Abstract
Rodent skeletal muscle has high levels of nitrate ions and this endogenous nitrate reservoir can supply nitrite / nitric oxide (NO) for functional hyperemia and/or for other physiological processes in muscle during exercise. Mice with a NOS1 knockout have markedly reduced muscle nitrate levels, suggesting NO production by NOS and its reaction with oxymyoglobin as a source of nitrate. However, oxygen levels are normally low in most internal organs, which raises the possibility that nitrate-derived NO pathway is physiologically important even at “normoxia”, and muscle nitrate reservoir is the main endogenous NO backup when exogeneous (dietary) nitrate intake is low. Using dietary nitrate manipulations, we explore the importance of diet for maintaining and renewal of muscle nitrate reservoir and its levels in other tissues. We found that skeletal muscle nitrate is extensively used when nitrate in diet is low. One week of nitrate starvation leads to dramatic nitrate depletion in skeletal muscle and a substantial decrease in liver. Nitrate depleted from skeletal muscle during starvation is quickly recovered from new dietary sources, with an unexpected significant “overload” compared with animals not subjected to nitrate starvation. Our results suggest the importance of dietary nitrate for nitrate reserves in muscle and in other tissues, when compared with endogenous NOS-derived sources. This requires an active transport mechanism for sequestering nitrate into cells, stimulated by lack of dietary nitrate or other enzymatic changes. These results confirm the hypothesis that muscle is a major storage site for nitrate in mammals.
Keywords: Nitrate, nitrite, diet, skeletal muscle, liver
1. Introduction
Nitric oxide (NO) is important for a large array of basic physiological functions, including the determination of vascular tone, affecting platelet activation, being a volume neurotransmitter and maintaining some of the body immunological functions. NO is a reactive molecule with a half-life in the blood in the order of milliseconds. NO is now known to be the active product of both the L-arginine-citruline nitric oxide synthase metabolic pathway (oxygen-dependent) and the reductive nitrate-nitrite-NO cycle (oxygen-independent). The nitric oxide synthase (NOS) enzymes are found in endothelium (NOS3 or eNOS); brain, skeletal muscle and other tissues (NOS1 or nNOS); or are induced upon inflammation (NOS2 or iNOS). NO produced by the NOS enzymes is always used directly at the production site with the excess oxidized to nitrate by oxy-heme proteins (oxyhemoglobin, oxymyoglobin or other still unknown heme proteins). At physiological normoxic conditions, nitrate is a relatively inert ion and can be considered as an effective storage molecule for NO production. However, enterobacteria can reduce nitrate to nitrite at low oxygen conditions and nitrate can be slowly reduced by Mo-containing proteins such as xanthine oxidoreductase (XOR) and aldehyde oxidase (AO) back to nitrite and then either further reduced by XOR or AO or deoxy-heme proteins (mainly deoxyhemoglobin (deoxyHb) or deoxymyoglobin (deoxyMb)) to NO [1–7].
Previously, we have shown that nitrate is highly concentrated in skeletal muscle, with a concentration gradient from muscle to blood to internal organs, while nitrite is more homogeneously distributed among internal organs, blood and skeletal muscle in Wistar rats [8]. We proposed that nitrate in skeletal muscle at least in part originates from oxidation of NO produced by NOS1 by oxymyoglobin and that this nitrate is likely one of the the main sources of endogenous nitrate in the rat. We also showed that skeletal muscle nitrate is accessible and used for generating nitrite and likely NO during vigorous exercise [9].
Here we present data in rats of nitrate and nitrite organ levels after nitrate deprivation and following nitrate intake reintroduction. Following dietary nitrate deprivation, endogenous nitrate in skeletal muscle decreases greatly, possibly serving as nitrite and NO source. In contrast, when an excess of dietary nitrate is consumed, skeletal muscle nitrate levels are increased above levels found in animals consuming standard diet and nitrate values in the liver are much higher than previously measured. Surprisingly, when excess nitrate is reintroduced after a period of nitrate starvation, nitrate uptake into skeletal muscle exceeds greatly the value observed after simple elevation of dietary nitrate.
We believe that this high nitrate “overload” suggests that nitrate is an essential ion and that the array of metabolic pathways in which the nitrate/nitrite/NO pathway is involved is much wider than previously suspected. Our results also raise the possibility of influence of enzyme reduction and/or active transport mechanisms in organs, especially muscle during dietary nitrate alterations. In summary, our findings suggest that high nitrate diet is very important for achieving proper physiological levels of nitric oxide, regardless of physical activity and/or oxygen availability. Based on the results we can also hypothesize that the question about what is the optimal healty level of nitrate and nitrite in mammalian or human body is still unanswered. Based on the body of available literature, it is certain that even small changes in nitrite and nitrate content in the diet do have large physiological effects and health consequences. Our findings about nitrate and nitrite fluxes in rats, already confirmed in humans [10], may lead to understanding of factors that determine the availability of the nitrate/nitrite/NO reductase pathways in human physiology and lead to simple and effective preventive and supportive therapies for an array of cardiovascular as well as muscular diseases.
2. Materials and methods
2.1. Dietary interventions
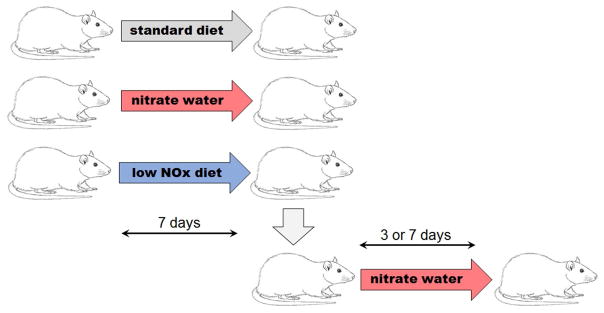
All experiments were done under NIDDK animal protocol K049-MMB-15 and in agreement with NIH animal care policies. Young Wistar rats (n=15, 250±50g) were divided into 3 groups (n=5 in each group) according to diet type. Control group rats were fed NIH standard rat diet (NIH07, nitrate 340.3±13.5 nmol/g, nitrite 7.4±0.1nmol/g); high nitrate diet groups consumed water containing 1g/l of sodium nitrate (Sigma, St. Louis, MO) and nitrate deprived groups were fed a low nitrate/nitrite diet (TD99366, Harlan Teklad, South Easton, MA, nitrate 23.4±0.7nmol/g, nitrite 5.3±0.7nmol/g). Low and high nitrate diets were maintained for 7 days. A separate group of rats was first given low NOx diet for 7 days, after which they were switched to high nitrate diet for another 3 or 7 days to determine if we could “rescue” nitrate levels after depletion. Figure 1 shows a flow chart of those experiments and nitrate and nitrite doses are in Table 1.
Figure 1.
Experimental setup. Rats were fed either standard diet (control rats) or had nitrate added into drinking water (high NOx diet) or fed low NOx diet for 7 days. After 7 days on low NOx diet, group of animals was switched to high NOx diet for 3 or 7 days.
Table 1.
Corresponding doses in mg/kg/day and μmol/kg/day used in experimental setup described in Figure 1. Calculations are done for 250g rat drinking 15ml of water and eating 25g of food per day. These doses are comparable to those reported in several human studies [17].
| DIET | NITRATE | NITRITE | ||
|---|---|---|---|---|
| (mg/kg/day) | (umol/kg/day) | (mg/kg/day) | (umol/kg/day) | |
| low NOx | 0.2 | 2.3 | 0.04 | 0.5 |
| standard | 2.9 | 34.1 | 0.05 | 0.7 |
| high nitrate | 60.2 | 708.3 | 0 | 0 |
2.2. Sample collection
Rats were enclosed in an anesthesia box and anesthetized using 5% isoflurane mixture with air. Anesthetized animals were placed on a pad in supine position and anesthesia continued through a nose cone. The thoracic cavity was opened and ~9–10 ml of blood collected by cardiac puncture; representing about two-thirds of total blood volume for a rat of this size. Heparin was used as an anticoagulant in nitrite and nitrate determinations. Immediately, blood was mixed with a solution containing potassium ferricyanide, NEM and detergent in final ratio 2:1 as described in [11] to conserve nitrite from oxidation by hemoglobin. Animals were then perfused using heparin-containing saline to flush the remaining blood out of the tissues. Perfusion continued until no blood was detected in outgoing saline and liver and kidneys were significantly discolored. Samples from liver and skeletal muscle from the hind legs were then collected and placed into 250μl of nitrite preserving solution containing potassium ferricyanide for chemiluminescence analysis. All samples were stored at −80°C until analysis. Animals were housed in a 12h light/dark cycle environment with access to food and drinking water ad libitum.
2.3. Determination of nitrite and nitrate
Nitrite and nitrate levels in whole blood were measured using a standard chemiluminescence method [11, 12]. For nitrite analysis, immediately after collection, fresh whole blood was mixed with nitrite preserving solution (K3Fe(CN)6, N-ethylmaleimide, water, Nonidet P-40) to maintain nitrite levels and kept frozen at −80°C until analysis. Samples were deproteinized by dilution with the same amount of cold methanol, then centrifuged for 5 min at 13,000 rpm and 4°C (AccuSpinMicroR, Fisher Scientific, Pittsburgh, PA), the supernatant was collected and immediately injected into the chemiluminescent nitric oxide analyzer (NOA, Sievers, Model 280 NO analyzer, Boulder, CO) using helium as the carrier gas for determination of NO. For nitrate analysis, samples were processed the same way as for nitrite determination and analyzed with NOA using the vanadium(III) chloride (VCl3) chemiluminescence assay.
Tissue samples were weighed, mixed with additional nitrite preserving solution and homogenized using GentleMacs (Miltenyi Biotec Inc, Auburn, CA). Proteins were precipitated using methanol (dilution 1:3 sample:methanol) and samples were centrifuged at 3,000 g for 45 min to separate most of the protein. Supernatants were collected and used to determine nitrite and nitrate content by chemiluminescence as described above for blood samples.
3. Results
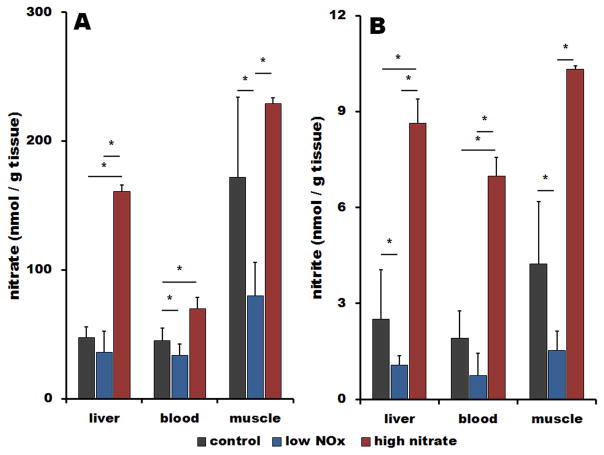
Figure 2 summarizes nitrate (A) and nitrite (B) concentrations measured in liver, blood and muscle in rats on three different diets – standard diet (black bars), high nitrate water (red bars) and low nitrate/nitrite diet (blue bars).
Figure 2.
Effect of diet on nitrate (A) and nitrite (B) levels in liver, blood and skeletal muscle of Wistar rats on standard diet (black bars), on low NOx diet for 7 days (blue bars) and on standard diet supplemented for 7 days by 1g/l of nitrate in drinking water (red bars), n=4 in each group, p<0. 05.
On standard diet, a large nitrate concentration gradient from muscle to blood is observed as we have reported previously [8]. We believe that high levels of nitrate in skeletal muscle are due to its synthesis in the muscle and perhaps also active transport from the blood. Nitrite is distributed almost homogeneously among organs, as previously reported in [8].
Consuming high nitrate water for 7 days causes increases in nitrate levels in muscle (1.3-fold increase), and largely increases in liver and blood, 3-fold and 1.5-fold, respectively, when compared to standard diet. A concentration gradient remains for nitrate in organs compared to blood levels. When rats consume high nitrate water, nitrite concentrations in muscle, liver and blood increase 3.5-, 3.7 and 2.5-fold, respectively.
When dietary nitrate/nitrite is restricted for one week, there is a significant depletion of nitrate from skeletal muscle and blood (0.4 and 0.7-fold of original values) while retaining liver nitrate concentration at 0.8-fold level of that at normal diet. Restriction of dietary nitrate leads to significant nitrite depletion in all tissues to about two-fifths of values on the standard diet (liver 0.42, blood 0.39 and muscle 0.36-fold of the original values).
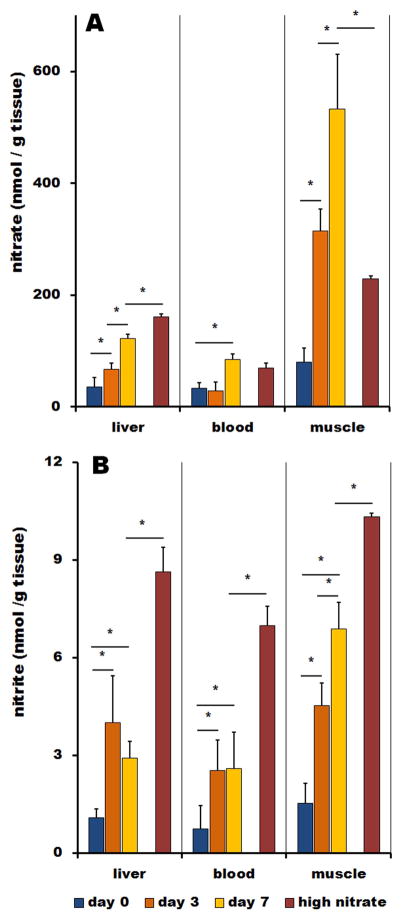
Figure 3 shows the time dependence of organ nitrate (A) and nitrite (B) reloading after reintroduction of high nitrate diet to nitrate-starved animals for 0, 3 and 7 days. Day 0 is the last day of a week-long nitrate starvation and these values are identical to corresponding values shown in Figure 1. For better comparison, Figure 3 also contains values of nitrate and nitrite measured in animals fed high nitrate water without the one-week period of nitrate starvation.
Figure 3.
Increase of nitrate (A) and nitrite (B) in liver, blood and skeletal muscle of Wistar rats on low NOx diet supplemented by 1g/l of nitrate in drinking water for 0 days (blue bars), 3 days (orange bars) and 7 days (brown bars). Zero days (blue bars) denotes rats that followed one-week low NOx diet these values are taken from the Figure 2. Values for rats at high NOx diet (red bars) are for rats that were drinking 1g/l of nitrate in water for 7 days without any prior dietary restrictions. These values are taken from the Figure 2.
When nitrate is added back to the diet after deprivation, there is a significant gradual increase of nitrate concentration through all studied organs, as expected. In liver, nitrate increases gradually over 7 days of treatment, reaching 2.6-times values of animals on standard diet, while nitrite is restored only to its original before-treatment levels. Nitrate in blood increased to 2-fold and nitrite to 1.4-fold of the levels on standard diet. Effect of nitrate reintroduction is strongest in muscle tissue. Nitrate level in skeletal muscle is increased 3-fold when compared to animals on standard diet and nitrite values rise up to 1.7-fold values of animals fed standard diet.
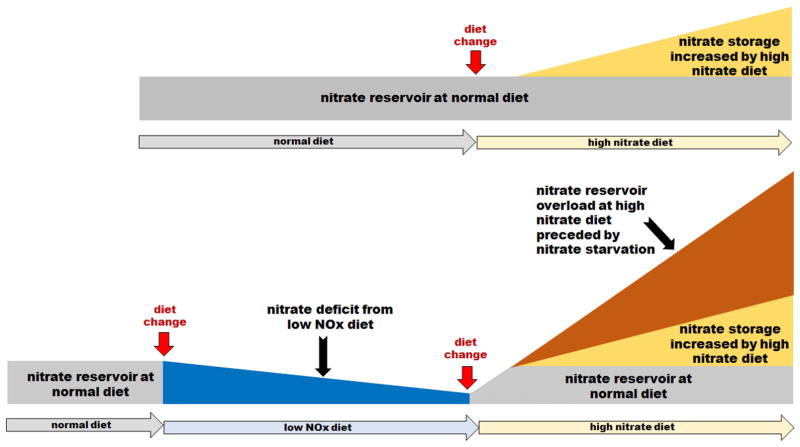
Figure 4 represents data from Figure 2 and 3 and other unpublished results schematically.
Figure 4.
Schematic representation of our experimental results on the effect of diets with different nitrate content on the nitrate levels in skeletal muscle. The upper scheme shows increase nitrate content when rats are switched from normal diet (gray area) to high nitrate diet (yellow area). The lower scheme shows nitrate depletion from skeletal muscle (blue area) after switch from normal diet to low NOx diet and then large increase of nitrate after introducing high nitrate diet. Interestingly, in this case, available nitrate in stored at levels much higher (orange area) than nitrate stored in rats on high nitrate diet without previous nitrate deprivation (yellow area).
4. Discussion
Skeletal muscle is one of the body’s largest organs. Until now, it was not commonly considered as an active member of nitrate/nitrite/NO metabolic pathways. We showed previously that there is a large nitrate pool present in this organ and that this pool supplies nitrate locally, such as during exercise, to create nitrite, and likely also NO. We hypothesized that there are both internal and external sources of the nitrate found in skeletal muscle. We hypothesized that NOS1 and likely NOS3 are the internal sources of nitrate. In the present study we focus on the external, dietary source of nitrate for the skeletal muscle reservoir. We aimed to answer the question wheather diet is an important source of skeletal muscle nitrate and how crucial is this source.
Effect of diet on nitrate and nitrite in liver, blood and skeletal muscle
Figure 2A and 2B show changes in nitrate and nitrite levels in muscle, blood and liver when rats follow three different diets: NIH standard rat chow, a nitrate depleted diet (low NOx diet) and nitrate-supplemented water (high nitrate diet).
When on standard diet, nitrate is distributed nonhomogenously, with the highest concentration found in skeletal muscle and lowest in liver. At the same time, nitrite is distributed almost equally through muscle, blood and liver. These results are similar to those we found previously [8].
Previously, we formulated the hypothesis, and supported it with data from the NOS1 (nNOS) knockout mouse and from exercise studies, that the nitrate reservoir in the skeletal muscle is a local source of NO for exercise-induced hyperemia and related processes [8, 9]. However, data collected from the NOS3 (eNOS) knockout mouse also showed decreased levels of nitrate in skeletal muscle and blood (unpublished data) suggesting that NOS1-derived NO is not the exclusive source of NO contributing to the skeletal muscle nitrate reservoir. Similar observation about decreased levels of nitrite and nitrate in plasma and heart of NOS3 KO mouse had been previously published [13].
In this study, we tested the contribution of dietary nitrate to blood and organ levels in order to see if the nitrate stock from the skeletal muscle is used systemically when there is a lack of dietary nitrate. We deprived rats of nitrate for 7 days and then measured the distribution of nitrite and nitrate ions through their bodies. As seen in Figure 2A (blue bars), there is a significant drop of nitrate in muscle and blood. After a week of nitrate/nitrite deprivation, we found depletion of the skeletal muscle nitrate reservoir, values dropping to about 1/3 of the original value. There were much less accompanying changes from the original values in blood and liver, only about one quarter to one fifth depletion of nitrate in each. The depletion pattern repeats itself for nitrite in Figure 2B – nitrate-deprived animals had levels of nitrite down by a quarter in blood and liver, with only half of original nitrite conserved in the muscle. These results indicate that dietary nitrate deprivation leads either to the use of the endogenous muscle nitrate reservoir or its redistribution. Our data are qualitatively similar to those reported for mice fed low NOx diet [13–15] where lack of dietary nitrate lead to significant depletion of nitrate and nitrite from plasma and heart muscle.
Next, we investigated the storage capacity of nitrate in skeletal muscle. Using high nitrate supplementation, we tried to see if skeletal muscle or liver sequester exogeneous dietary nitrate, once ingested, and to what degree. We fed rats high nitrate water for 7 days and then determined nitrate and nitrite distribution in liver, blood and muscle (see Figure 2A, B, red bars). As expected, nitrate in blood was ~1.5-fold higher when compared to standard diet, as the bloodstream is the primary absorber and distributor of nutrients from diet. Nitrate in liver dramatically increased 3-fold, but we saw only increases in skeletal muscle nitrate of about 1.3-fold of its original value. The same dose of nitrate in drinking water, in mice, led to elevated levels of nitrate and nitrite in the plasma and the heart [14].
These data suggest a modification of our original hypothesis. We previously hypothesized that nitrate stored in muscle originates primarily from oxidation of NOS1-derived NO by oxymyoglobin. In addition, we speculated about the possibility of the contribution of dietary nitrate, but our experimental setup at that time did not allow us to draw further conclusions about the existence and/or the role of active nitrate transport into the muscle or other tissues. In fact, the observation that nitrate levels in organs such as muscle and liver may be much higher than in blood itself, raises the possibility of active transport in various organs. The dramatic 3-fold increase of nitrate levels in liver when a surplus of nitrate is present, is likely due to nitrate sequesteration by liver cells for further use. According to this idea, most organs in the body would be able to store nitrate for later use and the levels of nitrate measured at “normal” conditions are equilibrium values between nitrate transport into the cells and the nitrate reductase activity of the cells of that organ. According to this view, low nitrate reductase activity in skeletal muscle [9] together with the presence of a robust nitrate-generating pathway contributes significantly to the high levels of nitrate observed in skeletal muscle. On the other hand, when excess nitrate is present in the diet, a significant reservoir of nitrate can build up in other organs (mainly the liver) because transport of nitrate into cells exceeds the reduction of nitrate by the somehow more active nitrate reduction pathway in these tissue [3, 7, 12]. This process is also likely to be dependent on other factors, most of which are still unknown.
All three organs reported on in this paper – muscle, blood and liver achieved comparable levels of nitrite, with muscle changing the least (2.5-fold increase), liver showing a 3.4-fold increase and blood changing the most (3.7-fold increase) when compared to standard diet (Figure 2B, red bars). We believe that the significant, almost 4-fold increase of nitrite in the blood and liver is due to increased activity of liver nitrate reductases and subsequent redistribution of this nitrite via bloodstream into other parts of body. The same phenomenon was observed in mice fed high nitrate water for a week, when nitrite in plasma increased only slightly but there was a 6-fold increase of nitrite levels in heart [16].
We also hypothesize that the action of oral bacteria nitrate reductases, the process described for humans by the Lundberg and Weitzberg groups [1] and confirmed by others [2] could contribute significantly to the high levels of blood nitrite observed. We realize, however, the existence of controversies about whether rodents have the oral bacterial systems that can reduce nitrate to nitrite with the same efficiency as in humans [16]. However, it is still possible that dietary nitrate in rats would follow the same metabolic pathway as in humans and that, in case of less efficient salivary bacteria nitrate reductase activity, gut bacteria, for example, could contribute significantly to this pathway in rats.
Reintroduction of nitrate into diet after its deprivation (“nitrate reloading”)
In the section above we showed that the rat body uses endogenous nitrate stored in skeletal muscle during periods of exogeneous (dietary) nitrate deprivation. Next, we investigated the reverse possibility – if exogeneous (dietary) nitrate can restore depleted endogenous nitrate from muscle and if so, how long and efficient would this process be.
Rats were first depleted of nitrate by a week of low NOx diet. Then, nitrate was reintroduced by using high nitrate water and groups of rats were killed at three different timepoints (0, 3 and 7 days after nitrate reintroduction) and their tissues and blood analyzed for nitrate and nitrite. Results shown in Figure 3 confirm that, indeed, dietary nitrate restores nitrate levels in depleted tissues and that the process is quite efficient. Three days of drinking high nitrate water were adequate to restore levels in all tissues to values equal or slightly higher than previously observed values for the control diet and seven days of supplying high nitrate brought its levels in muscle and blood to 2.3 and 1.2-fold above the ones measured for a group of rats on high nitrate water that were not previously starved of nitrate. Similarly, after 3 days of nitrate supplementation, nitrite levels in liver, blood and muscle exceeded the corresponding values in rats on standard diet by 1.2-, 1.4- and1.6-fold, respectively.
These findings also support the idea about the importance of nitrate for physiological processes in the mammalian body, so that in case of deprivation and depleted endogenous reservoir, excess nitrate (compared with the standard situation) is sequestered from the diet and stored for future needs. This situation could be somehow compared to calorie starvation, where food deprivation puts into action an array of mechanisms that encourage the body to hoard excess fat. At this point we do not understand the underlying processes and mechanisms by which this nitrate “overload” is executed. Among the mechanisms that might allow this to occur are active transport processes that could vary upon nitrate depletion or effect of depletion on NOS1 levels in muscle, for example. One of the most important speculations from our current results is that, even when providing diet assumed to be healthy and balanced for sedentary normoxic rats in arginine (NOS substrate), their NOS enzymes might not be delivering enough NO to various functions and they need to supply the rest from the nitrate reduction to nitrite and to NO, as follows up from the marked decrease of nitrate and nitrite stored in skeletal muscle when the exogeneous (dietary) nitrate supply is cut. As of now, is it unclear why normal NOS activity would be not sufficient to supply all NO at physiological normoxic conditions and why NO needs to be further supplied from dietary sources in healthy rats which are not subject to any hypoxia-related conditions. This subject is of crucial importance because it shows that NO generation is a multi-source complex process, not a straight line from arginine to NO (at normoxia) or nitrate to NO (at hypoxia). Our data suggest that both systems are complementary and active all the time, regardless of oxygen tension, and that hypoxic stress is not required to activate the nitrate reduction pathway, as has been largely presumed until now. Also, as follows from interesting studies using mice, dietary nitrite and nitrate likely follow the same metabolic/transport pathways. In their studies, the authors used low NOx diet to deplete nitrite and nitrate stores in plasma and heart and then supplemented nitrite in drinking water. After a week of supplementation, nitrite “overshoot” was observed in plasma and heart [14,15]. Importantly, this effect was dose-dependent, with the two different nitrite doses used [15].
A second conclusion, of equal importance, is that nitrate starvation and depletion of stored nitrate leads to dramatic nitrate sequestration from available exogenous (dietary) sources. This nitrate “overshoot” happens quickly, endogenous reservoirs of nitrate are filled after only three days when dietary nitrate becomes available again. It is worth noting here that muscle is not the only organ capable to sequester and store nitrate, levels in liver dramatically increased after excess of nitrate was present in diet, regardless of previous starvation or not. Levels of nitrate in skeletal muscle, on the other hand, are very sensitive to prior nitrate deprivation. When excess nitrate is introduced in the diet, nitrate levels in muscle do not increase that dramatically. Nitrate starvation, on the other hand, causes drastic increases in the amount of nitrate stored in the muscle when nitrate is reintroduced. Our summary of nitrate reservoir levels and direction of nitrate fluxes in and out of the skeletal muscle reservoir for diets with different nitrate levels is shown in Figure 4. A tentative model summarizing the findings and other information about the nitrate and nitrite fluxes among different organs (compartments) at different dietary nitrate accessibility is shown in Figure 5.
Figure 5.
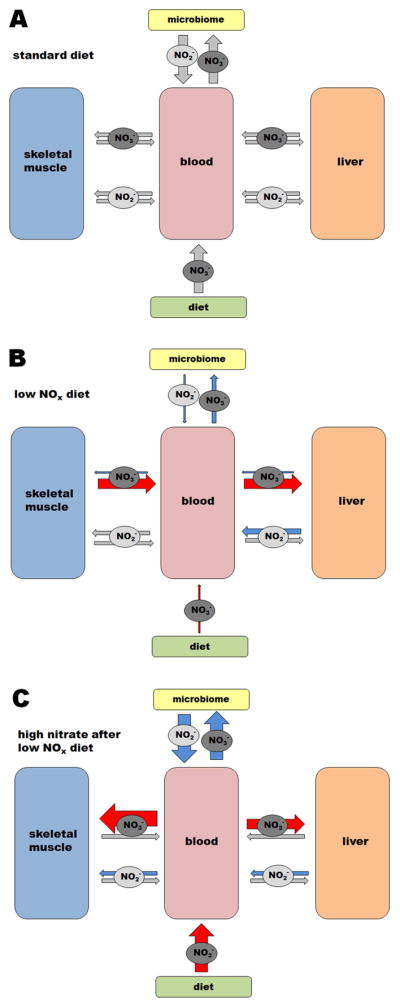
A tentative model of some of the organs nitrate-nitrite fluxes in the rat body with variation in nitrate diet levels: NIH standard diet (A), restricted nitrate/nitrite diet (B) and increased nitrate diet after one week of nitrate deprivation (C).
Panel A shows a simplified hypothesis for nitrate/nitrite fluxes among blood, muscle and liver when a standard diet is consumed. This model is based on our earlier, more detailed model proposed in [9]. Nitrate synthesized in skeletal muscle (and possibly, to a lesser extent, also in other organs) from oxidation of nitric oxide constitutes the “endogeneous” nitrate reservoir; diet is the source of “exogeneous” nitrate. Both, exogeneous and endogeneous nitrate, circulating in blood are reduced by the microbiome (especially salivary bacteria, but possibly also gut bacteria) to nitrite, which can be absorbed. A portion of circulating nitrate, both of dietary and endogeneous origin, is taken up by liver where it is further processed into nitrite by mammalian nitrate reductases, including xanthine oxidoreductase and aldehyde oxidase. This nitrite is then taken up by bloodstream and distributed into internal organs, as needed. In this hypothesis we presume the existence of bidirectional flow of nitrate and nitrite in and out of the internal organs and skeletal muscle.
Panel B shows how nitrate and nitrite fluxes change during dietary nitrate starvation. When dietary nitrate is restricted (as represented by thin red arrow), the skeletal muscle nitrate reservoir supplies the majority of nitrate needed for its conversion into nitrite by liver, as represented by the red arrows. Magnitude of these nitrate flows (out of the muscle and into the liver) exceeds the normal situation, as liver has to take up more nitrate to compensate for increased nitrate-to-nitrite conversion. Note that levels of nitrate in liver do not significantly change during the nitrate starvation (Figure 2A, blue bar). The microbiome pathway is likely to decrease due to restricted nitrate availability from the diet. We presume that the “backflow” of nitrate into muscle and liver is substantially diminished under these circumstances, we do not know about the extent of nitrite flows in and out of muscle.
Panel C shows the situation after high dietary nitrate is introduced to the diet after nitrate starvation. Unexpectedly, when high levels of nitrate become available after dietary nitrate deprivation for one week, the ability of skeletal muscle and internal organs to take up nitrate dramatically increases, as schematically shown by thick red arrows (these results are also shown for skeletal muscle in Figure 4). We also assume that the higher availability of dietary nitrate will translate into higher nitrite levels from microbiome activity (shown by blue arrows). Nitrite fluxes are also likely increased in the direction from the liver into muscle, as supported by data in Figure 3B.
The size of the grey arrows in the three panels are not representative of the magnitude of the fluxes, as this information is not available. The thickness of the arrows in panels B and C is proportional to changes in flows compared to standard diet, based on our experimental conditions and data in this paper (red arrows), hypothesized (blue arrows) or unknown (gray arrows, the same size as in panel A)
It should also be noted that when we refer to as a rat standard diet, our current state of knowledge about “normal” levels of nitrate does not allow us to tell with certainity that what we refer here as “standard” should be considered “normal” or ‘optimal” for rodents or other species.
The present results support our initial hypothesis that skeletal muscle nitrate is the largest body reservoir of nitrate and that this nitrate is also available for systemic use, should such a need arise. However, our data also show that skeletal muscle is not the exclusive nitrate storage organ. At times of increased nitrate supply, liver can sequester and store nitrate, too. It can be presumed that the steady state nitrate levels one measures in any organ are a balance between nitrate transport into the organ, endogenous nitrate synthesis within the organ (by oxidizing NO produced by NOS enzymes), if applicable, and nitrate reduction by nitrate reductases within that organ. However, three facts make skeletal muscle the prime emergency nitrate reservoir: 1. It does not rely exclusively on nitrate transport into its cells, because nitrate is also created onsite by oxidation of NOS1-derived NO by oxymyoglobin, 2. Nitrate reductase activity in skeletal muscle tissue is low, so only small amount of stored nitrate is locally used, and 3. Overall, muscle is one of the largest organs which leads to accumulation of a large quantity of nitrate to be used in times of need.
5. Conclusions
Our results strongly suggest that nitrate is necessary for proper physiological functioning and that in times of dietary starvation, skeletal muscle uses its nitrate reservoir to support physiology. This is likely even more important under the stress of hypoxic conditions. It also shows for the first time that nitrate deprivation will activate an array of pathways and mechanisms to aggressively take up all nitrate available, and to even “overload”, when it becomes available again. These facts strongly suggest that nitrate ions are important in physiology, likely much more than realized so far.
To translate these results into human physiology and further into development of therapeutic strategies, it is necessary to know human nitrate and nitrite concentrations and distribution and if current Western diets are “nitrate friendly” or lead to nitrate deficiency. These results suggest that more attention should be paid to determination and maintenance of optimal levels of dietary nitrate in human health, specifically endogenous levels in organs and total body nitrate levels, which are very sensitive to even short-term dietary changes. As shown previously, even small changes in nitrite levels in plasma and heart lead to important health benefits such as partial protection from myocardial infarction [14] and this infarction-protection effect was demonstrated at levels of nitrite well below those needed for more robust systemic changes such as blood pressure decrease [13].
Highlights.
Skeletal muscle nitrate reservoir is depleted by low nitrate diet.
Nitrate reintroduction leads to muscle nitrate reservoir recovery and overload.
Organ nitrate levels result from balance between local nitrate reduction and influx.
Main question: what should be considered “normal” organ nitrate levels?
Still unknown: What should be optimal dietary level of nitrate?
Acknowledgments
Authors would like to thank Dr Mark StClair for his advices and help with animal protocol and work.
Footnotes
Disclosure
Dr. Alan Schechter is listed as a co-inventor on several patents issued to the National Institutes of Health for the use of nitrite salts for the treatment of cardiovascular diseases. He receives royalties based on NIH licensing of these patents for clinical development but no other compensation. This does not alter the authors’ adherence to all the Nitric Oxide policies on sharing data and materials, as detailed online in the guide for authors. All other authors have no conflict of interest to declare.
Contributions
BP, JWP and ANS designed the experiments. ANS and BP wrote the manuscript. CNG, KSC, JKL and BP performed the research and analyzed the data. All authors contributed to data interpretation and commented on manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Govoni M, Jansson EA, Weitzberg E, Lundbetg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide. 2008;19:333–337. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Qu XM, Wu ZF, Pang BX, Jin LY, Qin LZ, Wang SL. From nitrate to nitric oxide: The role of salivary glands and oral bacteria. J Dental Research. 2016;95:1452–1456. doi: 10.1177/0022034516673019. [DOI] [PubMed] [Google Scholar]
- 3.Jansson EA, Huang L, Malkey R, Govoni M, Nihlen C, Olsson A, Stensdotter M, Petersson J, Holm L, Weitzberg E, Lundberg JO. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat Chem Biol. 2008;4:411–417. doi: 10.1038/nchembio.92. [DOI] [PubMed] [Google Scholar]
- 4.Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 5.van Faassen EE, Bahrami S, Feelisch M, Hogg N, Kelm M, Kim-Shapiro DB, Kozlov AV, Li H, Lundberg JO, Mason R, Nohl H, Rassaf T, Samouilov A, Slama-Schwok A, Shiva S, Vanin AF, Weitzberg E, Zweier J, Gladwin MT. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med Res Rev. 2009;29:683–741. doi: 10.1002/med.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, 3rd, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Cui H, Kundu TK, Alzawahra W, Zweier JL. Nitric oxide production from nitrite occurs primarily in tissues not in the blood: critical role of xanthine oxidase and aldehyde oxidase. J Biol Chem. 2008;283:17855–17863. doi: 10.1074/jbc.M801785200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piknova B, Park JW, Swanson KM, Dey S, Noguchi CT, Schechter AN. Skeletal muscle as an endogenous nitrate reservoir. Nitric Oxide. 2015;47:10–16. doi: 10.1016/j.niox.2015.02.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piknova B, Park JW, Lam JK, Schechter AN. Nitrate as a source of nitrite and nitric oxide during exercise hyperemia in rat skeletal muscle. Nitric Oxide. 2016;55–56:54–61. doi: 10.1016/j.niox.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nyakayiru J, Kouw IWK, Cermak NM, Senden JM, van Loon LJC, Verdijk LB. Sodium nitrate ingestion increases skeletal muscle nitrate content in humans. J Appl Physiol (1985) 2017;123:637–644. doi: 10.1152/japplphysiol.01036.2016. [DOI] [PubMed] [Google Scholar]
- 11.Piknova B, Schechter AN. Measurement of nitrite in blood samples using the ferricyanide-based hemoglobin oxidation assay. Methods Mol Biol. 2011;704:39e56. doi: 10.1007/978-1-61737-964-2_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinder AG, Rogers SC, Khalatbari A, Ingram TE, James PE. The measurement of nitric oxide and its metabolites in biological samples by ozone-based chemiluminescence. Methods Mol Biol. 2008;476:11e28. doi: 10.1007/978-1-59745-129-1_2. [DOI] [PubMed] [Google Scholar]
- 13.Bryan NS, Calvert JW, Gundewar S, Lefer DJ. Dietary nitrite restores NO homeostasis and is cardioprotective in endothelial nitric oxide-deficient mice. Free Radical Biol Med. 2008;45:468–474. doi: 10.1016/j.freeradbiomed.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. PNAS. 2007;104:19144–19149. doi: 10.1073/pnas.0706579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raat NJH, Noguchi AC, Liu VB, Raghavachari N, Liu D, Xu X, Shiva S, Munson P, Gladwin MT. Dietary nitrate and nitrite modulate blood and organ nitrite and the cellular ischemic stress response. Free Radical Biol Med. 2009;47:510–517. doi: 10.1016/j.freeradbiomed.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montenegro MF, Sundqvist ML, Nihlen C, Hezel M, Carlstrom M, Weitzberg E, Lundberg JO. Profound differences between humans and rodents in the ability to concentrate salivary nitrate: Implications for translational research. Redox Biol. 2016;10:206–210. doi: 10.1016/j.redox.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lara J, Ashor AW, Oggioni C, Ahluwalia A, Mathers JC, Siervo M. Effects of inorganic nitrate and beetroot supplementation on endothelial function: a systematic review and meta-analysis. Eur J Nutr. 2016;55:451–459. doi: 10.1007/s00394-015-0872-7. [DOI] [PubMed] [Google Scholar]