Abstract
Nonalcoholic fatty liver disease (NAFLD) is a disease of increasing interest, as its prevalence is on the rise. NAFLD has been linked to metabolic syndrome, which is becoming more common due to the Western diet. Because NAFLD can lead to cirrhosis and related complications including hepatocellular carcinoma, the increasing prevalence is concerning, and medical therapy aimed at treating NAFLD is of great interest. Researchers studying the effects of medical therapy on NAFLD use dietary mouse models. The two main types of mouse model diets are the methionine- and choline-deficient (MCD) diet and the Western-like diet (WD). Although both induce NAFLD, the mechanisms are very different. We reviewed several studies conducted within the last 5 years that used MCD diet or WD mouse models in order to mimic this disease in a way most similar to humans. The MCD diet inconsistently induces NAFLD and fibrosis and does not completely induce metabolic syndrome. Thus, the clinical significance of the MCD diet is questionable. In contrast, WD mouse models consisting of high fat, cholesterol, and a combination of high-fructose corn syrup, sucrose, fructose, or glucose not only lead to metabolic syndrome but also induce NAFLD with fibrosis, making these choices most suitable for research.
Key words: Nonalcoholic fatty liver disease (NAFLD), Nonalcoholic steatohepatitis, Fibrosis, Western diet, Methionine- and choline-deficient diet (MCD), Mouse model, Metabolic syndrome
INTRODUCTION
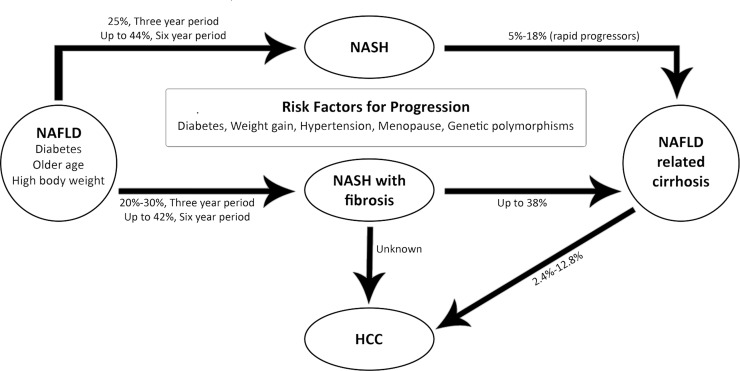
Nonalcoholic fatty liver disease (NAFLD) is defined as hepatic steatosis, the accumulation of fat in the liver, in the absence of heavy alcohol use. Research pertaining to NAFLD is of increasing interest because (i) it is the most common cause of elevated liver enzymes in the US, (ii) mortality among patients with NAFLD is significantly higher than the mortality of the general population, and (iii) end-stage liver disease secondary to nonalcoholic steatohepatitis (NASH) is currently the third most common indication for liver transplantation in the US1–4. NASH is even expected to surpass hepatitis C and alcoholic liver disease to become the most common indication in the upcoming years3. Additionally, up to 20% of patients with NASH will have progression of their disease to cirrhosis, and patients with cirrhosis also have an increased risk of developing hepatocellular carcinoma (HCC), which is the third most common cause of cancer-related deaths worldwide5,6. NAFLD encompasses a spectrum of liver diseases that can be further characterized as either nonalcoholic fatty liver (NAFL) or NASH based on the presence or absence of inflammation and liver cell injury7. NAFL is hepatic steatosis without inflammation and liver cell injury, whereas the hallmark of NASH is hepatic steatosis with evidence of inflammation and liver cell injury. The pathogenesis of hepatic steatosis is thought to be secondary to triglyceride accumulation due to both de novo lipogenesis and elevated peripheral fatty acids and is also associated with insulin resistance8,9. Additionally, NAFL can progress to NASH with or without fibrosis10. In the US, approximately one third of the population has NAFLD, and approximately 2%–5% have NASH1. Figure 1 provides an overview of the progression of NASH to NAFLD along with risk factors and statistical data regarding incidences of progression including the risk of HCC (reprinted from Calzadilla Bertot and Adams11).
Figure 1.
Overview of the progression of nonalcoholic steatohepatitis (NASH) to nonalcoholic fatty liver disease (NAFLD) and increased risk of hepatocellular carcinoma (HCC). Reprinted from Calzadilla Bertot and Adams11 per Creative Commons Attribution (CC-BY) license.
The progression from NAFL to NASH is not fully understood. One theory currently at the forefront is the “two-hit hypothesis”12,13. The accumulation of fat in the liver is the “first hit,” as it results in the liver being at increased susceptibility to further damage9. The “second hit” is the effect of oxidative stress on the liver and results in liver cell damage and inflammation, which signifies the transition from NAFL to NASH10.
The development of liver cirrhosis is dependent on the accumulation of progressive diffuse fibrosis and hepatic nodules, which cause the changes in liver architecture that characterize cirrhosis14. Although many cirrhotic patients are initially asymptomatic, they are at risk of decompensating and developing serious complications, including but not limited to portal hypertension, ascites, spontaneous bacterial peritonitis, hepatorenal syndrome, hepatic encephalopathy, and esophageal varices3,6. NASH-induced cirrhosis can also lead to end-stage liver disease, ultimately requiring liver transplantation.
Given these serious implications of NAFLD, the fact that the prevalence has increased is concerning. This increase is likely due to the rising occurrence of the components of metabolic syndrome, including hypertension, obesity, and diabetes mellitus2,4,15. According to the International Diabetes Federation, the national incidence of metabolic syndrome in the US is approximately 39%16. Many studies have shown links between metabolic syndrome and NAFLD8,17,18.
When researching NAFLD, it is important to understand what leads individuals to initially develop metabolic syndrome. The Western diet, which is a diet high in saturated fat, trans fat, and table sugar, is thought to play a role in the development of metabolic syndrome and has been shown to increase the risk of developing it19,20. Given the association between metabolic syndrome and NAFLD in humans, researchers attempting to better understand NAFLD have developed various mouse model diets in order to mimic the Western diet. Specifically, the interest in NAFLD mouse models lies within dietary models as opposed to knockout models since they better mimic the initial hepatic insult that causes the development of NAFLD. Furthermore, the use of dietary mouse models has increased recently, whereas the use of genetically altered models seems to be decreasing. Developing an appropriate mouse model is key to creating a study that could potentially help lead to a treatment of this rapidly growing disease. This article reviews several dietary mouse models used in studies within the last 5 years in an attempt to distinguish the diets that best mimic a Western diet and lead to NASH with hepatic fibrosis.
METHIONINE- AND CHOLINE-DEFICIENT (MCD) DIET
Mouse model diets can be divided into two major categories: (1) the MCD diet and (2) the Western-like diet (WD). Although both diets lead to hepatic steatosis in mice and can eventually lead to fibrosis, these processes occur through different mechanisms. In the MCD diet, the absence of methionine leads to hepatic injury, inflammation, and fibrosis, and the deficiency of choline leads to macrovesicular steatosis21. The MCD diet mouse model has been used in several studies to induce NASH with or without fibrosis. We review the effects of the MCD diet at the various levels of the progression to NASH and fibrosis.
Steatosis
The levels of intrahepatic triglycerides and lipid content can be used to measure hepatic steatosis, which is the hallmark of NAFL. Studies by Kim et al.22, Sutti et al.23, and Wehr et al.24 have shown that feeding wild-type (WT) mice the MCD diet for 4, 8, and 6 weeks, respectively, resulted in elevated intrahepatic triglycerides and lipid content, which indicates that the MCD diet led to hepatic steatosis in these mice. Although the livers of these mice become steatotic, the mechanism by which this occurs is not pathologically similar to human NAFL. This is one of the main criticisms of this dietary model in terms of relevance to human disease.
Inflammation
NASH is distinguished from NAFL by the presence of inflammation and liver cell injury. Histopathological evaluation of liver tissue and measurements of the serum levels of inflammatory cytokines, including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), are important markers of hepatic inflammation, but ultimately histological assessment is necessary to make this diagnosis. Liver cell injury is identified by hepatocyte balloon degeneration and is another manifestation indicative of the progression of NAFL to NASH25. In a study by Matsunaga et al., C57BL/6J mice were fed either a normal diet or an MCD diet for 8 weeks, and those fed the MCD diet developed increased hepatic inflammation as determined via histopathology in liver sections wherein increased fat droplets, increased inflammatory cell infiltration, and the presence of balloon degeneration in hepatocytes were found26. Similar findings were established in a study by Wang et al., which also showed the development of inflammation after mice were fed an MCD diet for only 2 weeks27. These findings were corroborated by serum analysis, where increased levels of TNF-α and IL-6 were noted in WT mice fed an MCD diet for 4, 8, or 2 weeks when compared to control diet in studies by Kim et al., Matsunaga et al., and Wang et al., respectively 22,26,27. Similarly, a study by Tosello-Trampont et al. showed that after only 10 days on the MCD diet, WT mice developed increased expression of TNF-α28, indicating that NASH may develop considerably early in this model.
Whereas NASH is largely identified by inflammation and liver cell injury, there is a subset of patients that develop NASH with fibrosis. A study by Rolla et al. examined the role of hepatic inflammation in the development of NASH with fibrosis in the MCD diet mouse model29. In that study, WT mice were fed an MCD diet for 8 weeks, subsequently developed NASH with fibrosis, and were shown to have increased infiltration of T helper 17 (Th17) and T helper 22 (Th22) cells, which play a role in inflammation. Infiltration of Th17 cells increased both at the beginning of the development of NASH and at the transition to NASH with fibrosis, and there was increased infiltration of Th22 cells between these two phases29. Considering that NAFL (which presents with little to no fibrosis) can progress to NASH with significant fibrosis30, this association between inflammation, inflammatory cell infiltration, and hepatic fibrosis development gives important insight into the pathophysiology of these diseases.
In NASH, inflammation is a reversible state as demonstrated by Itagaki et al. In their study, WT mice were fed an MCD diet, and after being on the diet for only 2 weeks developed severe steatohepatitis. Furthermore, after 16 weeks of MCD feeding, these mice exhibited hepatic fibrosis31. After completing 30 weeks on the MCD diet, mice were then switched back to the control diet for 16 weeks, after which time the degree of steatohepatitis returned to normal31. These findings are clinically relevant because weight loss through diet and exercise has been shown to improve hepatic steatosis, lobular inflammation, hepatocyte ballooning injury, and the NASH histological activity score (NAS) in humans32.
Fibrosis
In human patients with NASH, the severity of metabolic syndrome positively correlates with the degree of hepatic fibrosis and other adverse effects, including increased risk of cardiovascular disease and development of type 2 diabetes mellitus33. Since fibrosis is the precursor lesion of cirrhosis, it is important to understand the mechanisms underlying the development of fibrosis in NAFLD. Several studies have shown that an MCD diet can eventually induce hepatic fibrosis in WT mice23,24,29,31,34–39; however, the time required for mice to develop fibrosis while on an MCD diet varies. In studies by Katsura et al.35 and Simon et al.38, mice fed an MCD diet for 15 or 2 weeks, respectively, developed fibrosis. It is also unclear if longer feeding time on an MCD diet results in more severe fibrosis. In humans, hepatic fibrosis does not significantly change in response to weight loss through diet and exercise32, identifying that other interventions may be necessary to ameliorate hepatic fibrosis. The notion that hepatic fibrosis may not be ameliorated by diet change alone was shown in the study by Itagaki et al., in which WT mice were fed an MCD diet for 30 weeks, developed steatohepatitis with fibrosis, and were subsequently switched to a control diet for 16 weeks with the degree of steatohepatitis returning to normal but fibrosis persisting31.
Although fibrosis was found to occur in several studies wherein the MCD diet was utilized, many studies in which mice were fed an MCD diet for similar amounts of time did not develop hepatic fibrosis. For instance, in four studies, WT mice were fed the MCD diet for time periods varying from 2 to 8 weeks and developed hepatic steatosis or even severe steatohepatitis, but hepatic fibrosis was not noted22,26–28. The heterogeneity of hepatic fibrosis following consumption of an MCD diet may be due to varying strains and their response to the MCD diet.
Other Findings
Several studies have shown that feeding WT mice an MCD diet for as few as 2 weeks and up to 15 weeks resulted in elevated liver enzymes, including aspartate transaminase (AST) and alanine transaminase (ALT)23,24,31,35,38. In the study by Itagaki et al., WT mice were fed an MCD diet for 30 weeks total and developed increased levels of AST and ALT at only 2 weeks31. AST and ALT continued to increase and peaked at 16 weeks. At 30 weeks, AST and ALT decreased but remained elevated from normal. Interestingly, after completing 30 weeks on the MCD diet, mice were switched back to a control diet for 16 weeks, after which time the levels of AST and ALT returned to normal, indicating that the elevations in AST and ALT were reversible31. In one study by Katsura et al., alkaline phosphatase (ALP), which can also be used as a marker for liver disease, was found to be elevated in WT mice that were fed an MCD diet for 15 weeks35. These laboratory findings of elevations in serum levels of AST, ALT, and ALP can also be seen in humans with NAFLD but are not necessary for diagnosis40–43.
Although WD in humans leads to factors contributing to metabolic syndrome, including increased levels of serum triglycerides, cholesterol, and glucose, the MCD diet mouse model has had mixed results. Four studies showed that mice fed an MCD diet for as few as 2 weeks but up to 7 weeks developed no change or had decreased levels of serum triglycerides, cholesterol, and glucose27,37,38,44. In contrast, in a study by Kim et al., mice that were fed an MCD diet for 4 weeks developed elevated serum triglycerides and total cholesterol levels22. The reasoning for these differential changes noted in levels of serum triglycerides and total cholesterol between these studies is unknown, since feeding duration, age at start of feeding, and background of the mice are comparable22. Thus, between 2 and 7 weeks, the effects of the MCD diet on serum cholesterol and triglycerides is still variable, and further studies measuring these levels after a longer time period on the MCD diet may be beneficial.
In addition to causing hyperlipidemia, the WD is also linked to insulin resistance in humans. However, a study by Rinella and Green showed that mice fed an MCD diet for 10 or 28 days had significantly decreased fasting serum glucose than mice fed a normal chow diet45. This indicates that the MCD diet did not induce insulin resistance among these mice and again challenges the clinical relevance of the MCD diet.
Although weight gain secondary to WD contributes to developing metabolic syndrome, which is associated with NAFLD in humans, the MCD diet mouse model has consistently been shown to induce weight loss37,38,46. Loss in body mass resulting from the MCD diet is thought to be secondary to increased metabolic activity47. This signifies an important difference between the effects of the MCD diet mouse model and a typical WD consumed by humans.
It is important to note that many of the referenced studies use either the C57BL/6 mouse strain or substrains that are derived from the C57BL/6 background. It has previously been reported that mice with a C57BL/6 background are more susceptible to liver injury than other mouse strains48–50. Specifically, it has been shown that C57BL/6N mice are more susceptible to developing NASH than C3H/HeN mice following MCD feeding51. It is apparent that there are strain differences in susceptibility to MCD-induced damage, which can affect the outcome of the study.
As stated previously, the MCD diet has been shown to induce NAFLD and hepatic fibrosis in mice in several studies. However, the clinical relevance of this mouse model diet is questionable, as it does not mirror a diet consumed by humans. In addition, the MCD diet does not lead to features of metabolic syndrome in mice, despite metabolic syndrome playing an important role in the development of NAFLD in humans. It has also been noted that there is a high mortality among some strains of mice subjected to the MCD diet52. This questions the clinical significance of using the MCD diet mouse model to research NAFLD.
WESTERN-LIKE DIET (WD)
As previously stated, a Western diet is a diet high in saturated fat, trans-fat, and table sugar19,20. In humans, this type of diet has been shown to induce obesity, metabolic syndrome, NAFL, and, potentially, NASH19,20. In contrast to the MCD diet, researchers have turned to another type of dietary animal model wherein the composition of the diet more closely mimics a Western diet20. In the liver, a high-fat diet (HFD) leads to exaggerated free fatty acid levels, which induce hepatic insulin resistance, decreased fatty acid oxidation, and de novo lipogenesis in hepatocytes, causing weight gain and hepatic steatosis53. Specifically, excessive saturated fat consumption increases hepatocyte endoplasmic reticulum stress and hepatic steatosis54. Furthermore, a diet containing high cholesterol and high cholate induces atherogenesis, apoptosis, and inflammation, and high cholesterol alongside a HFD promotes hepatic inflammation55,56. These effects explain why the addition of certain dietary components is more likely to recapitulate pathogenesis associated with human NAFL progression to NASH.
The WD mouse model was designed to induce hepatic steatosis, NASH, and hepatic fibrosis in a pathophysiological manner similar to that observed in humans. Unlike the MCD diet mouse model, the composition of the WD used can vary greatly and is largely left to the preference of the researchers. For this reason, hepatic changes seen in mice fed a WD are highly variable depending on the content of the diet. For the sake of this review, we refer to this type of dietary model as a WD since some of these diets do not appropriately reflect the correct composition of a traditional, human Western diet. As well, it is worth noting that the terminology for this type of diet ranges from cafeteria diet to obesogenic diet to HFD, so currently there is no distinct term for this dietary model57–59. We review the effects of WD mouse models used in several studies based on the levels of advancement from NAFL to NASH with hepatic fibrosis.
Steatosis
Several WD models have been shown to lead to hepatic steatosis. In a study by Lian et al., WT mice that were fed a HFD diet (containing 60% calories from fat) for 16 weeks had increased liver weight, intrahepatic triglycerides, and hepatic steatosis on liver histology60. Aside from just administration of a HFD, some researchers have considered the role that added cholesterol has during NAFLD. In humans, dietary cholesterol is not a major contributor to serum cholesterol levels; however, the American Heart Association still recommends that humans consume less than 300 mg of dietary cholesterol per day61–63. In a study by Schierwagen et al., mice were fed a HFD (21% of fat from coconut oil and 19.5% from casein) supplemented with high cholesterol (1.25% of diet) for 7 weeks, and mice subsequently showed increased intrahepatic triglyceride levels, indicative of hepatic steatosis44.
Whereas the above dietary models more closely mimic human Western diet consumption and the development of NAFLD, they still do not encompass all aspects of a typical Western diet consumed by humans, primarily the added table sugar. The liver is known for its role in maintaining glucose homeostasis and metabolizing carbohydrates64; therefore, it is necessary to understand the impact that increased sugar consumption may have on liver injury. Specifically, sucrose (table sugar) has been highlighted for its impact on insulin sensitivity and lipid metabolism65. Several studies using a WD mouse model consisting of HFD, high cholesterol, and sucrose have been demonstrated to increase hepatic steatosis in WT mice66–68. In a study by Dorn et al., mice were fed a diet consisting of 15% pork lard, 15% beef tallow, 4% palmitic acid, 4% stearic acid, 0.2% cholesterol, and 30% sucrose for 12 weeks and developed both micro- and macrovesicular steatosis66. In a study by Gariani et al., mice were also fed a HFD supplemented with sucrose and showed evidence of macrovesicular steatosis on histological staining, but microvesicular steatosis was not noted67.
Similar to WD models involving sucrose, studies on the impact that the addition of high-fructose corn syrup will have on liver injury have become an increased topic of interest. High-fructose corn syrup is considered more lipogenic than sucrose, thereby leading to increased development of NAFLD69. For these reasons, many studies have analyzed the impact of a WD containing HFD with high cholesterol and added high-fructose corn syrup on NASH in mice20,46,70. Interestingly, in a study by Mells et al., researchers compared WD mouse models containing 45% of its calories from fat, in which 30% of the fat consisted of partially hydrogenated vegetable oil, and high-fructose corn syrup with or without 0.2% cholesterol20. While mice fed the WD with and without cholesterol for 16 weeks resulted in hepatocyte ballooning, increased intrahepatic lipid content, and the presence of Mallory bodies consistent with NASH, there were some differences between the two groups20. For instance, mice fed the WD with cholesterol had significantly heavier livers (58% heavier than those without cholesterol)20. As well, hepatic steatosis was present in both groups but was primarily found as microvesicular fat in mice fed a WD with cholesterol versus macrovesicular fat in those fed a WD without cholesterol20.
Inflammation
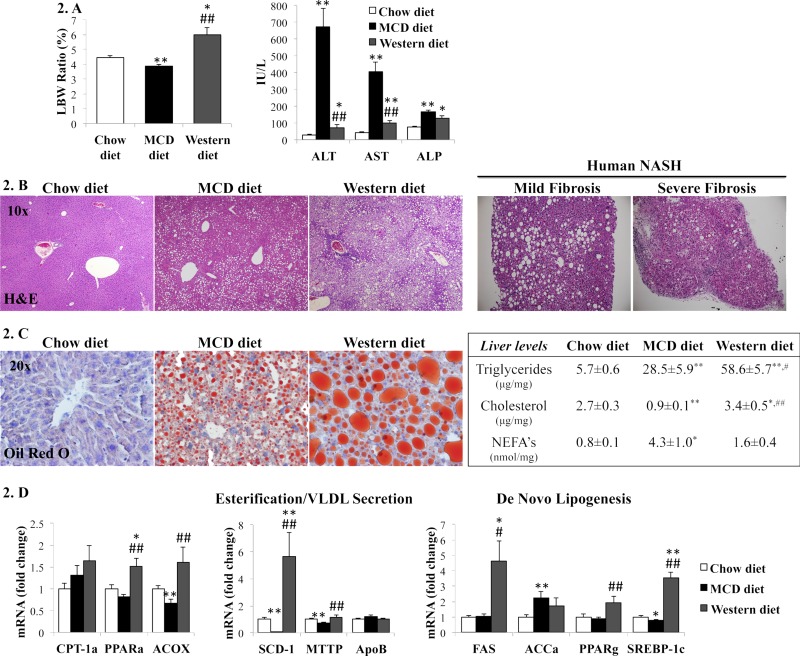
Many variations of the WD have also been shown to lead to hepatic inflammation. However, two studies mentioned previously that did not include added sugar were not noted to lead to hepatic inflammation in WT mice,44,47 indicating the significant role sugar may play in the inflammatory process. In the study by Dorn et al., mice were fed a HFD with cholesterol and sucrose (15% pork lard, 15% beef tallow, 4% palmitic acid, 4% stearic acid, 0.2% cholesterol, and 30% sucrose) for 12 weeks and developed hepatic inflammation as indicated by increased inflammatory cell inflitration66. HFD supplemented with two sugars was also shown to induce hepatic inflammation. In a study by Machado et al. (Fig. 2), mice fed a WD with high-fructose corn syrup (42 g/L glucose and fructose, 55% and 45%, respectively) for 16 weeks developed elevated liver enzymes and histological evidence of hepatic inflammation; however, these findings were significantly greater in MCD-fed mice46.
Figure 2.
(A) Liver-to-body weight (LBW) ratio, serum aminotransferases [alanine transaminase (ALT) and aspartate transaminase (AST)] and alkaline phosphatase (ALP) from mice fed chow, methionine- and choline-deficient (MCD), or Western diets. (B) Hematoxylin and eosin (H&E) staining of representative liver sections from mice (left) and NASH patients with mild or severe fibrosis (right). (C) Oil red O staining of representative liver sections from mice (left) and liver lipid levels (right). (D) qRT-PCR analysis of liver genes encoding lipid metabolic enzymes. The results were normalized to chow diet-fed mice and graphed as mean ± SEM. *p < 0.05 and **p < 0.005, control versus experimental diet; #p < 0.05 and ##p < 0.005, MCD versus Western diet. Reprinted with permission from Machado et al.46 per Creative Commons Attribution (CC-BY) license.
Fibrosis
Hepatic fibrosis was not seen in all previously mentioned WD models. In the study by Schierwagen et al., in mice fed a HFD (21% of fat from coconut oil and 19.5% from casein) supplemented with high cholesterol (1.25% of diet) for 7 weeks, hepatic fibrosis did not occur44. In contrast, in the study by Dorn et al., a HFD with cholesterol and sucrose led to NASH as well as the early stages of hepatic fibrosis66. This diet, which includes sucrose (table sugar), is clinically relevant as it closely mimics a typical human WD and induces metabolic syndrome, which likely plays a role in the development of NASH with fibrosis in both humans and mice.
Some studies used a WD mouse model containing HFD, cholesterol, and two types of sugar, fructose and glucose (a high-fructose corn syrup equivalent)46,71. In these two studies by Machado et al., mice that were fed a HFD (with 0.2% cholesterol diet from Teklad Research supplemented with high-fructose corn syrup equivalents) for 16 weeks developed NASH coupled with fibrosis46,71. However, in the study by Machado et al., consumption of this diet led to hepatic inflammation and fibrosis but to a lower degree than mice fed an MCD diet for only 8 weeks (Fig. 2)46. In a different study by Machado et al., this diet resulted in increased body weight and abdominal adipose tissue, elevated levels of serum cholesterol and triglycerides, glucose intolerance, and insulin resistance71. Mice were even noted to have developed overt type 2 diabetes mellitus46. The addition of fructose and glucose (a high-fructose corn syrup equivalent) to a HFD and high-cholesterol diet resulted in both NASH with fibrosis and metabolic syndrome, making this WD mouse model a useful tool due to its clinical relevance.
In one study by Jiang et al., mice fed WD (with 40% energy from fat, 12% saturated fat, 2% cholesterol, and supplemented with high-fructose corn syrup) developed hepatocyte ballooning, lipoapoptosis, necroinflammation, steatosis, fibrosis, and increased serum levels of ALT70. In addition, some studies have shown that after feeding WT mice the WD for 16 weeks, characteristics indicative of metabolic syndrome, including obesity and insulin resistance, were induced20,70. In the study by Mells et al., mice fed a HFD (with 45% calories from fat and 30% of fat was partially hydrogenated vegetable oil) with or without 0.2% cholesterol developed fibrosis, but increased markers of dense collagen deposition, which are indicators of fibrosis, and increased levels of profibrotic cytokines, leptin, and IL-6 were present in mice fed a WD with cholesterol versus WD without cholesterol20. Thus, a WD consisting of high fat, high-fructose corn syrup, and high cholesterol given to mice for 16 weeks resulted in increased degrees of fibrosis when compared to the same diet without cholesterol. Likewise, in a study by Ichimura et al., Sprague–Dawley rats fed a HFD and high-cholesterol diet (consisting of either 1.25% or 2.5% cholesterol) for 18 weeks developed NASH as well as cirrhosis, but rats fed a HFD without cholesterol for the same time period only developed hepatic steatosis and inflammation without fibrosis72. Interestingly, in humans, dietary cholesterol has been shown to not significantly impact serum levels of cholesterol, thus having less impact on metabolic syndrome61,62. The effect of dietary cholesterol on human NAFL disease progression remains unclear.
Other Findings
Although several models using the MCD diet did not reproduce metabolic syndrome in mice as mentioned previously, many WD models did. In a study by Lian et al., WT mice that were fed a HFD diet (containing 60% calories from fat) for 16 weeks had increased weight gain, which is an indicator of NAFLD and metabolic syndrome60. In a study by Schierwagen et al., a HFD (21% of fat from coconut oil and 19.5% from casein) supplemented with high cholesterol (1.25% of diet) was given to WT mice for 7 weeks44. Further, mice fed this diet developed increased levels of serum glucose but did not have significantly increased body weight44. In addition, in a study by Mells et al., researchers compared WD mouse models containing 45% of its calories from fat, in which 30% of the fat consisted of partially hydrogenated vegetable oil, and high-fructose corn syrup with or without 0.2% cholesterol20. Mice fed the WD with and without cholesterol for 16 weeks developed obesity, insulin resistance, higher serum blood glucose, and increased visceral fat20. This questions the significance of cholesterol in the development of metabolic syndrome in mice.
The impact of fructose consumption is an area of interest since it has been implicated in the development of type 2 diabetes mellitus and NAFLD73,74. In a study by Luo et al., researchers used a WD mouse model that consisted of high-fat content (40% of energy from fat with 30% of fat from lard, 30% from butterfat, and 30% from Crisco) and liquid fructose and sucrose (42 g/L total at a ratio of 55% fructose to 45% sucrose)75. Researchers found that this diet caused increased glucose intolerance, insulin resistance, and adipose tissue dysfunction75. In addition, there were increased levels of intrahepatic triglycerides, plasma ALT, liver weight, hepatic fibrosis, and hepatic inflammation75. This diet resulted in similar results as the WD consisting of high fat, cholesterol, and one type of sugar (either fructose or glucose), making it a reasonable mouse model option as it also induced NASH with fibrosis and features of metabolic syndrome.
Similar to the MCD section, many of the referenced studies that use WD models utilize either the C57BL/6 mouse strain or the substrains that are derived from the C57BL/6 background. As previously stated, mice with a C57BL/6 background are more susceptible to liver injury than other mouse strains48–50. Notably, in the choline- and folate-deficient diet model of NAFL, it was shown that sensitivity to liver injury was strain dependent, with the order being A/J ≈ C57BL/6J ≈ C3H/HeJ < 129S1/SvImJ ≈ CAST/EiJ < PWK/PhJ < WSB/EiJ49. While strain differences in susceptibility to a WD are currently unknown, it is possible that differences do exist based on the aforementioned studies.
FUTURE PERSPECTIVES AND CONCLUSIONS
Mouse model diets used to induce NASH with hepatic fibrosis are crucial to research involving the development of treatments of NAFLD and ways to slow progression to fibrosis. As discussed previously, mouse model diets can vary greatly, and the chosen model can result in differing outcomes (Fig. 3). It is important when choosing a mouse model to induce NASH with hepatic fibrosis that the model results in progression of disease in a similar manner as in humans. Aside from dietary models, there are multiple genetic mouse models used to mimic NAFL/NASH, such as ob/ob mice (mutation in the leptin gene), db/db mice (mutation in the leptin receptor gene), and foz/foz mice (mutation in Alms1 gene, essential for primary ciliary function)76. In humans, there are certain genetic polymorphisms that are strongly associated with susceptibility to NAFL/NASH, such as mutations in genes encoding for lipid metabolism, glucose metabolism, hypertension, or inflammation77. However, it is important to note that the genetic mouse models of NAFL/NASH do not overlap with these genetic polymorphisms. For this reason, we did not discuss the role that knockout models play in mimicking fatty liver, since these models do not appropriately reflect human disease. However, it is apparent that genetics play an important role in the development of NAFL/NASH and should not be discounted.
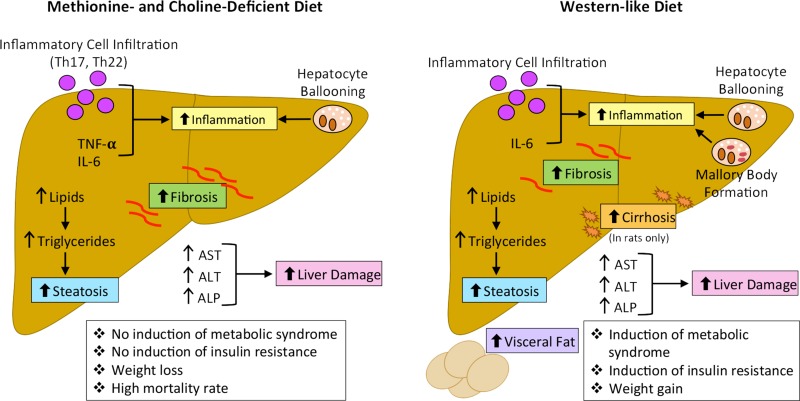
Figure 3.
The main mechanisms regulating pathogenesis of NAFLD and NASH with fibrosis in the MCD and Western-like diet (WD) animal models are depicted. Following MCD feeding for various times, it has been shown that mice had increased steatosis, inflammation, hepatic fibrosis, and liver damage, which mimic human NASH pathogenesis. However, mice fed the MCD diet did not develop metabolic syndrome or insulin resistance and showed weight loss and increased mortality, which are not similar to the human NASH phenotype. Following feeding of WD in mice, of varying compositions and durations, it is mainly noted that mice had increased steatosis, inflammation, fibrosis, liver damage, visceral fat formation, metabolic syndrome, insulin resistance, and weight gain, all of which closely mimic the pathogenesis of human NASH. Further, the development of cirrhosis has been shown in rats fed WD, which mimics complications associated with end-stage human NASH disease.
While an MCD diet mouse model is sometimes successful in causing NASH with fibrosis, its inability to induce features of metabolic syndrome and the fact that humans do not consume this type of diet make it a less-appealing model. In contrast, WD mouse models are based on the idea that by mimicking a Western diet that humans consume, mice fed a WD will develop NASH with fibrosis in a way parallel to humans. While the WD model more closely mimics human disease progression from NAFL to NASH, neither the MCD diet nor the WD mouse models have been shown to induce cirrhosis, which can be an end-stage complication in patients with severe, untreated NASH. The lack of cirrhosis development in mouse models of NAFL/NASH is a major downfall of murine models, and more research is necessary to evaluate this problem. However, one study discussed above involving Sprague–Dawley rats fed a WD was shown to progress to cirrhosis72. Changes in WD composition or duration of feeding should be implemented to produce cirrhosis in mouse models.
As described above, the definition of a WD can differ based on composition and the percentage and type of fat, cholesterol, and sugar used (Table 1). A typical human diet contains ∼33% of calories from fat78, while the fat content in the aforementioned WD models varies from 21% to 60%. Similarly, cholesterol content in a typical, human Western diet is ∼300–400 mg of cholesterol per day (i.e., less than 0.0001%), whereas the cholesterol content in the aforementioned WD models varies from 0.2% to 2.5%78. As well, duration of feeding for the WD studies ranges from 7 to 18 weeks; therefore, duration of feeding needs to be further studied. It is essential that the diet compositions are optimized to more closely mimic not only a human diet but also disease progression. Being able to appropriately address these areas of concern is proving difficult, and more research is necessary to help identify the most appropriate mouse dietary models of NAFL and NASH. Overall, we would recommend the WD for modeling NAFL/NASH, since it most closely mimics human pathophysiology; however, further work is necessary to further improve this model.
Table 1.
Comprehensive Table With Mouse Models of Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH) Including Diet Composition, Strains, and Outcomes
| Dietary Model of NAFLD | Composition | Strain | Treatment Length | Phenotypes/Outcomes |
|---|---|---|---|---|
| WD19 | 45% kcal fat (20% partially hydrogenated vegetable oil) + HFCS + 0.2% cholesterol | C57BL/6J | 16 weeks | Steatosis, increased triglycerides, increased liver weight, fibrosis |
| MCD21 | N/A | C57BL/6J | 4 weeks | Steatosis, elevated triglycerides |
| MCD22 | N/A | C57BL/6J | 8 weeks | Inflammation, steatosis, elevated triglycerides, increased serum liver enzymes |
| MCD23 | N/A | C57BL/6J | 6 weeks | Inflammation, steatosis, elevated triglycerides, increased serum liver enzymes |
| MCD25 | N/A | C57BL/6J | 8 weeks | Inflammation, steatosis |
| MCD26 | N/A | C57BL/6J | 2 weeks | Inflammation, steatosis |
| MCD27 | N/A | C57BL/6J | 10 days | Inflammation, steatosis |
| MCD28 | N/A | C57BL/6J | 8 weeks | Inflammation, steatosis, fibrosis |
| MCD30 | N/A | C57BL/6J | Up to 16 weeks | Steatohepatitis, increased serum liver enzymes, fibrosis, inflammation |
| MCD33 | N/A | C57BL/6J | 8 weeks | Steatohepatitis, increased serum liver enzymes, fibrosis, inflammation |
| MCD34 | N/A | C57BL/6J | 15 weeks | Steatohepatitis, increased serum liver enzymes, fibrosis, inflammation |
| MCD35 | N/A | C57BL/6J | 4 weeks | Steatohepatitis, increased serum liver enzymes, fibrosis, inflammation |
| MCD36 | N/A | C57BL/6J | 6 weeks | Steatohepatitis, increased serum liver enzymes, fibrosis, inflammation |
| MCD37 | N/A | C57BL/6J | 2 and 4 weeks | Fibrosis, elevated triglycerides, steatosis, increased serum liver enzymes |
| MCD38 | N/A | C57BL/6J | 2 weeks | Fibrosis, steatosis, increased serum liver enzymes |
| MCD43 | N/A | C57BL/6J | 7 weeks | Fibrosis, steatosis |
| HFD43 | 21% fat from coconut oil + 19.5% fat from casein + 1.25% cholesterol | 7 weeks | ||
| MCD44 | N/A | FVB/NJ | 10–28 days | Increased serum liver enzymes, fibrosis, steatohepatitis |
| MCD and WD45 | N/A and 45% kcal from fat (saturated fat) + 0.2% cholesterol + fructose and glucose (55% and 45%, respectively, w/w) | C57BL/6J | 8/16 weeks | Steatosis, inflammation, ductular reaction, fibrosis, type 2 diabetes (MCD induced features mimicking human NAFLD to NASH) |
| MCD50 | N/A | C57BL/6N and C3H/HeN | 6 weeks | Fibrosis, inflammation, steatosis, increased serum liver enzymes (not as severe as C57BL/6N) |
| HFD + HFHS54 | 44.6% kcal from fat (61% saturated fatty acids) + 40.6% kcal from carbohydrates (sucrose 340 g/kg) | C57BL/6J | Up to 18 weeks | Macrovesicular steatosis, fibrosis, inflammation |
| HFD59 | 60% kcal from fat | C57BL/6J | 16 weeks | Steatosis, increased liver weight and triglycerides |
| WD59 | 21% kcal fat + 0.2% cholesterol | C57BL/6J | 10–12 weeks | Steatosis, increased liver weight and triglycerides |
| MCD61 | N/A | C57BL/6J | 8 weeks | Insulin resistance, elevated triglycerides, glucose intolerance, increased adipose tissue |
| HFD + HFCS65–67 | 44.6% kcal from fat (61% saturated fatty acids) + 40.6% kcal from carbohydrates (sucrose 340 g/kg) | C57BL/6J | Up to 16 weeks | Steatosis |
| HFD + HFCS70 | 45% kcal from fat (saturated fat) + 0.2% cholesterol + fructose and glucose (55% and 45%, respectively, w/w) | C57BL/6J | 16 weeks | Steatosis and fibrosis coupled with NASH |
| HFD71 | +1.25% or 2.5% cholesterol | Sprague–Dawley | 18 weeks | NASH, cirrhosis, fibrosis, inflammation |
| HFD74 | 40% of energy from fat with 30% of fat from lard, 30% from butterfat, and 30% from Crisco and liquid fructose and sucrose (42 g/L total at a ratio of 55% fructose to 45% sucrose) | 8 weeks | Glucose intolerance, insulin resistance, adipose tissue dysfunction, increased levels of intrahepatic triglycerides, plasma ALT, liver weight, hepatic fibrosis, and inflammation |
HFD, high-fat diet [also consistent with Western-like diet (WD)]; HFCS, high-fructose corn syrup; MCD, methionine- and choline-deficient diet.
ACKNOWLEDGMENTS
Portions of this work were supported by (i) VA Merit Awards (1I01BX003031 to H.F. and 4I01BX000574 to G.A.) from the US Department of Veteran’s Affairs, Biomedical Laboratory Research and Development Service, (ii) R01 grants from the NIH NIDDK(DK108959 to H.F.), and (iii) the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Baylor Scott & White Health (G.A.). This material is the result of work supported with resources and the use of facilities at the Central Texas Veterans Health Care System, Temple, TX. The content is the responsibility of the author(s) alone and does not necessarily reflect the views or policies of the Department of Veterans Affairs or the US government. The authors declare no conflicts of interest.
REFERENCES
- 1. Vernon G, Baranova A, Younossi ZM. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34(3):274–85. [DOI] [PubMed] [Google Scholar]
- 2. Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98(5):960–7. [DOI] [PubMed] [Google Scholar]
- 3. Ratziu V, Bonyhay L, Di Martino V, Charlotte F, Cavallaro L, Sayegh-Tainturier MH, Giral P, Grimaldi A, Opolon P, Poynard T. Survival, liver failure, and hepatocellular carcinoma in obesity-related cryptogenic cirrhosis. Hepatology 2002;35(6):1485–93. [DOI] [PubMed] [Google Scholar]
- 4. Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology 2011;140(1):124–31. [DOI] [PubMed] [Google Scholar]
- 5. Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology 1999;116(6):1413–9. [DOI] [PubMed] [Google Scholar]
- 6. Heidelbaugh JJ, Sherbondy M. Cirrhosis and chronic liver failure: Part II. Complications and treatment. Am Fam Physician 2006;74(5):767–76. [PubMed] [Google Scholar]
- 7. Popescu M, Popescu IA, Stanciu M, Cazacu SM, Ianosi NG, Comanescu MV, Singer CE, Neagoe CD. Non-alcoholic fatty liver disease—Clinical and histopathological aspects. Rom J Morphol Embryol. 2016;57(4):1295–302. [PubMed] [Google Scholar]
- 8. Chitturi S, Abeygunasekera S, Farrell GC, Holmes-Walker J, Hui JM, Fung C, Karim R, Lin R, Samarasinghe D, Liddle C, Weltman M, George J. NASH and insulin resistance: Insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology 2002;35(2):373–9. [DOI] [PubMed] [Google Scholar]
- 9. Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115(5):1343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Caldwell SH, Crespo DM. The spectrum expanded: Cryptogenic cirrhosis and the natural history of non-alcoholic fatty liver disease. J Hepatol. 2004;40(4):578–84. [DOI] [PubMed] [Google Scholar]
- 11. Calzadilla Bertot L, Adams LA. The natural course of non-alcoholic fatty liver disease. Int J Mol Sci. 2016;17(5):774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of non-alcoholic fatty liver disease. QJM 2010;103(2):71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Day CP, James OF. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998;114(4):842–5. [DOI] [PubMed] [Google Scholar]
- 14. Anthony PP, Ishak KG, Nayak NC, Poulsen HE, Scheuer PJ, Sobin LH. The morphology of cirrhosis. Recommendations on definition, nomenclature, and classification by a working group sponsored by the World Health Organization. J Clin Pathol. 1978;31(5):395–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9(6):524–30.e1. [DOI] [PubMed] [Google Scholar]
- 16. Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care 2005;28(11):2745–9. [DOI] [PubMed] [Google Scholar]
- 17. Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, Omatsu T, Nakajima T, Sarui H, Shimazaki M, Kato T, Okuda J, Ida K. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143(10):722–8. [DOI] [PubMed] [Google Scholar]
- 18. Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N, Rizzetto M. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003;37(4):917–23. [DOI] [PubMed] [Google Scholar]
- 19. Hosseini Z, Whiting SJ, Vatanparast H. Current evidence on the association of the metabolic syndrome and dietary patterns in a global perspective. Nutr Res Rev. 2016;29(2):152–62. [DOI] [PubMed] [Google Scholar]
- 20. Mells JE, Fu PP, Kumar P, Smith T, Karpen SJ, Anania FA. Saturated fat and cholesterol are critical to inducing murine metabolic syndrome with robust nonalcoholic steatohepatitis. J Nutr Biochem. 2015;26(3):285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Caballero F, Fernandez A, Matias N, Martinez L, Fucho R, Elena M, Caballeria J, Morales A, Fernandez-Checa JC, Garcia-Ruiz C. Specific contribution of methionine and choline in nutritional nonalcoholic steatohepatitis: Impact on mitochondrial S-adenosyl-L-methionine and glutathione. J Biol Chem. 2010;285(24):18528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim SB, Kang OH, Lee YS, Han SH, Ahn YS, Cha SW, Seo YS, Kong R, Kwon DY. Hepatoprotective effect and synergism of bisdemethoycurcumin against MCD diet-induced nonalcoholic fatty liver disease in mice. PLoS One 2016;11(2):e0147745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sutti S, Jindal A, Locatelli I, Vacchiano M, Gigliotti L, Bozzola C, Albano E. Adaptive immune responses triggered by oxidative stress contribute to hepatic inflammation in NASH. Hepatology 2014;59(3):886–97. [DOI] [PubMed] [Google Scholar]
- 24. Wehr A, Baeck C, Ulmer F, Gassler N, Hittatiya K, Luedde T, Neumann UP, Trautwein C, Tacke F. Pharmacological inhibition of the chemokine CXCL16 diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. PLoS One 2014;9(11):e112327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bedossa P. Pathology of non-alcoholic fatty liver disease. Liver Int. 2017;37(Suppl 1):85–9. [DOI] [PubMed] [Google Scholar]
- 26. Matsunaga Y, Nakatsu Y, Fukushima T, Okubo H, Iwashita M, Sakoda H, Fujishiro M, Yamamotoya T, Kushiyama A, Takahashi S, Tsuchyia Y, Kamata H, Tokunaga F, Iwai K, Asano T. LUBAC formation is impaired in the livers of mice with MCD-dependent nonalcoholic steatohepatitis. Mediators Inflamm. 2015;2015:125380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y, Li J, Zhuge L, Su D, Yang M, Tao S, Li J. Comparison between the efficacies of curcumin and puerarin in C57BL/6 mice with steatohepatitis induced by a methionine- and choline-deficient diet. Exp Ther Med. 2014;7(3):663–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tosello-Trampont AC, Landes SG, Nguyen V, Novobrantseva TI, Hahn YS. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-alpha production. J Biol Chem. 2012;287(48):40161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rolla S, Alchera E, Imarisio C, Bardina V, Valente G, Cappello P, Mombello C, Follenzi A, Novelli F, Carini R. The balance between IL-17 and IL-22 produced by liver-infiltrating T-helper cells critically controls NASH development in mice. Clin Sci. (Lond) 2016;130(3):193–203. [DOI] [PubMed] [Google Scholar]
- 30. McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: Implications for prognosis and clinical management. J Hepatol. 2015;62(5):1148–55. [DOI] [PubMed] [Google Scholar]
- 31. Itagaki H, Shimizu K, Morikawa S, Ogawa K, Ezaki T. Morphological and functional characterization of non-alcoholic fatty liver disease induced by a methionine-choline-deficient diet in C57BL/6 mice. Int J Clin Exp Pathol. 2013;6(12):2683–96. [PMC free article] [PubMed] [Google Scholar]
- 32. Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, Fava JL, Wing RR. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010;51(1):121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ryan MC, Wilson AM, Slavin J, Best JD, Jenkins AJ, Desmond PV. Associations between liver histology and severity of the metabolic syndrome in subjects with nonalcoholic fatty liver disease. Diabetes Care 2005;28(5):1222–4. [DOI] [PubMed] [Google Scholar]
- 34. Baeck C, Wehr A, Karlmark KR, Heymann F, Vucur M, Gassler N, Huss S, Klussmann S, Eulberg D, Luedde T, Trautwein C, Tacke F. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut 2012;61(3):416–26. [DOI] [PubMed] [Google Scholar]
- 35. Katsura A, Morishita A, Iwama H, Tani J, Sakamoto T, Tatsuta M, Toyota Y, Fujita K, Kato K, Maeda E, Nomura T, Miyoshi H, Yoneyama H, Himoto T, Fujiwara S, Kobara H, Mori H, Niki T, Ono M, Hirashima M, Masaki T. MicroRNA profiles following metformin treatment in a mouse model of non-alcoholic steatohepatitis. Int J Mol Med. 2015;35(4):877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marcolin E, San-Miguel B, Vallejo D, Tieppo J, Marroni N, Gonzalez-Gallego J, Tunon MJ. Quercetin treatment ameliorates inflammation and fibrosis in mice with nonalcoholic steatohepatitis. J Nutr. 2012;142(10):1821–8. [DOI] [PubMed] [Google Scholar]
- 37. Okubo H, Sakoda H, Kushiyama A, Fujishiro M, Nakatsu Y, Fukushima T, Matsunaga Y, Kamata H, Asahara T, Yoshida Y, Chonan O, Iwashita M, Nishimura F, Asano T. Lactobacillus casei strain Shirota protects against nonalcoholic steatohepatitis development in a rodent model. Am J Physiol Gastrointest Liver Physiol. 2013;305(12):G911–8. [DOI] [PubMed] [Google Scholar]
- 38. Simon Y, Kessler SM, Gemperlein K, Bohle RM, Muller R, Haybaeck J, Kiemer AK. Elevated free cholesterol in a p62 overexpression model of non-alcoholic steatohepatitis. World J Gastroenterol. 2014;20(47):17839–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu W, Liu X, Peng X, Xue R, Ji L, Shen X, Chen S, Gu J, Zhang S. Bile acids override steatosis in farnesoid X receptor deficient mice in a model of non-alcoholic steatohepatitis. Biochem Biophys Res Commun. 2014;448(1):50–5. [DOI] [PubMed] [Google Scholar]
- 40. Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: An expanded clinical entity. Gastroenterology 1994;107(4):1103–9. [DOI] [PubMed] [Google Scholar]
- 41. Kocabay G, Telci A, Tutuncu Y, Tiryaki B, Ozel S, Cevikbas U, Okten A, Satman I. Alkaline phosphatase: Can it be considered as an indicator of liver fibrosis in non-alcoholic steatohepatitis with type 2 diabetes? Bratisl Lek Listy 2011;112(11):626–9. [PubMed] [Google Scholar]
- 42. Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, Sterling RK, Shiffman ML, Stravitz RT, Sanyal AJ. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 2003;37(6):1286–92. [DOI] [PubMed] [Google Scholar]
- 43. Sorbi D, Boynton J, Lindor KD. The ratio of aspartate aminotransferase to alanine aminotransferase: Potential value in differentiating nonalcoholic steatohepatitis from alcoholic liver disease. Am J Gastroenterol. 1999;94(4):1018–22. [DOI] [PubMed] [Google Scholar]
- 44. Schierwagen R, Maybuchen L, Zimmer S, Hittatiya K, Back C, Klein S, Uschner FE, Reul W, Boor P, Nickenig G, Strassburg CP, Trautwein C, Plat J, Lütjohann D, Sauerbruch T, Tacke F, Trebicka J. Seven weeks of Western diet in apolipoprotein-E-deficient mice induce metabolic syndrome and non-alcoholic steatohepatitis with liver fibrosis. Sci Rep. 2015;5:12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rinella ME, Green RM. The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. J Hepatol. 2004;40(1):47–51. [DOI] [PubMed] [Google Scholar]
- 46. Machado MV, Michelotti GA, Xie G, Almeida Pereira T, Boursier J, Bohnic B, Guy CD, Diehl AM. Mouse models of diet-induced nonalcoholic steatohepatitis reproduce the heterogeneity of the human disease. PLoS One 2015;10(5):e0127991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci USA 2002;99(17):11482–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shpyleva S, Pogribna M, Cozart C, Bryant MS, Muskhelishvili L, Tryndyak VP, Ross SA, Beland FA, Pogribny IP. Interstrain differences in the progression of nonalcoholic steatohepatitis to fibrosis in mice are associated with altered hepatic iron metabolism. J Nutr Biochem. 2014;25(12):1235–42. [DOI] [PubMed] [Google Scholar]
- 49. Tryndyak V, de Conti A, Kobets T, Kutanzi K, Koturbash I, Han T, Fuscoe JC, Latendresse JR, Melnyk S, Shymonyak S, Collins L, Ross SA, Rusyn I, Beland FA, Pogribny IP. Interstrain differences in the severity of liver injury induced by a choline- and folate-deficient diet in mice are associated with dysregulation of genes involved in lipid metabolism. FASEB J. 2012;26(11):4592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tsuchiya M, Ji C, Kosyk O, Shymonyak S, Melnyk S, Kono H, Tryndyak V, Muskhelishvili L, Pogribny IP, Kaplowitz N, Rusyn I. Interstrain differences in liver injury and one-carbon metabolism in alcohol-fed mice. Hepatology 2012;56(1):130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yamazaki Y, Kakizaki S, Takizawa D, Ichikawa T, Sato K, Takagi H, Mori M. Interstrain differences in susceptibility to non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2008;23(2):276–82. [DOI] [PubMed] [Google Scholar]
- 52. Rangnekar AS, Lammert F, Igolnikov A, Green RM. Quantitative trait loci analysis of mice administered the methionine-choline deficient dietary model of experimental steatohepatitis. Liver Int. 2006;26(8):1000–5. [DOI] [PubMed] [Google Scholar]
- 53. Wiernsperger N. Hepatic function and the cardiometabolic syndrome. Diabetes Metab Syndr Obes. 2013;6:379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology 2006;147(2):943–51. [DOI] [PubMed] [Google Scholar]
- 55. Rizzo M, Montalto G, Vinciguerra M. Editorial: Exploring lipid-related treatment options for the treatment of NASH. Curr Vasc Pharmacol. 2014;12(5):741–4. [DOI] [PubMed] [Google Scholar]
- 56. Vergnes L, Phan J, Strauss M, Tafuri S, Reue K. Cholesterol and cholate components of an atherogenic diet induce distinct stages of hepatic inflammatory gene expression. J Biol Chem. 2003;278(44):42774–84. [DOI] [PubMed] [Google Scholar]
- 57. Riordan JD, Nadeau JH. Modeling progressive non-alcoholic fatty liver disease in the laboratory mouse. Mamm Genome 2014;25(9–10):473–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sanches SC, Ramalho LN, Augusto MJ, da Silva DM, Ramalho FS. Nonalcoholic steatohepatitis: A search for factual animal models. Biomed Res Int. 2015;2015:574832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schattenberg JM, Galle PR. Animal models of non-alcoholic steatohepatitis: Of mice and man. Dig Dis. 2010;28(1):247–54. [DOI] [PubMed] [Google Scholar]
- 60. Lian J, Wei E, Groenendyk J, Das SK, Hermansson M, Li L, Watts R, Thiesen A, Oudit GY, Michalak M, Lehner R. Ces3/TGH deficiency attenuates steatohepatitis. Sci Rep. 2016;6:25747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hegsted DM, Ausman LM, Johnson JA, Dallal GE. Dietary fat and serum lipids: An evaluation of the experimental data. Am J Clin Nutr. 1993;57(6):875–83. [DOI] [PubMed] [Google Scholar]
- 62. Keys A. Serum cholesterol response to dietary cholesterol. Am J Clin Nutr. 1984;40(2):351–9. [DOI] [PubMed] [Google Scholar]
- 63. Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, Erdman JW Jr., Kris-Etherton P, Goldberg IJ, Kotchen TA, Lichtenstein AH, Mitch WE, Mullis R, Robinson K, Wylie-Rosett J, St Jeor S, Suttie J, Tribble DL, Bazzarre TL. AHA dietary guidelines: Revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Stroke 2000;31(11):2751–66. [DOI] [PubMed] [Google Scholar]
- 64. Raddatz D, Ramadori G. Carbohydrate metabolism and the liver: Actual aspects from physiology and disease. Z Gastroenterol. 2007;45(1):51–62. [DOI] [PubMed] [Google Scholar]
- 65. Grande F, Anderson JT, Keys A. Sucrose and various carbohydrate-containing foods and serum lipids in man. Am J Clin Nutr. 1974;27(10):1043–51. [DOI] [PubMed] [Google Scholar]
- 66. Dorn C, Engelmann JC, Saugspier M, Koch A, Hartmann A, Muller M, Spang R, Bosserhoff A, Hellerbrand C. Increased expression of c-Jun in nonalcoholic fatty liver disease. Lab Invest. 2014;94(4):394–408. [DOI] [PubMed] [Google Scholar]
- 67. Gariani K, Ryu D, Menzies KJ, Yi HS, Stein S, Zhang H, Perino A, Lemos V, Katsyuba E, Jha P, Vijgen S, Rubbia-Brandt L, Kim YK, Kim JT, Kim KS, Shong M, Schoonjans K, Auwerx J. Inhibiting poly ADP-ribosylation increases fatty acid oxidation and protects against fatty liver disease. J Hepatol. 2017;66(1):132–41. [DOI] [PubMed] [Google Scholar]
- 68. Verbeek J, Jacobs A, Spincemaille P, Cassiman D. Development of a representative mouse model with nonalcoholic steatohepatitis. Curr Protoc Mouse Biol. 2016;6(2):201–10. [DOI] [PubMed] [Google Scholar]
- 69. Mock K, Lateef S, Benedito VA, Tou JC. High-fructose corn syrup-55 consumption alters hepatic lipid metabolism and promotes triglyceride accumulation. J Nutr Biochem. 2017;39:32–9. [DOI] [PubMed] [Google Scholar]
- 70. Jiang JX, Chen X, Fukada H, Serizawa N, Devaraj S, Torok NJ. Advanced glycation endproducts induce fibrogenic activity in nonalcoholic steatohepatitis by modulating TNF-alpha-converting enzyme activity in mice. Hepatology 2013;58(4):1339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Machado MV, Michelotti GA, Jewell ML, Pereira TA, Xie G, Premont RT, Diehl AM. Caspase-2 promotes obesity, the metabolic syndrome and nonalcoholic fatty liver disease. Cell Death Dis. 2016;7:e2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ichimura M, Masuzumi M, Kawase M, Sakaki M, Tamaru S, Nagata Y, Tanaka K, Suruga K, Tsuneyama K, Matsuda S, Omagari K. A diet-induced Sprague-Dawley rat model of nonalcoholic steatohepatitis-related cirrhosis. J Nutr Biochem. 2017;40:62–9. [DOI] [PubMed] [Google Scholar]
- 73. Johnson RJ, Perez-Pozo SE, Sautin YY, Manitius J, Sanchez-Lozada LG, Feig DI, Shafiu M, Segal M, Glassock RJ, Shimada M, Roncal C, Nakagawa T. Hypothesis: Could excessive fructose intake and uric acid cause type 2 diabetes? Endocr Rev. 2009;30(1):96–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig RH. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol. 2010;7(5):251–64. [DOI] [PubMed] [Google Scholar]
- 75. Luo Y, Burrington CM, Graff EC, Zhang J, Judd RL, Suksaranjit P, Kaewpoowat Q, Davenport SK, O’Neill AM, Greene MW. Metabolic phenotype and adipose and liver features in a high-fat Western diet-induced mouse model of obesity-linked NAFLD. Am J Physiol Endocrinol Metab. 2016;310(6):E418–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lau JK, Zhang X, Yu J. Animal models of non-alcoholic fatty liver disease: Current perspectives and recent advances. J Pathol. 2017;241(1):36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Miyaaki H, Nakao K. Significance of genetic polymorphisms in patients with nonalcoholic fatty liver disease. Clin J Gastroenterol. 2017;10(3):201–7. [DOI] [PubMed] [Google Scholar]
- 78. Austin GL, Ogden LG, Hill JO. Trends in carbohydrate, fat, and protein intakes and association with energy intake in normal-weight, overweight, and obese individuals: 1971-2006. Am J Clin Nutr. 2011;93(4):836–43. [DOI] [PubMed] [Google Scholar]