Abstract
Amine functionalized polysaccharide hydrogels such as those based on chitosan are widely examined as biomaterials. Here we set out to develop a facile procedure for developing such hydrogels by crosslinking dextran with amino acid diamines. The dextran-amino acid gels were formed by the addition of the amino acid diamines to a dextran and epicholorohydrin solution once it became homogeneous. This was demonstrated with three amino acid diamines, lysine, lysine methyl ester, and cystine dimethyl ester. Hydrogel networks with albumin entrapped were also demonstrated. These hydrogels were characterized by FTIR, SEM, rotational rheometry, swelling studies and cell biocompatibility analysis. These hydrogels showed the unexpected pH-responsive behavior of greater swelling at more basic pH, similar to that of an anionic hydrogel. This is uncharacteristic for amine functionalized gels as they typically exhibit cationic hydrogel behavior. All hydrogels showed similar biocompatibility to that of dextran crosslinked without amino acids.
Keywords: Hydrogels, pH-responsive, viscoelastic properties, cell biocompatability
1. Introduction
Hydrogels are hydrophilic networked polymers that retain large quantities of water. Hydrogels are well researched and commercially available for a variety of biomedical applications including wound healing [1, 2], tissue engineering [3, 4] and drug delivery [5, 6]. The attractiveness of hydrogels depends on the application. For example in wound healing, hydrogels are used for their ability to absorb wound exudate while allowing transport of oxygen to the wound site [7]. In tissue engineering they are sought after for their ability to mimic the extracellular matrix and support cell growth [4]. For drug delivery, stimuli-responsive hydrogels vary swelling depending on an external stimulus causing the release of drug molecules [8]. Hydrogels can provide stimuli-responsive depending on the functional groups present. For example, pH-responsive hydrogels are formed by including groups ionizable by protonation or deprotonation.
With a multitude of publications based on chitosan, this amine functionalized polysaccharide has proven an important resource for developing biomaterials. In addition, recently an amine functionalized dextran hydrogel showed exemplary results as a wound dressing with superior neovascularization to those of a commercially available dressing [9–11]. Inspired by these important contributions of amine functionalized polysaccharide hydrogels, we set out to develop methods to rapidly prepare diverse amine functionialized polysaccharide hydrogels [12]. We recently reported a facile modular approach to rapidly prepare hydrogels by crosslinking polysaccharides with polyamines such as polyallylamine and polyethylene amine via epichlorohydrin [13]. Epichlorohydrin is commonly used as a crosslinker for both polyols and polyamines. Because of this it is an ideal crosslinker for a hybrid system incorporating both. This resulted in a method in which any polysaccharide and any polyamine with sufficient solubility can be combined to prepare hydrogels without cumbersome synthetic steps such as acrylation. These hydrogels were pH-responsive behaving as typical cationic hydrogels showing increased swelling at lower pH when the amines are protonated. These hydrogels however proved cytotoxic to mammalian cells. Biocompatibility can often be improved by utilizing biologically derived materials, and herein we describe the synthesis of polysaccharide-amino acid diamines networked gels crosslinked via epichlorhydrin. Cellular studies showed these hydrogels to be biocompatible and able to sustain fibroblast cell growth on the hydrogel surface and within its microenvironment. Swelling studies showed these hydrogels to be pH-responsive but surprisingly shows the opposite response to hydrogels from our previously reported study. Though containing amines, here hydrogels showed anionic hydrogel swelling behavior with greater swelling at basic pH. Rheological examinations confirmed the viscoelastic properties of the hydrogels to be dependent on the amino acid diamine source and its ratio to dextran.
Unpublished work within our lab has shown that semi-interpenetrating networks (semi-IPN) and entrapped nanoparticles can be made by adding polymers or nanoparticles to the gelation process. These polymers stayed incorporated within the network. Proteins have been demonstrated as a source for biocompatible hydrogel synthesis [14–17]. As such, hydrogels with the entrapped serum protein albumin were also evaluated.
2. Materials and Methods
2.1 Materials
All chemicals were used as is, Dextran (MW 500 kDa), L-Lysine methyl ester dihydrochloride 98% (LysOMe) and Cystine dimethyl ester dihydrochloride 98% (CysOMe) were obtained from Alfa Aesar. L-Lysine hydrate (LysOH) and bovine serum albumin were purchased from Sigma Aldrich. Epichlorohydrin was obtained from Acros Chemicals. Protein adsorption tests were completed using a Thermo Scientific BCA Assay kit. Spectroscopic measurements were obtained with a PerkinElmer Lamda 35 spectrometer and a Thermo Fisher Nicolet iS10 FTIR with an ATR accessory. For rotational rheology studies an AR-G2 rotational rheometer with a parallel plate attachment was utilized. To visually examine the hydrogel microstructures, the surfaces were evaluated using a HITACHI S-2700 SEM. The SEM was operated at 25 keV acceleration voltage. The SEM was equipped with and AMT digital camera system. The lyophilized hydrogel was mounted onto aluminum stub using carbon tape and then sputtered with gold prior to analysis to avoid charging.
2.2 Hydrogel synthesis with amino acid diamines
To a stirring dextran solution (20%, w/v; 500 μL), NaOH (5 M, 200 μL) was added, followed by Epichlorohydrin (75μL). The mixture was stirred until clear and the phase separation could no longer be seen (<25 min). The appropriate amount of amino acid was then added to prepare hydrogels with polysaccharride:amino acid mass ratios of 10:1, 5:1 and 2:1. The solutions were quickly stirred until homogeneous and allowed to set. Gels were allowed to sit for 24 h before being washed with water to remove excess NaOH.
2.3 Entrapped albumin hydrogels synthesis
Albumin ( up to 50 mg) was dissolved in 200 μL of water and placed in an ultrasonic bath to facilitate dissolution. Albumin was added slowly to a solution of dextran solution (20%, w/v; 500 μL), NaOH (5 M, 200 μL), Epichlorohydrin (75 μL) after it had been stirred for 25 minutes and was incorporated slowly via gentle stirring using the vortex genie. For the amino acid dextran gels, albumin was added last.
2.4 Swelling ratio
The circular gels were sectioned into quarters and immersed in 100 mL of PBS buffer or deionized water. For examining the effect of pH on swelling, phosphate buffers of pH 4.01, 7.41 and 10.4 were utilized. Gels were allowed to swell overnight before their swollen weight was recorded. They were then dried by a vacuum oven at 75 C for 24 hours. The swelling behavior was measured by the equation is the dried weight of the hydrogel and w is the wet weight.
2.5 Rotational rheometry
In order to examine the effects of hydrogel composition on the viscoelastic properties of the hydrogels, strain sweep and creep-recovery tests have been performed in a parallel plate setting on disc-shaped samples with thickness of ~5mm and 8mm diameter. Temperature was maintained at 15 °C to maintain hydration. Strain sweep oscillatory tests have been performed at an oscillatory frequency of 0.3149 rad/s, value within the linear viscoelasticity range of those hydrogels determined by frequency sweep tests (not shown); strain % range was kept between 0.01–100. The creep-recovery tests were performed by monitoring strain evolution first over a constant application of a shear stress set at 100Pa for 10 minutes, followed by 10 minute recovery, after the applied stress was removed.
2.6 Albumin release
Gels containing albumin (LysOH + Albumin and Dextran + Albumin) were cut into three 8 mm diameter pieces and were submerged in 2 mL of neutral PBS buffer for 1 week. At 1, 2, 3, and 7 days the buffer was removed and replaced with fresh buffer. The removed solution was then examined via BCA assay [18] for the presence of proteins.
2.7 Cell culture
NIH 3T3 cells, a generous gift provided by Dr. Lynne Chang (Nikon), were cultured in 90% DMEM (Life Technologies) containing 2 mM L-glutamine, penicillin (100 U/ml), and streptomycin (0.1 mg/ml), and supplemented with 10% fetal bovine serum (FBS). Cultures were maintained at 37°C with 5% CO2.
2.8 Cell viability assay
2 × 104 cells plated in 24-well plates containing polymer, were incubated for 24 hours and allowed to adhere. Polymers plus half (500 ul) of the initial culture media were then transferred to a new plate and incubated further for 24 hours. Cells adhered to bottom of original wells and those stuck on top of polymer were maintained in 1 mL culture media. Cell viability analysis was carried out according to Sarker et al (2014) [19], with some modifications. Cell cultures were incubated in 100 ul of WST-1 (Roche) for 3 hours following standard culture conditions. 100 ul of media was then transferred to 96-well plate and the absorbance was determined at 450 nm on a Synergy H1 Hybrid microplate reader (BioTek Instruments).
2.9 Light microscopy
Cells were fixed with 3.7 % formaldehyde (made in 1 × PBS) for 10 minutes, following removal of culture media by aspiration. After two brief rinses in 1× PBS, cells were stained with 0.2 mg/100 mL methylene blue (Allied Chemicals) for 10 minutes at room temperature and subsequently rinsed 3 X with tap water. Images of cells in the polymer microenvironment were captured using an Olympus DP70 Digital Camera coupled to a Zeiss Stemi SV II Apo Microscope.
3. Results and Discussion
3.1 Hydrogel Synthesis
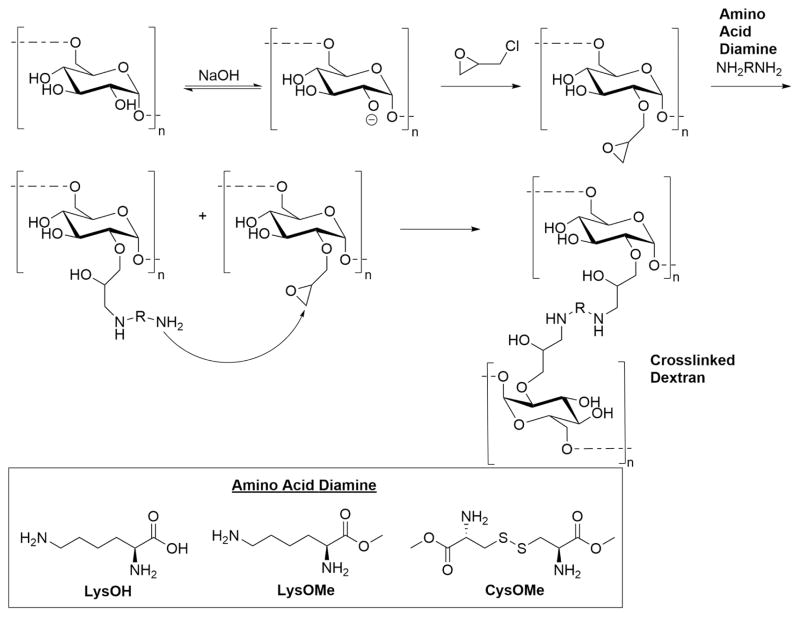
We previously reported the synthesis of polyscharride-polyamine hydrogels via crosslinking with epichlorohydrin [13]. In this process the polysaccharide was first reacted with epichlorohydrin until the reaction became homogenous. This indicated when all of the epichlorhydrin had been consumed and linked to the polysaccharide but crosslinking was not completed. The polyamine was then added quickly to complete the crosslinking process with the partially reacted epichlorohydrin. This methodology was demonstrated with polyallyl amine and polyethylene imine. Seeking to improve biocompatibility of these hydrogels we adapted this procedure replacing the synthetic polyamines with amino acid diamines. The rationale being that the naturally occurring amino acids would not have a deleterious biological effect and the diamines could insert into crosslinking by reacting with epichlorohydrin functionalized dextran (Fig. 1). In this methodology the crosslinking effect of epichlorohydrin is halved as two equivalents of epichlorhydrin are now required per crosslink. Commercially available lysine (LysOH), lysine methyl ester (LysOMe) and cystine methyl ester (CysOMe) were used, all diamines capable of inserting into the crosslinking process. Hydrogels with the protein albumin entrapped were also prepared by modifying this procedure. Protein based hydrogels have been demonstrated as a substrate for cellular growth and drug delivery [16]. Albumin is a readily available protein and has been demonstrated in various hydrogels to be biocompatible [14, 15]. Albumin is added to the dextran solution and the same methodology then followed as mentioned above.
Fig. 1.
Dextran hydrogel crosslinked with epichlorohydrin and amino acid diamines
3.2 Structure and morphology
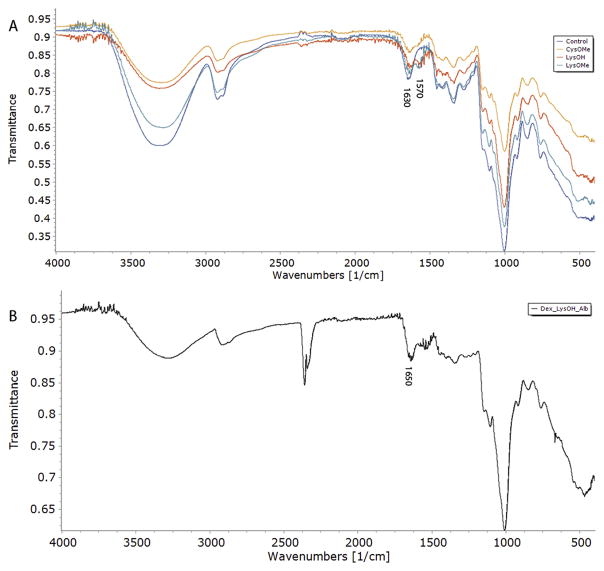
The hydrogels were all characterized by ATR-FTIR. Little difference was observed between the dextran hydrogel and diamine functionalized hydrogels indicating that the percentage incorporated into the hydrogels was in fact low. The most distinct difference can be noted in the spectra of a dextran:LysOMe hydrogels. A new peak at 1570 cm−1 is observed that is not present in the dextran hydrogel containing no LysOMe (Fig. 2a). This indicates a distinctively different material has been produced from the dextran hydrogel. This peak is adjacent to a peak of 1630 cm−1 which has been attributed to bound water in dextran [20]. Hydrogels with LysOH or CysOMe overlap between to two peaks makes it difficult to see the distinction. Unexpectedly no peaks in the region for esters were observed for gels made with LysOMe or CysOMe. Hydrogel synthesis occurs in a basic solution over 24 h and it is likely that the methyl esters were hydrolyzed in the process. FTIR spectra for hydrogels with albumin entrapped within also showed great similarity to that of the dextran hydrogel. Fig. 2b displays the spectrum for dextran-LysOMe-albumin hydrogel. A noticeable increase at 1650 cm−1 can be attributed to protein amides. This peak overlaps with water bound to dextran which also contributes to the peak intensity. The presence of protein was confirmed visually by a BCA assay and minimal amounts of protein was released from the gel when swollen in PBS buffer over 7 days.
Fig. 2.
FTIR spectra of hydrogels crosslinked with (a) 2:1 mass ratio of dextran to amino acid diamines CysOMe, LysOMe and LysOH (control with no amino acid diamine present) and (b) 2:1:1 mass ratio of dextran:LysOH:Albumin.
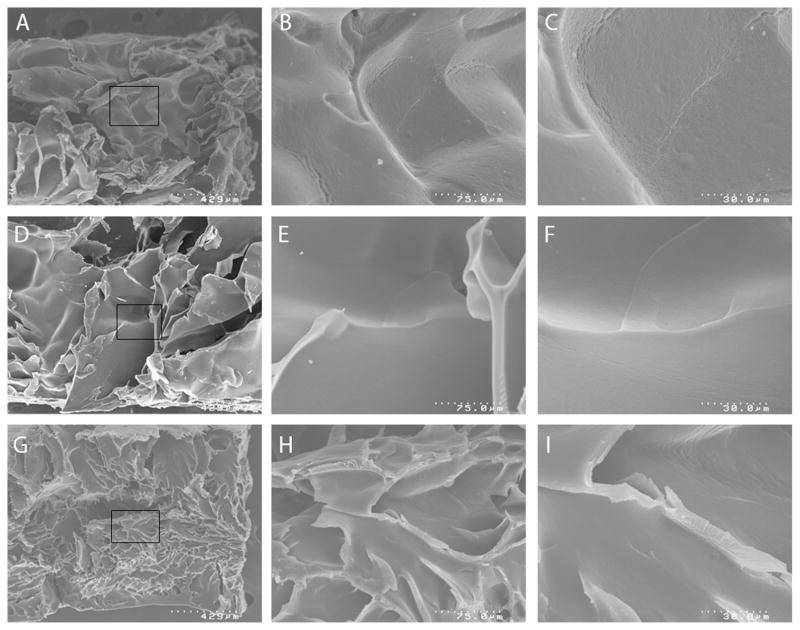
SEM micrographs revealed the morphology of the hydrogels to be dependent on the ratio of dextran to amino acid diamine used (Fig. 3). Hydrogels comprising of only dextran (Fig. 3g) showed smaller layered features than those of hydrogels with 2:1 and 5:1 dextan:LysOMe mass ratios (Fig. 3a,d). The variation in features is likely due to differences in crosslinking with lower crosslinking in gels with increased LysOMe. At increased magnification the layers were observed to have a granular (~75nm) type texture when LysOMe is used in the synthesis. This texture was most pronounced with the highest level of LysOMe (Fig. 3c) while the sheeted features in hydrogel made with only dextran appeared to lack this porosity (Fig. 3i).
Fig. 3.
SEM images of dextran:LysOMe hydrogels crosslinked at different mass ratios shown at increasing magnifications: (a) 2:1 at 70x, (b) 2:1 at 400x, (c) 2:1 at 1000x, (d) 5:1 at 70x, (e) 5:1 at 400x, (f) 5:1 at 1000x. For comparison a control dextran hydrogel with no amino acid crosslinker at various magnifications, (g) 70x, (h) 400× and (i) 1000x.
3.3 Swelling and rheology studies
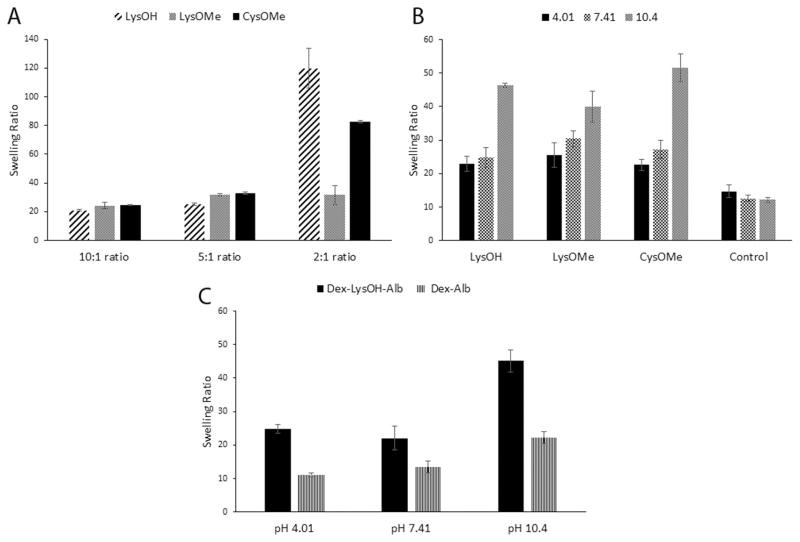
Hydrogels were prepared by varying mass ratio of dextran to amino acid and the effect on swelling in water was examined (Fig. 4a). Hydrogels with 10:1 dextran:amino acid mass ratios swollen in water averaged swelling ratios in the twenties, showing similar swelling independent of the amino acid used. Swelling ratios rose drastically when the amino acid was increased to a 2:1 dextran:amino acid mass ratio with hydrogels made with LysOH and CysOMe showing greater than 5 and 3 fold increases respectively. The amino acid is being inserted into the crosslinking process and requiring two equivalents of epichlorohydrin per diamine. This decrease in crosslinks would create a more flexible hydrogel capable of increased swelling. This is reinforced by the inability of a 1:1 dextran:amino acid mixture to consistently form hydrogel. Hydrogels prepared with LysOMe showed a more moderate increase of 1.3 fold when the ratio is changed. Also with LysOMe no significant difference was found between the swelling of gels with 5:1 and 2:1 mass ratios. The differences between amino acids at higher concentrations shows that there is an importance to the choice of the amino acid. The reaction occurs in basic media and the carboxylate of LysOH can participate in the reactions with epichlorhydrin. However the ester that would have been created may by hydrolyzed due to the prolonged 24 h synthetic procedure. This would decrease crosslinking and increase the swelling ratio of LysOH gels compared to the others. CysOMe has been found to decompose in alkaline solutions [21] and this would also attribute to decreased crosslinking and increased swelling. LysOMe may be inable of similar side reactions and thus has increased crosslinking and lower swelling at a 2:1 mass ratio compared to the other amino acids examined.
Fig. 4.
Swelling ratios for hydrogels with (a) dextran crosslinked with amino acid diamines at varying mass ratios (10:1, 5:1 and 2:1) in water, (b) 2:1 mass ratio of dextran to amino acid diamines CysOMe, LysOMe and LysOH in phosphate buffer of pH 4.0, 7.4 and 10.4, (c) dextran hydrogels with entrapped albumin crosslinked with and without LysOH.
Hydrogel with a 2:1 dextran:amino acid mass ratio were swollen in phosphate buffer solutions of pH 4.0, 7.4 and 10.4 to see the effect of pH on swelling (Fig. 3b). A surprising result of increased swelling at more basic pH was observed. Amine functionalized hydrogels are known to behave as cationic hydrogels, showing increased swelling at lower pH [8]. This is attributed to electrostatic repulsion and osmotic pressure. Independent of the amino acid, all hydrogels showed a significant increase with CysOMe containing hydrogels showing the largest increase, a greater than 2 fold increase. The dextran hydrogel with no amino acid showed no variation of swelling with change in pH. Thus these hydrogels are pH-responsive but unexpectedly behave as anionic hydrogels. From evidence in the FTIR, it was proposed that hydrolysis of the amino acid esters was occurring under the highly basic conditions. It is possible that the pH-dependent swelling is being driven by anionic carboxylate group.
Hydrogels with entrapped albumin also showed anionic pH-responsive behavior with increased swelling at higher pH (Fig. 3c). Albumin is known to have a net negative charge [22] and unsurprisingly a dextran-albumin hydrogel with and without LysOH as a crosslinker showed anionic hydrogel behavior with an increase of approximately two fold in both cases. However hydrogels with LysOH showed greater swelling than gels without. This is presumed to be due to increased swelling from diminished crosslinking when the amino acid is included in the formulation.
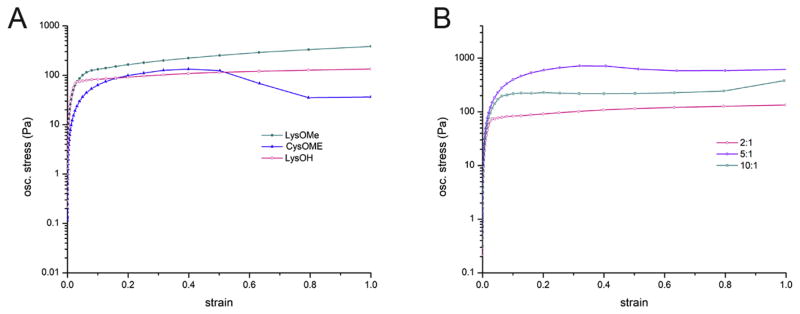
Rotational rheometry studies were performed to examine the effect of the amino acid and the ratio used in crosslinking on the viscoelastic properties of the hydrogels. Stress/strain curves of hydrogels with a mass ratio 2:1 dextran to amino acid were performed to examine the effect of the diamine on one of the properties of the gels, yield stress. Yield stress is defined as the critical stress beyond which a material will lose elasticity or reach a breaking point (Fig. 5). Hydrogels with LysOH and CysOMe reach the same yield stress value, 133.1 Pa while LysOMe hydrogels showed the largest yield stress value at 147.7Pa. Moreover, CysOMe appears to have a secondary yield stress value, which suggests a further breakdown of its original structure at 36Pa (Fig. 5). Stress/strain curves of hydrogels with varying mass ratios of 10:1, 5:1 and 2:1 dextran to LysOH showed 5:1 Dex:LysOH displays the highest yield stress (717 Pa). This value is considerably higher than for the other hydrogels, (219 Pa and 95Pa, respectively) and accounts for the largest range of elasticity for 5:1 Dex:LysOH. (Fig. 5b). The above-mentioned results demonstrate that yield stress values are influenced by both the mass ratio of dextran to amino acid and the amino acid itself.
Fig. 5.
Stress/Strain curves showing hydrogels with (a) 2:1 mass ratio of dextran to amino acid diamines CysOMe, LysOMe and LysOH, (b) dextran crosslinked with LysOH at varying mass ratios (10:1, 5:1 and 2:1).
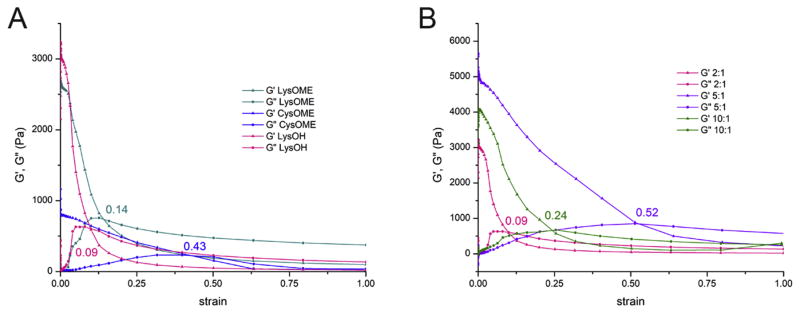
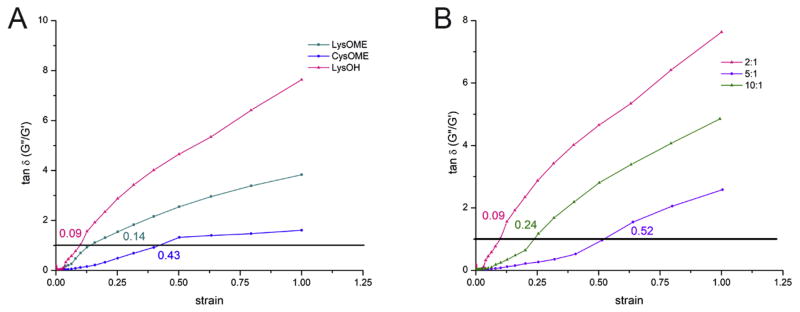
Further, the critical strain values have been evaluated from the elastic modulus G′ and viscous modulus G″ variation with applied strain (Fig. 6). In general, the rheological properties of a viscoelastic material are independent of strain up to a critical strain value, beyond which the material’s behavior is non-linear and the elastic modulus declines sharply. Critical strain values were evaluated at the crossing point between the G′ and G″ curves. It was identified that the critical strain values that samples can undergo without modifying the structure and/or elastic properties vary with both the amino acids (Fig. 6a) and the mass ratio of dextran to amino acid (Fig. 6b). The highest critical strain was identified for CysOMe (0.43), followed by LysOMe (0.14) and LysOH (0.09), the last value being extremely low, showing a very narrow range of linear viscoelasticity. The mass ratio of dextran to amino acid that contributed to the highest critical strain for the LysOH series was 5:1 (0.52), followed by 10:1 (0.24) and 2:1 (0.09). As G′ values decline and G″ values start to exceed the G′ ones, the hydrogels become progressively more fluid-like. The strength of colloidal forces inside the hydrogels are well evaluated using tan δ (G″/G′) (Fig. 7). Values of tan δ up to 1 suggest that the particles are highly associated due to colloidal forces, higher tan δ are an indication of particles that are largely unassociated. As it is shown in Fig. 6, tan δ = 1 exactly at the critical strain values for each sample, indicating that elastic range coincides with the presence of the colloidal forces in the hydrogel. Beyond the critical strain value, tan δ increases steadily and hydrogels are in the non-linear viscoelasticity range, their structure being irreversely affected by increased strain.
Fig. 6.
Elastic G′and viscous G″ moduli variation with strain curves showing hydrogels with (a) 2:1 mass ratio of dextran to amino acid diamines CysOMe, LysOMe and LysOH, (b) dextran crosslinked with LysOH at varying mass ratios (10:1, 5:1 and 2:1).
Fig. 7.
Tan delta variation with strain curves showing hydrogels with (a) 2:1 mass ratio of dextran to amino acid diamines CysOMe, LysOMe and LysOH, (b) dextran crosslinked with LysOH at varying mass ratios (10:1, 5:1 and 2:1).
At low strain values, the elastic component will play a major role in contributing to the materials behavior, thus it is important to be able to measure it. Creep is defined as the slow deformation of a material, usually measured under a constant stress. A creep test applies a small stress to a viscoelastic material and holds it constant for a period of time while measuring the resultant strain, and records from the moment we apply the stress, so the behavior initially observed will be from the elastic component alone. After a sample is allowed to creep under applied stress, the material’s elastic behavior can be evaluated by abruptly relieving the imposed stress and measuring the extent the sample recovers. Fig. 8 displays the strain values during creep and after recovery of the Dextran LysOH series. The lowest strain during creep step is displayed by the 2:1 mass ratio hydrogel of Dextran:LysOH (0.026), while the strain recovers to 0.011 after stress was removed. Meanwhile, the 10:1 mass ratio hydrogel of Dextran:LysOH displayed a higher strain value of 0.57, strain after recovery step becoming 0.46. The same trend is followed before and after the stress is removed, for sample with 5:1 ratio, but attained strain values are much higher, 2.5 and 2.05 respectively. Values indicate low elasticity for all samples, due to the small extrent that samples recover, and the strain values under the same applied stress differ. The higher the strain value the more succeptible a material is to be affected by the applied stress.
Fig. 8.
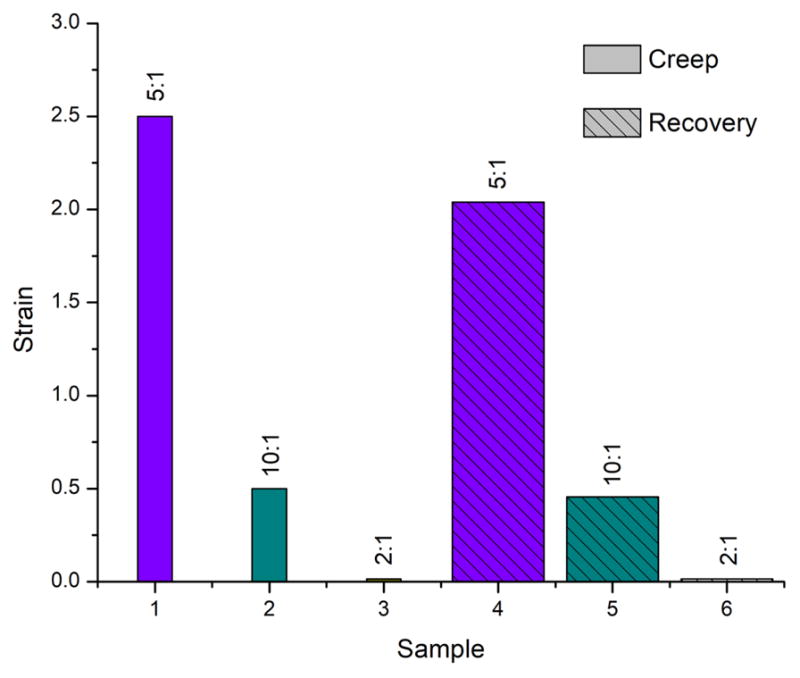
Creep/recovery strain values for dextran crosslinked with LysOH at varying mass ratios (10:1, 5:1 and 2:1).
3.4 Biocompatibility studies
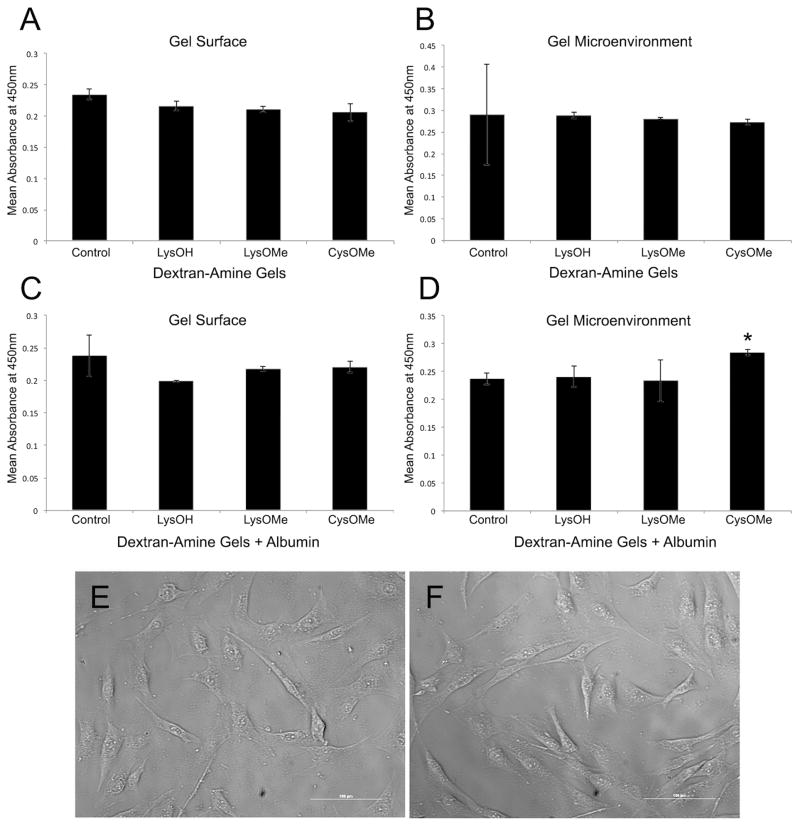
Biocompatibility was examined by cell viability assays with fibroblast cells on both the surface of the hydrogel and the microenvirnoment surrounding the hydrogel (Fig. 9). Cell viability studies were performed with hydrogels with a 2:1 mass ratio of dextran to amino acid diamines LysOH, LysOMe and CysOMe (Fig. 9a,b). As a control, a dextran hydrogel without amino acid crosslinkers was used. Examination of cell biocompatibility on the hydrogel surface and its microenvironment showed that hydrogels exhibited comparable cell viability properties compared to control. Cells on control gel did show a trend of improved viability compared to cells on gels made with LysOMe and CysOMe. No statistically significant difference was found in cell viability between microenviroments of gels comprised of diamines and control.
Fig. 9.
Cell Viability of hydrogels with 2:1 mass ratio of dextran to amino acid diamines CysOMe, LysOMe and LysOH (a) on the surface and (b) the microenvironment. Control was a dextran hydrogel. Cell Viability of hydrogels with 2:1 mass ratio of dextran to amino acid diamines CysOMe, LysOMe and LysOH with entrapped albumin (c) on the surface and (d) the microenvironment. Control was a dextran hydrogel with albumin entrapped. Microscopic images of fibroblast cells in the microenvironment of (e) control hydrogel of dextran with entrapped albumin and (f) CysOMe crosslinked dextran with entrapped albumin. Scale 100 μm.
Cell viability studies were also performed with hydrogels with the analogous 2:1 mass ratio of dextran to amino acid diamines with albumin (Fig. 9c,d). No statistically significant difference in cell viability was found between surfaces of experimental and control gel comprised of dextran with albumin. However, examination of the microenvironment found that hydrogels made with CysOMe showed a statistically significant increase in cell viability comapred to the control gel, P-value 0.02. In these studies this was the only instance of an improvement in vivability compared to the control hydrogel. The rationale for this is not known, however cystine methyl ester has been observed to degrade in aqueous and alkaline solutions [21, 23]. Hydrogels with albumin were found to have higher swelling than those without, and this might lead to an increase in the leaching of degradation products that might have an effect on cell culture.
4. Conclusion
In summary, amino acid diamines were successfully inserted into the epichlorohydrin crosslinking of dextran. The addition of amino acid diamines to the dextran hydrogel formulation resulted in changes in swelling, morphology and viscoelastic properties. These hydrogels exhibited anionic swelling behavior, with increased swelling at basic pH. This was attributed to hydrolysis of the amino acid methyl ester during hydrogel synthesis. Increasing the ratio of amino acid to dextran increases swelling as this results in a decrease in the crosslinking density. Varying the amino acid diamines to dextran ratio affected the morphology of the hydrogel with finer folds observed with less amino acid. On the other hand increased magnification showed granular texture (~75nm) in these features when the amino acid was increased. Viscoelastic properties of the hydrogels were found to be dependent on both the amino acid and the ratio in which it was used. Of comparative hydrogels, gels with LysOMe were observed to have the highest yield stress. While of gels of varying ratios of dextran to LysOH, gels with at 5:1 showed the yield stress. All hydrogels showed similar cell viability on their surface and in their microenvironment to that of a gel containing dextran only. An unexpected observation was increased cell viability in the microenviroment of CysOMe hydrogels with entrapped albumin.
Acknowledgments
This work was possible due to grants from the National Institute of Health and National Institue of Medical Sciences N.A.O. (5SC3GM111194), S.R. (5SC3GM113782), S.R. (5R21EY026752-02).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Corkhill PH, Hamilton CJ, Tighe BJ. Synthetic hydrogels. VI. Hydrogel composites as wound dressings and implant materials. Biomaterials. 1989;10(1):3–10. doi: 10.1016/0142-9612(89)90002-1. [DOI] [PubMed] [Google Scholar]
- 2.Murphy PS, Evans GR. Advances in wound healing: a review of current wound healing products. Plastic Surgery Inter. 2012;2012:190436. doi: 10.1155/2012/190436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jen AC, Wake MC, Mikos AG. Review: Hydrogels for cell immobilization. Biotechnol Bioeng. 1996;50(4):357–64. doi: 10.1002/(SICI)1097-0290(19960520)50:4<357::AID-BIT2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 4.Van Vlierberghe S, Dubruel P, Schacht E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules. 2011;12(5):1387–1408. doi: 10.1021/bm200083n. [DOI] [PubMed] [Google Scholar]
- 5.Chen YY, Wu HC, Sun JS, Dong GC, Wang TW. Injectable and thermoresponsive self-assembled nanocomposite hydrogel for long-term anticancer drug delivery. Langmuir. 2013;29(11):3721–9. doi: 10.1021/la400268p. [DOI] [PubMed] [Google Scholar]
- 6.Hu H, Yu L, Tan S, Tu K, Wang LQ. Novel complex hydrogels based on N-carboxyethyl chitosan and quaternized chitosan and their controlled in vitro protein release property. Carbohydr Res. 2010;345(4):462–8. doi: 10.1016/j.carres.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 7.Alper JC. Recent advances in moist wound healing. South Med J. 1986;79(11):1398–404. doi: 10.1097/00007611-198611000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Gupta P, Vermani K, Garg S. Hydrogels: from controlled release to pH-responsive drug delivery. Drug Discov Today. 2002;7(10):569–79. doi: 10.1016/s1359-6446(02)02255-9. [DOI] [PubMed] [Google Scholar]
- 9.Agnihotri SA, Mallikarjuna NN, Aminabhavi TM. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J Controlled Release. 2004;100(1):5–28. doi: 10.1016/j.jconrel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Kong M, Chen XG, Xing K, Park HJ. Antimicrobial properties of chitosan and mode of action: a state of the art review. Int J Food Microbiol. 2010;144(1):51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Kumar MNVR. A review of chitin and chitosan applications. React Funct Polym. 2000;46:1–27. [Google Scholar]
- 12.Sun G, Zhang X, Shen YI, Sebastian R, Dickinson LE, Fox-Talbot K, Reinblatt M, Steenbergen C, Harmon JW, Gerecht S. Dextran hydrogel scaffolds enhance angiogenic responses and promote complete skin regeneration during burn wound healing. Proc Natl Acad Sci, USA. 2011;108(52):20976–81. doi: 10.1073/pnas.1115973108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Connor NA, Abugharbieh A, Yasmeen F, Buabeng E, Mathew S, Samaroo D, Cheng HP. The crosslinking of polysaccharides with polyamines and dextran-polyallylamine antibacterial hydrogels. Int J Biol Macromol. 2015;72:88–93. doi: 10.1016/j.ijbiomac.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baler K, Michael R, Szleifer I, Ameer GA. Albumin hydrogels formed by electrostatically triggered self-assembly and their drug delivery capability. Biomacromolecules. 2014;15(10):3625–33. doi: 10.1021/bm500883h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raja ST, Thiruselvi T, Mandal AB, Gnanamani A. pH and redox sensitive albumin hydrogel: A self-derived biomaterial. Sci Rep. 2015;5:15977. doi: 10.1038/srep15977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watkins KA, Chen R. pH-responsive, lysine-based hydrogels for the oral delivery of a wide size range of molecules. Int J Pharm. 2015;478(2):496–503. doi: 10.1016/j.ijpharm.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Ma X, Sun X, Hargrove D, Chen J, Song D, Dong Q, Lu X, Fan TH, Fu Y, Lei Y. A Biocompatible and Biodegradable Protein Hydrogel with Green and Red Autofluorescence: Preparation, Characterization and In Vivo Biodegradation Tracking and Modeling. Sci Rep. 2016;6:19370. doi: 10.1038/srep19370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 19.Sarker B, Singh R, Silva R, Roether JA, Kaschta J, Detsch R, Schubert DW, Cicha I, Boccaccini AR. Evaluation of fibroblasts adhesion and proliferation on alginate-gelatin crosslinked hydrogel. PLoS One. 2014;9(9):e107952. doi: 10.1371/journal.pone.0107952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Velazquez G, Herrera-Gómez A, Martín-Polo MO. Identification of bound water through infrared spectroscopy in methylcellulose. J Food Eng. 2003;59(1):79–84. [Google Scholar]
- 21.Andrews JC. The alkaline decomposition of cystine. J Biol Chem. 1928;80:191–210. [Google Scholar]
- 22.Fogh-Andersen N, Bjerrum PJ, Siggaard-Andersen O. Ionic binding, net charge, and Donnan effect of human serum albumin as a function of pH. Clin Chem. 1993;39(1):48–52. [PubMed] [Google Scholar]
- 23.Coghill R. The Spontaneous Decomposition of Cystine Dimethyl Ester. J Biol Chem. 1936;114:419–424. [Google Scholar]