Abstract
AIM
To evaluate human lens epithelium cell apoptosis and epithelial to mesenchymal transition (EMT) induced by femtosecond laser in femtosecond laser assisted cataract surgery (FLACS).
METHODS
Sixty cataract patients with N2 to N3 stage according to the LOCS III were enrolled in this study and divided into three groups randomly: FLACS1 group (cataract surgery by FLACS with LenSx), FLACS2 group (cataract surgery by FLACS with LensAR) and manual group (cataract surgery by phacoemulsification). Patients in two FLACS groups performed anterior capsulotomy by LenSx or LensAR laser system. Patients in the manual group were performed continuous curvilinear capsulorrhexis (CCC) manually. The anterior capsules were fixed right after moved out of eye. Hematoxylin-eosine staining, immunofluorescence staining and real-time PCR were performed in order to observe human lens epithelium cells changes after cataract surgery.
RESULTS
The capsule cutting edge was shown irregularity and roughness in two FLACS groups and smooth edge in manual capsulotomy by pathologic staining. Irregularities of the cell configuration with partly swollen and destroyed nuclei were observed in two FLACS groups. Femtosecond laser could induce a significantly higher cell apoptosis in human lens epithelium cell than manually performed CCC (P<0.05). Lens epithelium cells apoptosis were correlated with femtosecond laser duration according to Pearson correlation analysis. Decreased N-cadherin expression, alpha-SMA and FSP-1 level in two FLACS groups showed the inhibition of cell EMT.
CONCLUSION
Femtosecond laser may affect the apoptosis and EMT of lens epithelium cells which are under the peeled central lens capsule.
Keywords: femtosecond lasers assisted cataract surgery, lens epithelium cell, apoptosis, epithelial mesenchymal transition
INTRODUTION
Posterior capsular opacification (PCO) was the main reason for the decrease of visual acuity after cataract surgery. The reasons for the formation of PCO include residual lens epithelial cells (LECs) proliferation, adhesion, migration and epithelial to mesenchymal transition (EMT). After the cataract surgery, part of the residual LECs moved to the central area of the posterior capsule and secreted a series of collagen fibers. However, the pathogenesis and prevention of PCO remains unclear.
Femtosecond laser technology, introduced clinically for ophthalmic surgery in 2001 as a new technique for creating lamellar flaps in laser in situ keratomileusis (LASIK), has recently been developed into a tool for cataract surgery[1]. In the past few years, femosecond laser assisted cataract surgery (FLACS) has become an accepted method of surgical intervention by ophthalmologists and patients. The most important features of FLACS are the creation of precise anterior capsulotomies, liquefaction of nucleus, construction of corneal wounds in any position and size, and treatment of preoperative astigmatism[2]–[3]. There are four kinds of femtosecond laser system in the clinical application. Two types of patient interface (PI) are available, which are named touch fluid filled interface and no-touch fluid filled interface.
Current femtosecond laser technology systems use neodymium (Nd): glass 1053 nm (near-infrared) wavelength light. This feature allows the light to be focused at a 3-µm spot size, with an accuracy of within 5 µm in the anterior segment[4]. The focused ultrashort pulses (10-15s) eliminate the collateral damage of surrounding tissues and the heat generation associated with slower excimer and Nd:YAG lasers. This attribute of femtosecond lasers is especially important for cataract surgery, wherein preservation of ocular structures like the cornea, iris, zonules, and capsular bag is critical for good visual outcomes.
The efficiency, safety and acceptable complication rate of FLACS have been demonstrated clinically by some investigations[5]–[6]. However, a significantly higher cell rate apoptosis in femtosecond laser-created corneal incisions was shown in one study, in comparison to that of manually performed incisions, indicating an upregulated postoperative wound-healing response[7]. Another study showed a rapid PCO formation after FLACS, which was considered as a result of thermomechanical damage to the LECs[8]. The aim of this study was to observe the morphological and histological changes of human LECs at anterior lens capsule after FLACS and traditional phacoemulsification cataract surgery, analyze LECs apoptosis and EMT after FLACS and traditional phacoemulsification cataract surgery, and discuss the effect of two different femtosecond laser system (LenSx and LensAR) on PCO.
SUBJECTS AND METHODS
The experimental study was approved by the local ethics committee and adhered to the tenets of the Declaration of Helsinki (ChiCTR-OCH-14005038). It included 60 patients with N2 to N3 cataract stage according to the LOCS III. Each patient had a complete ophthalmologic evaluation. Patients with previous ocular surgery, trauma, active ocular disease, poorly dilated pupils, substantial corneal opacity or known zonular weakness were excluded. Written informed consent was obtained from all patients and all clinical investigation conducted.
Patients were divided randomly into three groups: FLACS1 (cataract surgery by FLACS with LenSx), FLACS2 (cataract surgery by FLACS with LensAR) and the manual group (cataract surgery by phacoemulsification). One cataract surgeon (Zhang JS) performed all the procedures at China Medical University between May 2014 and July 2016. Patients with FLACS were performed with anterior capsulotomy, lens prefragmentation and corneal incision creation with two different femtosecond laser systems (LenSx or LensAR). Patients with phacoemulsification cataract surgery were performed with manual continuous curoilinear capsulorrhexis (CCC) and lens fragmentation. Infiniti vision system (Alcon, USA) was available for all the patients.
Surgical Technique
The LenSX laser system (Alcon, Inc., USA) is one kind of ultra-short pulse laser (10−15s) systems. This system has an infrared wavelength of 1030 nm and a touch fluid filled interface[9]. The LensAR laser system (LensAR, Inc., USA) has a no-touch fluid filled interface, which minimizes in intraocular pressure increases and corneal folds development during laser procures because of the absence of contact[10]. This 80 kHz femtosecond infrared laser has a pulse width of 600 to 800fs, a central laser wavelength of 1030 nm, and maximum pulse energy range from 5 to 15 µJ with a spot separation size of 5 µm for performing femtosecond laser-assisted anterior capsulotomy.
During the surgeries, the capsulotomy diameter was set to be 5.5 mm, the anterior/posterior safety zone for laser treatment was set to be 300 µm and a 2.8 mm clear corneal incision (CCI) was created superiorly or temporally. All surgeries in two FLACS groups were performed under topical anesthesia. Pranoprofen and phenylephrine hydrochloride 0.5% (Mydrin-P) were used preoperatively to maintain pupil dilation throughout the surgery. Laser treatment started with an anterior capsulotomy, followed by lens fragmentation. Sodium chondroitin sulfate-sodium hyaluronate (Discovisc) was injected into the anterior chamber, and the capsule button was removed with forceps.
Phacoemulsification cataract surgeries were performed under topical anesthesia. Pranoprofen and phenylephrine hydrochloride 0.5% (Mydrin-P) were used in all cases in the same way as in the FLACS groups. The surgeries were performed through a 2.8 mm clear corneal incision. A 5.0-5.5 mm capsulorrhexis was created after the anterior chamber was filled with Discovisc. The capsule was removed right after CCC. Infiniti System (Alcon, USA) was used and foldable intraocular lens (single-piece acrylic IOL) implanted using an IOL injector in both groups.
Histology Assay
All the anterior lens capsules with adherent LECs were placed in tubes filled with fortified balanced salt solution after moved from the eyes. Then the anterior capsules were transferred into 10% neutral-buffered formalin immediately. After fixation, 10 capsules of each group were stained directly with standard hematoxylin and eosine (HE) and the other 10 capsules were further dehydrated through graded alcohols, cleared in xylene and embedded in paraffin. Sections of 4 µm were cut for transferase deoxy-UTP-nick end labeling (TUNEL) fluorescein analysis, HE staining and immunohistochemical study.
Each section was inspected over its entire length using total magnifications of ×200, ×400, and ×1000. The expressions of all used assays were recorded. For apoptosis cell quantification, only cells with an illumination threshold of more than 70% in counterstained samples were visualized and enumerated. All the specimens were divided into two parts, central capsule and peripheral area (cutting edge). Five regions were selected from each part randomly and cells were counted under the ×400 microscopic field. The total number of cells in 5 non-overlapping full thickness columns extending from the anterior epithelial surface to the posterior stoma surface was manually counted for each specimen and the mean cell counts were averaged.
Transferase Deoxy-utp-nick End Labeling Analysis
The sections were rehydrated through graded alcohols. The terminal deoxynucleotidyl TUNEL assay was performed using a standard kit, the fluorescein DeadEnd™ Fluorometric TUNEL System (Promega, Madison, WI, USA). The TUNEL assay was used to detect the ends of DNA fragments formed during the apoptosis process. To differentiate between nonspecific cells, counterstaining was performed using 4′, 6-diamidino-2-phenylindole dihydrochloride (DAPI) in all the groups. All the tissue sections were covered with a mounting medium. Bright-field and fluorescence images of sections were taken by Olympus microscope (BX51, Olympus) with a SPOT camera.
Immunohistochemistry Assay
The anterior capsules were fixed right after moved out of eye. Fixed sections were rehydrated. Endogenous peroxidases were blocked with 3% H2O2. Epitope retrieval was performed in a 0.1 mol/L sodium citrate buffer (pH 6.8) at 100°C for 10min before adding blocking reagents. After the addition of primary antibodies, sections were incubated in a humidified chamber at 4°C overnight. E-cadherin and N-cadherin antibody (Life, Inc., USA) were used. Diaminobenzidine (DAB) (Life, San Francisco, CA, USA) compound for signal amplification.
Quantitative Analysis
Human lens anterior capsules were removed during the surgery, and washed there times with PBS. Total RNA from human LECs was extracted using Trizol® Reagent (Invitrogen, NY, USA) following the manufacturer's instructions. For realtime-polymerase chain reaction (realtime-PCR) analysis of alpha-SMA, FSP-1 and vimentin, RNA was reverse-transcribed with PrimerScript RT reagent kit (Takara, Dalian, China). The resulting cDNA was amplified using ALPHA-SMA, FSP-1 and vimentin primers with SYBR Premix Ex TaqTM II (Takara, Dalian, China) with the following parameters 95°C for 30s, followed by 40 cycles at 95°C for 5s, and at 60°C for 30s. Primers for alpha-SMA (human) were 5′-GCCTTGGTGTGTGACAATGG-3′ (forward) and 5′-AAAACAGCCCTGGGAGCAT-3′ (reverse)[11]. Primers for FSP-1 (human) were: 5′-GCTTCTTCTTTCTTGGTTTG-3′ (forward) and 5′-CTCCTTTAGTTCTGACTTGTTG-3′ (reverse)[11]. Primers for vimentin (human) were: 5′-CAATGAGTCCCTGGAACGCC-3′ (forward) and 5′-TCCAGATTAGTTTCCCTCAGGTTC-3′ (reverse)[12]. In order to ensure product specificity, melting curve analysis was performed at the end of the cycles. The relative quantity of alpha-SMA, FSP-1 and vimentin was calculated based on the equation RQ= 2−ΔΔCT.
Statistical Analysis
Data was evaluated by statistical software (SPSS version 13.0). Analysis of variance was used to compare the number of positive labeled cells in different lens capsule areas, apoptosis rates, E-cadherin, N-cadherin, alpha-SMA, FSP-1 levels in two FLACS groups and the manual group. Variations were expressed as standard errors of the mean. Pearson correlation analysis was conduct and all these analyses were performed using SPSS software. P<0.05 was considered to be significant.
RESULTS
Clinical Outcomes
Since all the patients undergoing cataract surgeries were divided into two FLACS groups and manual group randomly, there was no difference in demographics or baseline characteristics between each group (Table 1). All the surgical treatments were uneventful and a complete anterior lens capsule removal was possible in all the groups. In FLACS1 group, the overall time required of capsulotomy and lens pre-fragmentation was 29.91±5.68s. The energy was 6 µJ in capsulorrhexis and 12 µJ in lens fragmentation. In FLACS2 group, the overall time required of capsulotomy and lens pre-fragmentation was 12.54±2.19s. The energy was 7 µJ in capsulorrhexis and 12 µJ in lens fragmentation.
Table 1. Demographics of all patients according to age, gender.
| Patients | FLACS1 group | FLACS2 group | Manual group |
| Gender | |||
| F | 12 (60.0) | 11 (55.0) | 11 (55.0) |
| M | 8 (40.0) | 9 (45.0) | 9 (45.0) |
| Age (y) | 61.3±4.9 | 59.9±4.3 | 59.8±6.0 |
| Eye | |||
| Right | 13 (65.0) | 12 (60.0) | 9 (45.0) |
| Left | 7 (35.0) | 8 (40.0) | 11 (55.0) |
n (%)
Histopathologic Findings
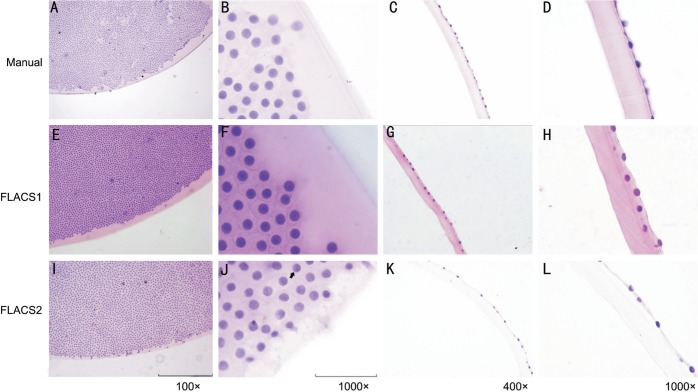
For pathologic staining of lens anterior capsule, the capsule cutting edge was shown irregularity and roughness in two FLACS groups and smooth edge in manual capsulotomy. HE staining revealed a regular distribution and shape of LECs in the manual group and no cell demarcation area (Figure 1A, 1B). In the peripheral part, cell distribution near the demarcation line of the anterior capsule seemed to be the same in three groups under lower magnifications (Figure 1A, 1E, 1I). Whereas, irregularities of the cell configuration with partly swollen and destroyed nuclei were observed in the FLACS groups under 1000× magnified immersion objective (Figure 1B, 1F, 1J). Lens epithelium cell dropped from anterior capsules severely after standard fixation, embedding and staining procedures and residual cell number significantly decreased in FLACS1 group (16.98±3.78) and FLACS2 group (15.07±5.74) with statistical significance compared with the manual group (27.46±8.83) (Figures 1D, 1H, 1L, 2M) (P<0.05). There were subtle differences between two FLACS groups. The lens anterior capsule edge in FLACS2 group was more irregular than that in FLACS1 group (Figure 1E, 1I). The exfoliated cells in FLACS1 group had a distance of approximately 3-4 LECs rows, while only 1-2 LECs rows distance could be found in FLACS2 group (Figure 1H, 1L).
Figure 1. HE staining of lens anterior capsule in manual group (A-D), FLACS1 group (E-H), and FLACS2 group (I-L).
The capsule cutting edge irregularity and roughness in two FLACS groups (E, I) and smooth edge in manual group (A). Lens epithelium cells showed a regular distribution and shape with even disperse chromatin in manual group (B) but irregularities of the cell configuration with partly swollen and destroyed nuclei were observed in two FLACS groups under 1000× magnified immersion objective (F, J). Lens epithelium cell dropped from anterior capsules severely after standard fixation, embedding and staining procedures and cell number significantly decreased in two FLACS groups (G, H, K, L) compared with the manual group (C, D). The lens anterior capsule edge in FLACS2 group was more irregular than that in FLACS1 group (E, I). The exfoliated cells in FLACS1 group had a distance of approximately 3-4 LECs rows, while only 1-2 LECs rows distance could be found in FLACS2 group (H, L).
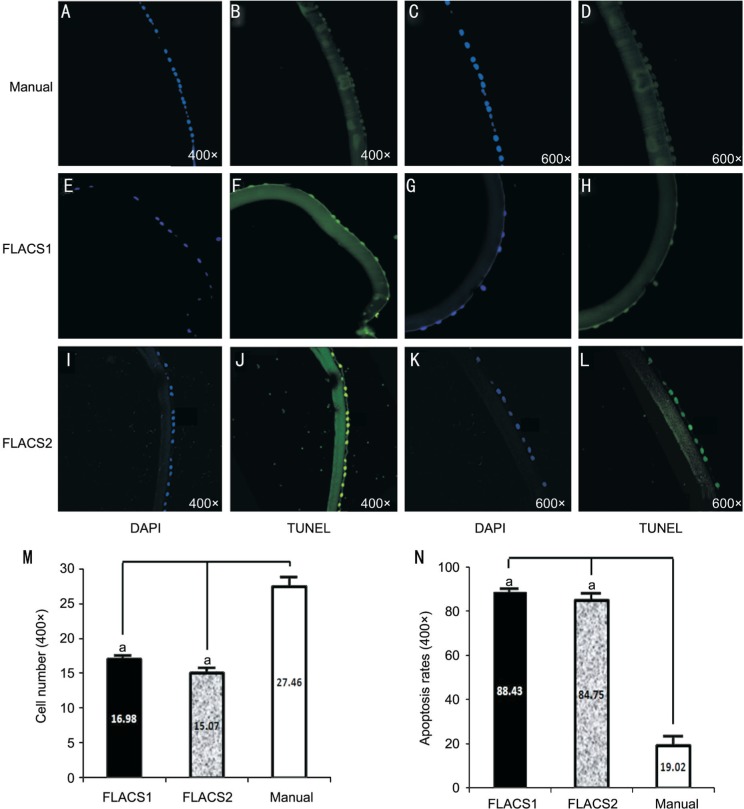
Figure 2. Apoptosis analysis by TUNEL staining.
Apoptosis cell was rarely found in the manual group (A-D). Apoptosis cell was found at every counting region in two FLACS groups (E-L), and there was significant difference of apoptosis cell rate between two FLACS groups and the manual group (N). The residual lens epithelium cell decreased severely in two FLACS groups after standard fixation, embedding and staining procedures compared with the manual group (M). aP<0.05.
Apoptosis Analysis
Apoptotic cells were detected in all specimens of three groups at central area and along the cutting edges. In manual group, apoptotic cells were rarely found at central area and randomly found at peripheral region (Figure 2A-2D). In two FLACS groups, apoptotic cells were found at every region counted and there was no statistic difference between central area and peripheral region (Figure 2E-2L). No marked difference was found between two FLACS groups. Comparing groups, the highest number of TUNEL positive cells was found in the FLACS groups at peripheral region followed by FLACS central area, manual group peripheral area and central area. Comparing groups, apoptosis cell rate was 88.43%±14.5% in FLACS1 group, 84.75%±18.9% in FLACS2 group and 19.02%±21.9% in the manual group, and there was significant statistic difference between two FLACS groups and the manual group (P<0.05) (Figure 2N). Furthermore, lens epithelium cell apoptosis rate correlated with femtosecond laser duration according to Pearson correlation analysis results in the FLACS groups (n=60, r=0.815).
Immunohistochemical Assay
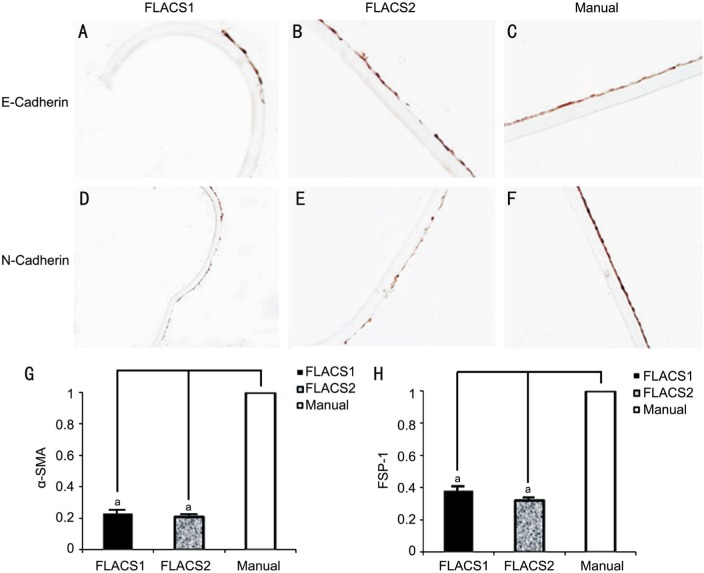
E-cadherin and N-cadherin were expressed in the LECs. DAB staining showed the expression of E-cadherin did not change obviously (Figure 3A, 3B), while the expression of N-cadherin decreased significantly (Figure 3D, 3E) in two FLACS groups compared with the manual group (Figure 3C, 3F). This suggested that the growth of lens epithelial cells was repressed and the ability of cell migration in the anterior lens epithelium was weakened. No difference could be found between two FLACS groups.
Figure 3. E-cadherin and N-cadherin expression in two FLACS groups and the manual group.
E-cadherin expression did not change obviously (A, B). N-cadherin expression decreased (D, E) in FLACS1 group and FLACS2 group compared with the manual group (C, F). No difference could be found between two FLACS groups. Alpha-SMA level decreased in FLACS1 group (0.23±0.15) and FLACS2 group (0.21±0.07) compared with the manual group (G). FSP-1 level decreased in FLACS1 group (0.38±0.17) and FLACS2 group (0.32±0.12) compared with the manual group (H). These differences were statistically significant. aP<0.05.
Realtime-polymerase Chain Reaction Analysis
Alpha-SMA level decreased in FLACS1 group (0.23±0.15) and FLACS2 group (0.21±0.07) compared with the manual group (Figure 3G). FSP-1 level decreased in FLACS1 group (0.38±0.17) and FLACS2 group (0.32±0.12) compared with the manual group (Figure 3H). These differences were statistically significant. There was no difference in vimentin level among three groups.
DISCUSSION
This prospective study demonstrated the morphological and histological changes, the occurrence of apoptosis and EMT in the LECs after FLACS. In research we discovered that apoptosis can be induced by femtosecond laser on human LECs in FLACS. A significant higher apoptosis rate of lens epithelium cell in two FLACS groups was found with less residual cells at the lens anterior capsules. Most prominent apoptosis areas were around the cutting edge in two femtosecond laser groups, and cell number decreased significantly in all anterior capsules because of cell dropping during fixation and embedding. These results were consistent with the previous study. Mayer et al[13] showed that femtosecond laser could induced LEC death by using LenSx femtosecond laser system and proved that cell death depends on the laser pulse energy settings by comparing the 5 µJ with 15 µJ laser energy setting results. We found the irregularity and roughness of capsule cutting edge in two FLACS groups (LenSx and LensAR) and smooth edge of manual capsulotomy as previously reported[14]. However, the distribution of the residual LECs around cutting edge and the demarcation area were almost the same in two FLACS groups and the manual group, which was different from Mayer et al's[13] results. We also found morphology changes of epithelial cells in two FLACS groups, such as the swelling of cell nucleus, hyperchromatic nuclei with chromatin granules, implying the LEC damage. Mayer et al[13] showed that the degree of cell death depends on the laser pulse energy settings. In the present study, the cell apoptosis rate also increased proportionally with laser duration. In addition, the standard apoptosis analysis performed in the embedded anterior capsule specimens showed more detached LECs from the capsule in two FLACS groups. DAPI stained cell number significantly decreased in two FLACS groups compared with manual group, suggesting less LEC adhesiveness and possible compromised subsequent LEC migration on the lens capsule. There were some differences between the two FLACS groups. FLACS with LensAR laser system was more destructive to the LECs on the edge of lens anterior capsule than the system of LenSx. The morphology changes of LECs in LensAR group were more obvious than that in LenSx group.
LECs EMT was another important index in the study of cataract surgery. Mochizuki et al[15] reported that E-cadherin and N-cadherin expressed in lens epithelium was closely related to lens fiber growth and cells movement. E-cadherin suppressed cell movement and EMT, while N-cadherin promoted cell movement and EMT by combining with α-catenin and actin filaments[16]. Expression of alpha-SMA, FSP-1 and vimentin could promote the transformation of LECs into fibroblasts or muscle fibroblasts. At present, there was no relative detailed report about LECs EMT after FLACS. In this study, markedly decreased N-cadherin, alpha-SMA, FSP-1 expression in two FLACS groups showed the inhibition of LECs EMT and movement compared with the manual group. It could be inferred that FLACS could reduce the incidence of PCO after femtosecond laser-assisted cataract surgery.
PCO is the most common complication after cataract surgery. The prevalence of PCO five years after surgery has been reported to be as high as 30%-40% in adults and 100% in children[17]. Histo-pathological data have indicated that the primary causes of PCO are the proliferation and transformation of the posterior capsule due to metaplasia and migration of the residual LECs of the anterior capsule or equator[18]. Although many methods for preventing PCO, such as antitumor medications, have been investigated, almost no effective measure has emerged except square edge shaped IOL design[19]–[20]. Therefore, the inhibition of LECs proliferation can possibly become an important development trend. The incidence of PCO after FLACS is still controversial in the previous study[21]–[23]. Aron-Rosa and Aron[24] used an Nd:YAG laser to perform anterior capsulotomies prior to conventional surgery. They found markedly decreased incidence of PCO in patients treated with laser capsulotomy (3.27% vs 50%). Rostami et al[8] showed a higher rate of early PCO following FLACS in his clinical study while Kovács et al[25] drew the opposite conclusion. Wertheimer et al[26] inferred that PCO formation might not be attributed to the type of surgery. In this study, the increased apoptosis rates and decreased EMT of LECs could contribute to a compromised LEC migration and reduced incidence of PCO in two FLACS groups. Further studies and long-term follow up of clinical patients who underwent FLACS are needed to confirm the effect of the femtosecond laser on the development of PCO over time.
Acknowledgments
Conflicts of Interest: Sun W, None; Liu J, None; Li J, None; Wu D, None; Wang J, None; Wang MW, None; Zhang JS, None; Zhao JY, None.
REFERENCES
- 1.Nagy Z, Takacs A, Filkorn T, Sarayba M. Initial clinical evaluation of an intraocular femtosecond laser in cataract surgery. J Refract Surg. 2009;25(12):1053–1060. doi: 10.3928/1081597X-20091117-04. [DOI] [PubMed] [Google Scholar]
- 2.Blehm C, Potvin R. Pseudophakic astigmatism reduction with femtosecond laser-assisted corneal arcuate incisions: a pilot study. Clin Ophthalmol. 2017;11:201–207. doi: 10.2147/OPTH.S127279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manning S, Barry P, Henry Y, Rosen P, Stenevi U, Young D, Lundström M. Femtosecond laser-assisted cataract surgery versus standard phacoemulsification cataract surgery: study from the European Registry of Quality Outcomes for Cataract and Refractive Surgery. J Cataract Refract Surg. 2016;42(12):1779–1790. doi: 10.1016/j.jcrs.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Kullman G, Pineda R., 2nd Alternative applications of the femtosecond laser in ophthalmology. Semin Ophthalmol. 2010;25(5-6):256–264. doi: 10.3109/08820538.2010.518507. [DOI] [PubMed] [Google Scholar]
- 5.Abell RG, Kerr NM, Vote BJ. Femtosecond laser-assisted cataract surgery compared with conventional cataract surgery. Clin Exp Ophthalmol. 2013;41(5):455–462. doi: 10.1111/ceo.12025. [DOI] [PubMed] [Google Scholar]
- 6.Conrad-Hengerer I, Al Juburi M, Schultz T, Hengerer FH, Dick HB. Corneal endothelial cell loss and corneal thickness in conventional compared with femtosecond laser-assisted cataract surgery: three-month follow-up. J Cataract Refract Surg. 2013;39(9):1307–1313. doi: 10.1016/j.jcrs.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 7.Mayer WJ, Klaproth OK, Hengerer FH, Kook D, Dirisamer M, Priglinger S, Kohnen T. In vitro immunohistochemical and morphological observations of penetrating corneal incisions created by a femtosecond laser used for assisted intraocular lens surgery. J Cataract Refract Surg. 2014;40(4):632–638. doi: 10.1016/j.jcrs.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Rostami B, Tian J, Jackson N, Karanjia R, Lu K. High rate of early posterior capsule opacification following femtosecond laser-assisted cataract surgery. Case Rep Ophthalmol. 2016;7(3):213–217. doi: 10.1159/000449124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts TV, Lawless M, Sutton G, Hodge C. Update and clinical utility of the LenSx femtosecond laser in cataract surgery. Clin Ophthalmol. 2016;17(10):2021–2029. doi: 10.2147/OPTH.S94306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu AY, Lin CX, Wang QM, Zheng MQ, Qin XY. Safety of femtosecond laser-assisted cataract surgery: assessment of aqueous humour and lens capsule. Acta Ophthalmol. 2016;94(7):e534–e540. doi: 10.1111/aos.13022. [DOI] [PubMed] [Google Scholar]
- 11.Pang XX, Bai Q, Wu F, Chen GJ, Zhang AH, Tang CS. Urotensin II induces er stress and emt and increase extracellular matrix production in renal tubular epithelial cell in early diabetic mice. Kidney Blood Press Res. 2016;41(4):434–449. doi: 10.1159/000443445. [DOI] [PubMed] [Google Scholar]
- 12.Luo T, Wang L, Wu P, Gong W, Chen W, Zhao H, Zheng Z. Downregulated vimentin and upregulated E-cadherin in T1 stage non-small-cell lung cancer: does it suggest a mesenchymal-epithelial transition? Neoplasma. 2017;64(5):693–699. doi: 10.4149/neo_2017_506. [DOI] [PubMed] [Google Scholar]
- 13.Mayer WJ, Klaproth OK, Ostovic M, Terfort A, Vavaleskou T, Hengerer FH, Kohnen T. Cell death and ultrastructural morphology of femtosecond laser-assisted anterior capsulotomy. Invest Ophthalmol Vis Sci. 2014;55(2):893–898. doi: 10.1167/iovs.13-13343. [DOI] [PubMed] [Google Scholar]
- 14.Donaldson KE, Braga-Mele R, Cabot F, Davidson R, Dhaliwal DK, Hamilton R, Jackson M, Patterson L, Stonecipher K, Yoo SH, ASCRS Refractive Cataract Surgery Subcommittee Femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. 2013;39(11):1753–1763. doi: 10.1016/j.jcrs.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Mochizuki T, Luo YJ, Tsai HF, Hagiwara A, Masai I. Cell division and cadherin-mediated adhesion regulate lens epithelial cell movement in zebrafish. Development. 2017;144(4):708–719. doi: 10.1242/dev.138909. [DOI] [PubMed] [Google Scholar]
- 16.Leonard M, Zhang L, Zhai N, Cader A, Chan Y, Nowak RB, Fowler VM, Menko AS. Modulation of N-cadherin junctions and their role as epicenters of differentiation-specific actin regulation in the developing lens. Dev Biol. 2011;349(2):363–377. doi: 10.1016/j.ydbio.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandez V, Fragoso MA, Billotte C, Lamar P, Orozco MA, Dubovy S, Willcox M, Parel JM. Efficacy of various drugs in the prevention of posterior capsule opacification: experimental study of rabbit eyes. J Cataract Refract Surg. 2004;30(12):2598–2605. doi: 10.1016/j.jcrs.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Kalauz M, Masnec S, Kordić R, Kuzman T, Vidas S, Škegro I, Jandroković S, Perić S. Posterior capsule opacification and Nd:YAG rates with two acrylic intraocular lenses after age-related cataract treatment: three-year results. Semin Ophthalmol. 2016:1–7. doi: 10.1080/08820538.2016.1247182. [DOI] [PubMed] [Google Scholar]
- 19.Apple DJ, Peng Q, Visessook N, Werner L, Pandey SK, Escobar-Gomez M, Ram J, Auffarth GU. Eradication of posterior capsule opacification: documentation of a marked decrease in Nd:YAG laser posterior capsulotomy rates noted in an analysis of 5416 pseudophakic human eyes obtained postmortem. Ophthalmology. 2001;108(3):505–518. doi: 10.1016/s0161-6420(00)00589-3. [DOI] [PubMed] [Google Scholar]
- 20.Nishi Y, Ikeda T, Nishi K, Mimura O. Epidemiological evaluation of YAG capsulotomy incidence for posterior capsule opacification in various intraocular lenses in Japanese eyes. Clin Ophthalmol. 2015;9:1613–1617. doi: 10.2147/OPTH.S89966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagy ZZ. New technology update: femtosecond laser in cataract surgery. Clin Ophthalmol. 2014;8:1157–1167. doi: 10.2147/OPTH.S36040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Packer M, Teuma E V, Glasser A, Bott S. Defining the ideal femtosecond laser capsulotomy. Br J Ophthalmol. 2015;99(8):1137–1142. doi: 10.1136/bjophthalmol-2014-306065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panthier C, Costantini F, Rigal-Sastourné JC, Brézin A, Mehanna C, Guedj M, Monnet D. Change of capsulotomy over 1 year in femtosecond laser-assisted cataract surgery and its impact on visual quality. J Refract Surg. 2017;33(1):44–49. doi: 10.3928/1081597X-20161028-01. [DOI] [PubMed] [Google Scholar]
- 24.Aron-Rosa DS, Aron JJ. Effect of preoperative YAG laser anterior capsulotomy on the incidence of posterior capsule opacification: ten year follow-up. J Cataract Refract Surg. 1992;18(6):559–561. doi: 10.1016/s0886-3350(13)80442-7. [DOI] [PubMed] [Google Scholar]
- 25.Kovács I, Kránitz K, Sándor GL, Knorz MC, Donnenfeld ED, Nuijts RM, Nagy ZZ. The effect of femtosecond laser capsulotomy on the development of posterior capsule opacification. J Refract Surg. 2014;30(3):154–158. doi: 10.3928/1081597X-20140217-01. [DOI] [PubMed] [Google Scholar]
- 26.Wertheimer C, Kreutzer TC, Dirisamer M, Eibl-Lindner K, Kook D, Priglinger S, Mayer WJ. Effect of femtosecond laser-assisted lens surgery on posterior capsule opacification in the human capsular bag in vitro. Acta Ophthalmol. 2017;95(2):e85–e88. doi: 10.1111/aos.13103. [DOI] [PubMed] [Google Scholar]