Abstract
AIM
To address the microstructure and biomechanical changes of the sclera of rabbits after negative lens application by spectacle frame apparatus.
METHODS
Five New Zealand rabbits of seven weeks post-natal were treated with -8 D lens monocularly over the course of two weeks. Refractive errors and axial length (AXL) were measured at the 1st, 7th and 14th days of the induction period. Ultrastructure of sclera was determined with electron microscopy. Biomechanical properties were tested by an Instron 5565 universal testing machine.
RESULTS
Lens-induced (LI) eyes elongated more rapidly compared with fellow eyes with AXL values of 15.56±0.14 and 15.21±0.14 mm (P<0.01). Fibril diameter was significantly smaller in the LI eyes compared with control ones in the inner, middle, and outer layers (inner layer, 63.533 vs 76.467 nm; middle layer, 92.647 vs 123.984 nm; outer layer, 86.999 vs 134.257 nm, P<0.01, respectively). In comparison with control eyes, macrophage-like cells that engulfed fibroblasts, dilated endoplasmic reticulum, and vacuoles in fibroblasts were observed in the inner and middle stroma in the LI eyes. Ultimate stress and Young's modulus were lower in the LI eyes compared with those in the control eyes.
CONCLUSION
Negative lens application alters eye growth, and results in axial elongation with changes in scleral ultrastructural and mechanical properties.
Keywords: negative lens, rabbit, biomechanics, sclera, ultrastructure
INTRODUCTION
An active emmetropization mechanism involves active ocular growth and scleral remodeling to match the eye's axial length (AXL) to its refractive power, producing eyes with retinal focus[1]–[3]. The biomechanical characteristics of the sclera play an essential role in influencing the refractive state of the eye[2]–[6].
Lens-induced (LI) compensation for imposed hyperopic defocus has been shown in guinea pigs[7], tree shrews[3],[8]–[10], chicks[11], marmosets[12], and monkeys[13]. Rabbits have large eyes, which grow as much as 12 mm until maturity and have similar microscopic retinal and scleral characteristics with humans[14]. Moreover, the rabbit eye is a good model for the pharmacokinetics of intraocular drugs, thus it should prove useful in applied pharmaceutical research[15]. Furthermore, rabbits are on a higher phylogenetic scale than chicks and are more practical than primates in terms of availability, spending, and management. Early experiments on the induction of myopia with rabbits were achieved by increasing intraocular pressure and temperature[16], lid sutures[4],[17]–[19], and photorefractive keratectomy (PRK)[20]. However, controlling the intraocluar pressure is difficult and requires specially designed suction cups[21]. Sutures used to induce deprivation myopia can be easily removed due to the unusually thick eyelids of rabbits[14],[17]–[19]. Furthermore, PRK-induced myopia was unstable[20]. LI compensation in rabbits has not been previously reported. In this study we attempted to use unilateral lenses with a soft frame to induce hyperopic compensation in rabbits.
Our study was to determine whether early development of the rabbit eye could be affected by wearing negative lenses and observe the microstructural and mechanical changes in the sclera of rabbits after lens application.
MATERIALS AND METHODS
Animal Care
Five seven-week-old New Zealand rabbits weighing from 1080-1255 g were obtained from the Animal Care Center of Eye & ENT (EENT) Hospital affiliated with Fudan University (Shanghai, China). The rabbits were raised under natural diurnal lighting, housed in individual cages and maternally reared, with water and food available ad libitum. All experiments were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the regulations of EENT Hospital for animal experimentation. The protocol was approved by the Animal Ethics Committee of EENT Hospital. All efforts were made to minimize suffering.
Lens-induced Anisometropia in Rabbits
Five New Zealand rabbits had anisometropia induced in the right eyes (LI eyes) by a defocusing method with a specially designed lens system (Figure 1), whereas the left eyes had no treatment (control eyes). A concave lens (4 cm diameter, -8.00 D, polymethyl methacrylate) was placed in front of each experimental eye at approximately 7 mm vertex distance. The contralateral eye in the same rabbit served as control. All of the animals were littermates to make sure that experiments can be performed on a single homogeneous sample during the 2-week induction period of wearing lenses. Presence of ocular lesions or deficiency of the dioptric mediums and fundus were excluded by a priori clinical examination. Throughout the experiment, the lens systems were inspected and adjusted if necessary at about 4h intervals throughout the day to ensure the sutures in the lens system were firmly in place and the lenses were clean and debris-free.
Figure 1. Spectacle frame.
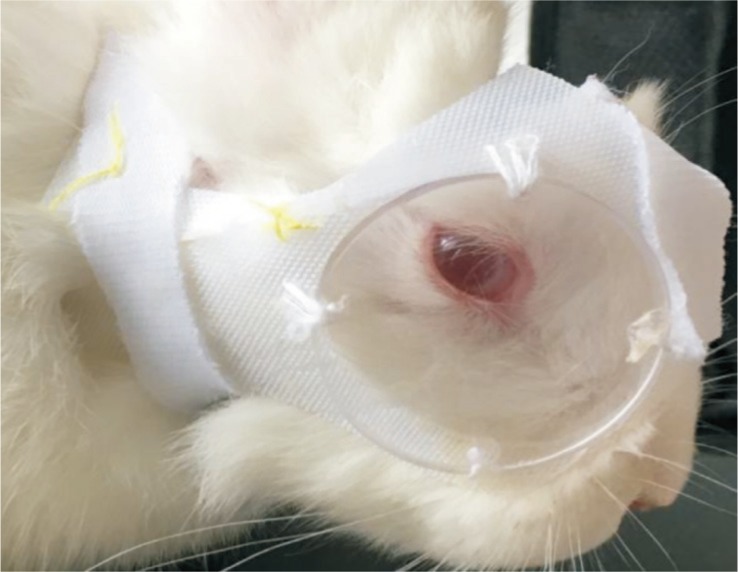
Lenses were attached to arcs made of Velcro and sutured by strings to the soft frame also made from soft Velcro, which circled the ear and neck of the rabbit.
Measurements of the Dioptric System
Measurements of ocular components were taken with A-scan ultrasonography (Opticon, Italian, software version: Opticon 2000SPA, instrument accuracy of ±0.036 mm). After topical anesthetic use of oxybuprocaine, the applanation of the probe on the cornea followed. Alignment of the probe was adjusted after monitoring the ultrasonic graph for signal strength and quality. Proper alignment was defined as strong ultrasonic wave reflection peaks of lens surfaces and retina. Five consecutive readings of ocular components, including depth of the anterior segment, lens thickness, AXL, and vitreous chamber length (VCL), were recorded and averaged after proper alignment was obtained.
Cycloplegic refractions were done in conscious animals under tropicamide-induced cycloplegia, using a streak retinoscope by an experienced optometrist, who was unaware which eye was induced. Four measurements with two axes of each were averaged as spherical equivalent of all eyes, with readings reported to the nearest 0.25 D. Measurements were performed at the 1st, 7th and 14th days of the induction period.
Specimen Preparation
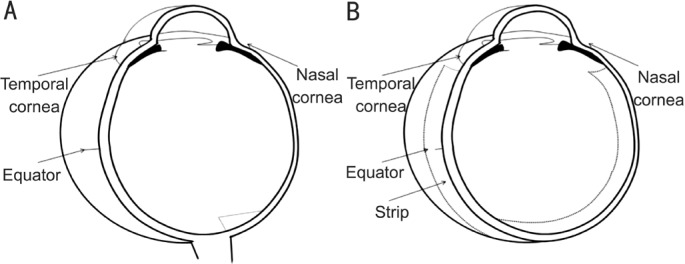
Lethal doses of ketamine and xylazine to animals were administered at the end of the experiment. The eyes were enucleated immediately after death and the globe was cleaned of residual orbital tissue. Figure 2 illustrates the specimen preparation. The superior part was marked by a suture at 2 mm behind the limbus at 11 o'clock, as well as the corresponding contralateral eye at the 1 o'clock position. The eyeball was split along the line from the marked spot to the optic nerve. Then, the lens and the vitreous were removed, with the nasal half of the eyeball containing the optic nerve head (ONH). Specimens were obtained from the two divided halves. To avoid cutting at a slanted angle, we made the cone outer segment parallel, thus setting the plane of section at a certain angle to the eyeball. For electron microscopy, six 3 mm ×1 mm strips of ocular tissue were taken from six eyes of three rabbits chosen randomly. To ensure that each specimen was properly located at the same area of each eye regardless of AXL, the 3 mm ×1 mm strips were cut from the edge of the ONH via a razor blade (Figure 2). The remaining nasal half was then fixed by perfusion of buffered aldehydes. A 4 mm wide strip was obtained from the temporal half for biomechanical tests. Figure 3 shows the perpendicularly arranged photoreceptors, suggesting the strips were cut squarely.
Figure 2. Illustration of scleral sample processing for biomechanical test and microscopy.
A: Nasal half of the eyeball; B: Temporal half of the eyeball.
Figure 3. Light microscopy of a semi-thin stained with toluidine blue from a strip excised from the edge of the ONH in the nasal direction of the nasal half.
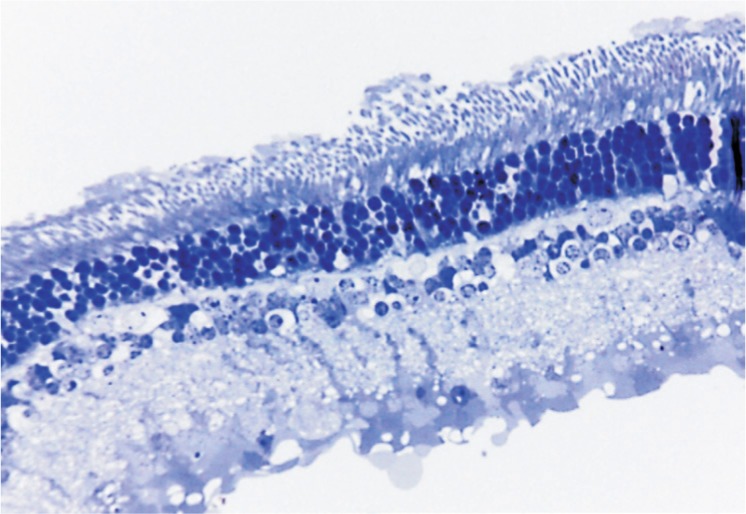
Image (×40) shows perpendicularly arranged photoreceptors, suggesting the strips were cut squarely, as per Figure 2.
Morphological Observations
Histopathological examination was performed on all the eyes by transmission electron microscopy. Buffered glutaraldehyde at 2.5% was used as fixative for tissues for 2h or more. Thin samples were carefully prepared by the ultramicrotome (LKB-I). Then, the sections were stained for transmission electron microscopy (Philips CM-120). We utilized Funata and Tokoro's[22] method for scleral lamination for analysis of the fibril diameter. Electron micrographs at ×42 000 of the outer layers (10 µm inward from the boundary between the episclera and sclera), the inner layers (10 µm outward from the boundary between the suprachoroid and sclera), and the middle layers (midway between the outer and inner layers) were generated. Eighteen micrographs were taken from the three layers of all samples, because researchers aimed to ensure that each graph consisted of different bundles. Diameter of the collagenous fibers and the number of collagenous fibers of the scleral sections were evaluated using an image processing software (ImageJ 1.51e, Wayne Rasband, National Institutes of Health, USA). The smaller diameter was measured when fibrils appeared to be oval. Approximately 300 fibrils were taken into account per eye at the edge of the ONH; scleral fibrils amounted to about 1200 total. These fibrils were all involved in the calculation of distribution of the fibrial diameter.
Biomechanical Measurements
Computer-controlled biomaterial tensile test have been proposed as a method to investigate the biomechanical response of the sclera[7],[23]. We used this tensile test in the present study to estimate the changing material properties of the rabbit sclera during negative lens compensation. Strips were dissected sagittally as described above. Biomechanical measurements were performed to the eight scleral specimens from four LI eyes and their four contralateral eyes.
Mechanical properties of the sclera were tested on Instron 5565 universal testing machine at 25°C±5°C and 50%±5% relative humidity. For tensile tests, samples were 4 mm in width and about 0.2 mm thick. During testing, the gauge length was determined by the equator, whereas the remainder was clamped by the fixture with a cross-head speed of 10 mm/min. We used the parameters from our previous study[7]. Ultimate stress (MPa) and ultimate strain (%) were used, and Young's modulus (MPa) was determined as the slope of the stress-strain graph at 10% strain.
Statistical Analysis
The data were analyzed using SPSS 24.0 (SPSS Inc., Chicago, IL, USA). Data were tested for normality by Shapiro-Wilk test and reported as the mean±standard deviation (SD) for normally distributed data. Otherwise, values were reported as the median (min, max). Nonparametric Kruskal-Wallis and Mann-Whitney tests were used to compare differences between the LI and control eyes.
RESULTS
Measurements of the Dioptric System
Results of ophthal-mological characteristics at different times were summarized in Table 1 and Figure 4. Variability was present in the average depth of the anterior segment and lens thickness but did not show significant difference between the LI and the control eye at any time point. At the outset of the experiment, refraction of all rabbits was slightly hyperopic. At the 14th day, the LI eyes had a longer AXL and a more myopic retinoscopy result than the control ones. The final measurements demonstrated that the AXL of LI eyes was 15.56±0.14 mm, whereas the control eyes was 15.21±0.14 mm. This difference was statistically significant (P=0.009). Similar changes in VCL were observed between the LI and control eyes, with a significant difference of 0.347 mm (6.90 vs 6.553 mm, P=0.047).
Table 1. Comparison of ocular dimension between the left control eye and the LI eye during defocus.
| Parameters (mm) | Control eye (n=5) |
LI eye (n=5) |
||||||
| Mean | SD | P (S-W test) | Mean | SD | P (S-W test) | Z | P (M-W test) | |
| Before | ||||||||
| AC | 2.214 | 0.057 | 0.356 | 2.229 | 0.061 | 0.098 | -0.731 | 0.465 |
| LENS | 6.126 | 0.28 | 0.282 | 6.100 | 0.24 | 0.183 | -0.313 | 0.754 |
| AXL | 14.70 | 0.078 | 0.505 | 14.69 | 0.094 | 0.187 | -0.313 | 0.754 |
| VCL | 6.357 | 0.30 | 0.813 | 6.366 | 0.24 | 0.670 | -0.104 | 0.917 |
| One week | ||||||||
| AC | 2.168 | 0.081 | 0.753 | 2.148 | 0.12 | 0.131 | -0.104 | 0.917 |
| LENS | 6.356 | 0.23 | 0.299 | 6.405 | 0.20 | 0.395 | -0.522 | 0.602 |
| AXL | 14.90 | 0.19 | 0.579 | 15.05 | 0.21 | 0.567 | -0.940 | 0.347 |
| VCL | 6.371 | 0.30 | 0.922 | 6.492 | 0.30 | 0.864 | -0.731 | 0.465 |
| Two weeks | ||||||||
| AC | 2.317 | 0.043 | 0.205 | 2.298 | 0.041 | 0.662 | -0.529 | 0.597 |
| LENS | 6.337 | 0.067 | 0.372 | 6.361 | 0.11 | 0.447 | -0.522 | 0.602 |
| AXL | 15.21 | 0.14 | 0.391 | 15.56 | 0.14 | 0.116 | -2.611 | 0.009a |
| VCL | 6.553 | 0.20 | 0.132 | 6.900 | 0.17 | 0.457 | -1.991 | 0.047a |
LI: Lens-induced; AC: Depth of the anterior segment; LENS: Lens thickness; AXL: Axial length; VCL: Vitreous chamber length; SD: Standard deviation. Shapiro-Wilk test (S-W test) was used to test normality. Mann-Whitney test (M-W test) was used to compare difference between the two groups. aP<0.05 for the significant difference between the control eye and the LI eye.
Figure 4. Results of lens-induced anisometropia in rabbits.
A, B, C: Anterior chamber, lens thickness and axial length via A-scan ultrasonography, respectively; D: Refractive error via retinoscopy. Solid lines and dashed lines stands for control eyes and LI eyes, respectively. Error bars represent standard error of the mean. LI: Lens-induced.
Refractive error was between +4.5 D and +3 D in all eyes throughout the experimental period (Table 2). Final refractive error measurements of the control and LI eyes were +3.75 D and +3.00 D, respectively (P=0.034).
Table 2. Comparison of refractive state between the control eyes and the LI eyes during defocus.
| Time | Control eye (n=5) |
LI eye (n=5) |
Z | P (M-W test) | ||||
| Median | Min, max | P (S-W test) | Median | Min, max | P (S-W test) | |||
| Before | 4.25 | 4.0, 4.25 | 0.006 | 4.00 | 4.0, 4.25 | 0.000 | -1.225 | 0.221 |
| One week | 3.50 | 3.0, 4.0 | 0.377 | 3.25 | 2.75, 3.75 | 0.967 | -1.057 | 0.290 |
| Two weeks | 3.75 | 3.0, 4.0 | 0.054 | 3.00 | 2.75, 3.50 | 0.421 | -2.115 | 0.034a |
LI: Lens-induced. Shapiro-Wilk test (S-W test) was used to test normality. Mann-Whitney test (M-W test) was used to compare the difference between the two groups. Data were expressed as median (min, max). aP<0.05 for significant difference between the control eye and the LI eye.
Morphological Observations
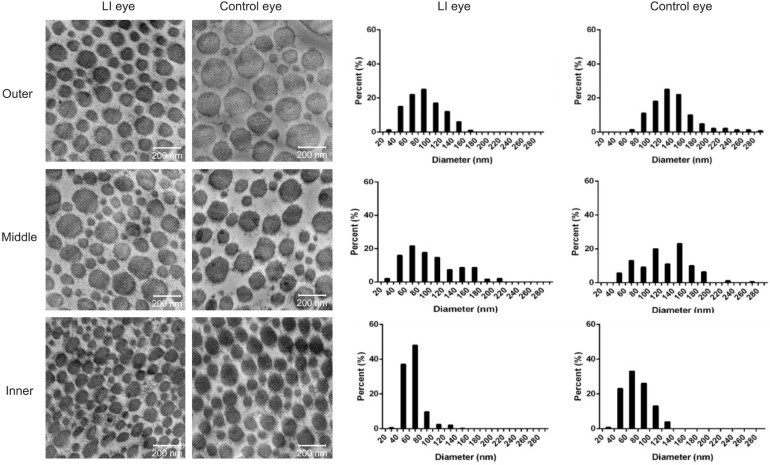
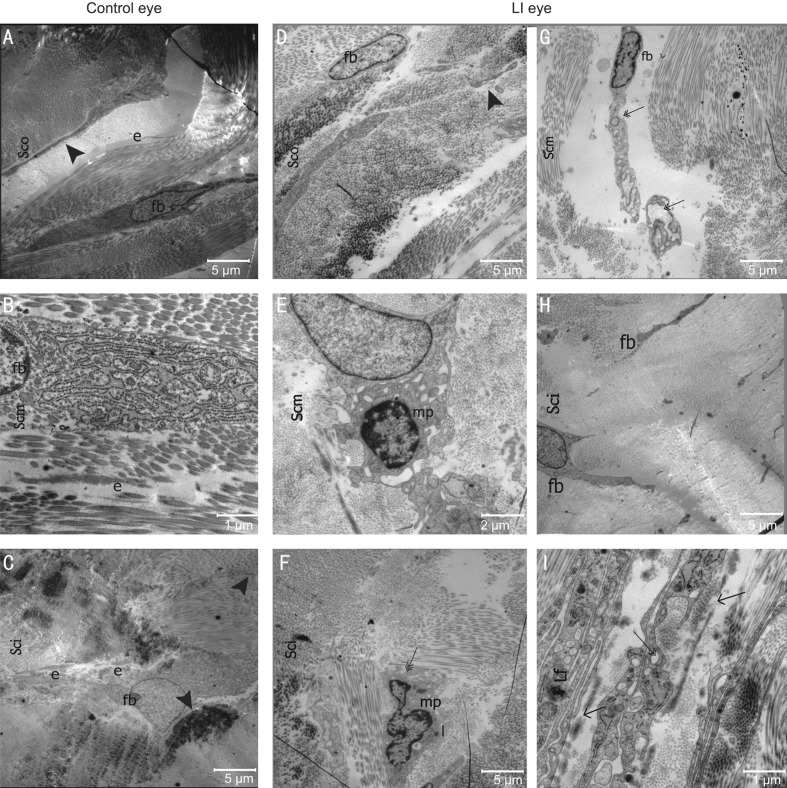
Main findings of the scleral ultrastructure of the rabbits from both eyes in the posterior region can be seen in Table 3, Figures 5 and 6. Collagen fibril diameter among the fibril bundles varied considerably ranging from 29.238-282.143 nm. Diameter of the fibril was significantly smaller in the LI eyes compared to the control eyes (inner layer: 63.533 vs 76.467 nm; middle layer: 92.647 vs 123.984 nm; outer layer: 86.999 vs 134.257 nm; P<0.01, respectively). The LI eyes had no normal fibril diametric gradient from the inner to outer layer, and fibril diameter of their outer and middle layer did not differ significantly (92.647 vs 86.999 nm, P=0.091). However, control eyes exhibited a normal gradient across the layers, with a significantly different fibril diameter between the three layers (P=0.000), and the middle and outer layers (123.984 vs 134.257 nm, P=0.004<0.017). In both eyes, the percentage of smaller diameter fibrils (10-100 nm) was largest in the inner layer (Figure 5). Elastic fibers were scattered around the fibril bundles (Figure 6A, 6C) or scleral cells (Figure 6B), whereas the elastic fibers found in the lamina fusca (Lf) of the LI eye were not consistent (Figure 6I). Scleral cells (mostly fibroblasts) and their processes were localized between or within collagen fibril bundles (Figure 6A-6D, 6G and 6H). Dilated endoplasmic reticulum and vacuoles in fibroblasts were observed in the inner and middle stroma in LI eyes (Figure 6G, 6I).
Table 3. Fibril diameter from different layers of the LI and control eyes at the edge of ONH.
| Layer (nm) | Control eye |
LI eye |
Z | P (M-W test) | ||||||
| n | Median | Min, max | P (S-W test) | n | Median | Min, max | P (S-W test) | |||
| Inner | 457 | 76.467 | 29.238, 152.313 | 0.000 | 545 | 63.533 | 36.362, 146.124 | 0.000 | -9.093 | 0.000a |
| Middle | 174 | 123.984 | 45.098, 261.905 | 0.012 | 245 | 92.647 | 35.900, 216.514 | 0.000 | -5.881 | 0.000a |
| Outer | 145 | 134.257 | 67.801, 282.143 | 0.000 | 300 | 86.999 | 34.131, 169.784 | 0.000 | -11.401 | 0.000a |
| χ2 | 336.952 | 217.865 | - | |||||||
| P (K-W test) | 0.000 | 0.000 | - | |||||||
LI: Lens-induced. Shapiro-Wilk test (S-W test) was used to test normality. Mann-Whitney test (M-W test) and Kruskal-Wallis test (K-W test) were used to compare difference between groups and layers. Data were expressed as median (min, max). P<0.05 for significant difference between the three layers. aP<0.05 for significant difference between the control and the LI eyes. P (control eye, inner vs middle)=0.000, P (control eye, middle vs outer)=0.004, P (control eye, inner vs outer)=0.000, P (LI eye, inner vs middle)=0.000, P (LI eye, middle vs outer)=0.091, P (LI eye, inner vs outer)=0.000.
Figure 5. Collagen fibril diameter taken from representative electron micrographs (×42 000) of the LI and control eyes.
The results showed sections taken from the outer, middle and inner layers of scleral stroma and the corresponding distribution of the diameter of collagen fibrils (shown as percentage). Small fibrils have diameters ranging from 10 to 100 nm, whereas large ones ranging from 110 to 380 nm. LI: Lens-induced.
Figure 6. Electronic micrographs representing the microstructure of the rabbit sclera in control eyes (A-C) and the LI eyes (D-I).
The collagen fibril bundles increased in size from the inner to outer stroma of the sclera. The number of cells decreased from inner to outer layers. In the outer scleral stroma (Sco), fibroblasts (fb) with thin processes (arrowhead) and elastic fibers (e) were scatterd within or between collagen fibril bundles. The inner scleral stroma (Sci) contain fibroblasts (fb) elastic fibers (e). Fibroblasts were observed in the outer scleral stroma of the LI eyes, but no fibers were observed (D). A fibroblast (fb) was surrounded by a macrophage-like cell (mp) (E). The phagocytes may contain collagen fibrils (triple arrow) (F). The cytoplasm of an abnormal fibroblast contained vacuoles (double arrow) and a dilated endoplasmic reticulum (G, I). In the LI eyes, some macrophage-like cells (mp) and lysosomes (l) were found in the middle scleral stroma (Scm). In the LI eyes, no consistency was observed in elastic fibers in the lamina fusca (Lf) (arrow) (I). LI: Lens-induced.
Biomechanical Measurements
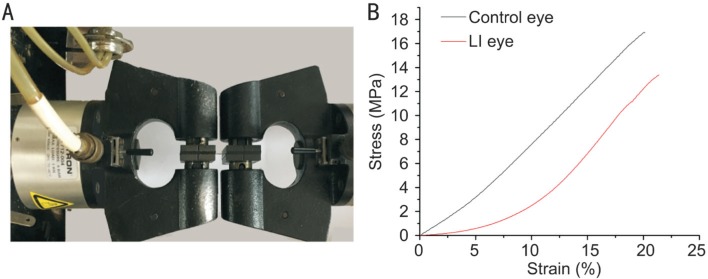
Table 4 and Figure 7 show statistically significant changes in biomechanical parameters from the control and LI eyes of the rabbit sclera. The ultimate stress decreased 27.6%, and the Young's modulus by 41.8%, whereas the ultimate strain increased 13.7%.
Table 4. Overview of biomechanical parameters between the control eye and the LI eye during defocus.
| Parameters | Control eye (n=4) |
LI eye (n=4) |
Z | P | |||||
| Mean | SD | P (S-W test) | Mean | SD | P (S-W test) | ||||
| Ultimate stress (MPa) | 18.65 | 1.40 | 0.979 | 13.53 | 1.60 | 0.912 | -2.309 | 0.021a | |
| Ultimate strain (%) | 29.38 | 9.11 | 0.492 | 33.40 | 9.82 | 0.879 | -0.866 | 0.386 | |
| Young modulus (MPa) | 0.8145 | 0.16 | 0.396 | 0.4740 | 0.19 | 0.747 | -2.021 | 0.043a | |
LI: Lens-induced; SD: Standard deviation. Shapiro-Wilk test (S-W test) was used to test normality. Mann-Whitney test (M-W test) was used to compare difference between the two groups. aP<0.05 for the difference between the control eye and the LI eye.
Figure 7. Illustration of biomechanical test.
A: Biomechanical test of scleral strips on an Instron 5565 universal testing machine; B: Stress-strain curve of tensile test. Two lines were chosen from one subject in this study. Ultimate stress of sclera in the LI eyes was lower than that in contralateral eyes (13.53 vs 18.65 MPa, P=0.021). Young's modulus was lower in the LI eyes than that in control eyes (0.4740 vs 0.8145 MPa, P=0.043). LI: Lens-induced.
DISCUSSION
Data from many studies suggest an active emmetropization mechanism that guides the postnatal development of the eye, matching the AXL to the focal plane. Hyperopic defocus caused by an infant's hyperopic refractive error modulates the axial growth of the eye to reduce refractive error. LI compensation for imposed hyperopic defocus has been shown in guinea pigs[7], tree-shrews[3],[8]–[10], chicks[11], marmosets[12], and monkeys[13]. Rabbits exhibited hyperopic refractive error compensation up to -6 D upon PRK induction[20]. In this study, 7-week-old rabbits were used due to previous findings that this was within the sensitive period where the rabbit eyes respond to hyperopic anisometropia. We used unilateral -8 D lens with a soft frame to induce defocused anisometropia, which was economically friendly and easy to manipulate and adjust by sutures using needle and strings in a soft frame made from Velcro. We demonstrated this effect when a hyperopic defocus stimulus was imposed by a -8 D lens, documenting that the eye elongated more rapidly in two weeks. The effect of spectacles on the axial elongation can be seen in Figure 4C. The divergence between the LI and control eyes increased steadily from baseline, with a significant difference of 0.35 mm in AXL (P=0.009). This differed a bit from another study, in which a -5 D PRK treatment was used, which took approximately 10wk to result in a difference of 0.56 mm in AXL. This difference may be due to corneal growth that diminishes the effects of PRK treatment in rabbits, which explains the regression of the effect[20]. This difference in axial length corresponds with the refraction changes (Table 2, Figure 4D). The lens thicknesses of LI and control eye were similar at any time point, which were coherent with existing studies of myopia models. This finding indicated that the crystalline lens have an almost nonexistent role in refractive error development, as demonstrated in other studies[24]. The effect of accommodation on the anterior segment of myopia remained unclear, and the role of the lens in myopia onset and progression is still controversial and requires further investigation.
After birth, the curvature of the rabbit cornea would decline for a year or more, which differs from that in tree shrew, whose curvature changes little after the first few weeks of visual exposure[14],[25]. It was suggested the rabbits' consecutively flattening cornea trend along with increasing AXL, resulting in a refractive error that is constant (0 to +3 D) during the rabbit's life[14]. This may explain why rabbits were only able to accommodate by 1.5-2.5 D[14]. Form deprivation or optical defocus had induced only low level myopia in rabbits[4],[20]. In our study, the refractive error was statistically significant between the control and LI eyes, with a median value of +3.75 D and +3.00 D, respectively. Decreased refractive error, along with elongated axial length was observed in both eyes during the experiment, which were consistent with a visual basis for emmetropization.
Biochemical and biomechanical characteristics of the sclera were essential in influencing the refractive status of the eye[2]–[3],[5]. Elongation of AXL may be controlled by a scleral remodelling mechanism, which modulates the collagen fibril creep and its rate[3]. Given that the changes in biomechanical properties of the human sclera can occur early in the development of myopia (within 24h)[5], this study demonstrated a weakening effect in the rabbit sclera of LI anisometropia after a 2-week-defocusing. Compared with the contralateral eye, the ultimate stress of the sclera in the induced rabbit eye decreased by 27.6%, and Young's modulus by 41.8%, while the ultimate strain increased 13.7% (Table 4, Figure 7B). The result of the biomechanical tests was consistent with McBrien et al[5] findings, which showed an increase in elasticity and scleral creep rate in myopic eyes and a reduction in the ultimate stress. A similar remarkable shift of the stress-strain curve was also reported by Murienne et al[6] recently in porcine sclera, and by Grytz and Siegwart[3] in tree shrew sclera. Changes in elastic modulus of the sclera indicates a dynamic biomechanical mechanism which underlies transient effects of stiffening in sclera[3]. Though the relationship between fibrillar shape and mechanical properties remains unclear, the diameter, distribution, and orientation of collagen fibrils have been suggested to significantly influence the biomechanical properties of the sclera[26]. In the present study, Young's modulus was determined as the slope of the stress-strain graph at a lower strain (10%), and fibril diameter in each layer was found to be significantly reduced in LI eyes compared with those in the control eyes, as suggested by electron microscopy (Table 3). The result was consistent with Christiansen et al's[27] hypothesis that lateral fusion of fibril subunits ensure sclera to resist deformation at low strain. Collagen fibrils of large diameter generate a high tissue tensile strength[28]. Additionally, the control eyes exhibited an increased gradient of the collagen fibrils diameter from the inner layer to the outer layer, whereas the LI eyes failed to have such a considerable size gradient. Therefore, the lower ultimate strength and Young's modulus can result from the large percentage of small-diameter fibrils and absence of normal gradient in the LI eyes. Densities of the elastic fibers and cells in the sclera decreased from the inner to outer scleral layers of both eyes (Figure 6A-6C, 6D-6I), whereas the elastic fibers were less in the outer layer of the LI eyes according to our observation. Compared with the control eyes, macrophage-like cells along with engulfed fibroblasts, dilated endoplasmic reticulum, and vacuoles (Figure 6E, 6F) were observed in the middle stroma in the LI eyes, indicated a degradative activity, which may degrade components of the extracellular matrix of sclera and thus result in the reduction of scleral stiffening. Apart from scleral biomechanics, a net loss of scleral tissue through reduced collagen synthesis and increased degradation, changes in the composition of the sclera[29], and remodeling of the scleral with decreased proteoglycan synthesis[8]–[10],[30] were associated with induced eye elongation. Reduced levels of glycosaminoglycans and proteoglycan of the sclera[8]–[10] might have altered the in vivo hydration of the sclera due to lens application. The similarity between myopia and autoimmune diseases was confirmed by C-reactive proteins and complement components found in human pathological myopia[31]. According to observations by electronic microscope, we therefore hypothesized that lens application induces alterations of normal metabolic process of scleral remodeling, and in turn represent a state of low-grade systemic or local inflammation, which are similar features in autoimmune diseases. We did not count scleral cells or proteoglycan levels here, although a high proportion of scleral cells have contractile potential[32]. However, Backhouse and Phillips[32] found that the proportion was unaffected by two weeks' monocular form deprivation in guinea pigs. The relationship between the total number and proportion of scleral cells, proteoglycan levels, and induced eye elongation needs further investigation.
Several limitations were observed when interpreting the results of the present research. We did not measure the corneal curvature of the rabbits. Cutting the scleral strips may alter the biomechanical characteristics of the sclera, which differs from its condition in vivo. Instead of being subjected to uniaxial tension in our mechanical tests, inflation test may offer us better estimates of scleral material properties in vivo[33]–[34]. To investigate lens-induced myopia on rabbits, experiments of more eyes in a longer term to study the period of recovery and in various degrees of lens, which includes positive lens due to the bidirectional visual compensatory mechanism, and analysis of general synthesis and degradation of scleral collagen are necessary. However, we did not modify the corneal curvature as previous researchers do by PRK induction[20], and corneal power was not significantly different between eyes with lens wearing and control eyes according to previous studies[13],[35]. Meanwhile, all tissue samples went through exactly the same process and tests while retinoscopy was conducted by the same optometrist who was masked to the experiment throughout the course; hence, reported trends on the differences of dioptric and material characteristics between the LI and the control eyes would remain even though the absolute values might differ.
To summarize, these experimental results demonstrated that ocular defocus by our spectacle frames resulted in significant differences in refractive error, AXL, and vitreous chamber depth. In addition, we identified changes of the material characteristics and microstructure of the rabbits sclera in the early-stage of LI compensation for defocusing. These results support the hypothesis that spectacle lenses can change development of children's eyes. The spectacle frame apparatus used in this study provides a method for the study of lens-induced myopia in rabbits and allows the use of form deprivation as well, which makes this apparatus attractive for future rabbit myopia experiments. Various reports using lens-induced myopia and form deprivation myopia paradigms have yielded contradicting conclusions regarding whether the ocular consequences of both develop from similar or different underlying mechanisms and the same method to block the development of myopia can result differently in different animals[1]; our study facilitates further research on lens-induced myopia in rabbits. Further experimental investigations are needed to gain more insights into scleral remodeling mechanisms, which underlie elongated AXL in myopia.
Acknowledgments
The authors would like to acknowledge Dr Richard L. Converse, Department of Biological Sciences, University of Cincinnati, United States, for his help in editing and proofreading the final paper.
Foundation: Supported by the Science and Technology Commission of Shanghai Municipality (No.134119a5100).
Conflicts of Interest: Lin X, None; Wang BJ, None; Wang YC, None; Chu RY, None; Dai JH, None; Zhou XT, None; Qu XM, None; Liu H, None; Zhou H, None.
REFERENCES
- 1.Morgan IG, Ashby RS, Nickla DL. Form deprivation and lens-induced myopia: are they different? Ophthal Physl Opt. 2013;33(3):355–361. doi: 10.1111/opo.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rada JA, Shelton S, Norton TT. The sclera and myopia. Exp Eye Res. 2006;82(2):185–200. doi: 10.1016/j.exer.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Grytz R, Siegwart JJ., Jr Changing material properties of the tree shrew sclera during minus lens compensation and recovery. Invest Ophthalmol Vis Sci. 2015;56(3):2065–2078. doi: 10.1167/iovs.14-15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin Z, Chen X, Ge J, Cui D, Wu J, Tang F, Tan J, Zhong X, Gao Q. Effects of direct intravitreal dopamine injection on sclera and retina in form-deprived myopic rabbits. J Ocul Pharmacol Ther. 2008;24(6):543–550. doi: 10.1089/jop.2008.0041. [DOI] [PubMed] [Google Scholar]
- 5.McBrien NA, Jobling AI, Gentle A. Biomechanics of the sclera in myopia: extracellular and cellular factors. Optom Vis Sci. 2009;86(1):23–30. doi: 10.1097/OPX.0b013e3181940669. [DOI] [PubMed] [Google Scholar]
- 6.Murienne BJ, Jefferys JL, Quigley HA, Nguyen TD. The effects of glycosaminoglycan degradation on the mechanical behavior of the posterior porcine sclera. Acta Biomater. 2015;12:195–206. doi: 10.1016/j.actbio.2014.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu S, Li S, Wang B, Lin X, Wu Y, Liu H, Qu X, Dai J, Zhou X, Zhou H. Scleral cross-linking using riboflavin UVA irradiation for the prevention of myopia progression in a guinea pig model: Blocked axial extension and altered scleral microstructure. PLoS One. 2016;11(11):e165792. doi: 10.1371/journal.pone.0165792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siegwart JJ, Jr, Strang CE. Selective modulation of scleral proteoglycan mRNA levels during minus lens compensation and recovery. Mol Vis. 2007;13:1878–1886. [PubMed] [Google Scholar]
- 9.Moring AG, Baker JR, Norton TT. Modulation of glycosaminoglycan levels in tree shrew sclera during lens-induced myopia development and recovery. Invest Ophthalmol Vis Sci. 2007;48(7):2947–2956. doi: 10.1167/iovs.06-0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao H, Frost MR, Jr, Siegwart JT, Norton TT. Patterns of mRNA and protein expression during minus-lens compensation and recovery in tree shrew sclera. Mol Vis. 2011;17:903–919. [PMC free article] [PubMed] [Google Scholar]
- 11.Hammond DS, Wallman J, Wildsoet CF. Dynamics of active emmetropisation in young chicks--influence of sign and magnitude of imposed defocus. Ophthalmic Physiol Opt. 2013;33(3):215–226. doi: 10.1111/opo.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benavente-Perez A, Nour A, Troilo D. The effect of simultaneous negative and positive defocus on eye growth and development of refractive state in marmosets. Invest Ophthalmol Vis Sci. 2012;53(10):6479–6487. doi: 10.1167/iovs.12-9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith ER, 3rd, Hung LF, Arumugam B, Huang J. Negative lens-induced myopia in infant monkeys: effects of high ambient lighting. Invest Ophthalmol Vis Sci. 2013;54(4):2959–2969. doi: 10.1167/iovs.13-11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prince JH. The rabbit in eye research. Springfield, Ill.: C.C. Thomas; 1964. [Google Scholar]
- 15.Ahn SJ, Hong HK, Na YM, Park SJ, Ahn J, Oh J, Chung JY, Park KH, Woo SJ. Use of rabbit eyes in pharmacokinetic studies of intraocular drugs. J Vis Exp. 2016;(113) doi: 10.3791/53878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maurice DM, Mushin AS. Production of myopia in rabbits by raised body-temperature and increased intraocular pressure. Lancet. 1966;2(7474):1160–1162. doi: 10.1016/s0140-6736(66)90476-4. [DOI] [PubMed] [Google Scholar]
- 17.Verolino M, Nastri G, Sellitti L, Costagliola C. Axial length increase in lid-sutured rabbits. Surv Ophthalmol. 1999;44(Suppl 1):S103–S108. doi: 10.1016/s0039-6257(99)00087-9. [DOI] [PubMed] [Google Scholar]
- 18.Dotan A, Kremer I, Gal-Or O, Livnat T, Zigler A, Bourla D, Weinberger D. Scleral cross-linking using riboflavin and ultraviolet-A radiation for prevention of axial myopia in a rabbit model. J Vis Exp. 2016;(110):e53201. doi: 10.3791/53201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nie HH, Huo LJ, Yang X, Gao ZY, Zeng JW, Trier K, Cui DM. Effects of 7-methylxanthine on form-deprivation myopia in pigmented rabbits. Int J Ophthalmol. 2012;5(2):133–137. doi: 10.3980/j.issn.2222-3959.2012.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bryant MR, Kampmeier J, Er H, Kasetsuwan N, Sanchez-DiMartino D, Shah SS, McDonnell PJ. PRK-induced anisometropia in the rabbit as a model of myopia. Graefes Arch Clin Exp Ophthalmol. 1999;237(2):161–165. doi: 10.1007/s004170050212. [DOI] [PubMed] [Google Scholar]
- 21.Tokoro T. Experimental myopia in rabbits. Invest Ophthalmol. 1970;9(12):926–934. [PubMed] [Google Scholar]
- 22.Funata M, Tokoro T. Scleral change in experimentally myopic monkeys. Graefes Arch Clin Exp Ophthalmol. 1990;228(2):174–179. doi: 10.1007/BF00935729. [DOI] [PubMed] [Google Scholar]
- 23.Wollensak G, Iomdina E. Crosslinking of scleral collagen in the rabbit using glyceraldehyde. J Cataract Refract Surg. 2008;34(4):651–656. doi: 10.1016/j.jcrs.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 24.Sivak JG. The role of the lens in refractive development of the eye: animal models of ametropia. Exp Eye Res. 2008;87(1):3–8. doi: 10.1016/j.exer.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Norton TT, McBrien NA. Normal development of refractive state and ocular component dimensions in the tree shrew (Tupaia belangeri) Vision Res. 1992;32(5):833–842. doi: 10.1016/0042-6989(92)90026-f. [DOI] [PubMed] [Google Scholar]
- 26.Girard MJ, Dahlmann-Noor A, Rayapureddi S, Bechara JA, Bertin BM, Jones H, Albon J, Khaw PT, Ethier CR. Quantitative mapping of scleral fiber orientation in normal rat eyes. Invest Ophthalmol Vis Sci. 2011;52(13):9684–9693. doi: 10.1167/iovs.11-7894. [DOI] [PubMed] [Google Scholar]
- 27.Christiansen DL, Huang EK, Silver FH. Assembly of type I collagen: fusion of fibril subunits and the influence of fibril diameter on mechanical properties. Matrix Biol. 2000;19(5):409–420. doi: 10.1016/s0945-053x(00)00089-5. [DOI] [PubMed] [Google Scholar]
- 28.Parry DA. The molecular and fibrillar structure of collagen and its relationship to the mechanical-properties of connective-tissue. Biophys Chem. 1988;29(1-2):195–209. doi: 10.1016/0301-4622(88)87039-x. [DOI] [PubMed] [Google Scholar]
- 29.Gentle A, Liu Y, Martin JE, Conti GL, McBrien NA. Collagen gene expression and the altered accumulation of scleral collagen during the development of high myopia. J Biol Chem. 2003;278(19):16587–16594. doi: 10.1074/jbc.M300970200. [DOI] [PubMed] [Google Scholar]
- 30.Rada JA, Nickla DL, Troilo D. Decreased proteoglycan synthesis associated with form deprivation myopia in mature primate eyes. Invest Ophthalmol Vis Sci. 2000;41(8):2050–2058. [PubMed] [Google Scholar]
- 31.Long Q, Ye J, Li Y, Wang S, Jiang Y. C-Reactive protein and complement components in patients with pathological myopia. Optom Vis Sci. 2013;90(5):501–506. doi: 10.1097/OPX.0b013e31828daa6e. [DOI] [PubMed] [Google Scholar]
- 32.Backhouse S, Phillips JR. Effect of induced myopia on scleral myofibroblasts and in vivo ocular biomechanical compliance in the guinea pig. Invest Ophthalmol Vis Sci. 2010;51(12):6162–6171. doi: 10.1167/iovs.10-5387. [DOI] [PubMed] [Google Scholar]
- 33.Grytz R, Fazio MA, Girard MJ, Libertiaux V, Bruno L, Gardiner S, Girkin CA, Downs JC. Material properties of the posterior human sclera. J Mech Behav Biomed Mater. 2014;29:602–617. doi: 10.1016/j.jmbbm.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fazio MA, Grytz R, Bruno L, Girard MJ, Gardiner S, Girkin CA, Downs JC. Regional variations in mechanical strain in the posterior human sclera. Invest Ophthalmol Vis Sci. 2012;53(9):5326–5333. doi: 10.1167/iovs.12-9668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Metlapally S, McBrien NA. The effect of positive lens defocus on ocular growth and emmetropization in the tree shrew. J Vis. 2008;8(3):1.1–12. doi: 10.1167/8.3.1. [DOI] [PubMed] [Google Scholar]