomes of simultaneous liver-kidney transplantation compared to liver transplant alone for candidates with renal dysfunction. Clin Transpl. 2015;29(1):34–43. doi: 10.1111/ctr.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma P, Goodrich NP, Zhang M, Guidinger MK, Schaubel DE, Merion RM. Short-term pretransplant renal replacement therapy and renal nonrecovery after liver transplantation alone. Clin J Am Soc Nephrol. 2013;8(7):1135–1142. doi: 10.2215/CJN.09600912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nadim MK, Davis CL, Sung R, Kellum JA, Genyk YS. Simultaneous liver-kidney transplantation: a survey of US transplant centers. Am J Transplant. 2012;12(11):3119–3127. doi: 10.1111/j.1600-6143.2012.04176.x. [DOI] [PubMed] [Google Scholar]
- 17.Massie AB, Kucirka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant. 2014;14(8):1723–1730. doi: 10.1111/ajt.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens LA, Schmid CH, Greene T, et al. Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis. 2010;56(3):486–495. doi: 10.1053/j.ajkd.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostatistics. 2009;10(1):1–2. doi: 10.1093/biostatistics/kxn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berg UB. Differences in decline in GFR with age between males and females. Reference data on clearances of inulin and PAH in potential kidney donors. Nephrol Dial Transplant. 2006;21(9):2577–2582. doi: 10.1093/ndt/gfl227. [DOI] [PubMed] [Google Scholar]
- 21.Tsai WC, Wu HY, Peng YS, et al. Risk Factors for Development and Progression of Chronic Kidney Disease: A Systematic Review and Exploratory Meta-Analysis. Medicine. 2016;95(11):e3013. doi: 10.1097/MD.0000000000003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Derose SF, Rutkowski MP, Crooks PW, et al. Racial differences in estimated GFR decline, ESRD, and mortality in an integrated health system. Am J Kidney Dis. 2013;62(2):236–244. doi: 10.1053/j.ajkd.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lunsford KE, Bodzin AS, Markovic D, et al. Avoiding Futility in Simultaneous Liver-kidney Transplantation: Analysis of 331 Consecutive Patients Listed for Dual Organ Replacement. Ann Surg. 2017;265(5):1016–1024. doi: 10.1097/SLA.0000000000001801. [DOI] [PubMed] [Google Scholar]
- 24.Wadei HM, Gonwa TA, Taner CB. Simultaneous Liver Kidney Transplant (SLK) Allocation Policy Change Proposal: Is It Really a Smart Move? Am J Transplant. 2016;16(9):2763–2764. doi: 10.1111/ajt.13844. [DOI] [PubMed] [Google Scholar]
- 25.Formica RN., Jr Simultaneous liver kidney transplantation. Curr Opin Nephrol Hypertens. 2016;25(6):577–582. doi: 10.1097/MNH.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 26.Formica RN., Jr Simultaneous Liver-Kidney Allocation: Let’s Not Make Perfect the Enemy of Good. Am J Transplant. 2016;16(9):2765. doi: 10.1111/ajt.13873. [DOI] [PubMed] [Google Scholar]
- 27.Constantinescu AR, Bitzan M, Weiss LS, et al. Non-enteropathic hemolytic uremic syndrome: causes and short-term course. Am J Kidney Dis. 2004;43(6):976–982. doi: 10.1053/j.ajkd.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Coulombe JT, Shih VE, Levy HL. Massachusetts Metabolic Disorders Screening Program. II. Methylmalonic aciduria. Pediatrics. 1981;67(1):26–31. [PubMed] [Google Scholar]
- 29.Stevens LA, Li S, Kurella Tamura M, et al. Comparison of the CKD Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) study equations: risk factors for and complications of CKD and mortality in the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2011;57(3 Suppl 2):S9–16. doi: 10.1053/j.ajkd.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


