Abstract
Background
The pathogenesis of severe asthma in childhood remains poorly understood.
Objective
To construct the immunological landscape in the airways of children with severe asthma.
Methods
Comprehensive analysis of multiple cell types and mediators was performed by flow cytometry and multiplex assay using bronchoalveolar lavage (BAL) specimens (n=68) from 52 highly characterized allergic and non-allergic children (0.5–17 years) with severe treatment-refractory asthma. Multiple relationships were tested by linear mixed-effects modeling.
Results
Memory CCR5+ Th1 cells were enriched in BAL fluid versus blood, and pathogenic respiratory viruses and bacteria were readily detected. IFN-γ+IL-17+ and IFN-γ−IL-17+ subsets constituted secondary Th types, and BAL CD8+ T cells were almost exclusively IFN-γ+. The Th17-associated mediators, IL-23 and MIP-3α/CCL20 were highly expressed. Despite low Th2 numbers, Th2 cytokines were detected and Th2-skewing correlated with total IgE levels. ILC2s and basophils were scarce in BAL fluid. Levels of IL-5, IL-33 and IL-28A/IFN-λ2, were increased in multi-sensitized children and correlated with IgE to dust mite, ryegrass and fungi, but not cat, ragweed or food sources. Additionally, levels of IL-5, but no other cytokine, increased with age and correlated with eosinophil numbers in BAL fluid and blood. Both plasmacytoid and IgE+FcεRI+ myeloid dendritic cells were present in BAL fluid.
Conclusions
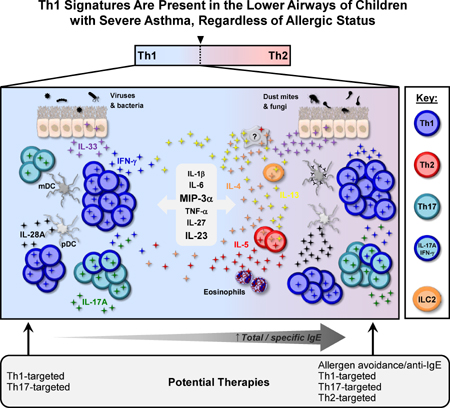
The lower airways of children with severe asthma display a dominant Th1 signature, and atypical cytokine profiles that link to allergic status. Our findings deviate from established paradigms, and warrant further assessment of the pathogenicity of Th1 cells in severe asthma.
Keywords: Severe asthma, allergic, IgE, IFN-γ, IL-4, IL-5, IL-23, IL-33, IL-28A, Th1 cells, Th2 cells, Th17 cells, ILC2
Graphical Abstract
Introduction
Asthma is an inflammatory disease of the airways characterized by intermittent airflow limitation beginning in childhood. Unfortunately, a subset of children experience severe symptoms and recurrent exacerbations, despite treatment 1. Such refractory asthma exacts a major health and economic toll on society and presents a clinical dilemma. Understanding the characteristic T-cell milieu in the lower airways of children with treatment-refractory asthma could fill gaps in the understanding of severe asthma and provide new opportunities for intervention.
CD4+ T cells are pivotal to the development and maintenance of inflammatory processes in the asthmatic lung. For many years, Th2 cells remained at the forefront, owing to their link to IgE production in allergic asthma and the pathogenic effects of IL-4 and IL-13 on airway inflammation 2, 3. However, asthma is now known to comprise heterogeneous phenotypes that reflect different underlying immune mechanisms 4. Severe asthma endotypes include IL-5-mediated eosinophilic disorders that may occur independently of IL-4 5, as well as lung neutrophilia promoted by Th17 cells 6, 7. However, the distinction between these disease entities is not clear-cut. Indeed, both eosinophils and neutrophils populate the airways of patients with severe asthma, pointing to complex immunopathology 8. In this context, the presence of T cells in the lungs that express multiple cytokines and do not adhere to conventional types is perhaps not surprising. For example, CD4+ T cells co-expressing IL-4 and IL-17A (“Th2/Th17”) have been linked to reduced lung function in adults with severe treatment-refractory asthma 9. By contrast, secretion of the Th1-associated cytokine, IFN-γ, as well as IL-13, was found to be a prominent feature of cultured cells isolated from the airways of adults with severe, but not mild, disease 10. Together, these findings indicate the persistence of complex T-cell types despite treatment.
Analyzing the T-cell landscape in children with severe asthma poses challenges owing to the scarcity and inaccessibility of T cells in the inflamed airways. Hence, the nature of T cells in the lower airways remains enigmatic. Moreover, T-cell phenotypes within the broader context of the cytokine milieu have not been evaluated. Here, we report a comprehensive assessment of T-cell signatures in bronchoalveolar lavage (BAL) specimens obtained from a well-characterized cohort of highly symptomatic asthmatic children. Through paralleled analysis of multiple cell types and secreted cytokines that are instrumental to adaptive immunity at the epithelial interface, we confirm a dominant Th1 signature in a mixed cytokine milieu, and identify novel relationships linked to allergic status. Our findings indicate the establishment of Th1 responses in the airways in early life, and support the view that regulation of this response might modify the development of severe asthma.
Methods
Human Subjects and Biological Specimens
Fifty-two children with severe, treatment refractory asthma (ages 6 months to 17 years) who underwent clinically-indicated bronchoscopy participated in the study. Strict definitions of asthma, asthma severity assignment, and symptom control by age at enrollment were followed (see Table EI in this article's Online Repository) 1, 11, 12. Demographic and clinical information were obtained for each participant by questionnaire and review of the medical record. Subjects were classified as allergic based on specific IgE >0.1 kUA/L to at least one of 9 allergens or else elevated total IgE (see Methods of Online Repository). Indications for bronchoscopy, procedure details, and subject features are detailed in the Online Repository. BAL specimens were obtained as previously described 13. In some cases as clinically indicated, two bronchial segments underwent lavage, and the BAL return was processed separately. Venous blood specimens were obtained for flow cytometry studies in a subset of subjects. Informed consent and subject assent, when indicated, were obtained. The study was approved by the University of Virginia Human Investigations Committee.
Detection of Respiratory Microbes
Detection of viruses and bacteria was assessed as described in the Online Repository.
Flow Cytometry Antibodies and Other Reagents
Details are provided in the Online Repository (Table EII).
Flow Cytometry
Cells were analyzed from BAL specimens and in available blood. Specimens were processed immediately to maximize cell viability and maintain phenotypic markers. BAL cells were isolated by centrifugation and supernatant fluid was frozen and stored at −80°C for later analysis. In cases where BAL samples were available from two lung regions, cells from the right middle lobe (n=17), lingula of left lung (n=2), or left lower lobe (n=1) were analyzed. PBMCs were isolated by established methods 14. Cell surface and intracellular markers were assessed by multi-color flow cytometry using established methods (see Online Repository)14–16. Data was acquired using FACS Diva software (version 8.0, BD Biosciences) and analyzed using FlowJo 9.6 (Tree Star). Analysis of complex cytokine-positive cell populations was performed using SPICE version 5.3., downloaded from http://exon.niaid.nih.gov 17.
Cytokine Assay
BAL supernatants were assayed for a panel of cytokines by cytometric bead assay (Millipore, Billerica, MA) using a Luminex MAGPIX System (Luminex, Austin, TX) (see Online Repository).
Statistical Analysis
Cytokine values in BAL supernatants were log transformed and absolute concentrations were determined with the R package nCal (https://CRAN.R-project.org/package=nCal)18 using a Bayesian random-effects model to fit the fluorescence intensities. Hierarchical clustering and mixed-effects modeling of cytokines was performed as described in the Online Repository. Additional details of statistical tests are also included in the Online Repository.
Results
Features of Study Participants
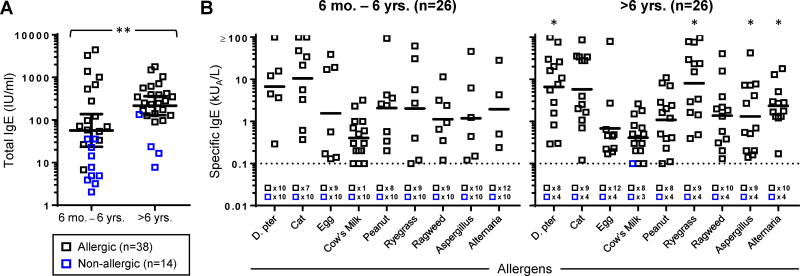
BAL specimens were available for analysis from 52 children with severe asthma, including 38 allergic and 14 non-allergic subjects (Table I, and see Table EI in the Online Repository). All subjects were receiving daily inhaled corticosteroid treatment at the time of bronchoscopy, and 18 subjects (34%) completed a course of oral corticosteroids within 3 months prior to bronchoscopy. Levels of serum total IgE were higher among asthmatics over 6 years of age versus those who were younger (Fig 1A). Among younger asthmatics, IgE antibodies to cow's milk (58%), cat (35%), and peanut (31%) were common, whereas IgE antibodies to indoor and outdoor inhalant allergens including fungi, increased with age (p<0.05 for dust mite, ryegrass, and Alternaria alternata, Fig. 1B). Allergic asthmatics had higher total IgE levels (gm=255 vs. 12 IU/ml, p<0.001) and an increased incidence of blood eosinophilia (70% vs. 21%, p<0.01) (Table I). The incidence of eosinophils in BAL fluid also trended higher among allergic versus non-allergic asthmatics (31% vs. 0%, p=0.09). Most asthmatics (92%) had evidence of current or past exposure to respiratory pathogens as judged by any past pneumonia (73%), positive PCR for enterovirus/human rhinovirus (38%) and/or positive bacterial cultures (29%) in BAL fluid (Table I). Detection of bacteria was more common in non-allergic asthmatics (p<0.05, Table I). Among those who were culture-positive, IgE levels trended lower (gm=46.22 IU/ml vs. 125.8 IU/ml, p=0.10), and the majority were ≤6 years of age (10/14, 71%)(Fig. E1A). Overall, 51% of children had any infectious microbes detected. Cultured bacteria included Streptococcus pneumoniae, Moraxella catarrhalis, Haemophilus influenzae, and Staphylococcus aureus. Four of 7 subjects colonized with S. pneumoniae were also colonized with H. influenzae and M. catarrhalis. Serum IgA and IgG levels were within normal range and did not differ according to bacterial culture status (Fig. E1A). BAL neutrophils (≥1% total cells) were a prominent feature among asthmatics, regardless of bacterial culture or allergic status (Table I and Fig. E1B).
Table I.
Characteristics of Study Participants.
| Total (N=52) | Allergic Asthma (N=38) | Non-allergic Asthma (N=14) | P value1 | |
|---|---|---|---|---|
|
| ||||
| Bronchoscopy age (year)2 | 5.7 [4.5–7.2] | 6.3 [4.8–8.3] | 4.3 [2.7–6.9] | 0.07 |
|
| ||||
| Male3 | 63% (33/52) | 71% (27/38) | 43% (6/14) | 0.06 |
|
| ||||
| African American3 | 29% (15/52) | 34% (13/38) | 14% (2/14) | 0.16 |
|
| ||||
| Total IgE (IU/ml)2 | 111 [66–188] | 255 [163–399] | 12 [6–23] | <0.001 |
|
| ||||
| Asthma Medications3 | ||||
| Inhaled corticosteroid | 100% (52/52) | 100% (38/38) | 100% (14/14) | 1.0 |
| Long-acting beta-agonist | 62% (32/52) | 68% (26/38) | 43% (6/14) | 0.13 |
| Anti-leukotriene | 60% (31/52) | 68% (26/38) | 36% (5/14) | <0.05 |
|
| ||||
| Inhaled corticosteroid dose (mcg)2 | 479 [382–602] | 571 [437–746] | 298 [205–433] | <0.01 |
|
| ||||
| Modified Asthma Predictive Index (≤ 5 yr.)3 | (N=26) | (N=16) | (N=10) | |
| Atopic dermatitis | 35% (9/26) | 44% (7/16) | 20% (2/10) | 0.21 |
| Family history of asthma | 88% (23/26) | 94% (15/16) | 80% (8/10) | 0.29 |
| Blood eosinophilia (≥4% of total WBC) | 44% (11/25) | 47% (7/15) | 40% (4/10) | 0.74 |
|
| ||||
| Asthma Control Test / Childhood Asthma Control Test2 (≥ 4 yr.) |
(N=40) 13 [11–15] |
(N=32) 13 [11–15] |
(N=8) 15 [12–18] |
0.59 |
|
| ||||
| Airflow limitation (>5 yr.) | ||||
| FEV1 (% predicted)2 |
(N=26) 80% [73–89%] |
(N=22) 80% [72–89%] |
(N=4) 82% [58–116%] |
0.88 |
| FEV1/FVC ratio4 |
(N=26) 0.75 [0.65–0.81] |
(N=22) 0.72 [0.60–0.81] |
(N=4) 0.76 [0.74–0.83] |
0.29 |
|
| ||||
| Bronchodilator response (%)4 |
(N=14) 16% [11–31%] |
(N=11) 13% [9–30%] |
(N=3) 29% [14–34%] |
0.90 |
|
| ||||
| Blood eosinophilia (≥4%)3 | 57% (29/51) | 70% (26/37) | 21% (3/14) | <0.01 |
| Absolute eosinophil count (cells per μL)4 | 420 [200–760] | 630 [320–830] | 270 [120–430] | <0.05 |
|
| ||||
| Echovirus / human rhinovirus3 | 38% (19/50) | 33% (12/36) | 50% (7/14) | 0.27 |
| Other viruses3, 5 | 0% (0/50) | 0% (0/36) | 0% (0/14) | 1.0 |
|
| ||||
| Any bacteria3 | 29% (14/49) | 20% (7/35) | 50% (7/14) | <0.05 |
| Streptococcus pneumoniae3 | 10% (5/49) | 9% (3/35) | 14% (2/14) | 0.55 |
| Other bacteria3, 6 | 24% (12/49) | 14% (5/35) | 50% (7/14) | <0.01 |
|
| ||||
| BAL Cells (≥1%)3 | (N=23) | (N=16) | (N=7) | |
| Eosinophils (%) | 22% (5/23) | 31% (5/16) | 0% (0/7) | 0.09 |
| Neutrophils (%) | 87% (20/23) | 88% (14/16) | 88% (6/7) | 0.91 |
| Lymphocytes (%) | 87% (20/23) | 88% (14/16) | 88% (6/7) | 0.91 |
|
| ||||
| Age at 1st wheeze (mo.)2 |
(N=39) 9.2 [6.1–14] |
(N=28) 8.9 [5.5–14] |
(N=11) 10 [3.7–27] |
0.53 |
|
| ||||
| Hospitalization for asthma within past 12 months (≥1 visit).3 |
(N=42) 67% (28/42) |
(N=32) 75% (24/32) |
(N=10) 40% (4/10) |
<0.05 |
|
| ||||
| Clinical History3, 7 | ||||
| Atopic dermatitis | 25% (9/36) | 36% (9/25) | 0% (0/11) | <0.05 |
| Food allergy | 60% (29/48) | 80% (29/36) | 0% (0/12) | <0.001 |
| Past pneumonia | 73% (36/49) | 69% (25/36) | 85% (11/13) | 0.06 |
Sample size varies based on available data.
Allergic versus non-allergic asthmatics.
Geometric mean [95% confidence interval].
Percentage of subjects (prevalence);
Median [interquartile range].
See online data supplement for details.
Haemophilus influenzae, Moraxella catarrhalis, Streptococcus pyogenes, and Staphylococcus aureus.
Based on documentation available in the medical record. Inhaled corticosteroid dose expressed as fluticasone equivalents.
Figure 1. Serum IgE Increases With Age in Children with Severe Asthma.
(A) Total serum IgE levels in children according to age. **p<0.01. (B) Levels of specific IgE antibodies in children according to age. Bars denote geometric means of positive values and dashed lines denote the limit of sensitivity of the assay. *p<0.05 as compared with children ≤6 years of age. D. pter., Dermatophagoides pteronyssinus (dust mite). The prevalence of allergy according to age was as follows: 6 months – 6 years, allergic (n=16) and non-allergic (n=10); >6 years, allergic (n=22) and non-allergic (n=4).
CCR5+ Memory T Cells are Enriched in BAL Fluid of Children with Severe Asthma
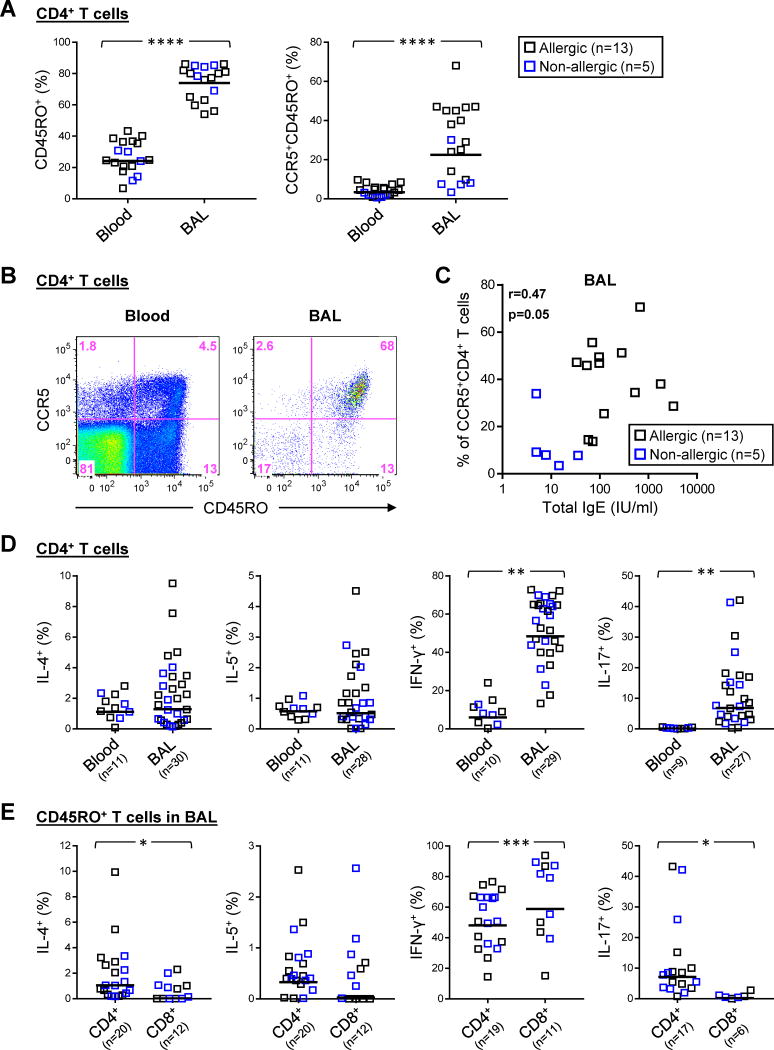
BAL specimens from 37 children who were representative of the study group contained a minimum of 1,000 live single T-cell events that were sufficient for analysis by flow cytometry (Fig. E2). BAL T cells were enriched for memory (CD45RO+) CD4+ T cells compared with peripheral blood (gm=74% vs. 24%, p<0.0001), and contained a higher proportion of memory T cells expressing CCR5 (gm=23% vs. 3%, p<0.0001) (Figs. 2A & B, and Fig. E3A). This Th1-associated chemoattractant receptor has been implicated in directing T-cell immunity to respiratory viruses19–23. Serum total IgE levels were positively correlated with numbers of CCR5+CD4+ T cells in BAL fluid (r=0.47, p<0.05)(Fig. 2C), and to a lesser extent in the blood (r=0.45, p=0.06), though statistical significance was not maintained after adjusting for subjects’ age and dose of inhaled corticosteroids (BAL: r=0.46, p=0.07). Analysis of total live T lymphocytes in BAL fluid showed a lower ratio of CD4+:CD8+ T cells in the non-allergic group that was not significant (gm=0.60 vs. 1.01, p=0.25)(Fig. E3B). Expression of CD45RO and CCR5 on CD8+ T cells in BAL fluid was comparable with CD4+ T cells (Fig. 2A, and Fig. E3C). Together, these findings suggest recruitment of Th1-like cells in severe asthma, irrespective of allergic status.
Figure 2. Surface Signature and Intracellular Cytokine Expression in CD4+ T Cells in the Peripheral Blood and BAL Fluid of Children with Severe Asthma.
(A) Comparison of the percentage of total CD4+ T cells expressing CD45RO and CCR5 in blood and BAL fluid specimens obtained within the same subject. Horizontal bars denote geometric mean values. (B) Representative scatter plots showing expression of CD45RO and CCR5 on CD4+ T cells. (C) Correlation between serum total IgE and percentages of CCR5+CD4+ T cells in BAL specimens from asthmatic subjects. (D) The percentage of IL-4+, IL-5+, IFN-γ+ and IL-17+ cells within total CD4+ T cells in BAL fluid compared with blood, based on intracellular cytokine staining. (E) Comparison of cytokine-positive CD4+ and CD8+ T cells within the memory subset in BAL fluid. Horizontal bars denote geometric mean values. *p≤0.05, **p<0.01, ***p<0.001, ****p<0.0001.
IFN-γ+ T Cells Dominate BAL Fluid of Children with Severe Asthma
Analysis of intracellular cytokines confirmed a dominant population of IFN-γ+CD4+ T cells in BAL fluid, and their marked augmentation compared with blood (gm=48% vs. 6%, p<0.01)(Fig 2D). Similarly, IL-17A+ cells were enriched in BAL fluid (gm=7% vs. 0.1%, p<0.01), and though generally less frequent than IFN-γ+ T cells, they constituted up to ~40% of CD4+ T cells in some asthmatics. By contrast, the percentage of Th2 cells (IL-4+ or IL-5+) comprised less than 10% of CD4+ T cells in both BAL and blood. Cytokine expression in memory CD4+ T cells in BAL fluid reflected the IFN-γ-dominated profile of total CD4+ T cells (Fig. 2E). Analysis of memory CD8+ T cells in BAL fluid revealed higher IFN-γ and lower expression of IL-4, IL-5 and IL-17A compared with CD4+ T cells, consistent with a dominant type 1 cytotoxic T (Tc1) phenotype (Fig. 2E, and Fig. E4).
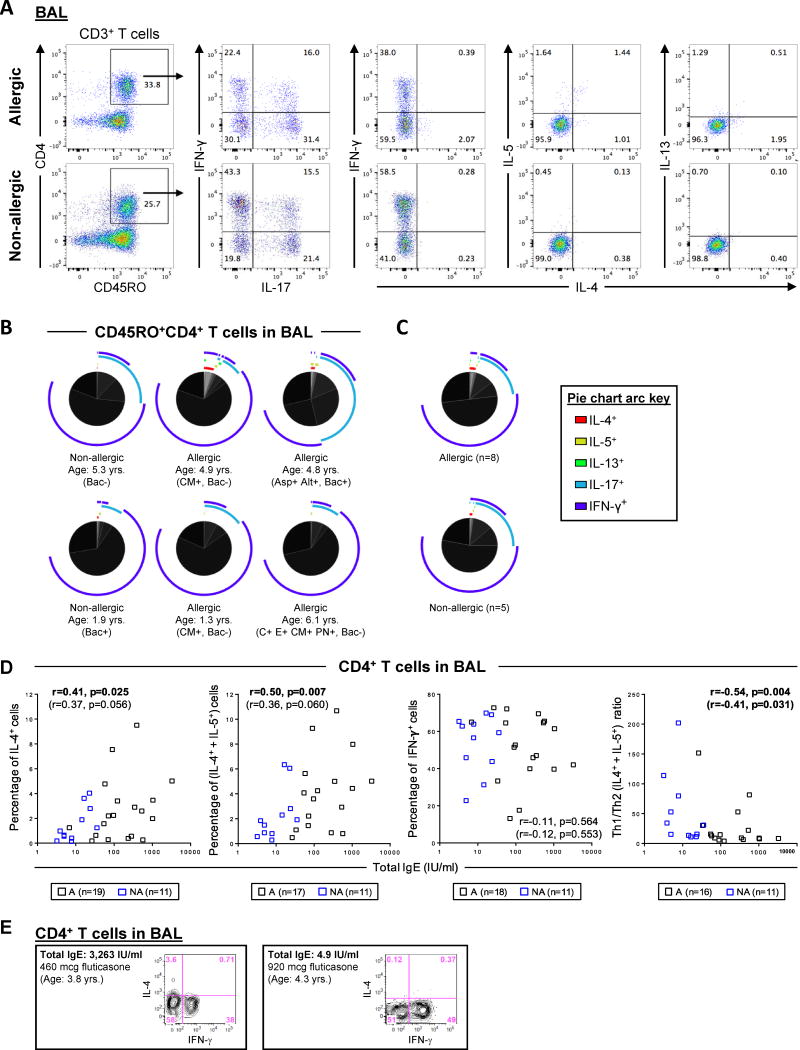
Expansion of the cytokine panel to include IL-13, which is pivotal to airway hyperresponsiveness in models of allergic asthma 24, revealed that IL-13+CD4+ T cells were infrequent (Fig. 3A). By using SPICE software 17 to visualize T-cell heterogeneity according to all 5 cytokines tested, the dominant memory CD4+ T cell subsets were either IFN-γ+ only, IL-17A+ only, or else IFN-γ+IL-17A+ only. This was consistent for subjects with variable age, allergic status, and bacterial culture status (Figs. 3B & C). Infrequent IL-5+ and IL-13+ T cells co-expressed IL-4, though rare cells expressing IL-5 or IL-13 either alone, or in combination with IL-17A or IFN-γ were also detectable (Figs. 3A & B). Collectively, these findings demonstrated that Th1 and Tc1 cells were the dominant cell types in the airway lumen of children with severe asthma.
Figure 3. Heterogeneity of Cytokine-Positive CD4+ T Cells in BAL Fluid and Relationship to Serum IgE.
(A) Representative scatter plots showing IL-4+, IL-5+, IL-13+, IL-17A+ and IFN-γ+ cells within the memory CD4+ T-cell subset in BAL fluid. (B) Pie charts comparing the distribution of cytokine positive subpopulations expressing various combinations of IL-4, IL-5, IL-13, IL-17A and IFN-γ within memory CD4+ T cells present in BAL. Pie charts show data from 6 subjects. Each slice of the pie denotes a single T-cell subpopulation within the memory T-cell subset, and different arc colors denote each cytokine expressed within a given subpopulation. Bacterial culture negative (Bac-) or positive (Bac+). Specific IgE to Cat (C) Aspergillus (Asp), Alternaria (Alt), cow's milk (CM), egg (E), and peanut (PN) is indicated. (C) Summary data for 8 allergic and 6 non-allergic subjects. (D) Correlations between serum total IgE and the percentage of CD4+ T cells expressing Th1 or Th2 cytokines, and Th1/Th2 ratio in BAL fluid. Correlation values in parentheses were adjusted for age and inhaled corticosteroid dose. A, allergic; NA, non-allergic. (E) Representative scatter plots from subjects with high and low IgE showing expression of IL-4 and IFN-γ in CD4+ T cells in BAL T cells.
Th2 Cell Numbers in BAL Fluid Relate to Age and Allergic Status
Serum total IgE was positively correlated with the percentage of IL-4+CD4+ T cells in BAL fluid (r=0.41, p=0.025), as well as total Th2 cells (IL-4+ and/or IL-5+, r=0.50, p=0.007). Moreover, total IgE was inversely correlated with Th1 skewing (r=−0.54, p=0.004). This reflected the increase in Th2 numbers, since IFN-γ+ T cells were not correlated with total IgE (r=−0.11, p=0.564) (Fig. 3D). The relationship with Th1 skewing was maintained when controlling for age and inhaled corticosteroid dose (r=−0.54, p=0.004). Thus, despite their low frequencies, Th2 cells were enriched in BAL fluid of allergic children with severe asthma. No relationship was identified between age or corticosteroid dose with Th types or Th skewing (Figs. 3D & E5).
Both Th1- and Th2-Associated Cytokines Are Elevated in BAL Fluid of Asthmatics Sensitized to Inhalant Allergens
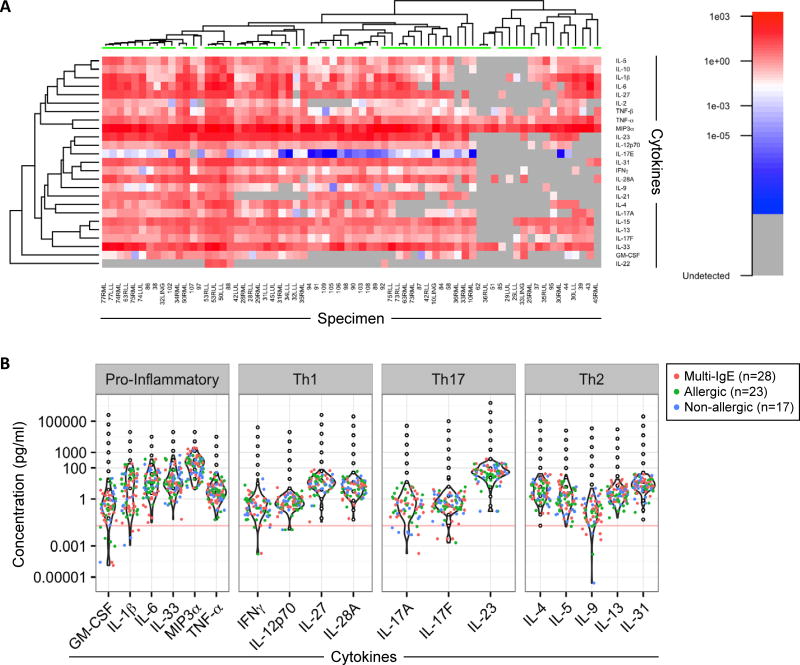
Sixty eight BAL specimens from 48 asthmatics (35 allergic and 13 non-allergic) were available for analysis of a broad array of secreted cytokines by bead-based multiplex assay (Fig. E2). These included specimens obtained from two different lung regions in 20 asthmatics. Heat map analysis revealed variations in cytokine profiles among different subjects (Fig. 4A). However, similar profiles were observed for different lung regions sampled within the same subject (Fig. E6). Pro-inflammatory cytokines expressed by epithelial cells (IL-1β, IL-6, IL-33, and the potent chemoattractant MIP3α/CCL20) were expressed at high levels (Fig. 4B). The most abundant T-cell-associated cytokines expressed were IL-23, IL-27, IL-28A/IFN-λ2, and canonical Th2 cytokines (IL-4, IL-5 and IL-13), as well as IL-31; however, IFN-γ levels were generally low (Fig. 4B). Cluster analyses did not reveal discrete profiles linked to higher levels of Th1- or Th2-associated cytokines.
Figure 4. Signatures of Secreted Cytokines in BAL Fluid of Asthmatics.
(A) Heat map of cytokines in BAL fluid from 68 specimens (includes specimens from 2 different lung regions in 20 subjects). LLL, left lower lobe; RLL, right lower lobe; LUL, left upper lobe; RUL, right upper lobe; RML, right middle lobe; LING, lingular lobe. Samples with undetectable levels are shown in grey. Samples with detectable signals below the standard curve are colored blue to white, while samples within standard curve ranges (>0.02 pg/ml) are scaled red. Green bars (top of heat map) denote samples from allergic subjects. (B) Violin plots of cytokine levels for BAL specimens and relationship to the standard curve (black open dots). Each dot corresponds to the “average” concentration for a given subject derived from all observed MFI values run in at least duplicates and interpolated from the standard curve. Red, green and blue dots correspond to BAL specimens from multi-sensitized (IgE to >3 allergens; 18 subjects), allergic (IgE to 1–3 allergens; 16 subjects) and non-allergic (14 subjects) asthmatics respectively. Horizontal line denotes the lowest value of all standard curves (0.02 pg/ml).
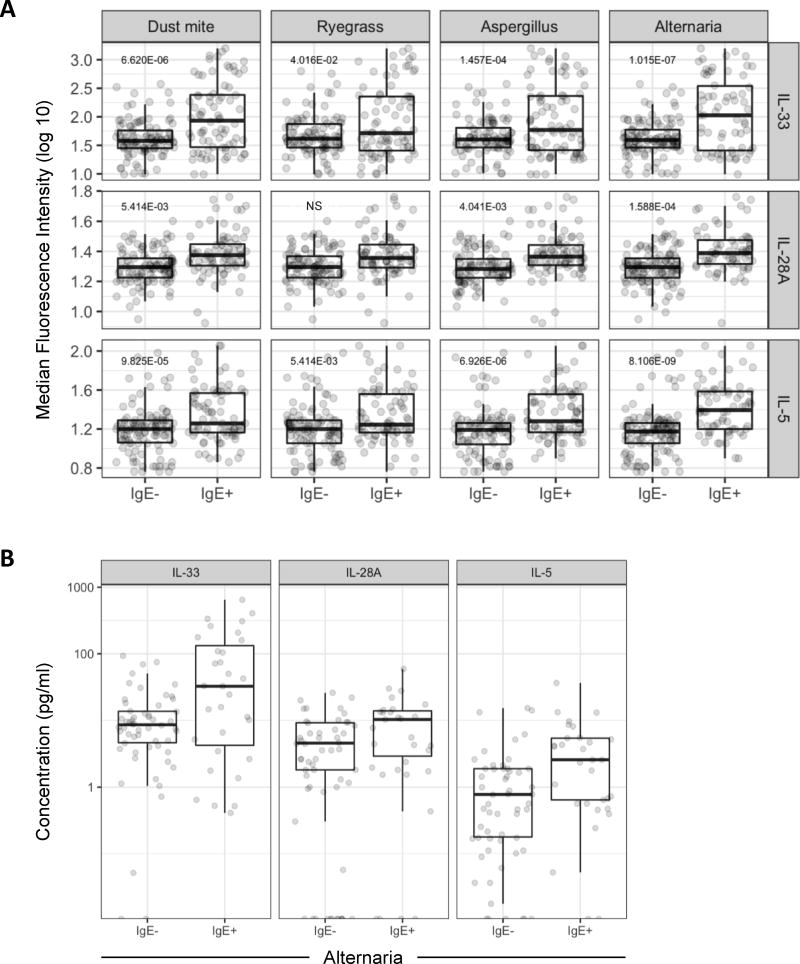
Next, we applied a sensitive and precise linear mixed-effects modeling approach based on median fluorescence intensity values for cytokines, in order to test relationships to patient characteristics (see Statistical Analysis in Online Repository for details) 25. By this strategy, no significant differences in cytokine levels were identified between allergic and non-allergic groups as a whole. By contrast, differences were observed based on the presence of specific IgE. Notably, levels of IL-33, IL-28A/IFN-λ2 and IL-5 were higher among asthmatics who had specific IgE to inhalant allergens including dust mite, ryegrass, Aspergillus fumigatus, and Alternaria alternata, as compared with those who did not, with consistently strong relationships identified for IL-33 and IL-5 (p≤0.0001)(Figs. 5A & B, and Table II). Moreover, specific IgE to Alternaria was associated with increased levels of a broader array of Th1/Th2-promoting cytokines compared with other sensitivities (Table II). No significant relationships were identified for cat, ragweed, or any of the food allergens tested (egg, cow's milk and peanut). Moreover, sensitization to multiple allergens (IgE to >3 allergens) was associated with higher levels of IL-5, IL-33, and IL-28A/IFN-λ2 compared with non-allergic asthmatics (Table II). Analysis of other relationships identified higher expression of the pro-inflammatory cytokines TNF-α and IL-1β, among asthmatics with positive versus negative bacterial cultures (p=0.002 and p=0.042 respectively), and higher levels of IL-5 among older children when testing age as a continuous variable (p<0.001). This latter relationship was maintained within the allergic, but not the non-allergic group (p=0.02 vs. p=0.65 respectively). IL-5 levels were also correlated with blood eosinophil counts (p=0.02) and were higher among those asthmatics who had detectable eosinophils in BAL fluid versus those who did not (p=0.001)(Fig. E7).
Figure 5. Levels of IL-33, IL-28A/IFN-λ2 and IL-5 are Increased in Asthmatics with Specific IgE to Inhalant Allergens.
(A) Box plots of MFI values for cytokines in BAL fluid from asthmatics without and with specific IgE to dust mite, ryegrass, and fungi (Aspergillus and Alternaria). Benjamin Hochberg adjusted P values are denoted. Samples were run in at least duplicates and all MFI values were plotted. (B) Absolute concentration of cytokines in allergic asthmatics with specific IgE to Alternaria. Values are shown for 68 BAL specimens from 48 subjects. Each dot corresponds to the “average” concentration for a given subject derived from all observed MFI values (≥ duplicate runs) and interpolated from the standard curve.
Table II.
Differential Expression of BAL Cytokines According to Allergic Status and Bacterial Culture Status.
| Comparison | Significant Relationships Identified1 |
|---|---|
|
| |
| Allergic Status (IgE+ vs. IgE−) | |
| - Dust mite (Dermatophagoides pteronyssinus) | IL-33****, IL-5****, IL-28A**, IL-17E* |
| - Ryegrass | IL-5**, IL-33* |
| - Aspergillus fumigatus | IL-5****, IL-33***, IL-28A**, TNF-β *, IL-9*, IL-17E* |
| - Alternaria alternata | IL-5****, IL-33****, IL-28A****, IL-6**, IL-17E**, IL-9*, IL-10*, IFN-γ*, IL-27*, IL-17F*, TNF-β* |
| Multi-IgE vs. Non-allergic | IL-5***, IL-33***, IL-28A* |
|
| |
| Bacteria-pos. vs. bacteria-neg. | TNF-α**, IL-1β* |
Based on positive fold change in fluorescence intensity values. Cytokines are listed in order of lowest to highest p value.
p≤0.0001,
p≤0.001,
p≤0.01,
p≤0.05 (adjusted P values).
Cytokines are listed in order of lowest to highest p value.
Type 2 Innate Lymphoid Cells are Present in BAL Fluid
Given our ability to detect IL-4, IL-5 and IL-13 in BAL fluid, despite low numbers of Th2 cells, we sought to identify type 2 innate lymphoid cells (ILC2s) as a potential alternative source. These lineage-negative cells, which reside at the epithelial interface, secrete Th2 cytokines in response to IL-33. Of nine BAL specimens analyzed, 7 had detectable innate lymphoid cells. Six of these specimens contained IL-4+ or IL-13+ ILC2s at low numbers (<2.5% of total live single-cell events) and expression of the ILC2 marker CRTH2, was a feature of these cells (Fig. E8).
Myeloid and Plasmacytoid Dendritic Cells are Present in BAL Fluid
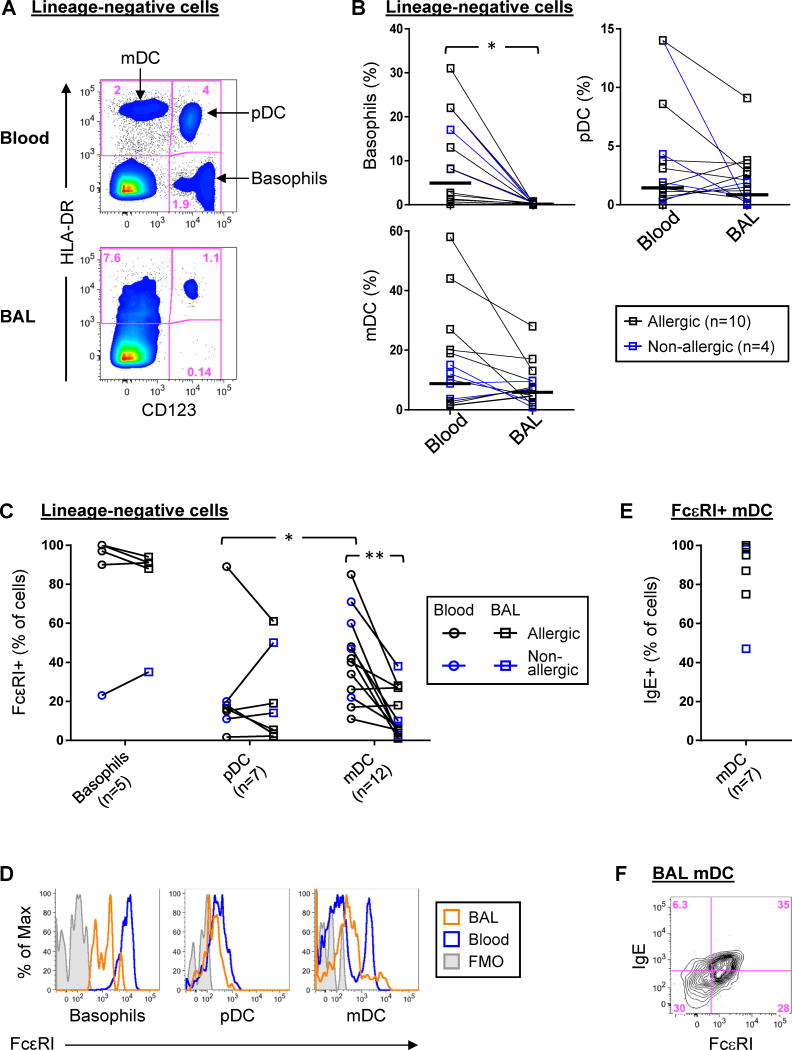
We sought to identify whether dendritic cell types necessary to promote distinct Th subsets were present in the airways. Whereas myeloid DCs (mDC) initiate and maintain Th2 responses in asthma 26, 27, plasmacytoid DCs (pDC) induce Th1 responses through the production of type I and type III interferons 28. Analysis of BAL fluid identified mDC and pDC based on expression of characteristic markers (Figs. 6A, and Fig. E9).29 Whereas the numbers of mDC and pDC were comparable between BAL and blood specimens, basophils, a major innate source of Th2 cytokines, were scarce in BAL fluid compared with blood (Figs. 6A & B). Both pDC and mDC in BAL fluid expressed FcεRI (Figs. 6C & D). Those FcεRI+ mDC in BAL fluid bound surface IgE, despite their lower expression of IgE receptor compared with blood (p=0.002)(Figs. 6C, E & F).
Figure 6. Plasmacytoid and Myeloid DCs are Present in BAL Fluid.
(A) Representative density plots of basophils and DC types within lineage-negative cells in BAL fluid and blood. (B) Percentages of basophils and dendritic cell types within lineage-negative cells of blood and BAL. Horizontal bars denote geometric means. (C & D) Collective data and representative histograms comparing expression of FcεRI on each cell type in blood and BAL. (E) Percentage of FcεRI+ mDC that expressed IgE. Insufficient cell events were available to reliably phenotype pDC. (F) Representative data showing co-expression of IgE with IgE receptor on BAL mDC. Sample sizes varied based on sufficient cell events for analysis. *p≤0.05. **p=0.002. ND, not done owing to insufficient basophils in BAL. FMO, fluorescence minus one control.
Discussion
In a highly characterized cohort of children with severe asthma, we confirm a Th1 signature in the airways, regardless of allergic status. The predominance of Th1 cells in a milieu of cytokines that are commonly ascribed to both Th1- and Th2-mediated responses, deviates from conventional T-cell paradigms. Analysis of cytokine-positive T cells provided a sensitive approach for evaluating Th profiles in BAL fluid, and enrichment of Th1 cells, as well as Tc1 cells, was verified through parallel analysis of blood samples. As potent inducers of IFN-γ, respiratory pathogens are prime candidates for driving Th1/Tc1 responses in children with severe asthma. T-cell expression of the Th1-associated chemokine receptor CCR5, which has been implicated in directing T-cell responses to respiratory viruses, was notable in this regard 21–23. The assessment of infectious microbes in the lower airways was a differentiating feature of our study. Bronchoscopy provides a reliable method for sampling microbes in the lower respiratory tract, and evidence of current or past exposure to respiratory pathogens was common in our study, consistent with the severe asthma phenotype 30,31. This feature may reflect a highly selected patient sample based on treatment failure and poor symptom control. No asthmatics had signs or symptoms of an active infection at the time of bronchoscopy; thus, detection of microbes may indicate a carrier state. Nonetheless, given the epidemiological link between childhood asthma and exposure to respiratory viruses in particular 32, establishment of an IFN-γ-dominated T-cell profile in childhood implies a pathogenic role in the development of severe disease. Moreover, our findings confirm that a Th1 signature is a feature of severe asthma, even in the face of Th2-promoting elements and allergic sensitization. This notion aligns with proof-of-concept work recently reported in mice wherein co-administration of bacterial components with allergen induced severe asthma in a Th1-mediated fashion 10.
After Th1 cells, IL-17+IFN-γ− and IL-17+IFN-γ+ T cells constituted the most abundant CD4+ subsets detected, regardless of allergic status. The role of such cells in severe asthma remains enigmatic; however, there is intriguing new evidence to support an association between “Th17-high” asthma and an altered neutrophil phenotype among adults with severe asthma 33. IL-17+IFN-γ+ T cells have been previously linked to diseases with autoimmune underpinnings 34, 35. These cells can be generated in vitro under Th17-promoting conditions, are more plastic than their single-positive counterparts, and can promote the production of inflammatory mediators by epithelial cells 36. The Th17-promoting cytokine IL-23 has been linked to asthma severity in adults 37. Its high levels in BAL fluid in the present study suggest that the inflammatory milieu might favor the induction of IL-17+IFN-γ+ T cells in situ. Other notable Th1/Th17-associated cytokines detected included IL-27 and IL-28/IFN-λ2. IL-27 is associated with asthma risk and disease severity at both the genetic and protein level 38, 39, and is integral to interferon-mediated networks in virus-induced exacerbations 40. Similarly, the type III interferon, IL-28A/IFN-λ2, which was increased among subjects sensitized to dust mite and fungi in our study, has anti-viral properties and is a potent inducer of IFN-γ in human T cells 41, 42.
A major strength of our study was the use of linear mixed-effects modeling to analyze multiple relationships between cytokine profiles in BAL fluid, allergic status, microbial colonization and age. By applying the algorithm to fluorescence intensity data as opposed to protein concentration values, our approach provided an accurate and sensitive analytical method, while correcting for biological and technical factors that can impact variations in multiplex assay systems 25, 43, 44. Older children in our cohort displayed a high prevalence of sensitization to dust mite, ryegrass, Aspergillus and Alternaria. Increased levels of IL-33 and IL-5 (as well as IL-28A) in BAL fluid from asthmatics sensitized to inhalant, but not food, allergens was a striking finding, suggesting a role for key allergens in driving their secretion, though cat and ragweed were notable exceptions. Exposure to multiple fungal allergens including Alternaria, is emerging as an important factor in asthma hospitalization in children and adolescents with asthma 45. Notably, severe childhood asthma with fungal sensitization has been linked to IL-33 expression in the airways 46, 47. This cytokine, which is secreted by epithelial and endothelial cells in response to tissue damage, is not only a potent Th2-promoting mediator, but contributes to the differentiation and function of Th1 cells 48, 49. Whether IL-33 drives Th1 responses among asthmatic patients sensitized to fungi warrants further study. On the other hand, the Th2 cytokine IL-5 is chemotactic for eosinophils, a feature of the exacerbation-prone asthma phenotype in severe asthma 50. The correlation between BAL IL-5 levels and eosinophil counts in the present study implicate IL-5 in disease pathogenesis, while the rise in IL-5 levels with age is consistent with the evolution of Th2 responses over time. From a broader perspective, our findings highlight the need to re-examine the interplay between immune components that have traditionally been characterized according to the Th1/Th2 dogma.
Despite the robust Th1 signature in BAL fluid, levels of secreted IFN-γ were low, but within the range previously reported in children and adults with severe asthma 51–53. Interestingly, those studies found similar or else lower levels of IFN-γ compared with healthy controls or asthmatics with milder disease 51, 52. Detection of IFN-γ by multiplex assay is context-dependent 54, and may be undermined in BAL fluid by several factors including degradation by the metalloproteinase ADAM17, which is highly expressed in the inflamed airways 55, 56; rapid uptake by airway macrophages 57; and limited dispersion compared with other Th cytokines 58. T-cell exhaustion might also be a factor owing to chronic antigenic activation; however, the ability for IFN-γ+ BAL T cells from adults with severe asthma to secrete high levels of IFN-γ after in vitro stimulation argues against this notion.10 In relation to Th2 cytokines, our ability to detect secreted IL-4 was a notable difference from previous work. ILC2s, which were detected in low numbers and have recently been implicated in severe asthma 5, 47, 59, are unlikely to be a major source of IL-4 in the face of infrequent Th2 cells and scarce basophils. Instead, more abundant cell types such as alternatively activated macrophages, might provide a source 60. Nonetheless, our ability to detect ILCs suggests that their further evaluation is warranted in severe asthma. A key question remains regarding the machinery required for Th1/Tc1 and Th17 cell enrichment in the lungs. We confirmed the presence of mDC and pDC in BAL, both of which can promote differentiation of various T-cell types. The epithelial cell-derived chemoattractant, MIP-3α, which was present at high levels in BAL fluid, may be pivotal to the recruitment of airway dendritic cells and Th17 cells via CCR6 61, 62. Though pDC are principal instigators of IFN-γ responses, the contributions of mDC and the IgE/interferon axis in particular, merit further study 63.
Our study was limited by several constraints. For example, endobronchial biopsies were not available that could yield insight into Th1 abundance in inflamed tissues 64. Similarly, while induced sputum provides a useful non-invasive proxy for studying inflammatory processes in the lungs, its procurement is not feasible in children of pre-school age. Additionally, our study lacked healthy controls, owing to barriers that preclude obtaining BAL specimens for research purposes in children. Asthmatic children not receiving corticosteroids were also not included; however, these subjects would by definition, have milder disease, and thus, constitute a distinct endotype. While an effect of corticosteroids on molecular signatures cannot be excluded in our study, there is scant data to support their Th1 skewing effects 65–68. Indeed, our findings argue against global suppression of Th2 pathways by corticosteroids based on the detection of a variety of canonical Th2 cytokines in both BAL fluid and T cells. On the other hand, Th1 cells can actually mediate corticosteroid resistance in mouse models of asthma 69, thereby supporting their pathogenicity in persistent airway inflammation. From a clinical standpoint, the presence of pro-inflammatory profiles in BAL fluid of children with severe asthma that is resistant to corticosteroids, questions the continued use of this therapy in this context.
In summary, we have confirmed a dominant Th1 signature in BAL fluid of a subset of children with severe asthma that exists from an early age. Nevertheless, the immune phenotypes observed encompassed a spectrum that deviates from established paradigms. Our findings support the consideration of a multi-pronged treatment strategy that may involve modifying effects of exposures to inhalant allergens and respiratory microbes by dual blockade of Th1 and Th2 pathways in early childhood, depending on allergic status. Controlled studies of much larger numbers of children will be necessary to fully elucidate these aspects.
Supplementary Material
Key Messages.
Th1 cells dominate the lower airways of children with severe asthma, regardless of allergic status.
The association between elevated levels of IL-5, IL-33 and IL-28A/IFN-λ2 in the airways, and specific IgE to inhalant allergens, but not foods, implicates key allergens in promoting atypical cytokine profiles.
Th1 cells might provide a therapeutic target for severe asthma in children.
Acknowledgments
Funding: This work was supported by NIH/NIAID R01 AI-052196 and NIH/NIAMS R01 AR-059058 (J. Woodfolk); NIH/NIAID T32 AI-100799 (L. Muehling); and NIH/NHLBI U10 HL 109250 and the University of Virginia Ivy Foundation (W. Gerald Teague).
The authors gratefully acknowledge the efforts of our study coordinator, Denise Thompson-Batt, RRT, for her assistance recruiting and consenting families of children undergoing clinical bronchoscopy and procurement of fresh leftover BAL specimens for this study. The authors thank Joanne Lannigan, MS, and Michael Solga, MS, for technical expertise related to flow cytometry.
Abbreviations
- BAL
bronchoalveolar lavage
- ILC2s
type 2 innate lymphoid cells
- mDC
myeloid dendritic cells
- pDC
plasmacytoid dendritic cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–73. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 2.Chung KF. Targeting the interleukin pathway in the treatment of asthma. Lancet. 2015;386:1086–96. doi: 10.1016/S0140-6736(15)00157-9. [DOI] [PubMed] [Google Scholar]
- 3.Romeo MJ, Agrawal R, Pomes A, Woodfolk JA. A molecular perspective on TH2-promoting cytokine receptors in patients with allergic disease. J Allergy Clin Immunol. 2014;133:952–60. doi: 10.1016/j.jaci.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gauthier M, Ray A, Wenzel SE. Evolving Concepts of Asthma. Am J Respir Crit Care Med. 2015;192:660–8. doi: 10.1164/rccm.201504-0763PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith SG, Chen R, Kjarsgaard M, Huang C, Oliveria JP, O'Byrne PM, et al. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol. 2016;137:75–86. e8. doi: 10.1016/j.jaci.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 6.Chakir J, Shannon J, Molet S, Fukakusa M, Elias J, Laviolette M, et al. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol. 2003;111:1293–8. doi: 10.1067/mai.2003.1557. [DOI] [PubMed] [Google Scholar]
- 7.Al-Ramli W, Prefontaine D, Chouiali F, Martin JG, Olivenstein R, Lemiere C, et al. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol. 2009;123:1185–7. doi: 10.1016/j.jaci.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 8.Holgate ST. Innate and adaptive immune responses in asthma. Nat Med. 2012;18:673–83. doi: 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- 9.Irvin C, Zafar I, Good J, Rollins D, Christianson C, Gorska MM, et al. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol. 2014;134:1175–86. e7. doi: 10.1016/j.jaci.2014.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raundhal M, Morse C, Khare A, Oriss TB, Milosevic J, Trudeau J, et al. High IFN-gamma and low SLPI mark severe asthma in mice and humans. J Clin Invest. 2015;125:3037–50. doi: 10.1172/JCI80911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guilbert TW, Morgan WJ, Krawiec M, Lemanske RF, Jr, Sorkness C, Szefler SJ, et al. The Prevention of Early Asthma in Kids study: design, rationale and methods for the Childhood Asthma Research and Education network. Control Clin Trials. 2004;25:286–310. doi: 10.1016/j.cct.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162:1403–6. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- 13.Fitzpatrick AM, Higgins M, Holguin F, Brown LA, Teague WG, et al. National Institutes of Health/National Heart L. The molecular phenotype of severe asthma in children. J Allergy Clin Immunol. 2010;125:851–7. e18. doi: 10.1016/j.jaci.2010.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wisniewski JA, Commins SP, Agrawal R, Hulse KE, Yu MD, Cronin J, et al. Analysis of cytokine production by peanut-reactive T cells identifies residual Th2 effectors in highly allergic children who received peanut oral immunotherapy. Clin Exp Allergy. 2015;45:1201–13. doi: 10.1111/cea.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baumgarth N, Roederer M. A practical approach to multicolor flow cytometry for immunophenotyping. J Immunol Methods. 2000;243:77–97. doi: 10.1016/s0022-1759(00)00229-5. [DOI] [PubMed] [Google Scholar]
- 16.Maecker HT, Trotter J. Flow cytometry controls, instrument setup, and the determination of positivity. Cytometry A. 2006;69:1037–42. doi: 10.1002/cyto.a.20333. [DOI] [PubMed] [Google Scholar]
- 17.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A. 2011;79:167–74. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fong Y, SK, Yu X. nCal: Nonlinear calibration. 2015 R pacakge version 2015.3–3. [Google Scholar]
- 19.Campbell JJ, Brightling CE, Symon FA, Qin S, Murphy KE, Hodge M, et al. Expression of chemokine receptors by lung T cells from normal and asthmatic subjects. J Immunol. 2001;166:2842–8. doi: 10.4049/jimmunol.166.4.2842. [DOI] [PubMed] [Google Scholar]
- 20.Lazarski CA, Ford J, Katzman SD, Rosenberg AF, Fowell DJ. IL-4 attenuates Th1-associated chemokine expression and Th1 trafficking to inflamed tissues and limits pathogen clearance. PLoS One. 2013;8:e71949. doi: 10.1371/journal.pone.0071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galkina E, Thatte J, Dabak V, Williams MB, Ley K, Braciale TJ. Preferential migration of effector CD8+ T cells into the interstitium of the normal lung. J Clin Invest. 2005;115:3473–83. doi: 10.1172/JCI24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohlmeier JE, Miller SC, Smith J, Lu B, Gerard C, Cookenham T, et al. The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections. Immunity. 2008;29:101–13. doi: 10.1016/j.immuni.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neyt K, GeurtsvanKessel CH, Lambrecht BN. Double-negative T resident memory cells of the lung react to influenza virus infection via CD11c(hi) dendritic cells. Mucosal Immunol. 2016;9:999–1014. doi: 10.1038/mi.2015.91. [DOI] [PubMed] [Google Scholar]
- 24.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–61. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 25.Clarke DC, Morris MK, Lauffenburger DA. Normalization and statistical analysis of multiplexed bead-based immunoassay data using mixed-effects modeling. Mol Cell Proteomics. 2013;12:245–62. doi: 10.1074/mcp.M112.018655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, et al. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity. 2013;38:322–35. doi: 10.1016/j.immuni.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 27.Froidure A, Vandenplas O, D'Alpaos V, Evrard G, Pilette C. Persistence of asthma following allergen avoidance is associated with proTh2 myeloid dendritic cell activation. Thorax. 2015;70:967–73. doi: 10.1136/thoraxjnl-2014-206364. [DOI] [PubMed] [Google Scholar]
- 28.Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat Immunol. 2000;1:305–10. doi: 10.1038/79747. [DOI] [PubMed] [Google Scholar]
- 29.Agrawal R, Wisniewski J, Yu MD, Kennedy JL, Platts-Mills T, Heymann PW, et al. Infection with human rhinovirus 16 promotes enhanced IgE responsiveness in basophils of atopic asthmatics. Clin Exp Allergy. 2014;44:1266–73. doi: 10.1111/cea.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bassis CM, Erb-Downward JR, Dickson RP, Freeman CM, Schmidt TM, Young VB, et al. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. MBio. 2015;6:e00037. doi: 10.1128/mBio.00037-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–13. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson DJ, Gern JE, Lemanske RF., Jr The contributions of allergic sensitization and respiratory pathogens to asthma inception. J Allergy Clin Immunol. 2016;137:659–65. doi: 10.1016/j.jaci.2016.01.002. quiz 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shikotra A, Choy DF, Siddiqui S, Arthur G, Nagarkar DR, Jia G, et al. A CEACAM6-High Airway Neutrophil Phenotype and CEACAM6-High Epithelial Cells Are Features of Severe Asthma. J Immunol. 2017 doi: 10.4049/jimmunol.1600606. [DOI] [PubMed] [Google Scholar]
- 34.Shimizu J, Takai K, Fujiwara N, Arimitsu N, Ueda Y, Wakisaka S, et al. Excessive CD4+ T cells co-expressing interleukin-17 and interferon-gamma in patients with Behcet's disease. Clin Exp Immunol. 2012;168:68–74. doi: 10.1111/j.1365-2249.2011.04543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kebir H, Ifergan I, Alvarez JI, Bernard M, Poirier J, Arbour N, et al. Preferential recruitment of interferon-gamma-expressing TH17 cells in multiple sclerosis. Ann Neurol. 2009;66:390–402. doi: 10.1002/ana.21748. [DOI] [PubMed] [Google Scholar]
- 36.Boniface K, Blumenschein WM, Brovont-Porth K, McGeachy MJ, Basham B, Desai B, et al. Human Th17 cells comprise heterogeneous subsets including IFN-gamma-producing cells with distinct properties from the Th1 lineage. J Immunol. 2010;185:679–87. doi: 10.4049/jimmunol.1000366. [DOI] [PubMed] [Google Scholar]
- 37.Newcomb DC, Cephus JY, Boswell MG, Fahrenholz JM, Langley EW, Feldman AS, et al. Estrogen and progesterone decrease let-7f microRNA expression and increase IL-23/IL-23 receptor signaling and IL-17A production in patients with severe asthma. J Allergy Clin Immunol. 2015;136:1025–34. e11. doi: 10.1016/j.jaci.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chae SC, Li CS, Kim KM, Yang JY, Zhang Q, Lee YC, et al. Identification of polymorphisms in human interleukin-27 and their association with asthma in a Korean population. J Hum Genet. 2007;52:355–61. doi: 10.1007/s10038-007-0123-8. [DOI] [PubMed] [Google Scholar]
- 39.Xie M, Mustovich AT, Jiang Y, Trudeau JB, Ray A, Ray P, et al. IL-27 and type 2 immunity in asthmatic patients: association with severity, CXCL9, and signal transducer and activator of transcription signaling. J Allergy Clin Immunol. 2015;135:386–94. doi: 10.1016/j.jaci.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bosco A, Ehteshami S, Panyala S, Martinez FD. Interferon regulatory factor 7 is a major hub connecting interferon-mediated responses in virus-induced asthma exacerbations in vivo. J Allergy Clin Immunol. 2012;129:88–94. doi: 10.1016/j.jaci.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li B, Xie C, Lin X, Su SB. Interleukin-28A promotes IFN-gamma production by peripheral blood mononuclear cells from patients with Behcet's disease. Cell Immunol. 2014;290:116–9. doi: 10.1016/j.cellimm.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Koltsida O, Hausding M, Stavropoulos A, Koch S, Tzelepis G, Ubel C, et al. IL-28A (IFN-lambda2) modulates lung DC function to promote Th1 immune skewing and suppress allergic airway disease. EMBO Mol Med. 2011;3:348–61. doi: 10.1002/emmm.201100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kreutz C, Bartolome Rodriguez MM, Maiwald T, Seidl M, Blum HE, Mohr L, et al. An error model for protein quantification. Bioinformatics. 2007;23:2747–53. doi: 10.1093/bioinformatics/btm397. [DOI] [PubMed] [Google Scholar]
- 44.Won JH, Goldberger O, Shen-Orr SS, Davis MM, Olshen RA. Significance analysis of xMap cytokine bead arrays. Proc Natl Acad Sci U S A. 2012;109:2848–53. doi: 10.1073/pnas.1112599109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tham R, Vicendese D, Dharmage SC, Hyndman RJ, Newbigin E, Lewis E, et al. Associations between outdoor fungal spores and childhood and adolescent asthma hospitalizations. J Allergy Clin Immunol. 2017;139:1140–7. e4. doi: 10.1016/j.jaci.2016.06.046. [DOI] [PubMed] [Google Scholar]
- 46.Saglani S, Lui S, Ullmann N, Campbell GA, Sherburn RT, Mathie SA, et al. IL-33 promotes airway remodeling in pediatric patients with severe steroid-resistant asthma. J Allergy Clin Immunol. 2013;132:676–85. e13. doi: 10.1016/j.jaci.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castanhinha S, Sherburn R, Walker S, Gupta A, Bossley CJ, Buckley J, et al. Pediatric severe asthma with fungal sensitization is mediated by steroid-resistant IL-33. J Allergy Clin Immunol. 2015;136:312–22. e7. doi: 10.1016/j.jaci.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Komai-Koma M, Wang E, Kurowska-Stolarska M, Li D, McSharry C, Xu D. Interleukin-33 promoting Th1 lymphocyte differentiation dependents on IL-12. Immunobiology. 2016;221:412–7. doi: 10.1016/j.imbio.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baumann C, Bonilla WV, Frohlich A, Helmstetter C, Peine M, Hegazy AN, et al. T-bet- and STAT4-dependent IL-33 receptor expression directly promotes antiviral Th1 cell responses. Proc Natl Acad Sci U S A. 2015;112:4056–61. doi: 10.1073/pnas.1418549112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Denlinger LC, Phillips BR, Ramratnam S, Ross K, Bhakta NR, Cardet JC, et al. Inflammatory and Co-Morbid Features of Patients with Severe Asthma and Frequent Exacerbations. Am J Respir Crit Care Med. 2016 doi: 10.1164/rccm.201602-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bossley CJ, Fleming L, Gupta A, Regamey N, Frith J, Oates T, et al. Pediatric severe asthma is characterized by eosinophilia and remodeling without T(H)2 cytokines. J Allergy Clin Immunol. 2012;129:974–82. e13. doi: 10.1016/j.jaci.2012.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Blic J, Tillie-Leblond I, Tonnel AB, Jaubert F, Scheinmann P, Gosset P. Difficult asthma in children: an analysis of airway inflammation. J Allergy Clin Immunol. 2004;113:94–100. doi: 10.1016/j.jaci.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 53.Hosoki K, Ying S, Corrigan C, Qi H, Kurosky A, Jennings K, et al. Analysis of a panel of 48 cytokines in BAL fluids specifically identifies IL-8 levels as the only cytokine that distinguishes controlled asthma from uncontrolled asthma, and correlates inversely with FEV1. PLoS One. 2015;10:e0126035. doi: 10.1371/journal.pone.0126035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Breen EC, Reynolds SM, Cox C, Jacobson LP, Magpantay L, Mulder CB, et al. Multisite comparison of high-sensitivity multiplex cytokine assays. Clin Vaccine Immunol. 2011;18:1229–42. doi: 10.1128/CVI.05032-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dreymueller D, Uhlig S, Ludwig A. ADAM-family metalloproteinases in lung inflammation: potential therapeutic targets. Am J Physiol Lung Cell Mol Physiol. 2015;308:L325–43. doi: 10.1152/ajplung.00294.2014. [DOI] [PubMed] [Google Scholar]
- 56.Kanzaki H, Shinohara F, Suzuki M, Wada S, Miyamoto Y, Yamaguchi Y, et al. A-disintegrin and metalloproteinase (ADAM) 17 enzymatically degrades interferon-gamma. Sci Rep. 2016;6:32259. doi: 10.1038/srep32259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Celada A, Schreiber RD. Internalization and degradation of receptor-bound interferon-gamma by murine macrophages. Demonstration of receptor recycling J Immunol. 1987;139:147–53. [PubMed] [Google Scholar]
- 58.Huse M, Lillemeier BF, Kuhns MS, Chen DS, Davis MM. T cells use two directionally distinct pathways for cytokine secretion. Nat Immunol. 2006;7:247–55. doi: 10.1038/ni1304. [DOI] [PubMed] [Google Scholar]
- 59.Nagakumar P, Denney L, Fleming L, Bush A, Lloyd CM, Saglani S. Type 2 innate lymphoid cells in induced sputum from children with severe asthma. J Allergy Clin Immunol. 2016;137:624–6. e6. doi: 10.1016/j.jaci.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 60.Draijer C, Boorsma CE, Robbe P, Timens W, Hylkema MN, Ten Hacken NH, et al. Human asthma is characterized by more IRF5+ M1 and CD206+ M2 macrophages and less IL-10+ M2-like macrophages around airways compared with healthy airways. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2016.11.020. In Press. [DOI] [PubMed] [Google Scholar]
- 61.Alcaide P, Maganto-Garcia E, Newton G, Travers R, Croce KJ, Bu DX, et al. Difference in Th1 and Th17 lymphocyte adhesion to endothelium. J Immunol. 2012;188:1421–30. doi: 10.4049/jimmunol.1101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hong GH, Kwon HS, Moon KA, Park SY, Park S, Lee KY, et al. Clusterin modulates allergic airway inflammation by attenuating CCL20-mediated dendritic cell recruitment. J Immunol. 2016;196:2021–30. doi: 10.4049/jimmunol.1500747. [DOI] [PubMed] [Google Scholar]
- 63.Henault J, Riggs JM, Karnell JL, Liarski VM, Li J, Shirinian L, et al. Self-reactive IgE exacerbates interferon responses associated with autoimmunity. Nat Immunol. 2016;17:196–203. doi: 10.1038/ni.3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andersson CK, Adams A, Nagakumar P, Bossley C, Gupta A, De Vries D, et al. Intra-epithelial Neutrophils in Paediatric Severe Asthma are Associated with Better Lung Function. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaur M, Reynolds S, Smyth LJ, Simpson K, Hall S, Singh D. The effects of corticosteroids on cytokine production from asthma lung lymphocytes. Int Immunopharmacol. 2014;23:581–4. doi: 10.1016/j.intimp.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 66.Loza MJ, Foster S, Peters SP, Penn RB. Interactive effects of steroids and beta-agonists on accumulation of type 2 T cells. J Allergy Clin Immunol. 2008;121:750e1–5e3. doi: 10.1016/j.jaci.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 67.Jirapongsananuruk O, Melamed I, Leung DY. Additive immunosuppressive effects of 1,25-dihydroxyvitamin D3 and corticosteroids on TH1, but not TH2, responses. J Allergy Clin Immunol. 2000;106:981–5. doi: 10.1067/mai.2000.110101. [DOI] [PubMed] [Google Scholar]
- 68.Leung DY, Martin RJ, Szefler SJ, Sher ER, Ying S, Kay AB, et al. Dysregulation of interleukin 4, interleukin 5, and interferon gamma gene expression in steroid-resistant asthma. J Exp Med. 1995;181:33–40. doi: 10.1084/jem.181.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang M, Kumar RK, Foster PS. Pathogenesis of steroid-resistant airway hyperresponsiveness: interaction between IFN-gamma and TLR4/MyD88 pathways. J Immunol. 2009;182:5107–15. doi: 10.4049/jimmunol.0803468. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.