Abstract
CD4+Foxp3+ regulatory T cells (Treg cells) are essential regulators of immune responses. Perturbation of Treg cell homeostasis or function can lead to uncontrolled inflammation and autoimmunity. Therefore, understanding the molecular mechanisms involved in Treg cell biology remains an active area of investigation. It has been shown previously that the NF-κB family of transcription factors, in particular, the canonical pathway subunits, c-Rel and p65, are crucial for the development, maintenance and function of Tregs. However, the role of the alternative NF-κB pathway components, p100 and RelB, in Treg biology remains unclear. Here we show that conditional deletion of the p100 gene, nfkb2, in all T cells, or only in Tregs, resulted in massive inflammation due to impaired suppressive function of nfkb2-deficient Tregs. Surprisingly, mice lacking RelB in Treg cells did not exhibit the same phenotype. Instead, deletion of both relb and nfkb2 rescued the inflammatory phenotype demonstrating an essential role for p100 as an inhibitor of RelB in Tregs. Our data therefore illustrates a new role for the alternative NF-κB signaling pathway in Tregs that has implications for the understanding of molecular pathways driving tolerance and immunity.
Introduction
Maintenance of immune homeostasis and tolerance is achieved through multiple feedback mechanisms. CD4+Foxp3+ regulatory T cells (Treg cells) represent 5–15% of the CD4+ T cell population and develop both in the thymus (nTreg) and the periphery (pTreg). They play a pivotal role in the control of innate and adaptive immune responses (1) and are generally characterized by the constitutive expression of CD25 and the forkhead-box transcription factor Foxp3, which is required for their suppressive activity (2). Perturbations in the homeostasis and/or function of these cells are associated with the development of autoimmune diseases such as Type 1 Diabetes and Rheumatoid Arthritis (3). Depletion of Treg in mice leads to a lethal multifocal inflammation; moreover, mutations in the foxp3 locus are responsible for the Scurfy phenotype in mice and the IPEX (Immunodysregulation, Polyendocrinopathy, and Enteropathy, X-linked) syndrome in humans (4). Thus, Treg cell development, maintenance and function must be tightly regulated through various molecular mechanisms. In recent years, several studies have demonstrated a crucial role for the NF-κB transcription factor in the development of Treg cells and the expression of Foxp3 (5–7).
NF-κB signaling can be separated in two main pathways. The canonical pathway leads to the phosphorylation and degradation of IκBα/β, and consequent nuclear translocation of the NF-κB1 p50 protein bound to either c-Rel or p65; whereas the alternative pathway leads to activation of NF-κB-inducing kinase (NIK) which promotes processing of NF-κB2 from the full-length precursor protein p100 to the p52 form, which results in formation of transcriptionally active p52:RelB complexes (8, 9). The biological processes regulated by these two NF-κB pathways are distinct. The canonical p65 and c-Rel subunits are well-defined inducers of inflammation and have clear roles in the activation of B and T cells, whereas the alternative NF-κB RelB:p52 heterodimer has primarily been implicated in lymphoid tissue organogenesis and cell migration (9, 10). NF-κB2 and RelB are important regulators of immune tolerance, as NIK−/−, relb−/− and nfkb2−/− mice develop spontaneous autoimmunity (reviewed in (11)). Importantly, the two NF-κB pathways are triggered by different receptors and signaling pathways. Upon engagement of the TCR/CD28 antigen receptor, the canonical pathway is activated and NF-κB complexes containing c-Rel can regulate de novo expression of Foxp3 in developing thymocytes, and therefore c-Rel-deficient animals exhibit fewer nTregs (5–7, 12–15). Recently, we and others have demonstrated a central role for the NF-κB p65 subunit in the maintenance of mature Treg identity and in the prevention of autoimmunity (16, 17). On the other hand, c-Rel, but not p65, is required for the homeostasis of Treg cells during anti-tumor responses (18). These observations highlight canonical NF-κB signaling as a master regulator of Treg development and function, and demonstrate the discrete functions of individual NF-κB subunits in Treg-dependent immune tolerance.
The alternative NF-κB signaling pathway, by contrast, is largely inactive during normal T-cell homeostasis. In lymphocytes NF-κB2 resides predominantly in the cytoplasm in its unprocessed p100 form, where it is believed to function as an inhibitor of other NF-κB proteins (9). However, activation of a subset of TNF receptor family members expressed in lymphocytes, including OX-40, CD40 or LT-βR, leads to activation of the alternative pathway. Specifically, these receptors direct the recruitment and inhibition of the TRAF2/3/cIAP1/2 ubiquitin ligase complex, which allows the stabilization and activation of NIK. The alternative pathway also appears to be indirectly involved in Treg cell development. For example, NIK−/− mice and NIK mutant aly/aly mice, have reduced numbers of Treg cells (19, 20). The same observation was made more recently in mice when IKKα was conditionally deleted in T cells (21). Finally, constitutive expression of NIK in T cells induced the peripheral expansion of poorly functional Treg cells (22). However, enforced expression of NIK can activate both alternative and canonical pathways (23) and therefore the specific role for the alternative NF-κB pathway remained unclear. Likewise, germline deletions of RelB or NF-κB2 were associated with developmental and autoimmune defects associated with changes with lymphoid organogenesis, obscuring analysis of their functional role in Treg cells (9). To overcome these difficulties, we have now used conditional deletion of nfkb2 and/or relb in both total T cells, and Treg cells, and demonstrated a critical function for NF-κB2 in maintaining Treg homeostasis. Surprisingly, rather than functioning as a crucial component of alternative pathway-dependent transcriptional complexes, we found that the key function of p100 in Tregs was as a negative regulator of p52-independent, RelB-containing complexes. Hence, our studies suggest that p100 and RelB have critical and unexpected functions in Treg cell homeostasis and suppressive function.
Material and Methods
Animals and cell lines
Nfkb2-floxed and relb-floxed mice were generated by U. Klein (Columbia University, New York) (24). CD4cre (Tg(CD4-cre)1Cw1), Foxp3CRE-YFP (Foxp3tm4(YFP/cre)Ayr), CD45.1 (Ptprca Pepcb/BoyJ) and RAG1−/−, all on a C57Bl/6J background, were originally purchased by the Jackson Laboratory and maintained in our animal facility. C57Bl/6J mice were purchased from the Jackson Laboratory. All mice were kept in specific pathogen-free conditions in the animal care facility at Columbia University (New York, NY). All mouse experiments were approved by Institutional Animal Care and Use Committee of Columbia University.
Flow cytometry
Cells were isolated from thymus, spleen and lymph nodes by mechanical desegregation in PBS+FBS 3%. Colon lamina propria lymphocytes were isolated upon digestion and Percoll for intracellular cytokines analyses, cell suspensions were incubated 3 hours with PMA (Sigma, 50 ng/mL), ionoymycin (Sigma, 1 μg/mL) in the presence of Golgi Plug (BD). Cells were then stained with mAbs purchased by EBioscience or Tonbo Bioscience. Foxp3 and cytokine staining were performed using the eBioscience kit and protocol. Cells were acquired on a LSR II (BD Biosciences) and analyzed with FlowJo (Tree Star) software. Cell-sorting was achieved on a BD ARIA II.
In vitro Treg differentiation
CD4+CD44lowCD25− naïve T cells were FACS-sorted from splenocytes suspensions. 105 T cells were cultured in complete RPMI (Gibco) with 105 T-cell depleted, mitomycin C-treated WT splenocytes and 2.5 μg/mL anti-mCD3 (BioXCell), in the presence of 10 ng/mL mIL-2 (Peprotech) and grading doses of human TGF-β1 (Peprotech), for 4 days at 37°C. Cells were then stained for flow cytometry analysis.
Suppression assays
For in vitro assays, LN-derived CD45.1+ naïve conventional CD4+ T cells were magnetically isolated (Miltenyi) and labelled with CellTrace Violet Proliferation Tracker- (CTV, Life Technologies). They were cultured with T-cell depleted, mitomycin C-treated WT splenocytes and 2.5 μg/mL anti-mCD3, in the presence or not of FACS-sorted WT or nfkb2−/− CD4+YFP+ Treg cells. Proliferation of CD4+CD45.1+ T cells was assessed by FACS at D4. The % of suppression was calculated as described. For in vivo assays, 4.105 naïve CD45.1+ Tconv cells were isolated as above and transferred with or without 1.105 Treg cells, to the retro-orbital sinus of 6–9 week-old RAG1−/− mice. Recipients were then weighed every week and euthanized when weight loss was >30%.
Western Blotting
Total lysates were extracted using RIPA buffer and protease inhibitors with SDS. Cytosolic and nuclear fractions were fractionated using the classical NARA/NARC protocol. 20 μg protein extracts were ran in polyacrylamide gels and transferred onto PVDF membranes. Membranes were incubated with polyclonal anti-NF-κB2 (K-27), RelB (C-19), p65 (C-20), c-Rel (C, sc-71), HDAC-1 (H51) (Santa-Cruz) and monoclonal anti-GAPDH (Fitzgerald, 10R-G109a) Abs, followed by HRP-coupled secondary Abs from Jackson Immunoresearch.
RT-qPCR and RNA-sequencing
Total RNA was extracted using a Qiagen Rneasy Mini Kit with DNase treatment. For qPCR, RNA was reverse transcribed by Superscript III (Invitrogen). cDNAs were used for PCR with SYBR Green reagents (Quanta Biosciences, Gaithersburg, MD) on a C1000 Touch thermal cycler (Bio Rad, Hercules, CA). The data was normalized to GAPDH expression. Primers sequences can be sent under request. For RNA-sequencing, RNA was extracted from Treg cells isolated from 3–4 pooled mice/genotype. Libraries were prepared using an Illumina TruSeq Library Kit and sequenced by an Illumina 2500 instrument. Upon sequencing, raw FASTQ files were aligned on the mm10 genome using STAR aligner with default parameters (25). Aligned fragments were then counted and annotated using Rsamtools v3.2 and the TxDb.Mmusculus.UCSC.mm10.knownGene version 3.1.2 transcript database respectively. Normalized FPKM (fragments per kilobase per million mapped reads) were obtained using the robust FPKM estimate function of DeSeq2 v1.10.1. Differentially expressed genes were obtained using the DESeqResults function of the same package. For gene set enrichment analysis, we acknowledge our use of the GSEA software, and Molecular Signature Database (MSigDB) including the ImmuneSigDB (C7 collection) (26, 27). We also used the Panther Database for further function analysis (28). GEO accession number: GSE108532 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE108532).
Histology
Colons were washed and fixed in 4% formalin acetate. 5μM sections were then prepared from the middle section of the colon; 3–4 sections, each separated by 300μM, were prepared for each sample. Tissues were then stained with H&E. Quantification of abscesses was performed in a double-blind fashioned. The mean number of abscesses among the sections is shown.
Statistical analyses
Experimental groups were compared statistically using the nonparametric Mann-Whitney test, or the unpaired, two-tailed Student’s t-test.
Results
Nfkb2 restrains the Treg cell pool size
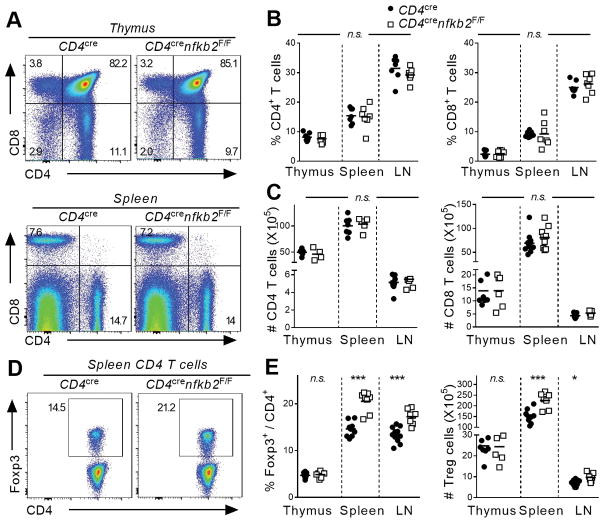
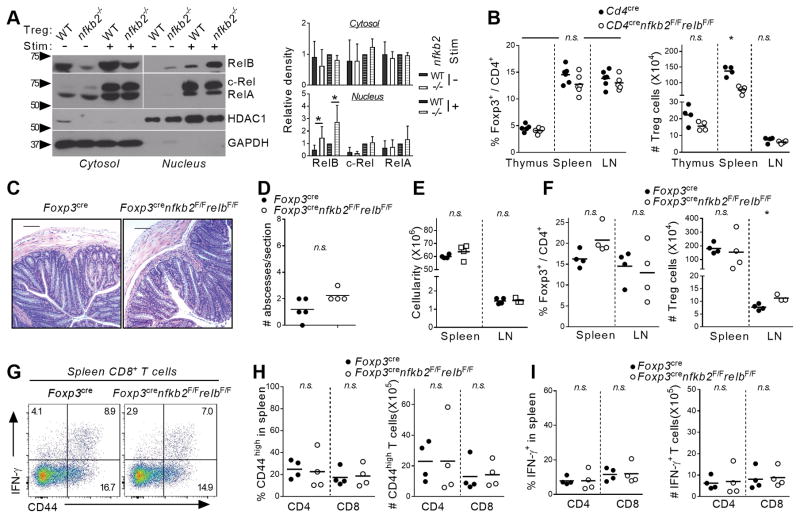
To investigate the T-cell-intrinsic role of NF-κB2 p100/p52 in the differentiation and function of CD4+ T-cell subsets, we first ablated nfkb2 in total T cells using the CD4cre deleter strain. This led to the efficient depletion of p100 in splenic CD4+ T cells. As expected, p100/p52 expression was not observed at steady-state in WT cells (Figure S1A and data not shown). Deletion of nfkb2 was not associated with significant changes in the proportion and numbers of CD4 and CD8 T cells in both primary and secondary lymphoid organs (Figure 1A–C). Next, we examined the differentiation of Foxp3+ regulatory T cells. The proportion and number of immediate Foxp3− Treg precursors and mature FoxP3+ Treg cells were similar in the thymus of WT and KO animals (Figure 1E and Figure S1B). However, we observed a significant increase in the percentage of Treg cells in the spleen and peripheral lymph nodes (pLNs) upon nfkb2 deletion (Figure 1D, E). This increase in the fraction of Tregs corresponded to an increase in the absolute number of Treg cells in these tissues (Figure 1E), consistent with there being an expansion of Treg cell numbers rather than a decrease in the Tconv populations. Moreover, the activation state of nfkb2-deficient Treg cells was altered, as demonstrated by significantly increased expression of CD44 and Ki67 relative to WT Treg cells (Figure S1C). Interestingly, we also observed a trend toward decreased expression of Foxp3 in nfkb2−/− Treg cells (Figure S1D). Thus, ablation of nfkb2 in T cells led to a selective expansion of the peripheral Treg cell population without apparent effects on the thymic differentiation of Treg cells.
Figure 1. T-cell specific ablation of nfkb2 drives peripheral Treg cell expansion.
Thymus, spleen and peripheral lymph nodes of 6–8 weeks-old CD4cre and CD4crenfkb2F/F mice were stained for FACS. (A) Dot plot showing CD4 and CD8 expression in gated total thymus (top) and spleen (bottom) live cells. Numbers indicate the % in each quadrant. (B, C) Proportion (B) and absolute numbers (C) of live TCR-β+CD4+ and CD8+ in the indicated tissues. (D) Expression of Foxp3 in gated spleen TCR-β+CD4+ live cells. Numbers indicate the percentage in gate. (E) % in TCR-β+CD4+ and absolute numbers of Treg cells. Data is representative (A, D) or the pool (B–C, E) of 3 experiments. In B, C and D, each dot represents a mouse. *p<0.05, ***p<0.001, n.s.: non-significant.
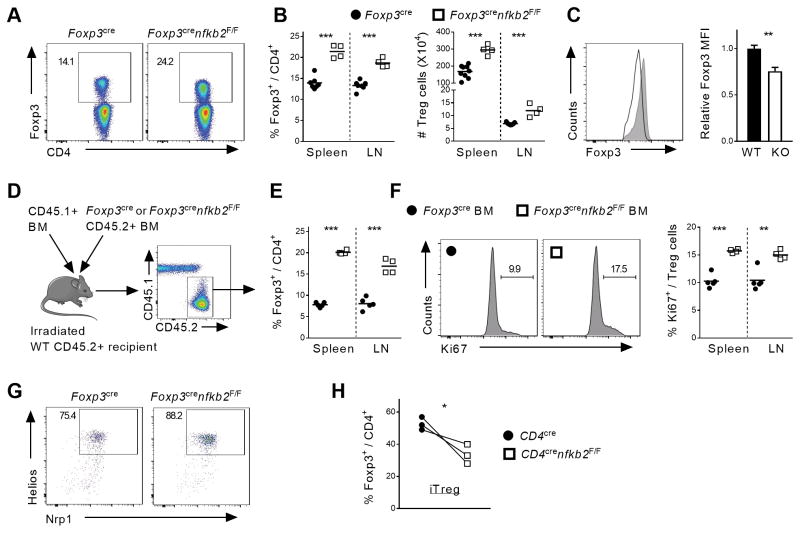
Although we did not observe any changes in CD4 or CD8 T cell phenotype, we wished to confirm that these changes in Treg homeostasis were due to intrinsic changes in p100/p52 function in Tregs. We therefore, selectively deleted NFκB2 in Tregs using the Foxp3CRE deleter mice. In 6 to 8-week-old animals, there were no obvious signs of systemic inflammation or autoimmunity. Consistent with the lack of gross phenotypic changes in these mice, there were no changes in thymic Treg differentiation or in the distribution or effector function of conventional CD4 (Tconv) and CD8 T cells in the mutant mice (data not shown). However, consistent with the results obtained with Cd4CRE mice, the Treg population was significantly expanded in the spleen and LN of Foxp3CREnfkb2F/F animals when compared to littermate controls (Figure 2A, B). Again, nkfb2-deficient Tregs displayed significantly impaired expression of Foxp3, whereas Treg activation markers were upregulated in nfkb2 deleted Tregs (Figure 2C and data not shown). Taken together, these results demonstrate an important role for p100, and or p52, in the regulation of Treg homeostasis.
Figure 2. Intrinsic NF-κB2 p100/p52 signaling restricts the peripheral Treg cell pool.
(A–C) Spleen and peripheral lymph nodes of 6–8 weeks-old Foxp3cre and Foxp3crenfkb2F/F mice were stained for FACS. (A) Expression of Foxp3 in gated spleen TCR-β+CD4+ live cells. Numbers indicate the percentage in gate. (B) % in TCR-β+CD4+ and absolute numbers of Treg cells. (C) Expression of Foxp3 and pooled MFI in gated spleen TCR-B+CD4+Foxp3+ Treg cells. (D–F) The cell-intrinsic role of nfkb2 in Treg cells was evaluated in mixed BM chimeras. (D) Experimental design. (E) % of Treg cells in TCR-β+CD4+CD45.2+ cells. (F) Representative Ki67 expression in gated spleen in TCR-β+CD4+CD45.2+Foxp3+ Treg cells, and pooled % of Ki67+ in Treg cells. Numbers indicate the percentage in gate. (G) Expression of Nrp-1 and Helios in gated spleen in TCR-β+CD4+Foxp3+ Treg cells from Foxp3cre and Foxp3crenfkb2 F/F animals. (G) Naïve T cells from CD4cre and CD4crenfkb2 F/F LN were cultured under iTreg polarization conditions for 4 days. The % of Treg in each genotype in 3 experiments is shown. Data is representative (A, C, D–G) or the pool (B, H) of 2–3 experiments. In B, E and F, each dot represents a mouse. *p<0.05,** p<0.005, ***p<0.001.
Although these results demonstrate a role for p100/p52 in Treg cells, we wondered whether nfkb2 controlled Treg homeostasis in a cell-intrinsic manner or indirectly e.g. by regulating production of a factor that was driving Treg expansion. Therefore, to formally demonstrate a Treg-autonomous role for nfkb2, we performed mixed-bone marrow (BM) chimera experiments. In such an experimental setting, any cell extrinsic defect in the KO BM would either be compensated for by the presence of WT BM, or be revealed by an effect on WT Tregs. Lethally irradiated WT recipients were transplanted with a 1:1 mixture of congenic WT bone marrow (BM) and either Foxp3CRE or Foxp3CREnfkb2F/F BM (Figure 2D). 8 weeks after BM reconstitution the proportion of Tregs in KO spleen and LN compartments were dramatically increased when compared to their WT counterparts (Figure 2E). Consistent with the increase in deleted Tregs, upregulation of the Ki67 proliferation marker was also selectively observed in Treg cells in which nfkb2 was deleted. Thus, nfkb2 has a cell-intrinsic function in restricting Treg proliferation in the periphery. Next, we assessed whether these expanded Tregs were of thymic origin or derived from a peripheral conversion of Tconv cells. The percent expression of two genes associated with nTregs, the membrane receptor Nrp-1, and the transcription factor Helios, were not affected by loss of nfkb2. This suggested that the enhanced nfkb2−/− Treg expansion was occurring in nTreg cells of thymic origin (Figure 2G). We also measured the effect of ablating nfkb2 on the in vitro conversion of Tconv into Foxp3+ Tregs, which mimic pTregs. After 4 days of culture, the proportion of Foxp3+ cells was slightly reduced in the absence of nfkb2 (Figure 2H). Therefore, nfkb2 does not have effects on either nTreg or pTreg development that explain the expansion of the peripheral Treg population. Instead, the data suggests that nfkb2 is a key, cell autonomous regulator of nTreg homeostasis.
Mild autoimmune syndrome in aged Foxp3CREnfkb2F/F mice
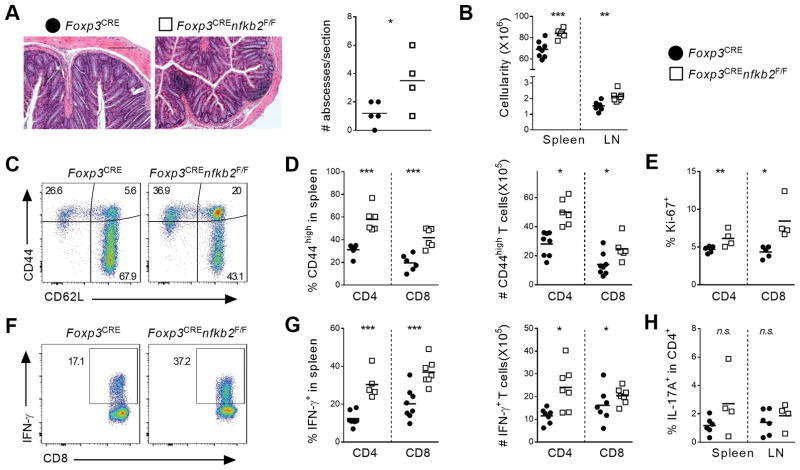
As described above, young Foxp3CREnfkb2F/F mice did not display abnormal inflammation or autoimmunity. However, beginning around 12 months of age, we began to observe variable weight loss and hunched posturing in the Foxp3CREnfkb2F/F animals. Histological examination revealed increased immune infiltrate in the colons of Foxp3CREnfkb2F/F mice, while other tissues (lungs, liver, and kidney) were unaffected (Figure 3A and data not shown). Of note, these localized colonic infiltrates were not associated with significant histological changes at the mucosa (Figure 3A). FACS analysis revealed increased numbers of IFN-γ producing CD4 and CD8 T cells in the colon lamina propria of nfkb2-deficient mice compared to WT controls, whereas the IL-17A+ cell population was unchanged (Figure S2A,B). Next, the lymphoid tissue composition was analyzed. The cellularity of both spleen and peripheral lymph nodes was significantly increased in Foxp3CREnfkb2F/F animals (Figure 3B). Interestingly, the CD4+ T-cell compartment was significantly expanded, leading to an increased CD4/CD8 ratio (Figure S2C). Moreover, we observed enhanced activation of both CD4+ Tconv and CD8+ T cells, as shown by the increased expression of CD44 and Ki67 (Figure 3C–E). Strikingly, we observed a nearly 2-fold increase in the proportion of CD4 and CD8 T cells expressing IFN-γ (Figure 3F–G). However, the expression of IL-17A, TNF and IL-2 was not significantly different between WT and KO mice (Figure 3H and Figure S2D). Finally, we could not detect any change in the levels of serum TNF and total IgG (data not shown). Thus, the expression of NF-κB2 by Treg cells prevents the late development of a mild autoimmune syndrome, likely mediated by Th1 CD4 and Tc1 CD8 T cells.
Figure 3. Uncontrolled inflammation in aged Foxp3crenfkb2F/F mice.
Tissues of 12-month-old Foxp3cre and Foxp3crenfkb2F/F mice were analyzed by histology and FACS. (A) (left) Representative section of colon stained with H/E. Bars: 200μm; original magnification 10X. (right) Quantification of abscesses (immune infiltrates) in each colon section. (B) Gross live cell count in spleen and LN. (C) CD44 and CD62L expression in gated spleen TCR-β+CD8+ cells. Numbers indicate the % in each quadrant. (D) % (in TCR-β+CD4+Foxp3− (CD4) and TCR-β+CD8+ (CD8)) and absolute numbers of CD44high T cells. (E) % of Ki67+ cells in spleen CD4 and CD8 cells. (F) Expression of IFN-γ upon PMA-ionomycin restimulation in gated spleen TCR-β+CD8+ cells. Numbers indicate the percentage in gate. (G) % (in CD4 and CD8) and absolute numbers of IFN-γ+ T cells. (H) % of IL-17A+ cells in CD4 cells. Data is representative (A, C, F) or the pool (A, B, D, E, G, H) of 3 experiments. In A, B, D, E, G, H, each dot represents a mouse. *p<0.05,** p<0.005, ***p<0.001, n.s.: non-significant.
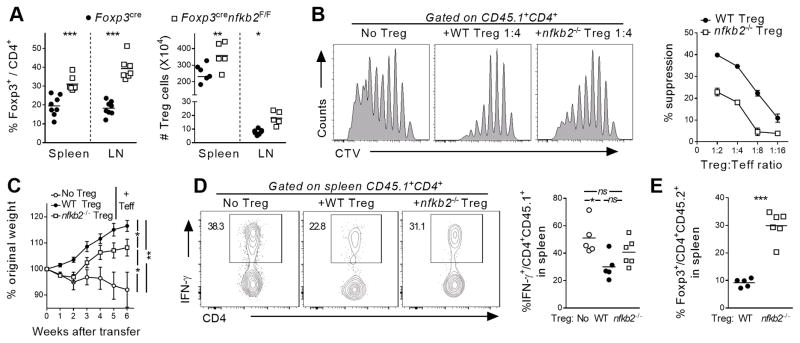
Given the expansion of Tregs in young Foxp3CREnfkb2F/F animals, we hypothesized that the observed autoimmune phenotype in aged animals was likely the consequence of an impaired Treg suppressive function. Indeed, FACS analysis revealed that the inflammatory syndrome observed in aged Foxp3CREnfkb2F/F mice was associated with continued expansion of Tregs (Figure 4A). Therefore, we next examined the suppressive capacity of nfkb2-deficient Tregs in vitro and in vivo. For the in vitro assay, the proliferative capacity of responder CD4 T cells was measured in the absence or presence of varying ratios of WT or KO Tregs. Nfkb2-deficient Tregs exhibited reduced inhibitory activity at each ratio relative to WT Tregs (Figure 4B). We next assessed the activity of WT and nfkb2−/− Tregs in vivo using a T-cell transfer colitis model (29). While WT Tregs effectively prevented weight loss and reduced IFN-γ expression by splenic Tconv cells transferred into RAG1−/− mice, nfkb2−/− Tregs were considerably less effective (Figure 4C, D). This was not due to a loss of the Treg population per se, since the proportion of nfkb2−/− Tregs 5 weeks after transfer was 3 times higher than that of WT Treg cells (Figure 4E). To better understand this loss of Treg suppressive function, we measured the mRNA expression of several well-characterized genes involved in Treg function. While the expression of Tgfb1, Lag3 and CD73 was unaffected in Treg cells lacking NF-κB2, Ctla4 mRNA was strongly down-regulated (Figure S2E). This was associated with a decreased expression of intracellular CTLA-4 in nfkb2−/− Treg cells, both in unmanipulated Foxp3CREnfkb2F/F mice, as well as in the T-cell transfer colitis model (Figure S2F). Taken together, these results demonstrate that the expression of NF-κB2 is required to restrict Treg proliferation and also to maintain optimal suppressive capacity.
Figure 4. Impaired suppressive function of nfkb2-deficient Treg cells.
(A) Spleen and peripheral lymph nodes of 12-month-old Foxp3cre and Foxp3crenfkb2F/F mice were analyzed by FACS. The % of Treg cells among CD4 T cells and their absolute numbers is shown. (B) In vitro suppressive function of Treg sorted from Foxp3cre (WT) and Foxp3crenfkb2F/F (nfkb2 −/−) animals was assessed as described in the Material and Methods section. (left) representative proliferation of responder T cells at D4; (right) Mean +/− SEM of suppression of responder T cells proliferation by Treg cells at different ratios. (C–E) In vivo suppression assay, as described in the Material and Methods section. (C) Mean +/− SEM % of original weight. (D) Representative (left) and pooled (right) expression of IFN-γ in gated CD45.1+CD4+ T cells at D50. (E) % of Foxp3+ Treg cells among live CD45.2+CD4+ cells. In A, C, E, data is the pool of 3 pooled experiments. In B, data is from 1 out of 3 experiments. *p<0.05,** p<0.005, ***p<0.001, n.s.: non-significant.
NF-κB RelB hyperactivity in nfkb2-deficient cells alters Treg homeostasis
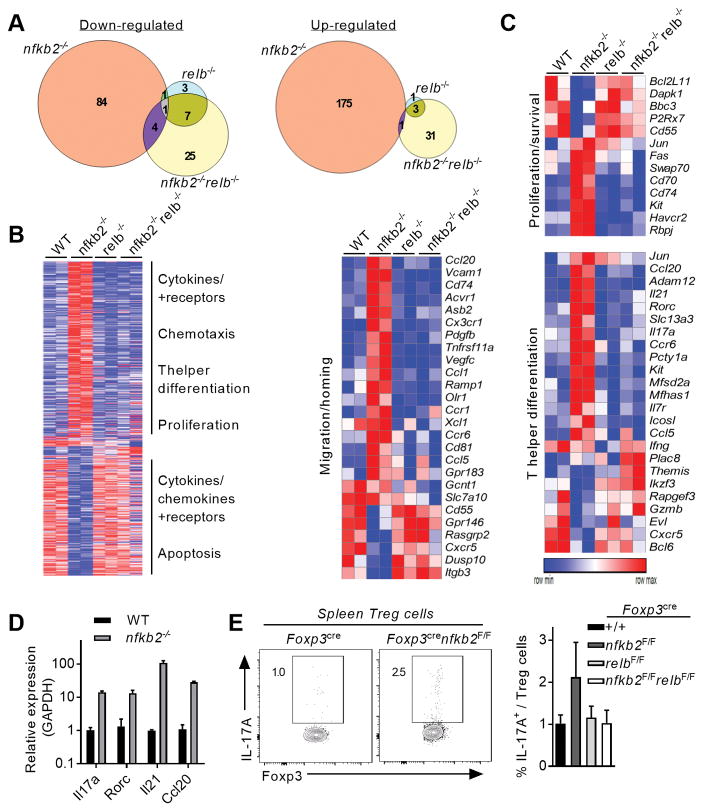
We next explored the molecular mechanism driving dysregulated Treg homeostasis in the absence of NF-κB2. NF-κB2 can act as both a negative regulator of other Rel-proteins, by sequestering them through interaction via its ankyrin domains, and as a positive regulator by generating the p52 subunit, which can associate with other NF-κB subunits to form transcriptionally active NF-κB heterodimers. Therefore, we first sought to examine the expression and activation state of the transcriptionally active NF-κB subunits – RelB, p65, and c-Rel. Both the expression and stimulation induced nuclear localization of the canonical NF-κB subunits RelA and c-Rel were unaffected in nfkb2−/− Tregs (Figure 5A). In contrast, there was a dramatic increase in the nuclear accumulation of RelB in TCR/CD28 stimulated nfkb2−/− Tregs (Figure 5A). Therefore the data suggested that NF-κB2 predominantly functions as an inhibitor of RelB activation in Tregs. To test the hypothesis that the primary function of NF-κB2 in Tregs is to regulate RelB activation, we deleted relb in both total T cells (using CD4cre) and Tregs (using Foxp3cre). RelB protein was undetectable in splenic T cells of CD4crerelbF/F mice (Figure S3A). Surprisingly, Relb deletion did not have a significant effect on either the overall distribution of CD4 and CD8 T cells in lymphoid organs or the corresponding number of Treg cells (Figure S3B, C). Strikingly, ablation of relb in the CD4crenfkb2F/F background restored a normal proportion and number of peripheral Tregs (Figure 5B). Hence, loss of NF-κB2 resulted in increased RelB activation and the Treg expansion observed in nfkb2-deficient animals was dependent on RelB.
Figure 5. Overactivation of RelB in the absence of NF-κB2 drives uncontrolled Treg expansion and inflammation.
(A) Treg cells were sorted from Foxp3cre and Foxp3crenfkb2F/F spleen and LN and activated overnight with anti-CD3/CD28 Abs and IL-2. Cytosolic and nuclear lysates were separated by SDS-PAGE and membranes were blotted with the indicated Ab. (B) Thymus, spleen and peripheral lymph nodes of 6–8 weeks-old CD4cre and CD4crenfkb2F/FrelbF/F mice were stained for FACS. The % in TCR-β+CD4+ and absolute numbers of Treg cells is shown (C–G) Tissues of 12-month-old Foxp3cre and Foxp3crenfkb2F/FrelbF/F mice were analyzed by histology and FACS. (C) Representative section of colon stained with H/E. Bars: 200μm; original magnification 10X. (D) Quantification of abscesses (immune infiltrates) in each colon section. (E) Gross live cell count in spleen and LN. (F) % in TCR-β+CD4+ and absolute numbers of Treg cells. (G–I) Cells were restimulated with PMA-ionomycin prior to FACS analysis. (G) CD44 and IFN-γ expression in gated spleen TCR-β+CD8+ cells. Numbers indicate the % in each quadrant. (H, I) % (in CD4 and CD8 cells) and absolute numbers of CD44high and IFN-γ+ T cells, respectively. Each dot represents a mouse. Data is representative (A, C, G) or pooled (B, D–G, H–I) from 2 to 4 experiments. *p<0.05, n.s.: non-significant.
We next sought to determine whether the altered in vivo suppressive function observed in Foxp3CREnfkb2F/F mice were also dependent on RelB. Therefore we next measured inflammation/autoimmunity symptoms in Treg-specific, Foxp3CREnfkb2F/FrelbF/F, double KO mice. Consistent with the normalization of Treg homeostasis upon RelB loss, no increase in colonic abscesses was evident in 10 to 12-month old Foxp3CREnfkb2F/FrelbF/F mice (Figure 5C, D). Moreover, the global cellularity of spleen and LN was also normal (Figure 5E). As in the CD4cre deleter strain, Foxp3CREnfkb2F/FrelbF/F mice also displayed normal numbers of peripheral Tregs (Figure 5F). Finally, we examined the activation state of conventional CD4 and CD8+ T cells. Again, the level of CD44 and IFN-γ expression remained unchanged in KO mice when compared to WT littermates (Figure 5G–I and Figure S3D–F). Taken together, these data indicate that p100 is an essential negative regulator of RelB in Tregs and that the uncontrolled expansion of Tregs, and the subsequent autoimmunity that develops after deletion of nfkb2, is secondary to enhanced RelB activation.
NF-κB2 regulates the Treg transcriptome
To further explore how NF-κB2 controls Treg homeostasis, we compared the transcriptomes of WT, nfkb2−/−, relb−/− and nfkb2−/−relb−/− Treg cells by RNA-sequencing. To ensure an optimal activation of the alternative NF-κB pathway, Tregs were stimulated with anti-CD3, anti-CD28, anti-OX-40 and IL-2. We initially analyzed global changes in gene expression between the genotypes (Figure 6A, B). Surprisingly, we found that loss of NF-κB2 resulted in a far broader effect on the Treg transcriptome than did loss of RelB. Specifically, the analysis of the nfkb2−/− Treg cells transcriptome was notable for the large number of upregulated transcripts (175 up vs 84 downregulated), reinforcing the idea the NF-κB2 predominantly affects Treg function through its IκB-like repressor activity. Interestingly, the dysregulated gene set in nfkb2−/− Treg showed only minor overlap with other genotypes. Furthermore, Relb deletion did not impair gene expression, which was at odds with its role in B-cells (24) but was consistent with the lack of a detectable phenotype in Foxp3CRErelbF/F mice. Remarkably however, dysregulated expression of the vast majority of genes in nfkb2-deficient Tregs was rescued upon relb ablation (Figure 6B). Therefore, this data further supports the idea that NF-κB2 primarily acts as an inhibitor of RelB-mediated transcription. Interestingly, neither partial nor complete ablation of the alternative NF-κB pathway drove a change in the expression of other NF-κB subunits genes (Figure S4A). Gene Set Enrichment Analysis (GSEA), and other gene ontology analyses, revealed significant changes in the expression of genes related to proliferation, survival and migration, as well as cytokine expression (Figure 6B and S4B). We observed a dramatic increase in the expression of genes involved in cell proliferation and anti-apoptotic functions in nfkb2-only deleted cells (Figure 6C). The expression of the pro-apopotic gene Bcl2l11, which encodes Bim, was down-regulated in nfkb2−/− Tregs, whereas the kinase Kit was up-regulated. These genes were restored to the WT expression levels upon additional ablation of relb. This perturbed expression profile may explain the specific accumulation of Tregs in the tissues of nfkb2-deficient mice.
Figure 6. NF-κB2 represses aberrant expression of inflammatory and homing genes in Treg cells.
Treg cells were sorted from Foxp3cre, Foxp3crenfkb2F/F, Foxp3crerelbF/F mice and Foxp3crenfkb2F/FrelbF/F mice (3 mice/genotype), activated overnight and processed for RNA-seq. (A) Venn diagrams showing genes with a fold-change <0.6 and >1.5 and a p-value< 0.05 compared to WT Treg cells. (B) Unsupervised hierarchical row clustering. Only genes with changed expression in at least 1 genotype is shown. The biological functions indicate selected enriched pathways upon GSEA and Metacore analysis. (C) Heatmaps showing the relative expression of selected genes enriched in different biological processes. (D) qRT-PCR quantification of selected genes. Mean +/− SEM of expression relative to GAPDH is shown. (E) Spleens of 12-month-old mice were analyzed by FACS. (left) relative expression of IL-17A in gated Treg cells; numbers indicate the % in the gate. (right) Mean +/− SEM of IL-17A expression in spleen Treg cells. Data is from two (A–C) or 3 (D–E) experiments.
It has previously been suggested that the alternative pathway may control the expression of migration and homing molecules in stromal cells and innate immune cells. In Tregs, the expression of numerous chemokines and chemokine receptors were modified by the absence of NF-κB2, but not RelB (Figure 6C). However, most of these genes were up-regulated and not down-regulated, revealing a previously unknown facet of the alternative NF-κB pathway as an inhibitor of expression of homing molecules in Tregs.
Finally, we observed changes in the expression of many genes related to inflammation and T-helper cell differentiation. Nfkb2−/− Treg cells displayed a significant overexpression of inflammatory molecules, in particular cytokines and transcription factors that are hallmarks of Th17 effector cells, such as Il17a, Il21, Ccr6 and Rorc (Figure 6C and Figure S4B). This was further confirmed by RT-PCR and flow cytometry analyses (Figure 6D, E). Moreover, the aberrant expression of most of these genes was “rescued” by knocking-out relb, underscoring a deleterious role for uncontrolled RelB activation on the Treg-associated molecular profile. These results are consistent with a predominantly inhibitory function of NF-κB2 in the expression of cell cycle and pro-inflammatory genes. As we recently demonstrated, the canonical NF-κB subunits p65 and c-Rel are critical for maintaining the expression of Treg hallmark genes (16). However, there was not a significant positive or negative correlation in gene expression between Foxp3CRERelaF/Fc-RelF/F and Foxp3CREnfkb2F/F Tregs (Figure S4C), suggesting that perturbations in gene expression in these two genotypes were distinct, and further supporting the idea that the inhibitory effect of NF-κB2 is on RelB-containing complexes. Therefore, taken together, our observations demonstrate a novel and unique function of NF-κB2 in maintaining Treg homeostasis and function.
Discussion
Recently, significant progress has been made in understanding the intrinsic role of the canonical NF-κB pathway in the biology of Tregs (16–18). Indeed, we have shown that conditional deletion of p65 in Treg cells drove a systemic autoimmune syndrome, which was further aggravated by deletion of c-Rel. c-Rel itself exhibited a critical function in maintaining Treg homeostasis specifically during anti-tumor immunity(18). We now demonstrate that the alternative NF-κB pathway, acting through its NF-κB2 subunit, is also crucial for the maintenance of Treg homeostasis and function. Forced activation of the alternative pathway by deleting TRAF3 (Traf3−/− mice), or overexpressing NIK (NIK-Tg mice), leads to increased numbers of Tregs (22, 30). Based on these findings it would have been predicted that loss of nfkb2 would result in impaired Treg expansion. Instead, surprisingly, we find that deletion of nfkb2 drives a cell-autonomous increase in Treg numbers in secondary lymphoid organs. This is likely due to the expansion of nTreg cells in the periphery as iTreg generation was only slightly impaired.
Interestingly, Treg cell numbers returned to baseline upon additional deletion of RelB (i.e., nfkb2−/−relb−/− double KO). Of note, in both Foxp3CRErelbF/F and Foxp3CREnfkb2F/FrelbF/F mice, the relative proportion of Tregs were not decreased compared to WT mice. This suggested that NF-κB2 functions to prevent aberrant RelB activation, which otherwise has no role in Treg expansion at steady-state. Previous studies had indirectly addressed the role of the alternative NF-κB pathway in Treg expansion. For instance, several reports showed a cell-intrinsic requirement for NIK in the peripheral homeostasis of Tregs (20, 31). Our data suggest that this result is likely due to NIK’s effect on the function of canonical NF-κB. A similar decrease of Tregs were observed in conditional IKKα-KO mice (21); but once again, as IKKα can also activate the canonical NF-κB pathway (32), this suggests that the effects of NIK or IKKα relied more on impaired p65 or c-Rel activation, rather than on a diminished activation of the alternative NF-κB pathway.
Nfkb2 encodes for both p100 and p52. Here we show that deletion of nfkb2 can drive uncontrolled Treg activation; however, it is not clear whether this effect results from the ablation of p100, p52 or both. Previous studies have suggested that p100 can act as a repressor of TCR-triggered signaling events; for instance, overexpression of p100 in cell lines decreased initial NF-κB activation and IL-2 transcription upon TCR triggering (33). Similarly, constitutive activation of NIK in c-IAPH570A mutant mice, or in NIK-Tg animals, led to over-activation of CD4+ T cells (22, 34). Moreover, T cells isolated from mice carrying a germline deletion of nfkb2, were also more proliferative in vitro (34). Finally, NIK-deficient T cells were slightly hyporesponsive in vitro and in vivo (19, 31). In sum, these studies suggest that Treg expansion in nfkb2- conditional KO animals was likely due to the absence of the inhibitor p100, rather than the absence of transcriptionally active p52-containing complexes. Mechanistically, several studies have demonstrated the role of p100 as a potent inhibitor of both canonical and alternative NF-κB signaling. For example, the p100 C-terminal ankyrin domains have been shown to physically interact and inhibit the translocation of RelB, p65 and c-Rel (19, 33, 35). Most compelling, however, are studies highlighting RelB as the critical target of p100 in the cytosol of mouse embryonic fibroblasts (MEFs), Hela cells, dendritic cells and T cells (36–39). Consistent with this, our studies in Tregs revealed that the nuclear translocation of RelB, but not p65 or c-Rel, was significantly increased in the absence of nfkb2. This suggested that an aberrant RelB activation was responsible for the accumulation of Treg cells in vivo. This hypothesis was confirmed by abolishment of nfkb2-deficient Treg expansion upon the additional ablation of relb.
Analysis of the transcriptional landscape associated with our knockout lines further confirmed the specific role of NF-κB2 in restraining the activity of the other NF-κB subunits. Indeed, many genes whose expression was increased in nfkb2−/− Treg cells were normalized in the nfkb2−/−relb−/− cells. As we did not observe a correlation of the nfkb2−/− Treg gene signature with Rela−/−Rel−/− Treg gene signature, it supported our hypothesis that nfkb2 deletion did not affect the function of the canonical NF-κB transcription factors. To our surprise, only a small number of genes were affected by the loss of RelB, or both NF-κB2 and RelB, suggesting that transcription by alternative NF-κB subunits was mainly expendable for the maintenance of Treg identity in the steady-state. However, it remains possible that p52 and/or RelB could display a more prominent function in Treg biology during acute or chronic inflammation, which is known to drive the engagement of a number of TNFR family members and therefore leads to alternative NF-κB activation and p100 processing(11, 40).
Previous studies have shown a role for the alternative pathway in the expression of chemokines and adhesion molecules by stromal cells upon LTβR triggering (9). This pattern was only partially confirmed in BAFF and CD40–stimulated nfkb2−/− B cells (24). In our experiments, we actually observed an increase in the expression of migration/homing genes, upon CD3/CD28/OX-40 stimulation of nfkb2−/− Treg cells, but not in double knockout (nfkb2−/−relb−/−, DKO) Treg cells. Another striking difference between nfkb2−/− and DKO Treg cells is the significant enrichment of a Th17 cells signature in the absence of p100 alone. The increased expression of RORc or IL17a might explain why these p100-deficient Treg cells partially lose their inhibitory function, even when the expression of suppressive cytokines or co-stimulation markers remain intact. IL-17-producing Foxp3+ Treg cells have been implicated in the development of certain autoimmune and chronic inflammatory conditions including psoriasis (41, 42). Interestingly, high numbers of IL-17+Foxp3+ were found in the lamina propria and blood of patients with inflammatory bowel disease and mouse models of colitis (43, 44). Therefore, it is possible that development of colonic inflammation in aging Foxp3CREnfkb2F/F animals relies on the aberrant expression of inflammatory cytokines by Treg cells.
Even though aging Foxp3CREnfkb2F/F mice had an expanded Treg cell population in their peripheral lymph nodes, the conventional CD4 and CD8 T cells exhibited increased activation and secretion of inflammatory cytokines, leading to undesired infiltration in non-lymphoid tissues. This suggested that despite a large increase in numbers, these Treg cells had impaired suppressive activity. This functional deficit was confirmed both in vitro and in vivo. Mice with conditional deletion of Ctla4 in Treg cells also develop a severe autoimmune syndrome despite a significant increase in their number of Treg cells (45) and we observed reduced CTLA-4 expression by nfkb2−/− Treg cells. This may partially explain both Treg expansion and their lack of suppressive function in Foxp3CREnfkb2F/F mice. Moreover, uncontrolled activation of RelB might be deleterious for Treg cells. This possibility has been previously addressed indirectly. For instance, the triggering of the GITR receptor, which induces activation of the alternative pathway (46), led to increased T-cell survival and proliferation (47). This drove a significant Treg accumulation in GITRL-transgenic animals (48). Meanwhile, Treg suppressive function was impaired by GITR signaling (49). These data also fit our model. Therefore, all these observations may help unmask a specific role for the alternative pathway in Treg homeostasis.
Based on the previous analyses of TNFR superfamily members in Tregs and the data presented here, we propose a model in which p100 is a crucial regulator RelB and Treg homeostasis and function. Following TCR stimulation, induced synthesis of p100 results in the inhibition of RelB containing complexes. However, upon activation of the alternative NF-κB pathway, e.g. through OX40 or GITR, increased processing of p100 results in the activation of RelB containing complexes leading to Treg expansion coupled with loss of Treg suppressive function. This model is consistent with recent reports analyzing OX40 and GITR function and provides a molecular explanation for the ability of these alternative NF-κB pathway stimuli to inhibit Treg function and tumor tolerance. Likewise, these data provide further insight into how constitutive activation of the canonical and alternative NF-κB pathways might drive pathology in patients with chronic inflammation and autoimmune disease (11, 40, 50). Therefore, taken together, our data demonstrate that the adequate balance of NF-kB activity, regulated by NF-kB2 p100, is essential for maintaining optimal Treg function and immune tolerance.
Supplementary Material
Acknowledgments
Funding: This work was supported by grants from the NIH (R01-AI068977) and from the Herbert Irving Cancer Center at Columbia University, to S.G. Y.G.B. was supported by a postdoctoral fellowship from the Cancer Research Institute.
We thank Dev Bhatt, Alexis Desrichard, Alice Lepelley, Gaelle H. Martin, and Thomas S. Postler for technical help and constructive discussions on the project.
Footnotes
Author contributions
Y.G.B., M.S.H and S.G. wrote the paper. Y.G.B. and S.G. conceived experiments. Y.G.B, R.C. and J.J.S. performed experiments. Y.G.B., C.S., M.S.H and S.G analysed the data. N.D.S. and U.K. provided mice and reagents. S.G. secured funding.
References
- 1.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nature immunology. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annual review of immunology. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isomura I, Palmer S, Grumont RJ, Bunting K, Hoyne G, Wilkinson N, Banerjee A, Proietto A, Gugasyan R, Wu L, McNally A, Steptoe RJ, Thomas R, Shannon MF, Gerondakis S. c-Rel is required for the development of thymic Foxp3+ CD4 regulatory T cells. J Exp Med. 2009;206:3001–3014. doi: 10.1084/jem.20091411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long M, Park SG, Strickland I, Hayden MS, Ghosh S. Nuclear factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity. 2009;31:921–931. doi: 10.1016/j.immuni.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 7.Ruan Q, Kameswaran V, Tone Y, Li L, Liou HC, Greene MI, Tone M, Chen YH. Development of Foxp3(+) regulatory t cells is driven by the c-Rel enhanceosome. Immunity. 2009;31:932–940. doi: 10.1016/j.immuni.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 9.Sun SC. The noncanonical NF-kappaB pathway. Immunological reviews. 2012;246:125–140. doi: 10.1111/j.1600-065X.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerondakis S, Siebenlist U. Roles of the NF-kappaB pathway in lymphocyte development and function. Cold Spring Harbor perspectives in biology. 2010;2:a000182. doi: 10.1101/cshperspect.a000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown KD, Claudio E, Siebenlist U. The roles of the classical and alternative nuclear factor-kappaB pathways: potential implications for autoimmunity and rheumatoid arthritis. Arthritis research & therapy. 2008;10:212. doi: 10.1186/ar2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grigoriadis G, Vasanthakumar A, Banerjee A, Grumont R, Overall S, Gleeson P, Shannon F, Gerondakis S. c-Rel controls multiple discrete steps in the thymic development of Foxp3+ CD4 regulatory T cells. PloS one. 2011;6:e26851. doi: 10.1371/journal.pone.0026851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Visekruna A, Huber M, Hellhund A, Bothur E, Reinhard K, Bollig N, Schmidt N, Joeris T, Lohoff M, Steinhoff U. c-Rel is crucial for the induction of Foxp3(+) regulatory CD4(+) T cells but not T(H)17 cells. European journal of immunology. 2010;40:671–676. doi: 10.1002/eji.200940260. [DOI] [PubMed] [Google Scholar]
- 14.Deenick EK, Elford AR, Pellegrini M, Hall H, Mak TW, Ohashi PS. c-Rel but not NF-kappaB1 is important for T regulatory cell development. European journal of immunology. 2010;40:677–681. doi: 10.1002/eji.201040298. [DOI] [PubMed] [Google Scholar]
- 15.Vang KB, Yang J, Pagan AJ, Li LX, Wang J, Green JM, Beg AA, Farrar MA. Cutting edge: CD28 and c-Rel-dependent pathways initiate regulatory T cell development. Journal of immunology. 2010;184:4074–4077. doi: 10.4049/jimmunol.0903933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh H, Grinberg-Bleyer Y, Liao W, Maloney D, Wang P, Wu Z, Wang J, Bhatt DM, Heise N, Schmid RM, Hayden MS, Klein U, Rabadan R, Ghosh S. An NF-kappaB Transcription-Factor-Dependent Lineage-Specific Transcriptional Program Promotes Regulatory T Cell Identity and Function. Immunity. 2017;47:450–465e455. doi: 10.1016/j.immuni.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Messina N, Fulford T, O’Reilly L, Loh WX, Motyer JM, Ellis D, McLean C, Naeem H, Lin A, Gugasyan R, Slattery RM, Grumont RJ, Gerondakis S. The NF-kappaB transcription factor RelA is required for the tolerogenic function of Foxp3 regulatory T cells. Journal of autoimmunity. 2016 doi: 10.1016/j.jaut.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Grinberg-Bleyer Y, Oh H, Desrichard A, Bhatt DM, Caron R, Chan TA, Schmid RM, Klein U, Hayden MS, Ghosh S. NF-kappaB c-Rel Is Crucial for the Regulatory T Cell Immune Checkpoint in Cancer. Cell. 2017;170:1096–1108e1013. doi: 10.1016/j.cell.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishimaru N, Kishimoto H, Hayashi Y, Sprent J. Regulation of naive T cell function by the NF-kappaB2 pathway. Nature immunology. 2006;7:763–772. doi: 10.1038/ni1351. [DOI] [PubMed] [Google Scholar]
- 20.Murray SE. Cell-intrinsic role for NF-kappa B-inducing kinase in peripheral maintenance but not thymic development of Foxp3+ regulatory T cells in mice. PloS one. 2013;8:e76216. doi: 10.1371/journal.pone.0076216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X, Willette-Brown J, Wu X, Hu Y, Howard OM, Hu Y, Oppenheim JJ. IKKalpha is required for the homeostasis of regulatory T cells and for the expansion of both regulatory and effector CD4 T cells. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2015;29:443–454. doi: 10.1096/fj.14-259564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray SE, Polesso F, Rowe AM, Basak S, Koguchi Y, Toren KG, Hoffmann A, Parker DC. NF-kappaB-inducing kinase plays an essential T cell-intrinsic role in graft-versus-host disease and lethal autoimmunity in mice. The Journal of clinical investigation. 2011;121:4775–4786. doi: 10.1172/JCI44943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karin M. How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene. 1999;18:6867–6874. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- 24.De Silva NS, Silva K, Anderson MM, Bhagat G, Klein U. Impairment of Mature B Cell Maintenance upon Combined Deletion of the Alternative NF-kappaB Transcription Factors RELB and NF-kappaB2 in B Cells. Journal of immunology. 2016;196:2591–2601. doi: 10.4049/jimmunol.1501120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godec J, Tan Y, Liberzon A, Tamayo P, Bhattacharya S, Butte AJ, Mesirov JP, Haining WN. Compendium of Immune Signatures Identifies Conserved and Species-Specific Biology in Response to Inflammation. Immunity. 2016;44:194–206. doi: 10.1016/j.immuni.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mi H, Muruganujan A, Casagrande JT, Thomas PD. Large-scale gene function analysis with the PANTHER classification system. Nature protocols. 2013;8:1551–1566. doi: 10.1038/nprot.2013.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. Journal of immunology. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 30.Yi Z, Lin WW, Stunz LL, Bishop GA. The adaptor TRAF3 restrains the lineage determination of thymic regulatory T cells by modulating signaling via the receptor for IL-2. Nature immunology. 2014;15:866–874. doi: 10.1038/ni.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Wang H, Zhou X, Xie X, Chen X, Jie Z, Zou Q, Hu H, Zhu L, Cheng X, Brightbill HD, Wu LC, Wang L, Sun SC. Cell intrinsic role of NF-kappaB-inducing kinase in regulating T cell-mediated immune and autoimmune responses. Scientific reports. 2016;6:22115. doi: 10.1038/srep22115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nature immunology. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 33.Legarda-Addison D, Ting AT. Negative regulation of TCR signaling by NF-kappaB2/p100. Journal of immunology. 2007;178:7767–7778. doi: 10.4049/jimmunol.178.12.7767. [DOI] [PubMed] [Google Scholar]
- 34.Giardino Torchia ML, Conze DB, Jankovic D, Ashwell JD. Balance between NF-kappaB p100 and p52 regulates T cell costimulation dependence. Journal of immunology. 2013;190:549–555. doi: 10.4049/jimmunol.1201697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee CE, Fulcher DA, Whittle B, Chand R, Fewings N, Field M, Andrews D, Goodnow CC, Cook MC. Autosomal-dominant B-cell deficiency with alopecia due to a mutation in NFKB2 that results in nonprocessable p100. Blood. 2014;124:2964–2972. doi: 10.1182/blood-2014-06-578542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lovas A, Weidemann A, Albrecht D, Wiechert L, Weih D, Weih F. p100 Deficiency is insufficient for full activation of the alternative NF-kappaB pathway: TNF cooperates with p52-RelB in target gene transcription. PloS one. 2012;7:e42741. doi: 10.1371/journal.pone.0042741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solan NJ, Miyoshi H, Carmona EM, Bren GD, Paya CV. RelB cellular regulation and transcriptional activity are regulated by p100. The Journal of biological chemistry. 2002;277:1405–1418. doi: 10.1074/jbc.M109619200. [DOI] [PubMed] [Google Scholar]
- 38.Speirs K, Lieberman L, Caamano J, Hunter CA, Scott P. Cutting edge: NF-kappa B2 is a negative regulator of dendritic cell function. Journal of immunology. 2004;172:752–756. doi: 10.4049/jimmunol.172.2.752. [DOI] [PubMed] [Google Scholar]
- 39.Tucker E, O’Donnell K, Fuchsberger M, Hilton AA, Metcalf D, Greig K, Sims NA, Quinn JM, Alexander WS, Hilton DJ, Kile BT, Tarlinton DM, Starr R. A novel mutation in the Nfkb2 gene generates an NF-kappa B2 “super repressor”. Journal of immunology. 2007;179:7514–7522. doi: 10.4049/jimmunol.179.11.7514. [DOI] [PubMed] [Google Scholar]
- 40.Xiao G, Rabson AB, Young W, Qing G, Qu Z. Alternative pathways of NF-kappaB activation: a double-edged sword in health and disease. Cytokine & growth factor reviews. 2006;17:281–293. doi: 10.1016/j.cytogfr.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 41.Jung MK, Kwak JE, Shin EC. IL-17A-Producing Foxp3(+) Regulatory T Cells and Human Diseases. Immune network. 2017;17:276–286. doi: 10.4110/in.2017.17.5.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L, Yang XQ, Cheng J, Hui RS, Gao TW. Increased Th17 cells are accompanied by FoxP3(+) Treg cell accumulation and correlated with psoriasis disease severity. Clinical immunology. 2010;135:108–117. doi: 10.1016/j.clim.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 43.Feng T, Cao AT, Weaver CT, Elson CO, Cong Y. Interleukin-12 converts Foxp3+ regulatory T cells to interferon-gamma-producing Foxp3+ T cells that inhibit colitis. Gastroenterology. 2011;140:2031–2043. doi: 10.1053/j.gastro.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li L, Boussiotis VA. The role of IL-17-producing Foxp3+ CD4+ T cells in inflammatory bowel disease and colon cancer. Clinical immunology. 2013;148:246–253. doi: 10.1016/j.clim.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 46.Lu LF, Gondek DC, Scott ZA, Noelle RJ. NF kappa B-inducing kinase deficiency results in the development of a subset of regulatory T cells, which shows a hyperproliferative activity upon glucocorticoid-induced TNF receptor family-related gene stimulation. Journal of immunology. 2005;175:1651–1657. doi: 10.4049/jimmunol.175.3.1651. [DOI] [PubMed] [Google Scholar]
- 47.Esparza EM, Arch RH. Glucocorticoid-induced TNF receptor functions as a costimulatory receptor that promotes survival in early phases of T cell activation. Journal of immunology. 2005;174:7869–7874. doi: 10.4049/jimmunol.174.12.7869. [DOI] [PubMed] [Google Scholar]
- 48.van Olffen RW, Koning N, van Gisbergen KP, Wensveen FM, Hoek RM, Boon L, Hamann J, van Lier RA, Nolte MA. GITR triggering induces expansion of both effector and regulatory CD4+ T cells in vivo. Journal of immunology. 2009;182:7490–7500. doi: 10.4049/jimmunol.0802751. [DOI] [PubMed] [Google Scholar]
- 49.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nature immunology. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 50.Starkey JM, Haidacher SJ, LeJeune WS, Zhang X, Tieu BC, Choudhary S, Brasier AR, Denner LA, Tilton RG. Diabetes-induced activation of canonical and noncanonical nuclear factor-kappaB pathways in renal cortex. Diabetes. 2006;55:1252–1259. doi: 10.2337/db05-1554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.