Abstract
Background
Despite concerns about adverse neurocognitive events raised by prior trials, pharmacologic PCSK9 inhibition was not associated with neurocognitive effects in a recent phase 3 randomized trial. PCSK9 loss-of-function (LOF) variants that result in life-long exposure to low LDL-C can provide information on the potential long-term effects of low LDL-C on neurocognitive impairment and decline.
Methods
We investigated the association between PCSK9 LOF variants and neurocognitive impairment and decline among African-American REasons for Geographic and Racial Differences in Stroke (REGARDS) study participants with (n=241) and without (n=10,454) C697X or Y142X LOF variants. Neurocognitive tests included Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) battery (Word List Learning, Delayed Recall, Animal Fluency) and Six Item Screener (SIS) assessments, administered longitudinally during follow-up. Neurocognitive impairment was defined as a score ≥ 1.5 standard deviations (SD) below age, sex, and education-based stratum-specific means on 2 or 3 CERAD assessments, or, separately, a score <5 on any SIS assessment at baseline or during follow-up. Neurocognitive decline was assessed using standardized continuous scores on individual neurocognitive tests.
Results
The mean sample age was 64 years (SD 9), 62% were women, and the prevalence of neurocognitive impairment at any assessment was 6.3% by CERAD and 15.4% by SIS definitions. Adjusted odds ratios (ORs) for neurocognitive impairment for participants with versus without PCSK9 LOF variants were 1.11 (95% CI 0.58, 2.13) using the CERAD battery and 0.89 (95% CI 0.61, 1.30) using the SIS assessment. Standardized average differences in individual neurocognitive assessment scores over the 5.6 year (range 0.1, 9.1) study period ranged between 0.07 (95% CI −0.06, 0.20) and −0.07 (95% CI −0.18, 0.05) among participants with versus without PCSK9 LOF variants. Patterns of neurocognitive decline were similar between participants with and without PCSK9 LOF variants (all p > 0.10). ORs for neurocognitive impairment per 20 mg/dL LDL-C decrements were 1.02 (95% CI 0.96, 1.08) and 0.99 (95% CI 0.95, 1.02) for the CERAD and SIS definitions of impairment, respectively.
Conclusion
These results suggest life-long exposure to low PCSK9 levels and cumulative exposure to lower LDL-C are not associated with neurocognitive effects in African Americans.
Keywords: PCSK9, variants, LDL-C, neurocognitive, impairment, decline
Introduction
Statin therapy has been associated with neurocognitive dysfunction in some but not all studies.1–3 The US Food and Drug Administration (FDA) issued a warning about potential adverse treatment effects of statin therapy in 2012.4 Additionally, short-term trials of pharmacologic PCSK9 inhibition to lower LDL-C suggested a possible association with neurocognitive adverse events.5, 6 Over 26 months of follow-up, the Phase 3 Evaluating PCSK9 Binding Antibody Influence on Cognitive Health in High Cardiovascular Risk Subjects (EBBINGHAUS) trial found no difference in change over time in neurocognitive function between participants randomized to evolocumab versus a placebo in addition to standard of care.7 Also, there were no differences in neurocognitive function between participants who achieved and did not achieve an LDL-C < 25 mg/dL during follow-up.7
Sequence variation in the PCSK9 gene is a major determinant of circulating levels of LDL-C.8 Loss-of-function (LOF) variants in the PCSK9 gene, occurring in 1% to 3% of African-American adults, are associated with low circulating LDL-C concentration.9 In a recent meta-analysis of 9 studies, 2.3% of African-American participants had nonsense mutations in PCSK9 which were associated with a 35 mg/dL lower LDL-C level while 3.1% of white participants had sequence variation in PCSK9 that was associated with a 13 mg/dL lower LDL-C level.10
Studying the association of PCSK9 LOF variants with neurocognitive function can provide insights into the long-term association between low LDL-C and neurocognitive function as well as into the potential for neurocognitive side effects with PCSK9 inhibitors. In the current study, we examined the association of PCSK9 LOF variants and LDL-C with neurocognitive impairment and longitudinal decline in cognition among African-American participants aged 45 years and older in the REasons for Geographic And Racial Differences in Stroke (REGARDS) study.
Methods
The data, analytic methods, and study materials will not be made publicly available to other researchers for purposes of reproducing the results or replicating the procedure. However, with review and approval, the information is available from the REGARDS study under established data sharing procedures.
Study population
The REGARDS study is a prospective cohort study that enrolled African-American and white adults ≥45 years of age between January 2003 and 2007.11 By design, the study oversampled African-American participants. Approximately half of the sample was recruited from the eight southern US states referred to as the “stroke buckle” (coastal plain region of North Carolina, South Carolina, and Georgia) and “stroke belt” (remainder of North Carolina, South Carolina, and Georgia, plus Alabama, Mississippi, Tennessee, Arkansas, and Louisiana) with the remaining participants recruited from the rest of the continental US. Trained and certified health professionals conducted in-home study visits at baseline that included a physical examination, blood pressure measurements, electrocardiogram, the collection of a blood sample, and a urine sample. Of relevance to the current analysis, telephone interviews with REGARDS study participants were conducted every six months after baseline. Starting in December 2003, brief neurocognitive screening assessments were administered annually, and starting in January 2006, expanded neurocognitive assessments were administered at regular intervals on a different schedule than the screening assessments (Supplemental Figure 1). All neurocognitive assessments were administered during the telephone interviews.
We included African-American REGARDS participants who were genotyped for PCSK9 LOF variants and had blood collected during an in-home visit (n=10,695). The population for the current analysis was restricted to African Americans. Only a small sample of white REGARDS participants were genotyped, and distinct PCSK9 variants are found in African-American and white populations; the PCSK9 LOF variants observed in African-American populations result in lower LDL-C levels than the PCSK9 variants observed in white populations.10 The Institutional Review Boards of all participating institutions approved this study. All participants provided written informed consent for participation including neurocognitive testing and genotyping.
Data collection
Covariates
At baseline, trained interviewers conducted computer-assisted telephone interviews and an in-home examination to obtain information on participants’ demographics (age, sex, region of residence, education), cigarette smoking, alcohol consumption, waist circumference, physical activity, and history of stroke, atrial fibrillation and coronary heart disease (CHD). Blood samples were used to measure fasting total cholesterol, high-density lipoprotein cholesterol (HDL-C), and triglycerides. LDL-C was calculated using the Friedewald equation.12 High-sensitivity C-reactive protein (hs-CRP) was measured by particle enhanced immunonephelometry using the BNII nephelometer. The Chronic Kidney Disease Epidemiology Collaboration equation was used to calculate estimated glomerular filtration rate (eGFR).13 Urinary albumin and creatinine measures were used to calculate the albumin-to-creatinine ratio (ACR). Depressive symptoms were measured using the Center for Epidemiologic Studies Depression-4 (CESD-4) scale. Antihypertensive, statin, antidiabetes/insulin, benzodiazepine, antipsychotic, and antidepressant medication use was identified during a medication inventory.
Genotyping
Genotyping of the variants Y142X (rs67608943) and C679X (rs28362286) single nucleotide polymorphisms was performed at the University of Vermont using a Taqman assay on DNA that was extracted from packed white blood cells. Participants with at least one of either LOF variant were categorized as being carriers.
Neurocognitive Function Assessment
Verbal learning, verbal memory, and semantic fluency were assessed using tests drawn from the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) battery (Word List Learning (WLL), Word List Delayed Recall (WLD), and Semantic (Animal) Fluency (AF), respectively). Global cognitive function was examined using the Six item screener (SIS) assessment. As described previously, CERAD and SIS tests were administered by telephone at 18–24 month and 12-month intervals, respectively.14 Neurocognitive assessment data were available through August 31, 2012. WLL is the number of words recalled on a 10-item list, administered three times (score 0–30). WLD is the number of words remembered after a delay (score 0–10). AF records the number of animals named in one minute. Each has been validated in the identification of mild cognitive impairment15, dementia and Alzheimer’s disease16. To create REGARDS-specific population norms, means and standard deviations on each test were calculated within 32 strata defined by age group (45 to 54, 55 to 64, 65 to 74, and 75+ years), sex (female, male), and education category (less than high school, high school graduate, some college, and college graduate and above).17 The SIS was administered at baseline and annually thereafter. It is a test of global cognitive function18 that assesses recall ability based on a three-item word list as well as orientation to year, month, and day of the week, and scores range from 0 to 6.
To identify neurocognitive impairment, we required participants to have completed at least one CERAD battery (n=7,409) or SIS neurocognitive assessment (n=10,461) during the course of the study (Figure 1). We applied definitions of neurocognitive outcomes previously used in REGARDS and other cohort studies.19,4 Neurocognitive impairment was defined as a score ≥1.5 standard deviations below the stratum-specific mean on 2 or 3 of the verbal learning (WLL), memory (WLR) and semantic fluency (AF) tests from the CERAD battery at first assessment or during any follow-up assessment.20 In a separate analysis using the SIS, neurocognitive impairment was defined as a score < 5 during any assessment.21 In a previous study, sensitivity and specificity were 74.2% and 80.2%, respectively, to identify clinically-confirmed neurocognitive impairment or dementia in a community-based cohort using a SIS score < 5.21
Figure 1. Participant Exclusion Flow Chart.
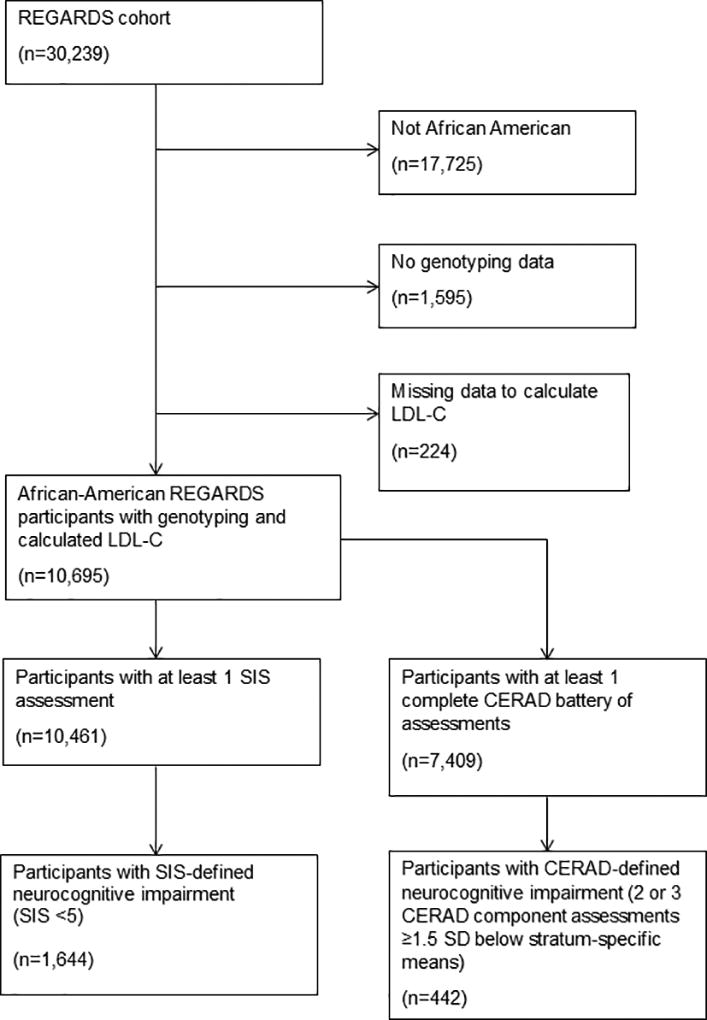
Abbreviations: CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; LDL-C, low-density lipoprotein-cholesterol; REGARDS, REasons for Geographic And Racial Differences in Stroke; SD, standard deviation.
A meta-analysis of randomized controlled trials conducted by Lipinski and colleagues estimated that PCSK9 inhibitors were associated with an increased risk of neurocognitive adverse events (odds ratio [OR] 2.43, 95% CI [1.11–4.93]).22 While designing the current study, we conducted a priori power calculations assuming 8,000 participants with ≥ 1 complete neurocognitive assessment, 5% prevalence of CERAD-defined neurocognitive impairment, 10% prevalence of SIS-defined neurocognitive impairment, 2.3% prevalence of PCSK9 LOF variants and ORs of 2.0 and 2.5 (Supplemental Table 1). Using a chi-square test with an alpha of 0.05, we estimated that we would have 74% and 92% power to detect ORs of 2.0 and 2.5, respectively, when examining CERAD-defined neurocognitive impairment and 91% and 99% power to detect ORs of 2.0 and 2.5, respectively, when examining SIS-defined neurocognitive impairment.
Statistical Analysis
We calculated means and standard deviations or number and proportions for participant characteristics in the overall population and among those with and without PCSK9 LOF variants. Tests of statistical significance for differences between groups were conducted using t-tests for means and chi-square tests for proportions. The presence of neurocognitive impairment at any assessment was estimated for participants with and without PCSK9 LOF variants for the CERAD- and SIS-based definitions of neurocognitive impairment, separately. Baseline characteristics of participants by CERAD-defined and, separately, SIS-defined neurocognitive impairment are provided in Supplemental Tables 2 and 3, respectively. ORs for neurocognitive impairment at any assessment associated with PCSK9 LOF variants were estimated using logistic regression with progressive adjustment. First, we adjusted for age at baseline and the total number of neurocognitive assessments performed. Second, we adjusted for the variables in the first model and a neurocognitive impairment risk score. Adjusting for a single score allowed us to account for potential confounders without over-fitting the regression models.23 To create this score, we calculated the probability of neurocognitive impairment at any assessment for each participant from logistic regression models including gender, region of residence, education, waist circumference, smoking, alcohol use, physical activity, systolic and diastolic blood pressure, use of antihypertensive medication, use of benzodiazepines, antipsychotics, or antidepressants, eGFR < 60 mL/min/1.73m2, albuminuria, diabetes, atrial fibrillation and depressive symptoms. In a third model, we additionally adjusted for statin use, history of CHD or stroke and hs-CRP.
To assess differences in neurocognitive decline between participants with versus without PCSK9 LOF variants, we modeled each of the three CERAD and the SIS neurocognitive function assessments as separate continuous variables. These measures were standardized as z-scores with a mean=0 and standard deviation=1, where positive values indicate better performance and negative values indicate worse performance on assessments. Using a generalized linear model, we examined baseline differences in each neurocognitive domain score by PCSK9 variant status using progressive adjustment as described above. Next, using linear repeated measures modeling with a compound symmetry covariance structure and empirical variance estimators, we examined neurocognitive changes over time for each continuous neurocognitive domain. We included a time variable that represented the length of time from the first assessment to each follow-up assessment. Associations between PCSK9 LOF variants and neurocognitive changes from these models used all available assessments. To determine if the rate of neurocognitive decline differed by PCSK9 variant status, we included an interaction term between time and PCSK9 variant status. Initially, time was modeled as a quadratic spline. Because these time trends appeared linear, time was modeled linearly in the final analyses. We tested whether the association between PCSK9 LOF variants and neurocognitive impairment and neurocognitive decline varied by statin use and LDL-C by including cross-product terms in the models.
Using logistic and linear regression we also examined the association between LDL-C and neurocognitive impairment and decline, respectively, as described above. Initially, LDL-C was modeled as a continuous variable using quadratic splines. There was no evidence that the association between LDL-C and neurocognitive impairment was nonlinear. Therefore, we modeled LDL-C as a linear continuous variable. For ease of interpretation, results are presented per 20 mg/dL LDL-C decrements. We tested whether the association between LDL-C and neurocognitive impairment and neurocognitive decline differed by statin use and PCSK9 variant status by adding cross product terms to the models. All analyses were conducted using SAS 9.4 (Cary, NC).
Results
Population characteristics
The mean age of the population at baseline was 64.1 (SD 9.3) years, and 61.7% (n=6,599) were female (Table 1). Over half of the population (51.3%) lived in the stroke belt/buckle region of the US, and 80.5% (n=8,612) of the population had at least a high school education. The CERAD battery was administered an average of 2.3 (Range 1, 4) times and the SIS was administered an average of 4.9 (Range 1, 11) times over a mean follow-up of 5.6 years. Overall, 2.3% (n=241) of participants had PCSK9 LOF variants. Among participants with versus without PCSK9 LOF variants, the prevalence of diabetes was higher (35.5% vs 29.5%, p=0.05), and mean LDL-C (85 mg/dL vs 118 mg/dL, p<0.001) and the prevalence of statin use (13.3% vs 29.7%, p<0.001) were lower.
Table 1.
Characteristics of REGARDS study participants overall and by PCSK9 loss of function variant status
| PCSK9 LOF Variant | ||||
|---|---|---|---|---|
|
| ||||
| Overall (n=10,695) |
No (n=10,454) |
Yes (n=241) |
p-value | |
|
| ||||
| Age, mean (SD) | 64.1 (9.3) | 64.1 (9.3) | 63.7 (9.2) | 0.55 |
| Female, n (%) | 6,599 (61.7) | 6,451 (61.7) | 148 (61.4) | 0.93 |
| Region, n (%) | ||||
| Belt | 3,596 (33.6) | 3,513 (33.6) | 83 (34.4) | 0.94 |
| Buckle | 1,888 (17.7) | 1,848 (17.7) | 40 (16.6) | -- |
| Non-belt/buckle | 5,211 (48.7) | 5,093 (48.7) | 118 (49.0) | ref |
| Education, n (%) | ||||
| Less than High School | 2,083 (19.5) | 2,026 (19.4) | 57 (23.6) | 0.29 |
| High School Graduate | 2,960 (27.7) | 2,909 (27.8) | 51 (21.2) | -- |
| Some College | 2,846 (26.6) | 2,777 (26.6) | 69 (28.6) | -- |
| College Graduate | 2,806 (26.2) | 2,742 (26.2) | 64 (26.6) | ref |
| Current Smoker, n (%) | 1,832 (17.1) | 1,784 (17.1) | 48 (19.9) | 0.25 |
| Current Alcohol Use, n (%) | 4,483 (41.9) | 4,378 (41.9) | 105 (43.6) | 0.65 |
| Depressive Symptoms, n (%) | 1,472 (13.8) | 1,432 (13.7) | 40 (16.6) | 0.20 |
| Physical Activity | ||||
| ≥4 times per week, n (%) | 2,826 (26.4) | 2,760 (26.4) | 66 (27.4) | 0.62 |
| 1–3 times per week, n (%) | 3,899 (36.5) | 3,809 (36.4) | 90 (37.3) | -- |
| 0 times per week, n (%) | 3,970 (37.1) | 3,885 (37.2) | 85 (35.3) | ref |
| Diabetes, n (%) | 3,165 (29.6) | 3,080 (29.5) | 85 (35.5) | 0.05 |
| History of CHD, n (%) | 1,664 (15.6) | 1,635 (15.6) | 29 (12.0) | 0.13 |
| History of Stroke, n (%) | 826 (7.7) | 814 (7.8) | 12 (5.0) | 0.11 |
| Atrial Fibrillation, n (%) | 835 (7.8) | 820 (7.8) | 15 (6.2) | 0.36 |
| Albuminuria, n (%) | 2,069 (19.3) | 2,025 (19.4) | 44 (18.3) | 0.67 |
| SBP, mean (SD) | 130.8 (17.4) | 130.8 (17.4) | 130.3 (16.1) | 0.65 |
| DBP, mean (SD) | 78.5 (10.1) | 78.5 (10.1) | 78.1 (9.4) | 0.53 |
| Waist Circumference, mean (SD) | 98.2 (15.4) | 98.3 (15.4) | 97.4 (15.5) | 0.40 |
| LDL-C, mean (SD) | 117 (37) | 118 (36) | 85 (32) | <0.001 |
| CRP, median (25th, 75th percentiles) | 2.9 (1.2, 6.6) | 2.9 (1.2, 6.7) | 2.9 (1.3, 5.3) | 0.18 |
| eGFR < 60 ml/min/1.73 m2, n (%) | 1,299 (12.1) | 1,266 (12.1) | 33 (13.7) | 0.46 |
| Antihypertensive Medication use, n (%) | 7,042 (65.8) | 6,892 (65.9) | 150 (62.2) | 0.23 |
| Statin use, n (%) | 3,137 (29.3) | 3,105 (29.7) | 32 (13.3) | <0.001 |
| Psychiatric Medication use, n (%) | 1,220 (11.4) | 1,193 (11.4) | 27 (11.2) | 0.92 |
| Number of neurocognitive tests, mean (Range) | ||||
| CERAD (WLL, WLD, AF) | 2.3 (1, 4) | 2.3 (1, 4) | 2.2 (1, 4) | 0.62 |
| SIS | 4.9 (1, 11) | 4.9 (1, 11) | 4.7 (1, 9) | 0.13 |
| Follow-up years, mean (SD) | 5.6 (2.1) | 5.6 (2.1) | 5.4 (2.2) | 0.39 |
| Neurocognitive impairment*, n (%) | ||||
| CERAD definition | 442 (6.0) | 432 (6.0) | 10 (6.2) | 0.91 |
| SIS definition | 1,644 (15.7) | 1,608 (15.7) | 36 (15.2) | 0.84 |
n(%) among participants with at least one CERAD battery or, separately, one SIS assessment at baseline or during any follow-up examination
Abbreviations: AF, animal fluency; CHD, coronary heart disease; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; CRP, c-reactive protein; DBP, diastolic blood pressure; LDL-C, low-density lipoprotein-cholesterol; LOF, loss of function; PCSK9, proprotein convertase subtilisin/kexin type-9; REGARDS, REasons for Geographic and Racial Differences in Stroke; SD, standard deviation; SIS, Six-Item Screener; WLD, word list delayed recall; WLL, word list learning
PCSK9 and neurocognitive impairment
The prevalence of neurocognitive impairment by the CERAD definition was 6.2% and 6.0% for individuals with and without PCSK9 LOF variants, respectively (Table 2). For the SIS definition, the prevalence of neurocognitive impairment comparing individuals with versus without PCSK9 LOF variants was 15.2% and 15.7%, respectively. In unadjusted models, the ORs for neurocognitive impairment comparing participants with versus without PCSK9 LOF variants were 1.04 (95% CI 0.54–1.98) and 0.97 (95% CI 0.67–1.38) using the CERAD and SIS definitions, respectively. After full multivariable adjustment, ORs for neurocognitive impairment were 1.11 (95% CI, 0.58–2.13) and 0.89 (95% CI, 0.61–1.30) when using the CERAD and SIS definitions of impairment, respectively. CERAD and SIS neurocognitive z-scores were not statistically significantly different between participants with versus without PCSK9 LOF variants at baseline (Supplemental Table 4). In multivariable adjusted models, average standardized differences over follow-up between participants with versus without PCSK9 LOF variants were −0.02 lower (95% CI −0.12, 0.08) for SIS, −0.04 lower (95% CI −0.16, 0.09) for WLL, 0.05 higher (95% CI −0.08, 0.18) for WLD, and −0.06 lower (95% CI −0.17, 0.06) for AF (Table 3). There was no evidence that the trajectory in neurocognitive decline differed by PCSK9 variant status (i.e., p>0.10 for the interaction between follow-up time and PCSK9 variant status for each neurocognitive test). The association between PCSK9 LOF variants and neurocognitive impairment and, separately, neurocognitive decline did not vary by statin use or LDL-C (all p-values >0.05).
Table 2.
Odds ratios for neurocognitive impairment associated with PCSK9 loss of function variants.
| Outcome: Impairment on CERAD battery |
Outcome: Impairment on the SIS |
|||
|---|---|---|---|---|
| PCSK9 LOF variant | No | Yes | No | Yes |
| Prevalence of neurocognitive impairment, % | 6.0 | 6.2 | 15.7 | 15.2 |
| Odds ratio (95% CI) | Odds ratio (95% CI) | |||
| Crude | 1.04 (0.54, 1.98) | 0.97 (0.67, 1.38) | ||
| Model 1 | 1.04 (0.55, 2.00) | 0.95 (0.65, 1.38) | ||
| Model 2 | 1.01 (0.52, 1.93) | 0.90 (0.62, 1.32) | ||
| Model 3 | 1.11 (0.58, 2.13) | 0.89 (0.61, 1.30) | ||
Abbreviations: CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; CI, confidence interval; LOF, loss of function; PCSK9, proprotein convertase subtilisin/kexin type-9; SIS, Six-Item Screener
Model 1 adjusted for age and number of neurocognitive assessments
Model 2 adjusted for Model 1 covariates + propensity score (gender, region of residence, education, waist circumference, smoking, alcohol use, physical activity, systolic and diastolic blood pressure, antihypertensive medication use, psychiatric medication use, estimated glomerular filtration rate, albuminuria, diabetes, atrial fibrillation, and depressive symptoms)
Model 3 adjusted for Model 2 covariates + statin use, history of coronary heart disease, history of stroke, and high-sensitivity C-reactive protein.
Table 3.
Differences in average z-scores for neurocognitive tests by PCSK9 loss of function variants
| Neurocognitive Score Differences between participants with versus without PCSK9 LOF Variants |
||||
|---|---|---|---|---|
|
| ||||
| PCSK9 LOF Variant | ||||
|
| ||||
| No | Yes | Average difference* |
p- difference† |
|
| SIS | Z-score (95% CI) | |||
|
| ||||
| Crude | −0.23 (−0.24, −0.21) | −0.27 (−0.38, −0.16) | −0.04 (−0.15, 0.07) | 0.45 |
| Model 1 | −0.23 (−0.25, −0.22) | −0.28 (−0.38, −0.18) | −0.05 (−0.15, 0.05) | 0.32 |
| Model 2 | −0.22 (−0.24, −0.21) | −0.25 (−0.35, −0.15) | −0.03 (−0.12, 0.07) | 0.59 |
| Model 3 | −0.32 (−0.35, −0.29) | −0.34 (−0.44, −0.24) | −0.02 (−0.12, 0.08) | 0.70 |
|
| ||||
| CERAD Word List Learning | Z-score (95% CI) | |||
|
| ||||
| Crude | −0.24 (−0.26, −0.22) | −0.30 (−0.44, −0.15) | −0.06 (−0.20, 0.09) | 0.46 |
| Model 1 | −0.24 (−0.26, −0.22) | −0.30 (−0.43, −0.17) | −0.06 (−0.19, 0.07) | 0.38 |
| Model 2 | −0.23 (−0.25, −0.21) | −0.25 (−0.37, −0.12) | −0.02 (−0.14, 0.11) | 0.81 |
| Model 3 | −0.42 (−0.46, −0.38) | −0.46 (−0.59, −0.33) | −0.04 (−0.16, 0.09) | 0.59 |
|
| ||||
| CERAD Word List Delay Recall | Z-score (95% CI) | |||
|
| ||||
| Crude | −0.21 (−0.23, −0.19) | −0.18 (−0.32, −0.03) | 0.03 (−0.12, 0.18) | 0.68 |
| Model 1 | −0.21 (−0.23, −0.19) | −0.18 (−0.31, −0.04) | 0.03 (−0.11, 0.16) | 0.70 |
| Model 2 | −0.20 (−0.22, −0.18) | −0.13 (−0.26, −0.00) | 0.07 (−0.06, 0.20) | 0.30 |
| Model 3 | −0.37 (−0.42, −0.33) | −0.32 (−0.45, −0.19) | 0.05 (−0.08, 0.18) | 0.44 |
|
| ||||
| CERAD Animal Fluency | Z-score (95% CI) | |||
|
| ||||
| Crude | −0.35 (−0.37, −0.33) | −0.40 (−0.52, −0.27) | −0.05 (−0.17, 0.08) | 0.47 |
| Model 1 | −0.35 (−0.37, −0.33) | −0.42 (−0.52, −0.30) | −0.07 (−0.18, 0.05) | 0.28 |
| Model 2 | −0.35 (−0.37, −0.33) | −0.39 (−0.51, −0.28) | −0.04 (−0.16, 0.07) | 0.46 |
| Model 3 | −0.49 (−0.53, −0.46) | −0.55 (−0.67, −0.43) | −0.06 (−0.17, 0.06) | 0.34 |
Z-score differences among participants with versus without LOF variants were calculated using all available assessments
p-value testing for the average differences in neurocognitive z-scores comparing participants with versus without PCSK9 LOF variants
Abbreviations: CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; CI, confidence interval; LOF, loss of function; PCSK9, proprotein convertase subtilisin/kexin type-9; SIS, Six-Item Screener
Model 1 includes adjustment for age
Model 2 includes adjustment for variables in Model 1 plus a propensity score(gender, region of residence, education, waist circumference, smoking, alcohol use, physical activity, systolic and diastolic blood pressure, antihypertensive medication use, psychiatric medication use, estimated glomerular filtration rate, albuminuria, diabetes, atrial fibrillation, and depressive symptoms)
Model 3 includes adjustment for variables in Model 2 plus statin use, history of coronary heart disease, history of stroke/transient ischemic attack, and high-sensitivity C-reactive protein
LDL-C and neurocognitive impairment
In unadjusted models ORs for neurocognitive impairment per 20 mg/dL lower LDL-C were 1.05 (95% CI 1.00, 1.11) and 1.02 (95% CI 0.99, 1.05) for the CERAD and SIS definitions of impairment, respectively (Table 4). After multivariable adjustment, ORs for neurocognitive impairment were 1.02 (95% CI 0.96, 1.08) and 0.99 (95% CI 0.95, 1.02) for the CERAD and SIS definitions of impairment, respectively. There were no statistically significant associations between LDL-C and baseline z-scores for any CERAD or SIS assessment (Supplemental Table 5). There was no evidence that trajectory in neurocognitive decline varied by LDL-C (p>0.10 for the interaction between follow-up time and LDL-C for each neurocognitive test). In multivariable adjusted models, average standardized differences over follow-up for 20 mg/dL lower LDL-C were 0.01 higher (95% CI 0.00, 0.02) for SIS and 0.00 (95% CI −0.01, 0.01) for each CERAD assessment (WLL, WLD, and AF) (Table 5). The association between each 20 mg/dL lower LDL-C and neurocognitive impairment, and separately, neurocognitive decline did not vary by statin use or PCSK9 LOF variant status (all p-values >0.05).
Table 4.
Odds ratios for neurocognitive impairment associated with 20 mg/dL lower low-density lipoprotein-cholesterol.
| Neurocognitive Impairment defined by CERAD battery |
Neurocognitive Impairment defined by SIS |
|||
|---|---|---|---|---|
| Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | |
| Crude | 1.05 (1.00, 1.11) | 0.07 | 1.02 (0.99, 1.05) | 0.12 |
| Model 1 | 1.05 (0.99, 1.11) | 0.09 | 1.00 (0.96, 1.03) | 0.78 |
| Model 2 | 1.04 (0.98, 1.10) | 0.16 | 0.98 (0.95, 1.01) | 0.25 |
| Model 3 | 1.02 (0.96, 1.08) | 0.51 | 0.99 (0.95, 1.02) | 0.45 |
Abbreviations: CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; CI, confidence interval; LDL-C, low-density lipoprotein-cholesterol; SIS, Six-Item Screener
Model 1 adjusted for age and number of neurocognitive assessments
Model 2 adjusted for Model 1 covariates + propensity score (gender, region of residence, education, waist circumference, smoking, alcohol use, physical activity, systolic and diastolic blood pressure, antihypertensive medication use, psychiatric medication use, estimated glomerular filtration rate, albuminuria, diabetes, atrial fibrillation, and depressive symptoms)
Model 3 adjusted for Model 2 covariates + statin use, history of coronary heart disease, history of stroke, and high-sensitivity C-reactive protein.
Table 5.
Average differences in neurocognitive test z-scores associated with 20mg/dL lower low-density lipoprotein-cholesterol
| SIS | CERAD Word List Learning | CERAD Word List Delay | CERAD Animal Fluency | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Z-score difference* (95% CI) |
p- value† |
Z-score difference* (95% CI) |
p- value† |
Z-score difference* (95% CI) |
p- value† |
Z-score difference* (95% CI) |
p-value† | |
| Crude | −0.01 (−0.02, 0.00) | 0.03 | −0.03 (0.02, 0.05) | <0.01 | −0.03 (−0.04, −0.02) | <0.01 | −0.02 (−0.03, −0.01) | <0.01 |
| Model 1 | 0.00 (−0.01, 0.01) | 0.77 | −0.01 (0.01, 0.03) | <0.01 | −0.02 (−0.03, 0.00) | 0.01 | −0.01 (−0.02, 0.00) | 0.04 |
| Model 2 | 0.01 (0.00, 0.02) | 0.01 | −0.01 (0.00, 0.02) | 0.19 | 0.00 (−0.01, 0.01) | 0.71 | 0.00 (−0.01, 0.01) | 0.55 |
| Model 3 | 0.01 (0.00, 0.02) | 0.10 | 0.00 (−0.01, 0.01) | 0.58 | 0.00 (−0.01, 0.01) | 0.67 | 0.00 (−0.01, 0.01) | 0.93 |
Z-score differences among participants with versus without LOF variants were calculated using all available assessments
p-value testing for the average differences in neurocognitive z-scores associated with 20 mmHg lower LDL-C
Abbreviations: CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; CI, confidence interval; SIS, Six-Item Screener
Model 1 includes adjustment for age
Model 2 includes adjustment for variables in Model 1 plus a propensity score for having neurocognitive impairment
Model 3 includes adjustment for variables in Model 2 plus statin use, history of coronary heart disease, history of stroke/transient ischemic attack, and high-sensitivity C-reactive protein
Discussion
In the current study of middle and older-aged African Americans, there was no association between having PCSK9 LOF variants and neurocognitive impairment based on CERAD battery and SIS assessments. PCSK9 LOF variants were not associated with longitudinal changes in neurocognitive function. Additionally, LDL-C was not associated with neurocognitive impairment or decline after adjusting for risk factors for neurocognitive impairment. The results from the current study provide evidence in a contemporary population that PCSK9 LOF variants and resulting life-long exposure to low LDL-C levels are not associated with neurocognitive impairment and decline.
Findings from the current study are consistent with a secondary analysis of the PROspective Study of Pravastatin in the Elderly at Risk (PROSPER) trial, which reported that lower cholesterol levels due to PCSK9 LOF variants were not associated with cognitive performance.24 However, differences between the PROSPER trial and the current study should be noted. PROSPER enrolled men and women between the ages of 70–82 years of age25, while the REGARDS study enrolled men and women ≥ 45 years. Additionally, the current analysis was restricted to African Americans due to a small number of white participants in the REGARDS study who have been genotyped and have PCSK9 LOF variants (n=21).10 The PROSPER trial also administered different cognitive function assessments than were administered in the REGARDS study. In the PROSPER trial, cognitive tests assessed verbal learning, delayed recall, selective attention and processing speed, while in the REGARDS study, verbal learning, verbal memory, semantic fluency and global cognition were assessed. The PROSPER trial did not enroll individuals with a low cognitive function score at baseline.26 The REGARDS study did not exclude individuals with low cognitive scores at baseline but did exclude individuals who, in the judgement of an interviewer, could not participate meaningfully in study interviews (e.g., those with impaired comprehension). Finally, different PCSK9 variants are present in white compared with African-American populations and were examined in PROSPER. Despite these differences, the overall conclusions are comparable between the two studies.
Evidence from observational studies on the association between low LDL-C and worse neurocognitive function has been conflicting.27, 28 For example, in an older cohort, high LDL-C was associated with better overall memory scores27. A secondary analysis of two Danish general population studies found that low LDL-C levels were associated with an increased risk of Alzheimer’s disease.29 However, low cholesterol levels due to PCSK9 variants had no association with the risk of Alzheimer’s disease. In contrast, older adults in the Northern Manhattan Study with high LDL-C had an increased risk of vascular dementia.30 The association between low LDL-C and neurocognitive function in older populations is difficult to interpret as aging is associated with both higher LDL-C and lower cognition.31 The current study provides contemporary evidence for these associations in a population of middle-aged and older adults.
PCSK9 has an important role in regulating circulating LDL-C levels. Sequence variations in the PCSK9 gene causing LOF mutations are associated with lower levels of LDL-C, and a reduced risk of CHD.8, 10 In several case reports/series, reductions in LDL-C following the initiation of statin therapy have been associated with neurocognitive impairment;1–3 however, these were often based on consumer reporting and rarely accompanied by formal neurocognitive testing. Neurocognitive symptoms in people taking statins have usually been mild and reversible, and these deficits resolved after treatment discontinuation.1 In the current study, there was no association between PCSK9 LOF variants and neurocognitive impairment or decline in participants taking or not taking statins. There was also no association between LDL-C and neurocognitive impairment or decline in this subgroup. This is consistent with trials monitoring adverse neurocognitive events associated with statin therapy alone25, 32 or in combination with PCSK9 inhibitors.33, 34
Imbalances in neurocognitive events including delirium, cognitive and attention disorders and disturbances, dementia and amnestic conditions, disturbances in thinking and perception, and mental impairment disorders were reported in post-hoc analyses of short-term Phase 2 and 3 clinical trials of PCSK9 inhibitors.5, 6 A combined analysis of the open-label, shorter duration evolocumab trials (median follow-up time 11.1 months) reported a higher frequency of neurocognitive adverse events in participants randomized to the treatment arms (n=27; 0.9%) compared with their counterparts randomized to placebo (n=4; 0.3%).5 However participants in the treatment arms received more face-to-face follow-up visits, providing additional opportunity to detect adverse events. In the ODYSSEY LONG TERM trial (78 week duration), participants in the alirocumab versus placebo arm had statistically non-significant higher rates of neurocognitive adverse events (1.2% vs 0.5%).6 The results from these two clinical trials could be due to chance since the total number of neurocognitive outcomes was small (31 in evolocumab trials and 22 in alirocumab trials). Additionally, neurocognitive events were not verified against standard definitions. A meta-analysis of 17 randomized clinical studies with a maximum duration of 102 weeks found an increased risk of neurocognitive adverse events among those randomized to receive PCSK9 inhibitors versus ezetimibe or placebo.22 In contrast, a pooled analysis of data from 14 randomized trials of PCSK9 inhibitors found that alirocumab treatment compared with ezetimibe or placebo did not result in an imbalance of neurocognitive adverse events over a median follow-up of 1.5 years.33 Neurocognitive impairment was not more common in participants achieving low levels of LDL-C (<25 mg/dL vs. ≥ 25 mg/dL).33
EBBINGHAUS7 was a 26-month (median follow-up time 19.8 months), Phase 3 trial that examined neurocognitive function in a subset of patients enrolled in the FOURIER34 cardiovascular outcomes trial of evolocumab, 140 mg every 2 weeks or 420 mg monthly, versus placebo. Using the Cambridge Neuropsychological Test Automated Battery (CANTAB) to examine working memory, memory function, and psychomotor speed there was no evidence of an association between pharmacologic PCSK9 inhibition or low LDL-C with neurocognitive decline.7 The results from the current study complement results from EBBINGHAUS by examining the cumulative effects of exposure to low levels of LDL-C during a lifetime, despite using different tests of neurocognitive function.
Strengths of the current study include the use of validated and detailed CERAD assessments of neurocognitive function across domains20 that have been consistently associated with performance on cognitively demanding activities of daily living.35, 36 The SIS is also a validated measure.21 The positive predictive value and negative predictive value for neurocognitive impairment and dementia as determined by a SIS score <5 are 100% and 76.6%, respectively.21 Additionally, the REGARDS study had few exclusion criteria and enrolled participants without regard to LDL-C levels, statin use, and prevalent CVD. We also acknowledge some limitations. This analysis was restricted to African-American participants limiting the generalizability of the findings. Even considering the older mean age of the population at baseline (64 years), the average duration of participant follow-up (5.6 years) may limit our ability to detect neurocognitive impairment occurring later in life. As neurocognitive assessments were administered in approximate 1 year (SIS) or 2 year (CERAD) cycles and some participants had neurocognitive impairment at the first assessment, we were unable to determine the time at which a participant became impaired. Additionally, the number of neurocognitive assessments administered varied across participants. The prevalence of PCSK9 LOF variants was low (2.3%), and few participants with PCSK9 LOF variants were categorized as having either CERAD or SIS-defined neurocognitive impairment which limited our power to detect small to moderate differences in risk of neurocognitive impairment. Although validated, the CERAD battery and SIS may miss subtle but clinically important neurocognitive deficits. This study examined neurocognitive impairment and decline; we do not have information on the full range of neurocognitive adverse events reported in the clinical trials of PCSK9 inhibitors. We relied on several self-reported covariates that may result in misclassification. Finally, exam measurements and laboratory data were available from only a single study visit, so we could not account for day-to-day variation in covariates.
In conclusion, PCSK9 LOF variants were not associated with neurocognitive impairment among a general population sample of middle and older-aged African Americans. No association was present for the overall population and for participants taking and not taking statins. Also, lower LDL-C levels were not associated with neurocognitive impairment. The results of this study suggest that life-long exposure to low PCSK9 levels and the resulting low levels of LDL-C do not have major effects on neurocognitive outcomes.
Supplementary Material
Clinical Perspective.
What is new?
In this general population sample of African American adults with extensive data collection and follow-up, there was no association between PCSK9 loss of function (LOF) variants and neurocognitive impairment or longitudinal neurocognitive decline.
There was no association between lower LDL-C levels and neurocognitive impairment or decline during follow-up.
The current study provides evidence in a contemporary population that PCSK9 LOF variants and resulting life-long exposure to low LDL-C levels are not associated with neurocognitive impairment and decline.
What are the clinical implications?
The results of this study suggest that life-long exposure to low PCSK9 levels and the resulting low levels of LDL-C do not have major effects on neurocognitive outcomes.
Acknowledgments
Funding Source: The REGARDS research project is supported by a cooperative agreement U01NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org
This research, including design and conduct of the study, analysis and interpretation of the data, and preparation of the manuscript, was supported through an academic collaboration between University of Alabama at Birmingham and Amgen Inc. The funders provided comments on the design and interpretation of this work. The academic authors conducted all analyses and maintained the rights to publish this manuscript.
Robert S. Rosenson receives research support from Akcea, Amgen, Eli Lilly, Medicines Company, and Sanofi. He has participated in Advisory Boards for Amgen, Eli Lilly, Regeneron and Sanofi. He consults for CVS Caremark and Easy Vitals. He receives honoraria from Kowa and royalties from UpToDate. Michael E. Farkouh receives research support from Amgen. Vera Bittner receives research support from Amgen, Astra-Zeneca, DalCor, Eli-Lilly, Esperion, Sanofi-Regeneron, and Bayer Healthcare. Monika Safford receives research support from Amgen. She has participated in Advisory Boards for Amgen. Ransi Somaratne works in Clinical Development at Amgen and is a stockholder of Amgen. Keri L. Monda works in the Center for Observational Research at Amgen and is a stockholder of Amgen. Paul Muntner receives research support from Amgen. Emily B. Levitan receives research support from Amgen. She has participated in Advisory Boards for Amgen and has consulted for Novartis.
Footnotes
Disclosures: Matthew T. Mefford has no disclosures.
Mary Cushman has no disclosures.
Leslie McClure has no disclosures. Virginia Wadley has no disclosures. Marguerite R. Irvin has no disclosures.
References
- 1.Wagstaff LR, Mitton MW, Arvik BM, Doraiswamy PM. Statin-associated memory loss: analysis of 60 case reports and review of the literature. Pharmacotherapy. 2003;23:871–880. doi: 10.1592/phco.23.7.871.32720. [DOI] [PubMed] [Google Scholar]
- 2.Orsi A, Sherman O, Woldeselassie Z. Simvastatin-associated memory loss. Pharmacotherapy. 2001;21:767–769. doi: 10.1592/phco.21.7.767.34577. [DOI] [PubMed] [Google Scholar]
- 3.King DS, Wilburn AJ, Wofford MR, Harrell TK, Lindley BJ, Jones DW. Cognitive impairment associated with atorvastatin and simvastatin. Pharmacotherapy. 2003;23:1663–1667. doi: 10.1592/phco.23.15.1663.31953. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson TA. NLA Task Force on Statin Safety–2014 update. J Clin Lipidol. 2014;8:S1–S4. doi: 10.1016/j.jacl.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, Ballantyne CM, Somaratne R, Legg J, Wasserman SM, Scott R, Koren MJ, Stein EA Open-Label Study of Long-Term Evaluation against LDLCI. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500–1509. doi: 10.1056/NEJMoa1500858. [DOI] [PubMed] [Google Scholar]
- 6.Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ ODYSSEY LONG TERM Investigators. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–1499. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- 7.Giugliano RP, Mach F, Zavitz K, Kurtz C, Im K, Kanevsky E, Schneider J, Wang H, Keech A, Pedersen TR, Sabatine MS, Sever PS, Robinson JG, Honarpour N, Wasserman SM, Ott BR EBBINGHAUS Investigators. Cognitive Function in a Randomized Trial of Evolocumab. N Engl J Med. 2017;377:633–643. doi: 10.1056/NEJMoa1701131. [DOI] [PubMed] [Google Scholar]
- 8.Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, protection against coronary heart disease. N Engl J Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 9.Huang CC, Fornage M, Lloyd-Jones DM, Wei GS, Boerwinkle E, Liu K. Longitudinal association of PCSK9 sequence variations with low-density lipoprotein cholesterol levels: the Coronary Artery Risk Development in Young Adults Study. Circ Cardiovasc Genet. 2009;2:354–361. doi: 10.1161/CIRCGENETICS.108.828467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kent ST, Rosenson RS, Avery CL, Chen YI, Correa A, Cummings SR, Cupples LA, Cushman M, Evans DS, Gudnason V, Harris TB, Howard G, Irvin MR, Judd SE, Jukema JW, Lange L, Levitan EB, Li X, Liu Y, Post WS, Postmus I, Psaty BM, Rotter JI, Safford MM, Sitlani CM, Smith AV, Stewart JD, Trompet S, Sun F, Vasan RS, Woolley JM, Whitsel EA, Wiggins KL, Wilson JG, Muntner P. PCSK9 Loss-of-Function Variants, Low-Density Lipoprotein Cholesterol, and Risk of Coronary Heart Disease and Stroke: Data From 9 Studies of Blacks and Whites. Circ Cardiovasc Genet. 2017;10:e001632. doi: 10.1161/CIRCGENETICS.116.001632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 12.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy RE, Wadley VG, McClure LA, Letter AJ, Unverzagt FW, Crowe M, Nyenhius D, Kelley BJ, Kana B, Marceaux J, Kurella Tamura M, Howard V, Howard G. Performance of the NINDS-CSN 5-minute protocol in a national population-based sample. J Int Neuropsychol Soc. 2014;20:856–867. doi: 10.1017/S1355617714000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nutter-Upham KE, Saykin AJ, Rabin LA, Roth RM, Wishart HA, Pare N, Flashman LA. Verbal fluency performance in amnestic MCI and older adults with cognitive complaints. Arch Clin Neuropsychol. 2008;23:229–241. doi: 10.1016/j.acn.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wicklund AH, Johnson N, Rademaker A, Weitner BB, Weintraub S. Word list versus story memory in Alzheimer disease and frontotemporal dementia. Alzheimer Dis Assoc Disord. 2006;20:86–92. doi: 10.1097/01.wad.0000213811.97305.49. [DOI] [PubMed] [Google Scholar]
- 17.Thacker EL, Gillett SR, Wadley VG, Unverzagt FW, Judd SE, McClure LA, Howard VJ, Cushman M. The American Heart Association Life's Simple 7 and incident cognitive impairment: The REasons for Geographic And Racial Differences in Stroke (REGARDS) study. J Am Heart Assoc. 2014;3:e000635. doi: 10.1161/JAHA.113.000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Glasser SP, Wadley V, Judd S, Kana B, Prince V, Jenny N, Kissela B, Safford M, Prineas R, Howard G. The association of statin use and statin type and cognitive performance: analysis of the reasons for geographic and racial differences in stroke (REGARDS) study. Clin Cardiol. 2010;33:280–288. doi: 10.1002/clc.20758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schinka JA, Loewenstein DA, Raj A, Schoenberg MR, Banko JL, Potter H, Duara R. Defining mild cognitive impairment: impact of varying decision criteria on neuropsychological diagnostic frequencies and correlates. Am J Geriatr Psychiatry. 2010;18:684–691. doi: 10.1097/JGP.0b013e3181e56d5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40:771–781. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Lipinski MJ, Benedetto U, Escarcega RO, Biondi-Zoccai G, Lhermusier T, Baker NC, Torguson R, Brewer HB, Jr, Waksman R. The impact of proprotein convertase subtilisin-kexin type 9 serine protease inhibitors on lipid levels and outcomes in patients with primary hypercholesterolaemia: a network meta-analysis. Eur Heart J. 2016;37:536–545. doi: 10.1093/eurheartj/ehv563. [DOI] [PubMed] [Google Scholar]
- 23.Rosenbaum PRRDB. Reducing bias in observational studies using subclassification on the propensity score. Journal of the American Statistical Association. 1984;79:516–524. [Google Scholar]
- 24.Postmus I, Trompet S, de Craen AJ, Buckley BM, Ford I, Stott DJ, Sattar N, Slagboom PE, Westendorp RG, Jukema JW. PCSK9 SNP rs11591147 is associated with low cholesterol levels but not with cognitive performance or noncardiovascular clinical events in an elderly population. J Lipid Res. 2013;54:561–566. doi: 10.1194/jlr.M033969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Meinders AE, Norrie J, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey C, Westendorp RG PROSPER Study Group. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 26.Shepherd J, Blauw GJ, Murphy MB, Cobbe SM, Bollen EL, Buckley BM, Ford I, Jukema JW, Hyland M, Gaw A, Lagaay AM, Perry IJ, Macfarlane PW, Meinders AE, Sweeney BJ, Packard CJ, Westendorp RG, Twomey C, Stott DJ. The design of a prospective study of Pravastatin in the Elderly at Risk (PROSPER). PROSPER Study Group. PROspective Study of Pravastatin in the Elderly at Risk. Am J Cardiol. 1999;84:1192–1197. doi: 10.1016/s0002-9149(99)00533-0. [DOI] [PubMed] [Google Scholar]
- 27.West R, Beeri MS, Schmeidler J, Hannigan CM, Angelo G, Grossman HT, Rosendorff C, Silverman JM. Better memory functioning associated with higher total and low-density lipoprotein cholesterol levels in very elderly subjects without the apolipoprotein e4 allele. Am J Geriatr Psychiatry. 2008;16:781–785. doi: 10.1097/JGP.0b013e3181812790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katsumata Y, Todoriki H, Higashiuesato Y, Yasura S, Ohya Y, Willcox DC, Dodge HH. Very old adults with better memory function have higher low-density lipoprotein cholesterol levels and lower triglyceride to high-density lipoprotein cholesterol ratios: KOCOA Project. J Alzheimers Dis. 2013;34:273–279. doi: 10.3233/JAD-121138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benn M, Nordestgaard BG, Frikke-Schmidt R, Tybjaerg-Hansen A. Low LDL cholesterol, PCSK9 and HMGCR genetic variation, and risk of Alzheimer's disease and Parkinson's disease: Mendelian randomisation study. BMJ. 2017;357:j1648. doi: 10.1136/bmj.j1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reitz C, Tang MX, Luchsinger J, Mayeux R. Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch Neurol. 2004;61:705–714. doi: 10.1001/archneur.61.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harada CN, Natelson Love MC, Triebel KL. Normal cognitive aging. Clin Geriatr Med. 2013;29:737–752. doi: 10.1016/j.cger.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Everett BM, Mora S, Glynn RJ, MacFadyen J, Ridker PM. Safety profile of subjects treated to very low low-density lipoprotein cholesterol levels (<30 mg/dl) with rosuvastatin 20 mg daily (from JUPITER) Am J Cardiol. 2014;114:1682–1689. doi: 10.1016/j.amjcard.2014.08.041. [DOI] [PubMed] [Google Scholar]
- 33.Robinson JRRS, Farnier M, Chaudhari U, Sasiela WJ, Merlet L, Miller K, Kastelein JJP. Safety of very low low-density lipoprotein cholesterol levels with alirocumab: Pooled data from randomized trials. J Am Coll Cardiol. 2017;69:471–482. doi: 10.1016/j.jacc.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 34.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR. Fourier Steering Committee and Investigators. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 35.Vaughan L, Giovanello K. Executive function in daily life: Age-related influences of executive processes on instrumental activities of daily living. Psychol Aging. 2010;25:343–355. doi: 10.1037/a0017729. [DOI] [PubMed] [Google Scholar]
- 36.Greenaway MC, Duncan NL, Hanna S, Smith GE. Predicting functional ability in mild cognitive impairment with the Dementia Rating Scale-2. Int Psychogeriatr. 2012;24:987–993. doi: 10.1017/S1041610211002717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


