Abstract
Objective
To analyze patient risk factors and processes of care associated with secondary surgical site infection (SSI) after coronary artery bypass grafting (CABG).
Methods
Data were collected prospectively between February and October 2010 for consenting adult patients undergoing CABG with saphenous vein graft (SVG) conduits. Patients who developed a deep or superficial SSI of the leg or groin within 65 days of CABG were compared to those who did not develop a secondary SSI.
Results
Among 2,174 patients identified, 65 (3.0%) developed a secondary SSI. Median time to diagnosis was 16 days (interquartile range [IQR]: 11 - 29) with the majority (86%) diagnosed after discharge. Gram-positive bacteria were most common. Readmission was more common in patients with a secondary SSI (34% vs 17%, p < 0.01). After adjustment, an open SVG harvest approach was associated with an increased risk of secondary SSI (adjusted hazard ratio [HR]: 2.12, 95% confidence interval [CI]: 1.28, 3.48). Increased body mass index (BMI) (adjusted HR: 1.08, 95% CI: 1.04, 1.12) and packed red blood cell (PRBC) transfusions (adjusted HR: 1.13, 95% CI: 1.05, 1.22) were associated with a higher risk of secondary SSI. Antibiotic type, antibiotic duration, and post-operative hyperglycemia were not associated with risk of secondary SSI.
Conclusions
Secondary SSI following CABG continues to be an important source of morbidity. This serious complication often occurs after discharge and is associated with open SVG harvesting, larger BMI, and blood transfusions. Patients with a secondary SSI have longer lengths of stay and are readmitted more frequently.
Introduction
Despite improvements in medical care and the increased emphasis on quality improvement programs to reduce postoperative infections, nearly 5% of patients experience major infection after cardiac surgery.1 Recently published findings from a prospective multi-institutional cohort study of infections associated with cardiac surgery observed substantial increases in morbidity, mortality, and costs associated with these events. 1,2 However, secondary surgical site infections in patients who undergo coronary artery bypass grafting (CABG) with saphenous vein graft (SVG) harvesting were one infection of interest not analyzed in this initial report.
Surgical site infections (SSI) of the SVG harvest site following CABG affect 1-4% of patients and are associated with substantial morbidity and cost. 3-5 In rare cases, these infections can even lead to limb loss.5 While numerous outcome studies after saphenous vein harvesting have been reported, few focus on infection as the primary endpoint and those that do frequently lack follow-up past discharge or 30 days postoperatively, are non-adjudicated, and are frequently retrospective single-center analyses. 4,6-20
In this study, using data previously collected by the Cardiothoracic Surgical Trials Network (CTSN), we aim to characterize secondary surgical site infections of the leg or groin in patients undergoing CABG with saphenous vein conduits and to analyze patient risk factors and processes of care associated with these infections.
Methods
Patient Population
As previously published, all consenting adult cardiac surgery patients at CTSN sites without an active systemic infection were enrolled in the prospective observational study “Management Practices and the Risk of Infections following Cardiac Surgery” (see appendix for list of sites). We selected all patients in the CTSN study who underwent a CABG procedure (with or without concomitant procedures) with saphenous vein conduit harvesting (n = 2,174, Figure 1).
Figure 1.
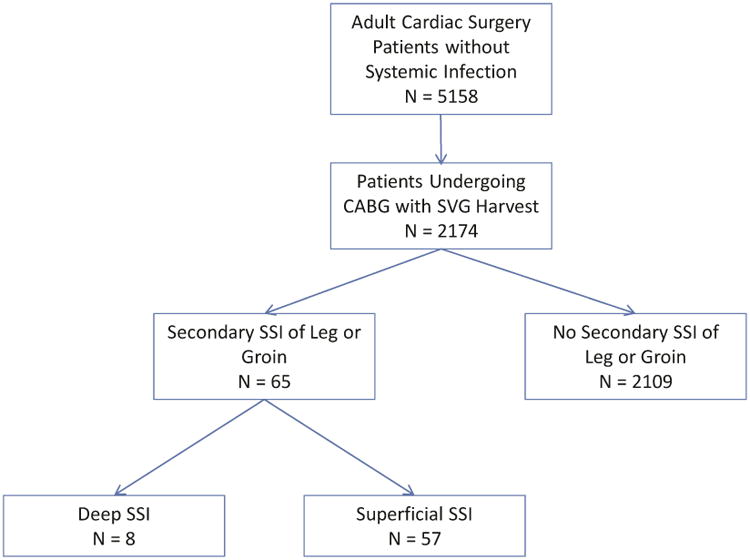
CONSORT diagram
Study Design
Baseline and operative characteristics, process of care data, and postoperative complications were captured up to 65 days post-procedure as part of the CTSN infection study. The primary outcome of interest in this analysis was the incidence of surgical site infection (SSI) of the secondary incision site in the leg or groin (e.g. saphenous vein harvest site, perfusion cannulation site). Secondary SSIs were categorized as deep or superficial. A secondary SSI was defined as an infection that occurred within 65 days of the procedure, involved the deep soft tissues of the incision (deep SSI) or the skin and subcutaneous tissue (superficial SSI), and had at least one of the following: purulent discharge; a spontaneous incisional dehiscence or an incision opened in response to fever or localized pain/tenderness and with a positive culture or without a culture in the presence of fever, localized pain, and/or tenderness; an abscess or other evidence of infection found on direct examination, during reoperation or by histopathologic or radiologic examination; or a diagnosis of an incisional SSI by the physician. Data related to the time to onset of secondary SSI, risk factors potentially affecting the incidence of SSIs, and microbial isolates related to the SSIs were also analyzed.
Statistical Analysis
Patients who did and did not develop a secondary SSI were compared with regards to baseline characteristics, operative characteristics, postoperative management, and outcomes. Continuous variables were compared using a t-test (for means) or Wilcoxon-Mann-Whitney test (for medians) while categorical variables were compared using Chi-square test or Fisher's exact test as appropriate. A Cox proportional hazards regression model of time to secondary SSI was built using backwards stepwise regression; death was accounted for as a competing risk.21 Variables found to be associated with a secondary SSI (at p < 0.20) on univariate analysis were considered for inclusion in the multivariate model; all variables in the final model were significant at the p ≤ 0.05 level. Complete case analysis was used for all adjusted models. Although frequency of use of vein graft harvest technique varied widely by site with most open harvests in Canada, we adjusted for patient-level risk factors and management practices rather than by site in order to avoid obscuring these risks.
All statistical analyses were performed using SAS statistical software (version 9.4, Cary, NC).
Results
Incidence of Surgical Site Infections
A total of 2,174 patients underwent CABG with saphenous vein graft harvesting, of whom 65 (3.0%) developed a secondary SSI within 65 days of the index operation. The cumulative incidence of secondary SSIs of the leg or groin by time from index procedure is shown in Figure 2. Median time to secondary SSI was 16 days (interquartile range [IQR]: 11- 29). The majority of these infections were diagnosed after discharge from the index hospitalization (n=56, 86%), most within 30 days of discharge (n=46, 82%).
Figure 2.
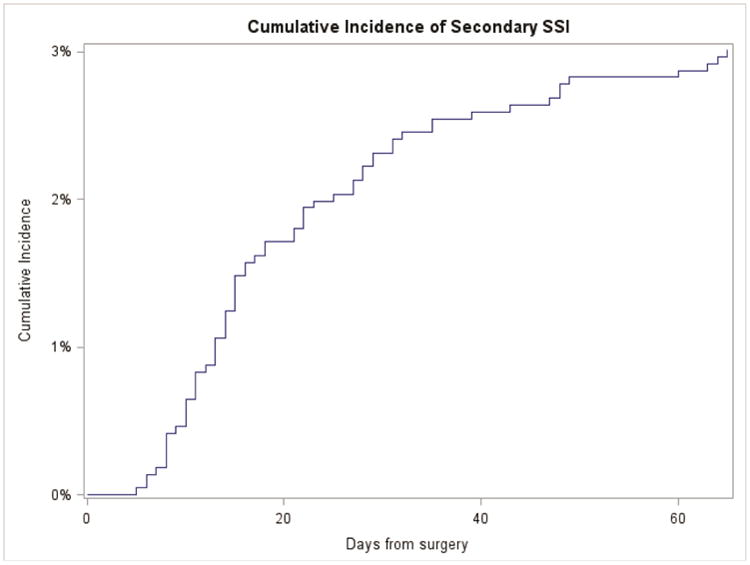
Cumulative incidence of secondary SSI of the leg or groin in patients undergoing CABG with saphenous vein harvesting by time from index procedure.
Of the secondary SSIs, 8 (12%) were defined as deep while 57 (88%) were defined as superficial. The median time to a deep secondary SSI was 27 days (IQR: 14 - 44) while the median time to a superficial secondary SSI was 15 days (IQR: 11 - 28). No patient with a superficial infection progressed to a deep infection.
Factors Associated with Surgical Site Infections
Baseline characteristics between those with and without a secondary SSI are shown in Table 1. With the exception of body mass index (BMI), baseline demographics and cardiac and non-cardiac morbidities were similar between groups. BMI was significantly associated with a higher rate of secondary SSI (median BMI among patients who developed and those who did not develop a secondary SSI: 31 vs. 29 kg/m2, p < 0.01).
Table 1. Baseline characteristics of patients undergoing CABG with SVG harvesting with and without secondary SSIa.
| Overall (n=2174) | Secondary SSI (n=65) | No Secondary SSI (n=2109) | P-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 67 (10.5) | 68 (9.9) | 67 (10.5) | 0.53 |
| Male | 1632 (75.1) | 46 (70.8) | 1586 (75.2) | 0.42 |
| White | 1754 (80.8) | 56 (86.2) | 1698 (80.6) | 0.26 |
| Body mass index (kg/m2) | 29 (26, 33) | 31 (28, 37) | 29 (26, 33) | <0.01 |
| Cardiac morbidity | ||||
| Heart failure | 578 (26.6) | 23 (35.4) | 555 (26.3) | 0.10 |
| Ejection fraction | 55 (45, 60) | 55 (45, 60) | 55 (45, 60) | 0.83 |
| Previous cardiac surgery | 231 (10.6) | 9 (13.9) | 222 (10.5) | 0.39 |
| Peripheral vascular disease | 309 (14.2) | 13 (20.0) | 296 (14.0) | 0.17 |
| Noncardiac morbidity | ||||
| Smoking history | 1169 (53.8) | 41 (63.1) | 1128 (53.5) | 0.13 |
| Hypertension | 1825 (84.0) | 58 (89.2) | 1767 (83.8) | 0.24 |
| Hyperlipidemia | 1696 (78.1) | 57 (87.7) | 1639 (77.8) | 0.06 |
| Diabetesb | 724 (33.3) | 26 (40.0) | 698 (33.1) | 0.24 |
| Renal insufficiency | 306 (14.1) | 13 (20.0) | 293 (13.9) | 0.16 |
| Corticosteroids | 68 (3.1) | 4 (6.3) | 64 (3.0) | 0.14 |
Continuous variables are expressed as mean (STD) or median (IQR), as appropriate, and categorical variables as count (%).
Insulin or oral medications.
Operative characteristics including type of procedure (isolated CABG, CABG + valve, CABG + other), urgency of surgery, operative time, cardiopulmonary bypass time, and number of vein anastomoses, were not significantly different between groups (Table 2). Most patients (n=2,171, 99.9%) had a sternotomy as the primary incision. Endoscopic vein harvesting was less frequently used in patients with secondary SSI (60.0% vs. 75.6%, p < 0.01).
Table 2. Operative and process of care characteristics of patients undergoing CABG with SVG harvesting with and without secondary SSIa.
| Overall (n=2174) | Secondary SSI (n=65) | No Secondary SSI (n=2109) | P-value | |
|---|---|---|---|---|
| Operative | ||||
| Procedure | 0.32 | |||
| Isolated CABG | 1486 (68.4) | 48 (73.9) | 1438 (68.2) | |
| CABG + valve | 566 (26.0) | 12 (18.5) | 554 (26.3) | |
| Other | 122 (5.6) | 5 (7.7) | 117 (5.5) | |
| Surgery Type | 0.71 | |||
| Elective | 1411 (64.9) | 42 (64.6) | 1369 (64.9) | |
| Urgent | 692 (31.8) | 22 (33.9) | 670 (31.8) | |
| Emergent | 71 (3.3) | 1 (1.5) | 70 (3.3) | |
| Operative time (hours) | 4.5 (3.5, 5.6) | 4.5 (3.4, 5.7) | 4.5 (3.5, 5.6) | 0.71 |
| Cardiopulmonary bypass | 1914 (88.0) | 59 (90.8) | 1855 (88.0) | 0.49 |
| Bypass time (minutesb) | 107 (80, 138) | 106 (73, 138) | 107 (80, 138) | 0.89 |
| Sternotomy | 2171 (99.9) | 65 (100.0) | 2106 (99.9) | 1.00 |
| Endoscopic SVH | 1633 (75.1) | 39 (60.0) | 1594 (75.6) | <0.01 |
| # Vein anastomoses | 2 (1, 2) | 2 (1, 2) | 2 (1, 2) | 0.58 |
| Process of Care | ||||
| Packed red blood cells (units) | 1 (0, 3) | 2 (0, 4) | 1 (0, 3) | 0.05 |
| Surgical site scrub with chlorhexidine | 1721 (80.4) | 55 (84.6) | 1666 (80.3) | 0.38 |
| 2nd generation cephalosporin | 1000 (46.0) | 28 (43.1) | 972 (46.1) | 0.63 |
| Continuation of antimicrobials | 0.78 | |||
| 0-24 hours | 1253 (57.6) | 40 (61.5) | 1213 (57.5) | |
| 24-48 hours | 791 (36.4) | 22 (33.9) | 769 (36.5) | |
| >48 hours | 130 (6.0) | 3 (4.6) | 127 (6.0) | |
| Post-operative hyperglycemiac | 1070 (49.2) | 36 (55.4) | 1034 (49.1) | 0.31 |
Continuous variables are expressed as median (IQR) and categorical variables as count (%).
88% of patients had on-pump surgical procedures.
Post-operative hyperglycemia is defined as a blood glucose >180 mg/dL within 48 hours after surgery.
Key: CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; SD, standard deviation
Furthermore, patients who developed a secondary SSI were significantly more likely to have received a larger transfusion of packed red blood cells (PRBC) during their index hospitalization than patients who did not develop an SSI (median [IQR] 2 units [0 - 4] vs. 1 unit [0 - 3], p = 0.05). Preoperative anemia did not affect risk of infection (p = 0.87) or modify the risk associated with transfusion (p = 0.69).There were no significant differences in infection rates with regards to type of surgical scrub (chlorhexidine or other), type of antibiotic given (2nd generation cephalosporin or other), duration of antimicrobial therapy postoperatively, or presence or absence of post-operative hyperglycemia.
Factors associated with the development of a secondary SSI in multivariable analysis treating death as a competing risk are shown in Figure 3. The use of an open approach (adjusted hazard ratio [HR]: 2.12, 95% confidence interval [CI]: 1.28, 3.48) remained associated with secondary SSIs. Increased body mass index (adjusted HR per kg/m2: 1.08, 95% CI: 1.04, 1.12) and PRBC transfusion (adjusted HR per unit: 1.13, 95% CI: 1.05, 1.22) both remained associated with a higher incidence of secondary SSIs. Neither the duration of postoperative antibiotics, type of antibiotics given, nor presence or absence of post-operative hyperglycemia was associated with a significant increase or decrease in secondary SSIs with multivariable modeling. A sensitivity analysis of U.S. patients alone with predominantly endoscopic vein harvest confirmed the risk associated with increased BMI and PRBC transfusion.
Figure 3.
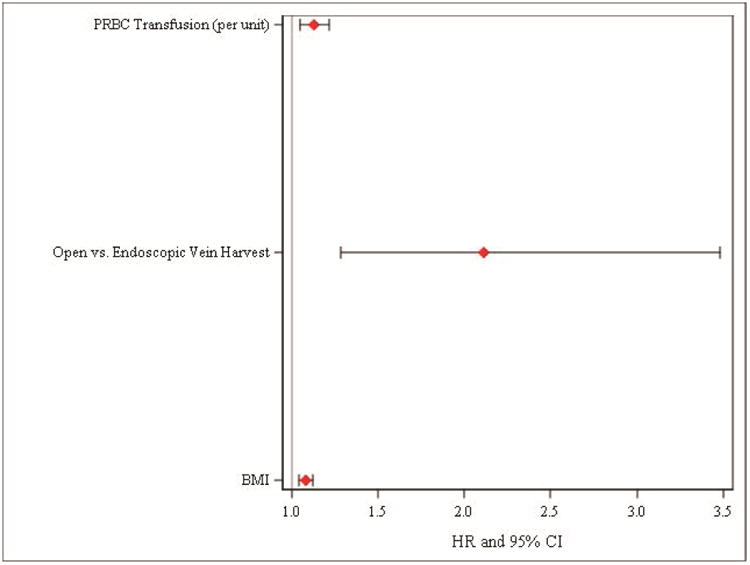
Factors associated with the development of a secondary SSI of the leg or groin in patients undergoing CABG with saphenous vein harvesting after multivariable adjustment.
Organisms Responsible for Secondary Surgical Site Infections
Among patients with a deep secondary SSI, 7 (87.5%) of patients had culture data. Equal numbers of Gram-positive and -negative organisms were isolated; one patient had both Gram-positive and Gram-negative bacteria isolated. Bacteria isolated were Coagulase negative staphylococcus (MRSE), Enterobacter cloacae, Enterococcus (n = 2), Proteus mirabilis, Pseudomonas aeruginosa (n = 2), Staphylococcus aureus (Methicillin resistant), and Staphylococcus aureus (Methicillin sensitive). There were no fungal deep secondary SSIs. Five patients had a single isolate, while two had multiple organisms isolated.
Among patients with a superficial secondary SSI, 22 (38.6%) had culture data. Gram-positive bacteria were most common (68%) though Gram-negative bacteria were not uncommon (23%). Candida/mixed skin flora were isolated in one patient, and both candida and Gram-negative bacteria were isolated in one patient. Staphylococcus aureus was the most common isolate found in 12 patients (2/3 were methicillin resistant and 1/3 were methicillin sensitive). Candida albicans, Coagulase negative staphylococcus (n = 2), Corynebacterium striatum, Enterobacter, Enterococcus (n = 2), Escherichia coli, mixed skin flora, Moranella morganii, Serratia marcescens, Staphylococcus epidermididis (Methicillin resistant), Stenotrophomas, and Streptococcus species were also isolated.
Outcomes
More patients who developed a secondary SSI were readmitted within 65 days of surgery than patients who did not develop a secondary SSI (34% vs 17%, p < 0.01). Of 27 readmissions in 23 patients with a secondary SSI who were readmitted, 21 (77.8%) readmissions were due to the secondary SSI. The median length of stay during the re-hospitalization was 7 days (IQR: 5 - 9). There was no significant difference in a low rate of 65-day mortality among patients with and without a secondary SSI (0.0% vs 1.9%, p = 0.63).
Discussion
In this large prospective multi-institutional cohort study, we demonstrated that secondary SSIs after CABG with SVG conduit are not uncommon, are often diagnosed after initial discharge, and are associated with more frequent hospital readmissions. In addition, SSI occurs with more frequency in those who undergo open saphenous vein harvest, receive more units of PRBCs, and have a higher BMI. Common widely endorsed process of care measures, specifically, duration of antibiotic therapy, type of antibiotic prophylaxis, and post-operative glycemic control, were not associated with secondary SSI in this analysis.
The incidence of secondary SSI in this study was 3.0% at 65 days of follow-up, which while similar to other recent large observational studies is greater than the most common major infection reported in the CTSN infection study. 1,3,4 The initial publication from the CTSN reported that nearly 5% of patients experience a major infection after cardiac surgery, with pneumonia (2.38%), blood stream infections (1.09%), and C. difficile colitis (0.97%) being the most common.1 Other surgical site infections reported included deep surgical site infections of the chest and groin (0.56 and 0.21% respectively) and superficial infections of the chest and groin (2.93 and 1.56% respectively).1 While secondary leg and groin site infections were not included in the original report, the overall incidence is clearly not trivial. The prospective, multicenter nature of this cohort study provides a unique opportunity to characterize these infections.
Like other recent reports, our results show that endoscopic saphenous vein harvesting is associated with a significantly lower incidence of secondary SSI in patients undergoing coronary artery bypass grafting. Endoscopic saphenous vein harvesting was first introduced in the mid-1990's and is currently estimated to be the method of choice in over 70% of CABG procedures in the United States.6,7,8 Three-quarters of patients undergoing CABG in this study had endoscopic vein harvesting, of whom 2.4% developed a secondary SSI which is less than half the proportion seen in the open vein harvest group (4.8%, p < 0.01).
Two large recent studies have examined the association of long-term outcomes following endoscopic vein harvesting after coronary artery bypass grafting. While both of these studies were conducted to primarily look at long term consequences of endoscopic versus open saphenous vein harvest in CABG, both also included an examination of saphenous vein graft site SSIs. Williams et al conducted a retrospective observational study of 235,394 Medicare patients undergoing isolated CABG between 2003 and 2008 at 934 surgical centers in which data from the Society of Thoracic Surgeons (STS) national database was linked to Medicare files to allow longitudinal assessment.3 In this study 52% of patients received endoscopic vein-graft harvesting during CABG surgery. Endoscopic vein-graft harvest was associated with a lower harvest site wound complication rate relative to open vein-graft harvesting at 30 days (2.97% compared to 3.60%, risk adjusted HR 0.83, 95% CI 0.77-0.89, p < 0.001). Dacey et al reported on 8,542 patients who underwent isolated CABG between 2001 and 2004 in which 52.5% of patients received endoscopic vein-graft harvesting for the Northern New England Cardiovascular Disease Study Group.4 Endoscopic vein harvesting was also associated a decreased risk of leg wound infections (0.2 vs 1.1%, p<0.001).
Our results support the hypothesis that the introduction of endoscopic saphenous harvesting has decreased the overall occurrence of these non-trivial secondary surgical infections. Importantly, our analysis suggests that this benefit extends not only to patients undergoing isolated CABG but also combined and emergent/urgent procedures as these patients were not excluded in our analysis.
BMI and the need for transfusion of more units of PRBCs were strongly associated with risk of developing a secondary SSI on multivariable modeling with those of higher BMI being more likely to develop a secondary SSI. This is not surprising given that patients with a higher BMI are generally thought to be at greater risk of postoperative infection and poor wound healing. 22,23 There are numerous theories as to why this relationship occurs, including reduced wound circulation and oxygenation, as well as increased wound tension. The need for transfusion of PRBCs was also associated with risk of developing a secondary SSI (HR 1.13 per unit, p < 0.01) and is consistent with others studies such as that reported by Horvath et al, in which there was an incremental crude risk for major infection of 29% for each unit of pack red blood cells transfused in cardiac surgery.24 Previous studies have demonstrated blood transfusion may lead to immunomodulatory effects, which may be responsible for the increased incidence of SSI.25 Alternatively, blood transfusion may be a surrogate for pre-operative anemia, which may also increase the risk of SSI due to reduced oxygen delivery to the tissues.
Other reported risk factors such as female gender, peripheral vascular disease, tobacco use, diabetes mellitus, renal failure, type of surgery and urgency, preoperative ejection fraction, need for an intra-aortic balloon pump, and postoperative hyperglycemia were not associated with a statistically significant increased risk of infection in our study, however, this should not be construed as evidence those factors are not associated with infection particularly given the low number of infections in the cohort. 25-28
As other studies have reported a risk modification of hyperglycemia by diabetes, we also investigated a possible interaction between diabetes and hyperglycemia but were likely limited by power. 29-33 BMI, which is included in the multivariable model, is correlated with diabetes but as a continuous variable provides more power than diabetes. Differences in the definition of hyperglycemia in the literature may also partly explain why we did not find an association between hyperglycemia and risk of infection; in this study, we used a broad definition of hyperglycemia: a single occurrence of blood glucose above 180 mg/dL within 48 hours of surgery.
While the organism data presented is limited by a low reporting rate, the nature of organisms isolated from these infections is nonetheless interesting for several reasons. First there is paucity of data of this nature reported in the literature. Second, one often makes the assumption that a secondary SSI will be the result of skin flora and very likely a Gram-positive organism, however, based on these limited data, we have shown that Gram-negative organisms and occasionally yeast are also isolated. This suggests that routine culture of these wounds should be considered to guide appropriate antibiotic coverage and that initial coverage should be appropriate for at least both Gram-positive and -negative organisms. Third, Staphylococcus aureus was the most commonly isolated organism despite the fact that it was tested and identified preoperatively in 1194 (55%) patients. The majority of these patients (71%) received S. aureus prophylaxis.
A major interest of ours was to investigate the recently instituted process of care measures endorsed by numerous regulatory agencies, including the Centers for Medicare and Medicaid Services and the Joint Commission, on the incidence of secondary SSIs. In 2002, the Centers for Medicare and Medicaid Services implemented the Surgical Infection Prevention Project to decrease morbidity and mortality associated with postoperative surgical site infections. The key measures implemented to decrease surgical site infections as related to cardiac surgery include 1) administration of parenteral antimicrobial prophylaxis within 1 hour of incision (2 hours for vancomycin or fluoroquinolones), 2) discontinuation of prophylactic antimicrobials within 48 hours of cardiac surgery, 3) proper removal of hair, and 4) appropriate blood glucose control.34
There were not large enough differences in the timing of parenteral antimicrobial prophylaxis or proper removal of hair to allow comparison in this cohort as virtually all patients had hair clipped and had prophylactic antibiotics administered appropriately. However, there was enough variability in duration of perioperative antibiotic therapy, type of antibiotic prophylaxis, and post-operative glycemic control to allow multivariable modeling that showed that practice variations in these factors were not associated with the development of a secondary SSI. While one can argue that we were simply underpowered to show a difference in these practice variations, there is an alternative interpretation – even if these process of care measures are adhered to rigidly, the incidence of secondary SSI will never be zero. This lends support to the concept that in major cardiac surgery, certain “never” events will never have a zero incidence, or that we are not looking at the correct combinations of process of care measures that will consistently prevent SSI events.
Perhaps the most interesting finding of our study is that, with patient follow up to 65 days after the index operation, we were able to determine that the majority of secondary SSI were diagnosed after discharge from index hospitalization, and a quarter were diagnosed more than 30 days after the index operation. This later group would typically be missed in the STS Adult Cardiac Surgery Database as it only captures events to 30 days after the index operation. In addition to the late occurrence of secondary SSIs, of those who developed a secondary SSI, the proportion of readmissions within 65 days of surgery was significantly higher than in those who did not develop a secondary SSI (34% vs 17%, p < 0.01) – and of those with a secondary SSI who were readmitted, over 70% were readmitted because of the secondary SSI.
Although this study carries the strength of being a prospective cohort, there are several important limitations. First, while limiting the population to those patients with CABG and SVG conduit would imply that the secondary SSI reported was a saphenous vein graft harvest site infection, the data point collected regarding secondary SSI did not differentiate between groin and leg. It is possible that what we presume to be SVG harvest site infections were actually groin cannulation sites (also not captured explicitly). The patients most likely in question would be the 9 infections in those with a previous sternotomy. Unfortunately, it is impossible to discern this subtle difference from this dataset, however, we are confident that all reported secondary SSIs were of the groin or leg in patients that underwent CABG with SVG harvesting. Second, although prospective, this study was not powered to examine the endpoint of secondary SSI, so its predictive power is limited. In this regard, our inability to show a benefit of any of the process of care variables could be due to lack of power. In addition, patients were not randomized to receive or not receive these measures, so there could be unobserved factors that were not measured or analyzed that explain our results. Third, our study unfortunately lacked data on method or outcome of treatment for secondary SSI. There was also limited data collected on vein harvest – we had no detail about who performed the vein harvest, the technique used for an open harvest, or whether an endoscopic harvest was attempted before converting to an open harvest. Use of drains, skin closure techniques, and type of dressing were not collected for the secondary surgical incision, and these factors may affect the risk of infection. No scoring system, such as that developed by Elahi et al35, was used to assess the severity of the secondary SSIs. Last, although overall follow-up was longer than in most registries and was 98% complete, events that occurred after 65 days would not have been identified. Occasionally follow up was conducted by phone if patients refused to return for the follow up visit. However, for the 2% of patients who did not have complete follow up, a medical record search was conducted and any documented infectious complications were reported. Serious infections reported by phone or found in a medical record search were all adjudicated by an Event Adjudication Committee of experts in infectious diseases.
Despite close adherence to commonly practiced process of care measures, both deep and superficial secondary SSIs continue to be an important source of morbidity in CABG patients, frequently resulting in hospital readmission. While we can identify patients at higher risk for these infections, such as those who undergo open harvesting of the saphenous vein, have a higher BMI, or receive more units of packed red blood cells, it is unlikely that the incidence of secondary SSI will ever be zero. Developing a better understanding of both patient factors as well as modifiable processes of care associated with secondary SSIs will help guide future endeavors focused on reducing the incidence of these morbid and costly complications.
Supplementary Material
Acknowledgments
Sources of Funding: A cooperative agreement (U01 HL088942) funded by the National Heart, Lung, and Blood Institute and the National Institute of Neurological Disorders and Stroke of the NIH and the Canadian Institutes of Health Research.
Abbreviations
- CABG
Coronary artery bypass grafting
- CI
Confidence interval
- CIHR
Canadian Institutes for Health Research
- COPD
Chronic obstructive pulmonary disease
- CTSN
Cardiothoracic Surgical Trials Network
- HR
Hazard ratio
- IQR
Interquartile range
- MRSA
Methicillin-resistant Staphylococcus aureus
- MRSE
Methicillin-resistant Staphylococcus epidermidis
- NHLBI
National Heart, Lung, and Blood Institute
- PRBC
Packed red blood cells
- SCIP
Surgical Care Improvement Project
- SSI
Surgical site infection
- SD
Standard deviation
- SVG
Saphenous vein graft
Footnotes
Clinical Trial Registry Number: NCT01089712
Conflicts of Interest: All authors have no conflicts of interest to disclose.
Central Message: Secondary surgical site infections after coronary artery bypass grafting are neither uncommon nor trivial and often require hospital readmission.
Perspective Statement: Deep and superficial secondary surgical site infections after coronary artery bypass grafting are serious complications that often occur after initial hospital discharge and require readmission. Patients who undergo open harvesting of the saphenous vein, have a higher BMI, and receive more packed red blood cells are at higher risk.
Central Picture Legend: Cumulative incidence of secondary SSI by time from index procedure.
Supplemental Material: Appendix EI: CTSN Members
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gelijns AC, Moskowitz AJ, Acker MA, et al. Management Practices and Major Infections After Cardiac Surgery. Journal of the American College of Cardiology. 2014;64(4):372–381. doi: 10.1016/j.jacc.2014.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greco G, Shi W, Michler RE, et al. Costs Associated With HealthCare-Associated Infections in Cardiac Surgery. Journal of the American College of Cardiology. 2017;65(1):15–23. doi: 10.1016/j.jacc.2014.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams JB, Peterson ED, Brennan JM, et al. Association Between Endoscopic vs Open Vein-Graft Harvesting and Mortality, Wound Complications, and Cardiovascular Events in Patients Undergoing CABG Surgery. JAMA. 2012;308:475–484. doi: 10.1001/jama.2012.8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dacey LJ, Braxton JH, Kramer RS, et al. Long-Term Outcomes of Endoscopic Vein Harvesting After Coronary Artery Bypass Grafting. Circulation. 2011;123(2):147–153. doi: 10.1161/CIRCULATIONAHA.110.960765. [DOI] [PubMed] [Google Scholar]
- 5.Paletta C, Huang D, Fiore A, Swartz M, Rilloraza F, Gardiner J. Major Leg Wound Complications After Saphenous Vein Harvest for Coronary Revascularization. The Annals of Thoracic Surgery. 2000 Jul;:492–497. doi: 10.1016/s0003-4975(00)01414-4. [DOI] [PubMed] [Google Scholar]
- 6.Allen K, Griffith G, Heimansohn D, et al. Endoscopic Versus Traditional Saphenous Vein Harvesting: A Prospective, Randomized Trial. 1998 Jul;:1–6. doi: 10.1016/s0003-4975(98)00392-0. [DOI] [PubMed] [Google Scholar]
- 7.Puskas JD, Wright CE, PK M, et al. A Randomized Trial of Endoscopic Versus Open Saphenous Vein Harvest in Coronary Bypass Surgery. 1999 Sep;:1–4. doi: 10.1016/s0003-4975(99)00952-2. [DOI] [PubMed] [Google Scholar]
- 8.Shahian DM, O'Brien SM, Filardo G, et al. The Society of Thoracic Surgeons 2008 Cardiac Surgery Risk Models: Part 1—Coronary Artery Bypass Grafting Surgery. ATS. 2009;88(S):S2–S22. doi: 10.1016/j.athoracsur.2009.05.053. [DOI] [PubMed] [Google Scholar]
- 9.Lopes R, Hafley G, Allen K, et al. Endoscopic versus Open Vein-Graft Harvesting in Coronary-Artery Bypass Surgery. 2009 Jul;:1–11. doi: 10.1056/NEJMoa0900708. [DOI] [PubMed] [Google Scholar]
- 10.Yun KL, Wu Y, Aharonian V, et al. Randomized trial of endoscopic versus open vein harvest for coronary artery bypass grafting: Six-month patency rates. The Journal of Thoracic and Cardiovascular Surgery. 2005;129(3):496–503. doi: 10.1016/j.jtcvs.2004.08.054. [DOI] [PubMed] [Google Scholar]
- 11.Kiaii B, Moon BC, Massel D, et al. A prospective randomized trial of endoscopic versus conventional harvesting of the saphenous vein in coronary artery bypass surgery. The Journal of Thoracic and Cardiovascular Surgery. 2002;123(2):204–212. doi: 10.1067/mtc.2002.118682. [DOI] [PubMed] [Google Scholar]
- 12.Andreasen JJ, Nekrasas V, Dethlefsen C. Endoscopic vs open saphenous vein harvest for coronary artery bypass grafting: a prospective randomized trial☆. Eur J Cardiothorac Surg. 2008;34(2):384–389. doi: 10.1016/j.ejcts.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 13.Schurr U, Lachat M, Reuthebuch O, et al. Endoscopic Saphenous Vein Harvesting for CABG ± a Randomized, Prospective Trial. 2017 Jul;:1–4. doi: 10.1055/s-2002-32412. [DOI] [PubMed] [Google Scholar]
- 14.Allen KB, Heimansohn DA, Robison RJ, Schier JJ, Griffith GL, Fitzgerald EB. Influence of endoscopic versus traditional saphenectomy on event-free survival: five-year follow-up of a prospective randomized trial. Heart Surg Forum. 2003;6(6):E143–E145. [PubMed] [Google Scholar]
- 15.Allen K, Cheng D, Cohn W, et al. Endoscopic Vascular Harvest in Coronary Artery Bypass Grafting Surgery: A Consensus Statement of the International Society of Minimally Invasive Cardiothoracic Surgery (ISMICS) 2005. Innovations. 2006;1:51–60. doi: 10.1097/01.gim.0000196315.32179.82. [DOI] [PubMed] [Google Scholar]
- 16.Markar SR, Kutty R, Edmonds L, Sadat U, Nair S. A meta-analysis of minimally invasive versus traditional open vein harvest technique for coronary artery bypass graft surgery. Interactive CardioVascular and Thoracic Surgery. 2010;10(2):266–270. doi: 10.1510/icvts.2009.222430. [DOI] [PubMed] [Google Scholar]
- 17.Ouzounian M, Hassan A, Buth KJ, et al. Impact of Endoscopic Versus Open Saphenous Vein Harvest Techniques on Outcomes After Coronary Artery Bypass Grafting. ATS. 2010;89(2):403–408. doi: 10.1016/j.athoracsur.2009.09.061. [DOI] [PubMed] [Google Scholar]
- 18.Alexander J, Hafley G, Harrington R, Peterson E, Ferguson T, Lorenz T. Efficacy and Safety of Edifoligide, an E2F Transcription Factor Decoy, for Prevention of Vein Graft Failure Following Coronary Artery Bypass Graft Surgery. 2005 Nov;:1–9. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 19.Shroyer A, Frederick G, Hattler B, et al. On-Pump versus Off-Pump Coronary-Artery Bypass Surgery. The New England Journal of Medicine. 2009 Oct;:1–12. doi: 10.1056/NEJMoa0902905. [DOI] [PubMed] [Google Scholar]
- 20.Zenati MA, Shroyer L, Collins JF, et al. Impact of endoscopic versus open saphenous vein harvest technique on late coronary artery bypass grafting patient outcomes in the ROOBY (Randomized On/Off Bypass) Trial. The Journal of Thoracic and Cardiovascular Surgery. 2011;141(2):338–344. doi: 10.1016/j.jtcvs.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Scrucca L, Santucci A, Aversa F. Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplantation. 2010;45(9):1388–1395. doi: 10.1038/bmt.2009.359. [DOI] [PubMed] [Google Scholar]
- 22.Rahmanian PB, Adams DH, Castillo JG, Chikwe J, Bodian CA, Filsoufi F. Impact of Body Mass Index on Early Outcome and Late Survival in Patients Undergoing Coronary Artery Bypass Grafting or Valve Surgery or Both. The American Journal of Cardiology. 2007;100(11):1702–1708. doi: 10.1016/j.amjcard.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Waisbren E, Rosen H, Bader AM, Lipsitz SR, Rogers SO, Eriksson E. Percent Body Fat and Prediction of Surgical Site Infection. Journal of the American College of Surgeons. 2010;210(4):381–389. doi: 10.1016/j.jamcollsurg.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Horvath KA, Acker MA, Chang H, et al. Blood Transfusion and Infection After Cardiac Surgery. ATS. 2013;95(6):2194–2201. doi: 10.1016/j.athoracsur.2012.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.cuyer LE, Murphy D, Little J, Fraser V. The Epidemiology of Chest and Leg Wound Infections following Cardiothoracic Surgery. Clinical Infectious Diseases. 2017;22:424–429. doi: 10.1093/clinids/22.3.424. [DOI] [PubMed] [Google Scholar]
- 26.DeLaria GA, Hunter JA, Goldin MD, Serry C, Javid H, Najafi H. Leg wound complications associated with coronary revascularization. The Journal of Thoracic and Cardiovascular Surgery. 1981;81(3):403–407. [PubMed] [Google Scholar]
- 27.Utley JR, Thomason ME, Wallace DJ, et al. Preoperative correlates of impaired wound healing after saphenous vein excision. The Journal of Thoracic and Cardiovascular Surgery. 1989;98(1):147–149. [PubMed] [Google Scholar]
- 28.Slaughter MS, Olson MM, Lee JT, Ward HB. A fifteen-year wound surveillance study after coronary artery bypass. ATS. 1993;56(5):1063–1068. doi: 10.1016/0003-4975(95)90014-4. [DOI] [PubMed] [Google Scholar]
- 29.Greco G, Ferket BS, D'Alessandro DA, et al. Diabetes and the Association of Postoperative Hyperglycemia With Clinical and Economic Outcomes in Cardiac Surgery. Dia Care. 2016;39(3):408–417. doi: 10.2337/dc15-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swenne CL, Lindholm C, Borowiec J, Schnell AE, Carlsson M. Peri-operative glucose control and development of surgical wound infections in patients undergoing coronary artery bypass graft. Journal of Hospital Infection. 2005;61(3):201–212. doi: 10.1016/j.jhin.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 31.Székely A, Levin J, Miao Y, et al. Impact of hyperglycemia on perioperative mortality after coronary artery bypass graft surgery. The Journal of Thoracic and Cardiovascular Surgery. 2011;142(2):430–437.e431. doi: 10.1016/j.jtcvs.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 32.Furnary A, YingXing W. Clinical Effects of Hyperglycemia in the Cardiac Surgery Population: The Portland Diabetic Project. 2006 Aug;:1–6. doi: 10.4158/EP.12.S3.22. [DOI] [PubMed] [Google Scholar]
- 33.Lazar HL, McDonnell M, Chipkin SR, et al. The Society of Thoracic Surgeons Practice Guideline Series: Blood Glucose Management During Adult Cardiac Surgery. ATS. 2009;87(2):663–669. doi: 10.1016/j.athoracsur.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Bratzler D, Hunt D. The Surgical Infection Prevention and Surgical Care Improvement Projects: National Initiatives to Improve Outcomes for Patients Having Surgery. Clinical Infectious Diseases. 2006;43:322–330. doi: 10.1086/505220. [DOI] [PubMed] [Google Scholar]
- 35.Elahi MM, Haesey AM, Graham KC, et al. Leg wound infections following cardiac surgery: a scoring system for assessment and management. Journal of wound care Jul. 2005;14(7):337–340. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


