Abstract
The acquisition of invasive functions by tumor cells is a first and crucial step toward the development of metastasis, which nowadays represents the main cause of cancer-related death. Cholangiocarcinoma (CCA), a primary liver cancer originating from the biliary epithelium, typically develops intrahepatic or lymph node metastases at early stages, thus preventing the majority of patients from undergoing curative treatments, consistent with their very poor prognosis. As in most carcinomas, CCA cells gradually adopt a motile, mesenchymal-like phenotype, enabling them to cross the basement membrane, detach from the primary tumor, and invade the surrounding stroma. Unfortunately, little is known about the molecular mechanisms that synergistically orchestrate this proinvasive phenotypic switch. Autocrine and paracrine signals (cyto/chemokines, growth factors, and morphogens) permeating the tumor microenvironment undoubtedly play a prominent role in this context. Moreover, a number of recently identified signaling systems are currently drawing attention as putative mechanistic determinants of CCA cell invasion. They encompass transcription factors, protein kinases and phosphatases, ubiquitin ligases, adaptor proteins, and miRNAs, whose aberrant expression may result from either stochastic mutations or the abnormal activation of upstream pro-oncogenic pathways. Herein we sought to summarize the most relevant molecules in this field and to discuss their mechanism of action and potential prognostic relevance in CCA. Hopefully, a deeper knowledge of the molecular determinants of CCA invasiveness will help to identify clinically useful biomarkers and novel druggable targets, with the ultimate goal to develop innovative approaches to the management of this devastating malignancy.
Key words: Liver cancer, Metastasis, Epithelial-to-mesenchymal transition (EMT), Tumor reactive stroma, S100A4
INTRODUCTION
Cholangiocarcinoma (CCA) is a malignancy of the biliary tract, arising from either the intrahepatic (iCCA) or the extrahepatic (eCCA) biliary tree, where the merging point of the second-order bile ducts represents the demarcation limit. CCA accounts for 15%–20% of the primary liver tumors, which are the second most common cause of cancer-related deaths worldwide, being responsible for around 9.1% of total deaths1,2. CCA still carries a dismal prognosis, with a median survival below 2 years and a survival rate of less than 10%3. Furthermore, the incidence of iCCA is markedly increasing, especially in Western countries4. A major problem to tackle when treating CCA is the strong and early invasiveness of the tumor. Indeed, most CCAs are diagnosed when intrahepatic or lymph node metastasization has already occurred, and about two thirds of the patients are thus prevented from undergoing surgical resection, which is currently the only potentially curative treatment available. Unfortunately, even in the surgical cases, recurrence rates are very high (49%–64%), and 5-year survival after resection is below 45%. Of note, the feasibility of orthotopic liver transplantation remains controversial. Indeed, promising results have been obtained, but in a few specialized centers, and only by applying highly restrictive patient selection criteria1,4. Patients who are not eligible for radical surgery are treated with systemic chemotherapy, the results of which are blunted by the known drug resistance of CCA5. To date, combined chemotherapy with gemcitabine and cisplatin represents the first-line treatment in the palliative setting, even though the median overall survival remains below 1 year4,6. Although comprehensive genomic profiling has led to the identification of recurrent molecular alterations driving cholangiocarcinogenesis (see below), there are still no molecular targeted therapies consistently effective in the treatment of CCA, either alone or in combination with conventional chemotherapy7,8.
CCA frequently develops on a background of noncirrhotic liver and often without known risk factors. Nevertheless, several pathological conditions associated with chronic liver inflammation carry an increased risk to develop CCA, including infection with hepatobiliary flukes, hepatolithiasis, primary sclerosing cholangitis, and congenital malformations of the bile ducts (e.g., Caroli’s disease, choledochal cysts). It has now become clear that patients with hepatitis B virus (HBV)- and HCV-related cirrhosis, as well as patients with metabolic syndrome, also are at a higher risk of developing iCCA3,4,9. Although CCA is commonly thought to originate from biliary epithelial cells (cholangiocytes), hepatic progenitor cells (HPCs) and mature hepatocytes have also been proposed as candidate cells of origin, particularly for the intrahepatic variant4,8. CCA pathogenesis is a complex, multistep process, marked by increasing genomic and epigenetic alterations, as well as by deregulation of various signaling networks. In particular, point mutations, copy number variations, and chromosome fusions have been reported to alter the expression of genes regulating key cellular processes, such as DNA repair (e.g., TP53), receptor tyrosine kinase signaling (e.g., KRAS, FGFR2, EGFR), and epigenetic regulation of gene expression (e.g., IDH1, IDH2, ARID1A). In addition, a number of inflammatory [e.g., interleukin-6 (IL-6), transforming growth factor-β (TGF-β)], proliferative [e.g., hepatocyte growth factor (HGF)], and developmental (e.g., Notch, Wnt/β-catenin) pathways have been found to be aberrantly activated since the early stages, supporting the progression from precancerous lesions to full-blown neoplasia4,8,9.
Despite the several efforts made in the last few years to understand the biology of CCA, the intricate network of molecular mechanisms responsible for the early and widespread dissemination of this neoplasm remains elusive. Although a unifying model for CCA invasiveness is still lacking, here we sought to summarize the most relevant findings in this field in an effort to provide the reader with a comprehensive overview of the phenotypic and functional changes experienced by CCA cells during the invasion process, as well as of the main molecular factors promoting the emergence of these proinvasive features.
MECHANISMS OF CANCER CELL INVASIVENESS
Invasiveness is a property of the cancer cell representing the first step toward metastasization. During the invasion–metastasis cascade, cancer cells gradually detach from the primary tumor, disseminate at distance through the vascular and/or lymphatic circulation, and then proliferate within the parenchyma of distant organs. In particular, to leave the primary site of growth, cancer cells must proteolytically perforate the basement membrane that encapsulates the tumor masses, migrate through the extracellular matrix (ECM) of the surrounding stroma, and finally penetrate the intratumoral (neovasculature) or peritumoral vessels10,11. The early stages of the invasion process, namely, the crossing of the basement membrane and the gradual detachment from the primary tumor mass, are typically carried on by large and cohesive cohorts of tumor cells, in the form of clusters, strands, sheets, or cords (collective migration). Although most of the cells within the invading cohort display an overt epithelial phenotype (including the maintenance of cell–cell adhesion), cells located at the leading front release matrix proteases and possess strong migratory abilities11–13. As cancer invasion proceeds, the migrating cell cohorts undergo a further dedifferentiation process, likely triggered by signals derived from the multiple mesenchymal and inflammatory cells populating the tumor stroma. Ultimately, this process results in a gradual loss of epithelial features and a concurrent acquisition of some mesenchymal traits. At this stage, a switch from collective to single-cell migration takes place, strikingly enhancing the efficiency of the invasion proces12,13. In other words, invasive cancer cells partly recapitulate an embryonic program known as epithelial-to-mesenchymal transition (EMT), through which epithelial cells dismantle cell–cell junctions, lose planar and apical–basal polarity, and eventually establish a front–rear axis, thus adopting an elongated, spindle-shaped morphology. These phenotypic changes, accompanied by the ability to degrade the matrix, confer pronounced migratory and invasive properties upon cancer cells14–16. It is worth noting that, despite assuming some mesenchymal properties, invasive cancer cells ultimately retain an overall, background epithelial identity, without actually undertaking a full lineage conversion program that should be based on a more focused, large-scale gene expression reprogramming17.
The mesenchymal mode of invasion is initiated by actin polymerization at the cell front, which induces the formation of sheet-like (lamellipodia) and finger-like (filopodia) cytoplasmic projections interacting with surrounding ECM components. These interactions occur at the level of large, multiprotein adhesive structures known as focal adhesions, which are based on transmembrane integrins, and mechanically connect the ECM to the actin cytoskeleton. The formation of cell protrusions and the continuous turnover of cell–matrix contacts generate strong traction forces that ultimately support cell movement13,18,19. In this context, it is worth mentioning that small GTPases belonging to the Rho family play a prominent role in orchestrating cell motility. Classically, the activation of these intracellular signaling molecules is mediated by guanine nucleotide exchange factors, which exchange protein-bound GDP for GTP, whereas GTPase-activating proteins stimulate GTP hydrolysis, thereby leading to protein inactivation. The most studied Rho proteins are RhoA, Rac1, and Cdc42. With regard to mesenchymal migration, Cdc42 is essential for establishing a front–rear polarity in the direction of movement and inducing filopodia extension, Rac1 promotes lamellipodia formation, and RhoA mediates the maturation of focal adhesions and drives the retraction of the cell rear18,19. In addition to lamellipodia and filopodia, the cell front is also equipped with other actin-rich extensions named invadopodia, wherein matrix proteases tend to cluster and exert their proteolytic activity13. Of note, both Cdc42 and RhoA are markedly involved in invadopodia assembly as well20.
Cancer-associated EMT is believed to play a paramount role in the early stages of carcinoma metastasization by conferring proinvasive features on tumor cells11. Furthermore, the EMT program is also capable of turning on stem cell attributes in cancer cells, including heightened resistance to cell death, as well as self-renewal and tumor-initiating abilities. Therefore, EMT may be necessary not only for the initial spreading of neoplastic cells from the primary bulk but also for a successful metastatic colonization at ectopic sites11,21. Of course, the events described above merely represent a schematic depiction of a gradually evolving and highly dynamic process, during which cancer cells go through a wide spectrum of readily interconvertible phenotypic states, overall enabling the progression from nonmotile to invasive carcinomas. EMT itself is a reversible process; indeed, metastatic colonies eventually tend to restore the expression of epithelial traits that are generally turned off throughout dissemination (e.g., cell–cell junctions) and to concomitantly lose the mesenchymal traits12,13.
THE TUMOR MICROENVIRONMENT
In CCA, the emergence of invasive features is thought to result not only from stochastic mutations occurring throughout the tumor dedifferentiation process but also from the influence of autocrine and paracrine signals permeating the tumor microenvironment17. Indeed, during CCA progression, in an effort to comply with the demanding functional needs of the neoplastic epithelium, the tumor stroma progressively undergoes an overwhelming remodeling that ultimately may favor the invasiveness of tumor cells. The so-called tumor reactive stroma (TRS) is predominantly composed of cancer-associated fibroblasts (CAFs) and tumor-associated macrophages (TAMs), which are embedded in an abnormally remodeled, collagen-rich ECM. Both CAFs and TAMs are able to severely impinge on cancer cell behavior by secreting a variety of cyto/chemokines, morphogens, and growth factors22–24. Although mounting evidence supports the notion that the tumor microenvironment overall promotes the progression to invasive tumor phenotypes (see below), it cannot be excluded that antitumorigenic signals may also come from the TRS, a possibility that should be carefully considered when designing TRS-oriented therapeutic approaches25,26.
CAFs exhibit a chronically activated phenotype and in CCAs are believed to originate from hepatic stellate cells (HSCs) and portal fibroblasts27. Several studies showed that coculture of CCA cells with either CAFs or HSCs resulted in increased cancer cell migration and/or invasiveness in vitro28–31. In addition, Clapéron et al. demonstrated that subcutaneous coinjection of CCA cells with liver myofibroblasts in immunodeficient mice enhanced both tumor growth and the incidence of intrahepatic micrometastases, compared with mice inoculated with cancer cells alone32. Among the plethora of CAF-derived signals able to boost CCA invasiveness, stromal cell-derived factor-1 (SDF-1) [also known as C-X-C chemokine ligand 12 (CXCL12)]30,33 and heparin-binding epidermal growth factor (HB-EGF)32 were proposed to play a major role. CAFs are recruited to the tumor mass by a wide range of soluble factors chronically released by both cancer and inflammatory cells, with platelet-derived growth factor-DD (PDGF-DD) arguably playing a prominent role22,34. The PDGF family consists of five dimeric ligands (PDGF-AA, -AB, -BB, -CC, and -DD) and two receptor tyrosine kinases (PDGFRα and PDGFRβ), which are predominantly involved in tissue repair and wound healing35. Recent studies from our group have shown that, unlike normal cholangiocytes, CCA cells secrete PDGF-DD at high levels, stimulated by the relative hypoxia within the tumor mass. Importantly, PDGF-DD stimulates the chemotaxis of fibroblasts by binding to PDGFRβ on their surface34. Furthermore, PDGF-DD released by CCA cells endows fibroblasts with prolymphangiogenic functions, triggering the secretion of vascular endothelial growth factor-A (VEGF-A) and VEGF-C36.
In CCA tissue, TAMs are predominantly localized at the tumor front37, and their number was shown to positively correlate with the presence of extrahepatic metastases38. Furthermore, incubation of CCA cells with conditioned medium from M2 macrophages increased cancer cell migration while promoting EMT-like changes38. In particular, TAMs are a rich source of matrix metalloproteinases (MMPs), especially MMP-939, and of tumor necrosis factor-α (TNF-α)40.
CYTOKINES AND GROWTH FACTORS FAVORING CHOLANGIOCARCINOMA INVASIVENESS
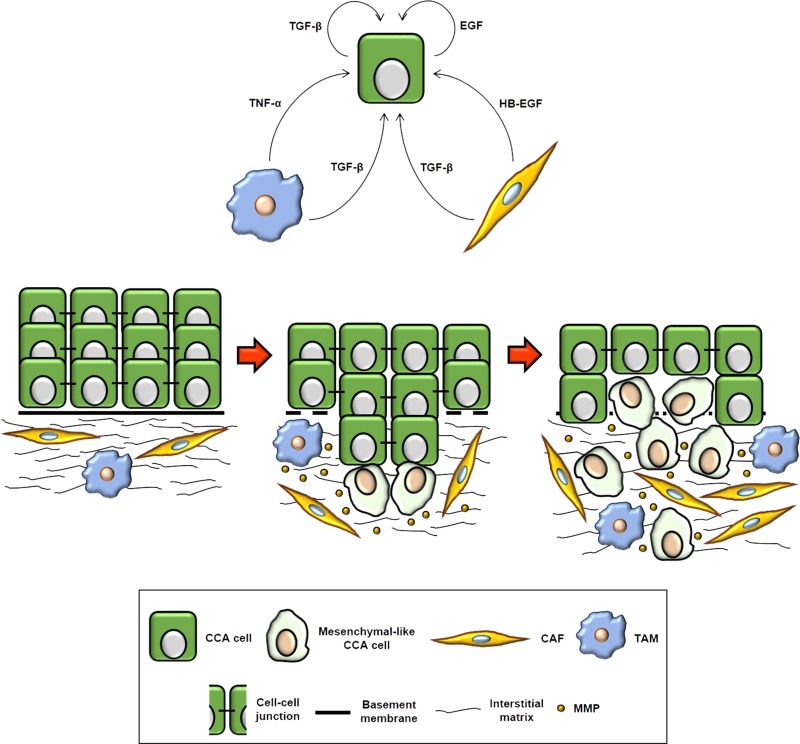
Regardless of the cellular source (i.e., reactive stromal or cancer cells), a wide range of soluble factors coupled with their respective cognate receptors are able to fuel the invasive attitude of CCA cells (as summarized in Table 1). Among them, the most extensively elucidated are TGF-β1, TNF-α, and EGF–like family members (Fig. 1).
Table 1.
Secreted Factors Promoting Cholangiocarcinoma (CCA) Cell Invasiveness
| Proinvasive Factors | References |
|---|---|
| Inflammatory cyto/chemokines | |
| CXCL12/SDF-1 | 30,33,66–68 |
| HMGB1 | 188 |
| IL-6 | 157,189 |
| TGF-β | 45,47–50 |
| TNF-α | 55–57,59,64,65 |
| Growth factors | |
| EGF-like family | 32,70,73,74 |
| FGF-19 | 156 |
| HGF | 51,52 |
| Hormones | |
| Adrenomedullin | 190 |
| Prostaglandin E2 | 191–193 |
| 17β-estradiol | 194 |
CXCL12, C-X-C motif chemokine ligand 12; SDF-1, stromal cell-derived factor-1; HMGB1, high-mobility group box 1; IL-6, interleukin-6; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α; EGF, epidermal growth factor; FGF-19, fibroblast growth factor-19; HGF, hepatocyte growth factor.
Figure 1.
Cellular mechanisms underlying cholangiocarcinoma (CCA) invasion and main soluble mediators involved in the process. Primary cancer lesions are lined by an altered yet intact basement membrane, which keeps CCA cells physically separate from the neighboring stroma, predominantly consisting of cancer-associated fibroblasts (CAFs), tumor-associated macrophages (TAMs), and endothelial cells, embedded in an abnormally stiff interstitial matrix. However, in the course of cancer progression, the basement membrane is progressively dismantled, and CCA cells start migrating collectively in a coordinated manner to pervade the surrounding tissue, thus reinforcing their interplay with reactive stromal cells. At this stage, despite an enhanced motility and a broad secretion of matrix metalloproteinases (MMPs), most of the cells within the invading cohort overall retain epithelial features, including cell–cell adhesion. As cancer invasion proceeds, autocrine and paracrine [i.e., tumor reactive stroma (TRS)-derived] signals permeating the tumor microenvironment lead CCA cells, especially those located at the leading front, to finally adopt a mesenchymal-like phenotype, which allows them to move individually and more efficiently across the stroma toward the blood or lymphatic vessels. In addition to directly providing CCA cells with proinvasive stimuli, CAFs and TAMs also aplenty produce MMPs, thereby further supporting the process of local invasion. EGF, epidermal growth factor; HB-EGF, heparin-binding EGF; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α.
Transforming Growth Factor-β1
TGF-β is a potent pleiotropic cytokine existing in three different isoforms (TGF-β1, -β2, and -β3), among which TGF-β1 is the best characterized. Upon being secreted as a latent complex and possibly stored within the ECM, TGF-β is locally activated by several factors (e.g., integrins, proteases), so that it can induce dimerization of type I and type II TGF-β receptors, eventually triggering Smad-dependent (canonical) or Smad-independent (noncanonical) pathways41. Besides participating in liver morphogenesis by intimately cooperating with key developmental pathways (e.g., Notch, Wnt, Hippo), TGF-β signaling is also indispensable for adult liver homeostasis and is actively involved in the regulation of tissue repair. Upon liver injury, TGF-β is markedly upregulated in different cell types (mainly HSCs and macrophages, but even reactive cholangiocytes), and activation of its signaling in chronic liver damage closely contributes to disease progression by stimulating persistent HSC activation and triggering hepatocyte death42–44. In liver cancer, TGF-β acts as an effective tumor suppressor during the early stages of carcinogenesis by inducing cell cycle arrest and apoptosis in nascent malignant cells. However, TGF-β behaves as a potent tumor promoter at later stages, when cancer cells become insensitive to its growth-inhibitory effects41,43.
In a panel of 78 CCA patients, neoplastic bile ducts were found to be TGF-β1+ in about half (37 cases), and high expression of TGF-β1 correlated with lymph node metastasis, lymphovascular invasion, distant metastasis, and tumor recurrence. Furthermore, multivariate analysis revealed that TGF-β1 expression was an independent predictor of overall survival45. It is worth noting that TGF-β is a prototypical EMT inducer46. Consistently, upon TGF-β1 treatment, CCA cells adopted a fibroblast-like morphology and acquired pronounced migratory and invasive capabilities, associated with the downregulation of epithelial markers [i.e., E-cadherin, cytokeratin 19 (CK19)] and the upregulation of mesenchymal-related genes (i.e., Snail, Twist, N-cadherin, vimentin, S100A4, MMP-2)47–49. Conversely, either knockdown or pharmacological inhibition of TGF-β1 significantly impaired CCA cell migration50. Furthermore, overexpression of TGF-β1 in a syngeneic rat model orthotopic of the human CCA resulted in larger tumor volume and increased number of metastatic foci50, whereas the administration of recombinant TGF-β1 in a CCA xenograft mouse model led to larger tumor mass and enhanced cancer cell dissemination47. Recently, it has been found that TGF-β1 negatively regulates the expression of microRNA-34a (miR-34a), thereby leading to the overexpression of the proto-oncogene c-Met50, whose activation by HGF may further stimulate CCA cell invasion51,52.
Tumor Necrosis Factor-α
TNF-α is a pleiotropic cytokine belonging to the TNF superfamily, predominantly expressed by macrophages, T and B lymphocytes, and natural killer cells. Soluble and membrane-bound TNF-α isoforms bind to either TNF receptor 1 (TNFR1) or TNFR2, whose activation modulates critical cell responses, including the balance between proliferation and apoptosis. The biological effects of TNF-α are thought to be mostly mediated by TNFR1, whose expression is almost ubiquitous. Conversely, TNFR2 is solely expressed by endothelial and immune cells. Physiologically, TNF-α acts as a proinflammatory cytokine, essential for innate immune responses and immune surveillance against cancer. However, aberrant TNF-α activity, due to either excessive production or increased cell responsiveness, can exacerbate a variety of disease conditions, including cancer development and progression53,54.
In CCA, TNF-α was originally reported to promote cancer cell invasiveness by enhancing the production of MMP-9 in a nuclear factor-κB (NF-κB)-dependent manner, through the activation of p38 mitogen-activated protein kinase (MAPK) and ERK1/2 (also known as p44/42 MAPK) signaling cascades55–58. In particular, focal adhesion kinase (FAK)59 and cyclooxygenase-2 (COX-2)56 were both indicated as downstream effectors of the proinvasive action of TNF-α. FAK is a nonreceptor tyrosine kinase predominantly localized to focal contacts, wherein it regulates adhesion-dependent cell motility downstream of integrins and growth factor receptors. In CCA cells, upon TNF-α stimulation, FAK is potently activated and likely acts as an upstream regulator of MAPKs signaling to induce MMP-9 expression59–61. On the other hand, COX-2 is a myeloperoxidase catalyzing the conversion of arachidonic acid into prostaglandin H2 (PGH2), which is then converted into five major prostanoids (e.g., PGE2) mediating several biological activities. Unlike COX-1, which displays a widespread constitutive expression, COX-2 is an inducible COX isoform, and its overexpression predicts poor outcome in several carcinomas62,63. In CCA cells, TNF-α strongly promotes COX-2 expression, thereby leading to the hypersecretion of PGE2, which eventually triggers MMP-9 production by binding to specific G-protein-coupled receptors (GPCRs)56. Besides enabling CCA cells to release active MMP-9, TNF-α was also reported to endow CCA cells with mesenchymal/proinvasive features by upregulating the transcription factors Snail and ZEB264,65. Furthermore, TNF-α treatment induced CCA cells to overexpress both TGF-β65 and CXCR440, the cognate receptor of SDF-1, thereby locking malignant cholangiocytes in a self-sustaining proinvasive loop30,33,66–68.
Epidermal Growth Factor-Like Family
EGFR, also known as ErbB1 or HER1, is a typical tyrosine kinase cell membrane receptor belonging to the ErbB family, together with ErbB2/HER2, ErbB3/HER3, and Erb4/HER4. EGFR supervises over a multitude of cellular processes and is often overexpressed or constitutively activated in several carcinomas, thus playing a prominent role during tumor development and progression69. In CCA, EGFR upregulation mediated by DNA copy number gain is a relatively frequent event, generally correlating with poor survival and tumor recurrence7. Moreover, EGFR overexpression in CCA cells is thought to be further supported by the cytoplasmic delocalization of ezrin–radixin–moesin-binding phosphoprotein (EBP)5070, a scaffold protein that is generally expressed at the apical region of epithelial cells, wherein it connects target transmembrane proteins (e.g., ion transporters, receptor tyrosine kinases, adherens junction proteins) to the cortical cytoskeleton71. In CCA specimens, membranous expression of EBP50 is frequently lost, and EBP50 cytoplasmic levels positively correlate with both EGFR expression and the incidence of intrahepatic metastases. Consistently, in CCA cells, EBP50 knockdown stimulated EGFR expression at the cell surface and enhanced cell motility in an EGFR-dependent fashion70. Importantly, CCA cells not only overexpress EGFR but also lose the ability to rapidly internalize it upon ligand binding, leading to a prolonged, overactivation of EGFR signaling72.
EGFR activation is well known to promote the emergence of EMT-like changes in cancer cells69. Indeed, treatment of CCA cells with the prototypical EGFR ligand EGF downregulated E-cadherin, membranous β-catenin, zonula occludens-1 (ZO-1), desmoplakin, and CK19, while upregulating N-cadherin, vimentin, S100A4, α-smooth muscle actin (α-SMA), fibronectin, MMP-1, and MMP-9. These changes were mediated by an ERK1/2- and signal transducer and activator of transcription 3 (STAT3)-dependent activation of Slug and ZEB1 and, overall, resulted in a more motile phenotype, as clearly evidenced by lamellipodia protrusion, enhanced focal adhesion turnover (via FAK activation), and higher migration speed70,73,74. Consistent with these in vitro findings, EGFR blockade by its ATP-competitive inhibitor gefitinib reduced tumor dissemination in mice bearing subcutaneous CCA xenografts, an effect associated to a membranous relocalization of E-cadherin in cancer cells32,74. Besides starting the EMT machinery, EGF may also foster CCA cell motility by inhibiting the nuclearization of the transcription factor forkhead box O4 (FOXO4) via activation of phosphoinositide 3-kinase (PI3K)/Akt signaling. Indeed, reduced nuclear import of FOXO4 results in an impaired expression of annexin A8 (ANXA8)73, a protein that modulates the interaction between the membrane and the cytoskeleton by binding to F-actin75. In CCA cells, EGF-induced ANXA8 downregulation enhanced the formation of actin stress fibers, leading to cell scattering and pronounced invasiveness, both in vitro and in vivo73.
Whether EGFR activation in CCA cells mainly relies on autocrine (i.e., from cancer cells)70 or paracrine (i.e., from CAFs)32 release of EGFR ligands remains yet to be determined. In this regard, it is worth mentioning that in addition to EGF and HB-EGF, which are abundantly expressed by CCA cells74 and CAFs32, respectively, several other growth factors can bind to EGFR, namely, TGF-α, amphiregulin, epiregulin, betacellulin, and epigen76.
NOVEL SIGNALING MECHANISMS AND TRASCRIPTION FACTORS PROMOTING THE MOTILITY AND INVASIVENESS OF CHOLANGIOCARCINOMA CELLS
A variety of intracellular mechanisms underpin the migratory and invasive properties of CCA cells, depending on the fine integration of a wide range of molecules mostly operating within the EMT machinery. Among them are transcription factors belonging to morphogenetic signaling pathways that are active during biliary development and become reactivated during liver repair and carcinogenesis, including Hedgehog77, Wnt68,78, and, notably, Notch79–83 signaling. Yes-associated protein (YAP)/TAZ are less-characterized transcriptional coactivators critically involved in organ development that are drawing much attention in cancer research and thus will be discussed in detail. Moreover, a wide range of additional putative proinvasive factors have been recently identified, though their upstream modulation, mechanism of action, and functional relationship with EMT are less understood. In some cases, it is even unclear whether their up- or downregulation is actually involved in the induction of an invasive phenotype or rather represents a mere surrogate of an otherwise ongoing mechanism. We must also be aware that chemotherapy may paradoxically reinforce the invasive functions of CCA cells by fueling the activation of proto-oncogenic signaling pathways, likely as part of cellular stress responses induced by DNA damage. Indeed, prolonged exposure of CCA cells to increasing concentrations of gemcitabine led to a substantial increase in migration and invasion, an effect coupled with the aberrant activation of the FAK/MAPK/NF-κB axis, which is eventually responsible for enhanced MMP-9 production84. Similarly, long-term exposure of CCA cell lines or xenografts to cisplatin induced the overactivation of EGFR and the upregulation of L1 cell adhesion molecule (L1CAM)85, a transmembrane glycoprotein supporting the migratory and invasive potential of neoplastic cholangiocytes86. Unfortunately, the modulatory effects of genotoxic therapies further complicate the characterization of the wide and heterogeneous range of molecular mechanisms through which CCA cells gradually adopt an invasive phenotype.
Herein we focus the discussion on those factors that are functionally linked to CCA invasiveness, bear prognostic significance, and potentially are amenable to therapeutic modulation.
Notch
The Notch system is composed of four single-pass transmembrane receptors (Notch-1, -2, -3, and -4) and five membrane-bound ligands, belonging to either the Serrate/Jagged (Jag-1 and -2) or the Delta-like (Dll-1, -3, and -4) family. Upon ligand-receptor binding, the γ-secretase complex unleashes the Notch intracellular domain (NICD) by proteolytic cleavage, thus allowing for its translocation into the nucleus. Here NICD regulates the transcription of Notch target genes, including hairy and enhancer of split 1 (Hes1) and Hes related with YRPW motif 1 (Hey1), before undergoing a rapid proteasomal degradation. Notch signaling is evolutionarily conserved and is mainly involved in the development of organs and tissues by directing the lineage commitment of progenitor cells. In the liver, Notch pathway is essential both in the embryo, by orchestrating the morphogenesis of intrahepatic bile ducts, and postnatally, by modulating progenitor cell-driven liver repair87–89. Defective Notch signaling is associated with Alagille syndrome (a developmental biliary disease characterized by bile duct paucity) and with altered biliary repair upon liver damage90,91. As chronic deregulation of tissue repair mechanisms is often a prerequisite of carcinogenesis, it is not surprising that persistent activation of Notch signaling is involved in the pathogenesis of several human epithelial cancers, including those of liver origin87–89. For instance, in experimental mouse models, combinatorial interaction between a sustained hepatic Notch1 activation and other pro-oncogenic stimuli (e.g., Akt overactivation, thioacetamide treatment) drives a pathological transdifferentiation of mature hepatocytes into cholangiocytes leading to CCA development, in a sort of “guilt in association”92–94. It is worth noting that in the adult liver, all Notch ligands and receptors are expressed at the transcriptional level, in both the epithelial and mesenchymal (e.g., HSCs) compartments, but their cell-specific localization is not always clear, and furthermore, their expression levels can markedly change during liver injury87–89.
In CCA tissue, bile ducts express Notch1 at higher levels compared with either peritumoral areas or normal liver, whereas Notch2, -3, and -4 are not differentially expressed80,81,95. Consistently, immunoblot analyses revealed that in normal cholangiocytes, the expression of Notch1 is generally lower than in CCA cell lines80. Inflammatory mediators such as inducible nitric oxide synthase may be partially responsible for Notch1 upregulation, via activation of JNK1/2 signaling95. It is also important to underline that in CCA cells, Notch signaling can be persistently activated even in lack of exogenous stimuli, as demonstrated by Hes1 and Hey1 expression in untreated cells, likely implying an autocrine stimulation of Notch receptors, which might be mediated by Jag-179,83. At the functional level, it was shown that forced overexpression of Notch1 significantly promoted CCA cell migration, along with prototypical EMT-like changes (i.e., downregulation of E-cadherin, upregulation of vimentin and α-SMA)80. Conversely, either Notch1 knockdown by siRNA80,81 or γ-secretase inhibition by DAPT79 {N-[N-(3,5-difluorophenacetyl)-1-alanyl]-S-phenylglycine t-butyl ester} prominently impaired the migratory and invasive abilities of CCA cells. In addition, upon DAPT treatment, CCA cells increased the expression of E-cadherin and β-catenin while reducing the expression of vimentin and Snail79. Of note, the pro-oncogenic role of Notch1 was attributed to its close cooperation with the transcription factor sex-determining region Y (SRY)-box 9 (Sox9), a well-known Notch target gene critical for biliary specification of HPCs87. Interestingly, progressive upregulation of nuclear Sox9 expression correlated with the evolution from precancerous lesions to full-blown CCA. Moreover, in CCA patients, high Sox9 expression in tumor cells was associated with ductal invasion and low survival rates. In CCA cells, forced Sox9 upregulation boosted cell migration and invasion, decreased E-cadherin expression, and increased vimentin and α-SMA expression, whereas Sox9 silencing dampened the migratory/invasive capabilities, similarly to what was observed with Notch1 manipulation82. Recently, aspartate β-hydroxylase (ASPH) has been identified as a critical upstream regulator of Notch1 transcriptional activity in CCA83. ASPH is a type 2 transmembrane protein that catalyzes the hydroxylation of EGF-like domains in several target proteins, including Notch1 and Jag-1, thereby modulating their function (e.g., by increasing protein stability)96. In CCA cells, shRNA-mediated downregulation of ASPH substantially blunted Notch signaling, as well as cell migration, both in vitro and in vivo83. Importantly, increased ASPH expression was noticed at the invasive front of CCA specimens, where high levels of ASPH expression correlated with vascular invasion and poor prognosis97.
Nuclear S100A4
S100A4 is a member of the S100 family of calcium-binding proteins, which interact with and modulate the function of substrate proteins without possessing any intrinsic enzymatic activity. S100A4 can be localized either in the cytoplasm or in the nucleus and predominantly exists as a homodimer. In addition, S100A4 oligomers can be released into the extracellular space, where they interact with specific surface receptors [e.g., receptor for advanced glycation end products (RAGE), ANXA2] to modulate intracellular signaling. In physiological contexts, S100A4 is almost exclusively expressed by motile cells, such as fibroblasts, macrophages, and leukocytes. Indeed, S100A4 is strongly involved in the regulation of cell movement, as exemplified by its close interaction with key cytoskeletal proteins such as actin, non-muscle myosin IIA (NMIIA), and tropomyosin98. In addition, recent evidence suggests that S100A4 may act independently as a transcription factor to stimulate the synthesis of matrix proteases99.
Aberrant expression of S100A4 by neoplastic cells was found to correlate with aggressive clinical parameters in several tumors and is currently regarded as an early event in cancer-associated EMT98. However, most of the studies in this field did not actually examine the subcellular localization of S100A4. In this regard, studies from our group showed that a large fraction of CCA expressed S100A4 in the nucleus of neoplastic cholangiocytes. In particular, this subset of patients was characterized by a worse outcome in terms of both metastasis development and survival after surgical resection100. Importantly, we further demonstrated that nuclearization of S100A4 is not merely a prognostic indicator, but rather a mechanistic determinant of the invasive phenotype of CCA cells. Indeed, either the downregulation of S100A4 by lentiviral silencing or the interference with its nuclear import by paclitaxel at nanomolar doses significantly reduced the migratory and invasive capabilities of CCA cells without affecting cell proliferation, viability, and apoptosis in vitro100,101. Furthermore, pharmacological inhibition of S100A4 nuclearization markedly blunted the activation of RhoA and Cdc42, the secretion of active MMP-9, and the expression of membrane-type 1 (MT1)-MMP101. Unlike secreted MMPs, MT1-MMP (also known as MMP-14) is tethered to the plasma membrane through a transmembrane domain and is capable of both degrading pericellular ECM components (e.g., fibrillar collagens) and proteolytically processing adhesion molecules, growth factors, and inactive precursors of other MMPs, including pro-MMP-2 and pro-MMP-13102. Interestingly, a massive upregulation of both MT1-MMP and secreted MMPs is required for efficient maturation of invadopodia, and moreover, the interplay between RhoA and Cdc42 is known to closely regulate the targeted delivery of MT1-MMPs to this intracellular location20,103. Overall, this suggests that the nuclear import of S100A4 might be of crucial importance for the assembly of functional invadopodia in CCA cells, even though the precise mechanisms underlying this phenomenon remain yet to be elucidated. In line with the in vitro findings, metronomic infusion of low-dose paclitaxel in mice bearing orthotopic CCA xenografts inhibited S100A4 nuclear entry in engrafted cancer cells, an effect associated with a decreased hematogenous metastasization to the lungs. Conversely, the growth of the primary tumor was not affected. Importantly, the selective reduction in S100A4 nuclear expression by low-dose paclitaxel was shown to rely on the inhibition of SUMOylation, a posttranslational modification that we identified as the main contributor to S100A4 nuclear import in CCA cells101. Indeed, the covalent attachment of SUMO modifiers essentially affects the intramolecular and intermolecular interactions of substrate proteins, thereby altering their activity, subcellular localization, or stability. Functionally, SUMOylation plays a paramount role in the regulation of gene transcription, since most of its targets are transcription factors, transcriptional coregulators, chromatin-remodeling proteins, and DNA repair enzymes104. As the expression of SUMO-conjugating enzymes is frequently deregulated in cancer105 and several EMT-inducing transcription factors are well-known targets of the SUMOylation machinery106, it is tempting to speculate that SUMOylation may be a driving force behind the plasticity of invasive carcinoma cells. Overall, these findings are paradigmatic of how the prognostic significance of a tumoral biomarker can be harnessed for therapeutic perspectives.
Sal-Like Protein 4
Sal-like protein 4 (SALL4) is a transcription factor abundantly expressed in the liver. Specifically, it is expressed by hepatoblasts during fetal development and by HPCs nearby the peribiliary glands in the adult tissue. Under physiological conditions, SALL4 is not expressed by mature hepatocytes and cholangiocytes107. In 175 CCA samples, SALL4 immunoreactivity was found in 102 cases and positively correlated with lymph node metastasis, vascular invasion, and perineural invasion. Moreover, high expression of SALL4 correlated with poor overall survival108. In CCA cells, SALL4 silencing significantly impaired cell migration and invasion and markedly counteracted the E/N-cadherin switch typically occurring during EMT, while repressing vimentin and fibronectin expression108,109. Interestingly, the proinvasive functions of SALL4 were shown to be dependent on the downregulation of the tumor suppressor phosphatase and tensin homolog (PTEN), as well as on the upregulation of the oncoprotein BMI-1109. PTEN is a tyrosine phosphatase that negatively regulates Akt activity, whereas BMI-1 is a ring finger protein that fosters the activation of Wnt signaling by both repressing the expression of Wnt inhibitors [i.e., members of the Dickkopf (DKK) family] and stimulating the expression of Wnt ligands (e.g., Wnt3a)110,111. Consistently, in CCA cells, both PI3K/Akt and Wnt/β-catenin signaling were turned off upon SALL4 silencing109. Importantly, both pathways promote CCA metastasization68,78,112.
Protein Tyrosine Phosphatases
The protein tyrosine phosphatase (PTP) superfamily encompasses 107 enzymes that are further divided into four classes according to the amino acid sequence of their catalytic domain. Class I is the most numerous and is composed of classical PTPs, which include both receptor (PTPRs) and nonreceptor (PTPNs) types, and the so-called Dsp family, which includes seven different subgroups, among which are PTENs, phosphatases of regenerating liver (PRLs), and MAPK phosphatases (MKPs). Class II, III, and IV consist of acid phosphatase 1 (ACP1), the cell division cycle 25 (CDC25) family, and the eyes absent homolog (EYA) family, respectively. PTPs govern a huge variety of biological processes mostly critical for oncogenesis, such as proliferation, apoptosis, differentiation, motility, and metabolic homeostasis, by directly modulating the function of receptor (e.g., EGFR) and nonreceptor (e.g., Src) tyrosine kinases, adaptor proteins [e.g., growth factor receptor-bound protein 2 (GRB2)], transcription activators (e.g., STAT3, β-catenin), cyclin-dependent kinases (CDKs), and focal adhesion-associated proteins (e.g., integrins, FAK). Genetic or epigenetic changes involving PTP genes are frequently reported in human malignancies, and several PTPs have been shown to function as oncogenes or tumor suppressors113.
In 124 CCA specimens, 51 harbored PTPN3 mutations, which correlated with a reduced recurrence-free survival of patients. In addition, a stark increase in the immunohistochemical expression of PTPN3 was observed in neoplastic bile ducts compared with the peritumoral biliary epithelium, and high PTPN3 protein levels were associated with increased tumor recurrence after surgery. Accordingly, in PTPN3 wild-type CCA cells, PTPN3 knockdown dramatically dampened cell migration, whereas forced overexpression of wild-type or, even more, mutant PTPN3 considerably stimulated cell motility. Overall, this has lent support to the notion that PTPN3 may act as a potent oncoprotein in CCA and that certain somatic mutations could further reinforce its prometastatic activity, though the underlying molecular mechanisms need to be unveiled114. In this regard, studies in breast cancer cells showed that PTPN3 promotes EGFR expression by inhibiting its proteasome-dependent degradation115. Interestingly, PTPN3 mutations harbored by CCA patients are not thought to result in increased gene expression, but rather to affect protein localization or substrate binding114.
In addition to PTPN3, two other PTPs, namely, PRL1 and EYA4, have been suggested to be involved in CCA progression. In particular, PRL1 is overexpressed by neoplastic bile ducts and promotes EMT-mediated invasion of CCA cells by enhancing the expression of Snail and ZEB1 in a PI3K/Akt-dependent manner. Consistently, in CCA patients, high expression of PRL1 correlated with lymph node metastasis and advanced tumor stage and was reported as an independent prognostic factor for overall and disease-free survival116. Conversely, EYA4 is downregulated in neoplastic biliary epithelium, and its forced overexpression suppressed CCA cell migration, exemplifying the repertoire of tumor suppressor activities that it is likely endowed with. Indeed, EYA4 protein levels were lower in CCA patients with lymph node metastasis, and low expression of EYA4 was an independent prognostic factor for overall and disease-free survival117. Of note, EYA4 downregulation in CCA tissue might result from gene promoter hypermethylation, as reported in other cancer types118,119. Although the precise antitumor function played by EYA4 in CCA requires further exploration, it is worth noting that in colorectal cancer, EYA4 keeps at bay the pro-neoplastic Wnt signaling by stimulating DKK1 expression119.
Mitogen-Activated Protein Kinase Kinase Kinase 4
MAPK signaling is typically driven by the sequential activation of three core protein kinases, namely, a MAPK kinase kinase (MAP3K or MAPKKK), a MAPK kinase (MAP2K or MAPKK), and, eventually, a MAPK. The initial activation of MAP3Ks can occur via receptor-dependent or receptor-independent mechanisms and ultimately results from the interaction with small GTPases and/or upstream protein kinases. Once activated, MAP3Ks directly phosphorylate and activate MAP2Ks, which then phosphorylate and activate MAPKs, thereby enabling them to orchestrate various biological responses by phosphorylating cytosolic and nuclear targets. The most characterized mammalian MAPKs are ERK1/2 (also called p44/42), JNK1/2/3, and p38 isoforms α/β/γ/δ. Specifically, ERK1/2 are mainly activated by growth factors and mitogens and regulate cell proliferation and differentiation, whereas JNKs and p38 MAPKs predominantly respond to environmental stressors and inflammatory cytokines and regulate apoptosis120. Overactivation of MAPK signaling is frequently reported in human cancers, owing either to an overwhelming stimulation by soluble ligands or stress factors or to the overexpression or uncontrolled activation of cell surface receptors, MAP3Ks, MAP2Ks, or MAPKs121.
Using whole exome sequencing, Gao et al. recently found that in CCA, the MAP3K gene family harbored recurrent somatic mutations114. In particular, MAP3K4 (also known as MEKK4 or MTK1), a primary MAP3K for p38 MAPK and JNK pathways, was found to be frequently mutated in CCA patients, especially in those with lymph node and intrahepatic metastases. Although these mutations did not affect MAP3K4 expression, but rather impaired its activity, downregulation of MAP3K4 was reported in most of the CCA samples, particularly at the invasive front, a feature correlated with vascular invasion, intrahepatic spreading, and lymph node metastasis. The tumor suppressor role of MAP3K4 is further implied by studies showing that its low expression was an independent prognostic factor for disease-free survival and that MAP3K4 genetic manipulation obviously enhanced (MAP3K4 knockdown) or reduced (MAP3K4 forced overexpression) CCA cell motility and invasiveness. These effects were shown to be closely dependent on the modulation of NF-κB, whose nuclear import is indeed promoted by MAP3K4 deficiency by relieving the inhibitory effect of p38 MAPK, a downstream target of MAP3K4. Once in the nucleus, NF-κB both promotes Snail expression and supports its nuclear localization by direct binding, thereby leading CCA cells to undergo EMT changes. In summary, MAP3K4 acts as a negative regulator of EMT-mediated CCA progression by counteracting the NF-κB/Snail axis via p38 MAPK. Inactivating mutations or reduced expression by epigenetic or posttranslational modifications has been proposed as possible mechanisms overcoming the tumor suppressor activity of MAP3K4 in CCA122.
F-Box and WD Repeat Domain-Containing 7
F-box and WD repeat domain-containing 7 (FBXW7) is an E3 ubiquitin ligase subunit that primes a wide range of substrates for ubiquitination-mediated proteolysis, a critical function in the modulation of cell homeostasis. Several oncoproteins [e.g., c-Myc, c-Jun, cyclin E, Notch1, myeloid cell leukemia-1 (Mcl-1)] can be targeted by FBXW7, thus suggesting that FBXW7 works as a potent tumor suppressor. FBXW7 is one of the most frequently mutated gene in human cancers, albeit the prognostic significance of FBXW7 mutations is not always clear123,124. A genetic screen for FBXW7 mutations performed in 1,556 primary human tumors of 15 different histotypes revealed that FBXW7 harbors somatic mutations in a variety of cancers, with an overall mutation frequency of about 6%, reaching the highest percentage in CCA (i.e., 35%)125. Recent studies also demonstrated that in CCA cells, the expression of FBXW7 is markedly lower compared with normal cholangiocytes. Furthermore, FBXW7 downregulation was associated with the presence of metastasis, higher tumor stage and grade, and poor prognosis126,127. In CCA cells, FBXW7 loss results in increased expression and activation of its substrate mammalian target of rapamycin (mTOR), which eventually leads to increased cell migration/invasion via ZEB1-induced EMT127,128. Of note, mTOR involvement in EMT is a well-established concept129,130, and mTOR inhibition by the FDA-approved drug everolimus was actually reported to reduce CCA cell invasion in vitro131. The role of the FBXW7/mTOR axis in CCA progression was confirmed in a xenograft model, where FBXW7 knockdown dramatically accelerated the dissemination of cancer cells, while mTOR inhibition with rapamycin potently dampened the metastatic potential of FBXW7-silenced CCA cells127.
14-3-3ζ
The 14-3-3 protein family consists of seven highly conserved, ubiquitous phosphoserine- and phosphothreonine-binding proteins that essentially function as adaptor molecules, physically interacting with a multitude of functionally divergent molecular targets132,133. In CCA specimens, 14-3-3ζ was upregulated compared to the peritumoral counterpart, and its expression level directly correlated with advanced tumor stage and lymphatic metastasis. Moreover, 14-3-3ζ was reported as an independent negative prognostic factor for overall survival134–136. In CCA cells, 14-3-3ζ silencing dramatically impaired motility and invasiveness, while substantially revoking EMT phenotypic traits, even when induced by TGF-β135,136. Interestingly, 14-3-3ζ is supposed to elicit the emergence of an EMT phenotype by promoting the overexpression and activation of atypical protein kinase C-ι (aPKC-ι), a member of the PKC family of serine/threonine protein kinases136. Specifically, 14-3-3ζ may act through PKC isozymes to inhibit the kinase activity of glycogen synthase kinase-3β (GSK-3β), thereby preventing Snail from undergoing proteasomal degradation136–138.
Yes-Associated Protein
Yes-associated protein (YAP) and its paralog, transcriptional coactivator with PDZ-binding motif (TAZ or WWTR1), are functionally redundant transcriptional coactivators shuttling between the cytoplasm and the nucleus, where they regulate gene expression by interacting with a range of cognate transcription factors, in particular TEA domain (TEAD) family members139. YAP/TAZ act in concert as intracellular transducers of fundamental structural features of the cell, including cell polarity and cytoskeletal organization. Thanks to these abilities, YAP/TAZ behave as critical determinants of organ size during embryonic development by controlling the balance between cell proliferation and apoptosis, as well as stem cell self-renewal140. In normal condition, YAP/TAZ activation is kept at bay by the Hippo pathway, which phosphorylates YAP/TAZ via large tumor suppressor homolog 1/2 (LATS1/2) (Hippo tumor suppressor network), so that they are retained in the cytoplasm to undergo proteasomal degradation141. Among morphogens, activation of Hippo pathway is peculiar, as it is not dependent on the interaction between extracellular ligands and surface receptors, but rather on the assembly of junctional and polarity complexes142. In addition to Hippo signaling, other Hippo-independent mechanisms work as gatekeepers of YAP/TAZ activity by regulating their nucleocytoplasmic shuttling. These include Wnt and GPCR signaling, as well as various metabolic inputs (e.g., energy stress, glycolysis, cholesterol biosynthesis). Furthermore, YAP/TAZ are finely modulated by cell mechanotransduction, that is, they act as intracellular sensors translating biophysical and mechanical stimuli into proper biological responses140,143. In this context, ECM stiffening, cell spreading, or tissue stretching may cause the nuclear accumulation of YAP/TAZ. Importantly, regulation of YAP/TAZ activity by mechanical stress occurs independently of the Hippo pathway and, more importantly, seems to dominate over it144,145.
In stark contrast with normal adult epithelia, where YAP/TAZ expression is generally faint or absent, several carcinomas exhibit an abnormal, widespread activation of YAP/TAZ143. However, activation of YAP/TAZ per se is not enough to confer a malignant phenotype. Indeed, oncologic effects of YAP/TAZ typically rely on the cooperation with ancillary tumor-associated players not directly involved in YAP/TAZ regulation, such as other transcription factors [e.g., activator protein-1 (AP-1) in mammary tumor cells]146. In this regard, hydrodynamic transfection of constitutively active YAP and PI3K in the mouse liver was shown to promote the early development of hepatic tumors, with histotypes ranging from CCA to hepatocellular carcinoma (HCC), including mixed HCC/CCA147. Similarly, intrabiliary injection of constitutively active YAP and Akt in mice resulted in the formation of advanced CCA, when combined with intraperitoneal administration of the proinflammatory cytokine IL-33148,149. Activation of the Notch pathway was also suggested as a putative partner of YAP-mediated cholangiocarcinogenesis in mice147,150.
In CCA samples, high nuclear expression of YAP by neoplastic bile ducts correlated with lymph node involvement and distant metastasis, as well as with high tumor stage and grade, and was even identified as an independent prognostic factor for overall survival151. Consistent with immunohistochemical findings151–153, CCA cell lines display high levels of nuclear (i.e., transcriptionally active) YAP, whereas in nonmalignant biliary epithelial cells, YAP is mainly sequestered in the cytoplasm154. Forced overexpression of YAP promoted the migratory and invasive capabilities of CCA cells in vitro and enhanced the metastatic dissemination of intraperitoneal CCA xenografts. Conversely, YAP knockdown dramatically impaired CCA cell invasiveness both in vitro and in vivo151. YAP may support the invasive functions of CCA cells either by promoting the expression of gankyrin (also known as PSMD10), an oncoprotein that positively regulates the IL-6/STAT3 axis151,155, or by upregulating a number of fibroblast growth factor receptor (FGFR) family members (i.e., FGFR1, FGFR2, FGFR4)154, overall providing CCA cells with pro-EMT inputs151,156,157. Interestingly, both gankyrin and FGFR (notably, FGFR2) could in turn support YAP expression, consistent with the presence of positive feedback loops involving YAP and its targets151,154. Given the ability of CCA cells to rapidly inactivate YAP upon high mechanical stress, as in high-density culture conditions153, it is also tempting to speculate that the increased ECM rigidity within the CCA-associated stroma may induce a cytoskeleton-dependent, Hippo-independent YAP/TAZ activation in CCA cells, thus further enhancing their malignant properties.
REGULATION OF CHOLANGIOCARCINOMA INVASIVENESS BY MicroRNAs
Evidence is mounting that microRNAs (miRNAs) play a significant role in human carcinogenesis by modulating the expression of proto-oncogenes or, alternatively, tumor suppressor genes158. In this regard, several miRNAs were found to be deregulated in CCA cell lines159 and tissues160–162, with the majority of them being underexpressed. miRNAs affect a variety of cell processes related to tumor biology, including cell motility. Specifically, miR-34a163, miR-122164, miR-140-5p165, miR-144162, miR-200c166, miR-204167, miR-212168, miR-214169, and miR-605170 negatively regulate CCA cell migration/invasion and, consistently, are frequently downregulated in neoplastic bile ducts. Conversely, miR-21161,171,172, miR-181c173, and miR-221174 are upregulated in CCA and promote the gain of invasive functions by cancer cells. Of note, the aberrant expression of miRNAs does not necessarily result from cytogenetic abnormalities in miRNA chromosomal sites, but it could also reflect the activation of concomitant pro-oncogenic pathways. For instance, the downregulation of miR-605 may occur downstream of p53 loss170, whereas the upregulation of miR-221 and miR-181c may be induced by the nuclearization of β-catenin174 and by the overproduction of the proinflammatory cytokine leukemia inhibitory factor (LIF)173, respectively.
The target genes of the above-mentioned miRNAs are shown in Table 2. They include, among others, Smad4, a common downstream effector of the TGF-β pathway41; septin 2 (SEPT2), a cytoskeletal protein controlling actin remodeling in cell migration175; platelet-activating factor acetylhydrolase 1b subunit 1 (PAFAH1B1; also known as lissencephaly-1), a microtubule-associated protein shaping the cytoskeleton fibers176; neural cell adhesion molecule 1 (NCAM1), an adhesion molecule promoting the assembly of focal adhesions177; and FOXA1, an EMT-inducing transcription factor15. Of note, the ability of miR-122 and miR-200c to target RhoA and ZEB1/2, respectively, though well recognized in many cancer types, is not yet documented in CCA178,179. Among target genes of upregulated miRNAs, tissue inhibitor of metalloproteinases 3 (TIMP3) is a broad-spectrum inhibitor of MMPs, reversion-inducing cysteine-rich protein with Kazal motif (RECK) is a membrane-anchored protein inhibiting MMP-2, MMP-9, MMP-14, and ADAM10180, and N-Myc downstream-regulated gene 2 (NDRG2) is a recently identified tumor suppressor of unknown function inhibiting metastasis in several human cancers, including HCC181, gallbladder cancer182, and CCA173.
Table 2.
MicroRNAs (miRNAs) Regulating CCA Cell Invasiveness
| miRNAs | Target Genes | References |
|---|---|---|
| Downregulated miRNAs | ||
| miR-34a | Smad4 | 163 |
| miR-122 | RhoA | 164,178 |
| miR-140-5p | SEPT2 | 165 |
| miR-144 | PAFAH1B1/LIS1 | 162 |
| miR-200c | NCAM1; ZEB1/2 | 166,179 |
| miR-204 | Slug | 167 |
| miR-212 | FOXA1 | 168 |
| miR-214 | Twist | 169 |
| miR-605 | PSMD10/gankyrin | 170 |
| Upregulated miRNAs | ||
| miR-21 | PTEN; RECK; TIMP3 | 159,161,171,172 |
| miR-181c | NDRG2 | 173 |
| miR-221 | PTEN | 174 |
SEPT2, septin 2; PAFAH1B1, platelet-activating factor acetylhydrolase 1b subunit 1; LIS1, lissencephaly 1; NCAM1, neural cell adhesion molecule 1; FOXA1, forkhead box A1; PTEN, phosphatase and tensin homolog; RECK, reversion-inducing cysteine-rich protein with Kazal motifs; TIMP3, tissue inhibitor of metalloproteinase 3; NDRG2, N-Myc downstream-regulated gene 2.
CONCLUSIONS AND FUTURE PERSPECTIVES
The main factor impairing the effectiveness of the already scarce treatment options for CCA is the pronounced tumor invasiveness, which precludes the feasibility of curative surgery by promoting early metastatic spread and/or favors tumor recurrence in resected patients183,184. In this context, lymphatic vessels, nerve fibers, and the venous system provide CCA cells with multiple routes of dissemination184. This highly invasive behavior, combined with a strong resistance to conventional chemotherapy, largely accounts for the poor prognosis of patients with CCA. Therefore, an in-depth understanding of the molecular mechanisms involved in CCA invasion is needed to identify predictive and prognostic biomarkers, as well as novel therapeutic targets. On the one hand, reliable biomarkers of early metastatic behavior would enable clinicians to stratify patients into prognostic classes and to rationally allocate them to the best curative and adjuvant therapies. Ultimately, this would improve patient outcome while avoiding redundant or toxic treatments184,185. On the other hand, targeted molecular therapies that selectively interfere with cancer invasion mechanisms may be particularly beneficial in the adjuvant setting, in an effort to prevent recurrence following surgical removal. Furthermore, pharmacological agents able to dampen cancer cell invasiveness could also be used as part of neoadjuvant strategies, to prevent tumor dissemination before surgery, or even in the context of palliative procedures, to slow the progression of the tumor183,186.
S100A4 is a paradigmatic example of these concepts. Nuclear expression of S100A4 first represents a useful tool in clinical decision making, allowing for a proper stratification of CCA patients into groups at high risk or low risk for disease recurrence and progression. Furthermore, the inhibition of S100A4 nuclear import by low-dose paclitaxel was able to inhibit metastasization in CCA100,101. Paclitaxel given at conventional doses is well known to promote microtubule polymerization and stabilization, thereby causing mitotic arrest and, consequently, cell death. In fact, high-dose paclitaxel exerted cytotoxic effects also on CCA cells by reducing cell proliferation and viability and increasing apoptosis. Conversely, paclitaxel at small doses did not affect cell viability or cytoskeletal integrity, but strongly reduced the nuclear import of S100A4 (through inhibition of its SUMOylation) and, more importantly, the invasive properties of CCA cells101. If confirmed in clinical studies, these observations may suggest the use of low-dose paclitaxel as an adjuvant therapy to prevent the metastatic spread of CCA after surgery, an exemplary case of drug repositioning187.
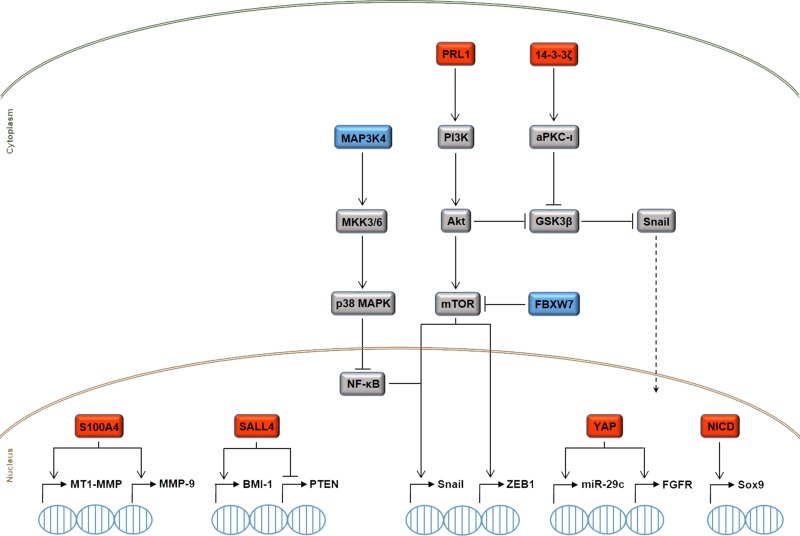
As we discussed above, there are several secreted (cyto/chemokines, growth factors, morphogens, and hormones) and intracellular (transcription factors, protein kinases and phosphatases, ubiquitin ligases, adaptor proteins, and miRNAs) factors that actually appear to determine CCA invasiveness, and recent extensive research has begun to unravel ”the plot” (Fig. 2). However, the bigger picture has yet to emerge. Indeed, while many genes, proteins, and miRNAs have been identified, their multifaceted interactions are still unclear and, moreover, there seems to be a high degree of redundancy. Therefore, a major effort should be devoted in finding the dominant mechanisms that are hierarchically above the others, with the ultimate goal of identifying single molecules serving as both prognostic biomarkers and targets for therapy.
Figure 2.
Simplified overview of the intracellular signaling mechanisms governing CCA cell motility and invasiveness. The upregulation (red boxes) or downregulation (blue boxes) of different transcription factors [S100A4, Sal-like protein 4 (SALL4), Yes-associated protein (YAP), Notch intracellular domain (NICD)], protein kinases [mitogen-activated protein kinase kinase kinase 4 (MAP3K4)] and phosphatases [phosphatases of regenerating liver 1 (PRL1)], ubiquitin ligases [F-box and WD repeat domain-containing 7 (FBXW7)], and adaptor proteins (14-3-3ζ) is responsible for the emergence of proinvasive features in CCA cells, due to the activation of deleterious transcriptional programs that are closely interwoven with the epithelial-to-mesenchymal transition (EMT) process. Protein tyrosine phosphatases protein tyrosine phosphatase nonreceptor 3 (PTPN3) and eyes absent homolog 4 (EYA4) are also endowed with protumorigenic and antitumorigenic functions in CCA, respectively, but the underlying molecular mechanisms have yet to be unveiled. Because of space limitations, we could not fully illustrate the extensive crosstalk among the depicted pathways. For instance, miR-29c, which is upregulated by YAP, can negatively regulate the expression of phosphatase and tensin homolog (PTEN), thereby allowing Akt to upregulate the expression of the oncoprotein gankyrin. Moreover, BMI-1 upregulation by SALL4 supports the activation of the prometastatic Wnt pathway. See text for further details. aPKC)-ι, atypical protein kinase C-ι; FGFR, fibroblast growth factor receptor; GSK-3β, lycogen synthase kinase-3β; mTOR, mammalian target of rapamycin; MMP-9, matrix metalloproteinase-9; MT1-MMP, membrane-type 1-MMP; NF-κB, nuclear factor-κB; Sox9, sex-determining region Y-box 9.
ACKNOWLEDGMENTS
M.S. was supported by the National Institutes of Health (RO1DK-079005-07, DK034989 Silvio O. Conte Digestive Diseases Research Centers–Clinical and Translational Core) and by Partners Seeking a Cure Foundation. L.F. was supported by Progetto di Ricerca Ateneo 2011 (CPD113799/11). The authors declare no conflicts of interest.
REFERENCES
- 1. Blechacz B. Cholangiocarcinoma: Current knowledge and new developments. Gut Liver 2017;11:13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr/Default.aspx [Google Scholar]
- 3. Brito AF, Abrantes AM, Encarnação JC, Tralhão JG, Botelho MF. Cholangiocarcinoma: From molecular biology to treatment. Med Oncol. 2015;32:245. [DOI] [PubMed] [Google Scholar]
- 4. Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes SJ, Fouassier L, Geier A, Calvisi DF, Mertens JC, Trauner M, Benedetti A, Maroni L, Vaquero J, Macias RI, Raggi C, Perugorria MJ, Gaudio E, Boberg KM, Marin JJ, Alvaro D. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13:261–80. [DOI] [PubMed] [Google Scholar]
- 5. Cadamuro M, Brivio S, Spirli C, Joplin RE, Strazzabosco M, Fabris L. Autocrine and paracrine mechanisms promoting chemoresistance in cholangiocarcinoma. Int J Mol Sci. 2017;18:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J, ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–81. [DOI] [PubMed] [Google Scholar]
- 7. Chong DQ, Zhu AX. The landscape of targeted therapies for cholangiocarcinoma: Current status and emerging targets. Oncotarget 2016;7:46750–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moeini A, Sia D, Bardeesy N, Mazzaferro V, Llovet JM. Molecular pathogenesis and targeted therapies for intrahepatic cholangiocarcinoma. Clin Cancer Res. 2016;22:291–300. [DOI] [PubMed] [Google Scholar]
- 9. Høgdall D, O’Rourke CJ, Taranta A, Oliveira DV, Andersen JB. Molecular pathogenesis and current therapy in intrahepatic cholangiocarcinoma. Dig Dis. 2016;34:440–51. [DOI] [PubMed] [Google Scholar]
- 10. Clark AG, Vignjevic DM. Modes of cancer cell invasion and the role of the microenvironment. Curr Opin Cell Biol. 2015;36:13–22. [DOI] [PubMed] [Google Scholar]
- 11. Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell 2017;168:670–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pandya P, Orgaz JL, Sanz-Moreno V. Modes of invasion during tumour dissemination. Mol Oncol. 2017;11:5–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gandalovičová A, Vomastek T, Rosel D, Brábek J. Cell polarity signaling in the plasticity of cancer cell invasiveness. Oncotarget 2016;7:25022–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009;139:871–90. [DOI] [PubMed] [Google Scholar]
- 15. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang RY, Guilford P, Thiery JP. Early events in cell adhesion and polarity during epithelial-mesenchymal transition. J Cell Sci. 2012;125:4417–22. [DOI] [PubMed] [Google Scholar]
- 17. Brivio S, Cadamuro M, Fabris L, Strazzabosco M. Epithelial-to-mesenchymal transition and cancer invasiveness: What can we learn from cholangiocarcinoma? J Clin Med. 2015;4:2028–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ridley AJ. Rho GTPases and cell migration. J Cell Sci. 2001;114:2713–22. [DOI] [PubMed] [Google Scholar]
- 19. Ridley AJ. Rho GTPase signalling in cell migration. Curr Opin Cell Biol. 2015;36:103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spuul P, Ciufici P, Veillat V, Leclercq A, Daubon T, Kramer IJ, Génot E. Importance of RhoGTPases in formation, characteristics, and functions of invadosomes. Small GTPases 2014;5:e28195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008;133:704–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cadamuro M, Morton SD, Strazzabosco M, Fabris L. Unveiling the role of tumor reactive stroma in cholangiocarcinoma: An opportunity for new therapeutic strategies. Transl Gastrointest Cancer 2013;2:130–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brivio S, Cadamuro M, Strazzabosco M, Fabris L. Tumor reactive stroma in cholangiocarcinoma: The fuel behind cancer aggressiveness. World J Hepatol. 2017;9:455–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cadamuro M, Stecca T, Brivio S, Mariotti V, Fiorotto R, Spirli C, Strazzabosco M, Fabris L. The deleterious interplay between tumor epithelia and stroma in cholangiocarcinoma. Biochim Biophys Acta 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Puré E, Lo A. Can targeting stroma pave the way to enhanced antitumor immunity and immunotherapy of solid tumors? Cancer Immunol Res. 2016;4:269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leyva-Illades D, McMillin M, Quinn M, Demorrow S. Cholangiocarcinoma pathogenesis: Role of the tumor microenvironment. Transl Gastrointest Cancer 2012;1:71–80. [PMC free article] [PubMed] [Google Scholar]
- 28. Heits N, Heinze T, Bernsmeier A, Kerber J, Hauser C, Becker T, Kalthoff H, Egberts JH, Braun F. Influence of mTOR-inhibitors and mycophenolic acid on human cholangiocellular carcinoma and cancer associated fibroblasts. BMC Cancer 2016;16:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Okabe H, Beppu T, Hayashi H, Horino K, Masuda T, Komori H, Ishikawa S, Watanabe M, Takamori H, Iyama K, Baba H. Hepatic stellate cells may relate to progression of intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2009;16:2555–64. [DOI] [PubMed] [Google Scholar]
- 30. Okamoto K, Tajima H, Nakanuma S, Sakai S, Makino I, Kinoshita J, Hayashi H, Nakamura K, Oyama K, Nakagawara H, Fujita H, Takamura H, Ninomiya I, Kitagawa H, Fushida S, Fujimura T, Harada S, Wakayama T, Iseki S, Ohta T. Angiotensin II enhances epithelial-to-mesenchymal transition through the interaction between activated hepatic stellate cells and the stromal cell derived factor-1/CXCR4 axis in intrahepatic cholangiocarcinoma. Int J Oncol. 2012;41:573–82. [DOI] [PubMed] [Google Scholar]
- 31. Kim Y, Kim MO, Shin JS, Park SH, Kim SB, Kim J, Park SC, Han CJ, Ryu JK, Yoon YB, Kim YT. Hedgehog signaling between cancer cells and hepatic stellate cells in promoting cholangiocarcinoma. Ann Surg Oncol. 2014;21:2684–98. [DOI] [PubMed] [Google Scholar]
- 32. Clapéron A, Mergey M, Aoudjehane L, Ho-Bouldoires TH, Wendum D, Prignon A, Merabtene F, Firrincieli D, Desbois-Mouthon C, Scatton O, Conti F, Housset C, Fouassier L. Hepatic myofibroblasts promote the progression of human cholangiocarcinoma through activation of epidermal growth factor receptor. Hepatology 2013;58:2001–11. [DOI] [PubMed] [Google Scholar]
- 33. Gentilini A, Rombouts K, Galastri S, Caligiuri A, Mingarelli E, Mello T, Marra F, Mantero S, Roncalli M, Invernizzi P, Pinzani M. Role of the stromal-derived factor-1 (SDF-1)-CXCR4 axis in the interaction between hepatic stellate cells and cholangiocarcinoma. J Hepatol. 2012;57:813–20. [DOI] [PubMed] [Google Scholar]
- 34. Cadamuro M, Nardo G, Indraccolo S, Dall’olmo L, Sambado L, Moserle L, Franceschet I, Colledan M, Massani M, Stecca T, Bassi N, Morton S, Spirli C, Fiorotto R, Fabris L, Strazzabosco M. Platelet-derived growth factor-D and Rho GTPases regulate recruitment of cancer-associated fibroblasts in cholangiocarcinoma. Hepatology 2013;58:1042–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Appiah-Kubi K, Wang Y, Qian H, Wu M, Yao X, Wu Y, Chen Y. Platelet-derived growth factor receptor/platelet-derived growth factor (PDGFR/PDGF) system is a prognostic and treatment response biomarker with multifarious therapeutic targets in cancers. Tumour Biol. 2016;37:10053–66. [DOI] [PubMed] [Google Scholar]
- 36. Cadamuro M, Vismara M, Brivio S, Bovo A, Strazzabosco M, Fabris L. Secretion of vascular endothelial growth factor-C by cancer-associated fibroblasts (CAF) is stimulated by platelet-derived growth factor-D (PDGF-D) and promotes lymphangiogenesis in cholangiocarcinoma. Gastroenterology 2016;150(Suppl 1):S1124. [Google Scholar]
- 37. Raggi C, Correnti M, Sica A, Andersen JB, Cardinale V, Alvaro D, Chiorino G, Forti E, Glaser S, Alpini G, Destro A, Sozio F, Di Tommaso L, Roncalli M, Banales JM, Coulouarn C, Bujanda L, Torzilli G, Invernizzi P. Cholangiocarcinoma stem-like subset shapes tumor-initiating niche by educating associated macrophages. J Hepatol. 2017;66:102–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thanee M, Loilome W, Techasen A, Namwat N, Boonmars T, Pairojkul C, Yongvanit P. Quantitative changes in tumor-associated M2 macrophages characterize cholangiocarcinoma and their association with metastasis. Asian Pac J Cancer Prev. 2015;16:3043–50. [DOI] [PubMed] [Google Scholar]
- 39. Subimerb C, Pinlaor S, Khuntikeo N, Leelayuwat C, Morris A, McGrath MS, Wongkham S. Tissue invasive macrophage density is correlated with prognosis in cholangiocarcinoma. Mol Med Rep. 2010;3:597–605. [DOI] [PubMed] [Google Scholar]
- 40. Ohira S, Sasaki M, Harada K, Sato Y, Zen Y, Isse K, Kozaka K, Ishikawa A, Oda K, Nimura Y, Nakanuma Y. Possible regulation of migration of intrahepatic cholangiocarcinoma cells by interaction of CXCR4 expressed in carcinoma cells with tumor necrosis factor-alpha and stromal-derived factor-1 released in stroma. Am J Pathol. 2006;168:1155–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Principe DR, Doll JA, Bauer J, Jung B, Munshi HG, Bartholin L, Pasche B, Lee C, Grippo PJ. TGF-β: Duality of function between tumor prevention and carcinogenesis. J Natl Cancer Inst. 2014;106:djt369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fabris L, Strazzabosco M. Epithelial-mesenchymal interactions in biliary diseases. Semin Liver Dis. 2011;31:11–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dooley S, Ten Dijke P. TGF-β in progression of liver disease. Cell Tissue Res. 2012;347:245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fabregat I, Moreno-Càceres J, Sánchez A, Dooley S, Dewidar B, Giannelli G, Ten Dijke P. TGF-β signalling and liver disease. FEBS J. 2016;283:2219–32. [DOI] [PubMed] [Google Scholar]
- 45. Chen Y, Ma L, He Q, Zhang S, Zhang C, Jia W. TGF-β1 expression is associated with invasion and metastasis of intrahepatic cholangiocarcinoma. Biol Res. 2015;48:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zavadil J, Böttinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene 2005;24:5764–74. [DOI] [PubMed] [Google Scholar]
- 47. Sato Y, Harada K, Itatsu K, Ikeda H, Kakuda Y, Shimomura S, Shan Ren X, Yoneda N, Sasaki M, Nakanuma Y. Epithelial-mesenchymal transition induced by transforming growth factor-β1/Snail activation aggravates invasive growth of cholangiocarcinoma. Am J Pathol. 2010;177:141–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Araki K, Shimura T, Suzuki H, Tsutsumi S, Wada W, Yajima T, Kobayahi T, Kubo N, Kuwano H. E/N-cadherin switch mediates cancer progression via TGF-β-induced epithelial-to-mesenchymal transition in extrahepatic cholangiocarcinoma. Br J Cancer 2011;105:1885–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Duangkumpha K, Techasen A, Loilome W, Namwat N, Thanan R, Khuntikeo N, Yongvanit P. BMP-7 blocks the effects of TGF-β-induced EMT in cholangiocarcinoma. Tumour Biol. 2014;35:9667–76. [DOI] [PubMed] [Google Scholar]
- 50. Huang CK, Aihara A, Iwagami Y, Yu T, Carlson R, Koga H, Kim M, Zou J, Casulli S, Wands JR. Expression of transforming growth factor β1 promotes cholangiocarcinoma development and progression. Cancer Lett. 2016;380:153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Leelawat K, Leelawat S, Tepaksorn P, Rattanasinganchan P, Leungchaweng A, Tohtong R, Sobhon P. Involvement of c-Met/hepatocyte growth factor pathway in cholangiocarcinoma cell invasion and its therapeutic inhibition with small interfering RNA specific for c-Met. J Surg Res. 2006;136:78–84. [DOI] [PubMed] [Google Scholar]
- 52. Menakongka A, Suthiphongchai T. Involvement of PI3K and ERK1/2 pathways in hepatocyte growth factor-induced cholangiocarcinoma cell invasion. World J Gastroenterol. 2010;16:713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Aggarwal BB. Signalling pathways of the TNF superfamily: A double-edged sword. Nat Rev Immunol. 2003;3:745–56. [DOI] [PubMed] [Google Scholar]
- 54. Tian T, Wang M, Ma D. TNF-α, a good or bad factor in hematological diseases? Stem Cell Investig. 2014;1:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tanimura Y, Kokuryo T, Tsunoda N, Yamazaki Y, Oda K, Nimura Y, Naing Mon N, Huang P, Nakanuma Y, Chen MF, Jan YY, Yeh TS, Chiu CT, Hsieh LL, Hamaguchi M. Tumor necrosis factor alpha promotes invasiveness of cholangiocarcinoma cells via its receptor, TNFR2. Cancer Lett. 2005;219:205–13. [DOI] [PubMed] [Google Scholar]
- 56. Itatsu K, Sasaki M, Yamaguchi J, Ohira S, Ishikawa A, Ikeda H, Sato Y, Harada K, Zen Y, Sato H, Ohta T, Nagino M, Nimura Y, Nakanuma Y. Cyclooxygenase-2 is involved in the up-regulation of matrix metalloproteinase-9 in cholangiocarcinoma induced by tumor necrosis factor-alpha. Am J Pathol. 2009;174:829–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Itatsu K, Sasaki M, Harada K, Yamaguchi J, Ikeda H, Sato Y, Ohta T, Sato H, Nagino M, Nimura Y, Nakanuma Y. Phosphorylation of extracellular signal-regulated kinase 1/2, p38 mitogen-activated protein kinase and nuclear translocation of nuclear factor-kappaB are involved in upregulation of matrix metalloproteinase-9 by tumour necrosis factor-alpha. Liver Int. 2009;29:291–8. [DOI] [PubMed] [Google Scholar]
- 58. Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer 2013;12:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mon NN, Hasegawa H, Thant AA, Huang P, Tanimura Y, Senga T, Hamaguchi M. A role for focal adhesion kinase signaling in tumor necrosis factor-alpha-dependent matrix metalloproteinase-9 production in a cholangiocarcinoma cell line, CCKS1. Cancer Res. 2006;66:6778–84. [DOI] [PubMed] [Google Scholar]
- 60. Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–26. [DOI] [PubMed] [Google Scholar]
- 61. Panera N, Crudele A, Romito I, Gnani D, Alisi A. Focal adhesion kinase: Insight into molecular roles and functions in hepatocellular carcinoma. Int J Mol Sci. 2017;18:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rizzo MT. Cyclooxygenase-2 in oncogenesis. Clin Chim Acta 2011;412:671–87. [DOI] [PubMed] [Google Scholar]
- 63. Schmitz KJ, Lang H, Wohlschlaeger J, Reis H, Sotiropoulos GC, Schmid KW, Baba HA. Elevated expression of cyclooxygenase-2 is a negative prognostic factor for overall survival in intrahepatic cholangiocarcinoma. Virchows Arch. 2007;450:135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Techasen A, Namwat N, Loilome W, Bungkanjana P, Khuntikeo N, Puapairoj A, Jearanaikoon P, Saya H, Yongvanit P. Tumor necrosis factor-α (TNF-α) stimulates the epithelial-mesenchymal transition regulator Snail in cholangiocarcinoma. Med Oncol. 2012;29:3083–91. [DOI] [PubMed] [Google Scholar]
- 65. Techasen A, Namwat N, Loilome W, Duangkumpha K, Puapairoj A, Saya H, Yongvanit P. Tumor necrosis factor-α modulates epithelial mesenchymal transition mediators ZEB2 and S100A4 to promote cholangiocarcinoma progression. J Hepatobiliary Pancreat Sci. 2014;21:703–11. [DOI] [PubMed] [Google Scholar]
- 66. Leelawat K, Leelawat S, Narong S, Hongeng S. Roles of the MEK1/2 and AKT pathways in CXCL12/CXCR4 induced cholangiocarcinoma cell invasion. World J Gastroenterol. 2007;13:1561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tan XY, Chang S, Liu W, Tang HH. Silencing of CXCR4 inhibits tumor cell proliferation and neural invasion in human hilar cholangiocarcinoma. Gut Liver 2014;8:196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhao S, Wang J, Qin C. Blockade of CXCL12/CXCR4 signaling inhibits intrahepatic cholangiocarcinoma progression and metastasis via inactivation of canonical Wnt pathway. J Exp Clin Cancer Res. 2014;33:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lindsey S, Langhans SA. Epidermal growth factor signaling in transformed cells. Int Rev Cell Mol Biol. 2015;314:1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Clapéron A, Guedj N, Mergey M, Vignjevic D, Desbois-Mouthon C, Boissan M, Saubaméa B, Paradis V, Housset C, Fouassier L. Loss of EBP50 stimulates EGFR activity to induce EMT phenotypic features in biliary cancer cells. Oncogene 2012;31:1376–88. [DOI] [PubMed] [Google Scholar]
- 71. Vaquero J, Nguyen Ho-Bouldoires TH, Clapéron A, Fouassier L. Role of the PDZ-scaffold protein NHERF1/EBP50 in cancer biology: From signaling regulation to clinical relevance. Oncogene 2017;36:3067–79. [DOI] [PubMed] [Google Scholar]
- 72. Yoon JH, Gwak GY, Lee HS, Bronk SF, Werneburg NW, Gores GJ. Enhanced epidermal growth factor receptor activation in human cholangiocarcinoma cells. J Hepatol. 2004;41:808–14. [DOI] [PubMed] [Google Scholar]
- 73. Lee MJ, Yu GR, Yoo HJ, Kim JH, Yoon BI, Choi YK, Kim DG. ANXA8 down-regulation by EGF-FOXO4 signaling is involved in cell scattering and tumor metastasis of cholangiocarcinoma. Gastroenterology 2009;137:1138–50. [DOI] [PubMed] [Google Scholar]
- 74. Clapéron A, Mergey M, Nguyen Ho-Bouldoires TH, Vignjevic D, Wendum D, Chrétien Y, Merabtene F, Frazao A, Paradis V, Housset C, Guedj N, Fouassier L. EGF/EGFR axis contributes to the progression of cholangiocarcinoma through the induction of an epithelial-mesenchymal transition. J Hepatol. 2014;61:325–32. [DOI] [PubMed] [Google Scholar]
- 75. Goebeler V, Ruhe D, Gerke V, Rescher U. Annexin A8 displays unique phospholipid and F-actin binding properties. FEBS Lett. 2006;580:2430–4. [DOI] [PubMed] [Google Scholar]
- 76. Roskoski R Jr. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol Res. 2014;79:34–74. [DOI] [PubMed] [Google Scholar]
- 77. El Khatib M, Kalnytska A, Palagani V, Kossatz U, Manns MP, Malek NP, Wilkens L, Plentz RR. Inhibition of hedgehog signaling attenuates carcinogenesis in vitro and increases necrosis of cholangiocellular carcinoma. Hepatology 2013;57:1035–45. [DOI] [PubMed] [Google Scholar]
- 78. Wang W, Zhong W, Yuan J, Yan C, Hu S, Tong Y, Mao Y, Hu T, Zhang B, Song G. Involvement of Wnt/β-catenin signaling in the mesenchymal stem cells promote metastatic growth and chemoresistance of cholangiocarcinoma. Oncotarget 2015;6:42276–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. El Khatib M, Bozko P, Palagani V, Malek NP, Wilkens L, Plentz RR. Activation of Notch signaling is required for cholangiocarcinoma progression and is enhanced by inactivation of p53 in vivo. PLoS One 2013;8:e77433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhou Q, Wang Y, Peng B, Liang L, Li J. The roles of Notch1 expression in the migration of intrahepatic cholangiocarcinoma. BMC Cancer 2013;13:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wu WR, Zhang R, Shi XD, Zhu MS, Xu LB, Zeng H, Liu C. Notch1 is overexpressed in human intrahepatic cholangiocarcinoma and is associated with its proliferation, invasiveness and sensitivity to 5-fluorouracil in vitro. Oncol Rep. 2014;31:2515–24. [DOI] [PubMed] [Google Scholar]
- 82. Matsushima H, Kuroki T, Kitasato A, Adachi T, Tanaka T, Hirabaru M, Hirayama T, Kuroshima N, Hidaka M, Soyama A, Takatsuki M, Kinoshita N, Sano K, Nishida N, Eguchi S. Sox9 expression in carcinogenesis and its clinical significance in intrahepatic cholangiocarcinoma. Dig Liver Dis. 2015;47:1067–75. [DOI] [PubMed] [Google Scholar]
- 83. Huang CK, Iwagami Y, Aihara A, Chung W, de la Monte S, Thomas JM, Olsen M, Carlson R, Yu T, Dong X, Wands J. Anti-tumor effects of second generation β-hydroxylase inhibitors on cholangiocarcinoma development and progression. PLoS One 2016;11:e0150336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wattanawongdon W, Hahnvajanawong C, Namwat N, Kanchanawat S, Boonmars T, Jearanaikoon P, Leelayuwat C, Techasen A, Seubwai W. Establishment and characterization of gemcitabine-resistant human cholangiocarcinoma cell lines with multidrug resistance and enhanced invasiveness. Int J Oncol. 2015;47:398–410. [DOI] [PubMed] [Google Scholar]
- 85. Yoon H, Min JK, Lee DG, Kim DG, Koh SS, Hong HJ. L1 cell adhesion molecule and epidermal growth factor receptor activation confer cisplatin resistance in intrahepatic cholangiocarcinoma cells. Cancer Lett. 2012;316:70–6. [DOI] [PubMed] [Google Scholar]
- 86. Kim H, Hwang H, Lee H, Hong HJ. L1 cell adhesion molecule promotes migration and invasion via JNK activation in extrahepatic cholangiocarcinoma cells with activating KRAS mutation. Mol Cells 2017;40:363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Morell CM, Fiorotto R, Fabris L, Strazzabosco M. Notch signalling beyond liver development: Emerging concepts in liver repair and oncogenesis. Clin Res Hepatol Gastroenterol. 2013;37:447–54. [DOI] [PubMed] [Google Scholar]
- 88. Morell CM, Strazzabosco M. Notch signaling and new therapeutic options in liver disease. J Hepatol. 2014;60:885–90. [DOI] [PubMed] [Google Scholar]
- 89. Geisler F, Strazzabosco M. Emerging roles of Notch signaling in liver disease. Hepatology 2015;61:382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Fabris L, Cadamuro M, Guido M, Spirli C, Fiorotto R, Colledan M, Torre G, Alberti D, Sonzogni A, Okolicsanyi L, Strazzabosco M. Analysis of liver repair mechanisms in Alagille syndrome and biliary atresia reveals a role for notch signaling. Am J Pathol. 2007;171:641–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Fiorotto R, Raizner A, Morell CM, Torsello B, Scirpo R, Fabris L, Spirli C, Strazzabosco M. Notch signaling regulates tubular morphogenesis during repair from biliary damage in mice. J Hepatol. 2013;59:124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fan B, Malato Y, Calvisi DF, Naqvi S, Razumilava N, Ribback S, Gores GJ, Dombrowski F, Evert M, Chen X, Willenbring H. Cholangiocarcinomas can originate from hepatocytes in mice. J Clin Invest. 2012;122:2911–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sekiya S, Suzuki A. Intrahepatic cholangiocarcinoma can arise from Notch-mediated conversion of hepatocytes. J Clin Invest. 2012;122:3914–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Strazzabosco M, Fabris L. Notch signaling in hepatocellular carcinoma: Guilty in association! Gastroenterology 2012;143:1430–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ishimura N, Bronk SF, Gores GJ. Inducible nitric oxide synthase up-regulates Notch-1 in mouse cholangiocytes: Implications for carcinogenesis. Gastroenterology 2005;128:1354–68. [DOI] [PubMed] [Google Scholar]
- 96. Cantarini MC, De La Monte SM, Pang M, Tong M, D’Errico A, Trevisani F, Wands JR. Aspartyl-asparagyl beta hydroxylase over-expression in human hepatoma is linked to activation of insulin-like growth factor and notch signaling mechanisms. Hepatology 2006;44:446–57. [DOI] [PubMed] [Google Scholar]
- 97. Maeda T, Taguchi K, Aishima S, Shimada M, Hintz D, Larusso N, Gores G, Tsuneyoshi M, Sugimachi K, Wands JR, De La Monte SM. Clinicopathological correlates of aspartyl (asparaginyl) beta-hydroxylase over-expression in cholangiocarcinoma. Cancer Detect Prev. 2004;28:313–8. [DOI] [PubMed] [Google Scholar]
- 98. Boye K, Maelandsmo GM. S100A4 and metastasis: A small actor playing many roles. Am J Pathol. 2010;176:528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Saleem M, Kweon MH, Johnson JJ, Adhami VM, Elcheva I, Khan N, Bin Hafeez B, Bhat KM, Sarfaraz S, Reagan-Shaw S, Spiegelman VS, Setaluri V, Mukhtar H. S100A4 accelerates tumorigenesis and invasion of human prostate cancer through the transcriptional regulation of matrix metalloproteinase 9. Proc Natl Acad Sci USA 2006;103:14825–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fabris L, Cadamuro M, Moserle L, Dziura J, Cong X, Sambado L, Nardo G, Sonzogni A, Colledan M, Furlanetto A, Bassi N, Massani M, Cillo U, Mescoli C, Indraccolo S, Rugge M, Okolicsanyi L, Strazzabosco M. Nuclear expression of S100A4 calcium-binding protein increases cholangiocarcinoma invasiveness and metastasization. Hepatology 2011;54:890–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Cadamuro M, Spagnuolo G, Sambado L, Indraccolo S, Nardo G, Rosato A, Brivio S, Caslini C, Stecca T, Massani M, Bassi N, Novelli E, Spirli C, Fabris L, Strazzabosco M. Low-dose paclitaxel reduces S100A4 nuclear import to inhibit invasion and hematogenous metastasis of cholangiocarcinoma. Cancer Res. 2016;76:4775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Itoh Y. Membrane-type matrix metalloproteinases: Their functions and regulations. Matrix Biol. 2015;44–46:207–23. [DOI] [PubMed] [Google Scholar]
- 103. Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278:16–27. [DOI] [PubMed] [Google Scholar]
- 104. Monribot-Villanueva J, Zurita M, Vázquez M. Developmental transcriptional regulation by SUMOylation, an evolving field. Genesis 2017;55. [DOI] [PubMed] [Google Scholar]
- 105. Lee JS, Choi HJ, Baek SH. Sumoylation and its contribution to cancer. Adv Exp Med Biol. 2017;963:283–98. [DOI] [PubMed] [Google Scholar]
- 106. Bogachek MV, De Andrade JP, Weigel RJ. Regulation of epithelial-mesenchymal transition through SUMOylation of transcription factors. Cancer Res. 2015;75:11–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Oikawa T, Kamiya A, Zeniya M, Chikada H, Hyuck AD, Yamazaki Y, Wauthier E, Tajiri H, Miller LD, Wang XW, Reid LM, Nakauchi H. Sal-like protein 4 (SALL4), a stem cell biomarker in liver cancers. Hepatology 2013;57:1469–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Deng G, Zhu L, Huang F, Nie W, Huang W, Xu H, Zheng S, Yi Z, Wan T. SALL4 is a novel therapeutic target in intrahepatic cholangiocarcinoma. Oncotarget 2015;6:27416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zhu L, Huang F, Deng G, Nie W, Huang W, Xu H, Zheng S, Yi Z, Wan T. Knockdown of Sall4 inhibits intrahepatic cholangiocarcinoma cell migration and invasion in ICC-9810 cells. Onco Targets Ther. 2016;9:5297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Blanco-Aparicio C, Renner O, Leal JF, Carnero A. PTEN, more than the AKT pathway. Carcinogenesis 2007;28:1379–86. [DOI] [PubMed] [Google Scholar]
- 111. Cho JH, Dimri M, Dimri GP. A positive feedback loop regulates the expression of polycomb group protein BMI1 via WNT signaling pathway. J Biol Chem. 2013;288:3406–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Yothaisong S, Dokduang H, Techasen A, Namwat N, Yongvanit P, Bhudhisawasdi V, Puapairoj A, Riggins GJ, Loilome W. Increased activation of PI3K/AKT signaling pathway is associated with cholangiocarcinoma metastasis and PI3K/mTOR inhibition presents a possible therapeutic strategy. Tumour Biol. 2013;34:3637–48. [DOI] [PubMed] [Google Scholar]
- 113. Julien SG, Dubé N, Hardy S, Tremblay ML. Inside the human cancer tyrosine phosphatome. Nat Rev Cancer 2011;11:35–49. [DOI] [PubMed] [Google Scholar]
- 114. Gao Q, Zhao YJ, Wang XY, Guo WJ, Gao S, Wei L, Shi JY, Shi GM, Wang ZC, Zhang YN, Shi YH, Ding J, Ding ZB, Ke AW, Dai Z, Wu FZ, Wang H, Qiu ZP, Chen ZA, Zhang ZF, Qiu SJ, Zhou J, He XH, Fan J. Activating mutations in PTPN3 promote cholangiocarcinoma cell proliferation and migration and are associated with tumor recurrence in patients. Gastroenterology 2014;146:1397–407. [DOI] [PubMed] [Google Scholar]
- 115. Ma S, Yin N, Qi X, Pfister SL, Zhang MJ, Ma R, Chen G. Tyrosine dephosphorylation enhances the therapeutic target activity of epidermal growth factor receptor (EGFR) by disrupting its interaction with estrogen receptor (ER). Oncotarget 2015;6:13320–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Liu LZ, He YZ, Dong PP, Ma LJ, Wang ZC, Liu XY, Duan M, Yang LX, Shi JY, Zhou J, Fan J, Gao Q, Wang XY. Protein tyrosine phosphatase PTP4A1 promotes proliferation and epithelial-mesenchymal transition in intrahepatic cholangiocarcinoma via the PI3K/AKT pathway. Oncotarget 2016;7:75210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Hao XY, Cai JP, Liu X, Chen W, Hou X, Chen D, Lai JM, Liang LJ, Yin XY. EYA4 gene functions as a prognostic marker and inhibits the growth of intrahepatic cholangiocarcinoma. Chin J Cancer 2016;35:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hou X, Peng JX, Hao XY, Cai JP, Liang LJ, Zhai JM, Zhang KS, Lai JM, Yin XY. DNA methylation profiling identifies EYA4 gene as a prognostic molecular marker in hepatocellular carcinoma. Ann Surg Oncol. 2014;21:3891–9. [DOI] [PubMed] [Google Scholar]
- 119. Kim SJ, Tae CH, Hong SN, Min BH, Chang DK, Rhee PL, Kim JJ, Kim HC, Kim DH, Kim YH. EYA4 acts as a new tumor suppressor gene in colorectal cancer. Mol Carcinog. 2015;54:1748–57. [DOI] [PubMed] [Google Scholar]
- 120. Morrison DK. MAP kinase pathways. Cold Spring Harb Perspect Biol. 2012;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene 2007;26:3279–90. [DOI] [PubMed] [Google Scholar]
- 122. Yang LX, Gao Q, Shi JY, Wang ZC, Zhang Y, Gao PT, Wang XY, Shi YH, Ke AW, Shi GM, Cai JB, Liu WR, Duan M, Zhao YJ, Ji Y, Gao DM, Zhu K, Zhou J, Qiu SJ, Cao Y, Tang QQ, Fan J. Mitogen-activated protein kinase kinase kinase 4 deficiency in intrahepatic cholangiocarcinoma leads to invasive growth and epithelial-mesenchymal transition. Hepatology 2015;62:1804–16. [DOI] [PubMed] [Google Scholar]
- 123. Wang Z, Inuzuka H, Zhong J, Wan L, Fukushima H, Sarkar FH, Wei W. Tumor suppressor functions of FBW7 in cancer development and progression. FEBS Lett. 2012;586:1409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Davis RJ, Welcker M, Clurman BE. Tumor suppression by the Fbw7 ubiquitin ligase: Mechanisms and opportunities. Cancer Cell 2014;26:455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Akhoondi S, Sun D, Von Der Lehr N, Apostolidou S, Klotz K, Maljukova A, Cepeda D, Fiegl H, Dafou D, Marth C, Mueller-Holzner E, Corcoran M, Dagnell M, Nejad SZ, Nayer BN, Zali MR, Hansson J, Egyhazi S, Petersson F, Sangfelt P, Nordgren H, Grander D, Reed SI, Widschwendter M, Sangfelt O, Spruck C. FBXW7/hCDC4 is a general tumor suppressor in human cancer. Cancer Res. 2007;67:9006–12. [DOI] [PubMed] [Google Scholar]
- 126. Enkhbold C, Utsunomiya T, Morine Y, Imura S, Ikemoto T, Arakawa Y, Kanamoto M, Iwahashi S, Saito Y, Ishikawa D, Shimada M. Loss of FBXW7 expression is associated with poor prognosis in intrahepatic cholangiocarcinoma. Hepatol Res. 2014;44:E346–52. [DOI] [PubMed] [Google Scholar]
- 127. Yang H, Lu X, Liu Z, Chen L, Xu Y, Wang Y, Wei G, Chen Y. FBXW7 suppresses epithelial-mesenchymal transition, stemness and metastatic potential of cholangiocarcinoma cells. Oncotarget 2015;6:6310–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Mao JH, Kim IJ, Wu D, Climent J, Kang HC, DelRosario R, Balmain A. FBXW7 targets mTOR for degradation and cooperates with PTEN in tumor suppression. Science 2008;321:1499–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lamouille S, Derynck R. Cell size and invasion in TGF-beta-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J Cell Biol. 2007;178:437–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Lamouille S, Connolly E, Smyth JW, Akhurst RJ, Derynck R. TGF-β-induced activation of mTOR complex 2 drives epithelial-mesenchymal transition and cell invasion. J Cell Sci. 2012;125:1259–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Moolthiya P, Tohtong R, Keeratichamroen S, Leelawat K. Role of mTOR inhibitor in cholangiocarcinoma cell progression. Oncol Lett. 2014;7:854–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Neal CL, Yu D. 14-3-3ζ as a prognostic marker and therapeutic target for cancer. Expert Opin Ther Targets 2010;14:1343–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Zhao J, Meyerkord CL, Du Y, Khuri FR, Fu H. 14-3-3 proteins as potential therapeutic targets. Semin Cell Dev Biol. 2011;22:705–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Wu Q, Liu CZ, Tao LY, Yu L, Liu W, Chen SS, He XD. The clinicopathological and prognostic impact of 14-3-3 protein isoforms expression in human cholangiocarcinoma by immunohistochemistry. Asian Pac J Cancer Prev. 2012;13:1253–9. [DOI] [PubMed] [Google Scholar]
- 135. Zhang C, Liu LX, Dong ZR, Shi GM, Cai JB, Zhang PF, Ke AW, Yu JX, Zhou J, Fan J. Up-regulation of 14-3-3ζ expression in intrahepatic cholangiocarcinoma and its clinical implications. Tumour Biol. 2015;36:1781–9. [DOI] [PubMed] [Google Scholar]
- 136. Yang Y, Liu Y, He JC, Wang JM, Schemmer P, Ma CQ, Qian YW, Yao W, Zhang J, Qi WP, Fu Y, Feng W, Yang T. 14-3-3ζ and aPKC-ι synergistically facilitate epithelial-mesenchymal transition of cholangiocarcinoma via GSK-3β/Snail signaling pathway. Oncotarget 2016;7:55191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Gao X, He Y, Gao LM, Feng J, Xie Y, Liu X, Liu L. Ser9-phosphorylated GSK3β induced by 14-3-3ζ actively antagonizes cell apoptosis in a NF-κB dependent manner. Biochem Cell Biol. 2014;92:349–56. [DOI] [PubMed] [Google Scholar]
- 138. Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–40. [DOI] [PubMed] [Google Scholar]
- 139. Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer 2015;15:73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Dupont S. Role of YAP/TAZ in cell-matrix adhesion-mediated signalling and mechanotransduction. Exp Cell Res. 2016;343:42–53. [DOI] [PubMed] [Google Scholar]
- 141. Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: Hippo signaling and beyond. Physiol Rev. 2014;94:1287–312. [DOI] [PubMed] [Google Scholar]
- 142. Johnson R, Halder G. The two faces of Hippo: Targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov. 2014;13:63–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Zanconato F, Battilana G, Cordenonsi M, Piccolo S. YAP/TAZ as therapeutic targets in cancer. Curr Opin Pharmacol. 2016;29:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature 2011;474:179–83. [DOI] [PubMed] [Google Scholar]
- 145. Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 2013;154:1047–59. [DOI] [PubMed] [Google Scholar]
- 146. Zanconato F, Forcato M, Battilana G, Azzolin L, Quaranta E, Bodega B, Rosato A, Bicciato S, Cordenonsi M, Piccolo S. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat Cell Biol. 2015;17:1218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Li X, Tao J, Cigliano A, Sini M, Calderaro J, Azoulay D, Wang C, Liu Y, Jiang L, Evert K, Demartis MI, Ribback S, Utpatel K, Dombrowski F, Evert M, Calvisi DF, Chen X. Co-activation of PIK3CA and Yap promotes development of hepatocellular and cholangiocellular tumors in mouse and human liver. Oncotarget 2015;6:10102–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Li J, Razumilava N, Gores GJ, Walters S, Mizuochi T, Mourya R, Bessho K, Wang YH, Glaser SS, Shivakumar P, Bezerra JA. Biliary repair and carcinogenesis are mediated by IL-33-dependent cholangiocyte proliferation. J Clin Invest. 2014;124:3241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Yamada D, Rizvi S, Razumilava N, Bronk SF, Davila JI, Champion MD, Borad MJ, Bezerra JA, Chen X, Gores GJ. IL-33 facilitates oncogene-induced cholangiocarcinoma in mice by an interleukin-6-sensitive mechanism. Hepatology 2015;61:1627–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Tschaharganeh DF, Chen X, Latzko P, Malz M, Gaida MM, Felix K, Ladu S, Singer S, Pinna F, Gretz N, Sticht C, Tomasi ML, Delogu S, Evert M, Fan B, Ribback S, Jiang L, Brozzetti S, Bergmann F, Dombrowski F, Schirmacher P, Calvisi DF, Breuhahn K. Yes-associated protein up-regulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma. Gastroenterology 2013;144:1530–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Pei T, Li Y, Wang J, Wang H, Liang Y, Shi H, Sun B, Yin D, Sun J, Song R, Pan S, Sun Y, Jiang H, Zheng T, Liu L. YAP is a critical oncogene in human cholangiocarcinoma. Oncotarget 2015;6:17206–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Li H, Wolfe A, Septer S, Edwards G, Zhong X, Abdulkarim AB, Ranganathan S, Apte U. Deregulation of Hippo kinase signalling in human hepatic malignancies. Liver Int. 2012;32:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Marti P, Stein C, Blumer T, Abraham Y, Dill MT, Pikiolek M, Orsini V, Jurisic G, Megel P, Makowska Z, Agarinis C, Tornillo L, Bouwmeester T, Ruffner H, Bauer A, Parker CN, Schmelzle T, Terracciano LM, Heim MH, Tchorz JS. YAP promotes proliferation, chemoresistance, and angiogenesis in human cholangiocarcinoma through TEAD transcription factors. Hepatology 2015;62:1497–510. [DOI] [PubMed] [Google Scholar]
- 154. Rizvi S, Yamada D, Hirsova P, Bronk SF, Werneburg NW, Krishnan A, Salim W, Zhang L, Trushina E, Truty MJ, Gores GJ. A Hippo and fibroblast growth factor receptor autocrine pathway in cholangiocarcinoma. J Biol Chem. 2016;291:8031–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Zheng T, Hong X, Wang J, Pei T, Liang Y, Yin D, Song R, Song X, Lu Z, Qi S, Liu J, Sun B, Xie C, Pan S, Li Y, Luo X, Li S, Fang X, Bhatta N, Jiang H, Liu L. Gankyrin promotes tumor growth and metastasis through activation of IL-6/STAT3 signaling in human cholangiocarcinoma. Hepatology 2014;59:935–46. [DOI] [PubMed] [Google Scholar]
- 156. Xu YF, Yang XQ, Lu XF, Guo S, Liu Y, Iqbal M, Ning SL, Yang H, Suo N, Chen YX. Fibroblast growth factor receptor 4 promotes progression and correlates to poor prognosis in cholangiocarcinoma. Biochem Biophys Res Commun. 2014;446(1):54–60. [DOI] [PubMed] [Google Scholar]
- 157. Zhou QX, Jiang XM, Wang ZD, Li CL, Cui YF. Enhanced expression of suppresser of cytokine signaling 3 inhibits the IL-6-induced epithelial-to-mesenchymal transition and cholangiocarcinoma cell metastasis. Med Oncol. 2015;32:105. [DOI] [PubMed] [Google Scholar]
- 158. Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12. [DOI] [PubMed] [Google Scholar]
- 159. Meng F, Henson R, Lang M, Wehbe H, Maheshwari S, Mendell JT, Jiang J, Schmittgen TD, Patel T. Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology 2006;130:2113–29. [DOI] [PubMed] [Google Scholar]
- 160. Chen L, Yan HX, Yang W, Hu L, Yu LX, Liu Q, Li L, Huang DD, Ding J, Shen F, Zhou WP, Wu MC, Wang HY. The role of microRNA expression pattern in human intrahepatic cholangiocarcinoma. J Hepatol. 2009;50:358–69. [DOI] [PubMed] [Google Scholar]
- 161. Selaru FM, Olaru AV, Kan T, David S, Cheng Y, Mori Y, Yang J, Paun B, Jin Z, Agarwal R, Hamilton JP, Abraham J, Georgiades C, Alvarez H, Vivekanandan P, Yu W, Maitra A, Torbenson M, Thuluvath PJ, Gores GJ, LaRusso NF, Hruban R, Meltzer SJ. MicroRNA-21 is overexpressed in human cholangiocarcinoma and regulates programmed cell death 4 and tissue inhibitor of metalloproteinase 3. Hepatology 2009;49:1595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Yang R, Chen Y, Tang C, Li H, Wang B, Yan Q, Hu J, Zou S. MicroRNA-144 suppresses cholangiocarcinoma cell proliferation and invasion through targeting platelet activating factor acetylhydrolase isoform 1b. BMC Cancer 2014;14:917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Qiao P, Li G, Bi W, Yang L, Yao L, Wu D. microRNA-34a inhibits epithelial mesenchymal transition in human cholangiocarcinoma by targeting Smad4 through transforming growth factor-beta/Smad pathway. BMC Cancer 2015;15:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Liu N, Jiang F, He TL, Zhang JK, Zhao J, Wang C, Jiang GX, Cao LP, Kang PC, Zhong XY, Lin TY, Cui YF. The roles of microRNA-122 overexpression in inhibiting proliferation and invasion and stimulating apoptosis of human cholangiocarcinoma cells. Sci Rep. 2015;5:16566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Yu J, Zhang W, Tang H, Qian H, Yang J, Zhu Z, Ren P, Lu B. Septin 2 accelerates the progression of biliary tract cancer and is negatively regulated by mir-140-5p. Gene 2016;589:20–6. [DOI] [PubMed] [Google Scholar]
- 166. Oishi N, Kumar MR, Roessler S, Ji J, Forgues M, Budhu A, Zhao X, Andersen JB, Ye QH, Jia HL, Qin LX, Yamashita T, Woo HG, Kim YJ, Kaneko S, Tang ZY, Thorgeirsson SS, Wang XW. Transcriptomic profiling reveals hepatic stem-like gene signatures and interplay of miR-200c and epithelial-mesenchymal transition in intrahepatic cholangiocarcinoma. Hepatology 2012;56:1792–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Qiu YH, Wei YP, Shen NJ, Wang ZC, Kan T, Yu WL, Yi B, Zhang YJ. miR-204 inhibits epithelial to mesenchymal transition by targeting slug in intrahepatic cholangiocarcinoma cells. Cell Physiol Biochem. 2013;32:1331–41. [DOI] [PubMed] [Google Scholar]
- 168. Zhu L, Huang F, Deng G, Nie W, Huang W, Xu H, Zheng S, Yi Z, Wan T. MicroRNA-212 targets FOXA1 and suppresses the proliferation and invasion of intrahepatic cholangiocarcinoma cells. Exp Ther Med. 2016;12:3790–6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 169. Li B, Han Q, Zhu Y, Yu Y, Wang J, Jiang X. Down-regulation of miR-214 contributes to intrahepatic cholangiocarcinoma metastasis by targeting Twist. FEBS J. 2012;279:2393–8. [DOI] [PubMed] [Google Scholar]
- 170. Li J, Tian F, Li D, Chen J, Jiang P, Zheng S, Li X, Wang S. MiR-605 represses PSMD10/Gankyrin and inhibits intrahepatic cholangiocarcinoma cell progression. FEBS Lett. 2014;588:3491–500. [DOI] [PubMed] [Google Scholar]
- 171. Huang Q, Liu L, Liu CH, You H, Shao F, Xie F, Lin XS, Hu SY, Zhang CH. MicroRNA-21 regulates the invasion and metastasis in cholangiocarcinoma and may be a potential biomarker for cancer prognosis. Asian Pac J Cancer Prev. 2013;14:829–34. [DOI] [PubMed] [Google Scholar]
- 172. Liu Z, Jin ZY, Liu CH, Xie F, Lin XS, Huang Q. MicroRNA-21 regulates biological behavior by inducing EMT in human cholangiocarcinoma. Int J Clin Exp Pathol. 2015;8:4684–94. [PMC free article] [PubMed] [Google Scholar]
- 173. Wang J, Xie C, Pan S, Liang Y, Han J, Lan Y, Sun J, Li K, Sun B, Yang G, Shi H, Li Y, Song R, Liu X, Zhu M, Yin D, Wang H, Song X, Lu Z, Jiang H, Zheng T, Liu L. N-myc downstream-regulated gene 2 inhibits human cholangiocarcinoma progression and is regulated by leukemia inhibitory factor/microRNA-181c negative feedback pathway. Hepatology 2016;64:1606–22. [DOI] [PubMed] [Google Scholar]
- 174. Li J, Yao L, Li G, Ma D, Sun C, Gao S, Zhang P, Gao F. miR-221 promotes epithelial-mesenchymal transition through targeting PTEN and forms a positive feedback loop with β-catenin/c-Jun signaling pathway in extra-hepatic cholangiocarcinoma. PLoS One 2015;10:e0141168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Poüs C, Klipfel L, Baillet A. Cancer-related functions and subcellular localizations of septins. Front Cell Dev Biol. 2016;4:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Lo FY, Chen HT, Cheng HC, Hsu HS, Wang YC. Overexpression of PAFAH1B1 is associated with tumor metastasis and poor survival in non-small cell lung cancer. Lung Cancer 2012;77:585–92. [DOI] [PubMed] [Google Scholar]
- 177. Frame MC, Inman GJ. NCAM is at the heart of reciprocal regulation of E-cadherin- and integrin-mediated adhesions via signaling modulation. Dev Cell 2008;15:494–6. [DOI] [PubMed] [Google Scholar]
- 178. Wang SC, Lin XL, Li J, Zhang TT, Wang HY, Shi JW, Yang S, Zhao WT, Xie RY, Wei F, Qin YJ, Chen L, Yang J, Yao KT, Xiao D. MicroRNA-122 triggers mesenchymal-epithelial transition and suppresses hepatocellular carcinoma cell motility and invasion by targeting RhoA. PLoS One 2014;9:e101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim Biophys Acta 2010;1803:55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Lee DC, Kang YK, Kim WH, Jang YJ, Kim DJ, Park IY, Sohn BH, Sohn HA, Lee HG, Lim JS, Kim JW, Song EY, Kim DM, Lee MN, Oh GT, Kim SJ, Park KC, Yoo HS, Choi JY, Yeom YI. Functional and clinical evidence for NDRG2 as a candidate suppressor of liver cancer metastasis. Cancer Res. 2008;68:4210–20. [DOI] [PubMed] [Google Scholar]
- 182. Lee DG, Lee SH, Kim JS, Park J, Cho YL, Kim KS, Jo DY, Song IC, Kim N, Yun HJ, Park YJ, Lee SJ, Lee HG, Bae KH, Lee SC, Shim S, Kim YM, Kwon YG, Kim JM, Lee HJ, Min JK. Loss of NDRG2 promotes epithelial-mesenchymal transition of gallbladder carcinoma cells through MMP-19-mediated Slug expression. J Hepatol. 2015;63:1429–39. [DOI] [PubMed] [Google Scholar]
- 183. Gores GJ. Cholangiocarcinoma: Preventing invasion as anti-cancer strategy. J Hepatol. 2003;38:671–3. [DOI] [PubMed] [Google Scholar]
- 184. Fabris L, Alvaro D. The prognosis of perihilar cholangiocarcinoma after radical treatments. Hepatology 2012;56:800–2. [DOI] [PubMed] [Google Scholar]
- 185. Goldberger NE, Hunter KW. A systems biology approach to defining metastatic biomarkers and signaling pathways. Wiley Interdiscip Rev Syst Biol Med. 2009;1:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer 2014;14:159–72. [DOI] [PubMed] [Google Scholar]
- 187. Entschladen F, Thyssen DA, Drell DW. Re-use of established drugs for anti-metastatic indications. Cells 2016;5(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Xu YF, Ge FJ, Han B, Yang XQ, Su H, Zhao AC, Zhao MH, Yang YB, Yang J. High-mobility group box 1 expression and lymph node metastasis in intrahepatic cholangiocarcinoma. World J Gastroenterol. 2015;21:3256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Yamada D, Kobayashi S, Wada H, Kawamoto K, Marubashi S, Eguchi H, Ishii H, Nagano H, Doki Y, Mori M. Role of crosstalk between interleukin-6 and transforming growth factor-beta 1 in epithelial-mesenchymal transition and chemoresistance in biliary tract cancer. Eur J Cancer 2013;49:1725–40. [DOI] [PubMed] [Google Scholar]
- 190. Zhou C, Zheng Y, Li L, Zhai W, Li R, Liang Z, Zhao L. Adrenomedullin promotes intrahepatic cholangiocellular carcinoma metastasis and invasion by inducing epithelial-mesenchymal transition. Oncol Rep. 2015;34:610–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Ma J, Chen M, Xia SK, Shu W, Guo Y, Wang YH, Xu Y, Bai XM, Zhang L, Zhang H, Zhang M, Wang YP, Leng J. Prostaglandin E2 promotes liver cancer cell growth by the upregulation of FUSE-binding protein 1 expression. Int J Oncol. 2013;42:1093–104. [DOI] [PubMed] [Google Scholar]
- 192. Sun B, Rong R, Jiang H, Zhang H, Wang Y, Bai X, Zhang M, Ma J, Xia S, Shu W, Zhang L, Leng J. Prostaglandin E2 receptor EP1 phosphorylate CREB and mediates MMP2 expression in human cholangiocarcinoma cells. Mol Cell Biochem. 2013;378:195–203. [DOI] [PubMed] [Google Scholar]
- 193. Du M, Shi F, Zhang H, Xia S, Zhang M, Ma J, Bai X, Zhang L, Wang Y, Cheng S, Yang Q, Leng J. Prostaglandin E2 promotes human cholangiocarcinoma cell proliferation, migration and invasion through the upregulation of β-catenin expression via EP3-4 receptor. Oncol Rep. 2015;34:715–26. [DOI] [PubMed] [Google Scholar]
- 194. Hunsawong T, Singsuksawat E, In-chon N, Chawengrattanachot W, Thuwajit C, Sripa B, Paupairoj A, Chau-in S, Thuwajit P. Estrogen is increased in male cholangiocarcinoma patients’ serum and stimulates invasion in cholangiocarcinoma cell lines in vitro. J Cancer Res Clin Oncol. 2012;138:1311–20. [DOI] [PubMed] [Google Scholar]