Abstract
This study used Magnetic Resonance Spectroscopy (MRS) to identify potential neurometabolitic markers of cognitive performance in male (n = 7) and female (n = 8) middle-aged (∼5 years old) common marmosets (Callithrix jacchus). Anesthetized marmosets were scanned with a 4.7 T/40 cm horizontal magnet equipped with 450 mT/m magnetic field gradients and a 20 G/cm magnetic field gradient insert, within 3 months of completing the CANTAB serial Reversal Learning task. Neurometabolite concentrations of N-Acetyl Asparate, Myo-Inositol, Choline, Phosphocreatine + creatine, Glutamate and Glutamine were acquired from a 3 mm3 voxel positioned in the Prefrontal Cortex (PFC). Males acquired the reversals (but not simple discriminations) faster than the females. Higher PFC Glx (glutamate + glutamine) concentration was associated with faster acquisition of the reversals. Interestingly, the correlation between cognitive performance and Glx was significant in males, but not in females. These results suggest that MRS is a useful tool to identify biochemical markers of cognitive performance in the healthy nonhuman primate brain and that biological sex modulates the relationship between neurochemical composition and cognition.
Keywords: Cognition, Magnetic resonance spectroscopy, Monkey, Primate, Sex differences
1. Introduction
In vivo magnetic resonance spectroscopy (MRS) is a noninvasive imaging technique that measures the biochemical composition of healthy and diseased brain tissues. MRS allows the regional measurement of several metabolites that include: N-acetyl aspartate (NAA), myo-Inositol containing compounds (mI), Choline containing compounds (Cho), Glutamate (Glu), Glutamine (Gln), and Phosphocreatine + Creatine (Cr). Cr levels are usually stable and may be used as an internal reference value, while the other metabolites are considered as specific biomarkers (see [1] for review). NAA, which is synthesized by mitochondria, is considered to be a neuronal marker. It is reliably decreased in several brain regions in neurodegenerative diseases. mI is considered to be a suitable marker for glial activity, and is elevated in disease states characterized by inflammation. Cho is a precursor of acetylcholine that is concentrated in phospholipids and is thought to be a marker for membrane turnover. High field MRS can also detect several neurotransmitters such as Glu. Glu is the most abundant excitatory neurotransmitter in the brain and plays a fundamental role in learning and memory. Glu released by pre-synaptic neurons is rapidly converted to Gln in astrocytes and Gln released from astrocytes is converted back to Glu, as part as a Glu/Gln cycle that is essential to the normal functioning of brain cells [2]. The combination of Glu and Gln concentrations is traditionally referred to as Glx. Alterations in the concentrations of Glu and Gln have been reported in numerous neurological and psychiatric diseases ([2]).
While 1H MRS is a useful neuroimaging method to identify neurochemical changes associated with a variety of disorders, it can also serve as a tool to investigate potential biomarkers of cognitive performance in the healthy brain [1,3]. For example, NAA in cortical tissues has been found to correlate with selective aspects of cognitive performance in healthy populations [3]. Glx concentration in the hippocampus has also been shown to predict verbal memory performance in healthy adult males [4] and delayed word list recall in older adults [5]. However, Patel et al. [3] caution that such approaches reveal brain/cognition associations that are typically small to moderate, and subject to a number of methodological biases.
We suggest that identifying neurochemical correlates of cognitive performance in a well characterized nonhuman primate (NHP) would provide further validation for the use of specific neurometabolites as biomarkers of cognitive processes. The present study used 1H MRS in the common marmoset (Callithrix jacchus), an NHP that is becoming increasingly attractive for neuroscience research [6], due to its small size (∼400 g), its potential as a genetic tool, and the breadth of its behavioral repertoire. Many neural features are well conserved between the marmoset and the human brain (e.g., [7]). The marmoset is able to perform complex cognitive tasks, including standard tasks of attention, memory and executive function that can be administered on automated touchscreen systems [8,9]. 1H MRS has successfully been used in this species [10–13]. However, none of these studies incorporated cognitive measures.
The present study focused on cognitive flexibility, as assessed by serial reversal learning. Previous studies in marmosets have determined that reversal learning depends on the integrity of the orbitofrontal cortex [14]. In addition, reversal learning is affected by a number of neurotransmitters, including serotonin, dopamine and glutamate [15]. We predicted that performance on reversal learning would depend on Glu (or Glx) concentrations in the prefrontal cortex (PFC), based on findings that glutamate blockade induces reversal learning impairments in the marmoset [16]. We did not expect performance to be related to other neurometabolites, as we tested healthy younger than 8 years old, when signs of aging begin to appear. With regards to sex differences, previous studies in humans have reported region-specific sex differences in a number of metabolites, including Glu and Gln [17]. However, our sample was too small to generate strong hypotheses with regards to sex differences.
2. Methods
2.1. Subjects
Fifteen marmosets (7 males, 8 females, mean age at scan = 4.96, SD = 0.60) were tested. The MRS scan occurred within 3 months of the onset of the cognitive test. The monkeys were housed in male/female pairs at the University of Massachusetts at Amherst (UMA) and maintained under a 12:12 light/dark cycle, at an ambient temperature of 80 F with a relative humidity of about 50%. The pairs were housed in steel mesh cages (101 × 76.2 × 78.7 cm) equipped with perches, hammock, nest boxes and branches. Male marmosets were vasectomized to prevent breeding. The marmosets were fed Teklad New World Primate Diet (Envigo, Madison, WI) supplemented with Zupreem marmoset diet, and a variety of fresh fruits, vegetables, nuts, and mealworms. The monkeys were provided with daily enrichment. The animals were cared for in accordance with the guidelines published in the Guide for the Care and Use of Laboratory Animals, 8th edition. The studies were approved by the Institutional Animal Care and Use Committees of UMA and UM Medical School (UMM).
2.2. Cognitive testing
The monkeys were tested on a serial reversal learning task included in the NHP version of the CAmbridge Neuropsychological Test Automated Battery (CANTAB) [8,9]. The apparatus consisted of a touch screen panel (37.78 cm) in a stainless steel frame (56 × 38 × 30 cm). A stainless steel sipper tube in the middle of the screen delivered the reward (banana milkshake).
2.2.1. General procedure
Marmosets were tested in their housing room in the presence of other marmosets. For testing, the marmoset voluntarily entered a transport box attached to the homecage and connected to the CANTAB.
2.2.2. Testing
The task consisted in the discrimination and reversal of three pairs of stimuli (Fig. 2). The first pair of stimuli was composed of a blue triangle and a white line. The second pair consisted either of 2 different white lines or two different pink shapes (order counterbalanced between monkeys). For each pair, monkeys had to perform a simple discrimination (SD), followed by a simple reversal (SR). The two stimuli appeared in any positions on the black background of the touch screen. Animals were given 40 trials a day to learn the stimulus-reward contingencies. Once they reached a 90% correct criterion on one pair (SD), the stimulus-reward contingencies were reversed (SR). Monkeys performed an SD and SR on each pair in succession until all 3 pairs were completed. The number of trials to reach criterion (TTC) was the dependent measure.
Fig. 2.
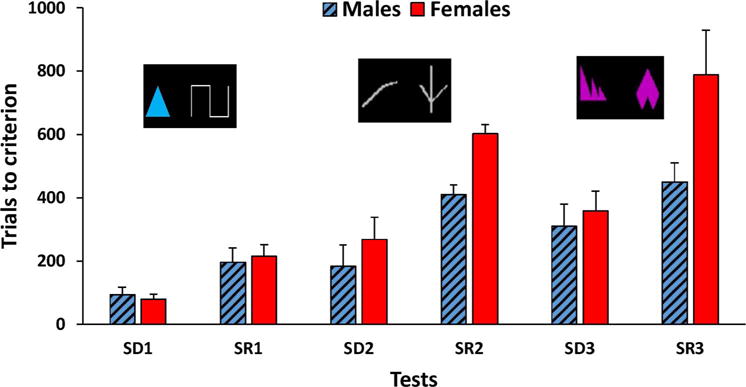
Mean trials to criterion (+ SEM) in male (blue hatched bars) and female (red bars) marmosets for the discrimination (SD) and reversal (SR) of the three pairs of stimuli. The order of presentation of pairs 2 and 3 was counterbalanced between monkeys.
2.3. Magnetic resonance spectroscopy
The monkeys were scanned at the Center for Comparative Neuroimaging at UMM. The monkeys were briefly anesthetized with ketamine (10 mg/kg, IM) to facilitate positioning. They were fitted with a sleeveless jacket (Lomir Biomedical, Inc), and earplugs were inserted in their ears for noise protection. A plastic semi-cylindrical cover made of Lexan was attached to the back of the marmoset’s jacket using plastic cable ties, as described in [18]. The marmoset was then placed in a prone position on an MR bed, which consisted of a cylindric tube of inner diameter 111 mm. A pressure pad (Biopac systems, INc.) was placed under the marmoset’s body to monitor respiratory changes. The cover was secured to the bed by screwing nylon thumb screws into the bars on the bed. The bed was then inserted into the scanner. During the experiment animals were ventilated with isoflurane (2–4%) via a face cone. Respiratory changes were monitored via the Biopac pressure sensor. Imaging was carried out on a high-field MRI system. The system incorporated a 4.7 T/40 cm horizontal magnet (Oxford, UK) equipped with 450 mT/m magnetic field gradients and a 20-G/cm magnetic field gradient insert (inner diameter = 11.5 cm; Bruker, Germany) with a digital interface to Bruker console, run by Paravision 6. Water suppressed (variable power and optimized relaxation delays: VAPOR) 1H MRS data were acquired using a Bruker volume headcoil and the PRESS localization sequence (90-153-153; bandwidth 4000 Hz; repetition time = 2500 ms; echo time = 16 ms; averages = 128;). A 3 mm × 3 mm × 3 mm voxel was positioned in the PFC guided by gradient echo localizer images. Un-suppressed water data were acquired for quantification purposes (repetition time = 2500 ms; echo time = 16 ms; averages = 16). Localized shimming was performed using FASTMAP. Shimming was performed until the Full Width Half Maximum (FWHM) of the water peak was at most less than 12 Hz. Data were transferred to a Linux workstation and metabolite concentrations, in institutional units, were fit using LCModel [19]. A representative marmoset spectrum is shown in Fig. 1.
Fig. 1.
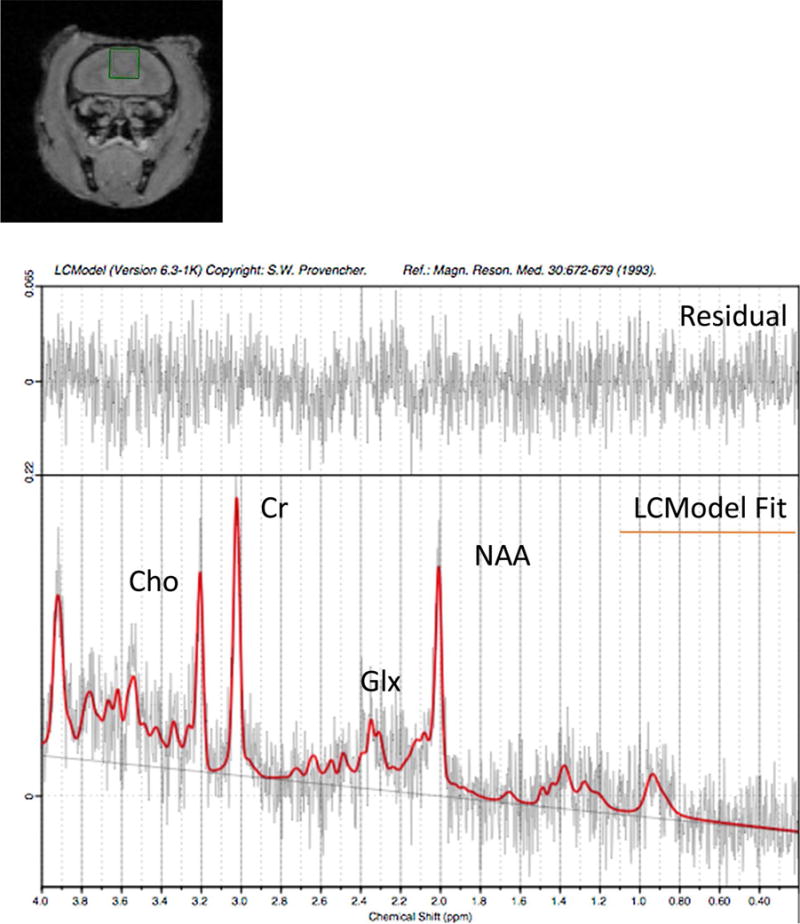
Location of the 3 × 3 × 3 mm voxel in the PFC and representative in vivo 1H–MRS spectrum of a marmoset brain at 4.7 T. The spectrum was not line broadened.
2.4. Statistical analyses
Statistical analyses were conducted with SPSS version 22. A repeated measures analysis of variance (ANOVA) with Test Type (Discrimination, Reversal) and Pair Number (1–3) as within-subjects factors, Sex as a between-subjects factor, and Sequence of tests (shape or line first) as covariate was conducted to analyze TTC on the Reversal Learning task. The Mann-Whitney U-test was used to determine whether metabolite concentrations differed as a function of sex. To examine associations between reversal learning performance and metabolite concentrations, a composite of performance, the Reversal Index SR/SD, was computed for each monkey by dividing the mean TTC across the 3 reversals (SR1, SR2, SR3) by the mean TTC on the 3 simple discriminations (SD1, SD2, SD3). This composite score reflected how many more trials the monkey had needed to perform the reversals relative to the simple discriminations. Associations between SR/SD and metabolite concentrations were assessed using Spearman rank correlations.
3. Results
3.1. Reversal learning performance
The TTC of male and female marmosets across the 3 pairs of stimuli can be seen in Fig. 2. Monkeys achieved the 3 discriminations and reversals in an average of 329.8 (± 29.8 SEM) trials. The ANOVA revealed a main effect of Test Type on TTC F(1, 12) = 7.41, p < .02), with monkeys achieving the simple discriminations faster (mean TTC = 216 ± 21.19 trials) than the reversals (mean TTC = 443.47 ± 43.32). The main effect of sex on TTC was of marginal significance Sex (F(1, 12) = 3.18, p = .10), but a significant Sex × Type interaction (F(1, 12) = 6.50, p < .05) revealed that females required more trials (mean = 535.05 ± 59.21) than the males (mean = 351.9 ± 63.30) to complete the reversals (t(13) = −2.19, p < .05), while there was no sex difference in the performance of simple discriminations (235.66 vs 196.28 trials; t(13) = −0.95, ns). As a result, SR/SD tended to be greater in females than in males (t (13) = −2.15, p = .051), reflecting poorer performance of the females in the reversals.
3.2. Correlations between performance and metabolite concentration
Metabolite concentrations did not differ significantly between males and females (Table 1). The Spearman’s rho correlation coefficient was negatively correlated with Glx (ρ = −0.643, p = .01, Fig. 3) but no other metabolite. This indicated that monkeys who acquired the reversals more quickly (i.e, lower SR/SD ratio) had higher Glx levels. As can be seen in Fig. 3, this correlation was driven by the males. Indeed, when only males were considered, a large correlation between SR/SD and Glx concentration (ρ = −0.821, p = .023) was revealed, whereas it was not significant among females (ρ = −0.476, p = .23). No other metabolite correlated significantly with SR/SD in either males or females.
Table 1.
PFC Metabolite concentrations, Cramér-Rao Lower Bounds (CRLB) and Reversal Learning performance (SR/SD) for each of the 15 marmosets.
| Monkeys | Metabolite concentrations in PFC
|
Reversal Learning performance | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glx | CRLB | Gln | CRLB | Glu | CRLB | mI | CRLB | tCho | CRLB | tNAA | CRLB | tCr | CRLB | SR/SD | |
| Males | |||||||||||||||
| 1 | 12.94 | 10 | 4.02 | 25 | 8.92 | 12 | 6.70 | 8 | 1.87 | 6 | 6.81 | 6 | 8.15 | 4 | 2.65 |
| 2 | 13.67 | 10 | 3.60 | 64 | 10.07 | 12 | 9.13 | 7 | 2.03 | 6 | 7.08 | 6 | 8.28 | 4 | 1.77 |
| 3 | 13.97 | 9 | 3.43 | 62 | 10.54 | 11 | 7.27 | 8 | 1.93 | 6 | 8.22 | 5 | 8.93 | 4 | 1.68 |
| 4 | 11.83 | 11 | 3.16 | 62 | 8.68 | 16 | 7.31 | 9 | 1.70 | 8 | 7.26 | 6 | 6.85 | 5 | 2.55 |
| 5 | 16.92 | 9 | 5.74 | 24 | 11.18 | 11 | 7.69 | 8 | 1.99 | 6 | 8.11 | 5 | 8.55 | 4 | 1.36 |
| 6 | 14.84 | 9 | 6.00 | 19 | 8.84 | 12 | 7.94 | 7 | 2.43 | 5 | 7.75 | 5 | 8.83 | 3 | 1.33 |
| 7 | 13.38 | 9 | 3.87 | 25 | 9.51 | 11 | 5.84 | 9 | 1.63 | 6 | 8.68 | 5 | 8.44 | 4 | 1.46 |
| Females | |||||||||||||||
| 1 | 11.79 | 11 | 4.04 | 25 | 7.76 | 13 | 7.87 | 7 | 2.39 | 5 | 7.65 | 5 | 8.32 | 4 | 2.71 |
| 2 | 9.61 | 14 | 3.35 | 35 | 6.26 | 18 | 7.48 | 8 | 2.26 | 5 | 8.07 | 5 | 8.66 | 4 | 2.56 |
| 3 | 12.99 | 10 | 3.66 | 33 | 9.33 | 13 | 8.20 | 7 | 1.92 | 6 | 9.02 | 5 | 8.12 | 4 | 1.97 |
| 4 | 14.98 | 11 | 4.43 | 42 | 10.55 | 15 | 7.33 | 9 | 1.76 | 8 | 7.57 | 7 | 8.44 | 5 | 2.40 |
| 5 | 11.70 | 11 | 3.86 | 28 | 7.84 | 14 | 8.42 | 7 | 1.93 | 5 | 8.47 | 5 | 8.79 | 4 | 2.56 |
| 6 | 16.11 | 9 | 5.54 | 25 | 10.57 | 11 | 7.64 | 7 | 1.99 | 6 | 7.14 | 6 | 9.22 | 4 | 2.37 |
| 7 | 12.24 | 11 | 5.19 | 22 | 7.05 | 15 | 7.83 | 7 | 2.02 | 5 | 6.55 | 6 | 7.81 | 4 | 1.78 |
| 8 | 13.83 | 11 | 5.05 | 27 | 8.78 | 14 | 7.30 | 9 | 1.39 | 9 | 8.77 | 5 | 7.88 | 5 | 2.20 |
Fig. 3.
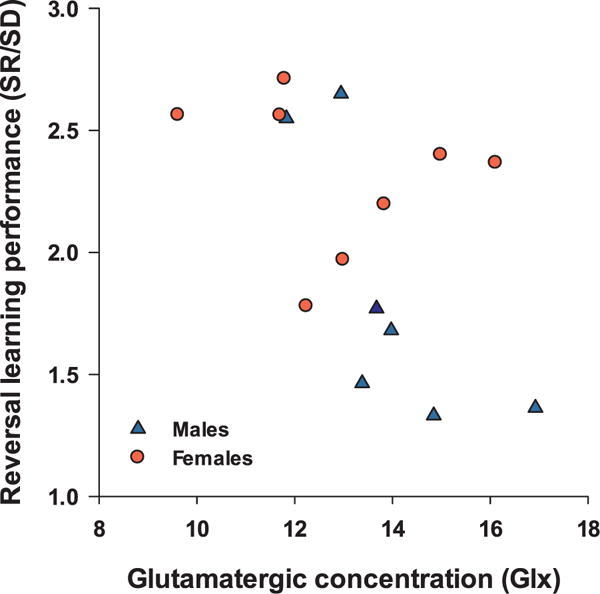
Scatterplot of the relationships between the reversal index (SR/SD) and PFC Glx concentration in 15 marmosets. Monkeys with better performance (lower reversal index) have greater Glx in PFC (Spearmanρ = −0.643, p = .01). The correlation is significant in males but not in females.
4. Discussion
This study in marmosets examined potential associations between cognitive flexibility, as assessed by serial Reversal Learning, and metabolite concentrations in the PFC, as measured by 1H MRS. We found a large correlation (ρ = −0.64) between cognitive flexibility and Glx concentrations in the PFC, indicating better performance on Reversal Learning (lower SR/SD ratio) with higher Glx concentrations in this region. Interestingly, the association between Glx and reversal learning was driven by the males, which also outperformed females on the reversals.
The MRS Glx signal reflects the combined concentrations of both Glu and Gln in brain cells located within the voxel of interest. Glu and Gln have complementary functions, with Glu being converted to Gln in astrocytes and Gln to Glu in neurons in a highly dynamic Glu/Gln cycle that is critical for the healthy functioning of neurons. Several studies support a role for Glx in cognitive processes. In a recent functional MRS study, an increase in medial PFC Glx concentration was found when participants were engaged in a task of visual imagery, relative to a control condition [20], suggesting greater Glu/Gln cycling or increased synaptic glutamate release in response to task demands. Other studies found that Glx predicted performance on a verbal memory task in both young men [4] and older adults [5]. Our study extends these results for the first time in an NHP, by showing an association between PFC Glx and cognitive flexibility. The results suggest that greater glutamate availability is associated with better reversal learning performance, consistent with the beneficial role of glutamate in reversal learning [15,16].
Interestingly, the relationship between Glx and reversal learning was driven by the males. These results suggest that males’ reversal learning performance may be more dependent on glutamate than that of females. Previous studies in rodents have established that the glutamatergic system is highly sensitive to sex hormones [21] and a few MRS studies in humans also report changes in Glu or Glx as a function of changes in sex steroids. For example, lower levels of Glu/Cr in the medial PFC were found in the luteal compared to the follicular phase in women [22] while the use of anabolic steroids was associated with an increase in Glx levels in the anterior cingulate in men [23].
At the cognitive level, males acquired the reversals faster than the females. This sex difference is consistent with our previous finding of an impairing effect of estradiol administration on reversal learning in OVX females [24]. In contrast, testosterone treatment did not affect reversal learning in males [25]. Although we did not monitor menstrual status of the females in the present study, we hypothesize that higher estradiol levels interferes with the ability of females to switch learned responses according to changing stimulus/reward contingencies.
One limitation of our study is that monkeys were anesthetized with ketamine, an NMDA antagonist, which, at anesthetic doses, has been shown to decrease extracellular glutamate levels in the rodent PFC, as assessed by microdialysis [26]. With the exception of one study in awake animals [12], MRS studies of the marmoset brain have used anesthetized preparations [10,11,13]. Although anesthesia-induced Glu decreases are not likely to be detected with MRS [27], it will be important to confirm our findings in awake marmosets to rule out a potential effect of ketamine on Glx. Additional improvements for data quality would include imaging monkeys with ultra-high magnetic fields MR scanners (7 T or higher) at a time more closely tied to task acquisition.
Despite these caveats, our study shows that PFC Glx is a reliable predictor of reversal learning ability in marmosets, and particularly so in males. Males also acquired the reversals faster than females. Altogether, these findings suggest that MRS is a useful tool to detect biochemical markers of cognitive function in healthy NHPs and that biological sex modulates the relationship between specific neurometabolites and cognitive function.
Acknowledgments
This research was supported by NIH grants AG046266 and S10 OD018132. We thank the UMA animal care staff and shop staff for their excellent support and UMA students for the acquisition of the cognitive data. We thank Dr. Afonso Silva for help with the neuroimaging set-up.
Footnotes
Competing financial interests
The authors report no competing financial interests.
References
- 1.Ross AJ, Sachdev PS. Magnetic resonance spectroscopy in cognitive research. Brain Res Rev. 2004;44:83–102. doi: 10.1016/j.brainresrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Ramadan S, Lin A, Stanwell P. Glutamate and glutamine: a review of in vivo MRS in the human brain. NMR Biomed. 2013;26:1630–1646. doi: 10.1002/nbm.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel T, Blyth JC, Griffiths G, Kelly D, Talcott JB. Moderate relationships between NAA and cognitive ability in healthy adults: implications for cognitive spectroscopy. Front Hum Neurosci. 2014;8:39. doi: 10.3389/fnhum.2014.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner G, Gussew A, Kohler S, de la Cruz F, Smesny S, Reichenbach JR, et al. Resting state functional connectivity of the hippocampus along the anterior-posterior axis and its association with glutamatergic metabolism. Cortex. 2016;81:104–117. doi: 10.1016/j.cortex.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 5.Nikolova S, Stark SM, Stark CEL. 3T hippocampal glutamate-glutamine complex reflects verbal memory decline in aging. Neurobiol Aging. 2017;54:103–111. doi: 10.1016/j.neurobiolaging.2017.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okano H, Hikishima K, Iriki A, Sasaki E. The common marmoset as a novel animal model system for biomedical and neuroscience research applications. Semin Fetal Neonatal Med. 2012;17:336–340. doi: 10.1016/j.siny.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Chaplin TA, Yu HH, Soares JGM, Gattass R, Rosa MGP. A conserved pattern of differential expansion of cortical areas in simian primates. J Neurosci. 2013;33:15120–15125. doi: 10.1523/JNEUROSCI.2909-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts AC, Robbins TW, Everitt BJ. The effects of intradimensional and extradimensional shifts on visual discrimination learning in humans and non-human primates. Q J Exp Psychol B. 1988;40:321–341. [PubMed] [Google Scholar]
- 9.Spinelli S, Pennanen L, Dettling AC, Feldon J, Higgins GA, Pryce CR. Performance of the marmoset monkey on computerized tasks of attention and working memory. Cogn Brain Res. 2004;19:123–137. doi: 10.1016/j.cogbrainres.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 10.van Vliet SA, Blezer EL, Jongsma MJ, Vanwersch RA, Olivier B, Philippens IH. Exploring the neuroprotective effects of modafinil in a marmoset Parkinson model with immunohistochemistry, magnetic resonance imaging and spectroscopy. Brain Res. 2008;1189:219–228. doi: 10.1016/j.brainres.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 11.t Hart BA, Vogels J, Bauer J, Brok HPM, Blezer E. Non-invasive measurement of brain damage in a primate model of multiple sclerosis. Trends Mol Med. 2004;10:85–91. doi: 10.1016/j.molmed.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Meyer JS, Brevard ME, Piper BJ, Ali SF, Ferris CF. Neural effects of MDMA as determined by functional magnetic resonance imaging and magnetic resonance spectroscopy in awake marmoset monkeys. Ann N Y Acad Sci. 2006;1074:365–376. doi: 10.1196/annals.1369.036. [DOI] [PubMed] [Google Scholar]
- 13.Michaelis T, Abaei A, Boretius S, Tammer R, Frahm J, Schlumbohm C, et al. Intrauterine hyperexposure to dexamethasone of the common marmoset monkey revealed normal cerebral metabolite concentrations in adulthood as assessed by quantitative proton magnetic resonance spectroscopy in vivo. J Med Primatol. 2009;38:213–218. doi: 10.1111/j.1600-0684.2009.00342.x. [DOI] [PubMed] [Google Scholar]
- 14.Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin card sorting test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci. 1996;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- 15.Izquierdo A, Brigman JL, Radke AK, Rudebeck PH, Holmes A. The neural basis of reversal learning: an updated perspective. Neuroscience. 2017;345:12–26. doi: 10.1016/j.neuroscience.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harder JA, Aboobaker AA, Hodgetts TC, Ridley RM. Learning impairments induced by glutamate blockade using dizocilpine (MK-801) in monkeys. Br J Pharmacol. 1998;125:1013–1018. doi: 10.1038/sj.bjp.0702178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hadel S, Wirth C, Rapp M, Gallinat J, Schubert F. Effects of age and sex on the concentrations of glutamate and glutamine in the human brain. J Magn Reson Imaging. 2013;38:1480–1487. doi: 10.1002/jmri.24123. [DOI] [PubMed] [Google Scholar]
- 18.Belcher AM, Yen CC, Stepp H, Gu H, Lu H, Yang Y, et al. Large-scale brain networks in the awake, truly resting marmoset monkey. J Neurosci. 2013;33:16796–16804. doi: 10.1523/JNEUROSCI.3146-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 20.Huang Z, Davis H, IV, Yue Q, Wiebking C, Duncan NW, Zhang J, et al. Increase in glutamate/glutamine concentration in the medial prefrontal cortex during mental imagery: a combined functional mrs and fMRI study. Hum Brain Mapp. 2015;36:3204–3212. doi: 10.1002/hbm.22841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barth C, Villringer A, Sacher J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front Neurosci. 2015;9:37. doi: 10.3389/fnins.2015.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Batra NA, Seres-Mailo J, Hanstock C, Seres P, Khudabux J, Bellavance F, et al. Proton magnetic resonance spectroscopy measurement of brain glutamate levels in premenstrual dysphoric disorder. Biol Psychiatry. 2008;63:1178–1184. doi: 10.1016/j.biopsych.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Kaufman MJ, Janes AC, Hudson JI, Brennan BP, Kanayama G, Kerrigan AR, et al. Brain and cognition abnormalities in long-term anabolic-androgenic steroid users. Drug Alcohol Depend. 2015;152:47–56. doi: 10.1016/j.drugalcdep.2015.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacreuse A, Chang J, Metevier CM, Laclair M, Meyer JS, Ferris CM. Oestradiol modulation of cognition in adult female marmosets (Callithrix jacchus) J Neuroendocrinol. 2014;26:296–309. doi: 10.1111/jne.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LaClair M, Lacreuse A. Reversal learning in gonadectomized marmosets with and without hormone replacement: are males more sensitive to punishment? Anim Cogn. 2016;19:619–630. doi: 10.1007/s10071-016-0966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chowdhury GM, Behar KL, Cho W, Thomas MA, Rothman DL, Sanacora G. 1) H-[(1) (3) C]-nuclear magnetic resonance spectroscopy measures of ketamine’s effect on amino acid neurotransmitter metabolism. Biol Psychiatry. 2012;71:1022–1025. doi: 10.1016/j.biopsych.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


