Abstract
Binge/moderate alcohol suppresses TLR4-MyD88 pro-inflammatory cytokines. However, alcohol effects on TLR-TRIF signaling, especially after in vivo exposure in humans, are unclear. We performed comparative analysis of TLR4-MyD88, TLR4-TRIF and TLR3-TRIF pathways in human monocytes following binge alcohol exposure. Mechanistic regulation of TLR-TRIF signaling by binge alcohol was evaluated by analyzing IRF3 and TBK-1, upstream regulator PP1 and immunoregulatory stress proteins HspA1A and XBP-1 in alcohol treated human and mouse monocytes/macrophages. Two approaches for alcohol exposure were employed – In vivo exposure of primary monocytes in binge alcohol consuming human volunteers OR in vitro exposure of human monocytes/murine macrophages to physiological alcohol concentrations (25-50mM ethanol), followed by LPS (TLR4) or polyI:C (TLR3) stimulation ex vivo. In vivo and in vitro binge alcohol exposure significantly inhibited TLR4-MyD88 cytokines TNFα and IL-6, and TLR4-TRIF cytokines/chemokines IFNβ, IP-10 and RANTES in human monocytes, but not TLR3-TRIF induced cytokines/chemokines, detected by qRT-PCR and ELISA. Mechanistic analyses revealed TBK1-independent inhibition of TLR4-TRIF effector IRF3 in alcohol-treated macrophages. While stress protein XBP-1, known to regulate IRF3-mediated IFNβ induction, was not affected by alcohol, HspA1A was induced by in vivo alcohol in human monocytes. Alcohol-induced HspA1A was required for inhibition of TLR4-MyD88 signaling but not TLR4-TRIF cytokines in macrophages. In contrast, inhibition of PP1 prevented alcohol-mediated TLR4-TRIF tolerance in macrophages. Collectively, our results demonstrate that in vivo and in vitro binge alcohol exposure in humans suppresses TLR4-MyD88 and TLR4-TRIF, but not TLR3-TRIF responses. While alcohol-mediated effects on PP1-IRF3 axis inhibits TLR4-TRIF pathway, HspA1A selectively suppresses TLR4-MyD88 pathway in monocytes/macrophages.
INTRODUCTION
Moderate or binge alcohol exposure suppresses host immune defenses to foreign pathogens (1, 2). Pathogens such as bacteria and viruses are recognized by Toll-like receptors (TLRs) on innate immune cells such as monocytes, macrophages and dendritic cells to mount an appropriate immune response (3). TLR4 can recognize both bacterial and viral components such as lipopolysaccharide (LPS) from gram-negative bacteria and fusion protein from respiratory syncytial virus (4–6). Upon recognition of ligand, early TLR4-MyD88 dependent signaling leads to activation of NF-κB and MAPK pathways resulting in production of pro-inflammatory cytokines such as TNFα and IL-6 (3). The divergent MyD88-independent branch of TLR4 signaling is mediated by adaptor molecules TRAM and TRIF and results in activation of TBK1, IKKε and IRF3 (7–9). IRF3, along with NF-κB, induces expression of type I interferon IFNβ and chemokines such as RANTES (CCL5) and IP-10 (CXCL10) downstream of TLR4-TRIF activation which can potently block viral replication (10–12). In addition to TBK1-IKKε mediated activation of IRF3, protein phosphatase 1 (PP1) has also been shown to exert regulatory effects on IRF3 by dephosphosphorylation (13). Endosomal TLR3 also utilizes the TRIF pathway to induce expression of type I IFNs via IRF3 (14).
Human and mouse studies have shown that alcohol-mediated suppression of innate immune function, specifically TLR-mediated responses, increases host susceptibility to infection. For example, alcohol significantly inhibited TLR4-induced cytokines IL-6 and IL-12 and impaired the ability of host to fight E. coli peritonitis in a binge-drinking mouse model (15, 16). Our own studies showed that binge/moderate alcohol exposure inhibited TLR4-induced NF-κB activation and reduced downstream pro-inflammatory cytokine production in human peripheral blood monocytes (17–20). Similarly, alcohol consumption also increases risk of viral infections such as influenza which are controlled by type I IFN responses (2, 21). For instance, TLR3-induced IFNβ, IL-6 and IL-12 expression in serum and peritoneal macrophages were suppressed in a murine model of binge alcohol consumption (oral gavage) (22, 23). PP1, which can exert regulatory effects on IRF3, is redox activated in response to alcohol exposure in airway cells and murine macrophages (24, 25). While the consequences of binge alcohol consumption in humans on TLR4-MyD88 signaling have been extensively studied, alcohol-mediated effects on the TRIF (MyD88 independent) pathway need further investigation. Here, we present the effects of alcohol on TLR4-MyD88, TLR4-TRIF and TLR3-TRIF signaling in human monocytes after binge drinking by human volunteers. We also employ in vitro ethanol exposure to study mechanisms related to alcohol mediated effects in human monocytes/macrophages. Understanding in vivo alcohol-mediated effects on the TLR4 and TLR3 signaling pathway will delineate the distinct mechanisms of immune regulation and help identify specific therapeutic targets for treatment in alcohol abusing patients.
Stress proteins, important in regulating cellular homeostasis, can mediate alcohol-induced immune tolerance due to their anti-inflammatory role in immune signaling (26). For example, splicing of ER stress protein X box binding protein-1 (XBP-1) is essential for polyI:C-induced IFNβ production and anti-viral responses by murine dendritic cells (27–29). Furthermore, XBP-1 was also required for TLR4 mediated IL-6 expression (30). Notably, previous studies from our lab have shown the regulatory role of stress chaperone proteins heat shock factor 1 (HSF1) and HspA1A (heat shock protein 70 - hsp70) in the mechanism of in vitro alcohol-mediated inhibition of TLR4-MyD88 pro-inflammatory responses (17, 19). HSF1 binding to TNFα promoter in alcohol-treated macrophages contributes to alcohol-mediated immunosuppression of TLR4-MyD88 responses since HSF1 can act as a transcriptional repressor (19). In addition, alcohol-induced HspA1A was shown to bind NF-κB p50 subunit and inhibit NF-κB promoter activity, indicating inhibition of NF-κB phosphorylation and nuclear translocation downstream of TLR4-MyD88 signaling in in vitro alcohol treated macrophages (17, 19). Here we seek to determine whether binge alcohol drinking (in vivo) in humans affects stress proteins XBP-1 and HspA1A and how this in turn regulates TLR4 or TLR3 signaling in human monocytes. Using monocytes from an UMMS-IRB approved study of human volunteers consuming binge alcohol to achieve BAC of 0.1g/dl and in vitro exposure of monocytes to a physiologically relevant alcohol concentration, we report that binge alcohol exposure leads to suppression of TLR4-TRIF signaling and downstream cytokine/chemokine responses but not TLR3-TRIF responses. Expression of spliced stress protein XBP-1 was not affected by alcohol exposure in monocytes. While HspA1A, induced by in vivo and in vitro alcohol, was required for alcohol-mediated inhibition of TLR4-MyD88 responses (17), HspA1A was not required or sufficient to cause inhibition of TLR4-TRIF responses, indicating selective regulation of TLR4 downstream pathways by alcohol-induced HspA1A. Alcohol-mediated inhibition of TLR4-TRIF responses was prevented by inhibition of PP1, indicating alcohol-mediated regulation of the PP1-IRF3 axis downstream of TLR4-TRIF. These results elucidate selective mechanisms involved in impaired immune responses after binge alcohol drinking and are clinically relevant as they can not only guide in targeted drug development for treatment of immune dysfunction in alcohol abuse patients but also advance our understanding of the pathophysiologic effects of binge drinking in humans.
MATERIALS AND METHODS
In vivo alcohol study
Seventeen healthy volunteers were included in the study which was approved by the Committee for Protection of Human Subjects in Research at UMass Medical School and the study was carried out in accordance with these approved guidelines. A questionnaire that incorporated the Alcohol Use Disorders Identification Test and CAGE tests was used to determine the alcohol use habits of the donors before recruitment into the study (31, 32). Informed consent was obtained from all subjects. Recruited males had fewer than nine drinks/week, females less than 6 drinks/week, and donors abstained from alcohol for 48 hours before participation. To study the in vivo effect of alcohol on monocyte function, the donors consumed alcohol (2 ml vodka/kg body weight in a total volume of 300 ml orange juice) over a period of 30 minutes while in the Clinical Research Center (CRC) at University of Massachusetts Medical Center. The individuals were monitored for approximately 6 hours or until their blood alcohol concentrations (BAC) decreased to 0.004 g/dl or less, after which the patients were allowed to leave the CRC. Blood samples were obtained before, 1 hour and 24 hours after the alcohol consumption and subjected to isolation of monocytes by adherence.
In vitro alcohol exposures
Twenty healthy individuals (ages 18 to 60, females and males) with no previous alcohol abuse history and who consumed less than 6 drinks/week were recruited in the study. Informed consent was obtained from all subjects. These individuals abstained from alcohol for 48 hours prior to study. Blood samples were obtained from these donors and subjected to isolation of monocytes by adherence. This study was reviewed and approved by University of Massachusetts Institutional Review Board and Department of Defense Human Research Protection Office. The study was performed at the Clinical Research Center (CRC) at University of Massachusetts Medical Center in accordance with these approved guidelines.
Cell stimulations
Peripheral blood from the healthy donors was subjected to Ficoll gradient separation to isolate mononuclear cells. Monocytes from these mononuclear cells were isolated by selective adherence as previously described (33). The adherent human monocytes were cultured in Iscove’s Modified Dulbecco’s Medium (Invitrogen Life Technologies) with 10% FBS (Serum Source International).
RAW 264.7 murine macrophages, purchased from American Type Culture Collection, were maintained in Dulbecco’s Modified Eagle Medium (Invitrogen Life Technologies) containing 10% FBS (Serum Source International).
Monocytes or macrophages plated at 1×106 cells/ml were treated with ethanol at 25 mM or 50 mM concentrations respectively and stimulated with 100 ng/ml Escherichia coli-derived LPS (Sigma-Aldrich) or 25 μg/ml high molecular weight (HMW) polyI:C (Invivogen). The 25 mM in vitro alcohol concentration approximates a blood alcohol level of 0.1 g/dl, which is above the legal limit of 0.08 g/dl BAC. In experiments involving PP1-specific inhibitor tautomycetin (R&D systems) (34), inhibitor was added to macrophages at 0.25 μM concentration along with ethanol (35). This concentration was selected based on efficient inhibition of PP1 and no cell death was observed at this concentration (data not shown). To heat shock for a positive control of HspA1A induction, cells were subjected to 42°C for 45 minutes.
RNA analysis and qRT-PCR
Total RNA was isolated from the monocytes/macrophages using the RNeasy Mini column purification kit (Qiagen) according to manufacturer’s instructions. Quantification of RNA was done by spectrophotometric analysis and OD 260/280 ratio was used to verify RNA quality. cDNA was synthesized using the Reverse Transcription system (Promega) according to the manufacturer’s instructions. The qRT-PCR reaction was set up using 6.25 μL iTAQ universal SYBR Green PCR master mix (Bio-Rad Laboratories), 0.25 μM each of forward and reverse primers, and 2.5 μL cDNA (corresponding to 25 ng RNA) for a total reaction volume of 12.5 μL. Real-time quantitative PCR was performed using the CFX96 real-time detection system (Bio-Rad Laboratories). The ΔCt of the gene was normalized to the ΔCt of housekeeping gene 18S rRNA and fold change of mRNA was expressed relative to untreated cells. Human IFNβ, IP-10, TNFα, IL-6, IL-1β, IKKε, spliced XBP-1, unspliced XBP-1 primers and mouse TNFα, RANTES, HspA1A and 18S primer pairs were synthesized by IDT Inc. and enumerated in Table I. Human RANTES primer pairs were purchased from SABiosciences.
Table I.
Sequences of primers used for qPCR
| Gene | Primer Sequence |
|---|---|
| hTNFα | Fwd, 5′-ATCTTCTCGAACCCCGAGTGA-3′ Rev, 5′-CGGTTCAGCCACTGGAGCT-3′ |
| hIL-6 | Fwd, 5′-CGAGCCCACCGGGAACGAAA-3′ Rev, 5′-GGACCGAAGGCGCTTGTGGAG-3′ |
| hIFNβ | Fwd, 5′- ACTGCCTCAAGGACAGGATG - 3′ Rev, 5′ – AGCCAGGAGGTTCTCAACAA – 3′ |
| hIP-10 | Fwd, 5′ - GAGCCTACAGCAGAGGAACC - 3′ Rev, 5′ – GAGTCAGAAAGATAAGGCAGC – 3′ |
| h sXBP-1 | Fwd, 5′ – CTGAGTCCGAATCAGGTGCAG - 3′ Rev, 5′ – ATCCATGGGGAGATGTTCTGG - 3′ |
| h usXBP-1 | Fwd, 5′ – CAGCACTCAGACTACGTGCA - 3′ Rev, 5′ – ATCCATGGGGAGATGTTCTGG - 3′ |
| hIKKε | Fwd, 5′ – CTGCTCATGAATGACAGTGA- 3′ Rev, 5′ – GGCGAGTGTATGTTATGCTT - 3′ |
| mTNFα | Fwd, 5′-GAAGTTCCCAAATGGCCTCC-3′ Rev, 5′-GTGAGGGTCTGGGCCATAGA-3′ |
| mHsp70 | Fwd, 5′-AACTACAAGGGCGAGAACCGGTC-3′ Rev, 5′-GATGATCCGCAGCACGTTCAGA-3′ |
| mRANTES | Fwd, 5′ - GCTGCTTTGCCTACCTCTCC -3′ Rev, 5′ TCGAGTGACAAACACGACTGC – 3′ |
| 18S | Fwd, 5′- GTAACCCGTTGAACCCCATT-3′ Rev, 5′- CCATCCAATCGGTAGTAGCG-3′ |
Enzyme-linked immunosorbent assay (ELISA)
Cell-free supernatants were collected from human monocyte or RAW macrophage cultures and analyzed for human RANTES (R&D systems) or murine TNFα (BD Biosciences) and RANTES (Peprotech Inc) according to the manufacturer’s instructions.
Preparation of nuclear, cytoplasmic and whole cell extracts
Nuclear and cytoplasmic extracts from macrophages were prepared as previously described (33). Briefly, at the end of the stimulation period, cells collected in ice-cold PBS were resuspended in cold hypotonic buffer A and incubated on ice for 20 min. Cells were then lysed with 0.6% Nonidet P-40 by vortexing for 20 seconds. The lysate was centrifuged at 12,000 × g for 1 minute to pellet the nuclei, and the supernatant was stored at −80°C as the cytoplasmic extract. The nuclear pellet was then resuspended in ice-cold buffer B (20 mM HEPES (pH 7.9), 400 mM KCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 1 mM PMSF, and 20% glycerol). All tubes were kept on a shaker at 4°C for 30 min. The lysate was then centrifuged at 12,000 × g for 10 min, and the supernatant was stored at −80°C as the nuclear extract.
For whole cell lysates, macrophages were resuspended in lysis buffer (10% glycerol, 1% Triton, 20 mM Tris, pH7.6, 150mM NaCl, 25 mM β-glycerophosphate, 50 mM NaF, 1 mM Na-orthovanadate, 1 mM EDTA and 1 mM DTT with protease inhibitors) and incubated for 20 minutes on ice. The lysates were then centrifuged at 12,000 × g for 10 min and the supernatant was collected for whole cell lysates.
Protein content in the whole cell, cytoplasmic or nuclear extracts was determined by the Bio-Rad protein assay dye reagent (Bio-Rad Laboratories).
Immunoblotting (Western blotting)
Whole cell, cytoplasmic or nuclear lysates (5–25 μg) were heat denatured and separated on 10% SDS-polyacrylamide gel and electroblotted onto nitrocellulose membranes. Membrane blots were blocked for nonspecific binding in TBS/5% nonfat dried milk/0.1% Tween 20 or TBS/5% BSA/0.1% Tween 20 followed by the antibodies indicated in each experiment. The mouse antibodies against HspA1A (HSPA1A), TBP-1 and β-actin were purchased from StressMarq Biosciences and Abcam respectively. The anti-human rabbit antibodies against phospho-TBK1, phospho-IRF3, total TBK1 and total IRF3 were from Cell Signaling Technology. These primary antibodies were detected using HRP-conjugated anti-mouse and anti-rabbit secondary antibodies (Santa Cruz Biotechnology and Abcam) and chemiluminescence assay reagents from Bio-Rad. The immunoreactive bands were quantified by densitometric analysis using a UVP System (Bio-Rad Laboratories).
Transient transfection of plasmids and siRNA
We received the HspA1A-CMV5 overexpression (pCMV5-HspA1A) plasmid as a gift from Dr. R. Morimoto (Northwestern University, Chicago, IL, USA) (36). RAW macrophages were transfected with 2 μg of plasmid at varying DNA:lipofectamine ratios in Opti-MEM for 6 hours using Lipofectamine 2000 (Invitrogen). We used ratios ranging from 1:1 to 1:3 for increasing HspA1A expression levels, with highest expression seen at 1:3 ratio (19). For knockdown experiments, RAW macrophages were transfected with 10 nM HspA1A siRNA (from Invitrogen Stealth Library) (synthesized by Invitrogen) or negative control in Opti-MEM for 24 hours using Lipofectamine 2000 as previously shown (37). siRNA knockdown efficiency was confirmed by qRT-PCR and Western blot for HspA1A. Twenty-four hours after transfection, cells were treated with ethanol or LPS as indicated and supernatants were collected.
Statistical analysis
Results are presented as mean ± SD. One way ANOVA analysis with Dunnett’s test or Sidak’s test for multiple comparisons was used to determine the statistical significance of differences between samples. Values of p < 0.05 were considered to represent statistical significant differences.
RESULTS
In vivo binge alcohol drinking in humans inhibits cytokine responses in peripheral blood monocytes downstream of TLR4-MyD88 dependent pathway
Previous studies have illustrated the suppressive effects of alcohol on LPS/TLR4 signaling with the TLR4-MyD88 signaling pathway being most studied (16, 20). Here we extend this observation to a physiologically relevant human model of alcohol consumption to determine effects of in vivo alcohol drinking on peripheral blood monocytes. As illustrated in Figure 1A, consumption of 2 ml/kg body weight of vodka by the volunteers resulted in BAC close to the legal limit of 0.08g/dl at 1 hour after alcohol consumption. At baseline, human monocytes stimulated with LPS showed robust cytokine responses downstream of TLR4-MyD88 signaling pathways (Figure 1B–C). However, monocytes isolated from blood of volunteers at 1 and 24 hours post-alcohol consumption showed significantly lower levels of TLR4-MyD88 cytokines TNFα (Figure 1B) and IL-6 (Figure 1C) mRNA compared to baseline LPS-stimulated monocytes. This is consistent with our previous studies that demonstrated human monocytes treated with alcohol in vitro for multiple time points (19) or in vivo (20) were severely impaired in TLR4-induced TNFα production. Supplementary Figure 1 illustrating significant inhibition of LPS-induced pro-inflammatory cytokines TNFα and IL-6 mRNA by monocytes after 24 hours of alcohol exposure in vitro provides a summary of our own previous findings (19). Taken together, here we show alcohol-mediated inhibition of TLR4-MyD88 induced IL-6 and TNFα which persists for 24 hours after binge alcohol consumption, suggesting an extended immunosuppressive effect of in vivo binge alcohol consumption in humans.
Figure 1. In vivo alcohol exposure significantly inhibits TLR4-MyD88 dependent pro-inflammatory cytokine mRNA expression by human monocytes.
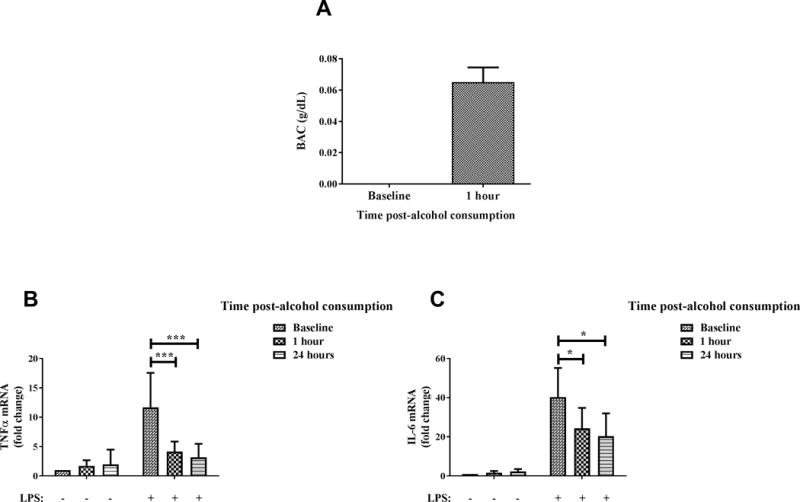
(A) Healthy human donors consumed alcohol (2 ml vodka/kg body weight in a total volume of 300 ml orange juice) over the period of an hour and their BAC was measured at baseline and 1 hour after alcohol consumption. Graph depicts mean ± SD of 10 independent experiments. (B-C) Primary human monocytes were isolated from blood of healthy human donors at baseline and 1 hour and 24 hours after alcohol consumption, following which the cells were stimulated with 100 ng/ml LPS for 2 hours. Levels of (B) TNFα and (C) IL-6 mRNA were analyzed by qRT-PCR. Fold change in expression of genes was calculated with respect to baseline. Data summarize mean ± SD of 6–8 experiments and statistical analysis was performed by one way ANOVA with Dunnett’s test for multiple comparisons. (ns p>0.05, * p<0.05, ** p<0.01, *** p<0.001, ****p=0.0001)
In vivo and in vitro binge alcohol inhibits TLR4-TRIF cytokine/chemokine production in human monocytes
To examine the effect of binge alcohol on TLR4-TRIF (MyD88 independent) pathway after in vivo alcohol exposure of human monocytes, we examined the expression of cytokines/chemokines which are characteristic of activation of TLR4-TRIF signaling in monocytes isolated from blood of volunteers after alcohol consumption. IFNβ (Figure 2A), RANTES (Figure 2B) and IP-10 (Figure 2C) mRNA were significantly inhibited in ex vivo LPS-stimulated monocytes collected 1 hour (high BAC) and 24 hours post-alcohol consumption compared to LPS-stimulated baseline (before alcohol consumption) monocytes. Consistent with mRNA results, Figure 2D shows that LPS-induced secreted RANTES was significantly inhibited in monocytes collected at both time points after alcohol consumption. These results indicate for the first time that in vivo alcohol exposure inhibits TLR4-TRIF cytokines/chemokines expression by human monocytes.
Figure 2. In vivo alcohol exposure significantly inhibits TLR4-TRIF cytokine/chemokine expression by human monocytes.
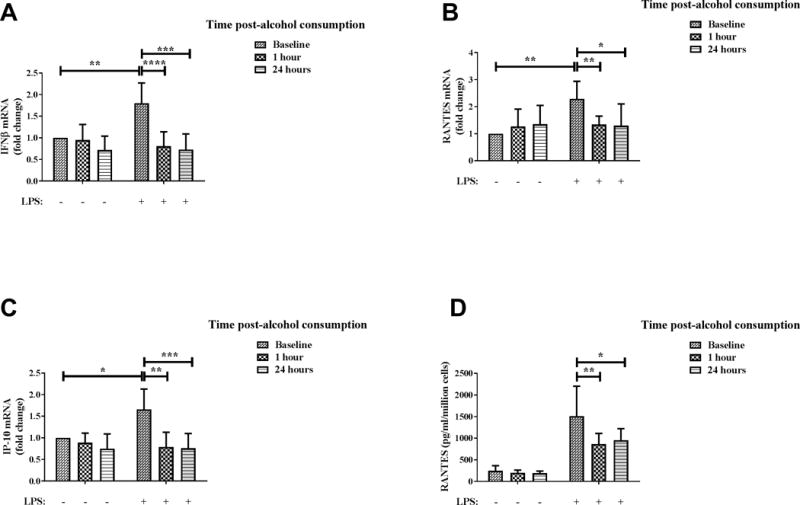
Primary human monocytes were isolated from blood of healthy human donors at baseline and 1 hour and 24 hours after alcohol consumption, following which the cells were stimulated with 100 ng/ml LPS. Levels of (A) IFNβ, (B) RANTES and (C) IP-10 mRNA after 2 hours of LPS stimulation were analyzed by qRT-PCR. Fold change in expression of genes was calculated with respect to baseline. (D) Secreted RANTES protein after overnight (18–22 hours) LPS stimulation was analyzed by ELISA. Data summarize mean ± SD of 6–9 independent experiments and statistical analysis was performed by one way ANOVA with Dunnett’s test for multiple comparisons. (ns p>0.05, * p<0.05, ** p<0.01, *** p<0.001, ****p=0.0001)
Next, in order to comprehensively examine the effect of alcohol on TLR4-TRIF pathway, we used the in vitro approach wherein the effect of alcohol exposure at multiple time points on the expression of LPS-induced TLR4-TRIF cytokines/chemokines in monocytes was analyzed. We used ethanol at a concentration of 25 mM (physiologically relevant because it approximates 0.1 g/dl BAC and is similar to the BAC in alcohol-drinking volunteers). As shown in Figure 3, monocytes stimulated with LPS significantly upregulated mRNA expression of type I interferon IFNβ (Figure 3A), chemokines RANTES (Figure 3B) and IP-10 (Figure 3C) at a magnitude consistent with previous studies (38). However, alcohol exposure at all time points up to 24 hours, even as early as 1 hour, exhibits significant downregulation of these LPS-induced TLR4-TRIF cytokine/chemokines in human monocytes (Figure 3A–C). Secreted RANTES protein levels, as examined by ELISA, were consistent with mRNA data wherein LPS-induced RANTES was significantly inhibited by exposure to alcohol in vitro for 1–24 hours in human monocytes (Figure 3D). Collectively, our observations illustrate that in vivo exposure by alcohol drinking by human volunteers or in vitro exposure (1–24 hours) exhibit inhibition of subsequent LPS stimulated cytokine/chemokine responses downstream of TLR4-TRIF signaling.
Figure 3. Exposure of human monocytes to in vitro alcohol significantly suppresses TLR4-TRIF cytokine/chemokine expression.
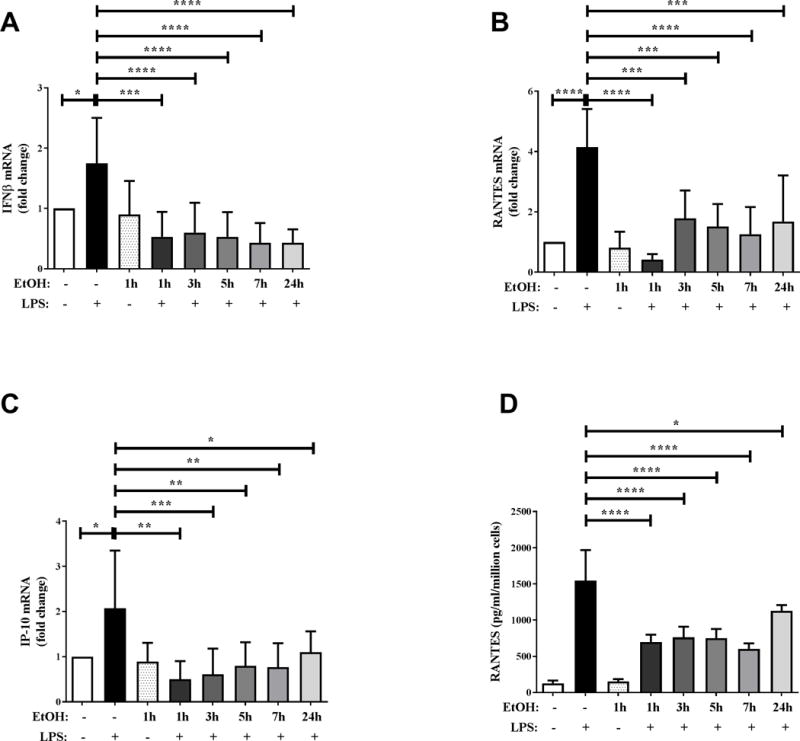
Adherence isolated human monocytes from healthy donors were treated with 100 ng/ml LPS or 25 mM alcohol (EtOH) for 2 hours or pre-exposed to 25 mM alcohol (EtOH) for 1, 3, 5, 7 or 24 hours followed by LPS stimulation for 2 hours. Levels of (A) IFNβ, (B) RANTES and (C) IP-10 mRNA after 2 hours of LPS stimulation were analyzed by qRT-PCR. Fold change in expression of genes was calculated with respect to untreated. (D) Secreted RANTES protein after overnight (18–22 hours) LPS stimulation was analyzed by ELISA. Data summarize mean ± SD of 6 independent experiments and statistical analysis was performed by one way ANOVA with Dunnett’s test for multiple comparisons. (ns p>0.05, * p<0.05, ** p<0.01, *** p<0.001, ****p=0.0001)
TLR3-TRIF responses are not suppressed by in vivo or in vitro binge alcohol exposure of human monocytes
Previous studies in mouse models have shown inhibition of TLR3-induced cytokines by binge alcohol exposure (22, 23). In addition, alcohol increases risk of viral infections which are targeted by type I IFN responses (2, 21). To examine the effect of in vivo binge alcohol exposure of human monocytes on TLR3-TRIF cytokine responses, we examined the expression of IFNβ, IP-10 and RANTES in polyI:C stimulated monocytes. As shown in Figure 4, expression of IFNβ (Figure 4A), RANTES (Figure 4B) and IP-10 (Figure 4C) mRNA was upregulated after polyI:C stimulation of baseline monocytes (before alcohol consumption). Secreted RANTES levels were also significantly induced by polyI:C stimulation (Figure 4D). However, IFNβ (Figure 4A), RANTES (Figure 4B) and IP-10 (Figure 4C) mRNA and secreted RANTES (Figured 4D) were not suppressed in monocytes collected 1 hour or 24 hours after alcohol consumption indicating TLR3-TRIF responses are not altered by in vivo binge alcohol exposure in human monocytes. To further test this, human monocytes from healthy donors were stimulated with polyI:C after exposure to alcohol for multiple time-points in vitro. Consistent with in vivo results, polyI:C stimulated IFNβ (Figure 5A), RANTES (Figure 5B) and IP-10 (Figure 5C) mRNA levels were not suppressed by in vitro alcohol exposure at any time-points tested. Secreted RANTES (Figure 5D) protein level after polyI:C stimulation was also unaffected by in vitro alcohol exposure. Therefore unlike TLR4 responses, in vivo and in vitro binge alcohol exposure does not inhibit polyI:C stimulated TLR3-TRIF cytokines/chemokines in human monocytes.
Figure 4. TLR3-TRIF cytokine/chemokine expression by human monocytes are not suppressed by in vivo binge alcohol consumption.
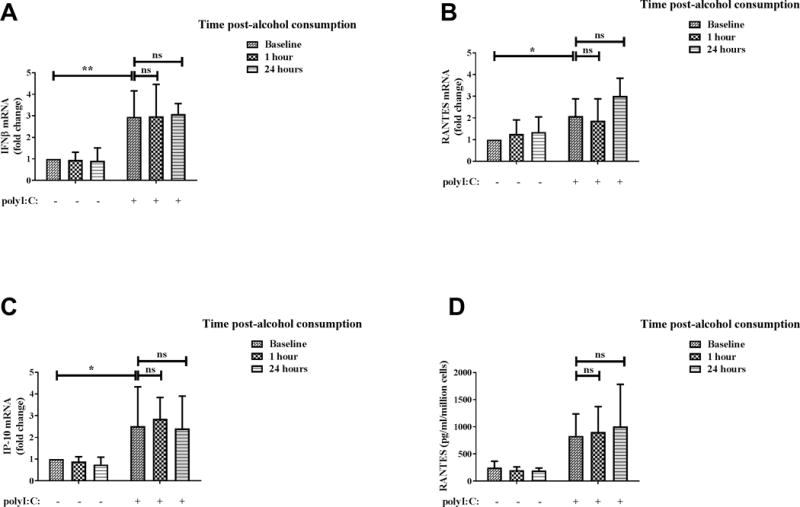
Primary human monocytes were isolated from blood of healthy human donors at baseline and 1 hour and 24 hours after alcohol consumption, following which the cells were stimulated with 25 μg/ml polyI:C. Levels of (A) IFNβ, (B) RANTES and (C) IP-10 mRNA after 2 hours of polyI:C stimulation were analyzed by qRT-PCR. Fold change in expression of genes was calculated with respect to baseline. (D) Secreted RANTES protein after overnight (18–22 hours) polyI:C stimulation was analyzed by ELISA. Data summarize mean ± SD of 6–9 independent experiments and statistical analysis was performed by one way ANOVA with Dunnett’s test for multiple comparisons. (ns p>0.05, * p<0.05, ** p<0.01, *** p<0.001, ****p=0.0001)
Figure 5. In vitro alcohol exposure of human monocytes does not inhibit TLR3-TRIF cytokine/chemokine expression.
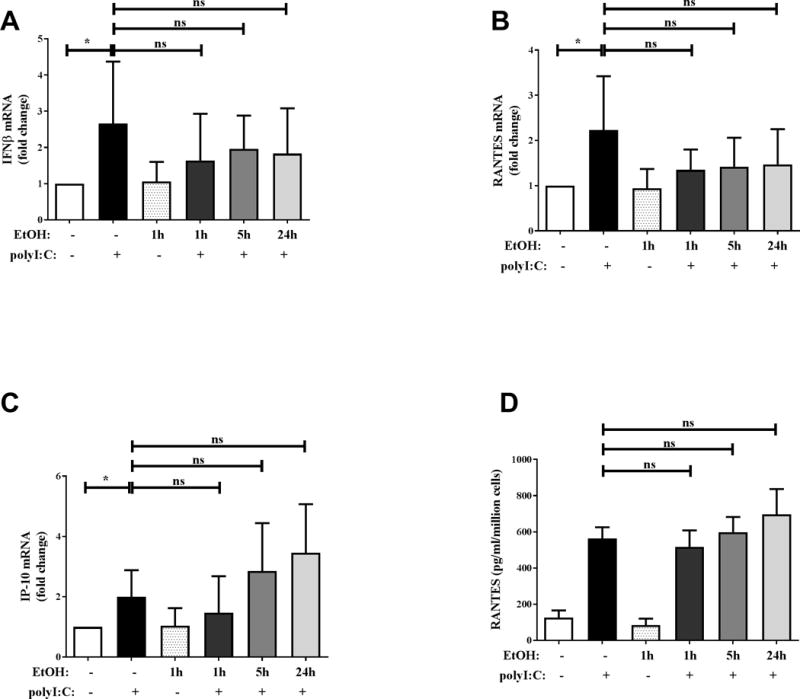
Adherence isolated human monocytes from healthy donors were treated with 25 μg/ml polyI:C or 25 mM alcohol (EtOH) for 2 hours or pre-exposed to 25 mM alcohol (EtOH) for 1, 5 or 24 hours followed by polyI:C stimulation for 2 hours. Levels of (A) IFNβ, (B) RANTES and (C) IP-10 mRNA after 2 hours of polyI:C stimulation were analyzed by qRT-PCR. Fold change in expression of genes was calculated with respect to untreated. (D) Secreted RANTES protein after overnight (18–22 hours) polyI:C stimulation was analyzed by ELISA. Data summarize mean ± SD of 6 independent experiments and statistical analysis was performed by one way ANOVA with Dunnett’s test for multiple comparisons. (ns p>0.05, * p<0.05, ** p<0.01, *** p<0.001, ****p=0.0001)
Alcohol exposure blocks activation of TLR4-TRIF downstream effectors
Expression of IFNβ, RANTES and IP-10 downstream of activation of the TLR4-TRIF pathway is regulated by signaling molecule TBK1 and transcription factor IRF3 (7, 39). Based on the suppressive effects of alcohol on TLR4-TRIF cytokine/chemokines, we performed mechanistic analysis to examine the effect of in vitro alcohol exposure on upstream signaling molecules in the TLR4-TRIF pathway using murine RAW264.7 macrophages. We have previously validated RAW macrophages as an acceptable model to examine alcohol effects on TLR responses and have used these cells due to limitations in monocyte cell numbers from human peripheral blood (19). As expected, LPS stimulation induced phosphorylation of TBK1 (Figure 6A) and IRF3 (Figure 6B), indicative of activation of downstream signaling molecules. Interestingly while alcohol pre-exposure did not significantly affect phosphorylation of TBK1 (Figure 6A), it significantly inhibited LPS-induced phosphorylation of IRF3 at the 1 hour time point (Figure 6B), but not at 24 hours. These studies indicate that alcohol exposure transiently suppressed phospho-activation of IRF3 downstream of TLR4-TRIF signaling, independent of TBK1. We also examined expression of IKKε, another regulator of IRF3 downstream of TLR4 activation (9) and found a statistically significant decrease in expression of IKKε by qRT-PCR (Supplementary Figure 2). However, the slower kinetics of LPS induced IKKε and its downstream IRF3 activation shown in previous studies (40) suggests alternative regulators of IRF3 activation are being affected during early alcohol exposure. Thus, it is imperative to evaluate alcohol mediated regulators of TLR4-TRIF-IRF3 signaling, independent of TBK-1.
Figure 6. In vitro alcohol exposure inhibits activation of TLR4-MyD88 independent signaling molecules in RAW macrophages.
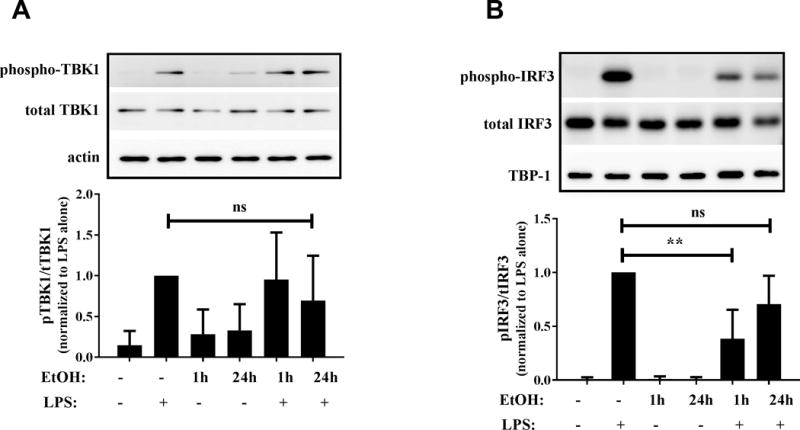
RAW macrophages were stimulated with 100 ng/ml LPS for 30 minutes, 50mM alcohol (EtOH) alone for 1 or 24 hours or pre-exposed to alcohol followed by LPS stimulation for 30 minutes. (A) TBK1 (phospho and total) and actin and (B) IRF3 (phospho and total) and TBP-1 were detected in cytoplasmic and nuclear lysates respectively by western blotting. Density of bands was measured and ratio of phospho to total protein was calculated. Graph represents the phospho/total ratio normalized to LPS alone condition and depicts mean ± SD of 4 independent experiments and statistical analysis was performed by one way ANOVA with Dunnett’s test for multiple comparisons. (ns p>0.05, * p<0.05, ** p<0.01, *** p<0.001, ****p=0.0001). Representative gels are shown with phospho-TBK1 or phospho-IRF3 (top) and total TBK1 or total IRF3 (middle) and actin or TBP-1 as loading controls (bottom).
ER stress has been shown to induce TBK1-independent IRF3 activation (41) and splicing of ER stress protein XBP-1 has also been shown to be essential in TLR-induced IFNβ responses (27–29) and upregulated by LPS stimulation (30). Analysis of spliced XBP-1 after in vitro alcohol exposure did not reveal any significant downregulation in the ratio of spliced to unspliced XBP-1 expression in alcohol pretreated monocytes stimulated with LPS compared to LPS alone (Supplementary Figure 3). Thus, our results show that XBP-1 may not be the candidate to regulate IRF3 mediated IFNβ inhibition during binge alcohol exposure in monocytes/macrophages.
Alcohol-mediated suppression of TLR4-TRIF signaling is dependent on PP1
Based on previous studies showing TBK-1 independent regulation of IRF3 by negative regulator PP1 (13) and its activation by alcohol (24, 25), we examined the effect of PP1 inhibition on TLR4-MyD88 and TLR4-TRIF responses in terms of TNFα and RANTES expression by qRT-PCR. While PP1 inhibitor tautomycetin did not affect LPS-stimulated TNFα expression significantly (Figure 7A), we observed a significant increase in expression of LPS-stimulated RANTES in the presence of PP1 inhibitor tautomycetin compared to cells that were not treated with the inhibitor (Figure 7B). Furthermore, alcohol-induced suppression of TLR4-TRIF RANTES was prevented in the presence of tautomycetin compared to alcohol pre-exposure alone, suggesting a role for PP1 in alcohol mediated regulation of TLR4-TRIF responses. Collectively, our results indicate that alcohol mediated suppression of TLR4 responses downstream of both TRIF and MyD88 branches are regulated discretely. HspA1A plays a selective role in alcohol-mediated suppression of TLR4-MyD88 responses but not TLR4-TRIF (MyD88 independent) signaling which is dependent on alcohol-mediated effects on the PP1-IRF3 axis.
Figure 7. PP1 inhibition reverses alcohol-mediated inhibition of TLR4-TRIF chemokine RANTES but not TNFα.
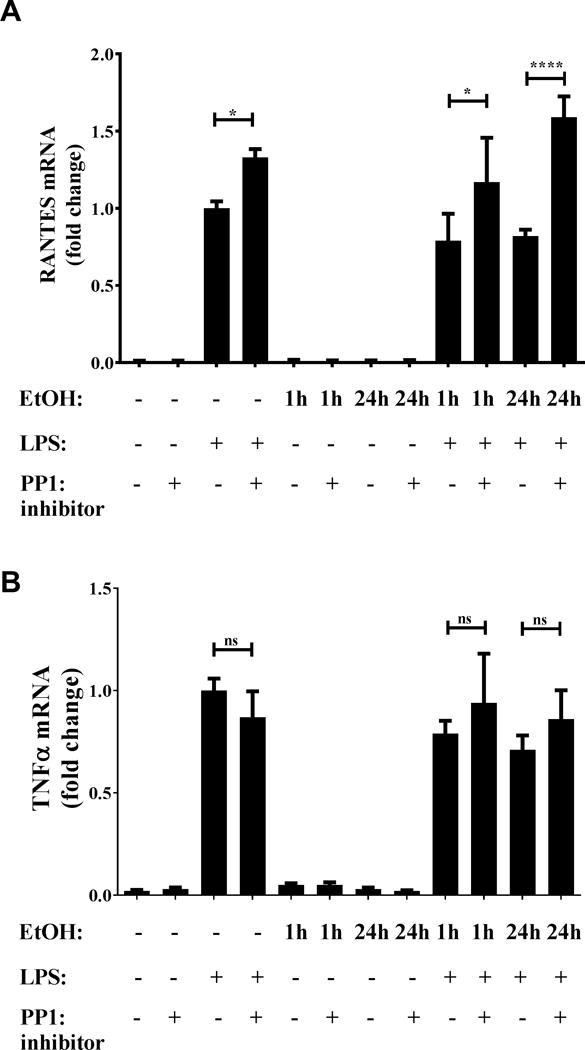
RAW macrophages were stimulated with 100 ng/ml LPS for 2 hours, 50 mM alcohol (EtOH) and 0.25 μM tautomycetin alone or in combination for 24 hours or pre-exposed to alcohol and tautomycetin followed by LPS stimulation for indicated times. (A) TNFα and (B) RANTES mRNA was analyzed by qRT-PCR and normalized to LPS stimulation alone. Graph depicts mean ± SD of 3 independent experiments and statistical analysis was performed by one way ANOVA with Sidak’s test for multiple comparisons. (ns p>0.05, * p<0.05, ** p<0.01, *** p<0.001, ****p=0.0001)
HspA1A is induced during binge alcohol consumption and its overexpression suppresses TLR4-MyD88 but not TLR4-TRIF responses
A previous study from our lab has shown induction of HspA1A mRNA after alcohol exposure in vitro in human monocytes, which played a mechanistic role in suppression of TLR4-MyD88 responses (19). Heat shock proteins have been shown to negatively regulate type I interferon responses and IRF3 activation in response to dengue and Sendai viral infections (42, 43). Here, we aimed to first delineate whether in vivo binge alcohol consumption induces HspA1A expression in human monocytes and determine whether HspA1A regulates TLR4-TRIF signaling. As shown in Figure 8A, we observed significant induction of HspA1A mRNA in human monocytes upon in vivo alcohol exposure at an early 1 hour time-point post-alcohol consumption, consistent with a trend to increase in HspA1A protein expression at a late 24 hour post-alcohol time-point (Figure 8B). Due to limitations in blood sampling post-alcohol consumption in binge alcohol drinking volunteers, we sought to use in vitro alcohol exposed monocytes to determine the kinetics of HspA1A protein induction in human monocytes. HspA1A protein in human monocytes exposed to alcohol directly in vitro was significantly upregulated as early as 6 hours and while there was some decrease compared to induction at 6 hours it remained higher than untreated at 15–20 hours (Figure 8C), consistent with monocytes after in vivo binge alcohol (Figure 8B) and our previously reported results (19). Thus it is plausible that maximal induction of HspA1A after in vivo binge alcohol drinking occurs at a time-point earlier than 24 hours in human monocytes.
Figure 8. In vitro and in vivo alcohol induces HspA1A expression in human monocytes.
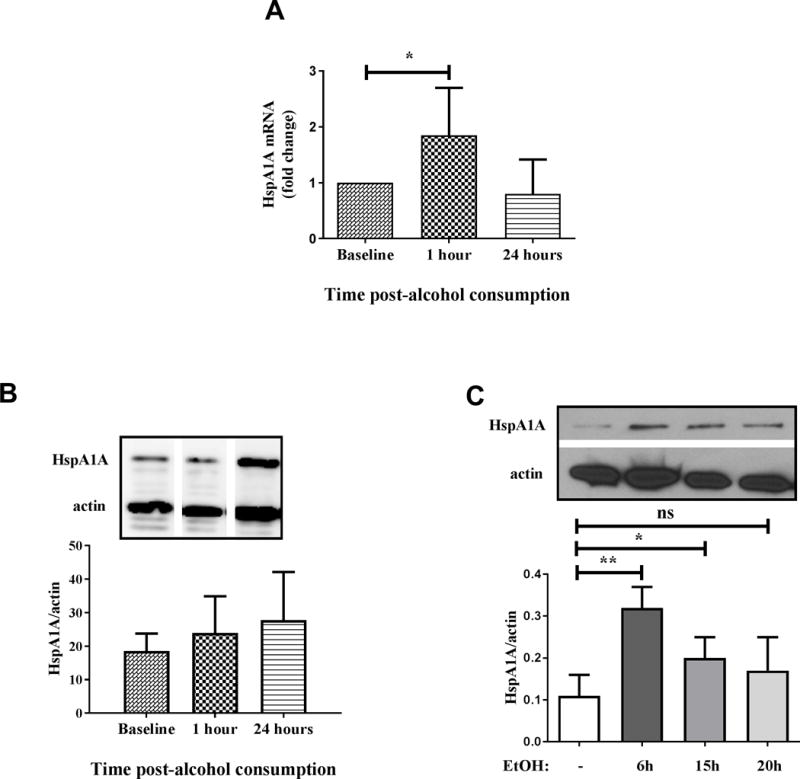
(A) Primary human monocytes were isolated from blood of healthy human donors at baseline and 1 hour and 24 hours after alcohol consumption and total RNA was analyzed for HspA1A mRNA by qRT-PCR. Graph depicts mean ± SD of 9 independent experiments. (B) Primary human monocytes were isolated from blood of healthy human donors at baseline and 1 hour and 24 hours after alcohol consumption and HspA1A protein was detected in whole cell lysates by western blotting. The densitometry graph represents quantitation of bands seen in the gel and depicts mean ± SD (n=2). Representative gels are shown with HspA1A (top) and loading control, β-actin (bottom). Gel pictures cropped from different lanes of a single gel for comparison. (C) Human monocytes isolated by adherence were exposed to 25 mM alcohol (EtOH) for 6–20 hours and HspA1A protein was detected in whole cell lysates by western blotting. The densitometry graph represents quantitation of bands seen in the gel and depicts mean ± SD (n=4) and statistical analysis was performed by one way ANOVA with Dunnett’s test for multiple comparisons. (ns p>0.05, * p<0.05, ** p<0.01, *** p<0.001, ****p=0.0001). Representative gels are shown with HspA1A (top) and loading control, β-actin (bottom).
Next, to determine functional implication of alcohol mediated HspA1A induction, we wanted to test if overexpression of HspA1A that mimics alcohol-mediated induction has any effect on TLR4-TRIF responses in RAW cells. For overexpression, RAW macrophages were transiently transfected with pCMV5-HspA1A plasmid, which resulted in a stepwise increase in expression of HspA1A as shown previously (19). Untransfected RAW cells respond to LPS stimulation with robust induction of TNFα (Figure 9A) and RANTES (Figure 9B) downstream of TLR4-MyD88 and TLR4-TRIF activation, respectively. While TNFα expression significantly reduced in a manner inversely correlated with HspA1A expression in LPS-stimulated transfected macrophages, RANTES expression remained significantly high, even showing a trend for increase, in LPS-stimulated HspA1A overexpressing cells. Overall, these results indicate that HspA1A does not play a mechanistic role in suppression of TLR4-TRIF responses but negatively regulates TLR4-MyD88 pathway.
Figure 9. Overexpression of HspA1A is sufficient to inhibit TLR4-MyD88 dependent cytokine TNFα but not TLR4-MyD88 independent chemokine RANTES.
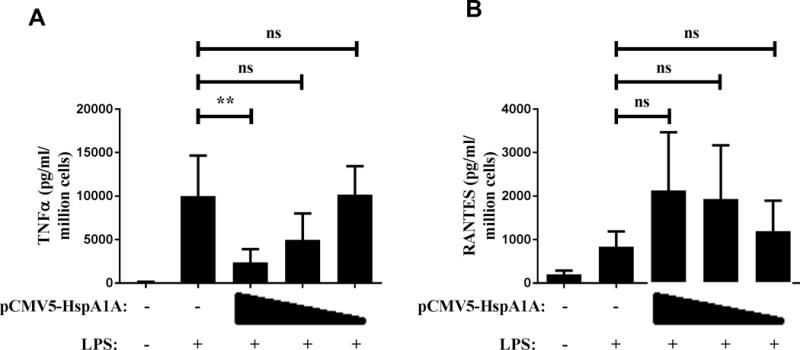
RAW macrophages were transiently transfected with pCMV5-HspA1A using 1:1, 1:2 and 1:3 ratios of 2 μg DNA to lipofectamine. 24 hours after transfection, macrophages were stimulated with 100 ng/ml LPS for 6 hours and culture supernatants were assayed for secreted (A) TNFα and (B) RANTES by ELISA. Graph depicts mean ± SD (n=4) and statistical analysis was performed by one way ANOVA with Dunnett’s test for multiple comparisons. (ns p>0.05, * p<0.05, ** p<0.01, *** p<0.001, ****p=0.0001)
Alcohol-mediated suppression of TLR4-TRIF signaling does not require HspA1A
To further evaluate if HspA1A is required for alcohol-mediated inhibition of TLR4-TRIF pathway, HspA1A was knocked down using siRNA in alcohol-treated RAW macrophages. As shown in Figure 10A, we observed 88% knockdown in HspA1A mRNA expression upon transfection with siRNA which was also confirmed at the protein level by Western blotting (Fig 10A). As expected, control transfected macrophages exposed to alcohol in vitro for 24 hours showed significant inhibition of LPS-induced TNFα and RANTES, downstream of TLR4-MyD88 and TLR4-TRIF activation respectively (Figure 10B and C). While alcohol-mediated inhibition of TNFα was abrogated in HspA1A silenced macrophages, alcohol-mediated suppression of RANTES expression was maintained even in the absence of HspA1A expression (Figure 10B and C). These results indicate that HspA1A is not required for alcohol-mediated suppression of TLR4-TRIF responses in contrast to its inhibitory effects on TLR4-MyD88 responses.
Figure 10. HspA1A is required for alcohol-mediated inhibition of TLR4-MyD88 dependent TNFα but not TLR4-MyD88 independent RANTES.
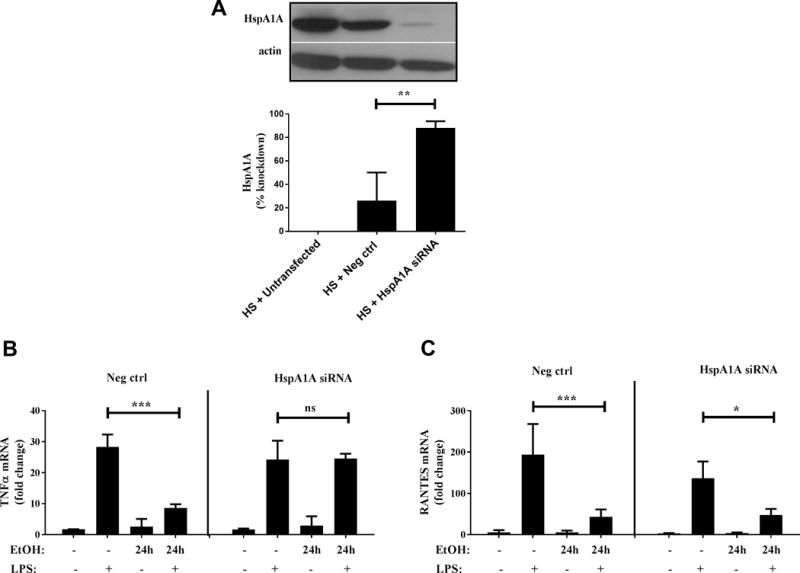
(A) RAW macrophages were transfected with 10 nM siRNA targeting HspA1A or negative control (Neg ctrl) siRNA. Cells were subjected to heatshock (HS) (42°C for 45 min) and total RNA was used for HspA1A mRNA determination by qRT-PCR. Percent knockdown in HS cells was calculated with respect to untransfected. Graph depicts mean ± SD (n=3). Representative gels are shown to illustrate knockdown at protein level with HspA1A (top) and loading control, β-actin (bottom). (B,C) RAW macrophages were transfected with 10 nM siRNA targeting HspA1A or negative control (Neg ctrl) siRNA. 24 hours after transfection, macrophages were stimulated with 100 ng/ml LPS for 2 hours, 50 mM alcohol (EtOH) alone for 24 hours or pre-exposed to alcohol followed by LPS stimulation for indicated times. (B) TNFα and (C) RANTES mRNA was analyzed by qRT-PCR. Graph depicts mean ± SD of 3 independent experiments and statistical analysis was performed by one way ANOVA with Sidak’s test for multiple comparisons. (ns p>0.05, * p<0.05, ** p<0.01, *** p<0.001, ****p=0.0001)
DISCUSSION
While the immunosuppressive effects of binge/moderate alcohol exposure on TLR4-MyD88 dependent signaling have been investigated, the effects of alcohol on TLR4-TRIF (MyD88 independent) and TLR3-TRIF signaling are not well defined. Furthermore the effects of in vivo binge alcohol consumption in humans on MyD88 and TRIF signaling have not been simultaneously studied. In this study, we describe for the first time the discrete suppressive effects of alcohol consumption on TLR4-MyD88 and TLR4-TRIF signaling, but not TLR3-TRIF signaling, using a model of human in vivo binge alcohol consumption. Additionally we employ in vitro alcohol exposure models to study mechanistic regulation of alcohol mediated cytokine responses in human monocytes. Moreover, we report for the first time that in vivo alcohol drinking in humans induces stress chaperone HspA1A in monocytes. We also show the negative regulatory role of HspA1A in alcohol-mediated immunosuppression of TLR4-MyD88 but not TLR4-TRIF signaling, whereas negative regulator PP1 mediates alcohol mediated TLR4-TRIF responses. These studies provide evidence for unique crosstalk mechanisms between inflammatory responses by TLR4-MyD88 signaling and cellular stress pathways, particularly induction of HspA1A, an emerging drug target (44), potentially for development of targeted modulation of immune responses. On the other hand, alcohol mediated TLR4-TRIF inhibition is not regulated by stress proteins but by protein phosphatase such as PP1.
We examined downstream cytokine/chemokine expression as readout of TLR-TRIF signaling to test the effect of alcohol on these pathways in human monocytes. It should be noted that although the magnitude of upregulation of TLR4-TRIF and TLR3-TRIF induced IFNβ, RANTES and IP-10 mRNA following LPS stimulation was modest compared to TLR4-MyD88 cytokines, it is consistent with cytokine mRNA levels observed by other groups examining human monocytes or monocyte-derived cells (45, 46). We were also able to corroborate RANTES protein levels by ELISA which was consistent with the corresponding significant changes in RANTES mRNA levels. Similar levels of IFNβ and RANTES mRNAs in monocytes from sepsis patients illustrates the biological significance of even such modest mRNA changes in a clinical in vivo model (38).
We utilized an IRB-approved, in vivo human alcohol binge drinking model to examine the effects of binge alcohol on TLR responses in primary human monocytes in this study. Utilizing blood from volunteers before and after alcohol consumption, we demonstrate that expression of LPS-stimulated TLR4-MyD88 dependent pro-inflammatory cytokines such as TNFα and IL-6 and TLR4-TRIF cytokines/chemokines IFNβ, RANTES and IP-10 in human monocytes is significantly inhibited after in vivo alcohol consumption. However, in vivo alcohol intake in humans did not suppress TLR3-induced IFNβ, RANTES and IP-10. To our knowledge, this is the first comprehensive report showing effects of in vivo alcohol consumption on TLR4-TRIF and TLR3-TRIF signaling in peripheral blood human monocytes.
Previous studies from our group showed suppression of TLR4-MyD88 cytokines upon in vitro alcohol exposure in human monocytes and the effect of moderate alcohol intake on TNFα and IL-1β in human monocytes (19, 20). Here we perform comprehensive time course analysis of in vitro alcohol exposure and show significant suppression of TLR4-TRIF mediated IFNβ, RANTES and IP-10 in primary human monocytes. This is consistent with Pang et al. (47), who have reported inhibition of TLR4-MyD88 independent IFNβ by human monocytes treated with LPS and alcohol together for 6 hours in vitro. LPS-induced RANTES and IP-10 production were also suppressed by acute alcohol exposure in murine Kupffer cells and alveolar macrophages respectively (48, 49), which is in line with our observations in human monocytes. Therefore, we can conclude that moderate alcohol exposure in vitro and in vivo, results in suppression of both branches of LPS-induced TLR4 signaling.
On the other hand, consistent with our in vivo findings, our in vitro data showed that alcohol exposure did not suppress polyI:C-induced TLR3 cytokines/chemokines IFNβ, RANTES or IP-10 in human monocytes,. This is not in line with previous studies by Pruett et al. (22, 23) where they showed suppression of TLR3 signaling after binge alcohol exposure (oral gavage) in murine models. This discrepancy may arise from the following differences in the study by Pruett et al. compared to our study: (1) alcohol dose of 4 g/kg body weight in mice resulting in a BAC of 0.2–0.3 g/dl (50) compared to a BAC of 0.1 g/dl in our study, (2) exposure to alcohol and polyI:C simultaneously compared to the alcohol pre-exposure model used in this study, and (3) the distinct set of cytokines examined including IFNα, IL-12 and IL-6 compared to this study. Our in vitro and in vivo results observed using human monocytes were in agreement and indicate TLR3-TRIF signaling is not altered during binge alcohol consumption in humans in contrast to TLR4 signaling.
To determine mechanism of alcohol-mediated inhibition of TLR4 responses, first we wanted to consider the temporal difference in alcohol mediated inhibition of the two branches of TLR4 signaling which activate different immune signaling pathways and play distinctive roles in host immunity (3, 7). While moderate alcohol pre-exposure in vitro suppressed both TLR4-MyD88 and TLR4-TRIF pathways, the comprehensive time course analysis reveals significant inhibition of TLR4-TRIF responses with as little as one hour alcohol pre-exposure unlike TLR4-MyD88 cytokines. This suggests that alcohol may regulate rapid and discrete immunosuppressive mechanisms to inhibit TLR4-TRIF versus TLR4-MyD88 pathway. Currently TLR4 signaling is proposed as the following sequential model; engagement of TLR4 on the plasma membrane activates the MyD88 dependent signaling pathway, resulting in production of pro-inflammatory cytokines such as TNFα downstream. TLR4 receptor complex is then endocytosed in a CD14 dependent manner and TLR4-TRIF signaling is induced from the endosome to produce IP-10 and IFNβ (51, 52). Since alcohol has been shown to delay TLR4 internalization and localization to early endosomes via clathrin (53) and also affect trafficking of proteins such as L1 adhesion molecule through lipid rafts (54), it may be plausible to suggest regulation of TLR4 localization as a mechanism of alcohol-mediated inhibition of TLR4-TRIF signaling at early time points.
Alcohol-mediated inhibition of TLR4-TRIF responses but not TLR3-TRIF suggests highly specific regulation of TLR signaling by moderate/binge alcohol. Here we focused on whether alcohol alters TRIF downstream signaling molecules TBK1, IKKε and IRF3. Consistent with alcohol-mediated suppression of TLR4-induced IFNβ, IP-10 and RANTES, we observed inhibition of phosphorylation of IRF3 in alcohol-treated LPS-stimulated cells. Interestingly, phosphorylation of upstream kinase TBK1 was not significantly inhibited by alcohol and only a modest decrease in IKKε expression was observed in macrophages suggesting alcohol-mediated early inhibition of IRF3 activation may occur in an IKKε/TBK1-independent mechanism. Cellular stress proteins with immunoregulatory function that are regulated by moderate alcohol were studied (19, 41). ER stress has been shown to induce TBK1-independent IRF3 activation (41) and ER stress protein XBP-1 (which can be induced by LPS (30)) was required for TLR-induced IFNβ production by dendritic cells (28) and IL-6 induction in macrophages (30, 41). However, we did not observe any significant change in immunoregulatory spliced XBP-1 mRNA indicating it does not play a mechanistic role in IRF3 mediated IFNβ inhibition upon alcohol exposure. Examination of IRF3 regulator PP1 (13), which has been shown to be activated by alcohol (24, 25), revealed alcohol-mediated TLR4-TRIF tolerance could be reduced by inhibition of the PP1-IRF3 axis. Further investigation will be necessary to fully discern the exact mechanism of alcohol-mediated suppression of TLR4-TRIF-IRF3 signaling.
In accordance with our previous in vitro study, we show here exciting and novel human data that moderate alcohol intake in vivo induces expression of immunoregulatory stress protein HspA1A at the mRNA and protein levels in monocytes (19). We also report that HspA1A is required for alcohol-mediated suppression of TLR4 induced TNFα and overexpression of HspA1A is sufficient to exert suppression of TLR4 induced responses. Previous studies using cell lines have shown that silencing of heat shock proteins HSPA8 (Hsc71), cognate or constitutive Hsp70 family member, or HspA1A resulted in increased expression of type I interferons in cells infected with Sendai virus/VSV or Dengue virus respectively (42, 43). Therefore, we postulated that HspA1A may also play a role in alcohol-mediated inhibition of IRF3 activation and type 1 IFN responses downstream of TLR4-TRIF activation. However, in our study, HspA1A was not required for alcohol-mediated suppression of TLR4-TRIF responses, suggesting its discrete inhibitory regulation of TLR4-MyD88 responses.
Overall, our comprehensive studies depicted in Figure 11, demonstrate for the first time that in vivo binge alcohol intake in humans alters TLR4-mediated MyD88 and TRIF signaling but not polyI:C-induced TLR3 signaling in primary human monocytes. Furthermore we unravel the selective regulatory role of stress protein HspA1A on alcohol-mediated suppression of TLR4-MyD88 signaling but not TLR4-TRIF signaling which is dependent on alcohol-mediated regulation of the PP1-IRF3 axis. The clinical implications for HspA1A targeting drugs, such as VER-155008 and YM-1 (55, 56), for restoration of normal immune function in binge alcohol abuse patients can be explored in the future. More importantly our study suggests that combination therapies targeting TLR4-TRIF pathway would have to be considered in treatment of alcoholic patients.
Figure 11. Binge alcohol inhibit TLR4-MyD88 and –TRIF signaling, but not TLR3-TRIF, via specific inactivation of HspA1A and IRF3 respectively.
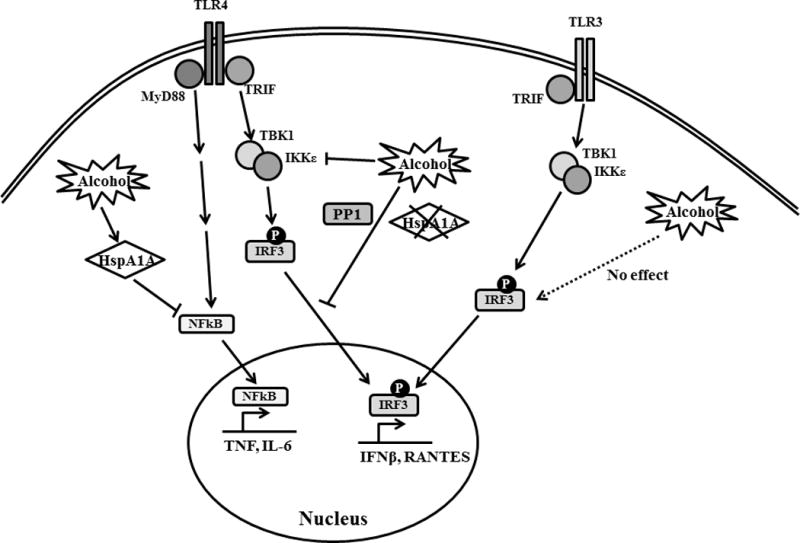
TLR4-MyD88 signaling results in NF-κB activation and production of pro-inflammatory cytokines such as TNFα and IL-6. TLR4- and TLR3-TRIF signaling results in TBK1-induced IRF3 phosphorylation and transcription of IFNβ and RANTES. Binge alcohol exposure inhibits both branches of signaling downstream of TLR4 but not TLR3-TRIF signaling. While alcohol-induced HspA1A suppresses TLR4-MyD88 signaling, HspA1A does not regulate alcohol-mediated suppression of TLR4-TRIF IRF3 activation which is dependent on protein phosphatase, PP1.
Supplementary Material
Footnotes
This work was supported by Department of Defense Grant W81XWH-11-1-0420 (to PM) and Public Health Service grant 2AA017986 (to P.M.) from the National Institute of Alcohol Abuse and Alcoholism, U.S. National Institutes of Health, Bethesda, MD, USA
Author contributions:
S.M – performed and supervised all experimental studies, wrote and edited the manuscript; A.L and D.C – performed experiments and data analysis; P.M – conceived the study, designed research, wrote and edited the manuscript.
Disclosure of conflicts of interest:
All authors declare no conflicts of interest in the publication of this article.
References
- 1.Szabo G. Monocytes, alcohol use, and altered immunity. Alcohol Clin Exp Res. 1998;22:216S–219S. doi: 10.1097/00000374-199805001-00002. [DOI] [PubMed] [Google Scholar]
- 2.Nelson S, Kolls JK. Alcohol, host defence and society. Nat Rev Immunol. 2002;2:205–209. doi: 10.1038/nri744. [DOI] [PubMed] [Google Scholar]
- 3.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 4.Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, Finberg RW. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 5.Haynes LM, Moore DD, Kurt-Jones EA, Finberg RW, Anderson LJ, Tripp RA. Involvement of toll-like receptor 4 in innate immunity to respiratory syncytial virus. J Virol. 2001;75:10730–10737. doi: 10.1128/JVI.75.22.10730-10737.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 7.Palsson-McDermott EM, O’Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doyle S, Vaidya S, O’Connell R, Dadgostar H, Dempsey P, Wu T, Rao G, Sun R, Haberland M, Modlin R, Cheng G. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity. 2002;17:251–263. doi: 10.1016/s1074-7613(02)00390-4. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, Coyle AJ, Liao SM, Maniatis T. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nature immunology. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 10.Schafer SL, Lin R, Moore PA, Hiscott J, Pitha PM. Regulation of type I interferon gene expression by interferon regulatory factor-3. The Journal of biological chemistry. 1998;273:2714–2720. doi: 10.1074/jbc.273.5.2714. [DOI] [PubMed] [Google Scholar]
- 11.Wathelet MG, Lin CH, Parekh BS, Ronco LV, Howley PM, Maniatis T. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-beta enhancer in vivo. Molecular cell. 1998;1:507–518. doi: 10.1016/s1097-2765(00)80051-9. [DOI] [PubMed] [Google Scholar]
- 12.Melchjorsen J, Paludan SR. Induction of RANTES/CCL5 by herpes simplex virus is regulated by nuclear factor kappa B and interferon regulatory factor 3. The Journal of general virology. 2003;84:2491–2495. doi: 10.1099/vir.0.19159-0. [DOI] [PubMed] [Google Scholar]
- 13.Gu M, Zhang T, lin W, Liu Z, Lai R, Xia D, Huang H, Wang X. Protein phosphatase PP1 negatively regulates the Toll-like receptor- and RIG-I-like receptor-triggered production of type I interferon by inhibiting IRF3 phosphorylation at serines 396 and 385 in macrophage. Cellular signalling. 2014;26:2930–2939. doi: 10.1016/j.cellsig.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 15.Pruett SB, Zheng Q, Fan R, Matthews K, Schwab C. Ethanol suppresses cytokine responses induced through Toll-like receptors as well as innate resistance to Escherichia coli in a mouse model for binge drinking. Alcohol. 2004;33:147–155. doi: 10.1016/j.alcohol.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Pruett SB, Fan R, Cheng B, Glover M, Tan W, Deng X. Innate immunity and inflammation in sepsis: mechanisms of suppressed host resistance in mice treated with ethanol in a binge-drinking model. Toxicol Sci. 2010;117:314–324. doi: 10.1093/toxsci/kfq215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandrekar P, Jeliazkova V, Catalano D, Szabo G. Acute alcohol exposure exerts anti-inflammatory effects by inhibiting IkappaB kinase activity and p65 phosphorylation in human monocytes. J Immunol. 2007;178:7686–7693. doi: 10.4049/jimmunol.178.12.7686. [DOI] [PubMed] [Google Scholar]
- 18.Bala S, Tang A, Catalano D, Petrasek J, Taha O, Kodys K, Szabo G. Induction of Bcl-3 by acute binge alcohol results in toll-like receptor 4/LPS tolerance. J Leukoc Biol. 2012;92:611–620. doi: 10.1189/jlb.0112050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muralidharan S, Ambade A, Fulham MA, Deshpande J, Catalano D, Mandrekar P. Moderate alcohol induces stress proteins HSF1 and hsp70 and inhibits proinflammatory cytokines resulting in endotoxin tolerance. J Immunol. 2014;193:1975–1987. doi: 10.4049/jimmunol.1303468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mandrekar P, Catalano D, White B, Szabo G. Moderate alcohol intake in humans attenuates monocyte inflammatory responses: inhibition of nuclear regulatory factor kappa B and induction of interleukin 10. Alcohol Clin Exp Res. 2006;30:135–139. doi: 10.1111/j.1530-0277.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- 21.Meyerholz DK, Edsen-Moore M, McGill J, Coleman RA, Cook RT, Legge KL. Chronic alcohol consumption increases the severity of murine influenza virus infections. J Immunol. 2008;181:641–648. doi: 10.4049/jimmunol.181.1.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pruett SB, Fan R, Zheng Q. Acute ethanol administration profoundly alters poly I:C-induced cytokine expression in mice by a mechanism that is not dependent on corticosterone. Life sciences. 2003;72:1825–1839. doi: 10.1016/s0024-3205(02)02507-9. [DOI] [PubMed] [Google Scholar]
- 23.Pruett SB, Schwab C, Zheng Q, Fan R. Suppression of innate immunity by acute ethanol administration: a global perspective and a new mechanism beginning with inhibition of signaling through TLR3. J Immunol. 2004;173:2715–2724. doi: 10.4049/jimmunol.173.4.2715. [DOI] [PubMed] [Google Scholar]
- 24.Price ME, Pavlik JA, Liu M, Ding SJ, Wyatt TA, Sisson JH. Alcohol drives S-nitrosylation and redox activation of protein phosphatase 1, causing bovine airway cilia dysfunction. American journal of physiology Lung cellular and molecular physiology. 2017;312:L432–L439. doi: 10.1152/ajplung.00513.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goral J, Kovacs EJ. In vivo ethanol exposure down-regulates TLR2-, TLR4-, and TLR9-mediated macrophage inflammatory response by limiting p38 and ERK1/2 activation. Journal of immunology. 2005;174:456–463. doi: 10.4049/jimmunol.174.1.456. [DOI] [PubMed] [Google Scholar]
- 26.Muralidharan S, Mandrekar P. Cellular stress response and innate immune signaling: integrating pathways in host defense and inflammation. J Leukoc Biol. 2013;94:1167–1184. doi: 10.1189/jlb.0313153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu F, Yu X, Wang H, Zuo D, Guo C, Yi H, Tirosh B, Subjeck JR, Qiu X, Wang XY. ER stress and its regulator X-box-binding protein-1 enhance polyIC-induced innate immune response in dendritic cells. European journal of immunology. 2011;41:1086–1097. doi: 10.1002/eji.201040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith JA, Turner MJ, DeLay ML, Klenk EI, Sowders DP, Colbert RA. Endoplasmic reticulum stress and the unfolded protein response are linked to synergistic IFN-beta induction via X-box binding protein 1. European journal of immunology. 2008;38:1194–1203. doi: 10.1002/eji.200737882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng L, Liu YP, Sha H, Chen H, Qi L, Smith JA. XBP-1 couples endoplasmic reticulum stress to augmented IFN-beta induction via a cis-acting enhancer in macrophages. J Immunol. 2010;185:2324–2330. doi: 10.4049/jimmunol.0903052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nature immunology. 2010;11:411–418. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ewing JA. Detecting alcoholism. The CAGE questionnaire. Jama. 1984;252:1905–1907. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- 32.Fleming MF, Barry KL. A three-sample test of a masked alcohol screening questionnaire. Alcohol and alcoholism. 1991;26:81–91. [PubMed] [Google Scholar]
- 33.Mandrekar P, Catalano D, Szabo G. Inhibition of lipopolysaccharide-mediated NFkappaB activation by ethanol in human monocytes. International immunology. 1999;11:1781–1790. doi: 10.1093/intimm/11.11.1781. [DOI] [PubMed] [Google Scholar]
- 34.Choy MS, Swingle M, D’Arcy B, Abney K, Rusin SF, Kettenbach AN, Page R, Honkanen RE, Peti W. PP1:Tautomycetin Complex Reveals a Path toward the Development of PP1-Specific Inhibitors. Journal of the American Chemical Society. 2017;139:17703–17706. doi: 10.1021/jacs.7b09368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitsuhashi S, Shima H, Tanuma N, Matsuura N, Takekawa M, Urano T, Kataoka T, Ubukata M, Kikuchi K. Usage of tautomycetin, a novel inhibitor of protein phosphatase 1 (PP1), reveals that PP1 is a positive regulator of Raf-1 in vivo. The Journal of biological chemistry. 2003;278:82–88. doi: 10.1074/jbc.M208888200. [DOI] [PubMed] [Google Scholar]
- 36.Michels AA, Kanon B, Konings AW, Ohtsuka K, Bensaude O, Kampinga HH. Hsp70 and Hsp40 chaperone activities in the cytoplasm and the nucleus of mammalian cells. The Journal of biological chemistry. 1997;272:33283–33289. doi: 10.1074/jbc.272.52.33283. [DOI] [PubMed] [Google Scholar]
- 37.Ambade A, Catalano D, Lim A, Mandrekar P. Inhibition of heat shock protein (molecular weight 90 kDa) attenuates proinflammatory cytokines and prevents lipopolysaccharide-induced liver injury in mice. Hepatology. 2012;55:1585–1595. doi: 10.1002/hep.24802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shalova IN, Kajiji T, Lim JY, Gomez-Pina V, Fernandez-Ruiz I, Arnalich F, Iau PT, Lopez-Collazo E, Wong SC, Biswas SK. CD16 regulates TRIF-dependent TLR4 response in human monocytes and their subsets. Journal of immunology. 2012;188:3584–3593. doi: 10.4049/jimmunol.1100244. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 40.Solis M, Romieu-Mourez R, Goubau D, Grandvaux N, Mesplede T, Julkunen I, Nardin A, Salcedo M, Hiscott J. Involvement of TBK1 and IKKepsilon in lipopolysaccharide-induced activation of the interferon response in primary human macrophages. European journal of immunology. 2007;37:528–539. doi: 10.1002/eji.200636090. [DOI] [PubMed] [Google Scholar]
- 41.Liu YP, Zeng L, Tian A, Bomkamp A, Rivera D, Gutman D, Barber GN, Olson JK, Smith JA. Endoplasmic reticulum stress regulates the innate immunity critical transcription factor IRF3. J Immunol. 2012;189:4630–4639. doi: 10.4049/jimmunol.1102737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Z, Wu SW, Lei CQ, Zhou Q, Li S, Shu HB, Wang YY. Heat shock cognate 71 (HSC71) regulates cellular antiviral response by impairing formation of VISA aggregates. Protein & cell. 2013;4:373–382. doi: 10.1007/s13238-013-3902-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Padwad YS, Mishra KP, Jain M, Chanda S, Ganju L. Dengue virus infection activates cellular chaperone Hsp70 in THP-1 cells: downregulation of Hsp70 by siRNA revealed decreased viral replication. Viral immunology. 2010;23:557–565. doi: 10.1089/vim.2010.0052. [DOI] [PubMed] [Google Scholar]
- 44.Evans CG, Chang L, Gestwicki JE. Heat shock protein 70 (hsp70) as an emerging drug target. Journal of medicinal chemistry. 2010;53:4585–4602. doi: 10.1021/jm100054f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Means TK, Hayashi F, Smith KD, Aderem A, Luster AD. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. Journal of immunology. 2003;170:5165–5175. doi: 10.4049/jimmunol.170.10.5165. [DOI] [PubMed] [Google Scholar]
- 46.Tamassia N, Le Moigne V, Calzetti F, Donini M, Gasperini S, Ear T, Cloutier A, Martinez FO, Fabbri M, Locati M, Mantovani A, McDonald PP, Cassatella MA. The MyD88-independent pathway is not mobilized in human neutrophils stimulated via TLR4. Journal of immunology. 2007;178:7344–7356. doi: 10.4049/jimmunol.178.11.7344. [DOI] [PubMed] [Google Scholar]
- 47.Pang M, Bala S, Kodys K, Catalano D, Szabo G. Inhibition of TLR8- and TLR4-induced Type I IFN induction by alcohol is different from its effects on inflammatory cytokine production in monocytes. BMC Immunol. 2011;12:55. doi: 10.1186/1471-2172-12-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bukara M, Bautista AP. Acute alcohol intoxication and gadolinium chloride attenuate endotoxin-induced release of CC chemokines in the rat. Alcohol. 2000;20:193–203. doi: 10.1016/s0741-8329(99)00100-7. [DOI] [PubMed] [Google Scholar]
- 49.Happel KI, Rudner X, Quinton LJ, Movassaghi JL, Clark C, Odden AR, Zhang P, Bagby GJ, Nelson S, Shellito JE. Acute alcohol intoxication suppresses the pulmonary ELR-negative CXC chemokine response to lipopolysaccharide. Alcohol. 2007;41:325–333. doi: 10.1016/j.alcohol.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carson EJ, Pruett SB. Development and characterization of a binge drinking model in mice for evaluation of the immunological effects of ethanol. Alcohol Clin Exp Res. 1996;20:132–138. doi: 10.1111/j.1530-0277.1996.tb01055.x. [DOI] [PubMed] [Google Scholar]
- 51.Zanoni I, Ostuni R, Marek LR, Barresi S, Barbalat R, Barton GM, Granucci F, Kagan JC. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell. 2011;147:868–880. doi: 10.1016/j.cell.2011.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pascual-Lucas M, Fernandez-Lizarbe S, Montesinos J, Guerri C. LPS or ethanol triggers clathrin- and rafts/caveolae-dependent endocytosis of TLR4 in cortical astrocytes. Journal of neurochemistry. 2014;129:448–462. doi: 10.1111/jnc.12639. [DOI] [PubMed] [Google Scholar]
- 54.Tang N, Farah B, He M, Fox S, Malouf A, Littner Y, Bearer CF. Ethanol causes the redistribution of L1 cell adhesion molecule in lipid rafts. Journal of neurochemistry. 2011;119:859–867. doi: 10.1111/j.1471-4159.2011.07467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goloudina AR, Demidov ON, Garrido C. Inhibition of HSP70: a challenging anti-cancer strategy. Cancer letters. 2012;325:117–124. doi: 10.1016/j.canlet.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 56.Sherman MY, V, Gabai L. Hsp70 in cancer: back to the future. Oncogene. 2015;34:4153–4161. doi: 10.1038/onc.2014.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


