Abstract
Women are twice as likely to be diagnosed with major depressive disorder. However, fewer studies in rodent models of depression have used female animals, leading to a relative lack of understanding of the female brain’s response to stress, especially at a neural circuit level. In this study, we utilized a 6-day subchronic variable stress (SCVS) mouse model and measured novelty suppressed feeding as behavioral criteria to evaluate susceptibility to SCVS in male and female mice. First, we showed that SCVS induced a decrease in latency to eat (susceptible phenotype) in female mice, but not in males (resilient phenotype). After determining behavioral phenotypes, we investigated the firing activities of dopamine (DA) neurons in the ventral tegmental area (VTA), as well as the neurons that project from lateral habenula (LHb) to the VTA and from locus coeruleus (LC) to the VTA. Utilizing retrograding lumafluor fluorescent tracers and electrophysiology techniques, we performed cell type- and circuit-specific measures of neuronal firing rates. Our data show that SCVS significantly increased the firing rate of LHb-VTA circuit neurons in female mice when compared to that of their female controls, an effect that was absent in SCVS-exposed males. Interestingly, SCVS did not induce significant firing alterations in VTA DA neurons and LC-VTA circuit neurons in either female mice or male mice when compared to their stress-naïve controls. Overall, our data shows sex differences in the LHb-VTA circuit responses to SCVS, and implicates a potential role of this projection in mediating vulnerability of female mice to stress-induced depression.
Keywords: sex difference, major depression, neuronal activity, ventral tegmental area, lateral habenula, locus coeruleus
Introduction
According to a WHO report (Piccinelli and Gomez Homen, 1997), women are twice as likely as men to be diagnosed with stress-related psychiatric disorders, including major depression and anxiety disorder (Kessler et al., 1994; Kendler et al., 1995; Kessler, 2003). It has been suggested that females may respond differently to stress and use distinct stress-coping strategies as compared to males (Kendler et al., 2001; Maciejewski et al., 2001; Klein and Corwin, 2002; Nemeroff et al., 2006). While widely used rodent models of depression have contributed enormously to unravel the neural mechanisms that underlie behavioral responses to stress, these animal models have largely used males only (Solomon et al., 2007; Dalla et al., 2008; Trainor et al., 2011; Ver Hoeve et al., 2013). Due to these limitations, the mechanisms underlying sex differences in stress vulnerability remain largely unknown.
The subchronic variable stress (SCVS) model is used to cause stress-induced depression by exposing mice to three alternating stressors across 6 days (LaPlant et al., 2009; Hodes et al., 2015). Historically, this paradigm induces a depressive phenotype in females, but not in males. Thus, the SCVS model provides an ideal model for exploring sex differences of vulnerability to stress. Based on this model, it has also been demonstrated that male and female mice show differential patterns of gene expression in the nucleus accumbens (NAc) (Hodes et al., 2015). Furthermore, Dnmt3a and NF-κB have been identified as important mediators that contribute to sex differences between male and female mice in stress susceptibility (LaPlant et al., 2009; Hodes et al., 2015). In contrast, less is known about the neurophysiological mechanisms that underlie sex differences in stress vulnerability.
The ventral tegmental area (VTA) dopamine (DA) system is a key part of the brain’s reward circuitry and plays an important role in mediating stress response (Chaudhury et al., 2013; Friedman et al., 2014). The lateral habenula (LHb) and the locus coeruleus (LC) both send substantial efferents to innervate VTA DA neurons (Omelchenko et al., 2009; Chandler et al., 2013; Juarez and Han, 2016). Accordingly, multiple lines of evidence have demonstrated that the VTA, LHb and LC brain areas all play crucial roles in mediating stress responses. The firing activities of neurons in these three brain regions are altered when exposed to various stressors (Ungless et al., 2004; Krishnan et al., 2007; Sartorius and Henn, 2007; Cao et al., 2010; Li et al., 2011; Valenti et al., 2012; Li et al., 2013; Isingrini et al., 2016). Furthermore, recent studies show that the firing activities of VTA DA neurons, LHb-VTA circuit neurons and LC-VTA circuit neurons determine susceptible versus resilient behaviors seen in male animals in a variety of stress-induced psychiatric disorders (Li et al., 2011; Chaudhury et al., 2013; Tye et al., 2013; Isingrini et al., 2016). However, how the activity of these neurons is affected following the SCVS paradigm remains unknown in both female and male mice.
Here, utilizing the SCVS model and cell type- and projection-specific in vitro electrophysiological recording techniques, we investigated the firing activity alterations of VTA DA neurons, LHb-VTA projecting neurons and LC-VTA projecting neurons in both male and female mice following SCVS. Our findings provide useful evidence that the hyper-activation of the LHb-VTA circuit may play an important role in mediating the vulnerability of female mice to stress-related disorders, as compared to males.
Experimental procedures
Animals
7-week-old C57BL/6J female and male mice (The Jackson Laboratory) were used to set up the SCVS paradigm. All mice were group-housed on a 12 h light/dark cycle with food and water available ad libitum. Following the last day of SCVS, all mice were singly housed for behavioral testing and in vitro recording. All procedures were approved by the Institutional Animal Care and Use Committee of the Icahn School of Medicine at Mount Sinai and in accordance with the National Institutes of Health guidelines. Twenty-eight female mice and twenty-five male mice were used in this study. Electrophysiological recordings were obtained from the same cohort of mice after behavioral tests (see below).
Subchronic variable stress
SCVS was performed as described previously (Hodes et al., 2015). Female and male mice were put through three unpredictable stressors over 6 days (Fig. 1A). To prevent habituation, mice were subjected to stress in the following order: 100 random foot shocks at 0.45 mA for 1 h (6–8 mice/chamber) on day 1 and day 4; tail suspension stress in which all mice were fixed to hang in an inverted position for 1 h on day 2 and day 5; and restraint stress, in which mice were placed inside a 50-ml falcon tube for 1 h within the home cage on day 3 and day 6. After each stressor, mice were returned to their home cage except on the last day of SCVS when they were then singly housed.
Fig. 1.
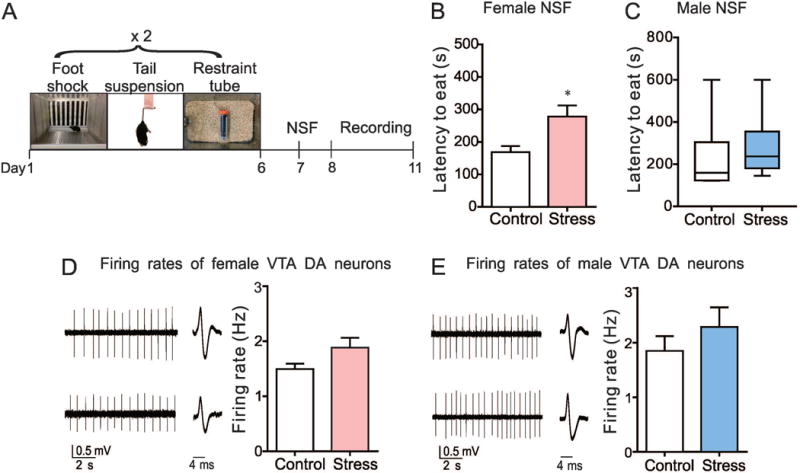
VTA dopamine (DA) neurons of female and male mice display firing rates comparable to that of their control mice after SCVS. (A) Experimental timeline of SCVS, novelty suppressed feeding (NSF) test and in vitro electrophysiological recording. (B) NSF data from female mice (SCVS female vs. control female: t14=2.83, *P<0.05, n=8 female mice/group). (C) NSF data from male mice (SCVS male vs. control male: Mann-Whitney U=10, P=0.23, n=6 male mice/group). (D) Sample traces and statistical data of neuronal activity of VTA DA neurons in female mice following SCVS (t14=1.88, P=0.08, n=8 mice/group). (E) Sample traces and statistical data of neuronal activity of VTA DA neurons in male mice following SCVS (t10=0.97, P=0.36, n=6 mice/group).
Novelty suppressed feeding (NSF)
It has been shown that the SCVS paradigm induces several consistent behavioral deficits in female mice, but not in males, specifically depression-associated behaviors (Hodes et al., 2015). To minimize the effect of behavioral test-related stress on our electrophysiological recordings, we chose less stressful NSF as a test to confirm the behavioral phenotypes before carrying out the electrophysiological experiments. The NSF test was carried out as previously described (Santarelli et al., 2003; Hodes et al., 2015). Briefly, mice were food deprived 24 h before testing. Water was offered ad libitum. On the day of testing, mice were transferred to the testing room 1 h prior to start of the experiment. Under red light conditions, corncob bedding was lightly distributed on the floor of a plastic box of 50×50×20 cm. A single food pellet was placed on a platform where a petri dish was covered with a white circle cut out from Whatman paper and the platform was positioned in the center of the box. Mice were then placed in the corner of the box and a timer was started. The latency for mice grasping the food pellet with their forepaws and biting was recorded with a limit up to 10 min during testing. As soon as the mice began to eat, or the 10-min time limit was reached, they were immediately transferred back to their home cage.
In vitro slice electrophysiology
The electrophysiological recording procedures were followed as previously described (Chaudhury et al., 2013; Friedman et al., 2014). Under blinded conditions, mice were anesthetized with isofluorane and perfused immediately for 40–60 s with ice-cold artificial cerebrospinal fluid (aCSF) containing (in mM): 128 NaCl, 3 KCl, 1.25 NaH2PO4, 10 D-glucose, 24 NaHCO3, 2 CaCl2 and 2 MgCl2 (oxygenated with 95% O2 and 5% CO2, pH 7.4, 295–305 mOsm). Acute brain slices (250 μm) containing VTA, LHb or LC were cut using a vibratome microslicer (DTK-1000, Ted Pella) in ice-cold sucrose aCSF, which was derived by fully replacing NaCl with 254 mM sucrose and saturated by 95% O2 and 5% CO2. Slices were maintained in holding chambers with aCSF for 1 h recovery at 37 °C. Brain slices were then transferred into the recording chamber fitted with a constant flow rate of aCSF equilibrated with 95% O2 and 5% CO2 at 34 °C (2.5 ml/min). Glasspatch pipettes (3–5 MΩ) for cell-attached recordings were filled with internal solution containing the following: 115 mM potassium gluconate, 20 mM KCl, 1.5 mM MgCl2, 10 mM phosphocreatine, 10 mM HEPES, 2 mM magnesium ATP and 0.5 mM GTP (pH 7.2, 285 mOsm). Putative VTA DA neurons were identified by their location and electrophysiological criteria: regular and spontaneous action potentials with triphasic waveforms (Ungless et al., 2004; Krishnan et al., 2007; Friedman et al., 2014). LHb-VTA and LC-VTA circuit neurons were visualized by lumafluor in brain slices containing LHb and LC, respectively. For cell-attached action potential recordings, signals were band-pass filtered at 300 Hz–1 kHz (Multiclamp 700B, Molecular Devices) and were then Bessel filtered at 10 kHz. Data acquisition and analysis were collected using a Digidata 1440A digitizer and pClamp 10.2 (Molecular Devices). The average electrophysiological firing activity per mouse was performed for statistical analyses.
Stereotaxic surgeries
All surgeries were performed under aseptic conditions. Mice were anesthetized with a mixture of ketamine (100 mg/kg) and xylaxine (10 mg /kg), and then placed in a small-animal stereotaxic apparatus (Kopf Instruments). The skull was exposed by scalpel incision. For lumafluor injections, 33-gauge syringe needles (Hamilton Co.) were used to bilaterally infuse 0.5 μL lumafluor into the VTA at a 7° angle (anterior/posterior, −3.3 mm, lateral/medial, +0.5 mm; dorsal/ventral, −4.4 mm) at a rate of 0.1 μL/min. Needles were slowly removed 5 min after injection to prevent backflow. After microinjection, the skin was sutured using 5-0 silk threads and covered with an antibiotic ointment. Red lumafluor was purchased from Lumafluor Inc.
Immunohistochemistry
Mice were transcardially perfused with 30 mL ice-cold PBS, followed by 30 mL 4% paraformaldehyde (PFA) under general anesthesia with 15% urethane (Chaudhury et al., 2013; Friedman et al., 2014). Brain tissue was removed and post-fixed in PFA overnight at 4°C, and then transferred to 30% sucrose in PBS to equilibrate at 4°C for 2 days. The brains were sectioned with a thickness of 30 μm. LHb and LC sections were directly imaged for lumafluor expression on a Zeiss LSM780 confocal microscope at the Icahn School of Medicine Microscope Core.
For cresyl violet staining, frozen sections were mounted on glass slides, air dried overnight, placed directly into 1:1 alcohol/chloroform overnight and rehydrated through 100% and 95 % alcohol to distilled water. Then, sections were stained in 0.1% cresyl violet solution (Millipore) for 5–10 min, rinsed quickly in distilled water, differentiated in 95% ethyl alcohol for 2–30 min, dehydrated in 100% alcohol 2×5 min, cleared in xylene 2×5 min and mounted with permanent mounting medium. The site of intracerebral infusion was examined under Olympus light microscopy.
Statistics
Data are represented as mean ± s.e.m or median ± interquartile range, depending on the data normality. Statistical analyses were performed by using GraphPad Prism 5.0 software (Graph-Pad Software Inc). If assumptions of normality (Shapiro–Wilk test) and equal variance (F test) were met, two-tailed unpaired Student’s t test was used to analyze the effects of SCVS on NSF test and firing rates of VTA DA, LHb-VTA and LC-VTA projecting neurons in both male and female mice. If the dataset were not normally distributed (Shapiro-Wilk test, P<0.05), then we performed the Mann-Whitney U test. A P value < 0.05 is considered statistically significant.
Results
Firing rate of VTA DA neurons in response to SCVS
The firing rate of VTA DA neurons is highly involved in mediating stress responses (Chaudhury et al., 2013; Friedman et al., 2014). To investigate the possible firing rate changes of VTA DA neurons in response to SCVS, we first used NSF as a behavioral test to evaluate the susceptibility or resilience of both male and female mice to SCVS (Fig. 1A). After the last stressor, mice were singly housed and food deprived overnight. 24 h later, mice were put into a novel environment where latency to eat was recorded. Consistent with our previous study (Hodes et al., 2015), the NSF test showed that the SCVS paradigm significantly increased the latency to eat in female mice (t14=2.83, P<0.05; stressed female versus female control) (Fig. 1B), while the latency did not change in male mice (Mann-Whitney U=10, P=0.23; stressed male versus male control) (Fig. 1C). Next, using in vitro cell-attached recording technique, we recorded from putative VTA DA neurons in this batch of mice and found that there was no significant alteration in the firing rate of VTA DA neurons in female (t14=1.88, P=0.08) (Fig. 1D) and male mice (t10=0.97, P=0.36) (Fig. 1E) when compared to their stress-naïve controls after the 6-d SCVS. These data suggest that the VTA may not be directly responsible for the susceptibility of female mice to SCVS treatment.
Firing rate of LHb-VTA circuit neurons in response to SCVS
The LHb is an important upstream structure of the VTA (Omelchenko et al., 2009; Juarez and Han, 2016) and the activity alteration of LHb-VTA projecting neurons has been shown in depressive-like states (Li et al., 2011). To investigate the firing activity of LHb-VTA circuit neurons in response to SCVS, we first injected retrograding red lumafluor into the VTA to label projection-specific neurons in the LHb (Fig. 2A-C). We also observed labeled neurons in the medial habenula (MHb), which is consistent with an earlier study that uncovered synaptic inputs from the MHb to the VTA (Watabe-Uchida et al., 2012). For this study, we focused on the stress-responsive LHb cells that project to the VTA.
Fig. 2.
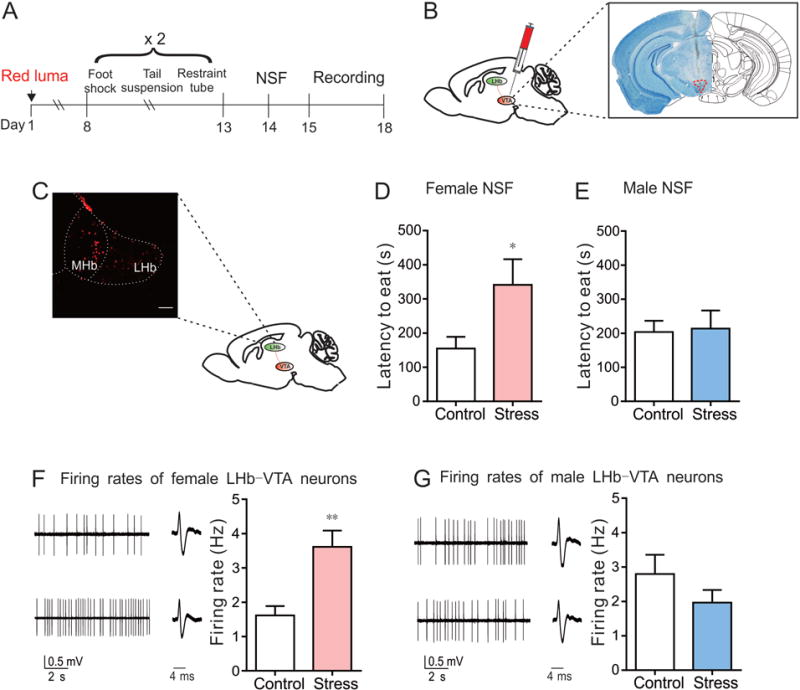
LHb-VTA circuit neurons display increased neuronal activity in SCVS female mice when compared to their control. (A) Experimental timeline. (B) Schematic showing retrograding red lumafluor (red luma) injected into VTA. (C) Red lumafluor-labeled LHb-VTA circuit cells (scale bar=100 μm). (D) NSF data from female mice (SCVS female vs. control female: t10 =2.35, *P<0.05, n=6 female mice/group). (E) NSF data from male mice (SCVS male vs. control male: t11=0.15, P=0.88, n=6-7 male mice/group). (F) Sample traces and statistical data of neuronal activity of LHb-VTA circuit neurons in female mice following SCVS (t10=4.08, **P<0.01, n=6 mice/group). (G) Sample traces and statistical data of neuronal activity of LHb-VTA circuit neurons in male mice following SCVS (t10=1.20, P=0.26, n=6 mice/group).
One week after the injection surgery, male and female mice underwent SCVS and significant behavioral differences in NSF were seen selectively in SCVS female mice as compared to control female mice (t10=2.35, P<0.05) (Fig. 2D and E). We next performed circuit-specific electrophysiological recordings from brain slices containing LHb and found that the firing rate of LHb-VTA circuit neurons was dramatically increased in female mice that underwent SCVS when compared to control female mice (t10=4.08, P<0.01) (Fig. 2F). This stress-induced effect was not seen between SCVS and control male mice (t10=1.20, P=0.26) (Fig. 2G). These findings indicate that the distinct alterations in the firing activity of LHb-VTA projecting neurons of male and female mice may potentially determine the different phenotypes after SCVS.
Firing rate of LC-VTA projecting neurons in response to SCVS
The LC is another upstream nucleus of the VTA (Chandler et al., 2013). Given the roles of LC-VTA circuit in modulating susceptibility and resilience to social stress (Zhang et al., 2015; Isingrini et al., 2016), we further investigated the firing activity of the LC-VTA circuit neurons in response to SCVS by injecting lumafluor into the VTA (Fig. 3A and B). Using the same batch of SCVS animals for the LHb recording (Fig. 3D and E), we cut LHb-containing slices as stated above and obtained LC-containing slices for LC-VTA circuit neuron recordings from the same brains. Our recording data showed that the LC-VTA circuit neurons of SCVS mice displayed firing rates comparable to their controls in both sexes (female: t10=1.11, P=0.30; male: t10=1.30, P=0.22) (Fig. 3C and D).
Fig. 3.
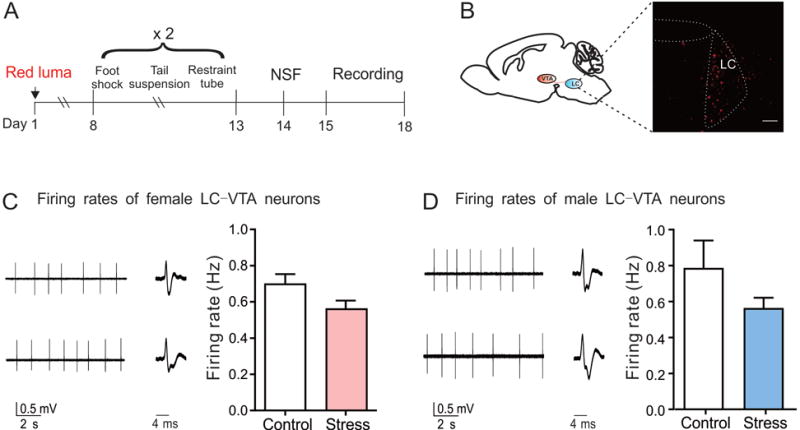
LC-VTA circuit neurons in female and male mice display firing rates comparable to that of their control mice after SCVS. (A) Experimental timeline. (B) Red lumafluor-labeled LC-VTA circuit neurons (scale bar=100 μm). (C) Sample traces and statistical data of neuronal activity of LC-VTA circuit neurons in female mice following SCVS (t10=1.11, P=0.30, n=6 mice/group). (D) Sample traces and statistical data of neuronal activity of LC-VTA circuit neurons in male mice following SCVS (t10=1.30, P=0.22, n=6 mice/group).
Discussion
In this study, a 6-d subchronic variable stress model was used to induce susceptible phenotypes in female mice and resilient phenotypes in males, as has been shown previously (Hodes et al., 2015). We then used cell type- or projection-specific electrophysiological recordings and found that the firing activity of LHb-VTA projecting neurons was dramatically increased in female mice that underwent SCVS when compared to their controls. This stress-induced effect on LHb-VTA circuit neurons was not seen in male mice. In contrast, we did not observe any differences in respect to the firing activity of VTA DA neurons and LC-VTA circuit neurons between SCVS female or SCVS male mice and their respective controls. To our knowledge, this is the first study that shows the sex differences in circuit-specific neuronal adaptations among the reward-related system in response to SCVS. This work demonstrates the potential functional role of the LHb-VTA circuit in mediating the vulnerability of females to stress-related affective disorders.
Current stress models including learned helplessness (LH), repeated social defeat stress (RSDS) and chronic mild stress (CMS) have been used to unravel novel mechanisms underlying behavioral deficits to variable stressors, but most of these studies have been conducted in males (Dalla et al., 2008; Chaudhury et al., 2013; Tye et al., 2013). There is an urgent need to understand stress responses in females because stress-related psychiatric disorders such as major depression have a higher incidence in women than men. Previous studies have shown that the SCVS paradigm can induce a depressive phenotype in female mice, whereas males display resilience in response to SCVS. This provides us an ideal stress model to investigate the sex difference in stress vulnerability. Utilizing this model, two studies have revealed interesting molecular mechanisms that underlie these sex differences (LaPlant et al., 2009; Hodes et al., 2015). Here, we replicated this 6-d stress paradigm and used an NSF test to confirm the different phenotypes before conducting electrophysiological studies.
VTA DA neurons in the brain’s reward circuit are highly involved in mediating stress responses (Nestler and Carlezon, 2006; Friedman et al., 2014; Friedman et al., 2016; Holly and Miczek, 2016). The firing activity of VTA DA neurons has also been implicated in determining susceptibility versus resilience in response to stress. It has been shown that CMS or physically aversive stimuli inhibit the activity of VTA DA neurons (Ungless et al., 2004; Valenti et al., 2012). Tye et al. found that selectively inhibiting VTA DA neurons induces a depression-like phenotype, and optogenetically activating VTA DA neurons reverses CMS-induced behavioral abnormities in tail suspension test, sucrose preference test and forced swim test (Tye et al., 2013). In contrast, RSDS enhances the activity of VTA DA neurons (Krishnan et al., 2007; Cao et al., 2010). Chaudhury et al. demonstrated that optogenetic activation of phasic, but not tonic, firing in VTA DA neurons during or after exposure to subthreshold defeat rapidly produces social avoidance and anhedonic behaviors, and optogenetic inhibition of VTA DA neuron firing reverses depressive behaviors in susceptible mice following RSDS exposure (Chaudhury et al., 2013). This divergence indicates that effects of stress on VTA DA neurons are highly complex and different stress protocols may cause distinct responses in VTA neurons (Valenti et al., 2012; Walsh and Han, 2014). It is also known that estrus or sex hormones regulate the firing activity of VTA DA neurons (Zhang et al., 2008; Calipari et al., 2017; Locklear et al., 2017), but the baseline firing of VTA DA neurons may have no differences between male and female animals (Locklear et al., 2017). Here we find that although 6-d SCVS segregates susceptible and resilient phenotypes from female and male mice, there are no alterations in the firing activity of VTA neurons both in female and male mice when compared to their controls, suggesting a divergent and model-dependent neuronal alteration of VTA DA neurons.
The LHb sends efferents to VTA DA neurons (Omelchenko et al., 2009; Juarez and Han, 2016) and several lines of evidence suggest heightened LHb activity in multiple depressive-like states (Sartorius and Henn, 2007; Li et al., 2011; Li et al., 2013). Aversive stimuli that induce anxiety- and depression-related phenotypes, such as foot shocks and physical restraint, will increase c-Fos expression and bursting action potentials in LHb neurons, respectively (Shumake et al., 2003; Brown and Shepard, 2013; Seo et al., 2017). Congenitally helpless rats that display helpless behavior in response to stress show elevated metabolism in the LHb (Shumake et al., 2003). In addition, a recent study using both acute learned helplessness (aLH) and congenital learned helplessness (cLH) models of depression also found that excitatory synapses onto LHb-VTA projecting neurons of helpless rats are potentiated (Li et al., 2011). An imaging study using positron emission tomography (PET) in humans showed that tryptophan depletion treatment, which will induce transient depressive relapses, increases blood flow in the habenula (Morris et al., 1999). Consistent with these previous studies, our data show that LHb-VTA projecting neurons exhibit an increased firing rate in susceptible female mice after SCVS, whereas the effect was not observed in resilient male mice after SCVS. These points raise the possibility that the activity of LHb plays a key role in mediating stress-related abnormalities.
In the last decade, progress has been made in treating depressive disorders by interfering with the activity of LHb neurons both in animals and humans. Yang et al. found that chemically lesioning the LHb reduces the immobility time and increases the climbing time during forced swim test in depressed rats that underwent CMS or clomipramine injection (Yang et al., 2008). Local infusion of GABAA receptor agonist muscimol, or the knocking-down of βCaMKII to silence LHb neurons, attentuates helpless behaviors in the cLH model (Winter et al., 2011; Li et al., 2013). More recently, Seo et al. reported that knockdown of p11 in LHb neurons, which abolishes LHb hyperexcitability, ameliorates depressive phenotypes in physically restrained mice (Seo et al., 2017). Indeed, it has been reported that DBS of the LHb suppresses excitatory synaptic transmission and ameliorates helpless behavior in rats (Li et al., 2011). In addition, DBS of the LHb also elevates numbers of crossings and rearings during the open-field test in rats subjected to CMS, which is accompanied by increased concentrations of monoamines in blood serum and the brain (Meng et al., 2011). In accordance with evidence from animals, a clinical case has been reported that bilateral DBS of the major afferent bundle of the LHb achieves a sustained full remission of major depression in a patient who was therapy-resistant to all standard treatments (Sartorius et al., 2010). Accordingly, deep brain stimulation (DBS) that can functionally suppress LHb neural activity may be a potent method for treating depression. Therefore, further work to investigate the relationship between activity alterations of LHb-VTA neurons and behavioral outcomes after SCVS would be of great interest.
The LC, through direct anatomical (Chandler et al., 2013) and functional (Guiard et al., 2008) connections to the VTA, is suggested to be an intermediate hub between external stressors and the regulation of the stress response. Studies focusing on sex differences in the LC structure from rodents have shown that the LC is a sexually dimorphic structure where the adult females have a larger volume and greater number of NE containing neurons than in males (Ohm et al., 1997; Pinos et al., 2001). Additionally, a morphological research focuses on LC reveals a greater dendritic extension and complexity in females than in males (Bangasser et al., 2011). Multiple reports have also shown that estrogen is able to increase norepinephrine (NE) synthesis and release in LC terminal regions and decrease NE degradation (Bangasser et al., 2016). Since corticotropin-releasing factor (CRF) is the primary mediator in response to emotional stress (Vamvakopoulos and Chrousos, 1994), a few studies have found that males and females have sex differences with regard to CRF regulation of LC physiology, CRF1 receptor signaling, coupling and CRF1 receptor trafficking, potentially explaining the heightened incidence of stress-related disorders in females (Bangasser et al., 2016). These findings suggest an important role of LC in mediating sex differences in response to stress.
Two recently reported studies investigated the roles of LC-VTA circuit in response to RSDS (Zhang et al., 2015; Isingrini et al., 2016). By using optogenetics and electrophysiological techniques, they showed that in male mice, LC-VTA projecting neurons exhibit increased firing in resilient mice when compared to control and susceptible mice, and NE neurotransmission from the LC to the VTA is both necessary and sufficient to promote resilience to social defeat. However, in our study we do not find any differences in the firing rate of LC-VTA projecting neurons between males and females as compared with their controls after SCVS. The disparities among the studies may due to different stress models or due to homeostatic resilience in response to SCVS. However, although no alteration is seen in activity of these neurons, there may be some other neurobiological intrinsic differences between sexes which mediate the different behavioral coping mechanisms with stress.
Our study, for the first time, depicts the phenomenon that SCVS increases the neuronal activity of LHb-VTA projecting neurons exclusively in female mice. No activity alterations of VTA DA neurons and LC-VTA projecting neurons were seen in female or male mice after SCVS. These results shed light on a potential important role of the LHb-VTA circuit in mediating sex differences in response to stress. Further understanding of the functional role of the LHb-VTA circuit is required to develop potential sex-specific treatment for stress-related psychiatric disorders such as major depression.
We replicated that SCVS induced a depressed susceptible phenotype in female but not male mice.
The firing activity of LHb-VTA circuit neurons was dramatically increased selectively in female mice that underwent SCVS.
VTA dopamine neurons and LC-VTA circuit did not display firing differences between stressed and control mice in both sexes.
Acknowledgments
Funding
This work was supported by the National Institute of Mental Health [R01 MH092306; R21 MH112081: M.H.H.], the National Institute on Alcohol Abuse and Alcoholism (R01 AA022445: M.H.H.), National Natural Science Foundation of China [NSFC81230025, 81070888, 81720108013: J.L.C.; NSFC81200862, 81300957: H.Z.], National Research Service Award [T32 MH096678, F31 MH108326: S.M.K.; T32 MH 087004, F31 AA022862: B.J.], Johnson & Johnson/IMHRO Rising Star Translational Research Award [M.H.H.], and NARSAD Independent Investigator Award [M.H.H.].
Statement of effort
Jun-Li Cao and Ming-Hu Han designed this study; Song Zhang, Hongxing Zhang, Stacy M. Ku, Barbara Juarez, Carole Morel performed the experiments and analyzed the data; Georgia E. Hodes, Anna Brancato and Scott J. Russo helped to establish the SCVS model, Nikos Tzavaras helped to microscope image acquisition. All authors have approved the final manuscript.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- CMS
chronic mild stress
- CRF
corticotropin-releasing factor
- RSDS
repeated social defeat stress
- DA
dopamine
- DBS
deep brain stimulation
- LC
locus coeruleus
- LH
learned helplessness
- LHb
lateral habenula
- MHb
medial habenula
- NAc
nucleus accumbens
- NE
norepinephrine
- NSF
novelty suppressed feeding
- SCVS
Subchronic variable stress
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have declared no conflict of interest.
References
- Bangasser DA, Wiersielis KR, Khantsis S. Sex differences in the locus coeruleus-norepinephrine system and its regulation by stress. Brain Res. 2016;1641:177–188. doi: 10.1016/j.brainres.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Zhang X, Garachh V, Hanhauser E, Valentino RJ. Sexual dimorphism in locus coeruleus dendritic morphology: a structural basis for sex differences in emotional arousal. Physiol Behav. 2011;103:342–351. doi: 10.1016/j.physbeh.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PL, Shepard PD. Lesions of the fasciculus retroflexus alter footshock-induced cFos expression in the mesopontine rostromedial tegmental area of rats. PLoS One. 2013;8:e60678. doi: 10.1371/journal.pone.0060678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Juarez B, Morel C, Walker DM, Cahill ME, Ribeiro E, Roman-Ortiz C, Ramakrishnan C, Deisseroth K, Han MH, Nestler EJ. Dopaminergic dynamics underlying sex-specific cocaine reward. Nat Commun. 2017;8:13877. doi: 10.1038/ncomms13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao JL, Covington HE, 3rd, Friedman AK, Wilkinson MB, Walsh JJ, Cooper DC, Nestler EJ, Han MH. Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J Neurosci. 2010;30:16453–16458. doi: 10.1523/JNEUROSCI.3177-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler DJ, Lamperski CS, Waterhouse BD. Identification and distribution of projections from monoaminergic and cholinergic nuclei to functionally differentiated subregions of prefrontal cortex. Brain Res. 2013;1522:38–58. doi: 10.1016/j.brainres.2013.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC, Pomeranz L, Christoffel DJ, Nectow AR, Ekstrand M, Domingos A, Mazei-Robison MS, Mouzon E, Lobo MK, Neve RL, Friedman JM, Russo SJ, Deisseroth K, Nestler EJ, Han MH. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C, Edgecomb C, Whetstone AS, Shors TJ. Females do not express learned helplessness like males do. Neuropsychopharmacology. 2008;33:1559–1569. doi: 10.1038/sj.npp.1301533. [DOI] [PubMed] [Google Scholar]
- Friedman AK, Juarez B, Ku SM, Zhang H, Calizo RC, Walsh JJ, Chaudhury D, Zhang S, Hawkins A, Dietz DM, Murrough JW, Ribadeneira M, Wong EH, Neve RL, Han MH. KCNQ channel openers reverse depressive symptoms via an active resilience mechanism. Nat Commun. 2016;7:11671. doi: 10.1038/ncomms11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman AK, Walsh JJ, Juarez B, Ku SM, Chaudhury D, Wang J, Li X, Dietz DM, Pan N, Vialou VF, Neve RL, Yue Z, Han MH. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science. 2014;344:313–319. doi: 10.1126/science.1249240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiard BP, El Mansari M, Blier P. Cross-talk between dopaminergic and noradrenergic systems in the rat ventral tegmental area, locus ceruleus, and dorsal hippocampus. Mol Pharmacol. 2008;74:1463–1475. doi: 10.1124/mol.108.048033. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, Magida J, Brancato A, Takahashi A, Flanigan ME, Menard C, Aleyasin H, Koo JW, Lorsch ZS, Feng J, Heshmati M, Wang M, Turecki G, Neve R, Zhang B, Shen L, Nestler EJ, Russo SJ. Sex Differences in Nucleus Accumbens Transcriptome Profiles Associated with Susceptibility versus Resilience to Subchronic Variable Stress. J Neurosci. 2015;35:16362–16376. doi: 10.1523/JNEUROSCI.1392-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly EN, Miczek KA. Ventral tegmental area dopamine revisited: effects of acute and repeated stress. Psychopharmacology (Berl) 2016;233:163–186. doi: 10.1007/s00213-015-4151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isingrini E, Perret L, Rainer Q, Amilhon B, Guma E, Tanti A, Martin G, Robinson J, Moquin L, Marti F, Mechawar N, Williams S, Gratton A, Giros B. Resilience to chronic stress is mediated by noradrenergic regulation of dopamine neurons. Nat Neurosci. 2016;19:560–563. doi: 10.1038/nn.4245. [DOI] [PubMed] [Google Scholar]
- Juarez B, Han MH. Diversity of Dopaminergic Neural Circuits in Response to Drug Exposure. Neuropsychopharmacology. 2016;41:2424–2446. doi: 10.1038/npp.2016.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Kessler RC, Walters EE, MacLean C, Neale MC, Heath AC, Eaves LJ. Stressful life events, genetic liability, and onset of an episode of major depression in women. Am J Psychiatry. 1995;152:833–842. doi: 10.1176/ajp.152.6.833. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Thornton LM, Prescott CA. Gender differences in the rates of exposure to stressful life events and sensitivity to their depressogenic effects. Am J Psychiatry. 2001;158:587–593. doi: 10.1176/appi.ajp.158.4.587. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Nelson CB, Hughes M, Swartz M, Blazer DG. Sex and depression in the National Comorbidity Survey. II: Cohort effects. J Affect Disord. 1994;30:15–26. doi: 10.1016/0165-0327(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Klein LC, Corwin EJ. Seeing the unexpected: how sex differences in stress responses may provide a new perspective on the manifestation of psychiatric disorders. Curr Psychiatry Rep. 2002;4:441–448. doi: 10.1007/s11920-002-0072-z. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- LaPlant Q, Chakravarty S, Vialou V, Mukherjee S, Koo JW, Kalahasti G, Bradbury KR, Taylor SV, Maze I, Kumar A, Graham A, Birnbaum SG, Krishnan V, Truong HT, Neve RL, Nestler EJ, Russo SJ. Role of nuclear factor kappaB in ovarian hormone-mediated stress hypersensitivity in female mice. Biol Psychiatry. 2009;65:874–880. doi: 10.1016/j.biopsych.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, Henn F, Malinow R. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470:535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Zhou T, Liao L, Yang Z, Wong C, Henn F, Malinow R, Yates JR, 3rd, Hu H. betaCaMKII in lateral habenula mediates core symptoms of depression. Science. 2013;341:1016–1020. doi: 10.1126/science.1240729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locklear MN, Michaelos M, Collins WF, Kritzer MF. Gonadectomy but not biological sex affects burst-firing in dopamine neurons of the ventral tegmental area and in prefrontal cortical neurons projecting to the ventral tegmentum in adult rats. Eur J Neurosci. 2017;45:106–120. doi: 10.1111/ejn.13380. [DOI] [PubMed] [Google Scholar]
- Maciejewski PK, Prigerson HG, Mazure CM. Sex differences in event-related risk for major depression. Psychol Med. 2001;31:593–604. doi: 10.1017/s0033291701003877. [DOI] [PubMed] [Google Scholar]
- Meng H, Wang Y, Huang M, Lin W, Wang S, Zhang B. Chronic deep brain stimulation of the lateral habenula nucleus in a rat model of depression. Brain Res. 2011;1422:32–38. doi: 10.1016/j.brainres.2011.08.041. [DOI] [PubMed] [Google Scholar]
- Morris JS, Smith KA, Cowen PJ, Friston KJ, Dolan RJ. Covariation of activity in habenula and dorsal raphe nuclei following tryptophan depletion. Neuroimage. 1999;10:163–172. doi: 10.1006/nimg.1999.0455. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Bremner JD, Foa EB, Mayberg HS, North CS, Stein MB. Posttraumatic stress disorder: a state-of-the-science review. J Psychiatr Res. 2006;40:1–21. doi: 10.1016/j.jpsychires.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Ohm TG, Busch C, Bohl J. Unbiased estimation of neuronal numbers in the human nucleus coeruleus during aging. Neurobiol Aging. 1997;18:393–399. doi: 10.1016/s0197-4580(97)00034-1. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Bell R, Sesack SR. Lateral habenula projections to dopamine and GABA neurons in the rat ventral tegmental area. Eur J Neurosci. 2009;30:1239–1250. doi: 10.1111/j.1460-9568.2009.06924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinelli M, Gomez Homen F. Gender differences in the epidemiology of affective disorders and schizophrenia. World Health Organization, Nations for Mental Health; 1997. [Google Scholar]
- Pinos H, Collado P, Rodriguez-Zafra M, Rodriguez C, Segovia S, Guillamon A. The development of sex differences in the locus coeruleus of the rat. Brain Res Bull. 2001;56:73–78. doi: 10.1016/s0361-9230(01)00540-8. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sartorius A, Henn FA. Deep brain stimulation of the lateral habenula in treatment resistant major depression. Med Hypotheses. 2007;69:1305–1308. doi: 10.1016/j.mehy.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Sartorius A, Kiening KL, Kirsch P, von Gall CC, Haberkorn U, Unterberg AW, Henn FA, Meyer-Lindenberg A. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010;67:e9–e11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- Seo JS, Zhong P, Liu A, Yan Z, Greengard P. Elevation of p11 in lateral habenula mediates depression-like behavior. Mol Psychiatry. 2017 doi: 10.1038/mp.2017.96. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumake J, Edwards E, Gonzalez-Lima F. Opposite metabolic changes in the habenula and ventral tegmental area of a genetic model of helpless behavior. Brain Res. 2003;963:274–281. doi: 10.1016/s0006-8993(02)04048-9. [DOI] [PubMed] [Google Scholar]
- Solomon MB, Karom MC, Huhman KL. Sex and estrous cycle differences in the display of conditioned defeat in Syrian hamsters. Horm Behav. 2007;52:211–219. doi: 10.1016/j.yhbeh.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Pride MC, Villalon Landeros R, Knoblauch NW, Takahashi EY, Silva AL, Crean KK. Sex differences in social interaction behavior following social defeat stress in the monogamous California mouse (Peromyscus californicus) PLoS One. 2011;6:e17405. doi: 10.1371/journal.pone.0017405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, Kim SY, Adhikari A, Thompson KR, Andalman AS, Gunaydin LA, Witten IB, Deisseroth K. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- Valenti O, Gill KM, Grace AA. Different stressors produce excitation or inhibition of mesolimbic dopamine neuron activity: response alteration by stress pre-exposure. Eur J Neurosci. 2012;35:1312–1321. doi: 10.1111/j.1460-9568.2012.08038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vamvakopoulos NC, Chrousos GP. Hormonal regulation of human corticotropin-releasing hormone gene expression: implications for the stress response and immune/inflammatory reaction. Endocr Rev. 1994;15:409–420. doi: 10.1210/edrv-15-4-409. [DOI] [PubMed] [Google Scholar]
- Ver Hoeve ES, Kelly G, Luz S, Ghanshani S, Bhatnagar S. Short-term and long-term effects of repeated social defeat during adolescence or adulthood in female rats. Neuroscience. 2013;249:63–73. doi: 10.1016/j.neuroscience.2013.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JJ, Han MH. The heterogeneity of ventral tegmental area neurons: Projection functions in a mood-related context. Neuroscience. 2014;282:101–108. doi: 10.1016/j.neuroscience.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Winter C, Vollmayr B, Djodari-Irani A, Klein J, Sartorius A. Pharmacological inhibition of the lateral habenula improves depressive-like behavior in an animal model of treatment resistant depression. Behav Brain Res. 2011;216:463–465. doi: 10.1016/j.bbr.2010.07.034. [DOI] [PubMed] [Google Scholar]
- Yang LM, Hu B, Xia YH, Zhang BL, Zhao H. Lateral habenula lesions improve the behavioral response in depressed rats via increasing the serotonin level in dorsal raphe nucleus. Behav Brain Res. 2008;188:84–90. doi: 10.1016/j.bbr.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Zhang D, Yang S, Yang C, Jin G, Zhen X. Estrogen regulates responses of dopamine neurons in the ventral tegmental area to cocaine. Psychopharmacology (Berl) 2008;199:625–635. doi: 10.1007/s00213-008-1188-6. [DOI] [PubMed] [Google Scholar]
- Zhang H, Chaudhury D, Juarez B, Friedman A, Ku S, Nectow A, Crumiller M, Jiang C, Zhang S, Morel C. Role of Locus Coeruleus-Ventral Tegmental Area Circuit in Mediating the Resilience to Social Stress. Neuropsychopharmacol. 2015;2015:S237–S238. [Google Scholar]


