Abstract
Allogeneic hematopoietic cell transplantation (allo-HCT) is a potential curative therapy for hematologic malignancies. Host antigen presenting cells (APCs) are pivotal to the desired graft-versus-tumor (GVT) effect. Recent studies have shown that β2-adrenergic receptor (β2AR) signaling can have an important impact on immune cell functions including dendritic cells (DCs). Here, we demonstrate that pretreatment of host mice with a β2AR blocker significantly increases the GVT effect of donor CD8+ T cells by decreasing tumor burden without increasing GVHD. β2AR-deficient host mice have a significantly increased effector memory and central memory CD8+ T cells and improved reconstitution of T cells including CD4+Foxp3+ regulatory T cells. Notably, β2AR deficiency induces increased CD11c+ dendritic cell (DC) development. Also, β2AR-deficient bone marrow-derived DCs (BMDCs) induce higher CD8+ T cell proliferation and improved tumor killing in vitro. Metabolic profiling shows that β2AR deficiency renders DCs more immunogenic through upregulation of mTOR activity and reducing STAT3 phosphorylation. All together, these findings demonstrate an important role of host β2AR signaling in suppressing T cell reconstitution and GVT activity.
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) is an established treatment for blood cancers including leukemia and lymphoma. With this treatment, donor T cells are able to attack host tumor cells, producing the beneficial graft-versus-tumor (GVT) effect (1). However, complications may arise in the form of graft-versus-host disease (GVHD) in which donor T cells target normal host tissues resulting in damage to the liver, skin, gastrointestinal tract and other organs (2). This is due to the fact that the donor and the host do not have identical histocompatibility antigens causing donor T cells to recognize the host as foreign (3). However, donor T cell engraftment and reconstitution are also important for protecting the host from infection (4). Therefore, it remains a challenge in allo-HCT to improve T cell reconstitution and GVT effect while achieving control of GVHD.
Host antigen presenting cells (APCs) especially dendritic cells (DCs) are instrumental in inducing GVT and GVHD (5, 6). Tissue damage after cytotoxic conditioning of the host induces DC activation through conditioning-released damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs). The activated DCs present host antigens to donor T cells and activate them causing T cell proliferation, differentiation, migration to GVHD target organs, and release of chemokines and cytokines including IFN-γ and IL-2, leading to target tissue damage. Cytotoxic T lymphocytes (CTLs) can directly cause target cell apoptosis during this process (7, 8). The β2-adrenergic receptor (β2AR) is found on APCs including DCs (9), B cells (10) and macrophages (11), T cells (12), and cancer cells (13) and acts as a means of communication between the nervous system and the immune system (14). β2AR activation on DCs results in enhanced anti-inflammatory cytokine production such as IL-10 and decreased IL-6 pro-inflammatory cytokine production in vitro (9). Also β2AR activation on bone marrow-derived DCs (BMDCs) shifts CD4+ T cell differentiation towards a Th2 response (15). Our previous report shows β2AR inhibition by β2AR blockers exacerbates GVHD induced by total T cells derived from donor bone marrow and splenocytes, and increased housing temperature also worsens GVHD through decreasing β2AR signaling, indicating an important anti-inflammatory role of β2AR signaling in allogeneic T cell response (16).
In this study, we investigated the effect of β2AR signaling on DC development and subsequent function in GVT effect. Since CD4+ T cells induce hyperacute lethal GVHD making it difficult for GVT study (17), this study has focused on how β2AR signaling in the host affects CD8+ T cell-mediated GVT effect. We demonstrate that β2AR inhibition changes host DC proliferation, function and metabolism which lead to increased T cell reconstitution and enhanced GVT effect without exacerbating GVHD.
Materials and methods
Animals and tumor cells
C57BL/6J (H-2b) and BALB/cJ (H-2d) mice were purchased from the Jackson Laboratory. β2 adrenergic receptor knockout (β2AR KO) mice on the BALB/cJ background were provided by J. David Farrar (University of Texas Southwestern Medical Center). All mice were maintained in SPF housing, and all experiments were performed according to the animal care guidelines at Roswell Park Cancer Institute, using protocols approved by the animal studies committee. Luciferase-expressing A20 cells were developed as previously described (18, 19).
Reagents and antibodies
Antibodies including anti-mouse TCRβ, CD4, CD8, CD44, CD62L, H-2Kb, H-2Kd, CD122, CD69, CD137, MHC-II, CD86, CD70, CD24, CD172a, and B220 were purchased from eBioscience. CD90.2 microbeads and negative mouse CD8+ T cells isolation kits were purchased from Miltenyi Biotec and Stem Cell Company respectively. The mouse CD11c+ cell isolation kits for CD11c+ negative selection were purchased from Stem Cell Company.
Donor cell preparation
Donor bone marrow (BM) cells were isolated from WT C57BL/6 mice. T cell depletion (TCD) was performed by using anti-CD90.2 microbeads (purity >92%). Donor CD8+ T cells were purified from the spleens of C57BL/6 WT by using mouse CD8+ isolation kits (purity >96%).
Bone marrow transplantation for GVT and GVHD
For GVT studies, β2AR KO and WT BALB/cJ hosts (H-2d) were irradiated with 900rad from a Cs-137 source at two split doses with 4 hours distance. One day later, the hosts were injected intravenously with 3×106 TCD-BM cells only or combined with 0.3×106 CD8+ T cells isolated from C57BL/6 (H-2b) WT mice. Host mice were injected intravenously with 0.1×106 luciferase expressing A20 tumor cells right before BM and T cell injection. Tumor burdens were measured by bioluminescence imaging every week and tumor mortality and overall survival were monitored. For β2AR blocking, we performed daily intraperitoneal injection of ICI 118,551, a selective β2AR blocker, before transplantation for 7 days. For GVHD studies, irradiated β2AR KO and WT BALB/cJ hosts (H-2d) were injected intravenously with 3×106 TCD-BM cells only or combined with 2×106 CD8+ T cells isolated from C57BL/6J (H-2b) WT mice. Then the host mice were weighed every three days and monitored for clinical GVHD score and survival.
Clinical GVHD scoring criteria
The clinical GVHD manifestations are weight loss; change in posture, activity, fur texture, hair loss and in some cases diarrhea. Clinical GVHD is evaluated comprehensively with a scoring system as published before (17, 20).
Bone marrow derived dendritic cells (BMDCs) and mixed lymphocyte reaction (MLR)
BMDCs as stimulators were generated from β2AR KO and WT BALB/cJ mice and cultured in 5% RPMI with 1% GM-CSF (GM-CSF releasing cell line supernatant) for 7 days. At day 6, LPS (100ng/ml) were added to mature DCs. Ef670 stained CD8+ T cells as responders were isolated from the spleens of C57BL/6 WT mice. 25×105 responders and 5×105 BMDCs as stimulators (ratio 5:1) were co-cultured in 300ul 10% RPMI/well in 96-well plate for 4 to 5 days. Cells were harvested and washed once with 1ml Dulbecco's Phosphate-Buffered Saline (DPBS) before staining for flow cytometry. In tumor killing assay, the luciferase-expressing A20 cells were added in two time points as indicated in the results section.
Histopathology scoring
30 days after allo-HCT, WT and β2AR KO host mice were sacrificed and the liver, large intestine, and small intestine were removed, fixed with formalin, sectioned, and stained with H&E. The intestinal tissue was examined using an established semi-quantitative scoring system (19, 21). Representative pictures were captured at 100×.
Real-time metabolic characterization
An XFe96 extracellular flux analyzer (Seahorse Bioscience) was used to analyze extracellular acidification rate (ECAR, mpH/min) and mitochondria oxygen consumption rate (OCR, O2 mpH/min) in WT and β2AR KO BMDCs. For ECAR analyses, WT or β2AR KO BMDCs were harvested, washed, and re-suspended in ECAR medium (DMEM base [no bicarbonate] with 2 mM L-glutamine, 143 mM NaCl, and 0.5% phenol red [pH 7.35]). The complete ECAR analysis consisted of four stages: basal (without drugs), glycolysis induction (10 mM glucose), maximal glycolysis induction (2 mM oligomycin), and glycolysis inhibition (100 mM 2-DG). For OCR analyses, WT and β2AR KO BMDCs were washed and re-suspended in OCR medium (DMEM base, 25 mM glucose, 1 mM pyruvate, 2 mM L-glutamine [pH 7.35]). Cells were plated in poly-D-lysine coated 96-well flat-bottom plates and incubated in a non-CO2 incubator for 1 hour at 37°C. A complete OCR study was performed with all different groups simultaneously in four consecutive stages: basal respiration (without drugs), mitochondrial complex V inhibition (2 mM oligomycin), maximal respiration induction [1 mM carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP)], and electron transportation chain inhibition (1 mM rotenone and 1 mM antimycin A). Immature BMDCs were activated with LPS for either 3 or 18 hours and metabolic analysis were conducted.
Statistical analysis
Data are presented as mean ± SEM. Difference between groups were analyzed using the unpaired Student’s t test and one way ANOVA for two and more than two groups respectively. Animal survival (Kaplan-Meier survival curves) was analyzed by log-rank test. The body weight and clinical score difference were analyzed using two way AVOVA and presented by each time point. P-value <0.05 was considered statistically significant. All statistical analyses were performed by using GraphPad Prism v7.
Results
Blockade of β2AR in the host increases GVT effect without exacerbating GVHD
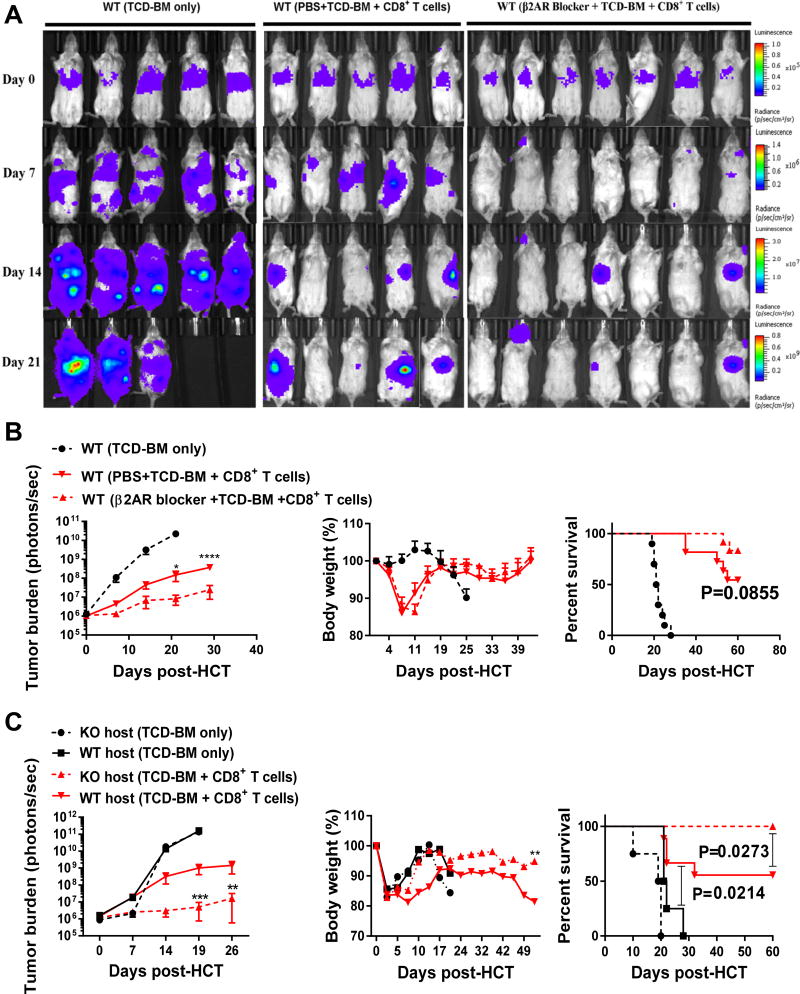
It has been shown that host DCs play a pivotal role in inducing GVT and GVHD (5). β2AR signaling contributes to regulating DC functions and subsequent T cell activation (22). Previous reports showed that β2AR inhibition increases inflammatory phenotype of DCs (23) and β2AR agonists bias DC function towards inducing an IL-17 immune response (24). In order to address the effect of β2AR deficiency in host DCs on GVT activity, we used two different methods: 1) pretreatment the host mice with the β2AR blocker (ICI 118,551) for 7 days before transplantation and 2) using WT and β2AR knockout (β2AR KO) BALB/cJ (H-2d) mice as hosts. C57BL/6J (H-2b) mice were used as donors. Host mice were lethally irradiated and inoculated with 0.1×106 A20 cells before transplanted with 3×106 TCD-BM and 0.3×106 CD8+ T cells from B6 donors. Representative images of tumor burden are shown in Figure 1A. Pretreatment was performed for 7 days, daily injection with 5mg/kg ICI-118,551 before transplantation. The group pretreated with the β2AR blocker had significantly decreased tumor burden than the PBS control group, and showed a strong trend of improved survival (Figure 1B). In parallel, weight loss of these mice was used as readout for GVHD. Both the blocker pretreatment group and the PBS control group were able to recover to their original body weight before some mice succumbed to tumor outgrowth. These observations were confirmed by using β2AR KO hosts, which showed significantly decreased tumor burden in comparison to WT hosts (Figure 1C). Interestingly, β2AR KO hosts also showed significantly improved bodyweight recovery in comparison to WT hosts (Figure 1C). Notably, β2AR KO hosts showed significantly improved survival (100%) compared to WT hosts (60%). As controls, both WT and KO groups receiving BM only showed uncontrolled tumor growth and 100% tumor-induced mortality. To understand the underlying mechanisms, we first examined donor-derived CD8+ T cells at different time points after allo-HCT (Supplemental Figure 1). At all three time points, days 10, 30 and 60, there were significantly higher numbers of CD8+ T cells in β2AR KO hosts compared to WT hosts (Supplemental Figure 1A). We used CD44 and CD62L to assess effector and memory phenotypes. At day 10, nearly 95 percent of CD8+ T cells were effector cells in both WT and β2AR KO hosts (Supplemental Figure 1B). However at day 30, compared to WT hosts, there was a significant increase in CD44+CD62L− effector memory cells in β2AR KO hosts (Supplemental Figure 1B). Interestingly, at day 60, the CD44+CD62L+ central memory cells were significantly increased in β2AR KO hosts compared to WT hosts. Together, these data demonstrate that β2AR deficiency in the host enhances CD8+ T cell expansion and memory formation, resulting in improved GVT effect without aggravating GVHD,
Figure-1. Blockade of β2AR in the recipients improves GVT effect.
BALB/cJ host mice were pretreated with β2AR blocker for 7 days before transplantation. At day −1, host mice were irradiated with 900 cGy and at day 0 mice were transplanted with 3×106 TCD-BM and 3×105 CD8+ T cells purified from C57BL/6J donor mice after IV injection of 1×105 luciferase-expressing A20 cells. Bioluminescence imaging was performed to measure tumor burden. (A) Representative images of tumor burden, and (B) summary data of tumor burden, body weight and survival rate were presented (n=10–13 for each group). (C) At day −1, BALB/cJ WT and β2AR KO mice were irradiated with 900 cGy and at day 0 were transplanted with 3×106 TCD-BM and 3×105 CD8+ T cells purified from C57BL/6J donor mice after IV injection of 1×105 A20 cells (n=10). Data are presented as mean ± SEM from two replicate experiments. **** P < 0.0001; *** P < 0.001; ** P < 0.01; * P < 0.05.
β2AR suppresses dendritic cell (DC) development and function
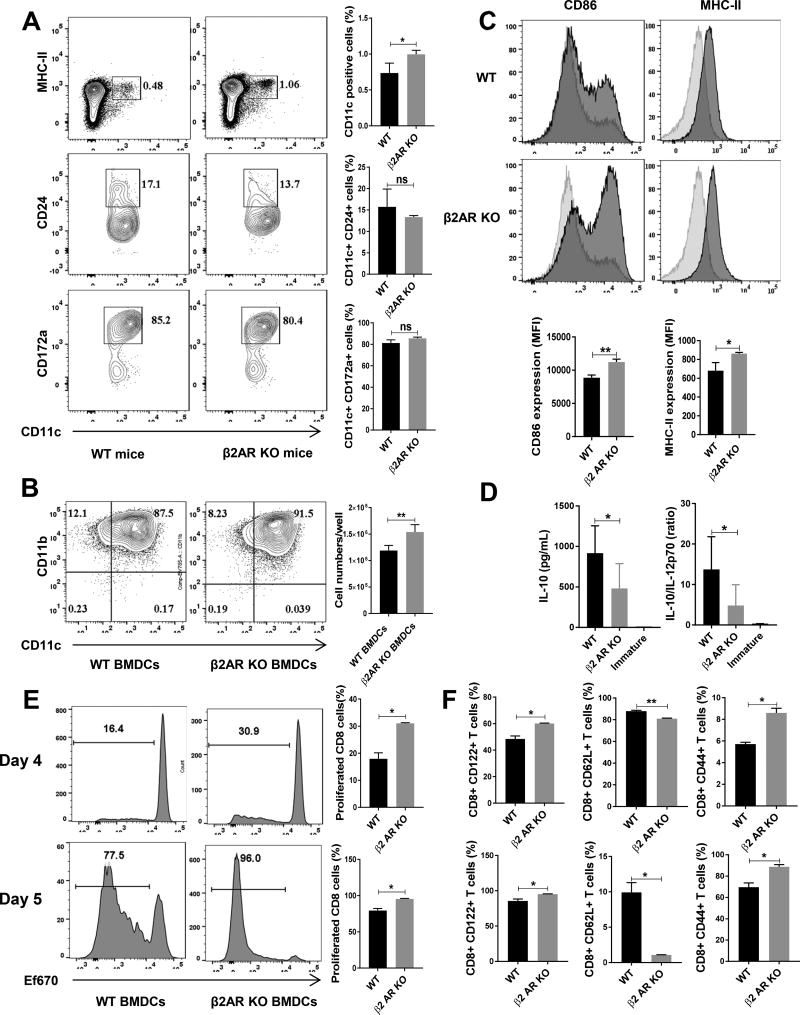
Catecholamine including dopamine, norepinephrine and epinephrine has both neural and endogenous sources in bone marrow and affects hematopoiesis (25). Almost all immune cells including macrophages, monocytes, T cells and B cells can synthesize and release catecholamine and change function via autocrine loop (26–28). Host APCs are crucial for inducing both GVHD and GVT (7). Furthermore, it has been demonstrated that different subpopulations of DCs have different roles in induction of GVHD and GVT. Host CD8+ DCs have a protective role in GVHD and are important for the optimal GVT effect (29). Also, some populations of plasmacytoid DCs exacerbate GVHD symptoms (30), yet CCR-9+ plasmacytoid DCs are able to suppress GVHD (31). Since our results indicate a role for β2AR in GVT, we investigated the effect of β2AR deficiency on CD11c+ DC development and function. β2AR KO mice have higher percentages of CD11c+ population but there was no difference in CD11c+ subpopulations including plasmacytoid DCs, CD11b+ DCs and CD8+ conventional DC identified with B220, CD172a and CD24 markers respectively (Figure 2A). To test the effect of β2AR deficiency on DC function, we developed BM-derived DCs (BMDCs). Interestingly, when we started with the same number of BM cells, β2AR KO BM cells yielded a higher number of BMDCs after 6 days of culture compared to WT BM cells. Yet there is no difference in CD11c+ percentage between WT and β2AR KO BMDCs (Figure 2B). When we examined the quality of the BMDCs, the KO BMDCs displayed significantly higher CD86 and MHC-II expression than the WT BMDCs (Figure 2C). Next we assessed cytokine production by BMDCs after LPS maturation. WT BMDCs released more IL-10 compared to KO BMDCs and that ratio of IL-10/IL-12 also was significantly higher in WT compared to KO BMDCs (Figure 2D). We then conducted a mixed lymphocyte reaction (MLR) using the BMDCs as stimulator cells and ef670-stained CD8+ T cells as responder cells. Compared to WT BMDCs, β2AR KO BMDCs induced a significant increase in T cell proliferation (Figure 2E). Also, we checked the expression of CD122 as an activation marker and there were a significantly higher percentage of CD122+ T cells activated by the β2AR KO DCs on day 4 and day 5 (Figure 2F). There were also significantly higher percentages of effector cells marked by CD44+CD62L− T cells activated by β2AR KO DCs on both days 4 and 5 compared to WT BMDCs (Figure 2F). Together, these data indicate that β2AR suppresses DC development and function.
Figure-2. β2AR suppresses DC proliferation and function.
(A) Representative flow cytometry plots of DC percentages and subpopulations in spleens of naïve WT and β2AR KO mice. The top two plots were gated on live splenocytes and the CD24 and CD172a plots were gated on live CD11c+ cells. Summary data are pooled from 2 repeated experiments with 3 mice in each group. (B) Flow cytometry plots of BMDCs developed from WT and β2AR KO mice as described in the Methods. Summary data are from 2 repeated experiments with 6 samples in each group. (C) Representative flow cytometry plots of CD86 and MHC-II expression in WT and β2AR KO BMDCs. Summary data are from 3 repeated experiments with 3 samples in each group. (D) Cytokine production by WT or β2AR KO BMDCs. Summary data are from 2 repeated experiments with 6 samples in each group. (E) The proliferation of CD8+ T cells, quantified by using ef670 proliferation dye, in MLR stimulated by WT or β2AR KO BMDCs for 4 and 5 days. Summary data are from 3 repeated experiments with 3 samples in each group. (F) Expression of CD122 as an activation marker, and effector phenotype markers CD44 and CD62L on CD8+ T cells in MLR stimulated by WT or β2AR KO BMDCs for 4 and 5 days. Summary data are from 3 repeated experiments with 3 samples in each group. ** P <0.01; *P <.05.
β2AR deficient BMDCs show decreased spare respiratory capacity and glycolytic capacity
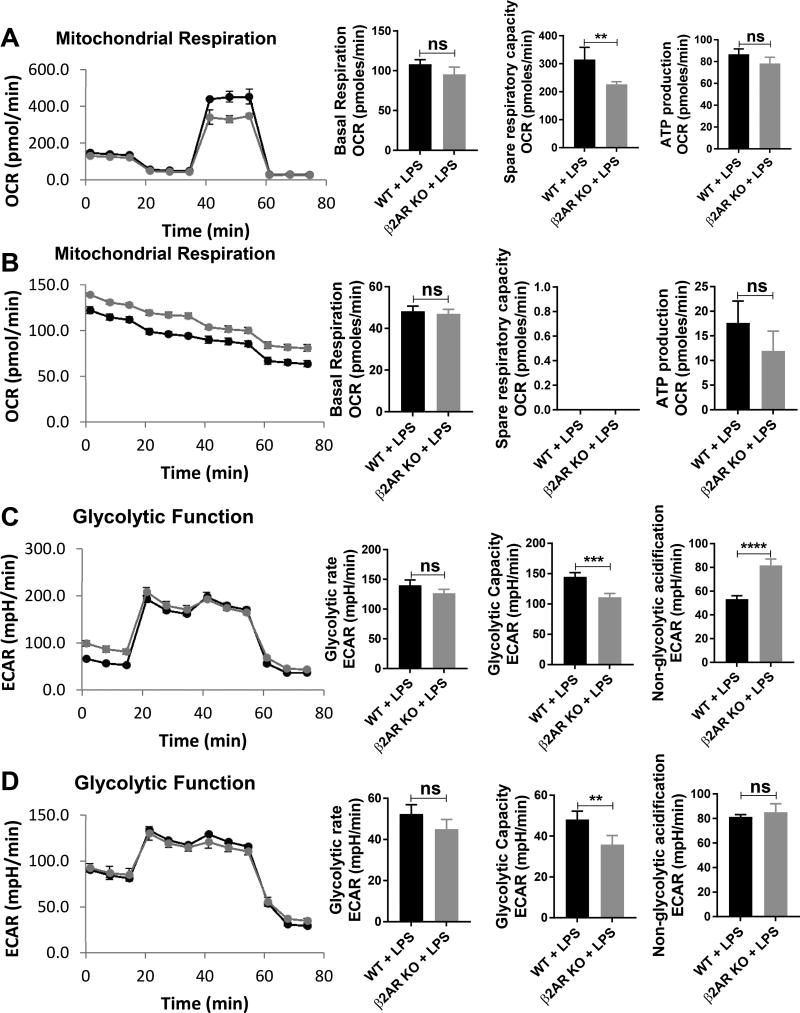
Thus far we showed that β2AR deficiency increases DC function, prompting us to elucidate the relevant mechanism. Recently, it has been shown that metabolism plays a crucial role in DC function (32). Glycolytic burst after LPS activation is signature for DC maturation and function (33). Therefore, we decided to address whether the β2AR KO DCs exhibit a metabolic profile similar to immunogenic DCs. We examined glycolysis and mitochondrial stress in WT and β2AR KO BMDCs at either 3 or 18 hours after LPS activation. In regards to mitochondrial stress, WT BMDCs had significantly higher spare respiratory capacity compared to β2AR KO BMDCs without difference in basal respiration and ATP production, indicating that β2AR DCs are more immunogenic DCs (Figure 3A). Same as previously reported (34), 18 hours after LPS activation, there was no response to FCCP and no difference between WT and β2AR KO BMDCs (Figure 3B). In regards to glycolysis, WT BMDCs had a higher glycolytic capacity compared to β2AR KO BMDCs at both 3 and 18 hours after LPS activation without any difference in glycolytic rate (Figure 3C and D respectively). Interestingly, the non-glycolytic acidification of β2AR KO BMDCs was significantly higher compared to WT BMDCs when no glucose was present in the media 3 hours after LPS activation yet without any difference at 18 hours after LPS activation. These data suggest that β2AR deficiency indeed changes the metabolic profiles of BMDCs and increases their immunogenicity.
Figure-3. β2AR deficiency in BMDCs decreases spare respiratory capacity and glycolytic capacity without changing ATP production and glycolytic rate.
(A and B) Representative kinetic study of mitochondria OCR (mpH/min) in WT (Blue) and β2AR KO (Red) BMDCs after adding LPS for (A) 3 hours and (B) 18 hours by using sequential addition of oligomycin (Olig), FCCP, and rotenone/antimycin A (Rot-AA). Summary bar graphs show basal respiration, spare respiratory capacity and ATP production in WT and β2AR KO BMDCs. OCR for spare respiratory capacity was zero because DCs did not response to FCCP 18 hours after LPS activation. (C and D) Representative kinetic study of glycolysis-dependent ECAR (mpH/min) in WT (Blue) and β2AR KO (Red) BMDCs, after adding LPS for (C) 3 hours and (D) 18 hours by using sequential addition of glucose (Gluc), oligomycin (Olig), and 2-DG. Summary bar graphs show glycolytic rate, glycolytic capacity, and non-glycolytic acidification in WT and β2AR KO BMDCs. Data were pooled from three independent experiments and presented as Mean ± SD. **** P < 0.0001; *** P < 0.001; ** P < 0.01.
β2AR deficiency in BMDCs reduces STAT3 phosphorylation and improves the tumor-killing efficacy of CD8+ T cells
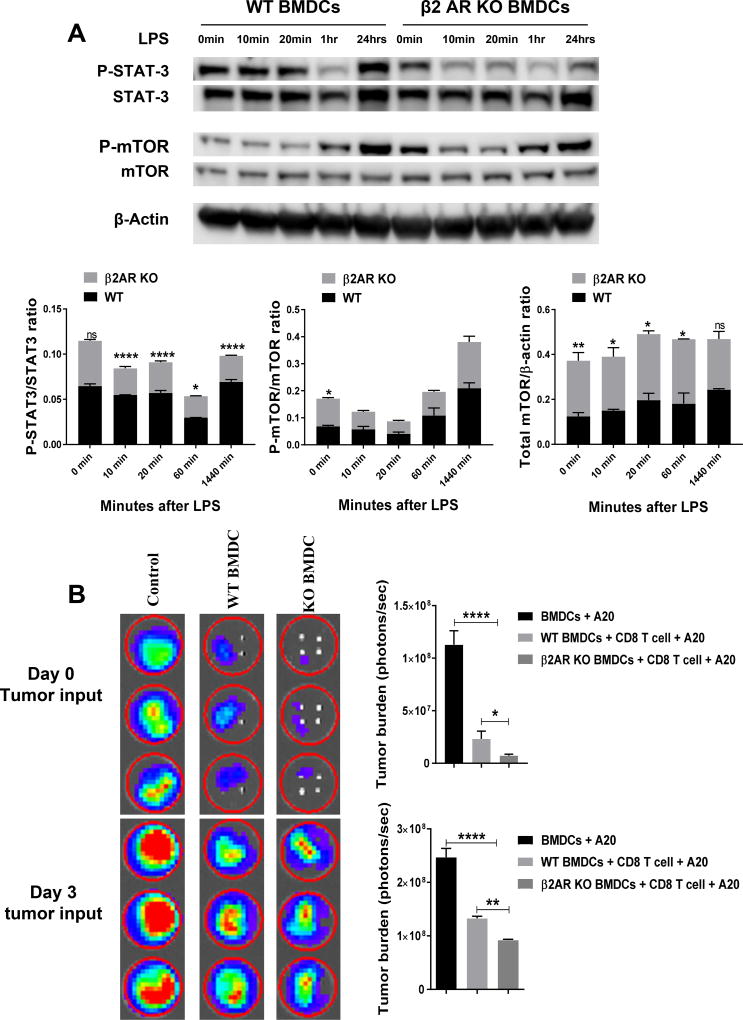
STAT3 signaling plays an important role suppressing immunogenic function of DCs through increasing IL-10 secretion and inhibition of NF-κB (35, 36). This pathway was previously connected with β2AR signaling (37). Also, it has recently been shown that STAT3 expression in host myeloid cells inhibits GVHD severity through suppressing DC function (38). However, previous studies demonstrated that β2AR agonists activate STAT3 signaling pathway in cardiomyocytes and macrophages and suppress NF-κB (36, 37). In addition, mTOR signaling is also important for glycolysis in DC and increases commitment of cells to anabolism (39). Therefore, we assessed the phosphorylated form of STAT3 and mTOR in WT and β2AR KO BMDCS at different time points (Figure 4A). STAT3 phosphorylation is significantly decreased in β2AR KO BMDCs after LPS activation. There was no significant difference in mTOR phosphorylation after LPS activation, but β2AR KO BMDCs expressed more mTOR protein when normalized to β-actin internal control (Figure 4A).
Figure-4. β2AR deficiency in BMDCs modulates STAT3 and mTOR activity and increases CD8+ T cell-mediated tumor killing.
(A) STAT3 and mTOR phosphorylation in WT and β2AR KO BMDCs after LPS activation. Representative western blots of P-STAT3 and P-mTOR proteins and quantification of P-STAT3/STAT3, P-mTOR/mTOR and total mTOR/β-actin ratios (n=3). Data are presented as mean ± SD. ****, P < 0.0001; ***, P = 0.001; **, 0.01; *, 0.05. (B) Mature BMDCs were generated from BALB/cJ WT and β2AR KO mice and co-cultured for MLR with CD8+ T cells purified from C57BL/6J mice in a ratio 1:5. At day 0 or day 3 of MLR, A20 tumor cells were added to MLR. Bioluminescence imaging was performed at day 4 to measure tumor burden in each well. Data are presented as mean ± SD. **** P < 0.0001; ** P <0.01; *P <0.05.
To examine how β2AR affects the immunogenicity of DCs in allogeneic CD8+ T cell response, we conducted MLR using WT or β2AR KO BMDCs with the addition of tumor cells. For MLR, WT and β2AR KO BMDCs were co-cultured with sorted CD8+ T cells for 4 days. To assess tumor killing activity in the MLR, luciferase-expressing A20 tumor cells were added either at the beginning or day 3 of culture. Bioluminescence imaging was performed at day 4 to measure tumor burden in the MLR culture. Our control group consisted of only BMDCs and A20 cells. Regardless of when tumor cells were added at day 0 or day 3 (Figure 4B), there was significantly decreased tumor signal detected in the β2AR KO DCs compared to WT DCs. These data indicate that β2AR deficiency in BMDCs improves the tumor-killing efficacy of alloreactive CD8+ T cells.
β2AR deficiency in the hosts ameliorates GVHD and improves CD4+Foxp3+ regulatory T cell (Treg) reconstitution
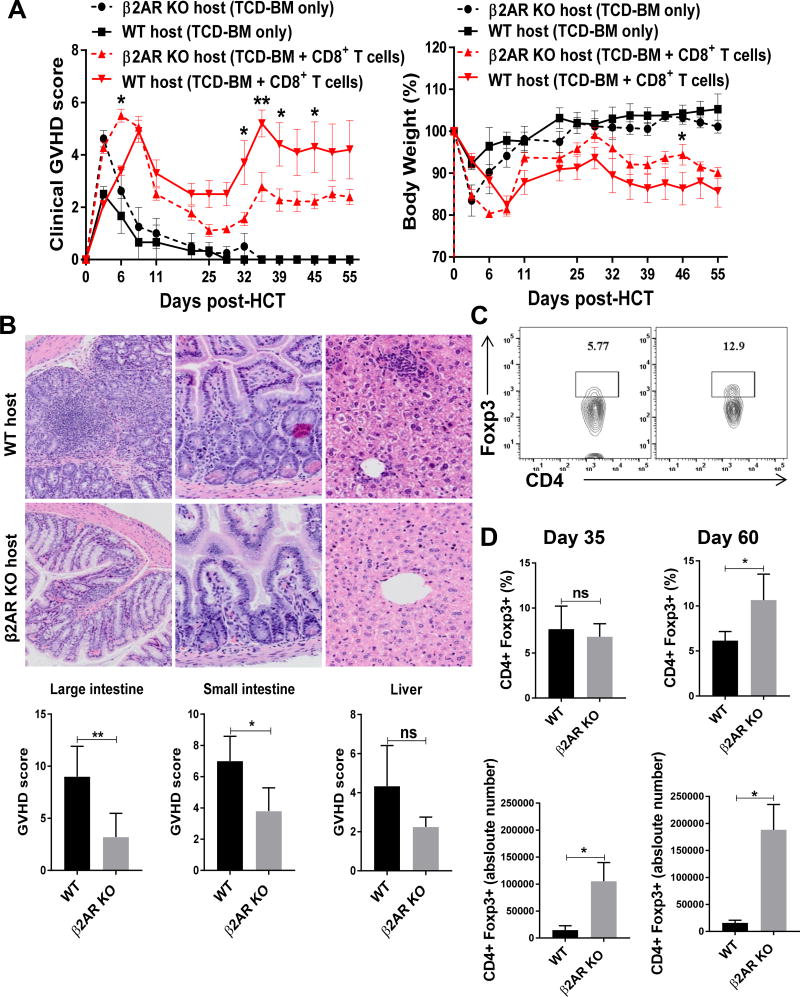
To define the effect of β2AR KO in the host on GVHD, we used MHC-mismatched GVHD model where the host mice were injected with allogeneic TCD-BM and CD8+ T cells. GVHD was measured using clinical GVHD score and body weight change (Figure 5A). As controls, WT and β2AR KO mice that received allogeneic TCD-BM only were able to recover and maintain their body weight and showed no sign of GVHD. In contrast, β2AR KO hosts that received TCD-BM and CD8+ T cells showed less weight loss accompanied with less severe GVHD than WT hosts. Histopathological analyses of target organs showed higher pathological GVHD scores in the large and small intestines with a similar trend in the liver in WT hosts compared to β2AR KO hosts (Figure 5B).
Figure 5. β2AR deficiency in the host decreases GVHD and improve Treg reconstitution.
At day −1, BALB/cJ WT or β2AR KO host mice were irradiated with 900 cGy. At day 0, host mice were transplanted with 3×106 TCD-BM and 2×106 CD8+ T cells purified from C57BL/6J mice. (A) Clinical GVHD score and body weight (n=17). (B) Representative histopathological images shown at 200× magnification, with pathological GVHD scores in the large intestine, small intestine and liver (n=5). (C) Representative flow cytometry plots for Tregs at day 60. (D) Percentages and absolute numbers of Tregs in the host spleens (n=8). Data are presented as Mean ± SD. ****, P < 0.0001; ***, P = 0.001; **, 0.01; *, 0.05.
To examine de novo T cell reconstitution in the hosts, we assessed donor-derived CD4+ T cell absolute numbers and percentages at day 35 and 60 in the spleen. β2AR KO mice have higher numbers of reconstituted CD3+CD4+ T cells (Supplemental Figure 1C). Because of the importance of reconstituted Tregs in controlling GVHD, we assessed Treg percentage and absolute number. There was no significant difference between WT and β2AR KO hosts regarding CD4+Foxp3+ Treg percentages at day 35, but Treg cell absolute numbers were noticeably higher in β2AR KO hosts (Figure 5D). At day 60, both Treg percentages and absolute numbers were significantly higher in β2AR KO hosts compared to WT hosts (Figure 5C and D). These results indicate that β2AR deficiency in the host induces not only higher expansion of transplanted donor CD8+ T cells (Supplemental Figure 1A), but also improved reconstitution of CD4+ T cells including Tregs from donor BM-derived progenitors and stem cells.
Discussion
Host APCs are a vital parameter in GVT and GVHD pathogenesis. In this study, we discovered that β2AR deficiency in the host increases GVT effect. We also showed that β2AR blockade with a selective blocker before BM transplantation resulted in significantly enhanced GVT effect. Our data demonstrated that β2AR plays a suppressive role on DC function via decreasing expression of co-stimulatory markers and increased IL-10 cytokine release. In agreement with our report, it has been shown that β2AR agonists such as isoprenaline and salbutamol suppress inflammatory phenotype of DCs (23, 24). Also our data showed that the increased immunogenic phenotype of β2AR-deficient DCs is associated with downregulation of STAT3 phosphorylation. β2AR signaling was previously connected with STAT3 activation (37), which led to increased IL-10 secretion and inhibition of NF-κB (35, 36). Also, it has recently been shown that STAT3 expression in host myeloid cells inhibits GVHD severity through suppressing DC function (38). Through MLR-based in vitro T cell activation and tumor cell killing experiments, we demonstrated that β2AR deficiency in DCs enhances alloreactive CD8+ T cell response in the tumor setting. However, our previous study showed that β2AR inhibition exacerbated GVHD induced by total T cells in which CD4+ T cells presumably play a dominant role. Put together, these findings suggest that β2AR signaling plays more complicated and differential roles in GVT and GVHD involving CD4+ versus CD8+ T cell responses and possibly other immune cells. Further study will be required to delineate the underlying mechanisms.
We show that β2AR deficiency in DCs decreases spare respiratory capacity and glycolytic capacity. Also β2AR KO DCs use glutamine as a source of energy more than WT DCs with a negative trend in glycolytic rate which is in agreement with previous reports indicating that high glucose can also suppress DC function marked by increased IL-10 secretion and down-regulation of costimulatory markers (40) and represses DC-induced T cell responses (41). Metabolic changes through mTOR signaling play a critical role in DC functions and cytokine release. High glycolysis with active mTOR signaling and less OXPHOS activation is a signature of activated DCs (39). In tolerogenic DCs, OXPHOS activation plays a major role in DC metabolism and energy generation through inhibition of mTOR signaling and activation of AMPK signaling (42). In line with previous reports, we showed that β2AR KO DCs express more mTOR proteins compared to WT DCs. It has also been shown that high mitochondrial respiration and glycolytic capacity is the phenotype of tolerogenic DCs (43), which is in agreement to what we found in WT DCs compared to β2AR KO DCs. Altogether, for the first time to our knowledge, our data confirm that β2AR deficiency shifts DC metabolism towards a more immunogenic phenotype.
It has been documented that an increased percentage of T cells including regulatory T cells (Tregs) after transplantation is a sign of improved reconstitution (44). After allogeneic transplantation, reconstituted Tregs play a crucial role in the control of DC co-stimulation and GVHD without affecting T cell expansion (45, 46). It has also been shown that occurrence of GVHD impairs immune cell reconstitution (47). Interestingly, the enhanced Treg reconstitution at day 60 in the β2AR KO hosts is associated with increased CD8+ T cell number and memory phenotype (Supplemental Figure 1A–B) and deceased GVHD. This result is also consistent with a previous report showing that Tregs promote memory CD8+ T cell maturation (48).
Finally, we summarized our finding in Supplemental Figure 2. In conclusion, our data demonstrate that β2AR signaling suppresses host DC function. Either blockade or knockout of host β2AR enhances GVT effect through modulating STAT3 and mTOR signaling in host DCs, resulting in up-regulation of co-stimulatory molecules and more immunogenic DCs. In addition, β2AR deficiency in the hosts induces enhanced T cell reconstitution and memory formation, which may translate into increased GVT activity without exacerbating GVHD.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant R21CA202358 (to X.C.) and used shared resources supported by the Roswell Park Cancer Institute Comprehensive Cancer Center Support Grant CA016056.
Footnotes
Disclosure of Conflicts of Interest
The authors have no special/competing interests to disclose.
References
- 1.Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, Wadleigh M, DeAngelo DJ, Stone RM, Sakamaki H. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. Jama. 2009;301:2349–2361. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramadan A, Paczesny S. Various forms of tissue damage and danger signals following hematopoietic stem-cell transplantation. Frontiers in immunology. 2015;6 doi: 10.3389/fimmu.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cutler C, Giri S, Jeyapalan S, Paniagua D, Viswanathan A, Antin JH. Acute and chronic graft-versus-host disease after allogeneic peripheral-blood stem-cell and bone marrow transplantation: a meta-analysis. Journal of Clinical Oncology. 2001;19:3685–3691. doi: 10.1200/JCO.2001.19.16.3685. [DOI] [PubMed] [Google Scholar]
- 4.Seggewiss R, Einsele H. Immune reconstitution after allogeneic transplantation and expanding options for immunomodulation: an update. Blood. 2010;115:3861–3868. doi: 10.1182/blood-2009-12-234096. [DOI] [PubMed] [Google Scholar]
- 5.Stenger EO, Turnquist HR, Mapara MY, Thomson AW. Dendritic cells and regulation of graft-versus-host disease and graft-versus-leukemia activity. Blood. 2012;119:5088–5103. doi: 10.1182/blood-2011-11-364091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shlomchik WD, Couzens MS, Tang CB, McNiff J, Robert ME, Liu J, Shlomchik MJ, Emerson SG. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 7.Duffner UA, Maeda Y, Cooke KR, Reddy P, Ordemann R, Liu C, Ferrara JL, Teshima T. Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease. The Journal of Immunology. 2004;172:7393–7398. doi: 10.4049/jimmunol.172.12.7393. [DOI] [PubMed] [Google Scholar]
- 8.Chan GW, Gorgun G, Miller KB, Foss FM. Persistence of host dendritic cells after transplantation is associated with graft-versus-host disease. Biology of Blood and Marrow Transplantation. 2003;9:170–176. doi: 10.1053/bbmt.2003.50006. [DOI] [PubMed] [Google Scholar]
- 9.Nijhuis LE, Olivier BJ, Dhawan S, Hilbers FW, Boon L, Wolkers MC, Samsom JN, de Jonge WJ. Adrenergic β2 receptor activation stimulates anti-inflammatory properties of dendritic cells in vitro. PLoS One. 2014;9:e85086. doi: 10.1371/journal.pone.0085086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohm AP, Mozaffarian A, Sanders VM. B cell receptor-and β2-adrenergic receptor-induced regulation of B7-2 (CD86) expression in B cells. The Journal of Immunology. 2002;168:6314–6322. doi: 10.4049/jimmunol.168.12.6314. [DOI] [PubMed] [Google Scholar]
- 11.Tan KS, Nackley AG, Satterfield K, Maixner W, Diatchenko L, Flood PM. β2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA- and NF-κB-independent mechanisms. Cellular Signalling. 2007;19:251–260. doi: 10.1016/j.cellsig.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Estrada LD, Ağaç D, Farrar JD. Sympathetic neural signaling via the β2-adrenergic receptor suppresses T-cell receptor-mediated human and mouse CD8+ T-cell effector function. European journal of immunology. 2016;46:1948–1958. doi: 10.1002/eji.201646395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H, Zhang W, Cheng X, Guo L, Xie S, Ma Y, Guo N, Shi M. β2-AR activation induces chemoresistance by modulating p53 acetylation through upregulating Sirt1 in cervical cancer cells. Cancer Science. 2017 doi: 10.1111/cas.13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calcagni E, Elenkov I. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Annals of the New York Academy of Sciences. 2006;1069:62–76. doi: 10.1196/annals.1351.006. [DOI] [PubMed] [Google Scholar]
- 15.Kim B-J, Jones HP. Epinephrine-primed murine bone marrow-derived dendritic cells facilitate production of IL-17A and IL-4 but not IFN-γ by CD4+ T cells. Brain, behavior, and immunity. 2010;24:1126–1136. doi: 10.1016/j.bbi.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leigh ND, Kokolus KM, O’Neill RE, Du W, Eng JW-L, Qiu J, Chen GL, McCarthy PL, Farrar JD, Cao X. Housing Temperature–Induced Stress Is Suppressing Murine Graft-versus-Host Disease through β2-Adrenergic Receptor Signaling. The Journal of Immunology. 2015;195:5045–5054. doi: 10.4049/jimmunol.1500700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du W, Leigh ND, Bian G, O'Neill RE, Mei L, Qiu J, Chen GL, Hahn T, Liu H, McCarthy PL. Granzyme B–Mediated Activation-Induced Death of CD4+ T Cells Inhibits Murine Acute Graft-versus-Host Disease. The Journal of Immunology. 2015;195:4514–4523. doi: 10.4049/jimmunol.1500668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bian G, Ding X, Leigh ND, Tang Y, Capitano ML, Qiu J, McCarthy PL, Liu H, Cao X. Granzyme B–Mediated Damage of CD8+ T Cells Impairs Graft-versus-Tumor Effect. The Journal of Immunology. 2013;190:1341–1350. doi: 10.4049/jimmunol.1201554. [DOI] [PubMed] [Google Scholar]
- 19.Ding X, Bian G, Leigh ND, Qiu J, McCarthy PL, Liu H, Aygun-Sunar S, Burdelya LG, Gudkov AV, Cao X. A TLR5 agonist enhances CD8+ T cell-mediated graft-versus-tumor effect without exacerbating graft-versus-host disease. The Journal of Immunology. 2012;189:4719–4727. doi: 10.4049/jimmunol.1201206. [DOI] [PubMed] [Google Scholar]
- 20.Leigh ND, O’Neill RE, Du W, Chen C, Qiu J, Ashwell JD, McCarthy PL, Chen GL, Cao X. Host-Derived CD70 Suppresses Murine Graft-versus-Host Disease by Limiting Donor T Cell Expansion and Effector Function. The Journal of Immunology. 2017:1502181. doi: 10.4049/jimmunol.1502181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai SF, Cao X, Hassan A, Fehniger TA, Ley TJ. Granzyme B is not required for regulatory T cell–mediated suppression of graft-versus-host disease. Blood. 2010;115:1669–1677. doi: 10.1182/blood-2009-07-233676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takenaka MC, Araujo LP, Maricato JT, Nascimento VM, Guereschi MG, Rezende RM, Quintana FJ, Basso AS. Norepinephrine Controls Effector T Cell Differentiation through β2-Adrenergic Receptor–Mediated Inhibition of NF-κB and AP-1 in Dendritic Cells. The Journal of Immunology. 2016;196:637–644. doi: 10.4049/jimmunol.1501206. [DOI] [PubMed] [Google Scholar]
- 23.Wu H, Chen J, Song S, Yuan P, Liu L, Zhang Y, Zhou A, Chang Y, Zhang L, Wei W. β2-adrenoceptor signaling reduction in dendritic cells is involved in the inflammatory response in adjuvant-induced arthritic rats. Scientific reports. 2016;6:24548. doi: 10.1038/srep24548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manni M, Granstein RD, Maestroni G. β2-Adrenergic agonists bias TLR-2 and NOD2 activated dendritic cells towards inducing an IL-17 immune response. Cytokine. 2011;55:380–386. doi: 10.1016/j.cyto.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maestroni GJ, Cosentino M, Marino F, Togni M, Conti A, Lecchini S, Frigo G. Neural and endogenous catecholamines in the bone marrow. Circadian association of norepinephrine with hematopoiesis? EXPERIMENTAL HEMATOLOGY-COPENHAGEN THEN EL PASO TEXAS THEN NEW YORK- 1998;26:1172–1177. [PubMed] [Google Scholar]
- 26.Cosentino M, Marino F, Kustrimovic N. [Accessed April 30, 2015];Endogenous catecholamines in immune cells: discovery, functions and clinical potential as therapeutic targets. 2013 Available from: http://brainimmune.com/endogenouscatecholamines in immune cells: discovery-functions-and-clinical-potentialas-pharmacotherapeutic-targets-3/
- 27.Freeman JG, Ryan JJ, Shelburne CP, Bailey DP, Bouton LA, Narasimhachari N, Domen J, Siméon N, Couderc F, Stewart JK. Catecholamines in murine bone marrow derived mast cells. Journal of neuroimmunology. 2001;119:231–238. doi: 10.1016/s0165-5728(01)00384-8. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen KD, Qiu Y, Cui X, Goh YS, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weber M, Rudolph B, Stein P, Yogev N, Bosmann M, Schild H, Radsak MP. Host-Derived CD8+ Dendritic Cells Protect Against Acute Graft-versus-Host Disease after Experimental Allogeneic Bone Marrow Transplantation. Biology of Blood and Marrow Transplantation. 2014;20:1696–1704. doi: 10.1016/j.bbmt.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Koyama M, Hashimoto D, Aoyama K, Matsuoka K-i, Karube K, Niiro H, Harada M, Tanimoto M, Akashi K, Teshima T. Plasmacytoid dendritic cells prime alloreactive T cells to mediate graft-versus-host disease as antigen-presenting cells. Blood. 2009;113:2088–2095. doi: 10.1182/blood-2008-07-168609. [DOI] [PubMed] [Google Scholar]
- 31.Hadeiba H, Sato T, Habtezion A, Oderup C, Pan J, Butcher EC. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nature immunology. 2008;9:1253–1260. doi: 10.1038/ni.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Neill LA, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. Journal of Experimental Medicine. 2016;213:15–23. doi: 10.1084/jem.20151570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Everts B, Amiel E, Huang SC-C, Smith AM, Chang C-H, Lam WY, Redmann V, Freitas TC, Blagih J, Van Der Windt GJ. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKK [epsiv] supports the anabolic demands of dendritic cell activation. Nature immunology. 2014;15:323–332. doi: 10.1038/ni.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, Cross JR, Jung E, Thompson CB, Jones RG. Toll-like receptor–induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–4749. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melillo JA, Song L, Bhagat G, Blazquez AB, Plumlee CR, Lee C, Berin C, Reizis B, Schindler C. Dendritic cell (DC)-specific targeting reveals Stat3 as a negative regulator of DC function. The Journal of Immunology. 2010;184:2638–2645. doi: 10.4049/jimmunol.0902960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rébé C, Végran F, Berger H, Ghiringhelli F. STAT3 activation: A key factor in tumor immunoescape. Jak-stat. 2013;2:e23010. doi: 10.4161/jkst.23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang H, Feng W, Liao W, Ma X, Han Q, Zhang Y. The gp130/STAT3 signaling pathway mediates β-adrenergic receptor-induced atrial natriuretic factor expression in cardiomyocytes. The FEBS journal. 2008;275:3590–3597. doi: 10.1111/j.1742-4658.2008.06504.x. [DOI] [PubMed] [Google Scholar]
- 38.Nieves EC, Toubai T, Peltier DC, Oravecz-Wilson K, Liu C, Tamaki H, Sun Y, Reddy P. STAT3 Expression in Host Myeloid Cells Controls Graft-versus-Host Disease Severity. Biology of Blood and Marrow Transplantation. 2017;23:1622–1630. doi: 10.1016/j.bbmt.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pearce EJ, Everts B. Dendritic cell metabolism. Nature Reviews Immunology. 2015;15:18–29. doi: 10.1038/nri3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferreira GB, Vanherwegen A-S, Eelen G, Gutiérrez ACF, Van Lommel L, Marchal K, Verlinden L, Verstuyf A, Nogueira T, Georgiadou M. Vitamin D3 induces tolerance in human dendritic cells by activation of intracellular metabolic pathways. Cell reports. 2015;10:711–725. doi: 10.1016/j.celrep.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 41.Lawless SJ, Kedia-Mehta N, Walls JF, McGarrigle R, Convery O, Sinclair LV, Navarro MN, Murray J, Finlay DK. Glucose represses dendritic cell-induced T cell responses. Nature Communications. 2017;8 doi: 10.1038/ncomms15620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Everts B, Pearce EJ. Metabolic control of dendritic cell activation and function: recent advances and clinical implications. Frontiers in immunology. 2014;5 doi: 10.3389/fimmu.2014.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malinarich F, Duan K, Hamid RA, Bijin A, Lin WX, Poidinger M, Fairhurst A-M, Connolly JE. High mitochondrial respiration and glycolytic capacity represent a metabolic phenotype of human tolerogenic dendritic cells. The Journal of Immunology. 2015;194:5174–5186. doi: 10.4049/jimmunol.1303316. [DOI] [PubMed] [Google Scholar]
- 44.Alpdogan O, Eng JM, Muriglan SJ, Willis LM, Hubbard VM, Tjoe KH, Terwey TH, Kochman A, van den Brink MR. Interleukin-15 enhances immune reconstitution after allogeneic bone marrow transplantation. Blood. 2005;105:865–873. doi: 10.1182/blood-2003-09-3344. [DOI] [PubMed] [Google Scholar]
- 45.Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, Del Papa B, Zei T, Ostini RI, Cecchini D. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117:3921–3928. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 46.Li Q, Zhai Z, Xu X, Shen Y, Zhang A, Sun Z, Liu H, Geng L, Wang Y. Decrease of CD4+ CD25+ regulatory T cells and TGF-β at early immune reconstitution is associated to the onset and severity of graft-versus-host disease following allogeneic haematogenesis stem cell transplantation. Leukemia research. 2010;34:1158–1168. doi: 10.1016/j.leukres.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 47.Bunting MD, Varelias A, Souza-Fonseca-Guimaraes F, Schuster IS, Lineburg KE, Kuns RD, Fleming P, Locke KR, Huntington ND, Blazar BR. GVHD prevents NK-cell–dependent leukemia and virus-specific innate immunity. Blood. 2017;129:630–642. doi: 10.1182/blood-2016-08-734020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laidlaw BJ, Cui W, Amezquita RA, Gray SM, Guan T, Lu Y, Kobayashi Y, Flavell RA, Kleinstein SH, Craft J. Production of IL-10 by CD4+ regulatory T cells during the resolution of infection promotes the maturation of memory CD8+ T cells. Nature immunology. 2015;16:871–879. doi: 10.1038/ni.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.