Abstract
Background
We have previously reported successful induction of renal allograft tolerance in nonhuman primates (NHP) following an initial posttransplant period of conventional immunosuppression (delayed tolerance) using a nonmyeloablative conditioning regimen consisting of anti-CD154 and anti-CD8 mAbs plus equine ATG (Atgam) and donor bone marrow transplantation (DBMT). Since these reagents are not currently clinically available, the protocol was revised to be applicable to human recipients of deceased donor allografts.
Method
Four cynomolgus monkeys received MHC-mismatched kidney allografts with conventional immunosuppression for 4 months. The recipients were then treated with a nonmyeloablative conditioning regimen consisting of thymoglobulin, belatacept, and DBMT. The results were compared with recipients treated with conditioning regimen consisting of Atgam and anti-CD154 mAb, with and without anti-CD8 mAb.
Results
In 4 consecutive NHP recipients treated with the modified conditioning regimen, homeostatic recovery of CD8+ TEM was delayed until after day 20 and multilineage chimerism was successfully induced. Three of the 4 recipients achieved long-term allograft survival (>728, >540, >449 days) without ongoing maintenance immunosuppression. Posttransplant MLR showed loss of anti-donor CD8+ T cell and CD4+ IFNγ responses with expansion of CD4+FOXP3+ regulatory T cells. However, the late development of DSA in NHP recipients confirms the need for additional anti-B cell depletion with agents, such as rituximab, as has been shown in our clinical trials.
Conclusion
This study provides proof of principle that induction of mixed chimerism and long-term renal allograft survival without immunosuppression after delayed donor bone marrow transplantation is possible with clinically available reagents.
Introduction
Long-term immunosuppression-free renal allograft survival has been successfully achieved in MHC-mismatched nonhuman primates (NHP) and humans through simultaneous kidney and donor bone marrow transplantation (SKBMT) and nonmyeloablative conditioning 1–6. To extend this approach to deceased donor transplantation, we previously reported the successful induction of renal allograft tolerance in NHP by delaying conditioning and donor bone marrow transplantation (DBMT) for several months after kidney transplantation (KTx) (delayed tolerance) 7,8. In this protocol, recipients were initially treated posttransplant with conventional immunosuppression, which included tacrolimus, mycophenolate mofetil (MMF), and prednisone, after which they received a conditioning regimen consisting of horse ATG (Atgam) plus anti-CD154 and anti-CD8 mAbs or LFA3-Ig for DBMT 9. Although anti-CD154 mAb and LFA3-Ig have been reported to have effective immunomodulatory properties 10,11, they are not available for clinical use. Therefore, we sought to revise the protocol using clinically available reagents in order to make this strategy applicable to deceased donor transplantation in humans. In the current study, we evaluated rabbit-ATG (thymoglobulin) in place of Atgam and anti-CD8 mAb, and CTLA4Ig (belatacept) in place of anti-CD154 mAb to induce delayed tolerance. The clinical impact of this approach is significant, since it would make tolerance induction possible, not only for current recipients of deceased donor organs, but also for previous transplant recipients whose donor hematopoietic stem cells are available.
Materials and Methods
Animals
Cynomolgus monkeys weighing between 4 and 8 kg were used (Charles River Primates, Wilmington, MA). All surgical procedures and postoperative care of animals were performed in accordance with National Institute of Health guidelines for the care and use of primates and approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee.
Cynomolgus MHC genotyping
MHC characterization was performed as previously described 12. Briefly, genomic DNA was prepared from peripheral blood mononuclear cells (PBMCs) and splenocytes. Panels of 17 microsatellite loci spanning approximately 5 Mb of the MHC region were amplified from the genomic DNA with fluorescent-labeled polymerase chain reaction primers, and fragment size analysis was determined. The microsatellite haplotypes for each animal were converted to predicted MHC genotypes based on previous cloning and sequencing work with cynomolgus monkeys.
Conditioning regimens
All recipients initially underwent KTx alone with a conventional triple drug immunosuppressive regimen consisting of tacrolimus (Astellas Pharma, Inc., Osaka, Japan) (starting with 0.1 mg/kg/day i.m. to maintain trough levels of 10–20 ng/dl), mycophenolate mofetil (Roche, Inc., Nutley, NJ) (200 mg/day), and prednisone (starting with 20 mg/day and tapering to a 1 mg/day maintenance dose in 1 week). Four months later, the recipients underwent conditioning and DBMT. Group 1 recipients have been previously reported. 7,8 Briefly, recipients in this group received low-dose total body irradiation (TBI; 1.5Gy × 2) on days -6 and -5 (relative to DBMT), thymic irradiation (TI; 7 Gy) on day -1, and T cell depletion by equine anti-thymocyte globulin (Atgam, Pharmacia and Upjohn, Kalamazoo, MI, 50 mg/kg/day on days -2, -1, and 0). After DBMT, anti-CD154 mAb (Nonhuman Primate Reagent Resource, Boston, MA) was administered at 20 mg/kg on days 0 and 2, 10 mg/kg on days 5, 7, 9, and 12 post-BMT, and cyclosporine A (CyA) was continued for 1 month to maintain serum trough levels of 200–300 ng/ml, after which no further immunosuppression was administered (Figure 1, Table 1). Group 2 received the same conditioning regimen administered to Group 1, but with the addition of anti-CD8 mAb (cM-T807 provided by Centocor Inc., Horsham, PA, USA, 5 mg/kg/day on days 0 and +2) as previously reported 7. In Group 3, the Thymoglobulin (Thymo)/belatacept (bela) regimen, Atgam and anti-CD8 mAb was replaced with rabbit-ATG (Thymoglobulin, Bridgewater, NJ, 10 mg/kg/day on day -2 and -1) and anti-CD154 mAb was replaced with CTLA4Ig (belatacept, Bristol-Myers Squib, New York, NY, 20 mg/kg X 4 on days 0, 2, 5, and 12). Flow cytometric analyses and detection of chimerism
Figure 1. Conditioning regimen for delayed tolerance.
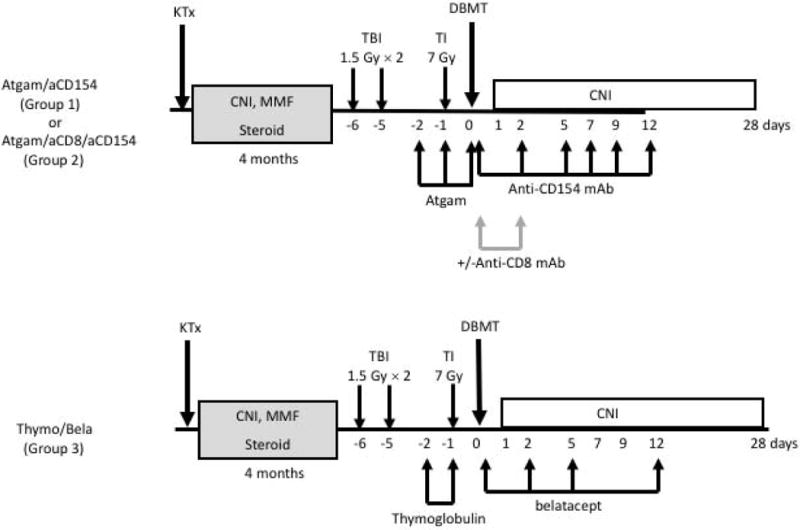
All recipients initially underwent KTx alone and received conventional triple drug immunosuppression including tacrolimus, mycophenolate mofetil, and prednisone. Three to 4 months later, the recipients underwent conditioning and DBMT. In the Atgam/aCD154 regimen (Groups #1 and #2), low-dose total body irradiation (1.5 Gy × 2) on days -6 and -5 (relative to DBMT), thymic irradiation (7 Gy) on day -1, and Atgam (50mg/kg X3) were administered before DBMT. After DBMT, a short course of anti-CD154 mAb and a 1-month course of cyclosporine A (CyA) were administered, after which no further immunosuppression was given. In the thymo/bela regimen (Group #3), anti-CD8 mAb, Atgam, and anti-CD154 mAb were replaced with thymoglobulin (10 mg/kg on days -2 and -1) and belatacept.
Table 1.
| MHC mismatch
|
Multi-lineage | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Class I | Class II | IS1 before DBMT | anti-T cell ab | CoB2 | chimerism | DSA | allograft Survival | |
| Group1 | 5 | haplo | haplo | Tacro+MMF+Pred | Atgam | aCD154 | − | + | 703 |
| haplo | haplo | Tacro+MMF+Pred | Atgam | aCD154 | − | − | 64 | ||
| full | haplo | Tacro+MMF+Pred | Atgam | aCD154 | − | − | 37 | ||
| full | full | Tacro+MMF+Pred | Atgam | aCD154 | − | − | 35 | ||
| haplo | haplo | Tacro+MMF+Pred | Atgam | aCD154 | − | − | 30 | ||
|
| |||||||||
| Group 2 | 12 | haplo | haplo | Tacro+MMF+Pred | Atgam/aCD8 | aCD154 | + | + | 1825 |
| haplo | haplo | Tacro+MMF+Pred | Atgam/aCD8 | aCD154 | + | − | 852 | ||
| full | full | Tacro+MMF+Pred | Atgam/aCD8 | aCD154 | + | + | 1260 | ||
| full | full | Tacro+MMF+Pred | Atgam/aCD8 | aCD154 | + | − | 999 | ||
| full | full | Tacro+MMF+Pred | Atgam/aCD8 | aCD154 | + | + | 791 | ||
| haplo | haplo | Tacro+MMF+Pred | Atgam/aCD8 | aCD154 | + | − | 721 | ||
| haplo | full | Tacro+MMF+Pred | Atgam/aCD8 | aCD154 | − | + | 107 | ||
| haplo | full | Tacro+MMF+Pred | Atgam/aCD8 | aCD154 | + | − | 75 | ||
| full | haplo | Tacro+MMF+Pred | Atgam/aCD8 | aCD154 | + | − | 75 (PTLD) | ||
| haplo | haplo | Tacro+MMF+Pred | Atgam/aCD8 | aCD154 | − | − | 67 | ||
| haplo | haplo | Tacro+MMF+Pred | Atgam/aCD8 | aCD154 | + | − | 32 (PTLD) | ||
| haplo | haplo | Tacro+MMF+Pred | Atgam/aCD8 | aCD154 | n/a3 | − | 14 (BK) | ||
|
| |||||||||
| Group 3 | 4 | full | full | Tacro+MMF+Pred | Thymo | belatacept | + | + | 728 |
| full | full | Tacro+MMF+Pred | Thymo | belatacept | + | +/− | 540 | ||
| full | full | Tacro+MMF+Pred | Thymo | belatacept | + | − | 449 | ||
| full | full | Tacro+MMF+Pred | Thymo | belatacept | + | − | 1084 | ||
immunosuppression,
costimulatory blockade,
died too early to determine chimerism,
died from an anesthesia complication w/o rejection.
PBMCs were analyzed via cell-surface staining using CD3 (SP34), CD4 (L200), CD8 (SK1), CD20 (2H7), CD25 (M-A251), and CD28 (CD28.8) (all BD Pharmingen, San Jose, CA); CD95 (DX2) and CD16 (NKP15) (all BD Biosciences, San Jose, CA); and NKG2a (Z199, Beckman Coulter, Brea, CA) antibodies. Each lymphocyte subset was determined by using the following surface markers: naïve T cells (CD3+CD95−CD28−), central memory T cells (TCM) (CD3+CD95−CD28−) and effector memory T cells (TEM) (CD3+CD95−CD28−), B cells (CD3-CD20+), and NK cells (CD3−CD8+CD16+NKG2a+). For the chimerism analyses, we used an anti–MHC class I HLA mAb (H38, One Lambda, Inc. Canoga Park, CA), which reacts specifically with an MHC class I antigen on donor but not recipient cells. To assess the intracellular protein expression of FOXP3 and INFγ, the cells were permeabilized using Fixation/Permeabilization solution (eBioscience, San Diego, CA) and then stained with anti-FOXP3 mAb (PCH101, BD Pharmingen, San Jose, CA) and anti-INFγ mAb (B27, BD Pharmingen, San Jose, CA). Cells were analyzed on a FACSverse (BD Biosciences, San Jose, CA) or Accuri Flow Cytometer (BD Biosciences) using FlowJo software (FLOWJO LLC, Ashland, OR).
Alloantibody Measurement
Anti-donor alloantibody was detected by flow cytometric analysis. First, donor PBMCs were incubated with recipient sera for 30 min. at 4°C. After washing with FACS medium, FITC-conjugated mouse anti-human IgG mAb was added and incubated for 30 min. at 4°C, then washed twice. The PBMCs were further incubated with PE-conjugated anti-CD20 mAb (Becton Dickinson, Mountain View, CA) for 30 min. at 4°C. After washing, the PBMCs were fixed with 2% paraformaldehyde. Cells were then acquired and analyzed with FACScan (Becton Dickinson, Mountain View, CA). A positive reaction of the T and B cells was defined as a shift greater than 10-channels in mean fluorescence (MCF) intensity of donor lymphocytes when using test sera compared with pretransplant serum control.
Mixed lymphocytes culture
T cells were purified by negative selection with a Pan T Cell Isolation Kit (Miltenyi Biotec, Somerville, MA). The isolated T cells were labeled with CFSE (Life Technologies, Waltham, MA) at a concentration of 3 µM per 107 cells at 37°C for 5 minutes and cultured in 96-well V-bottom plates with irradiated PBMCs. After 5 days, the cells were stained with antibodies and CFSE dilution was assessed by flow cytometry. In the staining of INFγ, leukocyte activation cocktail (BD Biosciences) was added, followed by an additional incubation of four hours.
Histological analyses
Protocol kidney allograft biopsies were obtained every 2 to 4 months in animals with stable function and whenever a rise in serum creatinine occurred. The samples were processed for routine microscopy and a portion of frozen tissue was saved. Other organs obtained surgically (lymph nodes, native kidney, spleen) were similarly processed. Following euthanasia, complete autopsies were performed for pathologic evaluation of the kidney allograft, lymph nodes, heart, lung, liver, pancreas, thymus, and skin. Immunohistochemical studies using a rabbit monoclonal antibody for C4d (A24-T, Biocare CA USA) were performed on formalin-fixed paraffin embedded tissues for all kidney allograft samples. Immunohistochemical staining for Foxp3 (FJK-16s, ThermoFisher/eBioscience) was done on kidney allograft samples with nodular mononuclear cell aggregates.
Statistical Analysis
All measured outcomes are summarized as mean ± standard error unless otherwise specified. Between-group pairwise comparison of means was performed by linear contrast in a random intercept longitudinal linear mixed effects model setting, for which the error covariance structure was specified with variance components. p<0.05 for an individual test was considered statistically significant. Renal allograft survival curves were created by the Kaplan and Meier method, and the results were compared using the log rank test (Prism version 7, Graphpad Software Inc., San Diego, CA).
Results
Sufficient deletion of CD8+ effector memory T cells is critical for induction of mixed chimerism in the delayed tolerance approach
Lymphocyte subset recovery after DBMT in recipients treated with the modified regimen (Group 3) was compared with the results observed in recipients treated with the Group 1 and 2 regimens (Figure 1). As previously reported 7, with the Group 1 regimen, rapid recovery of CD8+ effector memory T cells (TEM) was observed by day 12 (Figure 2, triangle), along with no induction of multi-lineage mixed chimerism (Table 1, Figure 3, triangle). Adding anti-CD8 mAb to the Group 1 regimen extended the duration of CD8+ TEM deletion to more than 30 days (Figure 2, open circle), and 9/12 recipients successfully developed mixed chimerism (1 recipient died too early to determine chimerism induction) (Table 1, Figure 3, open circle). With the Group 3 regimen, the deletion of CD8+ TEM was less intense than that observed in Group 2, but superior to that of Group 1 recipients up until day 20, which is a critical time for bone marrow cell engraftment (Figure 2, red circle). Although CD8+TCM was also depleted well up to day 20 in Group 3, rapid homeostatic proliferation of CD8+ TCM ensued after day 20. However, this homeostatic expansion of CD8+ TCM was not associated with loss of chimerism or rejection. This regimen provided successful induction of chimerism in 4 consecutive recipients (Figure 3, red circle). On the other hand, CD4+ central memory T cell (CD4+ TCM) depletion was more prominent in Group 3 than in Group 1 and Group 2 recipients, but it was not directly correlated with chimerism induction (Figure 2). There were no differences observed in the recovery of other T cell subsets, such as NK cells and B cells. These observations indicate that moderately prolonged deletion of CD8+ TEM is critical for induction of chimerism in the delayed tolerance approach.
Figure 2. Lymphocyte subsets after DBMT.
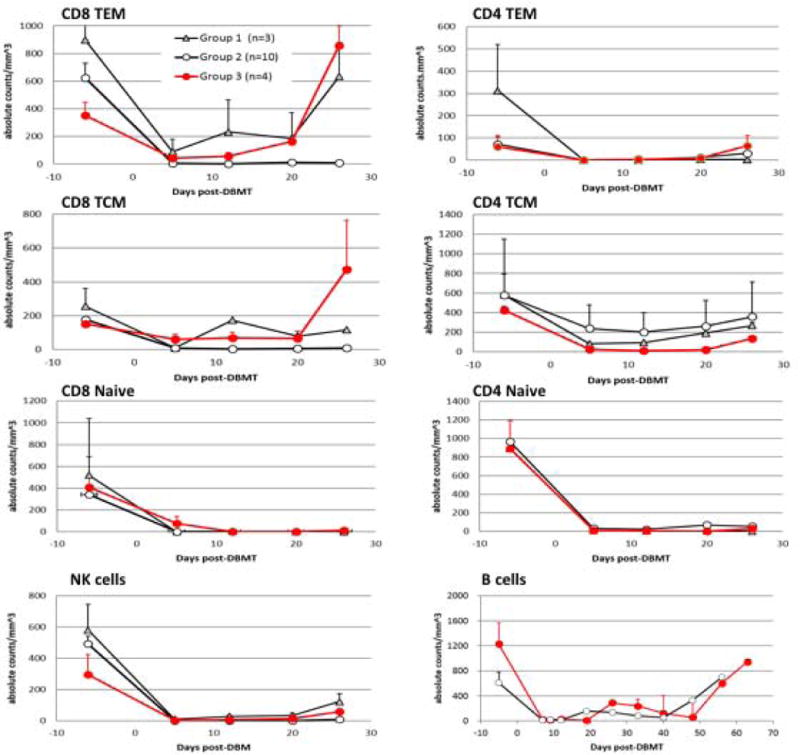
With the Atgam/aCD154 regimen, rapid recovery of CD8+ effector memory T cells (CD8+ TEM) and central memory T cells (CD8+ TCM) was observed by day 12 (triangle). With the addition of anti-CD8 mAb to the Atgam/aCD154 regimen, prolonged deletion of CD8+TEM and TCM was observed for more than 30 days (open circle). With the thymo/bela regimen, deletion of CD8+TEM and TCM was less intense than that observed with anti-CD8 mAb, but more prominent than Atgam alone (red circle). There was no difference observed in other T cell subsets, such as NK cells and B cells (B cell counts were not monitored in Group A).
Figure 3. Chimerism in the myeloid and lymphoid lineages after DBMT.
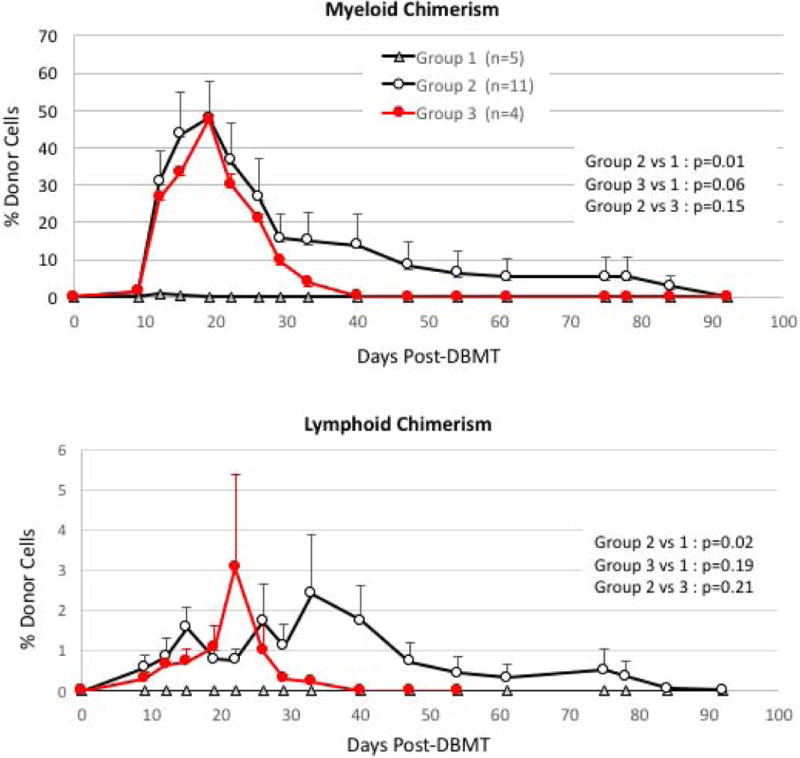
With the Atgam/aCD154 regimen (triangle), the recipients failed to develop chimerism (Figure 3, triangle). By adding anti-CD8 mAb to the Atgam/aCD154 regimen, 10/12 successfully developed mixed chimerism (Figure 3, open circle). With the thymo/bela regimen, all 4 recipients successfully developed chimerism in both lymphoid and myeloid lineages (red circle).
Long-term renal allograft survival without ongoing immunosuppression
In Group 1, all recipients failed to develop multi-lineage chimerism and rejected their renal allografts early (Figure 4A, gray dotted line). One recipient developed limited chimerism in the myeloid lineage only and had prolonged survival. With the addition of anti-CD8 mAb in Group 2, 10/12 achieved mixed chimerism; however, only 6/12 achieved long-term allograft survival (Table 1, Figure 4A, black line). The causes of death in the other recipients included viral infection or PTLD in 3 and rejection in 3 recipients (Table 1). In contrast, 4 consecutive monkeys treated with the Thymo/bela regimen tolerated the protocol well and successfully developed mixed chimerism. Although the duration of chimerism was shorter (up to day 40) than that observed in Group 2, no infectious or PTLD complications were observed in Group 3. Three of the recipients in Group 3 achieved long-term renal allograft survival without ongoing immunosuppression (Figures 4A and B). The last recipient in this group also did well, but died on day 108 following an anesthesia complication during the allograft biopsy (Table 1).
Figure 4.
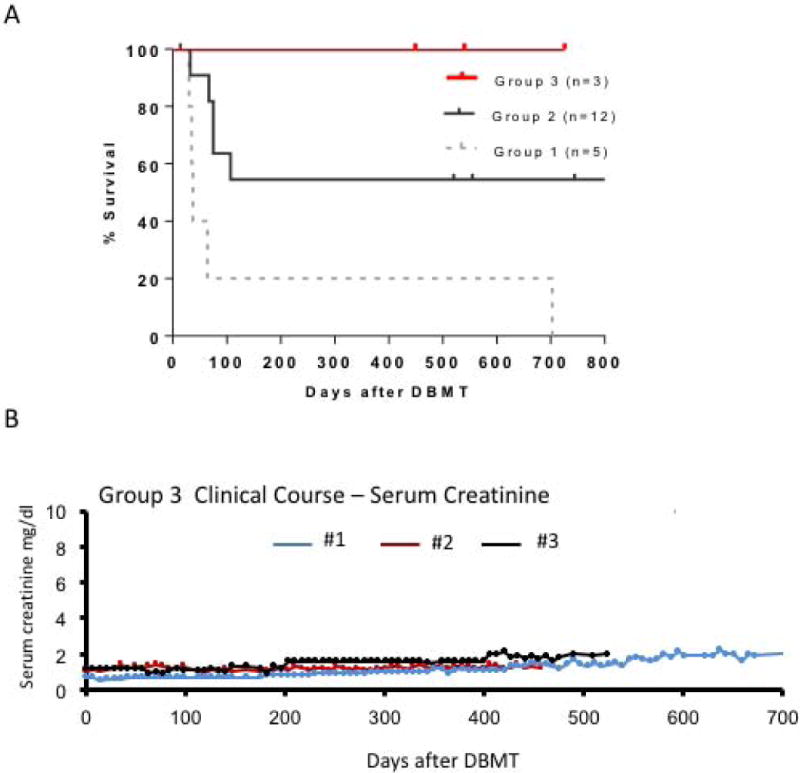
A. Renal allograft survival after DBMT
Renal allograft survival was significantly superior in recipients of the Thymo/bela regimen (red line), compared with recipients of the Atgam/aCD154 (p<0.03) regimen (dotted gray line). There was no statistically significant difference between thymo/bela and Atgam/aCD8/aCD154 (black line).
B. Serum creatinine levels after DBMT in Group 3 recipients
Serum creatinine was stable around 1.2 mg/dl during the entire observation period in Recipient #2, while Recipient #3 received his kidney from a relatively small recipient and his baseline creatinine levels were around 1.7 mg/dl. After the appearance of DSA, the baseline creatinine levels became slightly elevated (2.0 mg/dl). In Recipient #1, serum creatinine levels gradually increased from 1.0 to 2.1 mg/dl over 700 days.
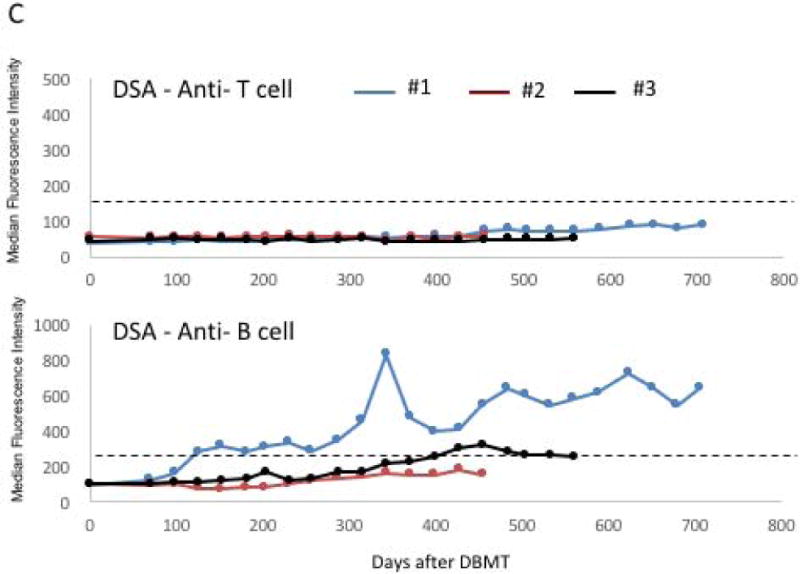
C. DSA titers after DBMT.
Anti-T cell DSA has never been detected in any of the 3 recipients. However, anti-B cell DSA, presumably against MHC-class II, became positive after day 100 post-DBMT in Recipient #1. Recipient #2 transiently developed weak DSA after day 400. The cut-off median fluorescent intensity (MFI) for positive DSA (dotted line) was defined as 3 times higher than the pretransplant DSA titer.
Although sequential biopsies initially showed no evidence of rejection (Figures 5A, B and F), Recipients #1 and #2 in Group 3 developed late onset anti-B cell DSA (Figure 4C), presumably against MHC class II. The third recipient transiently developed weak DSA after day 400 post-DBMT, and protocol allograft biopsy on day 411 showed evidence of transplant glomerulopathy (Figure 5C) with positive C4d (Figure 5D). The DSA titers of recipient #3 subsequently declined and are currently borderline (Figure 4C). In recipient #1, weakly (10%) positive C4d was identified in the protocol biopsy before DBMT (Figure 5E) but without serum DSA detection by flow cytometry. Although this recipient was presumably sensitized before DBMT, chimerism was induced and he survived long-term without immunosuppression, with biopsy on day 236 showing no rejection (Figure 5F) but persistent C4d staining. Anti-B cell DSA became detectable by flow cytometry after day 100 and chronic antibody-mediated rejection was diagnosed in the biopsy on day 391 (Figure 5G) with diffusely positive C4d staining (Figure 5H). The recipient was euthanized on day 728 post-DBMT despite relatively stable renal function. A consistent finding observed in all 3 long-term survivors included formation of T regulatory cell (Treg-rich organized lymphoid structures) (TOLS) in the renal allograft biopsies. (A representative biopsy from recipient #2 is shown in Figure 5I.)
Figure 5. Renal allograft biopsies in long-term survivors.
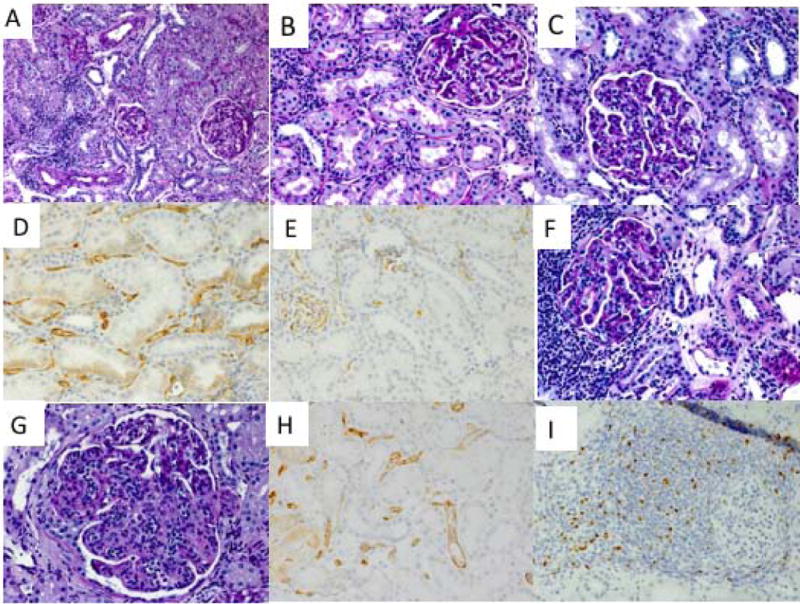
The renal allograft biopsy acquired from Recipient #2 on day 449 post-DBMT showed no evidence of rejection (A). The biopsy from Recipient #3 on day 299 showed no rejection but a subsequent biopsy on day 411 showed the development of transplant glomerulopathy (C) with positive C4d (D). A pre-DBMT protocol biopsy from Recipient #1 performed on day 72 after KTx (15 days before DBMT) showed positive C4d staining (E). Although the biopsy from Recipient #1 on day 236 showed no rejection with focal nodular aggregates (F), a biopsy on day 399 and later biopsies showed features of chronic antibody-mediated rejection (G) with persistent diffuse C4d staining (H). A representative biopsy with Foxp3 staining (brown) from Recipient #2 showed Treg-rich organized structures (TOLS).
Loss of anti-donor CD8+ T cell and Th1 cell responses after DBMT in long-term survivors
We have previously reported that loss of donor-specific CD8+ T cell responses and donor-specific expansion of CD4+ FOXP3+ regulatory T cells (Treg) appears to accompany long-term immunosuppression-free allograft survival in NHP recipients13. In the current study, T cell responses to donor antigens were similarly evaluated in 2 long-term survivors in Group 3 whose donor cells were available. Both recipients exhibited significant anti-donor CD8+ T cell responses before DBMT. These anti-donor responses were markedly decreased after conditioning and DBMT in both recipients. Although a reduction of anti-third-party CD8+ T cell responses was also observed, it was still 2 times higher than that observed with donor antigen stimulation. In contrast, substantial anti-donor CD4+ T cell proliferation persisted even after DBMT (Figures 6A, B and C; left panels). Among these proliferated CD4+ cells, marked reduction of Th1 (CD4+INF-γ) responses was observed after DBMT in both recipients, while there was an upward trend in donor reactive regulatory T cell (Treg) (CD4+FOXP3+) expansion after DBMT in both recipients. Treg expansion was also observed by third-party stimulation, but this was lower than that induced by donor stimulation (Figures 6A, B and C; right panels).
Figure 6. CFSE-MLR in 2 long-term survivors.
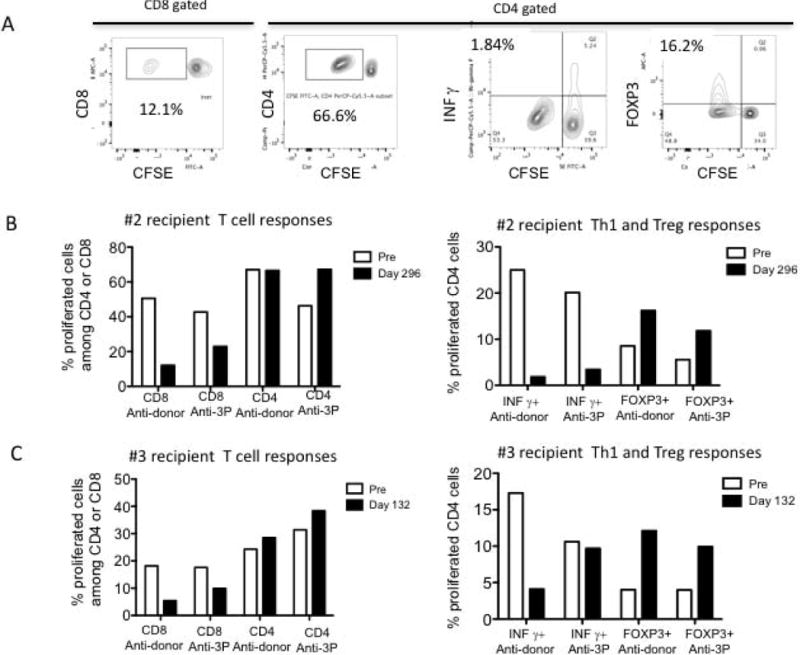
A: Representative flow cytometry dot plots on T cell (CD8 and CD4) responses to donor antigens after DBMT.
B: Recipient #2 T cell responses pre-and post DBMT: Recipient #2 exhibited a significant anti-donor CD8+ T cell response before DBMT. It was markedly decreased after conditioning and DBMT. Although a reduction of the anti-third-party CD8+ T cell response was also observed, it remained 2 times higher than that observed with donor antigen stimulation. In contrast, substantial anti-donor CD4+ T cell proliferation persisted even after DBMT (left panel). Among these proliferated CD4+ cells, a marked reduction of Th1 (CD4+INF-γ) responses was observed after DBMT (right panel), while there was an upward trend in donor reactive regulatory T cell (Treg) (CD4+FOXP3+) expansion after DBMT. Treg expansion was also observed by third-party stimulation, but it was lower than that achieved by donor stimulation (right panel).
C: Recipient #3 T cell responses: T cell (CD8+, CD4+, CD4+INF-γ and CD4+FOXP3+) responses similar to Recipient #2 were observed in Recipient #3.
Discussion
“Delayed tolerance” is associated with the theoretical disadvantage that donor-specific memory T cells (TMEM) might be elicited despite the administration of potent suppressive agents prior to conditioning and DBMT. Indeed, the original protocol (the Group 1 regimen), which was effective for SKBMT, failed to induce chimerism in the delayed approach. Since earlier homeostatic expansion of CD8 TEM was noted after DBMT in these recipients, anti-CD8 mAb was added to the original regimen (Group 2 regimen). With this modification, 10/12 recipients developed chimerism and 6 of those achieved long-term renal allograft survival without ongoing immunosuppression. However, 3 of the remaining 6 died from either CMV infection or EBV-related PTLD, and 3 lost their allografts as a result of acute rejection. We concluded that the anti-CD8 mAb was too toxic for clinical use in this protocol and, in fact, it is not clinically available. Therefore, we sought an alternative approach to overcome the TMEM response in the delayed tolerance approach. We initially evaluated alefacept (LFA3-Ig), a humanized chimeric fusion protein consisting of the extracellular CD2-binding portion of the human leukocyte function antigen-3 (LFA-3) adhesion molecule. LFA3-Ig is known to selectively deplete CD8+ effector memory T cells (TEM) while sparing naïve CD8+ T cells 14 and has been approved by the FDA for the treatment of psoriasis. In this study, chimerism was induced without significant depletion of CD8+ central memory T cells (CD8+ TCM), indicating that CD8+ TCM depletion is less important for chimerism induction than CD8+ TEM. The protocol, in which alefacept was substituted for anti-CD8 mAb, successfully induced mixed chimerism and long-term renal allograft survival 9. However, alefacept has also become clinically unavailable as a consequence of a marketing decision, which forced us to identify another clinically available regimen for delayed tolerance.
Thymoglobulin has been shown to have more potent T-cell-depleting properties than Atgam 15, and is the most commonly used agent for induction therapy in clinical transplantation 16. Therefore, we anticipated that Thymoglobulin might be a suitable substitute for Atgam and anti-CD8 mAb in a conditioning regime for delayed tolerance. Since anti-CD154 mAb is also not clinically available, we replaced it with belatacept, which we had shown was effective in inducing renal allograft tolerance following SKBMT3. Although the duration of CD8+ TEM depletion after thymoglobulin/belatacept treatment proved to be shorter than that following anti-CD8 mAb treatment, the combination of belatacept and thymoglobulin delayed the recovery of CD8+ TEM beyond day 20, which resulted in successful induction of chimerism without infectious complications. In contrast, CD8+ TEM recovery by day 12 was consistently associated with failure to induce chimerism in the Atgam/anti-CD154 regimen, and prolonged depletion was associated with significant mortality in the Atgam/aCD8/aCD154 protocol. In this study with thymoglobulin, significant depletion of CD8+ TCM was initially observed up to day 20, followed by rapid homeostatic expansion. Although this homeostatic expansion of CD8+ TCM was much more significant compared to Groups 1 and 2, it did not affect the chimerism induction. Together with the observation made in the previous study, in which chimerism was induced without significant depletion of CD8+ TCM 9, we conclude that CD8+ TCM may be less important in the induction or maintenance of mixed chimerism. In contrast, the importance of CD8+ TEM depletion in mixed chimerism induction has been consistently demonstrated, including in the current study with thymo/bela. There was no difference in NK cells among all regimens, indicating that NK cells are not involved in resistance to bone marrow stem cell engraftment in the delayed tolerance protocol. In contrast, CD4+ T cell depletion was most prominent with thymoglobulin, which may be responsible for the more consistent induction of mixed chimerism and better overall allograft survival.
In this study, T cell responses to donor antigens were evaluated in 2 long-term survivors whose donor cells were available. Observations in these recipients are consistent with the data provided in previous reports by us 13 and others17, suggesting that the mechanistic pathways to tolerance in NHPs are primarily regulatory 18 rather than deletional, since only transient mixed chimerism has been achieved 19. The recipients in the current study exhibited reduced anti-donor CD8+ T cell responses, while anti-donor CD4+ T cell responses were preserved. Although significant IFN-γ responses were observed among CD4+ T cells before DBMT, those responses were markedly reduced after DBMT. In contrast, the response of Treg following donor stimulation increased after DBMT, which has also been observed in our previous studies 13. Indeed, in our previous mechanistic studies, we showed that the expanded Treg population consisted of induced Treg (iTreg) 13. Furthermore, inhibition of Treg expansion by transforming growth factor-β (TGF-β) blockade was observed in tolerant recipients, resulting in restoration of anti-donor CD8+ T cell responses. Since some T cell subsets, such as Th3, have been shown to produce large amounts of TGF-β 20, we hypothesize that the specific T cell subsets (Thx) that promote conversion of Treg from non-Treg are induced during transient chimerism under costimulatory blockade. Upon encountering donor MHC class II antigens expressed on renal allograft endothelium 21, 22, these T cells may become activated and produce cytokines that convert non-Treg to Treg, resulting in local enrichment of Treg in the allograft (Figure 7). Such enrichment of Treg in the renal allograft has been observed in our previous studies 23,4. And the presence of these locally enriched Treg in the renal allograft may protect the allograft from rejection.
Figure 7. A hypothesis of renal allograft tolerance via transient mixed chimerism.
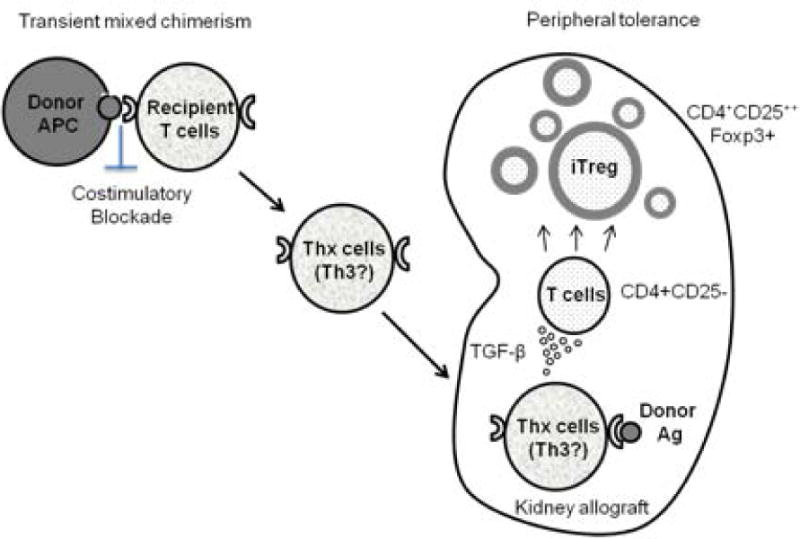
Chimeric donor cells provide significant donor-antigen presentation under co-stimulatory blockade. This may result in the generation of donor-specific memory T cells that can produce cytokines (eg Th3 produces TGF-β) upon stimulation by donor antigens. Upon encountering donor antigens in the graft, these T cells produce local cytokines that favor the conversion of non-Treg cells to Treg in the renal allograft.
Nevertheless, 2 of the recipients later developed low grade (up to 800 MFI) to borderline (300 MFI) DSA (1 monkey was presumably sensitized before DBMT) which was associated with the development of transplant glomerulopathy. In Group 2, half of the long-term survivors (3/6) also developed late onset anti-B cell DSA (Table 1). A marked feature in these NHP recipients has been the presence of antibody-mediated rejection without cellular rejection. We therefore hypothesize that enrichment of Treg in the renal allograft protects the allograft from cellular rejection but not from DSA. Therefore, when considering this strategy for clinical application, additional treatments against B cell lineages need to be considered. As previously reported in our clinical trials for tolerance induction in HLA-mismatched kidney transplantation, we initially observed 2 recipients that also developed DSA and late onset chronic rejection more than 5 to 7 years after transplantation5. However, the subsequent addition of intensified peritransplant treatment with rituximab effectively prevented this complication, in that none of the 4 recipients treated with intensified rituximab have developed chronic rejection after observation periods of 4 to 8 years5. Unfortunately, the addition of rituximab in our NHP protocols has proved to be poorly tolerated. Although 1 monkey received conditioning regimen with rituximab and survived beyond 700 days without DSA or rejection, seven other similarly treated recipients died owing to chronic gastrointestinal symptoms (diarrhea or anorexia) (unpublished results). In contrast, all human recipients of the intensified rituximab regimen, including a most recent recipient treated with exactly the same thymo/bela regimen but in the setting of SKBMT, have tolerated the treatment very well (unpublished results). We have thus concluded that the thymo/bela regimen can be effectively and safely applied to human renal allograft recipients.
In conclusion, although the number of animals studied in this report is limited, the current study provides important information on which to construct a clinical protocol to apply the delayed tolerance approach in human kidney transplantation.
Acknowledgments
We thank Ann S. Adams for editorial assistance.
Funding:
The present work was supported in part by Grant 5U19AI102405, part of the NIH NHP Transplantation Tolerance Cooperative Study Group and sponsored by the National Institute of Allergy and Infectious Diseases, the National Institute of Diabetes and Digestive and Kidney Diseases, and the Canadian Foundation for Innovation.
Abbreviations
- NHP
nonhuman primates,
- CyA
cyclosporine
- TMEM
memory T cells
- TEM
effector memory T cells
- TCM
central memory T cells
- KTx
kidney transplantation
- DBMT
donor bone marrow transplantation
- PTLD
posttransplant lymphoproliferative disease
- TBI
total body irradiation
- TI
thymic irradiation
- DSA
donor specific antibody
- TOLS
Treg-rich organized lymphoid structures
Footnotes
Authorship:
K.H. designed and performed the experiments, analyzed the data, and wrote the manuscript. T.O. contributed to the design of experiments and interpretation of results. A.B. performed pre- and posttransplant care of the animals. S.B. designed and performed the experiments and analyzed data. M.M. performed the experiments. I.R., R.N.S., and R.B.C. performed pathological studies. A.B.C designed the study and edited the manuscript. T.K. conceived and directed the study, performed experiments, and wrote the manuscript.
Disclosure:
The authors have no conflicts of interest to disclose.
References
- 1.Kawai T, Cosimi AB, Colvin RB, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59(2):256–262. [PubMed] [Google Scholar]
- 2.Kawai T, Sogawa H, Boskovic S, et al. CD154 blockade for induction of mixed chimerism and prolonged renal allograft survival in nonhuman primates. Am J Transplant. 2004;4(9):1391–1398. doi: 10.1111/j.1600-6143.2004.00523.x. [DOI] [PubMed] [Google Scholar]
- 3.Yamada Y, Ochiai T, Boskovic S, et al. Use of CTLA4Ig for Induction of Mixed Chimerism and Renal Allograft Tolerance in Nonhuman Primates. Am J Transplant. 2014;14(12):2704–2712. doi: 10.1111/ajt.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358(4):353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawai T, Sachs DH, Sprangers B, et al. Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am J Transplant. 2014;14(7):1599–1611. doi: 10.1111/ajt.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chhabra AY, Leventhal J, Merchak AR, Ildstad S. HSCT Based Approaches for Tolerance Induction in Renal Transplant. Transplantation. 2017 doi: 10.1097/TP.0000000000001837. [DOI] [PubMed] [Google Scholar]
- 7.Yamada Y, Boskovic S, Aoyama A, et al. Overcoming memory T-cell responses for induction of delayed tolerance in nonhuman primates. Am J Transplant. 2012;12(2):330–340. doi: 10.1111/j.1600-6143.2011.03795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koyama I, Nadazdin O, Boskovic S, et al. Depletion of CD8 memory T cells for induction of tolerance of a previously transplanted kidney allograft. Am J Transplant. 2007;7(5):1055–1061. doi: 10.1111/j.1600-6143.2006.01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee S, Yamada Y, Tonsho M, et al. Alefacept promotes immunosuppression-free renal allograft survival in nonhuman primates via depletion of recipient memory T cells. Am J Transplant. 2013;13(12):3223–3229. doi: 10.1111/ajt.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Neill NA, Zhang T, Braileanu G, et al. Comparative Evaluation of alphaCD40 (2C10R4) and alphaCD154 (5C8H1 and IDEC-131) in a Nonhuman Primate Cardiac Allotransplant Model. Transplantation. 2017;101(9):2038–2047. doi: 10.1097/TP.0000000000001836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo DJ, Weaver TA, Stempora L, et al. Selective targeting of human alloresponsive CD8+ effector memory T cells based on CD2 expression. Am J Transplant. 2012;11(1):22–33. doi: 10.1111/j.1600-6143.2010.03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Connor SL, Blasky AJ, Pendley CJ, et al. Comprehensive characterization of MHC class II haplotypes in Mauritian cynomolgus macaques. Immunogenetics. 2007;59(6):449–462. doi: 10.1007/s00251-007-0209-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hotta K, Aoyama A, Oura T, et al. Induced regulatory T cells in allograft tolerance via transient mixed chimerism. JCI insight. 2016;1(10) doi: 10.1172/jci.insight.86419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weaver TA, Charafeddine AH, Agarwal A, et al. Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates. Nat Med. 2009;15(7):746–749. doi: 10.1038/nm.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brennan DC, Flavin K, Lowell JA, et al. A randomized, double-blinded comparison of Thymoglobulin versus Atgam for induction immunosuppressive therapy in adult renal transplant recipients. Transplantation. 1999;67(7):1011–1018. doi: 10.1097/00007890-199904150-00013. [DOI] [PubMed] [Google Scholar]
- 16.Willoughby LM, Schnitzler MA, Brennan DC, et al. Early outcomes of thymoglobulin and basiliximab induction in kidney transplantation: application of statistical approaches to reduce bias in observational comparisons. Transplantation. 2009;87(10):1520–1529. doi: 10.1097/TP.0b013e3181a484d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duran-Struuck R, Sondermeijer HP, Buhler L, et al. Effect of Ex Vivo-Expanded Recipient Regulatory T Cells on Hematopoietic Chimerism and Kidney Allograft Tolerance Across MHC Barriers in Cynomolgus Macaques. Transplantation. 2017;101(2):274–283. doi: 10.1097/TP.0000000000001559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dons EM, Raimondi G, Cooper DK, Thomson AW. Non-human primate regulatory T cells: current biology and implications for transplantation. Transplantation. 2010;90(8):811–816. doi: 10.1097/TP.0b013e3181ebf782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada Y, Nadazdin O, Boskovic S, et al. Repeated Injections of IL-2 Break Renal Allograft Tolerance Induced via Mixed Hematopoietic Chimerism in Monkeys. Am J Transplant. 2015;15(12):3055–3066. doi: 10.1111/ajt.13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carrier Y, Yuan J, Kuchroo VK, Weiner HL. Th3 cells in peripheral tolerance. I. Induction of Foxp3-positive regulatory T cells by Th3 cells derived from TGF-beta T cell-transgenic mice. J Immunol. 2007;178(1):179–185. doi: 10.4049/jimmunol.178.1.179. [DOI] [PubMed] [Google Scholar]
- 21.Muczynski KA, Ekle DM, Coder DM, Anderson SK. Normal human kidney HLA-DR-expressing renal microvascular endothelial cells: characterization, isolation, and regulation of MHC class II expression. J Am Soc Nephrol. 2003;14(5):1336–1348. doi: 10.1097/01.asn.0000061778.08085.9f. [DOI] [PubMed] [Google Scholar]
- 22.Shiao SL, Kirkiles-Smith NC, Shepherd BR, McNiff JM, Carr EJ, Pober JS. Human effector memory CD4+ T cells directly recognize allogeneic endothelial cells in vitro and in vivo. J Immunol. 2007;179(7):4397–4404. doi: 10.4049/jimmunol.179.7.4397. [DOI] [PubMed] [Google Scholar]
- 23.Tonsho MBG, Boskovic S, Nadazdin O, Smith N, Colvin R, Sachs D, Cosimi A, Kawai T, Madsen J. Successful tolerance induction of cardiac allografts in nonhuman primates through donor kidney co-transplantation. Am J Transplant. 2013;13(Supple 5):181. [Google Scholar]


