Abstract
Background and Objectives
After the first acute myocardial infarction (AMI), a considerable proportion of patients are newly diagnosed with diabetes mellitus (DM). However, in AMI, controversy remains regarding the disparity in prognosis between previously diagnosed DM (known-DM) and newly diagnosed DM (new-DM).
Methods
The study included 10,455 patients with AMI (non-DM, 6,236; new-DM, 659; known-DM, 3,560) admitted to one of 15 participating centers in Korea between November 2011 and January 2016 (average follow-up, 523 days). We compared the characteristics and clinical course of patients with known-DM and those with new- or non-DM.
Results
Compared to patients with known-DM, those with new-DM or non-DM were younger, more likely to be male, and less likely to have hypertension, dyslipidemia, prior stroke, angina, or myocardial infarction. Compared to patients with new-DM or non-DM (reference), those with known-DM had higher risks of major adverse cardiac events (hazard ratio [HR], 1.20; 95% confidence interval [CI], 1.06–1.35; p=0.004), cardiac death (HR, 1.26; 95% CI, 1.01–1.57; p=0.042), and congestive heart failure (HR, 1.58; 95% CI, 1.20–2.08). Unlike known-DM, new-DM did not increase the risk of cardiac events (including death).
Conclusions
Known-DM was associated with a significantly higher risk of cardiovascular events after AMI, while new-DM had a similar risk of cardiac events as that noted for non-DM. There were different cardiovascular outcomes according to diabetes status in patients with AMI.
Keywords: Diabetes mellitus, Myocardial infarction, Cardiac death, Congestive heart failure
INTRODUCTION
Guidelines of the National Cholesterol Education Program consider that diabetes mellitus (DM) poses a risk of future cardiovascular events equivalent to that posed by coronary artery disease, therefore elevating patients with DM to the highest risk category.1)
Acute myocardial infarction (AMI) is a major cause of death and morbidity. While some studies have reported a decline in AMI-related mortality, generally attributed to closer adherence to guidelines for AMI management, mortality rates remain high among patients with AMI.2),3) Since the incidence of newly diagnosed DM (new-DM) is high in patients with AMI, it is recommended that all patients with AMI be screened for DM.4),5) Several studies evaluated the role of DM as a risk factor in patients with AMI. Among known risk factors, such as age, sex, and angiographic findings, DM is an important independent predictor of mortality in patients with AMI.6) Other studies suggested that DM is a major risk factor for mortality and AMI in patients with acute coronary syndrome, even after revascularization.7)
The VALsartan In Acute myocardial iNfarcTion (VALIANT) trial revealed that both new-DM and previously diagnosed DM (known-DM) were associated with poorer 1-year outcomes after AMI.4) Meanwhile, the Harmonizing Outcomes with RevasculariZatiON and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial also suggested that patients with new-DM have a similarly poor prognosis after AMI.8) However, Tian et al.5) reported that known-DM, but not new-DM, was an independent predictor for short-term (30-day) mortality and major adverse cardiac events (MACEs) in patients with ST-segment elevation myocardial infarction (STEMI). While DM status is recognized as an important factor affecting the risk of cardiovascular events in patients with AMI, controversy remains as to the exact role and extent of this influence. The VALIANT study was performed in 2003 and fewer than 20% of enrolled patients underwent percutaneous coronary intervention (PCI). The HORIZONS-AMI study only enrolled patients with STEMI, and Tian et al.'s study5) had relatively short-term follow-up.
In the present study, we aim to quantify the role of DM status in long-term clinical outcomes after AMI in Korean patients.
METHODS
Study design and population
In Korea, efforts have been made to collect nationwide data and standardize clinical practice regarding the management of AMI. Consecutive patients with AMI were selected from the database of the Korea Acute Myocardial Infarction Registry-National Institutes of Health (KAMIR-NIH). KAMIR-NIH is a prospective, multicenter, web-based observational cohort study aiming to develop a prognostic and surveillance index for Korean patients with AMI. KAMIR-NIH was initiated in November 2011 with the participation of 15 centers in Korea and with grant support from the Korea Centers for Disease Control and Prevention. The aims and protocols of the registries have been published elsewhere.9)
The protocol of the present study was reviewed and approved by the Institutional Review Board at each participating KAMIR-NIH center. Demographic data, procedural details, and clinical outcomes were collected from the KAMIR-NIH database. Invalid or incomplete data were excluded from the present analysis.
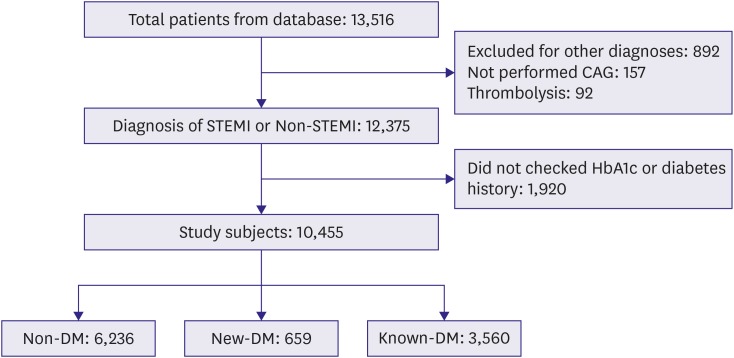
A total of 13,516 patients with AMI were selected from the database. Patients with any disease other than AMI (n=892), who had not undergone coronary angiography (CAG) (n=157), who had undergone thrombolysis (n=92), and without data regarding the levels of glycated hemoglobin (HbA1c) or any history of DM at admission for AMI were excluded (n=1,920). In total, 10,455 patients with AMI were included in the study and stratified according to DM status at admission for AMI (Figure 1).
Figure 1.
Study scheme. From nation-wide multicenter registries, 12,375 patients with myocardial infarction were analyzed in this study.
CAG = coronary angiography; DM = diabetes mellitus; HbA1c = glycated hemoglobin; known-DM = previously diagnosed diabetes mellitus; new-DM = newly diagnosed diabetes mellitus; STEMI = ST-segment elevation myocardial infarction.
Patients included in the study (i.e., after applying exclusion criteria) were stratified into 3 groups according to DM status at admission for AMI. Specifically, patients who had been diagnosed with DM prior to admission for AMI were included in the known-DM group. Patients who had not been diagnosed with DM prior to admission for AMI, but were found to have HbA1c levels of ≥6.5% at admission for AMI, were included in the new-DM group. In 2011, the World Health Organization concluded that HbA1c levels of ≥6.5% (48 mmol/mol) can be used as a diagnostic criterion for DM.10) Finally, patients who had not been diagnosed with DM prior to admission for AMI and who did not fulfill the HbA1c criterion for diagnosis at admission for AMI were included in the non-DM group. Electronic case report forms for data collection were developed and managed using internet-based Clinical Research and Trial management system (iCReaT), a data management system established by the Centers for Disease Control and Prevention, Ministry of Health and Welfare, Republic of Korea (iCReaT Study No. C110016).
CAG and PCI were performed using standard techniques.11) The choice of a particular stent was based on operator preference. Multivessel disease was defined as ≥70% stenosis in at least 2 major epicardial coronary arteries or ≥50% stenosis of the left main coronary artery.
Clinical outcomes
All deaths were considered cardiac death events unless a non-cardiac origin was documented. Recurrent AMI (re-AMI) was defined as recurrent AMI symptoms with new ST-segment elevation or re-elevation of cardiac markers to at least twice the upper normal limit. Revascularization was defined as any repeat intervention (surgical or percutaneous) of any vessel. MACEs were defined as a composite of cardiac death, re-AMI, stent thrombosis, revascularization via repeat PCI (re-PCI), and hospital admission related to congestive heart failure (CHF).
Statistical methods
All statistical analyses were performed using SPSS software, version 20.0 (SPSS Inc., Chicago, IL, USA). Baseline data are presented as the frequencies or mean±standard deviation. Continuous variables were compared using Student's t-test, and categorical variables were compared using χ2 test. The cumulative incidence of adverse events was estimated using the Kaplan-Meier method, and significance levels were assessed using log-rank tests. The association between DM status and outcomes was examined using univariable and multivariable Cox regression analysis. Cox regression analysis was also used to evaluate the prognostic significance of the following variables concerning clinical outcomes at follow-up: old age (>60 years old); male sex; body mass index (BMI); history of hypertension; final diagnosis (STEMI vs. non-STEMI); dyslipidemia; previous myocardial infarction; smoking status (current smoker); Killip class ≥II; multivessel disease; left ventricular (LV) dysfunction, characterized by left ventricular ejection fraction (LVEF) <50%; post-thrombolysis in myocardial infarction (TIMI) flow grade ≤2; and serum creatinine (Cr) levels >2.0 mg/dL. Patients lost to follow-up were considered at risk until the date of last contact, at which point their data were censored. The results of risk analyses are provided as hazard ratio (HR) with 95% confidence interval (CI).
RESULTS
Patient characteristics
Among 13,516 patients registered in the KAMIR-NIH database, data of 10,455 patients with AMI were included in our analysis (non-DM, 6,236 patients; new-DM, 659 patients; known-DM, 3,560 patients). The proportion of patients with new-DM was 6.3%. The mean follow-up duration was 523 days. Among patients with known-DM, the mean duration of DM was 11.2 years.
Patients with known-DM were older, more likely to be female, and less likely to be a smoker at the time of admission for AMI. Patients in the known-DM group also had higher incidence of hypertension, dyslipidemia, prior ischemic heart disease (angina, myocardial infarction), renal dysfunction, and LV dysfunction. Furthermore, compared to the new-DM and non-DM groups, the known-DM group had higher incidence of left main coronary artery disease and multivessel disease. The LVEF and Killip class were poorer in patients with known-DM. PCI was performed for 90.6%, 90.0%, and 87.4% of patients in the non-DM, new-DM, and known-DM groups, respectively. Glycoprotein IIb/IIIa inhibitors were more frequently used in non-DM patients.
Compared to patients with non-DM or known-DM, those with new-DM were younger, more likely to be male and to smoke, and had higher levels of low-density lipoprotein (LDL)-cholesterol. Additionally, compared to the non-DM group, the new-DM group had a higher incidence of previous stroke, prior angina, depressed LV systolic function (ejection fraction <50%), type B2/C lesion, and use of beta-blockers. Patient characteristics were described at Table 1.
Table 1. Baseline characteristics, clinical performance, and angiographic findings.
| Characteristics | Non-DM (n=6,236) | New-DM (n=659) | Known-DM (n=3,560) | ||
|---|---|---|---|---|---|
| Age (years) | 64.2±13.5 | 60.4±12.9* | 66.3±11.1*,† | ||
| Men | 74.4 | 78.1* | 68.3*,† | ||
| STEMI | 50.6 | 52.8 | 42.0*,† | ||
| Non-STEMI | 49.4 | 47.2 | 58.0*,† | ||
| Mean diabetes duration (years) | 11.2 | ||||
| Medical history | |||||
| Hypertension | 58.9 | 42.9 | 67.8*,† | ||
| Dyslipidemia | 10.6 | 9.6 | 14.9*,† | ||
| Current smoking | 40.6 | 53.4* | 31.4*,† | ||
| Prior stroke | 6.0 | 5.5* | 10.1*,† | ||
| Prior angina | 8.7 | 6.1* | 13.7*,† | ||
| Prior myocardial infarction | 6.1 | 6.8 | 11.5*,† | ||
| Renal dysfunction (Cr >2.0 mg/dL) | 2.9 | 1.4 | 12.0*,† | ||
| Depressed LV systolic function (<50%) | 35.2 | 39.1* | 44.2*,† | ||
| Laboratory finding | |||||
| HbA1c | 5.64±0.38 | 7.68±1.48* | 7.61±1.66* | ||
| Glucose | 142.1±49.3 | 216.4±90.4 | 227.2±105.4*,† | ||
| Cr (mg/dL) | 1.07±2.73 | 0.97±0.57* | 1.46±1.64*,† | ||
| LDL-cholesterol (mg/dL) | 113.9±38.1 | 122.9±41.5* | 100.3±40.1*,† | ||
| Peak CK-MB (ng/mL) | 119.6±179.2 | 111.6±137.7 | 83.4±125.2*,† | ||
| Peak troponin I (ng/mL) | 48.4±111.8 | 53.2±102.7 | 43.9±89.4 | ||
| Peak troponin T (ng/mL) | 5.7±18.3 | 7.4±23.5 | 4.7±6.3*,† | ||
| NT-proBNP | 1,989.1±5,625.9 | 2,301.1±9,500.0 | 4,732.8±13,992.4*,† | ||
| BNP | 254.6±606.5 | 258.3±554.2 | 557.2±1,086.2*,† | ||
| hs-CRP | 1.65±13.7 | 1.61±3.93 | 2.34±10.81* | ||
| BMI (kg/m2) | 24.8±49.8 | 24.9±3.7 | 24.5±15.9 | ||
| Ejection fraction (%) | 52.7±10.8 | 51.2±11.1* | 50.1±12.1*,† | ||
| Killip class (III, IV) | 10.9 | 12.6 | 17.8*,† | ||
| Angiographic finding | |||||
| 1 vessel disease | 53.8 | 50.7 | 41.2*,† | ||
| Left main related | 3.7 | 2.6 | 5.4*,† | ||
| Multivessel disease | 46.2 | 49.3 | 58.8*,† | ||
| Lesion type (type B2/C) | 86.6 | 90.2* | 86.6† | ||
| Culprit vessel | |||||
| Left main | 1.9 | 2.3 | 3.2* | ||
| Left anterior descending artery | 46.6 | 44.7 | 45.5* | ||
| Left circumflex artery | 17.6 | 17.2 | 16.7* | ||
| Right coronary artery | 33.9 | 35.8 | 34.6* | ||
| Initial-TIMI flow (0 or 1) | 59.8 | 60.0 | 52.4*,† | ||
| Post-TIMI flow (<3) | 3.5 | 3.1 | 3.6 | ||
| PCI | 90.6 | 92.0 | 87.4*,† | ||
| Medication during admission | |||||
| Aspirin | 99.6 | 100.0 | 99.1*,† | ||
| Clopidogrel | 78.5 | 78.1 | 81.7*,† | ||
| Ticagrelor | 22.5 | 20.3 | 18.7* | ||
| Prasugrel | 11.7 | 15.2* | 10.1*,† | ||
| Cilostazol | 9.7 | 12.0 | 12.8 | ||
| Beta-blocker | 81.3 | 85.4* | 80.5† | ||
| ACE inhibitor | 49.2 | 54.0* | 39.8*,† | ||
| ARB | 29.9 | 29.0 | 36.2*,† | ||
| Statin | 91.6 | 92.7 | 85.8*,† | ||
Data expressed as mean±standard deviation or percentage.
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; BMI = body mass index; BNP = b-type natriuretic peptide; CK-MB = creatine kinase-myocardial band; Cr = creatinine; DM = diabetes mellitus; HbA1c = glycated hemoglobin; hs-CRP = high-sensitivity C-reactive protein; LDL = low-density lipoprotein; LV = left ventricular; NT-proBNP = N-terminal pro b-type natriuretic peptide; PCI = percutaneous coronary intervention; STEMI = ST-segment elevation myocardial infarction; TIMI = thrombolysis in myocardial infarction.
*p<0.01 compared to patients without diabetes, †p<0.01 compared to patients with newly diagnosed diabetes.
Clinical outcomes
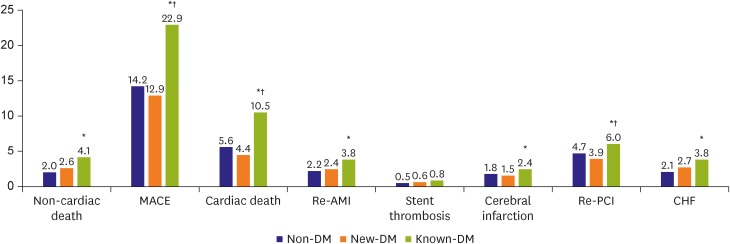
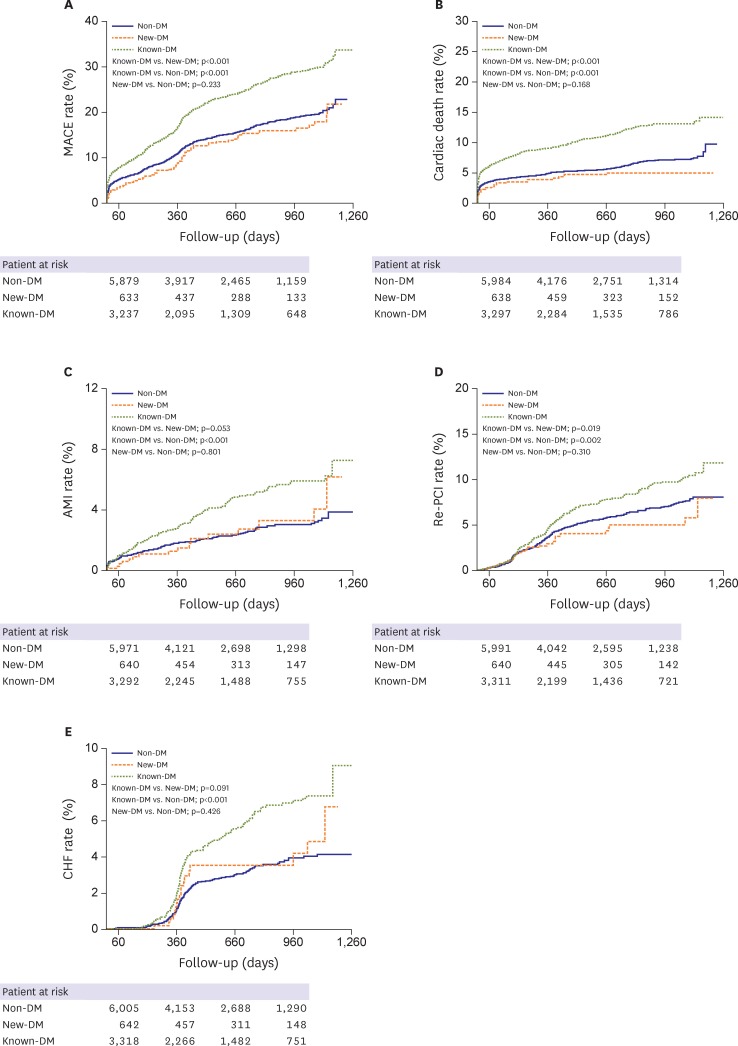
The incidence of all-cause death, cardiac death, re-AMI, re-PCI, CHF, and MACE were significantly higher in the known-DM group than in the non-DM and new-DM groups. However, the groups did not differ in terms of stent thrombosis or cerebral infarction rate (Figure 2). The cumulative incidence of MACEs (i.e., cardiac death, re-AMI, re-PCI, or hospitalization related to CHF) was greater in the known-DM group and similar between the new-DM and non-DM groups (Figure 3).
Figure 2.
Long-term clinical outcomes after AMI. Patients were stratified according to DM status at admission for AMI: non-DM, DM not diagnosed at the time of admission for AMI; known-DM, DM diagnosed prior to admission for AMI; new-DM, DM diagnosed at admission for AMI.
AMI = acute myocardial infarction; CHF = congestive heart failure; DM = diabetes mellitus; known-DM = previously diagnosed diabetes mellitus; MACE = major adverse cardiac event; new-DM = newly diagnosed diabetes mellitus; re-AMI = recurrent acute myocardial infarction; re-PCI = revascularization via repeat percutaneous coronary intervention.
*p<0.05 vs. non-DM, †p<0.05 for known-DM vs. new-DM.
Figure 3.
Kaplan-Meier curves for survival free of adverse events and reintervention after AMI. (A) Cumulative incidence of MACEs, (B) cardiac mortality, (C) re-AMI, (D) re-PCI, and (E) hospitalization related to CHF.
AMI = acute myocardial infarction; CHF = congestive heart failure; DM = diabetes mellitus; known-DM = previously diagnosed diabetes mellitus; MACE = major adverse cardiovascular event; new-DM = newly diagnosed diabetes mellitus; re-AMI = recurrent acute myocardial infarction; re-PCI = revascularization via repeat percutaneous coronary intervention.
Effect of DM status on clinical outcomes
Next, we examined whether DM status was an independent predictor of clinical outcomes after AMI. In univariate analysis, known-DM was associated with increased incidence of MACEs (HR, 1.60; 95% CI, 1.45–1.76), cardiac death (HR, 1.91; 95% CI, 1.65–2.21), and re-AMI (HR, 1.91; 95% CI, 1.65–2.21). In multivariate analysis, known-DM remained an independent predictor for MACEs (HR, 1.20; 95% CI, 1.06–1.35), cardiac death (HR, 1.26; 95% CI, 1.01–1.57), re-AMI (HR, 1.26; 95% CI, 1.01–1.57), and hospitalization for CHF (HR, 1.58; 95% CI, 1.20–2.08). However, there was no significant association between DM status and the incidence of stent thrombosis, cerebral infarction, or re-PCI (Table 2).
Table 2. Multivariate analysis for clinical outcomes.
| Clinical outcomes | Non-DM | New-DM | Known-DM | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | p value | HR (95% CI) | p value | ||
| MACE | 870 (14.2) | 83 (12.9) | 767 (22.3)*,† | |||
| Unadjusted | Reference | 0.87 (0.70–1.09) | 0.235 | 1.60 (1.45–1.76) | <0.001 | |
| Adjusted | Reference | 0.94 (0.72–1.23) | 0.648 | 1.20 (1.06–1.35) | 0.004 | |
| Cardiac death | 350 (5.6) | 29 (4.4) | 375 (10.5)*,† | |||
| Unadjusted | Reference | 0.77 (0.53–1.12) | 0.171 | 1.91 (1.65–2.21) | <0.001 | |
| Adjusted | Reference | 0.92 (0.55–1.54) | 0.761 | 1.26 (1.01–1.57) | 0.042 | |
| Re-AMI | 137 (2.2) | 16 (2.4) | 135 (3.8)* | |||
| Unadjusted | Reference | 0.77 (0.53–1.12) | 0.171 | 1.91 (1.65–2.21) | <0.001 | |
| Adjusted | Reference | 0.92 (0.55–1.55) | 0.761 | 1.26 (1.01–1.57) | 0.042 | |
| Stent thrombosis | 34 (0.5) | 4 (0.6) | 30 (0.8) | |||
| Unadjusted | Reference | 1.05 (0.37–2.96) | 0.924 | 1.54 (0.94–2.51) | 0.087 | |
| Adjusted | Reference | 0.83 (0.25–2.74) | 0.762 | 1.47 (0.86–2.50) | 0.158 | |
| Cerebral infarction | 111 (1.8) | 10 (1.5) | 87 (2.4)* | |||
| Unadjusted | Reference | 0.82 (0.43–1.57) | 0.558 | 1.40 (1.06–1.85) | 0.019 | |
| Adjusted | Reference | 1.00 (0.48–2.01) | 0.996 | 1.17 (0.84–1.63) | 0.367 | |
| Re-PCI | 292 (4.7) | 26 (3.9) | 213 (6.0)*,† | |||
| Unadjusted | Reference | 0.81 (0.54–1.21) | 0.308 | 1.31 (1.10–1.57) | 0.003 | |
| Adjusted | Reference | 0.78 (0.52–1.22) | 0.286 | 1.09 (0.90–1.33) | 0.362 | |
| CHF | 133 (2.1) | 18 (2.7) | 137 (3.8)* | |||
| Unadjusted | Reference | 1.22 (0.75–2.00) | 0.427 | 1.86 (1.46–2.36) | <0.001 | |
| Adjusted | Reference | 1.21 (0.68–2.16) | 0.522 | 1.58 (1.20–2.08) | 0.001 | |
Text describes multivariable models, data shown are number (%) not otherwise specified.
CHF = congestive heart failure; CI = confidence interval; DM = diabetes mellitus; HR = hazard ratio; known-DM = previously diagnosed diabetes mellitus; MACE = major adverse cardiovascular event; new-DM = newly diagnosed diabetes mellitus; re-AMI = recurrent acute myocardial infarction; re-PCI = revascularization via repeat percutaneous coronary intervention.
*p<0.01 compared to patients without diabetes, †p<0.01 compared to patients with newly diagnosed diabetes.
Subgroup analysis was performed according to several risk factors (Table 3). Known-DM was an independent predictor for MACEs in patients who were female (HR, 1.32; 95% CI, 1.08–1.62), elderly (HR, 1.21; 95% CI, 1.05–1.40), had one vessel disease (HR, 1.28; 95% CI, 1.04–1.58), STEMI (HR, 1.20; 95% CI, 1.00–1.43), non-STEMI (HR, 1.19; 95% CI, 1.00–1.41), or a high BMI (HR, 1.27; 95% CI, 1.05–1.54). However, analysis indicated that known-DM was not an independent predictor for male patients or those with young age, multivessel disease, or low BMI.
Table 3. Subgroup analysis for MACEs.
| MACEs | Non-DM | New-DM | Known-DM | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |||
| Gender | ||||||
| Female | Reference | 1.13 (0.70–1.82) | 0.613 | 1.32 (1.08–1.62) | 0.008 | |
| Male | Reference | 0.88 (0.64–1.21) | 0.426 | 1.14 (0.97–1.33) | 0.105 | |
| Age (years) | ||||||
| Young age (<60) | Reference | 0.92 (0.60–1.42) | 0.713 | 1.14 (0.89–1.47) | 0.296 | |
| Old age (≥60) | Reference | 0.95 (0.68–1.34) | 0.767 | 1.21 (1.05–1.40) | 0.008 | |
| Multivessel disease | ||||||
| One vessel disease | Reference | 1.13 (0.75–1.72) | 0.557 | 1.28 (1.04–1.58) | 0.022 | |
| Multivessel disease | Reference | 0.83 (0.59–1.18) | 0.303 | 1.14 (0.98–1.33) | 0.091 | |
| Final diagnosis | ||||||
| Non-STEMI | Reference | 0.80 (0.53–1.21) | 0.296 | 1.19 (1.00–1.41) | 0.047 | |
| STEMI | Reference | 1.08 (0.76–1.54) | 0.665 | 1.20 (1.00–1.43) | 0.046 | |
| BMI | ||||||
| Low | Reference | 0.89 (0.61–1.31) | 0.563 | 1.15 (0.98–1.35) | 0.097 | |
| High | Reference | 1.02 (0.71–1.48) | 0.903 | 1.27 (1.05–1.54) | 0.013 | |
BMI = body mass index; CI = confidence interval; DM = diabetes mellitus; HR = hazard ratio; known-DM = previously diagnosed diabetes mellitus; MACE = major adverse cardiac event; new-DM = newly diagnosed diabetes mellitus; STEMI = ST-segment elevation myocardial infarction.
Risk factors for MACEs
The predictors of MACEs in patients with AMI were analyzed (Table 4). Univariate analysis indicated that the HR with 95% CI for MACEs was 0.87 (95% CI, 0.70–1.09; p=0.235) and 1.60 (95% CI, 1.45–1.76; p<0.001) in new-DM and known-DM patients, respectively, relative to that of non-DM patients. Other significant predictors for MACEs were old age (HR, 2.16; 95% CI, 1.93–2.41; p<0.001), male sex (HR, 0.67; 95% CI, 0.60–0.73; p<0.001), BMI group (>24 kg/m2; HR, 0.71; p=0.001), current smoking (HR, 0.65; 95% CI, 0.58–0.72; p<0.001), low LVEF (HR, 1.92; 95% CI, 1.74–2.13; p<0.001), poor Killip class (HR, 3.15; 95% CI, 2.84–3.50; p<0.001), multivessel disease (HR, 1.83; 95% CI, 1.66–2.03; p<0.001), low post-TIMI (HR, 2.17; 95% CI, 1.75–2.68; p<0.001), hypertension (HR, 1.61; 95% CI, 1.45–1.79; p<0.001), dyslipidemia (HR, 0.76; 95% CI, 0.65–0.89; p=0.001), previous myocardial infarction (HR, 1.60; 95% CI, 1.38–1.86; p<0.001), and renal failure (HR, 3.39; 95% CI, 2.96–3.88; p<0.001).
Table 4. Unadjusted and adjusted risks for MACEs.
| Characteristics | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Non-DM | Reference | Reference | ||
| New-DM | 0.87 (0.70–1.09) | 0.235 | 0.94 (0.72–1.23) | 0.648 |
| Known-DM | 1.60 (1.45–1.76) | <0.001 | 1.20 (1.06–1.35) | 0.004 |
| Old age (>60 years) | 2.16 (1.93–2.41) | <0.001 | 1.38 (1.19–1.60) | <0.001 |
| Male sex | 0.67 (0.60–0.73) | <0.001 | 0.80 (0.70–0.92) | 0.001 |
| BMI group (>24 kg/m2) | 0.71 (0.65–0.79) | <0.001 | 0.86 (0.76–0.96) | 0.010 |
| STEMI | 0.96 (0.88–1.06) | 0.445 | 0.96 (0.86–1.09) | 0.536 |
| Current smoking | 0.65 (0.58–0.72) | <0.001 | 0.97 (0.85–1.12) | 0.715 |
| LVEF (<50%) | 1.92 (1.74–2.13) | <0.001 | 1.48 (1.31–1.66) | <0.001 |
| Killips class (III, IV) | 3.15 (2.84–3.50) | <0.001 | 1.55 (1.34–1.80) | <0.001 |
| Multivessel disease | 1.83 (1.66–2.03) | <0.001 | 1.54 (1.38–1.74) | <0.001 |
| Post-TIMI flow (TIMI ≤2) | 2.17 (1.75–2.68) | <0.001 | 1.52 (1.16–1.99) | 0.003 |
| Hypertension | 1.61 (1.45–1.79) | <0.001 | 1.32 (1.15–1.50) | <0.001 |
| Dyslipidemia | 0.76 (0.65–0.89) | 0.001 | 0.85 (0.70–1.02) | 0.078 |
| Previous myocardial infarction | 1.60 (1.38–1.86) | <0.001 | 1.41 (1.17–1.70) | <0.001 |
| Renal failure (Cr >2.0 mg/dL) | 3.39 (2.96–3.88) | <0.001 | 2.13 (1.77–2.57) | <0.001 |
BMI = body mass index; CI = confidence interval; Cr = creatinine; DM = diabetes mellitus; HR = hazard ratio; known-DM = previously diagnosed diabetes mellitus; LVEF = left ventricular ejection fraction; MACE = major adverse cardiac event; new-DM = newly diagnosed diabetes mellitus; STEMI = ST-segment elevation myocardial infarction; TIMI = thrombolysis in myocardial infarction.
Multivariate analysis also showed that the risk for MACEs was significantly higher in known-DM (HR, 1.20; 95% CI, 1.06–1.35; p=0.004) and not known-DM (HR, 0.94; 95% CI, 0.72–1.23; p=0.648). Multivariate analysis identified further risk factors for MACEs, such as old age (HR, 1.38; 95% CI, 1.19–1.60; p<0.001), male sex (HR, 0.80; 95% CI, 0.70–0.92; p=0.001), high BMI (HR, 0.86; 95% CI, 0.76–0.96; p=0.010), low LVEF (HR, 1.48; 95% CI, 1.31–1.66; p<0.001), poor Killip class (HR, 2.16; 95% CI, 1.93–2.41; p<0.001), multivessel disease (HR, 1.54; 95% CI, 1.38–1.74; p<0.001), low post-TIMI flow (HR, 1.52; 95% CI, 1.16–1.99; p=0.003), hypertension (HR, 1.32; 95% CI, 1.15–1.50; p<0.001), previous myocardial infarction (HR, 1.41; 95% CI, 1.17–1.70; p<0.001), and renal failure (HR, 2.13; 95% CI, 1.77–2.57; p<0.001).
DISCUSSION
In this multicenter, prospective, observational study, known-DM was a significant independent predictor of cardiac mortality, MACEs, re-AMI, and hospitalization for CHF during long-term follow-up after AMI. However, new-DM was not associated with increased risk of adverse clinical outcomes compared to the risk noted in non-DM patients. These results suggest that known-DM, but not new-DM, is strongly associated with worse clinical outcomes in patients with AMI.
The Multiple Risk Factor Intervention Trial and Framingham Heart Study reported on the importance of the association between DM and coronary heart disease (CHD).12)
The risk of infarction was reported to be greatest in patients with DM and prior myocardial infarction.13) Several studies reported that patients with AMI and known-DM have poor outcomes compared to those of patients with other comorbidities.14),15) Previous studies using data from Korean registries also suggested that DM has a high association with 1-year mortality, which exceeds the in-hospital mortality of patients with AMI who underwent successful PCI.16)
Several studies suggested that patients with DM may have poorer outcomes because of the greater prevalence of multivessel disease with atherosclerotic plaque burden, a pro-thrombotic state, and more neo-intimal proliferation.17),18) In the present study, multivessel disease and left main coronary artery disease were indeed more prevalent in the known-DM group.
Multiple mechanisms have been reported to lead to the increased incidence of MACEs in patients with DM. Specifically, a hypercoagulable state, plaque ulceration, and intracoronary thrombus were more frequently noted among DM patients,19) as were shear-induced platelet adhesion and subendothelium aggregation.20) Coronary tissue from patients with DM exhibits more lipid-rich atheromas, macrophage infiltration, and subsequent thrombosis.21) DM increases the risk of heart failure independent of CHD and hypertension, and may cause cardiomyopathy. Recent research in humans and animals has provided novel insights into the underlying molecular and pathophysiological mechanisms that increase the vulnerability of patients with DM to heart to failure, including mitochondrial dysfunction, altered substrate metabolism, and activation of the renin-angiotensin system.22)
Because the frequency of new-DM was higher in patients with AMI and since DM status at baseline affects clinical outcomes, prognosis evaluation should consider DM status.4),5) The prevalence of new-DM was reported to be 3.8%–17.0% among patients with AMI. Data sets from the Cleveland Clinic, Valliant, HORIZONS-AMI, and Tian et al.'s studies5) define 8.0%, 4.0%, 3.8%, and 17.0% of patients with AMI as new-DM, respectively.4),8),23) Therefore, all patients with AMI should be screened for DM. In our study, 6.3% of patients were newly diagnosed with DM upon admission for AMI based on HbA1c >6.5%, indicating a slightly higher prevalence of new-DM compared to what is expected based on previous reports.
Diabetes can be diagnosed using HbA1c criteria or plasma glucose criteria after either fasting or at 2 hours after a 75 g oral glucose tolerance test.24) Only HbA1c was used for diagnosis since we were restricted to tests that were included in the registry. As a result, diabetes may have been under-diagnosed. While DM is difficult to diagnose in AMI patients based solely on HbA1c because of temporarily increased glucose levels,25) we observed an incidence of 6.3% for new-DM using a single HbA1c-based criterion.
Several studies have reported prognosis after AMI according to DM status and classified patients as new-DM, known-DM, or non-DM. In the VALIANT cohort study, subjects with AMI were divided into 3 such groups. Patients with known-DM had poorer clinical outcomes, similar to patients that exhibited new-DM for at least 1 year.4) The HORIZONS-AMI trial database study suggested that patients with new-DM have a similarly poor prognosis after primary PCI in STEMI compared to those with known-DM for 3 years.8) However, Tian et al.5) reported that known-DM was an independent predictor for short-term mortality and MACEs in AMI patients, while new-DM was associated with similar outcomes as those noted for non-DM for 30 days. The observations from that study were similar to those in the present study. Meanwhile, the composite cardiovascular events were higher in the VALIANT (25% for 1 year) and HORIZON-AMI (20.9% for 3 years) studies in comparison to this study (16% for 17 months).
This study revealed that known-DM, not new-DM, resulted in increased risk of adverse outcomes in AMI, even after multivariate analysis.
In the VALIANT study, patients with cardiogenic shock or renal failure (Cr levels >2.5 mg/dL) were excluded. Moreover, the method of diagnosing new-DM was not described, and diagnosis was individually established by physician assessment. Finally, duration of DM was not indicated.4) The HORIZONS-AMI was a sub-study of an antiplatelet study in patients with STEMI. As a result, patients with multivessel disease, bifurcation lesion, and left main disease were excluded. Moreover, they did not suggest a diagnostic method for diabetes nor did they record the duration of DM. Conversely, the study by Tian et al.5) included all patients with AMI and used HbA1c levels to establish a cutoff in a manner similar to that performed in this study to establish DM diagnosis. Moreover, they did not exclude patients with cardiogenic shock or renal failure. We included patients with renal failure and cardiogenic shock, and these factors were entered into the multivariate analysis model.
Interestingly, the VALIANT study did not include information relating to several lab values, such as Cr and LDL-cholesterol. In the VALIANT study, Killip class was poorer in patients with new-DM and known-DM compared to non-DM patients. This is not in agreement with our findings or with those of Tian et al.,5) that Killip class of new-DM was similar to that of non-DM, but was better that of known-DM patients. Another study reported that Killip class was a major predictor of mortality and morbidity after AMI,26) and is likely related to the poor outcomes we found in patients with known-DM, but not in patients with new-DM or non-DM. In the HORIZON-AMI study, pre-TIMI flow and LVEF were poor in new-DM patients, while these factors were better in known-DM patients included in this study.
In new-DM patients, higher LDL-cholesterol levels have been successfully managed via guideline-based treatment, and it is likely that the younger age noted in new-DM patients partially accounts for the fact that clinical outcomes were similar to those of non-DM patients. The present study revealed that renal failure, LV dysfunction, high levels of brain natriuretic peptides, poor Killip class, low TIMI flow, multivessel disease, and left main coronary artery disease were more prevalent in patients with known-DM, but occurred at a similar incidence in new-DM and non-DM patients. Patients with known-DM had a higher incidence of comorbidities (previous hypertension, dyslipidemia, angina, myocardial infarction, stroke) and were more likely to be female, factors previously confirmed as independent predictors of cardiovascular events after AMI.18),27) However, the new-DM and non-DM groups generally did not differ with respect to these characteristics.
Previously, Kang et al.28) reported that smoking was associated with a 48% decrease in the risk of all-cause mortality after AMI. The rate of current smoking was higher in new-DM patients in this study, which may have influenced outcomes.
In the known-DM group, the mean duration of DM was 11.2 years. While this parameter could not be determined in patients with new-DM, DM duration was likely shorter in patients with new-DM than in those with known-DM. Previous studies in patients with AMI did not indicate DM duration. However, DM duration was independently associated with the risk of death related to CHD.29) Prolonged DM was associated with intravascular ultrasound-defined thin-cap fibroatheroma, a plaque phenotype associated with risk of rupture and CHD.30) Taken together, these results suggest that short-duration DM and improved baseline characteristics are the main factors responsible for better outcomes in patients with AMI and new-DM. Furthermore, we suspect that new-DM after AMI was managed via intensive medical therapy, which, combined with the relatively short-duration of DM, likely resulted in similar clinical outcomes as those noted for non-DM patients.
HbA1c level was similar between known-DM and new-DM patients. Unfortunately, there were no data regarding HbA1c level in patients with newly diagnosed diabetes who experienced an AMI. Although patients who were diagnosed with DM for the first time after AMI had relatively high HbA1c levels, this may be due to their history of AMI, advanced stage, and lack of medications.
Moreover, our subgroup analysis indicates that known-DM independently predicted MACEs in female patients or with those with old age, one vessel disease, STEMI, non-STEMI, or high BMI. Non-DM was not statically related to MACEs in all categories that were tested. The rate of PCI was higher in non-DM and new-DM patients compared to known-DM patients. Ultimately, we could not determine why patients did not undergo PCI; however, known-DM patients were associated with higher multivessel disease, older age, and unstable vital signs (poor Killip class), and these factors could contraindicate PCI.
Our study has several limitations. A major limitation is that the present study is based on registry data. Although rigorous adjustment analyses were performed, unmeasured hidden biases may remain, and a large number of patients were excluded due to lack of data regarding HbA1c level or diabetes history. The second drawback was a high rate of loss to follow-up. Since data collection was voluntary at each participating center, the follow-up data were partially incomplete. Third, glucose status and medication compliance could not be obtained from follow-up patients. The merits of this study include its large sample size, multicenter design, and prospective data collection. Furthermore, a single, unambiguous criterion was used to diagnose diabetes.
Known-DM was associated with a higher risk of long-term cardiac death, re-AMI, hospitalization related to CHF, and overall incidence of MACEs, and these trends held true even after multivariate adjustment. Our findings confirm similar previous observations; however, new-DM was not associated with increased risk of death or adverse cardiovascular events compared to the risk noted for non-DM. This observation may be related to differences in baseline characteristics and duration of DM. The outcome of patients with new-DM was similar to that of non-DM patients, which is explained by the fact that patients with new-DM likely received intensive medical therapy after AMI.
Footnotes
Funding: This research was supported by a grant (2016-ER6304-00) from the Research of Korea Centers for Disease Control and Prevention.
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Hwang JY.
- Data curation: Koh JS, Park JR, Jeong YH, Ahn JH, Jang JY, Kwak CH, Park Y, Jeong MH, Kim YJ, Cho MC, Kim CJ.
- Formal analysis: Park HW, Kang MG, Kim K.
- Investigation: Park HW, Kang MG.
- Methodology: Park HW, Hwang JY.
- Project administration: Hwang JY.
- Resources: Hwang JY.
- Supervision: Hwang JY.
- Validation: Hwang JY, Park HW.
- Visualization: Park HW, Kim K.
- Writing - original draft: Park HW.
- Writing - review & editing: Hwang JY.
References
- 1.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 2.Rogers WJ, Frederick PD, Stoehr E, et al. Trends in presenting characteristics and hospital mortality among patients with ST elevation and non-ST elevation myocardial infarction in the National Registry of Myocardial Infarction from 1990 to 2006. Am Heart J. 2008;156:1026–1034. doi: 10.1016/j.ahj.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 3.Wright RS, Anderson JL, Adams CD, et al. 2011 ACCF/AHA focused update of the guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American College of Emergency Physicians, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;57:1920–1959. doi: 10.1016/j.jacc.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Aguilar D, Solomon SD, Køber L, et al. Newly diagnosed and previously known diabetes mellitus and 1-year outcomes of acute myocardial infarction: the VALsartan In Acute myocardial iNfarcTion (VALIANT) trial. Circulation. 2004;110:1572–1578. doi: 10.1161/01.CIR.0000142047.28024.F2. [DOI] [PubMed] [Google Scholar]
- 5.Tian L, Wei C, Zhu J, et al. Newly diagnosed and previously known diabetes mellitus and short-term outcomes in patients with acute myocardial infarction. Coron Artery Dis. 2013;24:669–675. doi: 10.1097/MCA.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 6.Park HW, Yoon CH, Kang SH, et al. Early- and late-term clinical outcome and their predictors in patients with ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction. Int J Cardiol. 2013;169:254–261. doi: 10.1016/j.ijcard.2013.08.132. [DOI] [PubMed] [Google Scholar]
- 7.Norhammar A, Malmberg K, Diderholm E, et al. Diabetes mellitus: the major risk factor in unstable coronary artery disease even after consideration of the extent of coronary artery disease and benefits of revascularization. J Am Coll Cardiol. 2004;43:585–591. doi: 10.1016/j.jacc.2003.08.050. [DOI] [PubMed] [Google Scholar]
- 8.Ertelt K, Brener SJ, Mehran R, Ben-Yehuda O, McAndrew T, Stone GW. Comparison of outcomes and prognosis of patients with versus without newly diagnosed diabetes mellitus after primary percutaneous coronary intervention for ST-elevation myocardial infarction (the HORIZONS-AMI Study) Am J Cardiol. 2017;119:1917–1923. doi: 10.1016/j.amjcard.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Kim JH, Chae SC, Oh DJ, et al. Multicenter cohort study of acute myocardial infarction in Korea: interim analysis of the Korea acute myocardial infarction registry-national institutes of health registry. Circ J. 2016;80:1427–1436. doi: 10.1253/circj.CJ-16-0061. [DOI] [PubMed] [Google Scholar]
- 10.Thygesen K, Alpert JS, White HD Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Eur Heart J. 2007;28:2525–2538. doi: 10.1093/eurheartj/ehm355. [DOI] [PubMed] [Google Scholar]
- 11.Smith SC, Jr, Feldman TE, Hirshfeld JW, Jr, et al. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update 2001 Guidelines for Percutaneous Coronary Intervention) Circulation. 2006;113:e166–286. doi: 10.1161/CIRCULATIONAHA.106.173220. [DOI] [PubMed] [Google Scholar]
- 12.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 13.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 14.Mukamal KJ, Nesto RW, Cohen MC, et al. Impact of diabetes on long-term survival after acute myocardial infarction: comparability of risk with prior myocardial infarction. Diabetes Care. 2001;24:1422–1427. doi: 10.2337/diacare.24.8.1422. [DOI] [PubMed] [Google Scholar]
- 15.Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Impact of history of diabetes mellitus on hospital mortality in men and women with first acute myocardial infarction. The National Registry of Myocardial Infarction 2 Participants. Am J Cardiol. 2000;85:1486–1489. A7. doi: 10.1016/s0002-9149(00)00800-6. [DOI] [PubMed] [Google Scholar]
- 16.Park KH, Ahn Y, Jeong MH, et al. Different impact of diabetes mellitus on in-hospital and 1-year mortality in patients with acute myocardial infarction who underwent successful percutaneous coronary intervention: results from the Korean Acute Myocardial Infarction Registry. Korean J Intern Med. 2012;27:180–188. doi: 10.3904/kjim.2012.27.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 18.Creager MA, Lüscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation. 2003;108:1527–1532. doi: 10.1161/01.CIR.0000091257.27563.32. [DOI] [PubMed] [Google Scholar]
- 19.Silva JA, Escobar A, Collins TJ, Ramee SR, White CJ. Unstable angina. A comparison of angioscopic findings between diabetic and nondiabetic patients. Circulation. 1995;92:1731–1736. doi: 10.1161/01.cir.92.7.1731. [DOI] [PubMed] [Google Scholar]
- 20.Knobler H, Savion N, Shenkman B, Kotev-Emeth S, Varon D. Shear-induced platelet adhesion and aggregation on subendothelium are increased in diabetic patients. Thromb Res. 1998;90:181–190. doi: 10.1016/s0049-3848(98)00050-4. [DOI] [PubMed] [Google Scholar]
- 21.Moreno PR, Murcia AM, Palacios IF, et al. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation. 2000;102:2180–2184. doi: 10.1161/01.cir.102.18.2180. [DOI] [PubMed] [Google Scholar]
- 22.Boudina S, Abel ED. Mitochondrial uncoupling: a key contributor to reduced cardiac efficiency in diabetes. Physiology (Bethesda) 2006;21:250–258. doi: 10.1152/physiol.00008.2006. [DOI] [PubMed] [Google Scholar]
- 23.Aggarwal B, Shah G, Randhawa MS, Lincoff AM, Ellis SG, Menon V. Abstract 17910: patients with newly diagnosed diabetes have comparable long term mortality with known diabetics after ST segment elevation myocardial infarction. Circulation. 2014;130:A17910. [Google Scholar]
- 24.American Diabetes Association. (2) Classification and diagnosis of diabetes. Diabetes Care. 2015;38(Suppl):S8–S16. doi: 10.2337/dc15-S005. [DOI] [PubMed] [Google Scholar]
- 25.Tenerz A, Lönnberg I, Berne C, Nilsson G, Leppert J. Myocardial infarction and prevalence of diabetes mellitus. Is increased casual blood glucose at admission a reliable criterion for the diagnosis of diabetes? Eur Heart J. 2001;22:1102–1110. doi: 10.1053/euhj.2000.2445. [DOI] [PubMed] [Google Scholar]
- 26.Steg PG, Dabbous OH, Feldman LJ, et al. Determinants and prognostic impact of heart failure complicating acute coronary syndromes: observations from the Global Registry of Acute Coronary Events (GRACE) Circulation. 2004;109:494–499. doi: 10.1161/01.CIR.0000109691.16944.DA. [DOI] [PubMed] [Google Scholar]
- 27.Eagle KA, Lim MJ, Dabbous OH, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291:2727–2733. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 28.Kang SH, Suh JW, Choi DJ, et al. Cigarette smoking is paradoxically associated with low mortality risk after acute myocardial infarction. Nicotine Tob Res. 2013;15:1230–1238. doi: 10.1093/ntr/nts248. [DOI] [PubMed] [Google Scholar]
- 29.Fox CS, Sullivan L, D'Agostino RB, Sr, Wilson PW Framingham Heart Study. The significant effect of diabetes duration on coronary heart disease mortality: the Framingham Heart Study. Diabetes Care. 2004;27:704–708. doi: 10.2337/diacare.27.3.704. [DOI] [PubMed] [Google Scholar]
- 30.Lindsey JB, House JA, Kennedy KF, Marso SP. Diabetes duration is associated with increased thin-cap fibroatheroma detected by intravascular ultrasound with virtual histology. Circ Cardiovasc Interv. 2009;2:543–548. doi: 10.1161/CIRCINTERVENTIONS.109.876672. [DOI] [PubMed] [Google Scholar]