Abstract
More than any other organ, the heart is particularly sensitive to gene expression deregulation, often leading in the long run to impaired contractile performances and excessive fibrosis deposition progressing to heart failure. Recent investigations provide evidences that the protein phosphatases (PPs), as their counterpart protein kinases, are important regulators of cardiac physiology and development. Two main groups, the protein serine/threonine phosphatases and the protein tyrosine phosphatases (PTPs), constitute the PPs family. Here, we provide an overview of the role of PTP subfamily in the development of the heart and in cardiac pathophysiology. Based on recent in silico studies, we highlight the importance of PTPs as therapeutic targets for the development of new drugs to restore PTPs signaling in the early and late events of heart failure.
Keywords: Protein tyrosine phosphatase, PTP, Cardiac hypertrophy, Heart failure, Small-molecule inhibitor
Introduction
Cardiac physiology is heavily dependent on the intracellular signaling balance between protein kinases (PKs) and their counterparts, the protein phosphatases (PPs). Not surprisingly, the deregulation of such balance in the adult heart leads in most cases to heart dysfunction associated with impaired contractile performances and fibrosis deposition often leading to heart failure [1–4]. In the past decade, the signaling cascades of numerous PKs and their partners in mediating pathological cardiac hypertrophy and heart failure have been extensively studied and reviewed from investigations performed in cellular models and in genetically modified mice (reviewed in [5–7]). However, proportionally, the role of PPs in the development of myocardial disorders has been much less documented [8, 9]. This is mostly due to historical reasons as PPs were discovered 10 years after PKs [10, 11]. Over the past decades however, it has become clear that PPs are specific regulators that play active roles in coordinating with PKs the regulation of many physiological processes. The PPs can be divided into two main groups, which are the protein serine/threonine phosphatases (PPPs) and the protein tyrosine phosphatases (PTPs). The PPPs constitute the majority of the PPs and target phosphoproteins on their serine and/or threonine residues. The three most documented PPPs in the heart are protein phosphatase 1 (PP1), protein phosphatase 2A (PP2A), and protein phosphatase 2B (PP2B) also known as calcineurin. Mechanisms by which PPPs regulate Ca2+ homeostasis and cardiac function are well documented in human and animal models of heart failure [12–14]. Since PPPs have been the topic of many excellent reviews, they will not be discussed in this review [9, 15, 16]. The PTPs dephosphorylate tyrosyl residues in proteins [17, 18] with the majority of PTPs being active enzymes and as abundant as protein tyrosine kinases (PTKs) [10]. Here, we summarize the role of the PTPs in heart development and cardiovascular diseases, particularly their effect on cardiac hypertrophy and how their dysregulation progresses to heart failure. Finally, we discuss the inhibitors of PTPs and their therapeutic potential for the treatment of heart disease in human.
Protein tyrosine phosphatase family and substrate specificity
In human, more than 100 genes encode for the PTPs among which 81 are active phosphatases [10, 19, 20]. The PTPs constitute a large family of enzymes, many of which harbor a transmembrane domain and a variable ectodomain (for review, see [21–23]). All PTPs share a common signature motif (HCXXGXXR) responsible for the enzyme activity. The PTP superfamily can be divided into four classes based on their cellular localization/catalytic domains: the receptor-like PTPs (rPTPs), the non-receptor PTPs (nrPTPs), the low molecular weight PTP (LMWPTP), and the VH-1 and CDC-25 groups [10, 24–27]. A different classification of PTPs exists based on their amino acid sequence and catalytic domain, which groups them into four classes (reviewed in [10]). Class I includes rPTPs and nrPTPs also known as “classical” pTyr-specific PTPs. These comprise 38 PTPs, and VH1-like “dual-specificity” PTPs (DUSPs) which are very divers with 61 members able to dephosphorylate pTyr and pSer/pThr residues. Class II PTP has a single member, the LMWPTP that targets substrates specifically on their Tyr residues. Class III PTPs include three “dual-specific” Cdc25 enzymes, while class IV is represented by Eya proteins with pTyr or dual pTyr/pSer activity.
Tyrosine phosphorylation process mediates most if not all cell signaling processes including growth, differentiation, survival, and death [28]. In the early 1990s, the PTPs were mainly considered as housekeepers or passive players in the cell [10, 11]. After considerable efforts in the field, it is now recognized that PTPs are critical regulators of cell signaling. Deregulation of their expression or activity can compromise cell physiology and hence lead to diseases [29]. The importance of PTPs in regulating signaling pathways was first illustrated by the discovery of CD25, (a DUSP) and SHP2, which can positively regulate signaling by increasing the phosphorylated level of a tyrosyl site of PTK [30–33]. PTPs act as important regulators of tyrosine phosphorylation in many cell types including cardiac cells [34, 35].
Role of PTPs in cardiac development and diseases
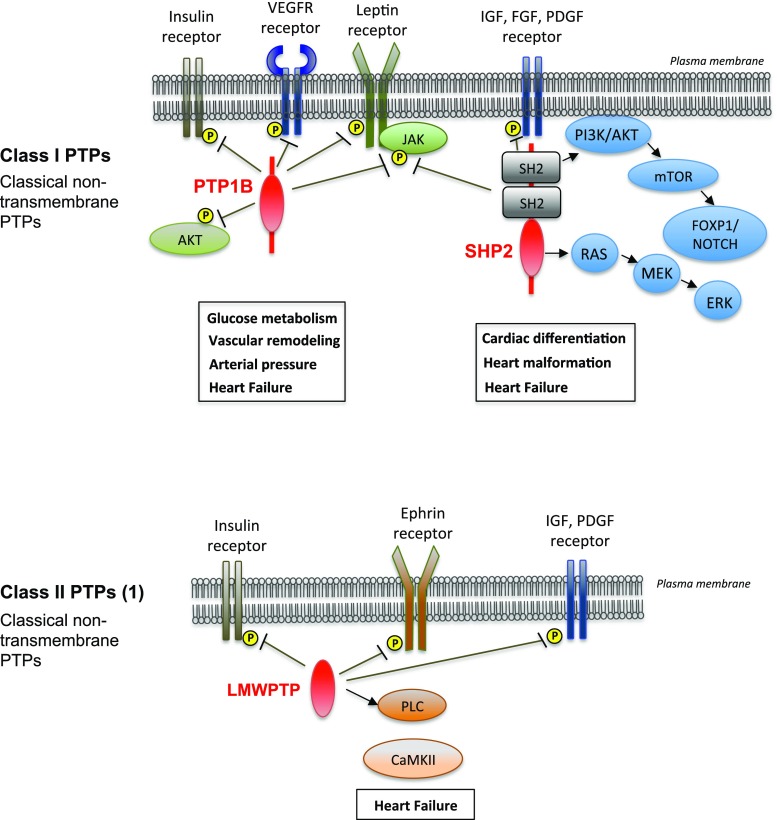
Out of the 107 human genes encoding PTPs, very few have been reported to have a role in the cardiovascular system. Up to this date, the PTPs implicated in cardiac development and disease include the protein-tyrosine phosphatase 1B (PTP1B), the Scr homology-2 (SH2) domain-bearing non-transmembrane protein tyrosine phosphatase (SHP2), and the LMWPTP (Fig. 1). Recent studies used genome-wide siRNA/shRNA screening and proteomics approach to identify novel roles and substrates of PTPs in human pathologies [36]. This powerful technology will be useful and adaptable for investigators to discover new PTPs implicated in human cardiovascular diseases. Below, we summarize mechanisms by which these PTPs regulate cardiac contractility and their role in heart development and disease (Table 1).
Fig. 1.
Protein tyrosine phosphatases (PTPs) playing a role in the cardiovascular system. Among the PTPs, three non-transmembrane PTPs have been described with roles in the cardiovascular system: protein tyrosine phosphatase 1B (PTP1B) and Scr-homology 2 domain containing phosphatase 2 (SHP2) belonging to class PTPs, and low molecular weight protein tyrosine phosphatase (LMWPTP), the sole member of class II PTPs
Table 1.
Summary of PTPs implicated in cardiac development and disease
| PTP | Animal model | Effect on the cardiovascular system | Comments | Reference |
|---|---|---|---|---|
| PTP1B (PTPN1 gene) | Global PTP1B deletion in Balb/c mice | PTP1B deficiency enhances the effects of leptin on arterial pressure | • Normal heart/body weight ratio, insulin, leptin, glucose, and triglyceride levels in fasting condition • Increased blood pressure at base line and after leptin infusion • Blunted response to phenylephrine • Reduction of aortic contraction after phenylephrine injection |
[37] |
| Global PTP1B deletion in Balb/c mice or PTP1B chemical inhibition | PTP1B deficiency protects against chronic heart failure after myocardial infarction | • No phenotype at baseline • Improvement of cardiac contractility and reduction of fibrosis and hypertrophy • Preserved endothelial function |
[38] | |
| Endothelial-specific deletion of PTP1B in mice | PTP1B deletion in endothelium improves angiogenesis and protects against pressure overload induced heart failure | • Improved systolic function and reduced cardiac hypertrophy of PTP1B-deficient mice • Preserved capillary density, reduced apoptosis and fibrosis • Improved cardiac VEGF signaling |
[39, 40] | |
| Global PTP1B deletion in Balb/c mice | PTP1B deletion enhances angiogenesis and arteriogenesis after myocardial infarction | • Increased capillary density • Increased VEGFR2 activation • Reduced diastolic dysfunction |
[41] | |
| SHP2 (PTPN11 gene) | Xenopus cardiac explants treated with the SPH2 inhibitor NSC-87877 | SHP2 is required for the maintenance of cardiac progenitor cells | • Reduction of MHC expression, lack of early cardiac markers and of and pharyngeal mesoderm in cardiac progenitors • SHP2 controls Ca2+ transient and oscillation in fibroblasts and in cardiac myocytes • SHP2 is positioned downstream of FGF |
[42] |
| Genetic deletion of SHP2 exon 3 in mice (SHP2Ex3−/−) | Gain-of-function/Noonan syndrome SHP2 mutants increase Ca2+ oscillations and impair NFAT signaling in fibroblasts and cardiomyocytes | • SHP2 is required for Ca2+ oscillations in response to FGF-2 in fibroblasts and cardiomyocytes • Gain-of-function SHP2 mutants disrupt the Ca2+ oscillatory control of NFAT |
[43] | |
| Cardiac specific deletion of SHP2 | SHP2 deletion in cardiac muscle causes dilated cardiomyopathy with no hypertrophy | • Defective ERK/MAPK activity and hyper-activation of Rho signaling after agonist treatment of cardiomyocytes and pressure overload | [44] | |
| Overexpression of Noonan syndrome SHP2-Q79R in mice | Congenital heart defects are rescued by ERK1/2 activation | • Impaired cycling activity, ventricular non-compaction, and septal defects • Activation of ERK1/2 |
[45] | |
| Muscle specific deletion of SHP2 | SHP2 deletion in skeletal muscle causes dilated cardiomyopathy, heart failure, and premature death | • Severe dilated cardiomyopathy resulting in heart failure and death • Associated with insulin resistance, glucose intolerance, and impaired glucose uptake in striated muscle cells • Upregulation of PI3K/Akt, ERK5, and STAT3 in cardiomyocytes |
[46] | |
| Knockin SHP2-Y279C mutation in mice | Recapitulation of LEOPARD syndrome by expression of the SHP2-Y279C mutation. Cardiomyopathy reversed by rapamycin treatment | • Increased binding of SHP2 to IRS1 • Decreased SHP2 catalytic activity, and blunted ERK/MAPK activation and increased AKT and mTOR after agonist treatment |
[18] | |
| Overexpression of SHP2-Q510E in mice | Early onset hypertrophic cardiomyopathy | • Impaired contractile function, thickening of the ventricular wall • Activation of AKT and mTOR • Rescue of cardiomyopathy by rapamycin treatment at the post-natal stage |
[3] | |
| Expression of LEOPARD and NOON SYNDROM mutations in zebrafish | Impaired early heart development and function | • Activation of MAPK in embryos expressing the mutations | [47] | |
| Knockin SHP2-Y279C mutation in mice | Expression of SHP2-Y279C causes developmental defects and adult onset HCM in mice originating from the endocardium | • Reduced trabeculation and valvular hyperplasia in embryonic hearts expressing SHP2-Y279C and in the heart of mice with endothelial-specific but not myocardial-specific SHP2-Y279C expression • Endothelial-specific expression of SHP2-Y279C induces cardiac hypertrophy in the adult heart • Myocardial-specific SHP2-Y279C expression causes ventricular septal defects • Abnormal AKT activity and decreased forkhead box P1 (FOXP1)/FGF and NOTCH1/EPHB2 signaling |
[48] | |
| LMWPTP (ACP1 gene) | Global deletion of ACP1 in mice | ACP1 deletion protects against pathological cardiac stress | • Attenuated fibrosis and preserved cardiac contractility • Increased IR phosphorylation, PKA, and Ephrin expression • Reduced CaMKII expression |
[4] |
PTP1B
The protein-tyrosine phosphatase 1B (PTP1B) is a non-transmembrane PTP with a wide tissue distribution and expressed mainly in the endoplasmic reticulum (ER) via its C-terminal domain [49]. PTP1B binds directly to PTK receptors including the insulin receptor (IR) and epidermal growth factor (EGF) receptor [50–53]. PTP1B is a key negative regulator of both insulin and leptin pathways ([54, 55] for review). Genetic deletion of PTP1B in mice results in insulin sensitivity and protects mice against high-fat diet-induced obesity [52, 56, 57]. High-fat diet-induced obesity increases the risk of hypertension and cardiovascular disorders, although the mechanism of action is unknown [58, 59]. Insulin resistance is strongly associated with oxidative stress, cardiac aging, and cardiomyocyte contractile dysfunction. Consistent with the effects of obesity and insulin on the cardiovascular system, PTP1B has emerged as a key regulator of obesity-induced cardiovascular disorders (recently reviewed in [39]).
Early studies using cardiac overexpression of antioxidant enzymes and dietary models documented reduced Akt expression and phosphorylation associated with increased PTP1B cardiac expression, suggesting a role of PTP1B in cardiac function [60, 61]. Enhancement of PTP1B was associated with impaired cardiac contractile and intracellular Ca2+ dysfunction [52, 60, 61]. As expected, PTP1B overexpression correlated with decreased phosphorylation of the IR (Tyr1146) and Akt after insulin stimulation in advanced cardiac aging and pre-diabetic insulin resistant hearts [60, 61]. Gomez and colleagues established the role of PTP1B in heart failure when they showed that mice with gene deletion or specific inhibition of PTP1B are protected against cardiac contractile dysfunction and heart failure after myocardial infarction. Improved heart function correlated with reduced fibrosis and hypertrophy while infarct size did not appear to change [38] (Table 1). In line with this study, recent findings showed that mice genetically lacking PTP1B resisted against chronic afterload-induced heart failure via a cardiac improvement of VEGF and angiogenesis signaling [39, 40]. Interestingly, eNOS, which modulates insulin secretion, was reduced in insulin resistant hearts or increased in PTP1B-deficient mouse hearts [38, 61]. Whether eNOS is directly associated with the insulin signaling is unclear, however the reduction of ROS generation alleviated insulin resistance-induced contractile dysfunction. PTP1B deletion enhanced capillary density and myocardial perfusion in mice 8-day post-myocardial infarction associated with increased VEGFR2 activity, although no reduction of infarct size was observed. This suggests that the beneficial effect of PTP1B ablation is mostly due to improved vascular remodeling possibly through nitric oxide (NO) [41]. Consistent with this, Panzhinskiy and collaborators reported that ER stress activates PTP1B via ROS-NFkB signaling resulting in insulin resistant in skeletal muscles under high-fat diet condition [52]. Also, endothelial dysfunction associated with diabetes did not occur in PTP1B-deficient mice mostly due to increased cyclooxygenase 2 expression [62]. Collectively, these studies are consistent with a protective role of PTP1B deletion against pathological cardiac and vascular remodeling. This is in contrast with the study by Belin de Chantemele and colleagues, where PTP1B knockout mice exhibited high blood pressure in response to leptin infusion [37]. This study indicates that PTP1B is a modulator of cardiovascular function through its capacity to negatively regulate leptin-induced hypertension possibly through the sympathetic nervous system. Overall, there is clear-cut evidence for a role of PTP1B in heart physiology and pathophysiology. More investigations are needed to define the underlying mechanisms by which PTP1B affects cardiovascular function. In this regard, a signaling of interest not yet elucidated is the alteration of Ca2+ handling through modulation of SERCA2a and NCX1 by PTP1B in the heart. Dysregulation of the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA2a) and NCX1 expression impair cardiac contractility. In cancer, targeting of Ca2+ signaling has been proposed as an alternative therapy to treat human cancer patients [63]. The use of animal models lacking or overexpressing PTP1B and cellular models with genetic siRNA targeting of PTP1B could be a starting point to decipher the SERCA2a-NCX1-PTP1B axis in altered Ca2+ signaling-driven heart failure.
SHP2
The Scr homology-2 (SH2) domain-bearing non-transmembrane protein tyrosine phosphatase SHP2 also known as protein-tyrosine phosphatase non-receptor type 11 (PTPN11) or protein tyrosine phosphatase 2C (PTP-2C) is encoded by the PTPN11 gene. SHP2 is ubiquitously expressed [64] and is important for the full activation of downstream partners (Ras/ERK/MEK) for most if not all the PTKs and cytokine receptors. SHP2 regulates important cellular events including differentiation, proliferation, and survival [64]. Key signaling pathways affected by SHP2 dysregulation include ERK1/2, insulin, AKT/GSK-3β, and mTOR pathways [3, 18, 44–46, 65] (Table 1). Not surprisingly, aberrant expression of SHP2 or changes within SHP2 activity are associated with human diseases and experimental animal models.
SHP2 is a key PTP required for early development. To avoid the embryonic lethality associated with SHP2 ablation, the role of SHP2 in early heart development was first addressed using Xenopus cardiac explants treated with the SHP-2-specific inhibitor NSC-87877 [42]. Results showed a reduction of myosin heavy chain expression, a lack of early cardiac markers of differentiation and of pharyngeal mesoderm. SHP2 interacted with FRS2 and that effect was associated with increased phosphorylation of SHP2 at both tyrosine 542 and 580. Collectively, this study positioned SHP2 downstream of FGF and showed that SHP2 is required for the maintenance of cardiac progenitors and survival in Xenopus embryonic hearts. Additional studies support the direct role of SHP2 in cell development and survival through the FGF signaling pathway [42, 66, 67]. Deletion of SHP2 in skeletal and cardiac muscle also causes cardiac dysfunction leading to dilated cardiomyopathy and premature death [46].
Germline mutations in SHP2 cause Noonan syndrome (NS) in human. This relatively common condition affects 1 in 1000–2000 children born with heart malformations including pulmonary valvular stenosis, septal defect, hypertrophic cardiomyopathy, and also abnormal facial characteristics and developmental delays [68, 69]. SHP2 mutations are also implicated in NS with multiple lentigines known as LEOPARD syndrome (LS). This rare genetic condition is associated with congenital heart malformations and also sensorineural deafness, growth retardation, and skin, craniofacial, and genital abnormalities [70, 71] (for reviews). Although NS and LS share common clinical features, SHP2 mutations are activating in NS due to increased phosphatase activity and inactivating in LS because of an inhibition of the catalytic activity of the phosphatase [72, 73]. However, while the majority of NS mutations have a gain-of-function phenotype, there is also documentation that LS-causing mutations reduce SHP2 phosphatase activity but prolong substrate turnover to produce a loss-of-function phenotype [74].
The effects of NS- and LS-associated SHP2 mutations on cardiac morphogenesis can be recapitulated in mice and are due to increased MAPK signaling. Indeed, independent investigations demonstrated that both Q79R SHP2 gain of function and lack of SHP2-induced hyperactivation of ERK1/2 and RhoA signaling, leading to impaired heart function and dilated cardiomyopathy [44, 45]. Expression of the LS mutant Q510E causing severe hypertrophic cardiomyopathy in infants inhibits the differentiation of P19CL6 cells in cardiomyocytes mostly due to increased Akt/GSK3β/β-catenin activity [3, 18, 44–46, 65], and induces hypertrophic cardiomyopathy in mice through mTOR pathway [3].
Gain-of-function SHP2 mutants (R465M, E76A, D61G) enhanced Ca2+ response in cardiomyocytes through RTKs mediated Ca2+ signaling pathway but not upon activation of G protein-coupled receptor [43], further supporting the requirement of SHP2 in the activation of most RTK signaling. Consistent with these findings, more recent data indicate that NS and LS SHP2 variants significantly enhanced ERK activity, which partly mediated defective early cardiac development in zebrafish [47]. Furthermore, expression of Shp2-Y279C, a mutation causing LS in human, recapitulated the phenotypic abnormalities seen in LS patients with signs of hypertrophic cardiomyopathy progressing to dilated cardiomyopathy and enhanced interaction of Shp2 with IRS1, and increased Akt/mTOR activity. These cardiac defects were totally reversed by treatment with the mTOR inhibitor rapamycin [18]. The developmental defects and adult-onset hypertrophic cardiomyopathy in Shp2-Y279C mutant mice correlated with increased AKT activity, inhibition of FOXP1/NOTCH1 pathways, and upregulation of NFAT activity. Dysregulated signaling originated from the endocardium indicating a reciprocal cross-talk between the endocardium and the myocardium, which is essential for heart development [48]. Such non-cell-autonomous mechanism was also triggered by overexpression of the related transcription enhancer factor-1 RTEF1 in endothelial cells, which induced cardiac hypertrophy in response to aortic constriction through an increase of VEGFB protein level [75]. Although studies of Lauriol and colleagues did not reveal enhanced expression of VEGFB mRNA level, they did not exclude a possible implication of SHP2 in NSML cardiac hypertrophy that employs this RTEF1-VEGF signaling mechanism [48].
LMWPTP
LMWPTP is a class 2 cys-based PTP encoded by the ACP1 gene located on the short arm of chromosome 2 (2p25) in the human genome and widely distributed within various tissues and organs including the heart [10, 76, 77]. LMWPTP controls a number of essential processes in mammalian cell physiology [10]. This 18 kDa protein tyrosine phosphatase has three isoforms generated by alternative splicing. The first two isoforms produce functional proteins while the third isoform is considered a pseudogene [76, 78–80]. A decrease of both LMWPTP isoforms leads to increase in phosphorylation of the insulin receptor, Akt and PI3-K activity in the liver [81].
LMWPTP has five tyrosine residues and can be phosphorylated by tyrosine kinases such as V-src, Lck, and Fyn. Depending on the tyrosine residue phosphorylated, LMWPTP has different phenotypes. The phosphorylation of Tyr131 residue leads to a 25-fold increase of LMWPTP level while the phosphorylation of Tyr132 mediates the recruitment of Grb2 protein [82]. On the other hand, like other PTPs, LMWPTP can be dephosphorylated by tyrosine phosphatases, and can by itself dephosphorylate various tyrosine kinases and their respective substrates [24, 76].
Studies suggested that its overexpression causes increased dephosphorylation of phosphotyrosine, which may repress tyrosine kinase oncogene malignant transformation and growth factor receptor signaling [10]. LMWPTP also modulates the JAK-STAT pathway by binding and dephosphorylating STAT5 [83]. Furthermore, LMWPTP oxidation prevents dephosphorylation and inactivation of STAT2 and JAK5 [83]. In addition, LMWPTP is regulated by ROS mediated oxidation [84]. LMWPTP tyrosine residues can be oxidized by exogenous oxidative stress by glucose oxidase or sodium pervanadate in vivo [24]. Alteration of LMWPTP levels causes a reduction in enzymatic binding, glycolysis, and erythrocyte plasticity in T cell signaling [76]. Furthermore, an increase in LMWPTP levels was associated with protection against several conditions such as allergy, asthma, and abortion [76].
LMWPTP also acts as a negative regulator of EphA2 tyrosine phosphorylation, which regulates tumor cell growth and survival [83, 85]. LMWPTP affects cellular proliferation through reduction of FGFR tyrosine phosphorylation [24, 82, 86] and modulates PDGF expression through its phosphatase activity [84]. Overexpression of LMWPTP markedly decreases cell growth rate as a secondary effect of PDGF reduction [24]. LMWPTP also acts as a negative regulator of insulin signaling as shown by the improved glucose and insulin tolerance of diet-induced obese mice injected with an LMWPTP antisense oligonucleotide [81].
Examination of genetic variations in the ACP1 gene revealed the presence of single-nucleotide polymorphisms (SNPs) that alter the enzyme activity and the ratio of the two main protein isoforms LMPTP-A and LMPTP-B [87]. Association studies in various populations indicated a role of ACP1 in metabolic syndrome and coronary artery disease [88–91]. In particular, the hypertrophic response of the myocardial wall is regulated by LMWPTP through activation of growth factors such as PDGF, IGF1, and insulin [76]. Moreover, increased LMWPTP activity reduces the metabolic rate and subsequently enhanced the demand of the hypertrophic response [76]. Consistent with this report, deletion of LMWPTP in mice confers a cardio-protective phenotype after long-term pressure overload hypertrophy [4]. The striking reduction of fibrosis and sustained cardiac function of LMWPTP knockout mice subjected to pathological stress are associated with upregulation of fetal cardiac genes, increased insulin receptor phosphorylation, and inactivation of Gαq/11/PLCβ/CaMKII pathways. LMWPTP levels are also high in the fetal murine heart, reduced in the post-natal heart, and increased in patients with end-stage heart failure indicating that LMWPTP is a positive regulator of pathological cardiac hypertrophy [4].
PTP inhibitors as potential new therapeutic for cardiac diseases
PTPs play a central role in modulating physiological cardiac development [35]. Hyper-activation of the catalytic domain of PTPs initiates cardiomyopathy through the deregulation of cellular processes like proliferation [43]. Therefore, the implication of PTPs in the development of cardiomyopathies supports PTPs as potential targets for signaling-based therapeutics for these diseases. Two decades ago, vanadate and pervanadate were widely used to inhibit PTPs including PTP1B [92] (Table 2). This inhibition revealed potent and selective to PTPs but unfortunately non-specific. Subsequently, several small molecules targeting PTPs have been developed or tested with the hope to treat human pathologies including heart failure, diabetes, and cancers. Among those, vanadyl sulfate protected against ischemia-reperfusion in rats via increased FLIP and decreased Fas ligand and Bim expression secondary to AKT activation [93]. However, when tested in diabetic patients, vanadyl sulfate altered the expression of proteins involved in early insulin signaling in skeletal muscle, with no effect on protein phosphatase activity [94]. Endothelial dysfunction of peripheral arteries after short-term ischemia-induced heart failure was improved in mice after administration of AS279, AS098, and AS713 PTP1B inhibitors [95]. Compound inhibitors of PTP1B have being developed in academic laboratories and by the pharmaceutical industry and tested in animal models of obesity. Some like trodusquemine and ertiprotafib have reached phase 2 clinical trials to treat obesity and diabetes, although ertiprotafib was discontinued for lack of efficacy [55, 99]. It remains to be seen whether PTP1B inhibition has long-term beneficial effects for the treatment of cardiovascular disorders such as congestive heart failure.
Table 2.
Inhibitors of PTPs with effects in the cardiovascular system
| Inhibitor | Targeted PTP | Effect on cardiovascular system | Model | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|---|
| Vanadate | PTPs | • Selective | • Non-specific | [92] | ||
| Vanadyl sulfate | PTPs | Cardioprotection against ischemia reperfusion | • Coronary occlusion in rats | • Reduction of infarct size and of left ventricular end diastolic pressure • Improvement of left ventricular developed pressure and contractility • Inhibition of apoptosis • Increased FLIP expression and decreased Fas ligand and Bim expression via activation of Akt |
• Gastrointestinal side effects | [38, 93] |
| Vanadyl sulfate | PTPs | Alteration of proteins involved in early insulin signaling in skeletal muscle. No global change in protein phosphatase activity | • Human with diabetes mellitus | • Increased basal levels of IR, Shc, and IRS-1 tyrosine phosphorylation and IRS-1 • No effect on insulin |
• Gastrointestinal side effects at 150 and 300 mg | [94] |
| AS279, AS098, AS713 | PTP1B and SHP2 | Improved endothelial dysfunction in peripheral mesenteric arteries | • Mice with coronary ligation | • Selective inhibitors • Transient early eNOS phosphorylation and AKT phosphorylation |
• Relatively good selectivity | [95] |
| AS279 | PTP1B | Protects against chronic heart failure after myocardial infarction | • Mice | • Reduced adverse ventricular remodeling • Improved LV function and cardiac contractility • Reduced fibrosis and cardiac hypertrophy • Preserved endothelial function • No effect on glucose level |
[38] | |
| NSC-87877 | SHP2 | SHP2 is required for the maintenance of cardiac progenitor cells | • Xenopus heart explants | • Reduction of MHC expression, lack of early cardiac markers and of and pharyngeal mesoderm in cardiac progenitors • SHP2 controls Ca2+ transient and oscillation in fibroblasts and in cardiac myocytes • SHP2 is positioned downstream of FGF |
[42] | |
| PHPS1 | SHP2 | Inhibits cardiac hypertrophy induced by SHP2-Q510E and SHP2-Y279C mutations | • Cardiomyocytes and mice | • Normalization of the size of cardiomyocytes expressing SHP2-Q510A and SHP2-Y279C • Restoration of AKT and mTOR levels |
• Specificity | [96] |
| Chromones | LMWPTP and PTP1B | • High activity | [97] | |||
| Compound 23 | LMWPTP | Small-molecule inhibitor reverses high fat diet-induced obesity | • Mice | • Improves glucose tolerance • Increases IR phosphorylation in the liver • Orally bioavailable • Uncompetitive mode of action • High selectivity |
• Not reported | [98] |
Chromones, which are derivatives of benzopyran, are a class of highly active inhibitors of LMWPTP. They also have selective inhibition towards PTP1B [97]. The findings that LMWPTP plays a role in cancer and heart failure and alters insulin signaling prompted interest to develop inhibitors of LMWPTP with high activity. A series of compounds were discovered which inhibit LMWPTP and also PTP1B [97]. Recently, several novel small-molecule inhibitors of LMWPTP with potency and strong selectivity were discovered [100]. Also, a non-competitive small-molecule inhibitor of LMWPTP with high selectivity over other phosphatase with the ability to reverse high-fat diet-induced obesity has been reported [98]. This new inhibitor is viewed as promising for the treatment of human diseases including type 2 diabetes and heart failure. Numerous efforts have been made to identify inhibitors of SHP2 since missense mutations cause NS and LS in human. High-throughput screening identified a small molecular weight compound PHPS1 with high specificity and cell permeability [101]. PHPS1 inhibits SHP2 catalytic domain and was found to prevent the hypertrophic effect of mutant SHP2-Q510E in isolated cardiomyocytes and in mice expressing the mutant protein [96, 101]. Since the SHP2-Q510E mutation causes an aggressive form of LS in human, it would be of interest to test whether the PHPS1 inhibitor confers cardioprotective effects in clinical settings. Compound inhibitors of SHP2 have also been identified using a computational approach [102]. One compound #220–324 proved efficient in inhibiting SHP2-mediated signaling and proliferation of cancer cells, but whether it is an ideal inhibitor to treat the cardiomyopathy associated with SHP2 mutations remains to be seen.
Challenges with PTP inhibitors
Although recent advances are encouraging, the use of PTP inhibitors faced significant technical challenges in the early days. These were mostly due to the lack of PTP inhibitors with both specificity and strong binding activity. This was in part due to the small size of PTPs in addition to sharing a common catalytic signature motif (HCXXGXXR) responsible for the enzyme activity. For instance, NSC-87877 inhibited both Shp2 and Shp1 in vitro with the same efficacy [103]. Another important challenge still remaining today is the need to optimize and develop safer PTP inhibitors for heart failure treatment avoiding undesirable effects, in particular, cardiotoxicity. Thus, solving these technical challenges is critical before PTP inhibitors enter extensive preclinical trials to treat human heart failure.
Complementary concepts from computer-aided drug design (CADD) to 3D QSAR/structure-based design have been used in experimental assays to identify potential drugs targeting the catalytic pocket of PTPs, ligand binding, and conformational change during inhibitor interactions [104–106]. Information on the 3D crystal structure on PTPs is a key step for in silico screening of abundant inhibitors available in databases. SHP2 and PTP-1B are shown to be “druggable” molecules for treating cardiac diseases and other pathologies. However, few 3D crystal structure PTPs are currently available, thus making the computer search approach to find PTP inhibitors difficult [107–109]. PTP-targeted therapy is still in its early phase, although novel drugs are emerging for cancer treatment [75, 110, 111]. Further testing is needed to determine whether these new compounds can be used for pharmacological treatment of cardiomyopathies.
Conclusion
Growing experimental data support the role of PTPs as activators of cardiac diseases that operate through different mechanisms. This extensive work led to consider PTP family as drug targets to treat the diverse forms of cardiomyopathies. Few PTP inhibitors are known compared to the number of existing compounds for the treatment of such diseases. With the discovery of new compounds based on new screening strategies combined with the information on the 3D crystal structure on PTPs, one hopes that the design of drugs targeting PTPs will open a door of opportunity to treat human heart failure.
Acknowledgments
This work was supported by a grant from King Abdulaziz City for Science & Technology (10-BIO 1347-20) awarded to CP.
List of abbreviations
- AKT
Protein kinase B
- CaMK
Ca2+/calmodulin-dependent protein kinase
- eNOS
Endothelial nitric oxide synthase
- ERK
Extracellular signal-regulated kinase
- FGFR
Fibroblast growth factor receptor
- FOXP1
Forkhead box protein P1
- FRS2
Fibroblast growth factor receptor substrate 2
- GSK-3
Glycogen synthase kinase 3
- HCM
Hypertrophic cardiomyopathy
- JAK2
Janus kinase 2
- JAK-STAT
Janus kinase/signal transducers and activators of transcription
- mTOR
Mammalian target of rapamycin
- NFAT
Nuclear factor of activated T cells
- NFkB
Nuclear factor-kappa B
- NOTCH1
Notch homolog 1, translocation-associated (Drosophila)
- P19CL6
Clone of the P19 mouse embryonal carcinoma cell line
- PLC
Phospholipase C
- PI3-K
Phosphoinositide 3-kinase
- PDGF
Platelet-derived growth factor
- RhoA
Ras homolog gene family, member A
- ROS
Reactive oxygen species
- RTKs
Receptor tyrosine kinases
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Oudit GY, Kassiri Z, Zhou J, Liu QC, Liu PP, Backx PH, Dawood F, Crackower MA, Scholey JW, Penninger JM. Loss of PTEN attenuates the development of pathological hypertrophy and heart failure in response to biomechanical stress. Cardiovasc Res. 2008;78(3):505–514. doi: 10.1093/cvr/cvn041. [DOI] [PubMed] [Google Scholar]
- 2.Shen YH, Zhang L, Gan Y, Wang X, Wang J, LeMaire SA, Coselli JS, Wang XL. Up-regulation of PTEN (phosphatase and tensin homolog deleted on chromosome ten) mediates p38 MAPK stress signal-induced inhibition of insulin signaling. A cross-talk between stress signaling and insulin signaling in resistin-treated human endothelial cells. J Biol Chem. 2006;281(12):7727–7736. doi: 10.1074/jbc.M511105200. [DOI] [PubMed] [Google Scholar]
- 3.Schramm C, Fine DM, Edwards MA, Reeb AN, Krenz M. The PTPN11 loss-of-function mutation Q510E-Shp2 causes hypertrophic cardiomyopathy by dysregulating mTOR signaling. Am J Phys Heart Circ Phys. 2012;302(1):H231–H243. doi: 10.1152/ajpheart.00665.2011. [DOI] [PubMed] [Google Scholar]
- 4.Wade F, Quijada P, Al-Haffar KM, et al. Deletion of low molecular weight protein tyrosine phosphatase (Acp1) protects against stress-induced cardiomyopathy. J Pathol. 2015;237(4):482–494. doi: 10.1002/path.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y. Mitogen-activated protein kinases in heart development and diseases. Circulation. 2007;116(12):1413–1423. doi: 10.1161/CIRCULATIONAHA.106.679589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swaminathan PD, Purohit A, Hund TJ, Anderson ME. Calmodulin-dependent protein kinase II: linking heart failure and arrhythmias. Circ Res. 2012;110(12):1661–1677. doi: 10.1161/CIRCRESAHA.111.243956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhalla NS, Muller AL. Protein kinases as drug development targets for heart disease therapy. Pharmaceuticals. 2010;3(7):2111–2145. doi: 10.3390/ph3072111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicolaou P, Kranias EG. Role of PP1 in the regulation of Ca cycling in cardiac physiology and pathophysiology. Front Biosci (Landmark Ed) 2009;14:3571–3585. doi: 10.2741/3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heijman J, Dewenter M, El-Armouche A, Dobrev D. Function and regulation of serine/threonine phosphatases in the healthy and diseased heart. J Mol Cell Cardiol. 2013;64:90–98. doi: 10.1016/j.yjmcc.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T. Protein tyrosine phosphatases in the human genome. Cell. 2004;117(6):699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Tonks NK. Protein tyrosine phosphatases--from housekeeping enzymes to master regulators of signal transduction. FEBS J. 2013;280(2):346–378. doi: 10.1111/febs.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugano Y, Lai NC, Gao MH, Firth AL, Yuan JXJ, Lew WYW, Hammond HK. Activated expression of cardiac adenylyl cyclase 6 reduces dilation and dysfunction of the pressure-overloaded heart. Biochem Biophys Res Commun. 2011;405(3):349–355. doi: 10.1016/j.bbrc.2010.12.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qian J, Vafiadaki E, Florea SM, Singh VP, Song W, Lam CK, Wang Y, Yuan Q, Pritchard TJ, Cai W, Haghighi K, Rodriguez P, Wang HS, Sanoudou D, Fan GC, Kranias EG. Small heat shock protein 20 interacts with protein phosphatase-1 and enhances sarcoplasmic reticulum calcium cycling. Circ Res. 2011;108(12):1429–1438. doi: 10.1161/CIRCRESAHA.110.237644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh JG, Kim J, Jang SP, Nguen M, Yang DK, Jeong D, Park ZY, Park SG, Hajjar RJ, Park WJ. Decoy peptides targeted to protein phosphatase 1 inhibit dephosphorylation of phospholamban in cardiomyocytes. J Mol Cell Cardiol. 2013;56:63–71. doi: 10.1016/j.yjmcc.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Fan WJ, van Vuuren D, Genade S, et al. Kinases and phosphatases in ischaemic preconditioning: a re-evaluation. Basic Res Cardiol. 2010;105(4):495–511. doi: 10.1007/s00395-010-0086-3. [DOI] [PubMed] [Google Scholar]
- 16.Wittkopper K, Dobrev D, Eschenhagen T, El-armouche A. Phosphatase-1 inhibitor-1 in physiological and pathological beta-adrenoceptor signalling. Cardiovasc Res. 2011;91(3):392–401. doi: 10.1093/cvr/cvr058. [DOI] [PubMed] [Google Scholar]
- 17.Cohen P. The origins of protein phosphorylation. Nat Cell Biol. 2002;4(5):E127–E130. doi: 10.1038/ncb0502-e127. [DOI] [PubMed] [Google Scholar]
- 18.Marin TM, Keith K, Davies B, Conner DA, Guha P, Kalaitzidis D, Wu X, Lauriol J, Wang B, Bauer M, Bronson R, Franchini KG, Neel BG, Kontaridis MI. Rapamycin reverses hypertrophic cardiomyopathy in a mouse model of LEOPARD syndrome-associated PTPN11 mutation. J Clin Invest. 2011;121(3):1026–1043. doi: 10.1172/JCI44972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7(11):833–846. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- 20.Sacco F, Perfetto L, Castagnoli L, Cesareni G. The human phosphatase interactome: an intricate family portrait. FEBS Lett. 2012;586(17):2732–2739. doi: 10.1016/j.febslet.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoker AW. Protein tyrosine phosphatases and signalling. J Endocrinol. 2005;185(1):19–33. doi: 10.1677/joe.1.06069. [DOI] [PubMed] [Google Scholar]
- 22.Kappert K, Peters KG, Bohmer FD, et al. Tyrosine phosphatases in vessel wall signaling. Cardiovasc Res. 2005;65(3):587–598. doi: 10.1016/j.cardiores.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 23.Senis YA. Protein-tyrosine phosphatases: a new frontier in platelet signal transduction. J Thromb Haemost: JTH. 2013;11(10):1800–1813. doi: 10.1111/jth.12359. [DOI] [PubMed] [Google Scholar]
- 24.Raugei G, Ramponi G, Chiarugi P. Low molecular weight protein tyrosine phosphatases: small, but smart. Cell Mol Life Sci: CMLS. 2002;59(6):941–949. doi: 10.1007/s00018-002-8481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paul S, Lombroso PJ. Receptor and nonreceptor protein tyrosine phosphatases in the nervous system. Cell Mol Life Sci: CMLS. 2003;60(11):2465–2482. doi: 10.1007/s00018-003-3123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boutros R, Lobjois V, Ducommun B. CDC25 phosphatases in cancer cells: key players? Good targets? Nat Rev Cancer. 2007;7(7):495–507. doi: 10.1038/nrc2169. [DOI] [PubMed] [Google Scholar]
- 27.Karisch R, Fernandez M, Taylor P, Virtanen C, St-Germain JR, Jin LL, Harris IS, Mori J, Mak TW, Senis YA, Östman A, Moran MF, Neel BG. Global proteomic assessment of the classical protein-tyrosine phosphatome and “Redoxome”. Cell. 2011;146(5):826–840. doi: 10.1016/j.cell.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graves JD, Krebs EG. Protein phosphorylation and signal transduction. Pharmacol Ther. 1999;82(2-3):111–121. doi: 10.1016/s0163-7258(98)00056-4. [DOI] [PubMed] [Google Scholar]
- 29.Ostman A, Hellberg C, Bohmer FD. Protein-tyrosine phosphatases and cancer. Nat Rev Cancer. 2006;6(4):307–320. doi: 10.1038/nrc1837. [DOI] [PubMed] [Google Scholar]
- 30.Koretzky GA, Picus J, Thomas ML, Weiss A. Tyrosine phosphatase CD45 is essential for coupling T-cell antigen receptor to the phosphatidyl inositol pathway. Nature. 1990;346(6279):66–68. doi: 10.1038/346066a0. [DOI] [PubMed] [Google Scholar]
- 31.Ostergaard HL, Shackelford DA, Hurley TR, Johnson P, Hyman R, Sefton BM, Trowbridge IS. Expression of CD45 alters phosphorylation of the lck-encoded tyrosine protein kinase in murine lymphoma T-cell lines. Proc Natl Acad Sci U S A. 1989;86(22):8959–8963. doi: 10.1073/pnas.86.22.8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas ML. The regulation of antigen-receptor signaling by protein tyrosine phosphatases: a hole in the story. Curr Opin Immunol. 1999;11(3):270–276. doi: 10.1016/s0952-7915(99)80044-2. [DOI] [PubMed] [Google Scholar]
- 33.Hanafusa H, Torii S, Yasunaga T, Matsumoto K, Nishida E. Shp2, an SH2-containing protein-tyrosine phosphatase, positively regulates receptor tyrosine kinase signaling by dephosphorylating and inactivating the inhibitor Sprouty. J Biol Chem. 2004;279(22):22992–22995. doi: 10.1074/jbc.M312498200. [DOI] [PubMed] [Google Scholar]
- 34.Mustelin T, Alonso A, Bottini N, Huynh H, Rahmouni S, Nika K, Louis-dit-Sully C, Tautz L, Togo SH, Bruckner S, Mena-Duran AV, al-Khouri AM. Protein tyrosine phosphatases in T cell physiology. Mol Immunol. 2004;41(6-7):687–700. doi: 10.1016/j.molimm.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 35.Lauriol J, Jaffre F, Kontaridis MI. The role of the protein tyrosine phosphatase SHP2 in cardiac development and disease. Semin Cell Dev Biol. 2015;37:73–81. doi: 10.1016/j.semcdb.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee H, Yi JS, Lawan A, Min K, Bennett AM. Mining the function of protein tyrosine phosphatases in health and disease. Semin Cell Dev Biol. 2015;37:66–72. doi: 10.1016/j.semcdb.2014.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belin de Chantemele EJ, Muta K, Mintz J, Tremblay ML, Marrero MB, Fulton DJ, Stepp DW. Protein tyrosine phosphatase 1B, a major regulator of leptin-mediated control of cardiovascular function. Circulation. 2009;120(9):753–763. doi: 10.1161/CIRCULATIONAHA.109.853077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomez E, Vercauteren M, Kurtz B, Ouvrard-Pascaud A, Mulder P, Henry JP, Besnier M, Waget A, Hooft van Huijsduijnen R, Tremblay ML, Burcelin R, Thuillez C, Richard V. Reduction of heart failure by pharmacological inhibition or gene deletion of protein tyrosine phosphatase 1B. J Mol Cell Cardiol. 2012;52(6):1257–1264. doi: 10.1016/j.yjmcc.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Thiebaut PA, Besnier M, Gomez E, Richard V. Role of protein tyrosine phosphatase 1B in cardiovascular diseases. J Mol Cell Cardiol. 2016;101:50–57. doi: 10.1016/j.yjmcc.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Gogiraju R, Schroeter MR, Bochenek ML, Hubert A, Münzel T, Hasenfuss G, Schäfer K. Endothelial deletion of protein tyrosine phosphatase-1B protects against pressure overload-induced heart failure in mice. Cardiovasc Res. 2016;111(3):204–216. doi: 10.1093/cvr/cvw101. [DOI] [PubMed] [Google Scholar]
- 41.Besnier M, Galaup A, Nicol L, Henry JP, Coquerel D, Gueret A, Mulder P, Brakenhielm E, Thuillez C, Germain S, Richard V, Ouvrard-Pascaud A. Enhanced angiogenesis and increased cardiac perfusion after myocardial infarction in protein tyrosine phosphatase 1B-deficient mice. FASEB J: Off Publ Fed Am Soc Exp Biol. 2014;28(8):3351–3361. doi: 10.1096/fj.13-245753. [DOI] [PubMed] [Google Scholar]
- 42.Langdon YG, Goetz SC, Berg AE, Swanik JT, Conlon FL. SHP-2 is required for the maintenance of cardiac progenitors. Development. 2007;134(22):4119–4130. doi: 10.1242/dev.009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uhlen P, Burch PM, Zito CI, et al. Gain-of-function/Noonan syndrome SHP-2/Ptpn11 mutants enhance calcium oscillations and impair NFAT signaling. Proc Natl Acad Sci U S A. 2006;103(7):2160–2165. doi: 10.1073/pnas.0510876103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kontaridis MI, Yang W, Bence KK, Cullen D, Wang B, Bodyak N, Ke Q, Hinek A, Kang PM, Liao R, Neel BG. Deletion of Ptpn11 (Shp2) in cardiomyocytes causes dilated cardiomyopathy via effects on the extracellular signal-regulated kinase/mitogen-activated protein kinase and RhoA signaling pathways. Circulation. 2008;117(11):1423–1435. doi: 10.1161/CIRCULATIONAHA.107.728865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakamura T, Colbert M, Krenz M, Molkentin JD, Hahn HS, Dorn GW, II, Robbins J. Mediating ERK 1/2 signaling rescues congenital heart defects in a mouse model of Noonan syndrome. J Clin Invest. 2007;117(8):2123–2132. doi: 10.1172/JCI30756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Princen F, Bard E, Sheikh F, Zhang SS, Wang J, Zago WM, Wu D, Trelles RD, Bailly-Maitre B, Kahn CR, Chen Y, Reed JC, Tong GG, Mercola M, Chen J, Feng GS. Deletion of Shp2 tyrosine phosphatase in muscle leads to dilated cardiomyopathy, insulin resistance, and premature death. Mol Cell Biol. 2009;29(2):378–388. doi: 10.1128/MCB.01661-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonetti M, Paardekooper Overman J, Tessadori F, Noël E, Bakkers J, den Hertog J. Noonan and LEOPARD syndrome Shp2 variants induce heart displacement defects in zebrafish. Development. 2014;141(9):1961–1970. doi: 10.1242/dev.106310. [DOI] [PubMed] [Google Scholar]
- 48.Lauriol J, Cabrera JR, Roy A, Keith K, Hough SM, Damilano F, Wang B, Segarra GC, Flessa ME, Miller LE, Das S, Bronson R, Lee KH, Kontaridis MI. Developmental SHP2 dysfunction underlies cardiac hypertrophy in Noonan syndrome with multiple lentigines. J Clin Invest. 2016;126(8):2989–3005. doi: 10.1172/JCI80396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frangioni JV, Beahm PH, Shifrin V, Jost CA, Neel BG. The nontransmembrane tyrosine phosphatase PTP-1B localizes to the endoplasmic reticulum via its 35 amino acid C-terminal sequence. Cell. 1992;68(3):545–560. doi: 10.1016/0092-8674(92)90190-n. [DOI] [PubMed] [Google Scholar]
- 50.Salmeen A, Andersen JN, Myers MP, Tonks NK, Barford D. Molecular basis for the dephosphorylation of the activation segment of the insulin receptor by protein tyrosine phosphatase 1B. Mol Cell. 2000;6(6):1401–1412. doi: 10.1016/s1097-2765(00)00137-4. [DOI] [PubMed] [Google Scholar]
- 51.Egawa K, Maegawa H, Shimizu S, Morino K, Nishio Y, Bryer-Ash M, Cheung AT, Kolls JK, Kikkawa R, Kashiwagi A. Protein-tyrosine phosphatase-1B negatively regulates insulin signaling in l6 myocytes and Fao hepatoma cells. J Biol Chem. 2001;276(13):10207–10211. doi: 10.1074/jbc.M009489200. [DOI] [PubMed] [Google Scholar]
- 52.Panzhinskiy E, Ren J, Nair S. Protein tyrosine phosphatase 1B and insulin resistance: role of endoplasmic reticulum stress/reactive oxygen species/nuclear factor kappa B axis. PLoS One. 2013;8(10):e77228. doi: 10.1371/journal.pone.0077228. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Feizi S. Protein tyrosine phosphatase-1B (PTP1B) regulates EGF-induced stimulation of corneal endothelial cell proliferation. J Ophthalmic Vis Res. 2009;4(2):127–128. [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng A, Dube N, Gu F, et al. Coordinated action of protein tyrosine phosphatases in insulin signal transduction. Eur J Biochem. 2002;269(4):1050–1059. doi: 10.1046/j.0014-2956.2002.02756.x. [DOI] [PubMed] [Google Scholar]
- 55.Cho H. Protein tyrosine phosphatase 1B (PTP1B) and obesity. Vitam Horm. 2013;91:405–424. doi: 10.1016/B978-0-12-407766-9.00017-1. [DOI] [PubMed] [Google Scholar]
- 56.Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283(5407):1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 57.Klaman LD, Boss O, Peroni OD, Kim JK, Martino JL, Zabolotny JM, Moghal N, Lubkin M, Kim YB, Sharpe AH, Stricker-Krongrad A, Shulman GI, Neel BG, Kahn BB. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol. 2000;20(15):5479–5489. doi: 10.1128/mcb.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rahmouni K, Correia ML, Haynes WG, et al. Obesity-associated hypertension: new insights into mechanisms. Hypertension. 2005;45(1):9–14. doi: 10.1161/01.HYP.0000151325.83008.b4. [DOI] [PubMed] [Google Scholar]
- 59.Kalil GZ, Haynes WG. Sympathetic nervous system in obesity-related hypertension: mechanisms and clinical implications. Hypertens Res: Off J Japn Soc Hypertens. 2012;35(1):4–16. doi: 10.1038/hr.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fang CX, Doser TA, Yang X, Sreejayan N, Ren J. Metallothionein antagonizes aging-induced cardiac contractile dysfunction: role of PTP1B, insulin receptor tyrosine phosphorylation and Akt. Aging Cell. 2006;5(2):177–185. doi: 10.1111/j.1474-9726.2006.00201.x. [DOI] [PubMed] [Google Scholar]
- 61.Dong F, Fang CX, Yang X, Zhang X, Lopez FL, Ren J. Cardiac overexpression of catalase rescues cardiac contractile dysfunction induced by insulin resistance: role of oxidative stress, protein carbonyl formation and insulin sensitivity. Diabetologia. 2006;49(6):1421–1433. doi: 10.1007/s00125-006-0230-7. [DOI] [PubMed] [Google Scholar]
- 62.Herren DJ, Norman JB, Anderson R, et al. Deletion of protein tyrosine phosphatase 1B (PTP1B) enhances endothelial cyclooxygenase 2 expression and protects mice from type 1 diabetes-induced endothelial dysfunction. PLoS One. 2015;10(5):e0126866. doi: 10.1371/journal.pone.0126866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cui C, Merritt R, Fu L, Pan Z. Targeting calcium signaling in cancer therapy. Acta Pharm Sin B. 2017;7(1):3–17. doi: 10.1016/j.apsb.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Neel BG, Gu H, Pao L. The 'Shp'ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28(6):284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 65.Ishida H, Kogaki S, Narita J, Ichimori H, Nawa N, Okada Y, Takahashi K, Ozono K. LEOPARD-type SHP2 mutant Gln510Glu attenuates cardiomyocyte differentiation and promotes cardiac hypertrophy via dysregulation of Akt/GSK-3beta/beta-catenin signaling. Am J Phys Heart Circ Phys. 2011;301(4):H1531–H1539. doi: 10.1152/ajpheart.00216.2011. [DOI] [PubMed] [Google Scholar]
- 66.Chan RJ, Li Y, Hass MN, Walter A, Voorhorst CS, Shelley WC, Yang Z, Orschell CM, Yoder MC. Shp-2 heterozygous hematopoietic stem cells have deficient repopulating ability due to diminished self-renewal. Exp Hematol. 2006;34(9):1230–1239. doi: 10.1016/j.exphem.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 67.Yang W, Klaman LD, Chen B, Araki T, Harada H, Thomas SM, George EL, Neel BG. An Shp2/SFK/Ras/Erk signaling pathway controls trophoblast stem cell survival. Dev Cell. 2006;10(3):317–327. doi: 10.1016/j.devcel.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 68.Noonan J, O'Connor W. Noonan syndrome: a clinical description emphasizing the cardiac findings. Acta Paediatr Jpn: Overseas Ed. 1996;38(1):76–83. doi: 10.1111/j.1442-200x.1996.tb03443.x. [DOI] [PubMed] [Google Scholar]
- 69.Tartaglia M, Mehler EL, Goldberg R, Zampino G, Brunner HG, Kremer H, van der Burgt I, Crosby AH, Ion A, Jeffery S, Kalidas K, Patton MA, Kucherlapati RS, Gelb BD. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 2001;29(4):465–468. doi: 10.1038/ng772. [DOI] [PubMed] [Google Scholar]
- 70.Lauriol J, Kontaridis MI. PTPN11-associated mutations in the heart: has LEOPARD changed its RASpots? Trends Cardiovasc Med. 2011;21(4):97–104. doi: 10.1016/j.tcm.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tafazoli A, Eshraghi P, Koleti ZK, Abbaszadegan M. Noonan syndrome—a new survey. Arch Med Sci: AMS. 2017;13(1):215–222. doi: 10.5114/aoms.2017.64720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Keilhack H, David FS, McGregor M, Cantley LC, Neel BG. Diverse biochemical properties of Shp2 mutants. Implications for disease phenotypes. J Biol Chem. 2005;280(35):30984–30993. doi: 10.1074/jbc.M504699200. [DOI] [PubMed] [Google Scholar]
- 73.Kontaridis MI, Swanson KD, David FS, Barford D, Neel BG. PTPN11 (Shp2) mutations in LEOPARD syndrome have dominant negative, not activating, effects. J Biol Chem. 2006;281(10):6785–6792. doi: 10.1074/jbc.M513068200. [DOI] [PubMed] [Google Scholar]
- 74.Yu ZH, Zhang RY, Walls CD, Chen L, Zhang S, Wu L, Liu S, Zhang ZY. Molecular basis of gain-of-function LEOPARD syndrome-associated SHP2 mutations. Biochemistry. 2014;53(25):4136–4151. doi: 10.1021/bi5002695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu M, Jin Y, Song Q, Wu J, Philbrick MJ, Cully BL, An X, Guo L, Gao F, Li J. The endothelium-dependent effect of RTEF-1 in pressure overload cardiac hypertrophy: role of VEGF-B. Cardiovasc Res. 2011;90(2):325–334. doi: 10.1093/cvr/cvq400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bottini N, Bottini E, Gloria-Bottini F, Mustelin T. Low-molecular-weight protein tyrosine phosphatase and human disease: in search of biochemical mechanisms. Arch Immunol Ther Exp. 2002;50(2):95–104. [PubMed] [Google Scholar]
- 77.Chernoff J, Li HC. A major phosphotyrosyl-protein phosphatase from bovine heart is associated with a low-molecular-weight acid phosphatase. Arch Biochem Biophys. 1985;240(1):135–145. doi: 10.1016/0003-9861(85)90016-5. [DOI] [PubMed] [Google Scholar]
- 78.Magherini F, Giannoni E, Raugei G, Cirri P, Paoli P, Modesti A, Camici G, Ramponi G. Cloning of murine low molecular weight phosphotyrosine protein phosphatase cDNA: identification of a new isoform. FEBS Lett. 1998;437(3):263–266. doi: 10.1016/s0014-5793(98)01241-1. [DOI] [PubMed] [Google Scholar]
- 79.Lazaruk KD, Dissing J, Sensabaugh GF. Exon structure at the human ACP1 locus supports alternative splicing model for f and s isozyme generation. Biochem Biophys Res Commun. 1993;196(1):440–446. doi: 10.1006/bbrc.1993.2269. [DOI] [PubMed] [Google Scholar]
- 80.Cirri P, Fiaschi T, Chiarugi P, Camici G, Manao G, Raugei G, Ramponi G. The molecular basis of the differing kinetic behavior of the two low molecular mass phosphotyrosine protein phosphatase isoforms. J Biol Chem. 1996;271(5):2604–2607. doi: 10.1074/jbc.271.5.2604. [DOI] [PubMed] [Google Scholar]
- 81.Pandey SK, Yu XX, Watts LM, et al. 2007 Reduction of low-molecular-weight protein tyrosine phosphatase expression improves hyperglycemia and insulin sensitivity in obese mice. J Biol Chem [DOI] [PubMed]
- 82.Ramponi G, Manao G, Camici G, Cappugi G, Ruggiero M, Bottaro DP. The 18 kDa cytosolic acid phosphatase from bovine live has phosphotyrosine phosphatase activity on the autophosphorylated epidermal growth factor receptor. FEBS Lett. 1989;250(2):469–473. doi: 10.1016/0014-5793(89)80778-1. [DOI] [PubMed] [Google Scholar]
- 83.Hoekstra E, Peppelenbosch MP, Fuhler GM. The role of protein tyrosine phosphatases in colorectal cancer. Biochim Biophys Acta. 1826;2012:179–188. doi: 10.1016/j.bbcan.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 84.Xing K, Raza A, Lofgren S, et al. Low molecular weight protein tyrosine phosphatase (LMW-PTP) and its possible physiological functions of redox signaling in the eye lens. Biochim Biophys Acta. 2007;1774(5):545–555. doi: 10.1016/j.bbapap.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kikawa KD, Vidale DR, Van Etten RL, Kinch MS. Regulation of the EphA2 kinase by the low molecular weight tyrosine phosphatase induces transformation. J Biol Chem. 2002;277(42):39274–39279. doi: 10.1074/jbc.M207127200. [DOI] [PubMed] [Google Scholar]
- 86.Park EK, Warner N, Mood K, Pawson T, Daar IO. Low-molecular-weight protein tyrosine phosphatase is a positive component of the fibroblast growth factor receptor signaling pathway. Mol Cell Biol. 2002;22(10):3404–3414. doi: 10.1128/MCB.22.10.3404-3414.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dissing J (1993) Human “red cell” acid phosphatase (ACP1) genetic, catalytic and molecular properties. PhD Thesis, Copenhagen University, Copenhagen, Denmark
- 88.Bottini N, MacMurray J, Peters W, Rostamkhani M, Comings DE. Association of the acid phosphatase (ACP1) gene with triglyceride levels in obese women. Mol Genet Metab. 2002;77(3):226–229. doi: 10.1016/s1096-7192(02)00120-8. [DOI] [PubMed] [Google Scholar]
- 89.Shu YH, Hartiala J, Xiang AH, Trigo E, Lawrence JM, Allayee H, Buchanan TA, Bottini N, Watanabe RM. Evidence for sex-specific associations between variation in acid phosphatase locus 1 (ACP1) and insulin sensitivity in Mexican-Americans. J Clin Endocrinol Metab. 2009;94(10):4094–4102. doi: 10.1210/jc.2008-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Banci M, Saccucci P, D’Annibale F, et al. ACP1 genetic polymorphism and coronary artery disease: an association study. Cardiology. 2009;113(4):236–242. doi: 10.1159/000203405. [DOI] [PubMed] [Google Scholar]
- 91.Bottini E, Gloria-Bottini F, Borgiani P. ACP1 and human adaptability. 1. Association with common diseases: a case-control study. Hum Genet. 1995;96(6):629–637. doi: 10.1007/BF00210290. [DOI] [PubMed] [Google Scholar]
- 92.Huyer G, Liu S, Kelly J, Moffat J, Payette P, Kennedy B, Tsaprailis G, Gresser MJ, Ramachandran C. Mechanism of inhibition of protein-tyrosine phosphatases by vanadate and pervanadate. J Biol Chem. 1997;272(2):843–851. doi: 10.1074/jbc.272.2.843. [DOI] [PubMed] [Google Scholar]
- 93.Bhuiyan MS, Takada Y, Shioda N, Moriguchi S, Kasahara J, Fukunaga K. Cardioprotective effect of vanadyl sulfate on ischemia/reperfusion-induced injury in rat heart in vivo is mediated by activation of protein kinase B and induction of FLICE-inhibitory protein. Cardiovasc Ther. 2008;26(1):10–23. doi: 10.1111/j.1527-3466.2008.00039.x. [DOI] [PubMed] [Google Scholar]
- 94.Goldfine AB, Patti ME, Zuberi L, Goldstein BJ, LeBlanc R, Landaker EJ, Jiang ZY, Willsky GR, Kahn CR. Metabolic effects of vanadyl sulfate in humans with non-insulin-dependent diabetes mellitus: in vivo and in vitro studies. Metab Clin Exp. 2000;49(3):400–410. doi: 10.1016/s0026-0495(00)90418-9. [DOI] [PubMed] [Google Scholar]
- 95.Vercauteren M, Remy E, Devaux C, Dautreaux B, Henry JP, Bauer F, Mulder P, Hooft van Huijsduijnen R, Bombrun A, Thuillez C, Richard V. Improvement of peripheral endothelial dysfunction by protein tyrosine phosphatase inhibitors in heart failure. Circulation. 2006;114(23):2498–2507. doi: 10.1161/CIRCULATIONAHA.106.630129. [DOI] [PubMed] [Google Scholar]
- 96.Schramm C, Edwards MA, Krenz M. New approaches to prevent LEOPARD syndrome-associated cardiac hypertrophy by specifically targeting Shp2-dependent signaling. J Biol Chem. 2013;288(25):18335–18344. doi: 10.1074/jbc.M113.483800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Forghieri M, Laggner C, Paoli P, Langer T, Manao G, Camici G, Bondioli L, Prati F, Costantino L. Synthesis, activity and molecular modeling of a new series of chromones as low molecular weight protein tyrosine phosphatase inhibitors. Bioorg Med Chem. 2009;17(7):2658–2672. doi: 10.1016/j.bmc.2009.02.060. [DOI] [PubMed] [Google Scholar]
- 98.Stanford SM, Aleshin AE, Zhang V, et al. 2017 Diabetes reversal by inhibition of the low-molecular-weight tyrosine phosphatase. Nat Chem Biol [DOI] [PMC free article] [PubMed]
- 99.Lantz KA, Hart SG, Planey SL, et al. Inhibition of PTP1B by trodusquemine (MSI-1436) causes fat-specific weight loss in diet-induced obese mice. Obesity. 2010;18(8):1516–1523. doi: 10.1038/oby.2009.444. [DOI] [PubMed] [Google Scholar]
- 100.He R, Wang J, Yu ZH, et al. (2016) Inhibition of low molecular weight protein tyrosine phosphatase by an induced-fit mechanism. J Med Chem [DOI] [PMC free article] [PubMed]
- 101.Hellmuth K, Grosskopf S, Lum CT, Wurtele M, Roder N, von Kries JP, Rosario M, Rademann J, Birchmeier W. Specific inhibitors of the protein tyrosine phosphatase Shp2 identified by high-throughput docking. Proc Natl Acad Sci U S A. 2008;105(20):7275–7280. doi: 10.1073/pnas.0710468105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu B, Liu W, Yu WM, Loh ML, Alter S, Guvench O, MacKerell AD, Tang LD, Qu CK. Targeting protein tyrosine phosphatase SHP2 for the treatment of PTPN11-associated malignancies. Mol Cancer Ther. 2013;12(9):1738–1748. doi: 10.1158/1535-7163.MCT-13-0049-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen L, Sung SS, Yip ML, Lawrence HR, Ren Y, Guida WC, Sebti SM, Lawrence NJ, Wu J. Discovery of a novel shp2 protein tyrosine phosphatase inhibitor. Mol Pharmacol. 2006;70(2):562–570. doi: 10.1124/mol.106.025536. [DOI] [PubMed] [Google Scholar]
- 104.Sippl W. Development of biologically active compounds by combining 3D QSAR and structure-based design methods. J Comput Aided Mol Des. 2002;16(11):825–830. doi: 10.1023/a:1023888813526. [DOI] [PubMed] [Google Scholar]
- 105.Yu WM, Guvench O, Mackerell AD, et al. Identification of small molecular weight inhibitors of Src homology 2 domain-containing tyrosine phosphatase 2 (SHP-2) via in silico database screening combined with experimental assay. J Med Chem. 2008;51(23):7396–7404. doi: 10.1021/jm800229d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sobhia ME, Paul S, Shinde R, et al. Protein tyrosine phosphatase inhibitors: a patent review (2002–2011) Expert Opin Ther Patents. 2012;22(2):125–153. doi: 10.1517/13543776.2012.661414. [DOI] [PubMed] [Google Scholar]
- 107.Hof P, Pluskey S, Dhe-Paganon S, Eck MJ, Shoelson SE. Crystal structure of the tyrosine phosphatase SHP-2. Cell. 1998;92(4):441–450. doi: 10.1016/s0092-8674(00)80938-1. [DOI] [PubMed] [Google Scholar]
- 108.Lee JO, Yang H, Georgescu MM, di Cristofano A, Maehama T, Shi Y, Dixon JE, Pandolfi P, Pavletich NP. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell. 1999;99(3):323–334. doi: 10.1016/s0092-8674(00)81663-3. [DOI] [PubMed] [Google Scholar]
- 109.Wiesmann C, Barr KJ, Kung J, Zhu J, Erlanson DA, Shen W, Fahr BJ, Zhong M, Taylor L, Randal M, McDowell RS, Hansen SK. Allosteric inhibition of protein tyrosine phosphatase 1B. Nat Struct Mol Biol. 2004;11(8):730–737. doi: 10.1038/nsmb803. [DOI] [PubMed] [Google Scholar]
- 110.Scott LM, Chen L, Daniel KG, Brooks WH, Guida WC, Lawrence HR, Sebti SM, Lawrence NJ, Wu J. Shp2 protein tyrosine phosphatase inhibitor activity of estramustine phosphate and its triterpenoid analogs. Bioorg Med Chem Lett. 2011;21(2):730–733. doi: 10.1016/j.bmcl.2010.11.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Scott LM, Lawrence HR, Sebti SM, et al. Targeting protein tyrosine phosphatases for anticancer drug discovery. Curr Pharm Des. 2010;16(16):1843–1862. doi: 10.2174/138161210791209027. [DOI] [PMC free article] [PubMed] [Google Scholar]