Abstract
This study is a new analysis to obtain novel metabolic data on the functional connectivity of prefrontal-limbic regions in Pavlovian fear acquisition and extinction of tone-footshock conditioning. Mice were analyzed with the fluorodeoxyglucose (FDG) autoradiographic method to metabolically map regional brain activity. New FDG data were sampled from the nuclei of the habenula and other regions implicated in aversive conditioning, such as infralimbic cortex, amygdala and periaqueductal gray regions. The activity patterns among these regions were inter-correlated during acquisition, extinction or pseudorandom training to develop a functional connectivity model. Two subdivisions of the habenular complex showed increased activity after acquisition relative to extinction, with the pseudorandom group intermediate between the other two groups. Significant acquisition activation effects were also found in centromedial amygdala, dorsomedial and ventrolateral periaqueductal gray. FDG uptake increases during extinction were found only in dorsal and ventral infralimbic cortex. The overall pattern of activity correlations between these regions revealed extensive but differential functional connectivity during acquisition and extinction training, with less functional connectivity found after pseudorandom training. Interestingly, habenula nuclei showed a distinct pattern of inter-correlations with amygdala nuclei during extinction. The functional connectivity model revealed changing interactions among infralimbic cortex, amygdala, habenula and periaqueductal gray regions through the stages of Pavlovian fear acquisition and extinction. This study provided new data on the contributions of the habenula to fear conditioning, and revealed previously unreported infralimbic-amygdala-habenula-periaqueductal gray interactions implicated in acquisition and extinction of conditioned fear.
Keywords: aversive conditioning, fear extinction, habenula, infralimbic cortex, amygdala, intercalated cells
Introduction
The neural mechanisms that underlie the process of Pavlovian fear extinction are intricate, controversial, and of great interest to those that study learning phenomena (Auchter et al., 2017) as well as its clinical applications (Zoellner et al., 2017). This paradigm is a simple model of how a memory is formed, then modified on the basis of experience. Extinction may be defined as the reduction of a previously learned response that occurs because a conditioned stimulus (CS) is no longer paired with an unconditioned stimulus (US). In Pavlovian fear conditioning of rodents, a tone CS is often paired with an aversive footshock US during acquisition, such that presentations of the CS alone will elicit freezing behavior, a defensive conditioned response (CR). During extinction, repeated presentation of the tone alone (in the absence of footshock) will cause the fear-evoked CR to decrease over time.
In terms of neural mechanisms, increased habenula fluorodeoxyglucose (FDG) uptake has been linked to the prediction of the US by a tone CS in an early Pavlovian conditioning study in rats with aversive electrical stimulation of the brain as US (Gonzalez-Lima and Scheich, 1986). Our subsequent FDG study in the mouse brain (Barrett et al., 2003) examined the metabolic effects of Pavlovian acquisition and extinction of tone-footstock conditioning, but there was no investigation of the habenula. Renewed interest in the habenula in recent years (Velasquez et al., 2014), such as new findings on the negative-reward-predicting properties of habenula neurons (Matsumoto and Hikosaka, 2007) have prompted us to obtain new data from the habenula from our FDG study of tone-footstock conditioning as well as obtain new data from other limbic regions related to acquisition and extinction. We hypothesized that habenula metabolism may be involved in the prediction of aversive events more generally, i.e., the withdrawal of a reward stimulus or the delivery of an aversive one (Shumake and Gonzalez-Lima, 2013). Therefore, we predicted that habenula metabolism will increase during acquisition and decrease during extinction of tone-footstock conditioning. In addition, the patterns of metabolic activity correlations found between these regions of interest were analyzed for the first time to inform the question of how interactions between the habenula and other brain regions implicated in fear conditioning change from acquisition to extinction training.
Limbic regions such as the amygdala may be specialized for fear acquisition processes in general (Balderston et al., 2014; Schultz et al., 2016), and expression of the freezing CR in particular. For example, many studies have documented the involvement of the central amygdala in CR expression (Helmstetter, 1992; Kim et al., 1993; LeDoux, 1995), in fear-potentiated startle (Davis et al., 1993), and avoidance learning (Poremba & Gabriel, 1997). The central nucleus is the main output zone of the amygdala, and its activity probably reflects the expression of the freezing CR at the time of sampling. After extinction training, the CR is no longer present; thus, the central amygdala may show opposite effects after acquisition vs. extinction. This might not reflect a general “unlearning” process, however; it is also consistent with the involvement of CR inhibition from outside the amygdala.
This CR inhibition is likely provided by the prefrontal cortex (PFC), which has been extensively implicated as playing a crucial role in extinction of conditioned fear (Quirk et al., 2006; Gilmartin et al., 2014). For example, lesions of medial PFC disrupt CR extinction in rats (Morgan et al., 1993), and Milad & Quirk (2002) showed that neurons in infralimbic cortex develop enhanced firing rates during extinction, which are correlated with the decrease in the freezing CR. If infralimbic cortex neurons inhibit the amygdala, metabolic activation of the infralimbic cortex would be present only after extinction, and not after acquisition or pseudorandom training in the Pavlovian fear conditioning paradigm.
Most lesion and stimulation studies of the effects of Pavlovian fear extinction on the rodent brain have focused on the basolateral amygdala and infralimbic cortex as the sites of acquisition and extinction of tone-footstock conditioning, respectively. However, other brain regions such as the habenula may be also involved in these learning processes in the tone-footstock conditioning paradigm. Our lab was the first to use the FDG autoradiographic method to metabolically map brain activity after other learning paradigms, including aversive conditioning (Gonzalez-Lima and Scheich, 1986), operant extinction (Nair & Gonzalez-Lima, 1999; Nair et al., 2001a, 2001b), blocking (Jones & Gonzalez-Lima, 2001a), differential inhibition (Jones & Gonzalez-Lima, 2001b), and habituation (Gonzalez-Lima et al., 1989a, 1989b). One advantage of the FDG autoradiographic technique is the ability to take optical density readings from any brain region. Thus, any region recently implicated in aversive conditioning, such as the intercalated cells of the amygdala (Asede et al., 2015, Strobel et al., 2015) or the nuclei of the habenula (Shumake and Gonzalez-Lima, 2013) can be sampled, and this activity can be correlated with that of other brain systems after fear acquisition or extinction to develop a functional connectivity model.
Therefore, the objective of this study was to obtain new FDG data on the habenula and other limbic regions implicated in aversive conditioning, and to construct a novel functional connectivity model of infralimbic-amygdala-habenula-periaqueductal gray interactions after Pavlovian fear acquisition and extinction.
Experimental Procedures
This manuscript is a new analysis that provides new FDG data from regions not previously investigated in the experiment described in Barrett et al. (2003). Images from the autoradiographic films obtained from those subjects were used to take new optical density readings from new brain regions of interest based on recent findings on the brain metabolic effects of Pavlovian fear extinction. All animal experimentation was carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996 and was approved by the University of Texas Institutional Animal Care and Use Committee. All efforts were made to minimize the number of animals used and their suffering.
A timeline diagram of the fear conditioning protocol and experimental design is provided in Figure 1. The methods for behavioral training and tissue processing for FDG autoradiography were those described in Barrett et al. (2003). Briefly, 32 male CBA/J mice, 5 weeks of age when delivered from Jackson Laboratory (Bar Harbor, ME), were handled for 7 days prior to training on Pavlovian acquisition (n=11), extinction (n=11), or pseudorandom (n=10) presentations of a tone CS (frequency-modulated tone, 1-2 kHz, 65 dB, 15 sec) and footshock US (0.5 mA, 0.75 sec). Phase I (acquisition training) occurred in context A. Since our objective was to map neural effects evoked by the tone rather than by context A, all subsequent steps (probe trials, extinction training, and FDG uptake) were conducted in context B to minimize the effects of excitatory conditioning to context A. After training, subjects were given an intraperitoneal injection of 18 μCi/100 gm body weight of 14C(U)-FDG, placed in context B, and exposed to the tone CS in a 5 sec on, 5 sec off cycle for 45 min. Subjects were then decapitated and brains were rapidly removed and frozen in isopentane at −40°C. Sections of the brain were cut at 40 μm, picked up on slides, dried, and exposed to autoradiographic film (along with standards of known 14C concentration) for 2 weeks. Films were developed, dried, and stored in protective covers.
Fig. 1.
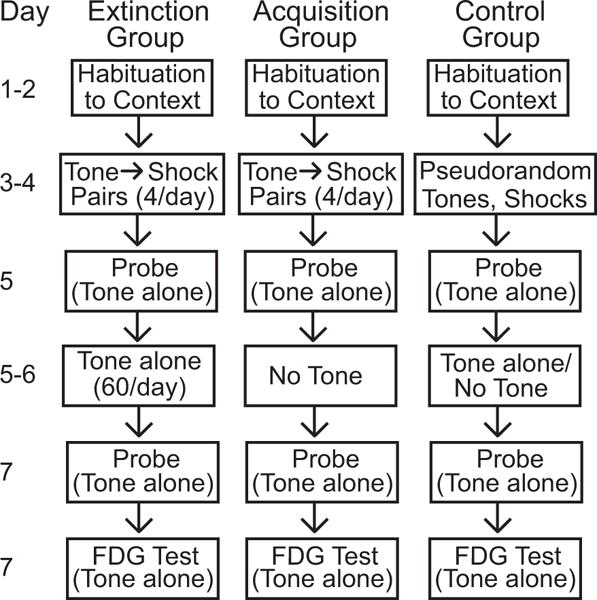
Timeline of the experimental procedures for each of the three groups.
Films were placed on a light box and artifact-free images were captured, digitized, and corrected for film background and optical distortions from the camera via background subtraction. Absolute gray levels of the 14C standards were used to create a calibration curve unique to each individual film. Optical density measurements from brain regions of interest were automatically expressed in terms of isotope incorporation per gram of brain tissue (nanocuries per gram). The Paxinos and Franklin (2001) mouse brain atlas was used to locate and delineate the boundaries of each region measured as illustrated in the diagrams shown in Figures 3–6. For the purpose of sampling regional FDG uptake, the infralimbic cortex was subdivided into two equal halves by a line at the center of the dorsal-ventral axis (Fig. 6). Each region was sampled from three adjacent sections, with four non-overlapping readings taken in each section; these measurements were averaged together to give one measurement per region per subject. The brain regions sampled were: Infralimbic cortex, dorsal (ILd); Infralimbic cortex, ventral (ILv); Anterior habenula (HBa); Nucleus reunions; Paraventricular hypothalamus; Amygdala, lateral nucleus (LA); Amygdala, basolateral nucleus (BLA); Amygdala, centrolateral nucleus (CeL); Amygdala, centromedial nucleus (CeM); Amygdala, intercalated cells, dorsal (ITCd); Amygdala, intercalated cells, ventral (ITCv); Posterior habenula, lateral (HBl); Posterior habenula, medial (HBm); Periaqueductal gray, anterior; Posterior thalamus; Posterior intralaminar nucleus; Dorsomedial periaqueductal gray (DMPAG); Ventrolateral periaqueductal gray (VLPAG); Pontine reticular formation. Bregma levels and FDG data for all regions are listed in the appendix table.
Fig. 3.
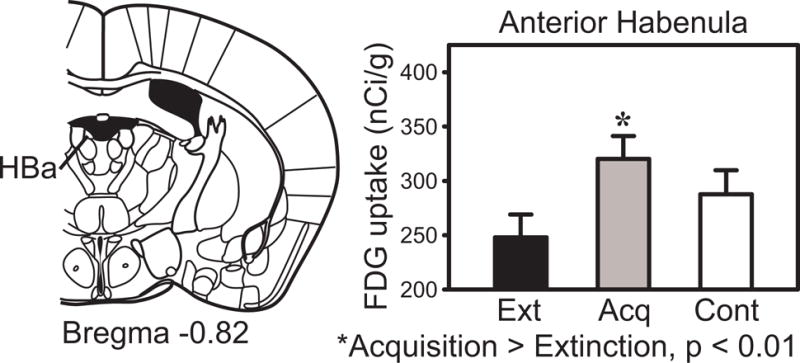
Anterior habenula FDG uptake in the extinction, acquisition and pseudorandom control groups.
Fig. 6.
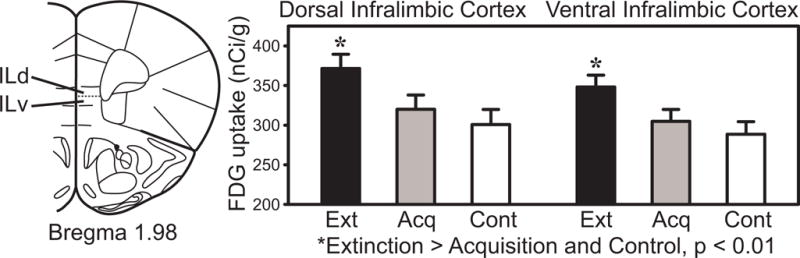
Infralimbic cortex FDG uptake in the extinction, acquisition and pseudorandom control groups.
Statistical analysis
We found normal distributions and equal variances in our sampled distributions. Mean brain activity was assessed as in Barrett et al. (2003). Briefly, to reduce variability resulting from individual differences in FDG uptake unrelated to the experimental paradigm, white matter readings were used as covariates in an analysis of covariance (ANCOVA). Because there was no statistically significant difference in the white matter readings between groups, the ANCOVA can use the covariate to compensate for small variations in isotope incorporation across individuals. Mean activity readings were then expressed in nanocuries per gram of tissue for each region of interest, adjusted by the covariate white matter readings, with 99% confidence intervals for each group. If the measurements for two groups were both outside the other’s 99% confidence interval, the effect was considered significant at uncorrected p < 0.01 and at corrected p < 0.05 adjusted for multiple comparisons among the groups with the Hochberg procedure, which is a sharper Bonferroni procedure for multiple tests of significance (Hochberg, 1988). Effect sizes were estimated using Cohen’s D [(Mean1-Mean2)/Pooled standard deviation]. A Cohen’s D larger than 0.8 is considered a large effect size (Cohen 1977).
Functional connectivity is an analytic approach that has been used for several decades to refer to the correlations of activity between neural elements. It was first introduced using electrophysiological data and glucose metabolic rates (Horwitz et al., 1984; Aertsen et al., 1987). Functional connectivity using autoradiographic FDG data was introduced by McIntosh and Gonzalez-Lima (1991, 1992). This approach was then introduced using fMRI data (Friston, 1994). To state that two neural elements (neurons or brain regions) display functional connectivity is to say that these elements show statistically significant correlated activity without reference to how that correlation is mediated by anatomical connections (McIntosh and Gonzalez-Lima, 1994). Because extinction of conditioned behavior is manifested as neural changes in functional connectivity of FDG data in our previous studies, the functional relationships among the regional brain activity data were analyzed in terms of pair-wise correlations within each group, exactly as done before in our FDG studies of extinction of instrumental behavior (Nair and Gonzalez-Lima, 1999) and Pavlovian fear conditioning (Barrett et al., 2003). Briefly, for the interregional correlation analysis, Pearson product–moment correlations were computed using pair-wise comparisons of each region. To ensure the statistical reliability of correlations, a jackknife procedure was performed to avoid inflated type 1 errors caused by the large number of interregional correlations computed relative to the sample sizes. In the jackknife procedure, each individual subject was dropped from a group, and then correlations were calculated again without that subject’s data. This procedure was iterated until each subject had been sequentially dropped and the analysis performed again. Correlations were considered to be reliably corrected as significant only if they remained significantly (p < 0.05) different from zero throughout all iterations. This is a conservative correction method sensitive to outliers that avoids inflated type 1 errors in the functional connectivity analysis of metabolic brain data (Sakata et al., 2000).
Results
Representative FDG autoradiographs are shown in Figure 2. Bregma levels with regions showing a significant mean difference in FDG uptake are shown in Figures 3–6. The means, standard errors, and confidence intervals of each region’s FDG uptake are listed in a table in the Appendix. Correlation coefficients, which remained significant at p < 0.05 after the jackknife procedure, are shown in Figure 7.
Fig. 2.
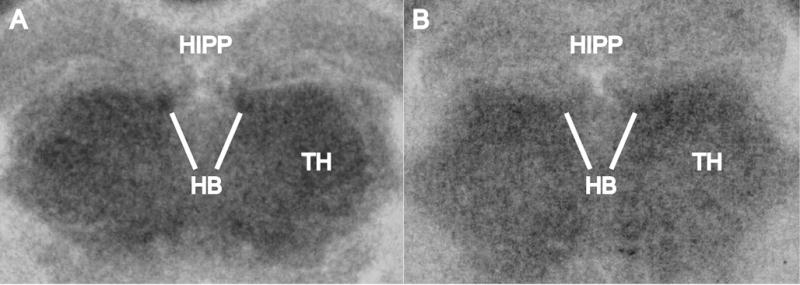
Representative FDG autoradiographs showing the habenula (HB), thalamus (TH), and hippocampus (HIPP) in images developed from coronal sections of the mouse brain at the level of HB. The white lines point to HB to illustrate the increase in FDG uptake in A as compared to B. However, the results reported in the paper are not based on visual inspection of autoradiographs; rather, they are based on quantitative densitometric analysis and group statistics that showed that HB had a statistically significant FDG increase in the acquisition group as compared to the extinction group.
Fig. 7.
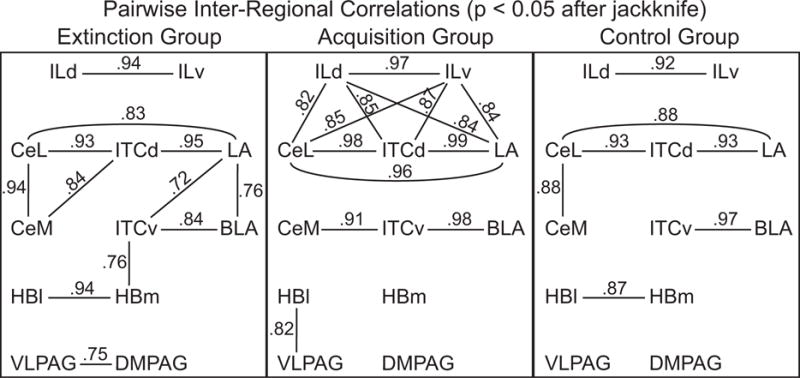
Functional connectivity models of infralimbic-amygdala-habenula-periaqueductal gray interactions based on pairwise inter-regional correlations of FDG uptake in the extinction group, acquisition group and pseudorandom control group. The lines with numbers indicate the inter-regional correlation coefficients that remained reliably significant at p < 0.05 after the jackknife procedure.
Behavior
Table 1 summarizes the percent freezing to the CS for the three groups. After acquisition training, freezing was significantly increased in both the acquisition group [F(1,19) = 40.4, p < 0.01] and extinction group [F(1,19) = 41.9, p < 0.01]. After extinction training, the extinction group had a significant decrease in freezing [F(1,20) = 123.0, p <0.01].
Table 1.
Percent freezing behavior in the three groups.
| Group | Percent Freezing | |
|---|---|---|
| Probe 1 (post-acquisition) | Probe 2 (post-extinction) | |
| Extinction | 83% | 21% |
| Acquisition | 82% | 83% |
| Control | 30% | 27% |
Mean FDG uptake changes
Table 2 shows the effect sizes (Cohen’s D) for each of the significant group differences in FDG uptake. Novel findings during Pavlovian tone-footshock conditioning were found in the habenula (HB, Fig. 2). Habenular regions showed increased activity after acquisition relative to extinction, with the pseudorandom group intermediate between the other two groups. Statistically significant (uncorrected p < 0.01 and corrected p < 0.05) acquisition effects (increased metabolic activity after acquisition training) were found in two subdivisions of the habenula complex: anterior habenula (HBa, Fig. 3) and medial habenula (HBm, Fig. 4); but the increase in lateral habenula did not reach uncorrected p < 0.01.
Table 2.
Summary of effect sizes (Cohen’s D) of significant comparisons.
| Brain Region | Comparison | Effect Size |
|---|---|---|
| Infralimbic cortex, dorsal | Extinction > Control | 1.23 |
| Infralimbic cortex, dorsal | Extinction > Acquisition | 0.90 |
| Infralimbic cortex, ventral | Extinction > Control | 1.26 |
| Infralimbic cortex, ventral | Extinction > Acquisition | 0.93 |
| Anterior habenula | Acquisition > Extinction | 1.09 |
| Amygdala, centromedial nucleus | Acquisition > Control | 0.94 |
| Posterior habenula, medial | Acquisition > Extinction | 1.09 |
| Dorsomedial periaqueductal gray | Acquisition > Control | 1.30 |
| Ventrolateral periaqueductal gray | Acquisition > Control | 1.03 |
Fig. 4.
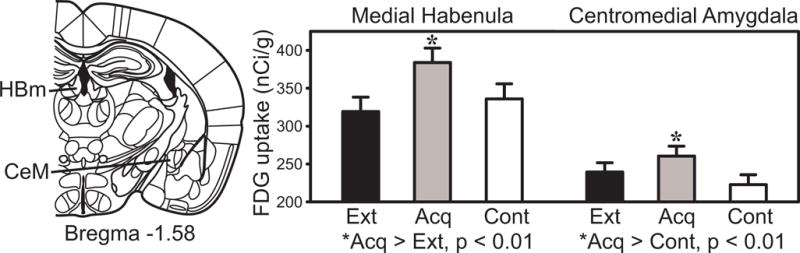
Medial habenula and centromedial amygdala FDG uptake in the extinction, acquisition and pseudorandom control groups.
Another significant acquisition effect was found in centromedial amygdala (CeM, Fig. 4). The acquisition group showed significantly greater metabolic activity relative to the pseudorandom group, with the extinction group showing an intermediate value. However, other amygdala subdivisions such as basal, lateral, centrolateral, or intercalated cells of the amygdala did not show this increase at uncorrected p < 0.01.
Significant acquisition effects were also found in dorsomedial and ventrolateral periaqueductal gray (DMPAG, VLPAG, Fig. 5). Both regions showed significantly greater metabolic activity after acquisition training, relative to the control group. The values for the extinction group were intermediate between acquisition and control groups.
Fig. 5.
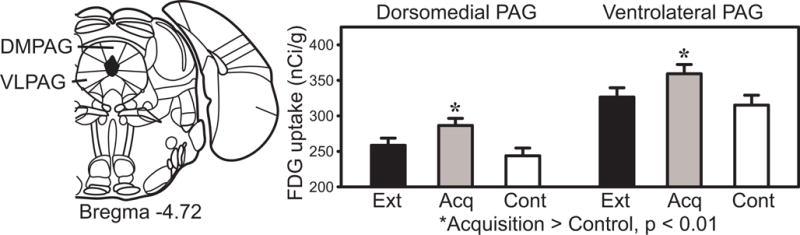
Periaqueductal gray FDG uptake in the extinction, acquisition and pseudorandom control groups.
Significant mean FDG uptake increases during extinction were found only in dorsal and ventral infralimbic cortex subdivisions (ILd, ILv, Fig. 6), among all the regions sampled. Both infralimbic subdivisions showed increased activity after extinction, but not acquisition or pseudorandom training.
Functional connectivity
Pairwise within-group correlations between FDG uptake of infralimbic cortex, amygdala, habenula and periaqueductal gray regions for each of the three groups showed that all significant correlation coefficients (p < 0.05 after jackknife) were positive (Fig. 7). This is indication that for each unit increase or decrease in FDG uptake in one region, the correlated region showed a corresponding increase or decrease in FDG uptake (i.e., positive functional connectivity). The overall pattern of significant correlations between these systems revealed extensive but differential functional connectivity after acquisition and extinction training, with less functional connectivity found after pseudorandom training.
Whereas infralimbic cortex, amygdala, habenula and periaqueductal gray showed various positive correlation coefficients in both pseudorandom and acquisition groups, their pattern of relationships was modified by extinction training. For example, infralimbic regions showed extensive positive functional connectivity with amygdala regions after acquisition training; however, extinction training reversed this trend, resulting in a decrease in the positive correlative activity between infralimbic cortex and amygdala, and an increase in intra-amygdala positive correlations.
Specifically, habenula regions showed a distinct pattern of inter-correlations with amygdala nuclei during extinction. The extinction pattern was characterized by a strong medial habenula-intercalated cell (HBm-ITCv) correlation; and the ITCv was positively correlated with the lateral amygdala (ITCv-LA). This pattern of habenula-amygdala inter-regional correlations was not found during acquisition or pseudorandom control training. In addition, metabolic activity in the habenula was positively correlated with that of the ventrolateral PAG (HBl-VLPAG) only in the acquisition group. Furthermore, the acquisition group was characterized by a network of infralimbic-amygdala functional connections in which ILd-ILv were strongly inter-correlated with CeL, ITCd and LA. In this network, the strongest intra-amygdala correlations were among CeL, ITCd and LA regions. The extinction group, however, showed a loss of ILd-ILv positive functional connectivity with CeL-ITCd-LA, a pattern more similar to that of the control group.
Therefore, the functional connectivity models revealed changing interactions among infralimbic cortex, amygdala, habenula and periaqueductal gray regions through the stages of Pavlovian acquisition and extinction.
Discussion
Current understanding of neural pathways relevant to auditory fear acquisition and extinction (Amano et al., 2011, Nair 2012, Gilmartin et al., 2014) suggest that: 1) tone and footshock information arrive at the lateral amygdala nucleus (LA) via thalamic and cortical pathways; 2) LA projects to the basolateral amygdala nucleus (BLA) and also to dorsal intercalated cells (ITCd) and centrolateral amygdala (CeL); 3) BLA fear acquisition neurons project to centromedial amygdala (CeM) and BLA fear extinction neurons project to ventral intercalated cells (ITCv); and 4) the central nucleus provides the amygdala output which projects to brainstem regions such as the periaqueductal gray (PAG) and other regions eliciting fear-related behavior. Consistent with the hypothesis that infralimbic cortex (IL) plays an active modulatory role in Pavlovian extinction (Quirk et al., 2006), both dorsal (ILd) and ventral (ILv) infralimbic cortex subdivisions showed significantly greater metabolic activity after extinction training; no other region sampled showed increased activity after extinction. Infralimbic cortex showed the single largest percentage increase in mean FDG uptake after extinction training in our previous study (Barrett et al., 2003). The pairwise correlations between infralimbic cortex and amygdala, which are significantly positive after acquisition but not extinction (Fig. 7), also indicate a shift in the role of infralimbic cortex after Pavlovian conditioning. In particular, the infralimbic-amygdala positive functional connectivity in which ILd-ILv were strongly inter-correlated with CeL, ITCd and LA during acquisition were not found during extinction. The extinction group showed a decrease in the positive correlative activity between infralimbic cortex and amygdala, and an increase in intra-amygdala correlations. The observed intra-amygdala activity correlations among CeL, ITCd and LA are consistent with direct projections from ITCd to both CeL and LA (Asede et al., 2015, Strobel et al., 2015).
The acquisition effect found in CeM supports this region’s important role in fear conditioning (Barrett et al., 2003). Other amygdala regions (ventral intercalated cells, lateral nucleus) also showed greater activity after acquisition relative to pseudorandom training, but only at the less stringent uncorrected p < 0.05 level. The stronger effect seen in CeM may be consistent with this region’s role as the response expression area of the amygdala. If activity in this region is more coupled to the expression of the conditioned response (freezing behavior), then the higher levels of conditioned freezing in the acquisition group may be driving the increase in metabolic activity in CeM. There are extensive reciprocal anatomical connections between the central amygdala and the periaqueductal gray (PAG) (Rizvi et al., 1991).
This argument, in which regions tied to the expression of conditioned freezing behavior show elevated activity after acquisition training, also applies to the changes seen in PAG. Anterior PAG showed an acquisition effect at uncorrected p < 0.05, but this effect was not significant at p < 0.01. However, posterior PAG (both dorsomedial and ventrolateral periaqueductal gray) showed significant acquisition effects at p < 0.01, in which metabolic activity was elevated by acquisition training, then attenuated by extinction training. In both of these regions, the level of FDG uptake was proportional to the levels of conditioned freezing exhibited by the three experimental groups. These two regions, however, may be differentiated by their specialized roles in the expression of extinguished freezing behavior, as the magnitude of FDG uptake was greater in ventrolateral than in dorsomedial periaqueductal gray in all three groups. This is consistent with the finding that naloxone (an opioid antagonist) injection into ventrolateral (but not dorsal) PAG impaired fear extinction (McNally et al, 2004). Ventrolateral PAG appeared therefore more involved than dorsomedial PAG in the functional connectivity model, as a final output mediating the expression of conditioned freezing behavior. Furthermore, FDG uptake in the ventrolateral PAG was better correlated with that of the habenula than to the amygdala during acquisition. In terms of anatomical circuits that may mediate functional connectivity between these brain regions, there is evidence by the HRP method that neurons in the ventral PAG project to the habenula (Herkenham and Nauta, 1977).
The extinction, acquisition and pseudorandom group received an equal number of tones and footshocks; but only the extinction group received additional tone-alone presentations. If limbic regions like the habenula mediate the prediction of aversive events signaled by the CS, then the acquisition group where the CS triggers a defensive CR would show habenula-PAG functional connectivity, but not the extinction and pseudorandom groups without CRs, as seen in the model. With extinction, the predictive negative value of the tone CS, presented repeatedly without footshock US, was gradually neutralized, decreasing the functional coupling between habenula-PAG pathways. Again, these changes provide multiple routes for the limbic system to adaptively modify behavior, by decreasing conditioned freezing after extinction training. Gonzalez-Lima and Scheich (1986) were the first to link metabolic changes in HBl activity to learning. HBl metabolism was selectively increased by pairings of a tone CS with an aversive US consisting of electrical stimulation to the midbrain reticular formation. Since the HBl was not activated by presentations of the CS alone or the US alone, this implied that the HBl responded to the prediction of the US by the CS. This early FDG finding not only anticipated the discovery of the negative-reward predicting properties of HBl neurons (Matsumoto and Hikosaka, 2007), but it is also consistent with the present FDG findings, suggesting that HBl neurons may be involved in the prediction of aversive events more generally.
The acquisition effect found in the habenula, at both anterior and posterior levels, is consistent with this region’s possible role in the prediction of aversive events that may support the persistence of fear memories (Shumake and Gonzalez-Lima, 2013). The congenital helpless rat, which our lab has extensively characterized in terms of baseline brain metabolism using cytochrome oxidase histochemistry (Shumake et al., 2001, 2002, 2003, 2004), shows increased habenula activity, which may play a role in the predisposition to develop affective disorders such as post-traumatic stress disorder (PTSD) and depression (Shumake and Gonzalez-Lima, 2003). The behavioral characteristics of the congenital helpless rat also show remarkable parallels with those of humans predisposed to the development of PTSD (Shumake et al., 2005), including a dramatic deficit in extinction learning, in which presentations of the tone CS during extinction actually result in “paradoxical enhancement” of the original fear memory and increased freezing behavior, instead of inhibition of the CS-evoked freezing response. This role of the habenula in the persistence of fear memories may be reflected in both the significant mean activity differences and in the significant pairwise correlation between lateral habenula and ventrolateral PAG, found only in the acquisition group (Fig. 7). If acquisition training results in increased functional coupling between these two regions, the habenula may be partially driving the increase in CS-evoked freezing behavior after acquisition.
The experience of fear learning and modifying an associative relationship between a CS and US seems to result in increased pairwise correlations between several brain systems (Barrett et al., 2003), as if Pavlovian conditioning drives these systems to become more interactive and more functionally coupled, at least in terms of FDG uptake. However, in terms of inhibitory influences on amygdala from infralimbic cortex, the pairwise correlations during extinction provided evidence for only weak negative correlations between infralimbic and amygdala regions that did not reach statistical significance after the jackknife procedure. The change from strong positive correlations during acquisition to weak correlations during extinction may be considered perhaps as uncorrelated infralimbic-amygdala activity during extinction.
The pattern of habenula-amygdala inter-regional correlations we found in the extinction group (but not in the acquisition or pseudorandom groups) together with our lab’s previous data (Barrett et al., 2003) suggests that infralimbic-amygdala interactivity alone may not account for extinction (Amano et al., 2011). Other regions may also contribute to extinction, such as habenular, thalamic and hippocampal regions. For example, the medial dorsal thalamus, which projects reciprocally to medial prefrontal cortex, showed greater activity, as well as a significant negative pairwise correlation with dorsal frontal cortex, after extinction but not after acquisition (Barrett et al., 2003). This thalamic region, when stimulated, can also modify CR extinction (Herry et al., 1999; Herry & Garcia, 2002), indicating that this thalamic region may also be involved in CR inhibition. Hippocampal regions also appear to be involved, and previous metabolic findings on the contextual modulation of extinction (Bruchey et al., 2007) support the importance of the hippocampus in this role.
In conclusion, the present FDG study provided new data on the contributions of the habenula to fear conditioning, and it revealed previously unreported infralimbic-amygdala-habenula-periaqueductal gray functional interactions during acquisition and extinction of tone-footshock conditioning. Regional metabolic mapping and functional connectivity modeling of FDG data served to reveal functional influences between brain regions that may be ultimately responsible for the adaptive behavioral changes seen after fear extinction learning.
Highlights.
A new analysis of brain effects of Pavlovian fear conditioning revealed increased fluorodeoxyglucose uptake in the habenula.
Metabolic increases were also found in infralimbic cortex in extinction, and amygdala and periaqueductal gray in acquisition.
Functional connectivity models revealed novel interactions between these regions during fear acquisition and extinction.
Acknowledgments
This research was supported by National Institutes of Health grant R01 NS37755 to FGL. DWB was responsible for optical densitometry and figure preparation. Both authors contributed to experimental design, statistical analysis, data interpretation, and writing the manuscript. FGL replied to the reviewers comments and revised the paper.
Abbreviations
- CeL
amygdala, centrolateral nucleus
- CeM
amygdala, centromedial nucleus
- CS
conditioned stimulus
- CR
conditioned response
- DMPAG
dorsomedial periaqueductal gray
- FDG
fluorodeoxyglucose
- HBa
anterior habenula
- HBl
posterior habenula, lateral
- HBm
posterior habenula, medial
- HRP
horseradish peroxidase
- ILd
infralimbic cortex, dorsal
- ILv
infralimbic cortex, ventral
- ITCd
amygdala, intercalated cells, dorsal
- ITCv
amygdala, intercalated cells, ventral
- PAG
periaqueductal gray
- PFC
prefrontal cortex
- PTSD
post-traumatic stress disorder
- US
unconditioned stimulus
- VLPAG
ventrolateral periaqueductal gray
Appendix Table.
Means, standard errors, and 99% confidence intervals from analysis of covariance.
| Region | Bregma Level | Group | Mean | Std. Error | 99% Confidence Interval | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Infralimbic cortex, dorsal (ILd) | 1.98 mm | Acquisition | 320.1 | 18.0 | 270.4 | 369.8 |
| Extinction, 1 | 371.5 | 18.0 | 321.7 | 421.3 | ||
| Control | 300.8 | 19.3 | 247.5 | 354.1 | ||
| Infralimbic cortex, ventral (ILv) | 1.98 mm | Acquisition | 304.8 | 14.8 | 263.9 | 345.7 |
| Extinction, 1 | 348.2 | 14.8 | 307.2 | 389.2 | ||
| Control | 288.5 | 15.9 | 244.7 | 332.4 | ||
| Anterior habenula (HBa) | −0.82 mm | Acquisition, 2 | 320.2 | 20.9 | 262.4 | 377.9 |
| Extinction | 248.0 | 21.0 | 190.1 | 305.9 | ||
| Control | 287.7 | 22.4 | 225.8 | 349.7 | ||
| Nucleus reunions | −0.82 mm | Acquisition | 324.4 | 17.9 | 274.9 | 373.8 |
| Extinction | 300.2 | 18.0 | 250.6 | 349.8 | ||
| Control | 275.4 | 19.2 | 222.3 | 328.5 | ||
| Paraventricular hypothalamus | −0.82 mm | Acquisition | 257.0 | 10.8 | 227.2 | 286.8 |
| Extinction | 238.3 | 10.8 | 208.5 | 268.2 | ||
| Control | 230.1 | 11.6 | 198.1 | 262.0 | ||
| Amygdala, lateral nucleus (LA) | −1.58 mm | Acquisition | 295.0 | 18.3 | 244.3 | 345.8 |
| Extinction | 290.1 | 17.3 | 242.2 | 338.0 | ||
| Control | 252.2 | 18.8 | 200.2 | 304.1 | ||
| Amygdala, basolateral nucleus (BLA) | −1.58 mm | Acquisition | 294.6 | 15.7 | 251.1 | 338.1 |
| Extinction | 302.5 | 14.8 | 261.4 | 343.5 | ||
| Control | 280.0 | 16.1 | 235.5 | 324.5 | ||
| Amygdala, centrolateral nucleus (CeL) | −1.58 mm | Acquisition | 252.9 | 18.4 | 202.1 | 303.8 |
| Extinction | 252.9 | 17.3 | 204.9 | 300.9 | ||
| Control | 218.3 | 18.8 | 166.3 | 270.4 | ||
| Amygdala, centromedial nucleus (CeM) | −1.58 mm | Acquisition, 3 | 260.5 | 12.8 | 225.0 | 296.1 |
| Extinction | 239.6 | 12.1 | 206.0 | 273.1 | ||
| Control | 222.8 | 13.1 | 186.4 | 259.2 | ||
| Amygdala, intercalated cells, dorsal (ITCd) | −1.58 mm | Acquisition | 274.8 | 18.7 | 223.0 | 326.5 |
| Extinction | 273.6 | 17.6 | 224.7 | 322.4 | ||
| Control | 239.9 | 19.1 | 186.9 | 292.9 | ||
| Amygdala, intercalated cells, ventral (ITCv) | −1.58 mm | Acquisition | 286.6 | 13.0 | 250.6 | 322.6 |
| Extinction | 271.8 | 12.3 | 237.8 | 305.7 | ||
| Control | 258.5 | 13.3 | 221.7 | 295.4 | ||
| Posterior habenula, lateral (HBl) | −1.58 mm | Acquisition | 435.2 | 22.2 | 373.8 | 496.5 |
| Extinction | 376.0 | 22.3 | 314.5 | 437.6 | ||
| Control | 401.7 | 23.8 | 335.9 | 467.5 | ||
| Posterior habenula, medial (HBm) | −1.58 mm | Acquisition, 2 | 384.0 | 18.8 | 332.1 | 436.0 |
| Extinction | 319.3 | 18.8 | 267.2 | 371.4 | ||
| Control | 335.9 | 20.2 | 280.2 | 391.7 | ||
| Periaqueductal gray, anterior | −3.08 mm | Acquisition | 353.9 | 15.9 | 309.8 | 398.0 |
| Extinction | 354.6 | 16.0 | 310.5 | 398.8 | ||
| Control | 312.7 | 17.1 | 265.5 | 360.0 | ||
| Posterior thalamus | −3.08 mm | Acquisition | 375.9 | 14.5 | 335.7 | 416.1 |
| Extinction | 367.0 | 14.6 | 326.7 | 407.3 | ||
| Control | 353.0 | 15.6 | 309.9 | 396.1 | ||
| Posterior intralaminar nucleus | −3.08 mm | Acquisition | 370.8 | 15.1 | 329.1 | 412.6 |
| Extinction | 358.9 | 15.1 | 317.1 | 400.7 | ||
| Control | 346.2 | 16.2 | 301.5 | 391.0 | ||
| Dorsomedial periaqueductal gray (DMPAG) | −4.72 mm | Acquisition, 3 | 286.4 | 10.3 | 258.0 | 314.8 |
| Extinction | 258.6 | 10.3 | 230.1 | 287.0 | ||
| Control | 243.7 | 11.0 | 213.3 | 274.2 | ||
| Ventrolateral periaqueductal gray (VLPAG) | −4.72 mm | Acquisition, 3 | 359.3 | 13.4 | 322.2 | 396.5 |
| Extinction | 326.5 | 13.5 | 289.2 | 363.7 | ||
| Control | 315.1 | 14.4 | 275.3 | 355.0 | ||
| Pontine reticular formation | −4.72 mm | Acquisition | 330.9 | 11.1 | 300.2 | 361.6 |
| Extinction | 308.4 | 11.1 | 277.6 | 339.1 | ||
| Control | 313.9 | 11.9 | 281.0 | 346.8 | ||
Regional FDG uptake values are expressed as nanocuries of isotope incorporation per gram tissue wet weight. Indicated group differences are significant at p < 0.01 after ANCOVA and at p < 0.05 after correction by the Hochberg procedure for multiple group comparisons. 1, Extinction group significantly greater than both acquisition and control. 2, Acquisition group significantly greater than extinction. 3, Acquisition group significantly greater than control.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors declare no conflict of interest.
References
- Aertsen A, Bonhoeffer T, Kruger J. Coherent activity in neuronal populations: analysis and interpretation. In: Caianiello ER, editor. Physics of cognitive processes. Singapore: World Scientific Publishing; 1987. pp. 1–34. [Google Scholar]
- Amano T, Duvarci S, Popa D, Paré D. The fear circuit revisited: contributions of the basal amygdala nuclei to conditioned fear. J Neurosci. 2011;31:15481–9. doi: 10.1523/JNEUROSCI.3410-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchter A, Shumake J, Gonzalez-Lima F, Monfils MH. Preventing the return of fear using reconsolidation updating and methylene blue is differentially dependent on extinction learning. Sci Rep. 2017;7:46071. doi: 10.1038/srep46071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asede D, Bosch D, Lüthi A, Ferraguti F, Ehrlich I. Sensory inputs to intercalated cells provide fear-learning modulated inhibition to the basolateral amygdala. Neuron. 2015;86:541–54. doi: 10.1016/j.neuron.2015.03.008. [DOI] [PubMed] [Google Scholar]
- Balderston NL, Shultz DH, Baillet S, Helmstetter FJ. Rapid amygdala responses during trace fear conditioning without awareness. PLoS One. 2014;9:e96803. doi: 10.1371/journal.pone.0096803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett D, Shumake J, Jones D, Gonzalez-Lima F. Metabolic mapping of mouse brain activity after extinction of a conditioned emotional response. J Neurosci. 2003;23:5740–9. doi: 10.1523/JNEUROSCI.23-13-05740.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchey AK, Shumake J, Gonzalez-Lima F. Network model of fear extinction and renewal functional pathways. Neuroscience. 2007;145:423–37. doi: 10.1016/j.neuroscience.2006.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for behavioral sciences. New York: Academic Press; 1977. (revised ed.) [Google Scholar]
- Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: a neural and pharmacological analysis. Behav Brain Res. 1993;58:175–98. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity in neuroimaging: a synthesis. Hum Brain Mapp. 1994;2:56–78. [Google Scholar]
- Gilmartin MR, Balderston NL, Helmstetter FJ. Prefrontal cortical regulation of fear learning. Trends Neurosci. 2014;37:455–64. doi: 10.1016/j.tins.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Finkenstädt T, Ewert JP. Learning-related activation in the auditory system of the rat produced by long-term habituation: a 2-deoxyglucose study. Brain Res. 1989a;489:67–79. doi: 10.1016/0006-8993(89)90009-7. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Finkenstädt T, Ewert JP. Neural substrates for long-term habituation of the acoustic startle reflex in rats: a 2-deoxyglucose study. Neurosci Lett. 1989b;96:151–6. doi: 10.1016/0304-3940(89)90049-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Scheich H. Classical conditioning of tone-signaled bradycardia modifies 2-deoxyglucose uptake patterns in cortex, thalamus, habenula, caudate-putamen and hippocampal formation. Brain Res. 1986;363:239–56. doi: 10.1016/0006-8993(86)91009-7. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ. Contribution of the amygdala to learning and performance of conditional fear. Physiol Behav. 1992;51:1271–6. doi: 10.1016/0031-9384(92)90320-2. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ. Afferent connections of the habenular nuclei in the rat. A horseradish peroxidase study, with a note on the fiber-of-passage problem. J Comp Neurol. 1977;173:123–146. doi: 10.1002/cne.901730107. [DOI] [PubMed] [Google Scholar]
- Herry C, Garcia R. Prefrontal cortex long-term potentiation, but not long-term depression, is associated with the maintenance of extinction of learned fear in mice. J Neurosci. 2002;22:577–583. doi: 10.1523/JNEUROSCI.22-02-00577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Vouimba RM, Garcia R. Plasticity in the mediodorsal thalamo-prefrontal cortical transmission in behaving mice. J Neurophysiol. 1999;82:2827–2832. doi: 10.1152/jn.1999.82.5.2827. [DOI] [PubMed] [Google Scholar]
- Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–802. [Google Scholar]
- Horwitz B, Duara R, Rapoport SI. Intercorrelations of glucose metabolic rates between brain regions: application to healthy males in a state of reduced sensory input. J Cereb Blood Flow Metab. 1984;4:484–99. doi: 10.1038/jcbfm.1984.73. [DOI] [PubMed] [Google Scholar]
- Jones DA, Gonzalez-Lima F. Mapping Pavlovian conditioning effects on the brain: blocking, contiguity and excitatory effects. J Neurophysiol. 2001a;86:809–823. doi: 10.1152/jn.2001.86.2.809. [DOI] [PubMed] [Google Scholar]
- Jones DA, Gonzalez-Lima F. Associative effects of Pavlovian differential inhibition of behaviour. Eur J Neurosci. 2001b;14:1915–1927. doi: 10.1046/j.0953-816x.2001.01810.x. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behav Neurosci. 1993;107:1093–8. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion: clues from the brain. Annu Rev Psychol. 1995;46:209–35. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–5. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez-Lima F. Structural modeling of functional neural pathways mapped with 2-deoxyglucose: effects of acoustic startle habituation on the auditory system. Brain Res. 1991;547:295–302. doi: 10.1016/0006-8993(91)90974-z. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez-Lima F. Structural modeling of functional visual pathways mapped with 2-deoxyglucose: effects of patterned light and footshock. Brain Res. 1992;578:75–86. doi: 10.1016/0006-8993(92)90232-x. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez-Lima F. Structural equation modeling and its application to network analysis in functional brain imaging. Hum Brain Mapp. 1994;2:2–22. [Google Scholar]
- McNally GP, Pigg M, Weidemann G. Blocking, unblocking, and overexpectation of fear: a role for opioid receptors in the regulation of Pavlovian association formation. Behav Neurosci. 2004;118:111–20. doi: 10.1037/0735-7044.118.1.111. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–4. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett. 1993;163:109–13. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Nair HP, Berndt JD, Barrett D, Gonzalez-Lima F. Metabolic mapping of brain regions associated with behavioral extinction in preweanling rats. Brain Res. 2001a;903:141–153. doi: 10.1016/s0006-8993(01)02469-6. [DOI] [PubMed] [Google Scholar]
- Nair HP, Berndt JD, Barrett D, Gonzalez-Lima F. Maturation of extinction behavior in infant rats: large-scale regional interactions with medial prefrontal cortex, orbitofrontal cortex, and anterior cingulate cortex. J Neurosci. 2001b;21:4400–4407. doi: 10.1523/JNEUROSCI.21-12-04400.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair HP, Gonzalez-Lima F. Extinction of behavior in infant rats: development of functional coupling between septal, hippocampal, and ventral tegmental regions. J Neurosci. 1999;19:8646–8655. doi: 10.1523/JNEUROSCI.19-19-08646.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SS. Auditory fear circuits in the amygdala – insights from computational models. In: Ferry B, editor. The amygdala – a discrete multitasking manager. Rijeka: InTech; 2012. pp. 141–170. Available from: https://www.intechopen.com/books/the-amygdala-a-discrete-multitasking-manager/auditory-fear-circuits-in-the-amygdala-insights-from-computational-models. [Google Scholar]
- Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates. 2. San Diego: Academic; 2001. [Google Scholar]
- Poremba A, Gabriel M. Amygdalar lesions block discriminative avoidance learning and cingulothalamic training-induced neuronal plasticity in rabbits. J Neurosci. 1997;17:5237–44. doi: 10.1523/JNEUROSCI.17-13-05237.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–43. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Rizvi TA, Ennis M, Behbehani MM, Shipley MT. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: topography and reciprocity. J Comp Neurol. 1991;303:121–131. doi: 10.1002/cne.903030111. [DOI] [PubMed] [Google Scholar]
- Sakata J, Coomber P, Gonzalez-Lima F, Crews D. Functional connectivity among limbic brain areas: Differential effects of incubation temperature and gonadal sex in the leopard gecko, Eublepharis macularius. Brain Behav Evol. 2000;55:139–151. doi: 10.1159/000006648. [DOI] [PubMed] [Google Scholar]
- Schultz DH, Balderston NL, Baskin-Sommers AR, Larson CL, Helmstetter FJ. Psychopaths show enhanced amygdala activation during fear conditioning. Front Psychol. 2016;7:348. doi: 10.3389/fpsyg.2016.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumake J, Barrett D, Gonzalez-Lima F. Behavioral characteristics of rats predisposed to learned helplessness: reduced reward sensitivity, increased novelty seeking, and persistent fear memories. Behav Brain Res. 2005;164:222–30. doi: 10.1016/j.bbr.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Shumake J, Conejo-Jimenez N, Gonzalez-Pardo H, Gonzalez-Lima F. Brain differences in newborn rats predisposed to helpless and depressive behavior. Brain Res. 2004;1030:267–76. doi: 10.1016/j.brainres.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Shumake J, Edwards E, Gonzalez-Lima F. Hypermetabolism of paraventricular hypothalamus in the congenitally helpless rat. Neurosci Lett. 2001;311:45–8. doi: 10.1016/s0304-3940(01)02142-5. [DOI] [PubMed] [Google Scholar]
- Shumake J, Edwards E, Gonzalez-Lima F. Dissociation of septo-hippocampal metabolism in the congenitally helpless rat. Neuroscience. 2002;114:373–7. doi: 10.1016/s0306-4522(02)00297-x. [DOI] [PubMed] [Google Scholar]
- Shumake J, Edwards E, Gonzalez-Lima F. Opposite metabolic changes in the habenula and ventral tegmental area of a genetic model of helpless behavior. Brain Res. 2003;963:274–81. doi: 10.1016/s0006-8993(02)04048-9. [DOI] [PubMed] [Google Scholar]
- Shumake J, Gonzalez-Lima F. Brain systems underlying susceptibility to helplessness and depression. Behav Cogn Neurosci Rev. 2003;2:198–221. doi: 10.1177/1534582303259057. [DOI] [PubMed] [Google Scholar]
- Shumake J, Gonzalez-Lima F. Functional opposition between habenula metabolism and the brain reward system. Front Hum Neurosci. 2013;7:662. doi: 10.3389/fnhum.2013.00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumake J, Poremba A, Edwards E, Gonzalez-Lima F. Congenital helpless rats as a genetic model for cortex metabolism in depression. Neuroreport. 2000;11:3793–8. doi: 10.1097/00001756-200011270-00040. [DOI] [PubMed] [Google Scholar]
- Strobel C, Marek R, Gooch HM, Sullivan RK, Sah P. Prefrontal and auditory input to intercalated neurons of the amygdala. Cell Rep. 2015;10:1435–1442. doi: 10.1016/j.celrep.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Velasquez KM, Molfese DL, Salas R. The role of the habenula in drug addiction. Front Hum Neurosci. 2014;8:174. doi: 10.3389/fnhum.2014.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoellner LA, Telch MJ, Foa EB, Farach FJ, McLean CP, Gallop R, Bluett EJ, Cobb A, Gonzalez-Lima F. Enhancing extinction learning in posttraumatic stress disorder with brief daily imaginal exposure and methylene blue: A randomized controlled trial. J Clin Psychiatry. 2017;78:e782–e789. doi: 10.4088/JCP.16m10936. [DOI] [PubMed] [Google Scholar]


