Abstract
Objectives
Investigate whether deficits in empathic accuracy (i.e., ability to recognize emotion in others) in patients with neurodegenerative disease are associated with greater depression in their caregivers.
Design
Two cross-sectional studies.
Setting
Academic Medical Center and Research University.
Participants
Two independent samples (N=172, N=63) of patients with a variety of neurodegenerative diseases and their caregivers; comparison group of healthy couples.
Measurement
Patients’ empathic accuracy was assessed in the laboratory using a novel dynamic tracking task (rating another person’s changing emotions over time) and more traditional measures (recognizing the emotion expressed in photographs of facial expressions and by characters in films). Caregivers completed self-report inventories of depression.
Results
Lower empathic accuracy in patients was associated with greater depression in caregivers in both studies. In Study 1, this association was found when empathic accuracy was measured using the dynamic tracking measure but not when measured using the more traditional photograph and film measures. In Study 2, we found preliminary support for our theoretical model wherein lower empathic accuracy in patients is associated with increased caregiver stress (loneliness, strain, and burden), which in turn is associated with greater caregiver depression.
Conclusions
Caring for a patient with deficits in empathic accuracy is associated with greater loneliness, strain, and burden for caregivers, and increased depression. Caregivers may benefit from interventions designed to compensate for the stress and interpersonal loss associated with patients’ declining empathic accuracy.
Keywords: caregiver, depression, empathic accuracy, neurodegenerative disease
Objective
Neurodegenerative diseases produce profound deficits in cognitive, emotional, and motor functioning. As these diseases progress, patients become increasingly impaired and dependent on caregivers for assistance. For many patients, close loved ones play a primary caregiving role. The psychiatric morbidity associated with caregiving is well-established (1), including up to four-fold increases in rates of depression (2). These elevations in caregiver depression are all the more striking given that caregiving most commonly occurs in late life, a time when depression rates normally drop (3).
Individual differences in caregiver vulnerability
Caregiving is challenging to all. Nonetheless, some caregivers move through the experience relatively intact, whereas others spiral downward in a trajectory of declining mental health. Understanding this variability has become an important part of caregiver research. A consistent theme emerging from two decades of reviews of adverse outcomes in caregivers of dementia patients (e.g., 4,5) has been that behavioral and psychological symptoms of dementia (BPSDs) are strongly associated with psychiatric morbidity in caregivers (even more so than cognitive and functional symptoms). BPSDs encompass a wide range of behaviors (e.g., aggression, agitation, sleep disturbance, wandering). There are considerable differences among investigators as to which behaviors BPSDs include and how they are best measured. This has led to calls for more research on specific patient symptoms associated with adverse caregiver outcomes and on the mechanisms that link patient symptoms with these outcomes (4).
Emotional symptoms in patients: Impact of deficits in emotion recognition
Among BPSDs, many have clear links to patients’ emotional functioning (e.g., agitation, depression). Deficits in emotional functioning are seen in a number of neurodegenerative diseases (6). These deficits can take a variety of forms including alterations in emotional reactivity (generating emotional responses), regulation (adjusting emotional responses to situational demands), and recognition (knowing what others are feeling). Among these, deficits in emotion recognition can be particularly difficult for caregivers, and are found in a variety of neurodegenerative diseases (e.g., 7,8). When patients are insensitive to others’ emotions, caregivers may feel increasingly unsupported, which impairs the quality of patient-caregiver relationships (9). The loss of a supportive relational partner has been associated with heightened stress and loneliness, factors that are well-established longitudinal contributors to the development of depression (10–12).
Measuring empathic accuracy
There are a number of ways of measuring emotion recognition. Although early measures were largely based on respondent’s self-assessments, contemporary approaches typically assess ability to recognize emotion using an external criterion for accuracy (empathic accuracy; 13) In the dementia literature, empathic accuracy has been measured by having patients identify emotions portrayed in static facial expressions (e.g., 14) or expressed by a character in a film (e.g., 15). These tests have proven quite useful, but have limits in their ecological validity. In the real world, emotion recognition typically involves: (a) integrating multiple types of information (visual, auditory, etc.); (b) processing information from multiple bodily regions (e.g., face, posture); (c) monitoring behaviors that occur in interpersonal situations; and (d) tracking continuously changing emotions as they ebb and flow over time. For these reasons, in research with neurologically healthy individuals, investigators increasingly measure empathic accuracy dynamically, using approaches that assess the ability to track changing emotions in others over time (16–18). We have applied a “dynamic tracking” approach previously with neurological patients (19), but it has not been used to study links between patient deficits and caregiver depression.
The Current Studies
We conducted two studies with independent samples of patients and caregivers to test the hypothesis that low levels of empathic accuracy in patients would be associated with high levels of depression in caregivers. In Study 1, we evaluated three methods for assessing empathic accuracy: (a) dynamically tracking changing emotions expressed by a character in a film; (b) identifying emotion displayed in photographs, and (c) identifying emotion expressed by a character in a series of films. We assess the robustness of these relationships by controlling for patient cognitive and functional symptoms. Study 2 expands upon study 1 by examining measures of caregivers’ personality and emotion regulation strategies that have previously been associated with depression (e.g., neuroticism and reappraisal; (20,21) and by examining measures of caregiver stress and loneliness hypothesized to account for the relationship between empathic accuracy and caregiver depression.
Study 1 Methods
Participants
Patients with neurodegenerative disease (N=172) and their caregivers (N=172) were recruited from the Memory and Aging Center at the University of California, San Francisco (UCSF). Caregivers were primarily spouses (N=169). A comparison group of neurologically healthy individuals (N=25) and their close relational partners were also recruited from UCSF, and these individuals were selected to be within a similar age range as the individuals with neurodegenerative disease. At UCSF patients participated in neurological examinations, neuropsychological testing, and neuroimaging, and received a diagnosis based on current research criteria (e.g., 22–24). Patient diagnoses are presented in Table 1.
Table 1.
Patient Diagnoses for Study 1 and Study 2.
| Diagnostic Group | Study 1 | Study 2 |
|---|---|---|
| Frontotemporal Dementia | 69 | 31 |
| Alzheimer’s Disease | 59 | 8 |
| Corticobasal Syndrome | 22 | 8 |
| Progressive Supranuclear Palsy | 10 | 9 |
| Amyotropic Lateral Sclerosis | 7 | -- |
| Other | 5 | 7 |
Procedure
Following their evaluation at UCSF, patients and individuals from the comparison group were scheduled for a daylong assessment of emotional functioning at the Berkeley Psychophysiology Laboratory at the University of California, Berkeley (typically within a month of their UCSF visit). This assessment provided a comprehensive evaluation of their emotional reactivity, regulation, and recognition (25), including our dynamic tracking and film tasks for assessing empathic accuracy (the photograph task was administered at the UCSF evaluation). All procedures were approved by the human subjects committees at UCSF and Berkeley.
Measures
Patient Empathic Accuracy
Dynamic tracking task
Participants were seated facing a color television monitor with a rating dial located near the dominant hand. The dial consisted of a small box with a rotating pointer that traversed a 180° path over a nine-point scale anchored by the legends “very bad” (depicted by a schematic frowning face) at the extreme left, “neutral” (depicted by a neutral face) in the middle, and “very good” (depicted by a smiling face) at the extreme right. The dial generated a position-dependent voltage that was sampled by a computer every 3 milliseconds and averaged every second.
Participants were instructed to adjust the rating dial as often as needed so that it always reflected the emotions of the target character in a film. Following the instructions, the experimenter asked several questions to ensure that the participant understood the task. The participant then viewed and rated an 80-second film clip in which the female target character has a conversation with a male dinner companion and expresses a range of positive and negative emotions.
Accuracy was calculated using time-lagged cross correlations to compute the association between each participants’ ratings of the film and ratings obtained previously from an expert panel of healthy individuals. To allow for processing and motor delays, the maximum correlation coefficient was selected for lags between −10 and +10 seconds (correlation coefficients ranged from −.26 to .92).
Emotion recognition in photographs
A subset of participants (N=107) were shown static photographs of 7 emotional facial expressions and asked to choose the correct emotion term (happy, sad, angry, etc.) from a list 7 terms (Comprehensive Affect Testing System;26). Correct responses were summed.
Emotion recognition in films
A subset of participants (N=196) viewed 11 short (approximately 40 sec) film clips each depicting a character experiencing an emotion (amusement, fear, embarrassment, etc.;15). After each film, participants were asked to indicate what emotion the character felt most strongly from a list of 11 emotions. Correct responses were summed and divided by the total number of responses.
Caregiver’s Report of Patient’s Empathic Accuracy
A subset of caregivers (N=140) reported on patients’ empathic accuracy using the Caregiver Assessment of Socioemotional Functioning (27). Caregivers rated the extent to which patients recognized and understood each of 10 basic emotions over the past month on a scale from 0 (not at all) to 4 (a lot). Emotions included anger, fear, disgust, sadness, amusement, shame, guilt, pride, and embarrassment (Cronbach’s alpha=.91).
Patient Cognitive and Functional Deficits
Mini-mental State Examination
A subset of participants (N=161) completed the Mini-mental State Examination (MMSE;28) an assessment of cognitive impairment with lower scores indicating greater impairment.
Clinical Dementia Rating Scale
A subset of participants (N=181) completed the Clinical Dementia Rating Scale (29) which uses semi-structured interviews with participants and their caregivers (or comparison group partners) to assesses participants’ functional impairment. The sum of boxes score was used (CDR-Box) with higher scores indicating greater dementia severity.
Caregiver Depression
Symptom Checklist-90-R
Caregivers (and partners of healthy individuals from the comparison group) completed the 13-item depression subscale of the Symptom Checklist-90-R (30) with higher scores indicating greater depression.
Statistical Analyses
Analyses were conducted using SPSS 22. To test our hypothesis that low levels of patient empathic accuracy would be associated with high levels of caregiver depression, we conducted regression analyses in which the four empathic accuracy tasks were entered together as predictors and caregiver depression was the dependent variable. Regressions only used data from the patient-caregiver couples.
Study 1 Results
Preliminary Analyses
Group means of measures are presented in Table 2. Additional demographic information and group analyses are presented in Supplemental Digital Content (See document, Supplemental Digital Content 1). Correlations among the measures of empathic accuracy were all statistically significant, and small to moderate in size (absolute values ranged from .16 – .45; See Table 3).
Table 2.
Demographic data for patients, comparison group members, and caregivers.
| Study: Group | n | Age | M:F | Cognitive Impairment (MMSE) |
Dementia Severity (CDR- Box) |
Empathic Accuracy (Tracking) |
Emotion Recognition (Photos) |
Emotion Recognition (Films) |
Empathic Accuracy (Care Report) |
Depressive Symptoms (SCL-90) or (CESD) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1: Patients | 172 | 66.99 (8.02) | 94:78 | 24.14 (4.97) | 4.62 (2.88) | .51(.30) | 10.93(2.77) | 7.40(2.62) | 1.93(1.08) | -- |
| 1: Comparison Group | 25 | 67.24 (11.96) | 12:13 | 29.3 (0.68) | 0.00 (0.00) | .83(.08) | 13.5(1.73) | 10.08(.76) | 3.16(0.79) | -- |
| 1: Caregivers | 172 | 61.68 (10.96) | 70:102 | -- | -- | -- | -- | -- | -- | 0.79 (0.66) |
| 1: Comparion Partners | 25 | 60.07 (14.37) | 11:14 | -- | -- | -- | -- | -- | 0.30 (0.32) | |
|
| ||||||||||
| 2: Patients | 63 | 62.89 (11.45) | 30:33 | 24.31 (5.58) | 3.98 (2.68) | .64(.24) | -- | -- | -- | -- |
| 2: Caregivers | 63 | 61.52 (9.99) | 30:33 | -- | -- | -- | -- | -- | -- | 11.44 (8.88) |
Table 3.
Intercorrelations of empathic accuracy variables.
| Study 1 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1. Empathic Accuracy: Dynamic Tracking | 1 | |||
| 2. Emotion Recogntion in Photographs | .41*** | 1 | ||
| 3. Emotion Recognition in Films | .37*** | .46*** | 1 | |
| 4. Caregiver Report of Patient’s Empathic Accuracy | .17* | .39*** | .26** | 1 |
| 5. Caregiver Depression | −.15* | −.12 | .01 | −.23** |
Note.
p<.05,
p<.01,
p < .001
Patient Empathic Accuracy and Caregiver Depression
Among the four measures of empathic accuracy, only the dynamic tracking task and caregiver’s report of patient’s empathic accuracy were significantly correlated with caregiver depression (See Table 3).
Regression results indicated that lower patient empathic accuracy measured using the dynamic rating task predicted higher caregiver depression, β=−.31, t(84)=−2.80, p=.006, and caregiver’s report of patients empathic accuracy was a marginally significant predictor, β=−.21, t(84)=−1.79, p=.077. Neither the emotion recognition in photographs task, β=.07, t(84)=.55, p=.58, nor the emotion recognition in films task predicted caregiver depression, β=.02, t(84)=.21, p=.83.
To determine whether this relationship held when controlling for patient cognitive and functional deficits, the analysis was repeated with patient MMSE and CDR-Box scores entered together on the first step and the four emotion recognition tasks entered together on the second step. Patient MMSE and CDR-Box were not significant predictors of caregiver depression, F(2,78)=1.48, p=.23. Adding the three emotion recognition tasks accounted for significant additional variance, change in R2 F(4,74) =2.95, p=.025. In this model which included all patient functioning and empathic accuracy measures, only lower patient empathic accuracy measured using the dynamic rating task was associated with greater caregiver depression, β=−.37, t(74)=−2.90, p=.005.
We continued to examine the robustness of the association between dynamic empathic accuracy and caregiver depression by adding caregiver age and gender as additional predictors in the second step of the existing model. Empathic accuracy on the dynamic tracking task remained significantly associated with caregiver depression, β=−.30, t(72)=−2.32, p=.023. Gender was the only other predictor approaching significance in this full model, with female caregivers having marginally greater depression than males, β=.22, t(72)=1.86, p=.067. Additionally, the relationship between empathic accuracy and depression did not seem to differ by diagnosis (See Supplemental Digital Content 1).
Study 2 Methods
Participants
An independent, non-overlapping sample of patients (N=63) and their caregivers was recruited from UCSF and assessed in the same way as in Study 1. Patient diagnoses are presented in Table 1.
Procedure
Procedures were the same as in Study 1 with the exception that a different measure of caregiver depression was used and five additional caregiver measures were included that were not assessed in Study 1 (see below).
Measures
Caregiver Neuroticism
Caregivers completed the 10-item version of the Big Five Inventory (31) with higher scores indicating higher neuroticism.
Caregiver Reappraisal
Caregivers completed the 6-item cognitive reappraisal subscale of the Emotion Regulation Questionnaire (ERQ;32) which captures individual tendencies to assess a situation in a different way to alter its emotional impact, with higher scores indicating greater reappraisal.
Caregiver Stress
We obtained three measures related to the stress of caregiving: (a) Loneliness was assessed using the UCLA Loneliness Scale (33), a 20-item measure that assesses subjective feelings of loneliness and isolation; (b) Strain was measured using the Caregiver Strain Index (34), a 13-item measure that assesses subjective and objective elements of caregiver stress; and (c) Burden was assessed using the 12-item version of the Zarit Burden Interview (35), which assesses subjective elements of caregiver stress.
Each of these three measures captures facets of caregiver stress and loneliness thought to longitudinally promote the development of depression (11,36). Given our hypothesis that caregiver stress and loneliness would account for the relationship between empathic accuracy and caregiver depression, and because these measures are highly correlated (Chronbach’s alpha= .86), we created an overall composite of “caregiver stress” by z-scoring each scale, and averaging the three scales such that higher scores indicated higher stress.
Caregiver Depression
Caregivers completed the Center for Epidemiologic Studies Depression measure (CESD;37), a 20-item self-report assessment of depressive symptoms. The presence of symptoms in the past week is rated on a 4-point scale ranging from “not at all” to “a lot”.
Statistical Analyses
Linear regression in SPSS 22 was again used to test robustness of associations between patient empathic accuracy and caregiver depression. As a preliminary examination of our hypothesized model wherein deficits in patient empathic accuracy are associated with increased stress and loneliness for caregivers (factors known to contribute to the onset of depression), we conducted a path analysis using bootstrapping (Mediation Analysis using PROCESS;38,39) in which indirect effects were computed for each of 5,000 bootstrapped samples and bias corrected bootstrap 95% confidence intervals were computed for caregiver’s stress.
Study 2 Results
Patient Empathic Accuracy and Caregiver Depression
As in Study 1, lower empathic accuracy in patients measured using the dynamic tracking task was associated with higher depression in caregivers, β=−.32, t(57)=−2.52, p=.015. To determine whether this relationship held when controlling for aspects of caregiver personality and emotion regulation that have previously been linked with caregiver depression, the analysis was repeated with caregiver neuroticism and reappraisal scores entered together on the first step and empathic accuracy entered on the second step. Caregiver neuroticism and reappraisal were not significant predictors of caregiver depression, F(2,51)=.55, p=.58. Adding the empathic accuracy task accounted for significant variance, change in R2 F(1,50) =4.47, p=.039 with lower patient empathic accuracy being associated with greater caregiver depression, β=−.28, t(50)=−2.11, p=.039.
We continued to examine the robustness of this association, by adding caregiver age and gender as additional predictors in the second step of the existing model, empathic accuracy remained marginally associated with caregiver depression, β=−.26, t(48)=−2.01, p=.051. Age was the only other predictor approaching significance in this full model, with younger caregivers having marginally greater depression, β=−.24, t(48)=−1.78, p=.082. Lastly, we added patient cognitive impairment and MMSE into the model, and the effect of patient empathic accuracy on caregiver depression was no longer significant, β=.079, t(44)=.58, p=.56. In this full model, caregiver age emerged as a significant predictor of caregiver depression, β=−.25, t(44)=−2.21, p=.032, as did patient cognitive impairment, β=−.31, t(44)=−2.45, p=.018, and functional impairment, β=.46, t(44)=3.40, p=.001.
The Potential Mediating Role of Caregiver Stress and Loneliness
There was a significant indirect effect of caregiver stress on caregivers’ depression, bootstrap CI95=[−.61, −.08], indicating lower levels of patients’ empathic accuracy were associated with higher levels of caregiver stress, which in turn were associated with higher levels of caregiver depression. Figure 1 illustrates these findings. Indirect effects are significant when strain, burden, or loneliness are used independently in the model (See Supplemental Digital Content 1).
Figure 1.
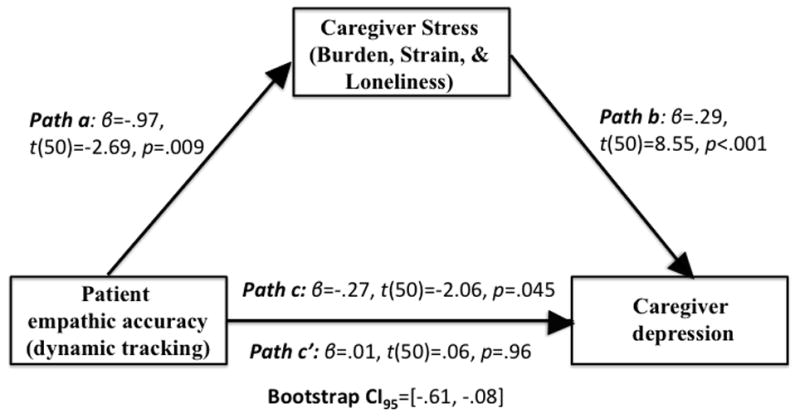
Preliminary path analysis of our hypothesized model wherein lower empathic accuracy in patients is associated with greater burden, strain, and loneliness for caregivers, which in turn is associated with greater caregiver depression.
Conclusions
Across two studies with independent samples of patients with a variety of neurodegenerative diseases and their caregivers, we found support for our hypothesis that lower empathic accuracy in patients was associated with higher depression in their caregivers. In Study 1, we found that this relationship held only when empathic accuracy was measured using the dynamic tracking task; it was not found using the more traditional tasks (identifying emotion in photographs or films). In Study 1, the relationship between patient empathic accuracy and caregiver depression was found after controlling for patient cognitive and functional symptoms, and in Study 2, the relationship was found after accounting for caregiver characteristics that have previously been associated with caregiver depression (e.g., neuroticism and reappraisal).
Empathic accuracy in patients and caregiver depression
Our findings are consistent with the growing consensus(1,4) about the important role that patients’ behavioral and psychological symptoms play in determining the degree to which caregivers experience the adverse effects of caregiving. In the present research, we focused on a particular aspect of patients’ emotional behavior, empathic accuracy. We conducted a preliminary examination of a model wherein caregivers of patients who have difficulty tracking changing emotions in others experience greater loneliness, burden, and strain, which in turn contributes to increases in depression. Of course, our data are not longitudinal, and thus we cannot establish the causal direction of these associations with certainty. However, other longitudinal research support stress and loneliness as a precursors for subsequent depression (10,11). It is also important to note that we are not suggesting caregiver stress or depression are exclusively driven by patient empathic accuracy. Our data suggest a diverse set of factors such as gender and dementia severity also have associations with caregiver depression. The robustness of these effects likely varies as a function of disease stage and diagnosis, as the types of challenges faced by caregivers differ across disease stages and diagnoses.
Measuring empathic accuracy in patients
Our finding in Study 1 that the association between patient empathic accuracy and caregiver depression was only found when empathic accuracy was assessed using the dynamic tracking task has several important implications. Identifying emotion in photographs and films has a long history of proven utility in distinguishing between types of dementia(15). These tasks also have the advantage of being easily administered (although we are developing a portable version of the dynamic tracking task). However, for uncovering associations between patient empathic accuracy and caregiver depression, the dynamic tracking task is arguably more sensitive to the kinds of patient deficits that are particularly stressful for caregivers and contribute to the development of depression.
To some extent, both the photograph and film tasks assess semantic knowledge about emotion (e.g., knowing that a smiling face is a “happy” face or that a yelling and gesticulating actor is “angry”). Patients who have this semantic knowledge may be more sensitive and responsive to their caregivers than those who do not. However, the emotion recognition challenges that confront patients on a daily basis are much more likely to resemble the dynamic tracking task, where emotions are changing over time. In previous work we have found that performance on dynamic tracking tasks relies on the integrity of orbitofrontal circuitry thought to be involved in updating value judgments(19). Caring for a patient who does not recognize emotional changes in others and does not update behaviors accordingly can be particularly isolating and stressful for caregivers, creating a fertile environment for increasing caregiver depression.
Implications
The current findings shed light on an important behavioral symptom in patients associated with increased depression in caregivers -- namely, lower empathic accuracy as measured using the dynamic tracking task. Findings add to our understanding of specific BPSDs contributing to adverse outcomes in caregivers, and provide preliminary support for the role that caregiver loneliness, strain and burden may play in explaining the association between low empathic accuracy in patients and greater depression in caregivers. Caregiver depression is a huge public health problem that may compromise caregivers’ ability to provide high quality care and contribute to patient mortality(40). Caregivers of patients with early deficits in empathic accuracy may be good candidates for preventative interventions designed to compensate for the associated loneliness, strain, and burden as one of many ways to stave off increases in depression. Of course, at this point, our findings are purely correlational; the efficacy of actual interventions remains to be determined.
Limitations
The present study was neither longitudinal nor experimental; thus, assertions concerning directionality and causality of found relationships are speculative. Other limitations include the measurement of caregiver depression by symptom inventories rather than clinical interviews and the focus on only one aspect of patients’ emotional functioning (i.e., empathic accuracy; patients’ ability to generate and regulate emotion were not included).
Conclusions
Patients with lower levels of empathic accuracy have caregivers who experience greater loneliness, strain, and burden, which, in turn, is associated with greater caregiver depression. Finding this relationship when measuring empathic accuracy with a dynamic tracking task but not when using more traditional tasks (i.e., identifying emotion in photographs or films) suggests that patients’ inability to track the changing emotions of others may be particularly stressful for caregivers. We suspect this greater stress results from the loss of emotional understanding, responsiveness, and support in an important life partner. Caregiving for a close loved one with a neurodegenerative disease will become even more common as the population ages. For this reason, it is critically important to continue to identify specific behavioral symptoms in patients that confer greater vulnerability to the negative effects of caregiving, to develop optimal ways of measuring these symptoms, and to understand the mechanisms that link patient symptoms with adverse caregiver outcomes.
Supplementary Material
Highlights.
Across two independent studies, lower ability of neurodegenerative patients to track changing emotions in others was associated greater depressive symptoms in caregivers
Associations between empathic accuracy and caregiver depressive symptoms were found for the dynamic tracking task but not for recognizing emotion in photographs or films
The association between lower patient empathic accuracy and greater caregiver depressive symptoms was accounted for by increased loneliness, burden, and strain in caregivers
Acknowledgments
Conflicts of Interest and Source of Funding: Preparation of this manuscript was supported by a National Institute on Aging grant awarded to Robert W. Levenson (R01AG041762), a National Institute on Mental Health pre-doctoral fellowship awarded to Casey L. Brown (T32MH020006), and a National Institute of Aging grant P01AG019724.
Abbreviations
- BPSDs
Behavioral and Psychological Symptoms of Dementia
- UCSF
University of California, San Francisco
- MMSE
Mini Mental State Examination
- CDR-Box
Clinical Dementia Rating- Boxscore
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schulz R, O’Brien AT, Bookwala J, Fleissner K. Psychiatric and Physical Morbidity Effects of Dementia Caregiving: Prevalence, Correlates, and Causes. Gerontologist. 1995;35(6):771–91. doi: 10.1093/geront/35.6.771. [DOI] [PubMed] [Google Scholar]
- 2.Cuijpers P. Depressive disorders in caregivers of dementia patients: a systematic review. Aging Ment Health. 2005;9(4):325–30. doi: 10.1080/13607860500090078. [DOI] [PubMed] [Google Scholar]
- 3.Blazer D, Burchett B, Service C, George LK. The Association of Age and Depression Among the Elderly: An Epidemiologic Exploration. J Gerontol Oxford University Press. 1991;46(6):M210–M215. doi: 10.1093/geronj/46.6.m210. [DOI] [PubMed] [Google Scholar]
- 4.Ornstein K, Gaugler JE. The problem with “problem behaviors”: a systematic review of the association between individual patient behavioral and psychological symptoms and caregiver depression and burden within the dementia patient–caregiver dyad. Int Psychogeriatrics. 2012;24(10):1536–52. doi: 10.1017/S1041610212000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulz R, O’Brien AT, Bookwala J, Fleissner K. Psychiatric and physical morbidity effects of dementia caregiving: Prevalence, correlates, and causes. Gerontologist. 1995;35(6):771–91. doi: 10.1093/geront/35.6.771. [DOI] [PubMed] [Google Scholar]
- 6.Levenson RW, Sturm VE, Haase CM. Emotional and Behavioral Symptoms in Neurodegenerative Disease: A Model for Studying the Neural Bases of Psychopathology. Annu Rev Clin Psychol Annual Reviews. 2014;10(1):581–606. doi: 10.1146/annurev-clinpsy-032813-153653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghosh BCP, Rowe JB, Calder AJ, Hodges JR, Bak TH. Emotion Recognition in Progressive Supranuclear Palsy. J Neurol Neurosurg Psychiatry. 2009;80(10):1143–5. doi: 10.1136/jnnp.2008.155846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumfor F, Sapey-Triomphe L-A, Leyton CE, Burrell JR, Hodges JR, Piguet O. Degradation of emotion processing ability in corticobasal syndrome and Alzheimer’s disease. Brain. 2014;137(11):3061–72. doi: 10.1093/brain/awu246. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh S, Irish M, Daveson N, Hodges JR, Piguet O. When one loses empathy: its effect on carers of patients with dementia. J Geriatr Psychiatry Neurol. 2013;26(3):174–84. doi: 10.1177/0891988713495448. [DOI] [PubMed] [Google Scholar]
- 10.Cacioppo JT, Hughes ME, Waite LJ, Hawkley LC, Thisted RA. Loneliness as a specific risk factor for depressive symptoms: Cross-sectional and longitudinal analyses. Psychol Aging American Psychological Association. 2006;21(1):140–51. doi: 10.1037/0882-7974.21.1.140. [DOI] [PubMed] [Google Scholar]
- 11.Aneshensel CS, Frerichs RR. J Community Psychol. 4. Vol. 10. John Wiley & Sons, Inc; 1982. Stress, support, and depression: A longitudinal causal model; pp. 363–76. [Google Scholar]
- 12.Fried EI, Bockting C, Arjadi R, Borsboom D, Amshoff M, Cramer AOJ, et al. From loss to loneliness: The relationship between bereavement and depressive symptoms. J Abnorm Psychol. 2015;124(2):256–65. doi: 10.1037/abn0000028. [DOI] [PubMed] [Google Scholar]
- 13.Ickes WJ. Empathic Accuracy. New York, NY: Guilford Press; 1997. [Google Scholar]
- 14.Rosen HJ, Perry RJ, Murphy J, Kramer JH, Mychack P, Schuff N, et al. Emotion comprehension in the temporal variant of frontotemporal dementia. Brain. 2002;125(10):2286–95. doi: 10.1093/brain/awf225. [DOI] [PubMed] [Google Scholar]
- 15.Goodkind MS, Sturm VE, Ascher EA, Shdo SM, Miller BL, Rankin KP, et al. Emotion recognition in frontotemporal dementia and Alzheimer’s disease: A new film-based assessment. Emotion. 2015;15(4):416–27. doi: 10.1037/a0039261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ickes W, Stinson L, Bissonnette V, Garcia S. Naturalistic social cognition: Empathic accuracy in mixed-sex dyads. J Pers Soc Psychol. 1990;59(4):730–42. [Google Scholar]
- 17.Levenson RW, Ruef AM. Physiological aspects of emotional knowledge and rapport. Empath accuracy. 1997:44–72.
- 18.Zaki J, Bolger N, Ochsner K. It takes two: The interpersonal nature of empathic accuracy: Research article. Psychol Sci. 2008;19(4):399–404. doi: 10.1111/j.1467-9280.2008.02099.x. [DOI] [PubMed] [Google Scholar]
- 19.Goodkind MS, Sollberger M, Gyurak A, Rosen HJ, Rankin KP, Miller B, et al. Tracking emotional valence: The role of the orbitofrontal cortex. Hum Brain Mapp. 2012;33(4):753–62. doi: 10.1002/hbm.21251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romero-Moreno R, Márquez-González M, Mausbach BT, Losada A. Variables modulating depression in dementia caregivers: a longitudinal study. Int Psychogeriatrics C Int Psychogeriatr Assoc. 2012;248:1316–24. doi: 10.1017/S1041610211002237. [DOI] [PubMed] [Google Scholar]
- 21.Campbell P, Wright J, Oyebode J, Job D, Crome P, Bentham P, et al. Int J Geriatr Psychiatry. 10. Vol. 23. John Wiley & Sons, Ltd; 2008. Determinants of burden in those who care for someone with dementia; pp. 1078–85. [DOI] [PubMed] [Google Scholar]
- 22.Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–14. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7(3):263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(9):2456–77. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levenson RW, Ascher E, Goodkind M, McCarthy M, Sturm V, Werner K. Laboratory testing of emotion and frontal cortex. Handbook of Clinical Neurology. 2008:489–98. doi: 10.1016/S0072-9752(07)88025-0. [DOI] [PubMed]
- 26.Froming K, Levy M, Schaffer S, Ekman P. The comprehensive affect testing system. Psychol software, Inc; 2006. [Google Scholar]
- 27.Ascher EA, Madan A, Yuan JW, Sturm VE, Eckart JA, Levenson RW. Caregiver Assessment of Socioemotional Functioning. Unpubl Instrum [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A practice method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int psychogeriatrics. 1997;9(1):173–176. doi: 10.1017/s1041610297004870. [DOI] [PubMed] [Google Scholar]
- 30.Derogatis LR, Savitz KL. The SCL–90–R and Brief Symptom Inventory (BSI) in primary care. Handbook of psychological assessment in primary care settings. 2000:297–334. [Google Scholar]
- 31.Rammstedt B, John OP. Measuring personality in one minute or less: A 10-item short version of the Big Five Inventory in English and German. Journal of Research in Personality. 2007;41(1):203–212. [Google Scholar]
- 32.Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J Pers Soc Psychol. 2003;85(2):348–62. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- 33.Russell DW. J Pers Assess. 1. Vol. 66. Lawrence Erlbaum Associates, Inc; 1996. UCLA Loneliness Scale (Version 3): Reliability, Validity, and Factor Structure; pp. 20–40. [DOI] [PubMed] [Google Scholar]
- 34.Robinson BC. Validation of a Caregiver Strain Index. J Gerontol Oxford University Press. 1983;38(3):344–8. doi: 10.1093/geronj/38.3.344. [DOI] [PubMed] [Google Scholar]
- 35.Bedard M, Molloy DW, Squire L, Dubois S, Lever JA, O’Donnell M. The Zarit Burden Interview: A New Short Version and Screening Version. Gerontologist Oxford University Press. 2001;41(5):652–7. doi: 10.1093/geront/41.5.652. [DOI] [PubMed] [Google Scholar]
- 36.Cacioppo JT, Hughes ME, Waite LJ, Hawkley LC, Thisted RA. Loneliness as a specific risk factor for depressive symptoms: Cross-sectional and longitudinal analyses. Psychol Aging American Psychological Association. 2006;21(1):140–51. doi: 10.1037/0882-7974.21.1.140. [DOI] [PubMed] [Google Scholar]
- 37.Radloff LS, Teri L. Use of the Cebter for Epidemiological Studies Depression Scale With Older Adults. Clin Gerontol. 1986;5(1–2):119–36. [Google Scholar]
- 38.Hayes A. Introduction to mediation, moderation, and conditional process analysis. New York, NY: Guilford; 2013. [Google Scholar]
- 39.Hayes AF. PROCESS: A Versatile Computational Tool for Observed Variable Mediation, Moderation, and Conditional Process Modeling. Judd & Kenny MacKinnon; 1986. Available from: http://www.afhayes.com/ [Google Scholar]
- 40.Lwi SJ, Ford BQ, Casey JJ, Miller BL, Levenson RW. Poor caregiver mental health predicts mortality of patients with neurodegenerative disease. Proc Natl Acad Sci U S A National Academy of Sciences. 2017;114(28):7319–24. doi: 10.1073/pnas.1701597114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


