Abstract
AIM
To evaluate the safety and efficacy profile of 27-gauge (27G) pars plana vitrectomy (PPV) for the treatment of various vitreoretinal diseases.
METHODS
The clinical outcomes of 61 eyes (58 patients) with various vitreoretinal diseases following 27G PPV were retrospectively reviewed.
RESULTS
Surgical indications included rhegmatogenous retinal detachment (n=24), full-thickness macular hole (n=12), diabetic retinopathy (n=11), vitreous hemorrhage (n=6), Eales disease (n=4), pathological myopia-related vitreous floater (n=2), and macular epiretinal membrane (n=2). The mean follow-up was 166.4±61.3d (range 98-339d). The mean logMAR best-corrected visual acuity (BCVA) improved from 1.7±1.1 [0.02 decimal visual acuity (VA) equivalent] preoperatively to 1.2±1.0 (0.06 decimal VA equivalent) at the last postoperative visit (P<0.001). The mean operative time was 49.9min. With the exception of complicated cataract in one eye, no intraoperative complications were encountered. No case required conversion to conventional 20-, 23- or 25G instrumentation in all surgical maneuvers except for silicone oil infusion, which required a 25G oil injection syringe. Postoperative complications included transient ocular hypertension, vitreous hemorrhage, persistent intraocular pressure elevation, subconjunctival oil leakage, and recurrent retinal detachment. No cases of hypotony, endophthalmitis, and sclerotomy-related tears were observed.
CONCLUSION
The current results suggest that 27G PPV system is a safe and effective treatment for various vitreoretinal diseases. When learning to perform 27G PPV, surgeons may encounter a learning curve and should gradually expand surgical indications from easy to pathologically complicated cases.
Keywords: 27-gauge, par plana vitrectomy, vitreoretinal disease
INTRODUCTION
Since pars plana vitrectomy (PPV) was first introduced in 1971, this technique has been widely used to treat posterior segment ocular diseases and progressively evolved with smaller and faster vitrectomy systems[1]. Within ten years or more, 25-gauge (25G) and 23-gauge (23G) microincision sutureless vitrectomy (MISV) instruments have dramatically simplified vitrectomy procedures and provided numerous advantages, including shorter operative time, self-sealing scleral wound, decreased postoperative pain and inflammation, decreased astigmatism and faster visual recovery, over traditional 20-gauge (20G) instruments[2]–[4]. However, compared with 20G vitrectomy, sutureless vitrectomy increased the incidence of sclerotomy leakage-related complications, such as postoperative hypotony, choroidal detachment and even sight-threatening endophthalmitis[5]–[6]. In 2010, a 27-gauge (27G) sutureless transconjunctival vitrectomy (STV) system was initially reported by Oshima et al[7]. The 27G PPV has been increasingly applied in the past few years, and its efficacy and safety are being evaluated[8]–[12]. However, most previous studies were either limited due to small sample sizes or focused on a particular disease. Only one published study in the US shed light on a large spectrum of posterior segment diseases with a sample size greater than 50 eyes treated with 27G PPV. However, these surgeries were completed by multiple surgeons whose surgical techniques may lack standardization. Here, we report sixty-one eyes with various vitreoretinal diseases treated with 27G PPV by the same surgeon and reveal the features of surgical efficiency improvement and the learning curve associated with 27G PPV. To the best of our knowledge, there is no large case series study regarding the outcomes of 27G microincision vitrectomy in a Chinese population. The purpose of this study is to review our initial experience, learning curve, efficacy and safety profile and clinical outcomes of 27G PPV for various vitreoretinal diseases.
SUBJECTS AND METHODS
Study Design and Patients
A retrospective review of all patients who underwent 3-port, 27G PPV at the Sichuan Provincial People's Hospital (SPPH, Chengdu, China) by the same surgeon (Zhong J) from June 2015 to March 2016 was performed. In total, 58 patients (61 eyes) were included in the study. All patients who underwent 27G PPV were reported, and no patients were excluded from the study. All patients underwent complete preoperative and postoperative ophthalmic examinations. This study has been proved by the Institutional Review Committee of SPPH. The procedures used conformed to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all patients before surgery.
Surgical Techniques
All surgeries were performed using a 27G Constellation Vision System (Constellation Vitrectomy 27+ Total Plus Pak, Alcon Laboratories, USA) and S88/OPMI Lumera T operation microscope with a RESIGHT wide-angle viewing operation system (Carl Zeiss Meditec AG, Germany). After retrobulbar anesthesia was induced with 2% lidocaine, and the conjunctiva was disinfected with povidone-iodine, three valve cannulas were initially placed inferotemporal, superotemporal and superonasal at a position 3.5 to 4 mm posterior to the limbus after sclerotomies were created by a 30° angle of entry insertion technique. The infusion cannula was connected to the inferotemporal cannula.
Surgical procedures varied depending on the diagnosis. If a significant cataract was observed, concurrent standard phacoemulsification and aspiration (PEA) and intraocular lens (IOL) implantation were performed before the scleral incision for vitrectomy. All cataract surgeries were performed though a 2.4-mm clear corneal incision. The vitrectomy surgical parameters were 7500 cuts per minute (cpm) and a vacuum of 0 to 650 mm Hg. Core vitrectomy was performed in all cases, and subsequent peripheral vitreous shaving was performed when necessary. Fibrovascular membrane peeling was performed utilizing a vitreous cutter or internal limiting membrane (ILM) forceps. A 27G vitrectomy instrument was used to dissect and/or trim fibrovascular tissue in areas of dense adhesions and when necessary, perform retinectomy. A chandler light source was not used in any case. A direct endolaser was utilized to treat retinal breaks and ischemic retinas and/or apply scatter photocoagulation. Transvitreal diathermy was used to mark retinectomy sites and achieve intraocular hemostasis. Perfluorocarbon liquid (PFCL, Fluoron) was used to stabilize the retina in cases of complex rhegmatogenous retinal detachment (RRD) and proliferative vitreoretinopathy (PVR). Membrane and/or ILM peeling was performed for epiretinal membrane (ERM) and macular hole indications. Indocyanine green (ICG, Dandong Yichuang Pharmaceutical Co., Ltd., China) was utilized for macular staining if necessary. Fluid-air exchange and subretinal fluid drainage were performed with a 27G vitrectomy cutter. A soft-tip cannula (Alcon) was used for PFCL removal. Silicone oil (5000 centistokes, Zeiss) was infused in cases of PVR and RRD. Since commercialized medical gas has been unavailable in mainland China since June 2015, we injected silicone oil for all RRD cases. When silicone oil injection was required, the inferotemporal 27G cannula was removed, and a 25G cannula was placed at the same sclerotomy site. Oil was injected through a 25G oil injection syringe. Scleral buckling was concurrently performed when RRD was induced by retinal breaks within the inferior quadrants or in eyes with severe PVR as determined by the surgeon. The peripheral retina was examined before cannula removal, and no sclerotomy site suture was placed.
Main Outcome Measurements
Information extracted from patient records included age, sex, ophthalmic history, anterior segment and fundus examination findings, pre- and postoperative best-corrected visual acuity (BCVA), pre- and postoperative intraocular pressure (IOP), and follow-up period. Intraoperative data including operative time, complications, method of wound construction, sclerotomy site leakage, and usage of tamponade were documented.
All patients were subjected to ocular examination with the Chinese E standard logarithmic visual acuity (VA) chart, non-contact tonometer, and slit-lamp biomicroscopy at 1d, 2wk, 1, and 2mo postoperatively, as well as at all subsequent postoperative visits. Postoperative complications, including hypotony, ocular hypertension, wound leakage, retinal detachment (RD), recurrent RD, endophthalmitis, and choroidal detachment, were also reported if present. For statistical analysis, decimal VA was converted into logarithm of minimal angle of resolution (logMAR). Counting fingers (CF) and hand motions (HM) vision were converted to 1.98 and 2.28, respectively, according to a previous study[13]. The primary outcome measures were changes in VA and IOP and the occurrence of intra- and postoperative complications.
Statistical Analysis
All data were tabulated and analyzed with Microsoft Excel (Microsoft Corp., Redmond, WA, USA). All statistical analyses were performed using paired Student's t-test or independent t-test (IBM SPSS Statistics 24.0, IBM Corporation, USA). Data are reported as the mean±standard deviation (SD) unless otherwise noted. P values <0.05 were considered statistically significant.
RESULTS
Sixty-one eyes (31 right eyes and 30 left eyes) of 58 patients were included. The mean age was 53.0y (SD 12.4y, range from 16 to 74y), and 34 patients were female. The mean follow-up period was 166.4d (the median was 140d, range from 98 to 339d). Fifty-seven eyes (93.4%) were phakic, and 4 eyes (6.6%) were pseudophakic. Three eyes (5.3%) underwent concurrent cataract PEA and IOL, and 4 eyes (6.6%) underwent concurrent scleral buckling during vitrectomy surgery. Relevant past ocular history included proliferative diabetic retinopathy (PDR) in 11 eyes (18.0%) and open-angle glaucoma in 2 eyes (3.2%) among which one eye had prior filtering surgery.
Surgical indications for vitrectomy included RRD (n=24), full-thickness macular hole (FTMH, n=12), diabetic retinopathy (DR, n=11), vitreous hemorrhage (VH, n=6), Eales disease (EALES, n=4), pathological myopia related vitreous floater (FLT/PM, n=2), and macular epiretinal membrane (MEM, n=2). Ten of 11 DR patients needed surgery due to VH, and the other needed surgery due to tractional RD.
With the exception of one eye with a complicated cataract induced by an extruding cutter probe on the posterior capsule, no intraoperative complications, including iatrogenic retinal breaks, sclerotomy-related breaks and subchoroidal hemorrhage, were found. At the end of surgery, 21 eyes (34.4%) were left with a fluid-filled vitreous cavity, 29 eyes (47.5%) were filled with silicone oil, and 11 eyes were (18.0%) infused with air. No eyes required sclerotomy sutures for wound closure. We determined that 27G instruments have sufficient rigidity to perform all maneuvers in one operation, including peripheral vitreous shaving, membrane peeling, endolaser treatment, and subretinal fluid extraction. No eyes required conversion to a conventional 23G or 25G instruments for any surgical maneuver except for oil infusion during the last stage due to the lack of a 27G oil injection syringe.
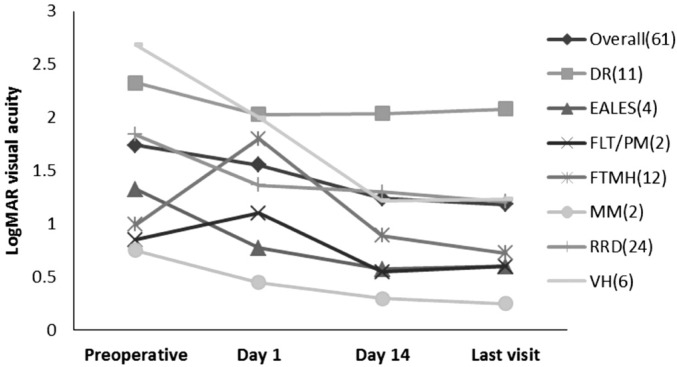
Primary outcomes are summarized in Table 1. The mean logMAR VA improved from 1.7±1.1 (0.02 decimal VA equivalent) preoperatively to 1.2±1.0 (0.06 decimal VA equivalent) at the last postoperative visit (P<0.001). VA outcomes were also evaluated by different surgical indications (Figure 1). Significant improvement in logMAR VA was noted for eyes with RRD (n=24, P<0.01) and FTMH (n=12, P=0.02) at the final postoperative visit. Improvements were also noted for patients with DR (n=11, P=0.43), EALES (n=4, P=0.39), FLT/PM (n=2, P=0.50), MEM (n=2, P=0.34), and VH (n=6, P=0.08) but did not reach statistical significance. The primary operative purpose, including anatomic reattachment of the retina, removal of pre-macular membranes, clearing of VH and floaters, and closure of macular holes, was achieved in every case.
Table 1. Summary of the primary outcomes of 61 eyes (58 patients) that underwent 27G vitrectomy.
| Diagnosis | n | Age (y) | Mean IOP (mm Hg) |
Mean logMAR VA |
Operative time (min) | Follow-up (d) | ||||
| Preop. | Day 1 | Last visit | Preop. | Last visit | P | |||||
| Overall | 61 | 53.0±12.4 | 12.5±3.4 | 14.2±9.1 | 15.5±3.3 | 1.7±1.1 | 1.2±1.0 | <0.001 | 49.9±24.7 | 166.4±61.3 |
| RRD | 24 | 49.5±15.2 | 10.8±2.9 | 14.9±6.9 | 14.3±3.2 | 1.8±1.1 | 1.2±0.9 | <0.01 | 54.2±23.2 | 174.6±66.6 |
| FTMH | 12 | 61.3±8.5 | 12.8±1.1 | 8.3±2.0 | 13.7±1.9 | 1.0±0.4 | 0.7±0.3 | 0.02 | 46.8±27.9 | 159.0± 67.9 |
| DR | 11 | 54.6±9.7 | 15.0±4.6 | 13.7±5.8 | 15.5±5.0 | 2.3±0.6 | 2.1±1.1 | 0.43 | 57.7±26.3 | 137.0±31.1 |
| VH | 6 | 61.3±8.5 | 12.8±1.1 | 8.3±2.0 | 13.7±1.9 | 2.7±1.3 | 1.2±1.4 | 0.08 | 46.8±27.9 | 179.5±79.7 |
| EALES | 4 | 49.8±7.9 | 11.8±3.4 | 26.5±17.3 | 12.3±1.0 | 1.3±1.2 | 0.6±0.5 | 0.39 | 33.8±25.0 | 190.5±71.4 |
| FLT/PM | 2 | 42.0±11.3 | 11.5±2.1 | 9.3±3.9 | 14.0±1.4 | 0.9±0.6 | 0.6±0.3 | 0.50 | 35.0±21.2 | 169.2±56.2 |
| MEM | 2 | 55.5±2.1 | 14.6±3.3 | 11.8±1.8 | 14.0±0.0 | 0.8±0.1 | 0.3±0.4 | 0.34 | 17.5±3.5 | 121.2±5.5 |
RRD: Rhegmatogenous retinal detachment; FTMH: Full-thickness macular hole; DR: Diabetic retinopathy; VH: Vitreous hemorrhage; EALES: Eales disease; FLT/PM: Pathological myopia-related floater; IOP: Intraocular pressure; MEM: Macular epiretinal membrane; SD: Standard deviation; VA: Visual acuity.
mean±SD
Figure 1. Outcomes of 27G vitrectomy surgery in 61 eyes: mean logMAR VA by diagnosis.
Compared with the mean preoperative IOP of 12.5±3.4 mm Hg (range 5.2-24.0 mm Hg), the mean IOP was 14.2±9.1 mm Hg (range 6.4-51.7 mm Hg) on postoperative day 1 (P=0.19), 17.0±7.1 mm Hg (range 10.0-41.0 mm Hg) on day 14 (P<0.001), and 15.5±3.3 mm Hg (range 10.0-25.2 mm Hg) at the last postoperative visit (P<0.001) across all eyes (Table 2). IOP outcomes were also compared among tamponade agents. Compared with preoperative values, the mean IOP was 14.8±3.0 mm Hg (P=0.43) in fluid-filled eyes, 16.5±3.5 mm Hg (P<0.001) in eyes with silicone oil, and 14.0±2.3 mm Hg (P=0.32) in eyes with air at the final postoperative visit.
Table 2. Outcomes of 27G vitrectomy surgery in 61 eyes: pre- and postoperative mean IOP by tamponade agent.
| Tamponade (n) | IOP (mm Hg) |
Transient hypotony | Transient ocular hypertension | ||||||
| Preoperative | Day 1 | Day 14 | Day 30 | Day 60 | Last visit | P (last visit) | |||
| Overall (61) | 12.5±3.4 | 14.2±9.1 | 17.0±7.1 | 14.7±4.1 | 14.8±3.6 | 15.5±3.3 | <0.001 | 0 | 20 (32.8) |
| Fluid filled (21) | 14.2±3.8 | 13.9±11.2 | 15.5±5.5 | 14.8±4.5 | 14.8±4.2 | 14.8±3.0 | 0.43 | 0 | 7 (33.3) |
| Silicone oil (29) | 11.2±3.1 | 15.9±8.5 | 18.5±8.2 | 14.9±4.5 | 15.5±3.4 | 16.5±3.5 | <0.001 | 0 | 12 (41.4) |
| Air (11) | 12.6±1.3 | 10.1±4.1 | 15.7±6.5 | 13.7±1.3 | 12.9±2.1 | 14.0±2.3 | 0.32 | 0 | 1 (9.1) |
n (%), mean±SD
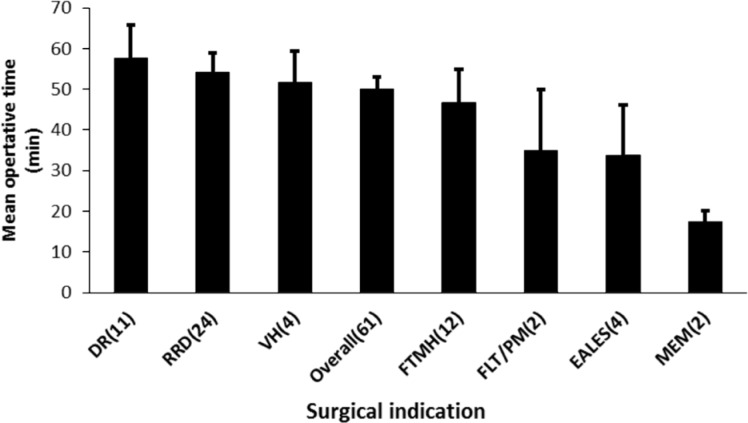
The total operative time was assessed for each diagnosis (Figure 2). Across all cases, the mean operative time was 49.9min (median 50min, range 15-110min). The three shortest operations (for FTMH, MEM and EALES) took 15min; the longest operation (110min) was for PDR. By surgical indication, the average operative time was shortest for MEM at 17.5min (range 15-20min) and longest for DR at 57.7min (range 25-110min).
Figure 2. Outcomes of 27G vitrectomy surgery in 61 eyes: mean operative time by diagnosis.
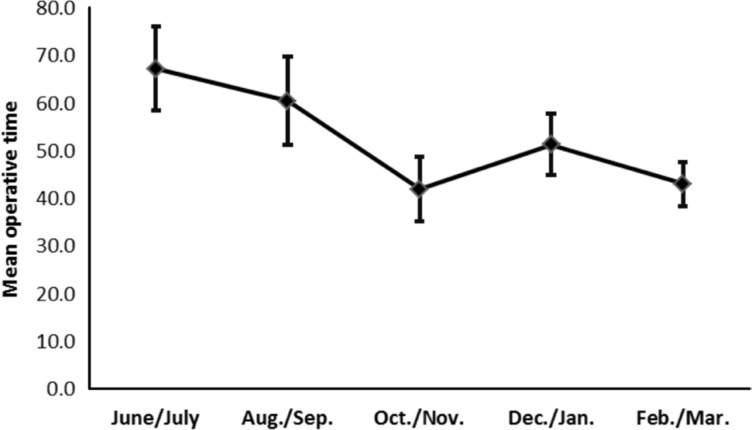
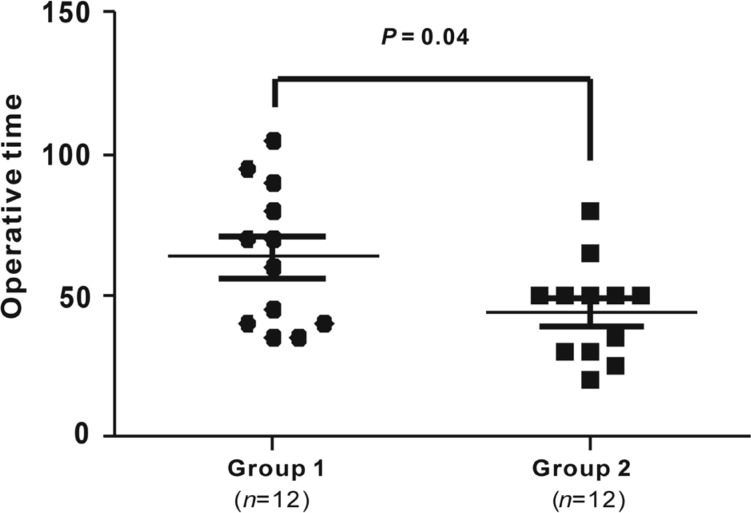
Operative time was evaluated every two months (Figure 3). The average operative time was 67.3±8.8min (range 25-90min) during the first two months (Jun./Jul. of 2015) when the surgeon (Zhong J) started to conduct 27G microincision vitrectomy. The average operative time was gradually shortened, and it decreased by 36% to 43.1±14.6min (range 20-80min) during the last two months (Feb./Mar. of 2016) of our study (Table 3). The mean operative time used to treat the first half of the RRD cases (63.8±7.2min, n=12) was significantly longer than that (44.5±5.0min, n=12) used to treat the second half (Figure 4).
Figure 3. Changes in the mean operative time.
The mean operative time of the same surgeon (Zhong J) using 27G microincision vitrectomy to treat various vitreoretinal diseases was gradually shortened.
Table 3. Changes in the composition of surgical indications and the operative time of 27G vitrectomy.
| Month | Eyes (n) | MS/total | RRD/total | Mean operative time (min) |
| Jun./Jul. | 7 | 2 (29) | 1 (14) | 67.3±8.8 |
| Aug./Sep. | 10 | 3 (30) | 3 (30) | 60.5±9.3 |
| Oct./Nov. | 15 | 5 (33) | 4 (27) | 42.0±6.7 |
| Dec./Jan. | 11 | 2 (18) | 7 (64) | 51.4±6.5 |
| Feb./Mar. | 18 | 2 (11) | 9 (50) | 43.1±4.5 |
MS: Macular surgery (including MEM and FTMH); RRD: Rhegmatogenous retinal detachment; SE: Standard error.
n (%), mean±SE
Figure 4. Operative time used to treat RRD.
Twenty-four eyes diagnosed with RRD were equally divided into two groups. The mean operative time used to treat the first half (group 1, n=12) of RRD cases was 63.8±7.2min, which was significantly shorter than that (44.5±5.0min) used to treat the second half (group 2, n=12), P=0.04.
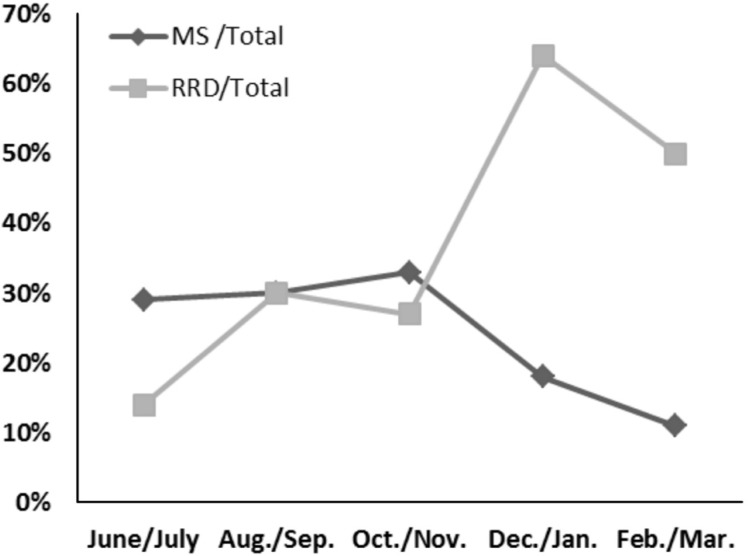
Surgical indications related to macular diseases, including FTMH and MEM, accounted for 29% (two eyes) of all surgical cases during the first two months. However, during the last two months of our retrospective review, macular-related surgeries decreased to 11% (two eyes). During the same period, the rate of RRD surgery increased from 14% (one eye) to 50% (9 eyes) (Figure 5).
Figure 5. Changes in the composition of the surgical indication.
Over time, the proportion of macular surgery (including MEM and FTMH) decreased; by contrast, the ratio of RRD treated with 27G vitrectomy increased dramatically. MS: Macular surgery; RRD: Rhegmatogenous retinal detachment.
Postoperative complications included transient ocular hypertension (new-onset IOP greater than 25 mm Hg) in 19 eyes (31.1%). No transient hypotony was found (new-onset IOP less than 6 mm Hg). Of the 19 eyes with transient ocular hypertension, twelve (63.2%) were silicone oil-filled eyes. Most (16 eyes, 84.2%) eyes with ocular hypertension were detected during the first two postoperative weeks. All cases of transient ocular hypertension were managed medically, and IOP returned to normal within a week. One eye with DR complicated by early postoperative VH exhibited persistent IOP elevation, which required drainage valve implantation. In total, post-vitrectomy VH occurred in 2 eyes (3.3%) with DR. One of the eyes underwent drainage valve implantation as mentioned above, and the other underwent vitreous cavity lavage one month after initial 27G vitrectomy. Within the follow-up period, recurrent RD, which was initially diagnosed as RRD, occurred in 3 of 24 eyes (12.5%); the detachments resulted from the development of PVR. Two of the eyes underwent the traditional PPV procedure, including silicone oil removal, proliferative membrane peeling, laser photocoagulation and silicone oil refilling; the other underwent two-port 27G vitrectomy, including membrane peeling and endolaser treatment without silicone oil removal maneuvers. All eyes achieved anatomic retinal reattachment. Subconjunctival silicone oil droplets were observed in 5 (17.9%) of the 29 oil-filled eyes. No cases of endophthalmitis, hyphema, choroidal detachment and sclerotomy-related tears were noted within the follow-up period.
DISCUSSION
Since the consecutive introduction of 25G and 23G transconjunctival sutureless vitrectomy (TSV) in the first few years of 21th century[2]–[3], these microincision vitrectomy systems have been widely used, and their indications have progressively expanded[4],[14]. These MISV systems dramatically simplified vitrectomy procedures and have numerous advantages including shorter operation time, faster VA recovery, improved patient comfort with faster wound healing and less postoperative astigmatism, over conventional 20G PPV. However, criticisms of microincision vitrectomy have increased[5]–[6],[15]. The most serious and common criticisms of 25G and 23G surgery focused on self-healing wound-related complications, including an increased incidence of postoperative hypotony, choroidal detachment and severe bacterial endophthalmitis.
Oshima et al[7] first described a 27G vitrectomy system in 2010. With smaller diameter instrumentation, 27G vitrectomy allows for a smaller incision. To date, several studies have evaluated the efficacy and safety profile of 27G vitrectomy and discussed the initial experience[7]–[9],[11]–[12],[16]. However, several of these studies focused either on RRD or macular-related diseases. Only two studies discussed the application of 27G vitrectomy in various vitreoretinal diseases. Rizzo et al[10] reported a series of 16 eyes that underwent 27G vitrectomy in Italy. Khan et al[9] described a multicenter retrospective study including 95 eyes with various vitreoretinal diseases that underwent 27G vitrectomy performed by multiple surgeons with different surgical maneuvers or techniques.
In our study, we described the complications and visual and anatomic outcomes of our initial 27G microincision vitrectomy surgeries in 61 eyes with an average follow-up period of 166.4d (range 98-339d). Overall, 27G vitrectomy fulfilled the surgical aim, including anatomic reattachment of the retina, removal of ERMs and vitreous opacities, reduction of vitreoretinal traction and closure of macular holes. We found that a 27G probe was rigid enough to perform all the surgical maneuvers in the operation, consistent with previous reports[9]–[11]. Our study further validated the feasibility of using 27G PPV to treat a wide variety of vitreoretinal surgical indications. Regarding visual function, the overall logMAR VA improved from 1.7±1.1 preoperatively to 1.2±1.0 at the last follow-up visit (P<0.001); this finding was comparable to the results of a previous 27G vitrectomy study and those achieved with 23G and 25G instrumentations[9]–[10],[14],[17].
No severe intraoperative complications were observed except for a complicated cataract due to an extruding cutter probe on the posterior capsule in one eye. At the end of surgery, the 27G sclerotomies showed good wound closure, which was determined by pressing a swab or forceps on the wound for 30s in eyes infused with balanced salt solution (BSS) or air. Overall, postoperative complications were limited. No transient (new-onset IOP less than 6 mm Hg) hypotony was documented. Transient ocular hypertension (new-onset IOP greater than 25 mm Hg) was a common (19 eyes, 31.1%) postoperative complication that was managed effectively with conservative measures. Postoperative VH was detected in two eyes with DR (3.3%). Both eyes required further surgical intervention-one underwent vitreous cavity lavage, and the other underwent drainage valve implantation due to secondary persistent ocular hypertension. Recurrent RD was observed in 3 eyes (12.5%), and additional surgical inventions were required for anatomic reattachment of the retina. Subconjunctival silicone oil droplets were detected in 5 eyes (17.9%). Since we used 25G sclerotomy to perform oil infusions, it is unclear whether these silicone oil droplets leaked from 27G or 25G sclerotomy sites. No endophthalmitis, hyphema, choroidal detachment and sclerotomy-related tears were noted in the follow-up period.
The complication rate in our study was partly comparable to that of previous studies of 27G vitrectomy, as well as 23G or 25G surgeries. Similar to previous reports, hypotony was not detected in our study[10],[12]. However, the hypotony rate was 5% in Khan et al's[9] study and ranged from 0 to 25.6% in 25G surgery studies[6],[18]. Transient ocular hypertension was much more common in our study (31.1%) than in Khan et al's[9] study (8%). The recurrent RD rate (12.5%) in this study was slightly higher than that in previous 27G studies, with rates ranging from 7% to 10%[9]–[10],[12]. The lack of intraoperative sutures in our study is comparable to that in most initial 27G studies[8],[10]–[12],[19]–[20] but different from that in 23G and 25G studies (0-7.1%)[4],[14],[17],[21]–[22].
When 27G vitrectomy was first introduced in 2010, surgical indications were limited to relatively easy cases, including macular hole, ERM, vitreomacular traction, macular edema, VH, focal tractional RD, and vitreous opacities. In further studies of 27G PPV, surgical indications progressively expanded to diabetic tractional RD, RRD, endophthalmitis, submacular hemorrhage, sub-silicone oil RD, retained lens material, and aqueous misdirection[9]–[12]. In our study, we pushed this boundary further by removing vitreous floaters using 27G vitrectomy in two eyes with pathological myopia. Both patients were satisfied with their visual quality improvement after the surgery. The use of 27G PPV to eliminate vitreous floaters was also mentioned by Dr. Sommerville[23] in his unpublished data. Furthermore, we treated one of three cases of recurrent RD using two-port 27G vitrectomy without a silicone oil removal strategy in this study. We have also used this “non-oil-removal” technique to treat several eyes with recurrent RD[24]. We began performing 27G surgeries with easy macular surgeries including MEM and FTMH). We then expanded surgical indications when the surgeon became more experienced and confident. The composition of surgical indications gradually shifted to more pathologically complicated cases such as RRD (Figure 5).
Operation efficiency remains one of the most common concerns regarding 27G instrumentation, which has a smaller diameter probe (0.42 mm) than the 25G instrumentation (0.52 mm)[7]. Flow rate is the key factor for operation efficiency. According to the Hagen-Poiseuille law, a decrease in the inner lumen radius by 20% in the 27G probe theoretically results in a decrease in the flow rate by approximately 60%. However, previous hydromechanics studies revealed that 27G dual-pneumatic probes enable control of duty cycles at an ultrahigh speed cut rate (7500 cpm) and maintain an efficient vitreous flow rate[19],[25]–[26]. In addition, Mitsui et al[8] revealed that the mean operative time for ERM by 27G vitrectomy was 20.2min, which was not significantly different from that by 25G vitrectomy. In several other studies, the mean operative time of 27G vitrectomy for more complicated surgical indications ranged from 32 to 36.3min[9]–[10],[12], which was comparable to initial experiences with 25G equivalents[2],[17]. Across all cases in the current study, the mean operative time was 49.9min, which is slightly longer than that in previous studies. This difference could have many explanations, including the time used to change to a 25G injection syringe for oil infusion, different surgical procedures and intraoperative cooperation. If we take only easy cases, including ERM, EALES and vitreous floaters, into account, then the mean operative time is reduced to 30min, and consistent with previous studies[9]–[10],[12]. Moreover, since all the operations were performed by the same surgeon, we could evaluate surgical efficiency improvement and depict the learning curve by comparing the surgical time and surgical indication composition every two months (Table 3, Figure 5). These data demonstrated that we began performing 27G vitrectomy at an average duration of 67min and ended with an average duration of 43min per eye during the final stage of our study, although more complicated surgical indications were gradually included across the study period. To rule out bias caused by different surgical indications, we compared the surgical time used the treat RRD alone. The time used to treat the second half of the RRD cases was significantly shorter than that used to treat the first half of the RRD cases (Figure 4). Thus, we cannot deny surgical efficiency that could be affected by decreased flow rate within the 27G probe; however, our study demonstrates that more efficient use of the cutter and surgical skill improvement could compensate for the reduced flow rate.
Similar to other surgeons, we experienced the many advantages of 27G instrumentation in this study[9]–[10],[12],[20]. For example, by tuning the “3D” mode provided by the constellation vitrectomy system, we could set a high cut rate with a low aspiration rate to reduce vitreoretinal traction from the probe tip, which would benefit peripheral shaving and possibly reduce iatrogenic retinal breaks. When performing the core vitrectomy, we can move to a more powerful aspiration rate to increase surgical efficiency. With its smaller diameter, the 27G probe can be more easily inserted into the tiny space between the membrane and the retina, which is helpful in membrane dissection in PVR and PDR surgery. In addition, the current 27G vitrectomy probe features a port placed 0.2 mm from the end of the probe. This feature could be used for BSS removal in the fluid-air exchange procedure. Therefore, the small, delicate, controlled, multifunctional 27G cutter saves time wasted due to changing instruments during challenging cases. However, shorter effective work length of 27G instrument compared to 25G, 23G, and 20G instruments, is not suitable for eyes with a long axis. Furthermore, 27G vitrectomy is not suitable for severe PVR, intraocular foreign body and lens dislocation. In addition, inducing posterior vitreous detachment (PVD) is more difficult with 27G instrumentation due to lower flow/suction, especially in younger patients whose posterior vitreous cortex is tightly adherent to the retina. When PVD induction was unsuccessful, we used ILM forceps to create a hole in the posterior vitreous cortex as a starting point to help induce PVD.
Our present study has several limitations because it is a retrospective, uncontrolled, noncomparative study from one center. Since commercialized medical gas was recalled by China's State Food and Drug Administration in 2015 due to reported adverse events, we could not include gas tamponade data. To perform silicone oil injection, we had to convert to a 25G oil injection syringe, which may also introduce bias and affect data with respect to IOP, wound healing, operative time, etc. Finally, a long-term follow-up period is needed to reveal long-term prognoses and complications. However, despite these limitations, most patients who underwent 27G PPV in our study experienced positive results, including visual function improvement and limited complications. Only a handful of intra- or postoperative complications, which are equally common in 25G or 23G TSV, were documented. No sight-threatening complications such as endophthalmitis were observed in our study. The current results increased our knowledge of the efficacy and safety profile of 27G vitrectomy for a variety of vitreoretinal diseases. However, similar to other MISV instrumentations, 27G instrumentation must be assessed by additional comparative, long-term, prospective, controlled studies to determine its potential merits and faults in application to the full spectrum of vitreoretinal diseases in the near future.
Acknowledgments
Authors' contributions: Li J, Dong WT, Li F, Zhou CH and Xu XD were responsible for collection of data. Li J, Liu SM and Zhong J performed the surgery and statistical analysis. Li J, Liu SM and Zhong J were responsible for interpretation of results. Li J and Zhong J participated in the design and wrote the first draft of the manuscript. All authors reviewed and approved the final manuscript.
Foundation: Supported by the National Natural Science Foundation of China (No.81700841).
Conflicts of Interest: Li J, None; Liu SM, None; Dong WT, None; Li F, None; Zhou CH, None; Xu XD, None; Zhong J, None.
REFERENCES
- 1.Machemer R, Buettner H, Norton EW, Parel JM. Vitrectomy: a pars plana approach. Trans Am Acad Ophthalmol Otolaryngol. 1971;75(4):813–820. [PubMed] [Google Scholar]
- 2.Fujii GY, De Juan E, Jr, Humayun MS, Pieramici DJ, Chang TS, Awh C, Ng E, Barnes A, Wu SL, Sommerville DN. A new 25-gauge instrument system for transconjunctival sutureless vitrectomy surgery. Ophthalmology. 2002;109(10):1807–1812. doi: 10.1016/s0161-6420(02)01179-x. [DOI] [PubMed] [Google Scholar]
- 3.Eckardt C. Transconjunctival sutureless 23-gauge vitrectomy. Retina. 2005;25(2):208–211. doi: 10.1097/00006982-200502000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Rizzo S, Genovesi-Ebert F, Murri S, Belting C, Vento A, Cresti F, Manca ML. 25-gauge, sutureless vitrectomy and standard 20-gauge pars plana vitrectomy in idiopathic epiretinal membrane surgery: a comparative pilot study. Graefes Arch Clin Exp Ophthalmol. 2006;244(4):472–479. doi: 10.1007/s00417-005-0173-6. [DOI] [PubMed] [Google Scholar]
- 5.Scott IU, Flynn HW, Jr, Dev S, Shaikh S, Mittra RA, Arevalo JF, Kychenthal A, Acar N. Endophthalmitis after 25-gauge and 20-gauge pars plana vitrectomy: incidence and outcomes. Retina. 2008;28(1):138–142. doi: 10.1097/IAE.0b013e31815e9313. [DOI] [PubMed] [Google Scholar]
- 6.Bamonte G, Mura M, Stevie Tan H. Hypotony after 25-gauge vitrectomy. Am J Ophthalmol. 2011;151(1):156–160. doi: 10.1016/j.ajo.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 7.Oshima Y, Wakabayashi T, Sato T, Ohji M, Tano Y. A 27-gauge instrument system for transconjunctival sutureless microincision vitrectomy surgery. Ophthalmology. 2010;11793(1):102. e2. doi: 10.1016/j.ophtha.2009.06.043. [DOI] [PubMed] [Google Scholar]
- 8.Mitsui K, Kogo J, Takeda H, Shiono A, Sasaki H, Munemasa Y, Kitaoka Y, Takagi H. Comparative study of 27-gauge vs 25-gauge vitrectomy for epiretinal membrane. Eye (Lond) 2016;30(4):538–544. doi: 10.1038/eye.2015.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan MA, Shahlaee A, Toussaint B, Hsu J, Sivalingam A, Dugel PU, Lakhanpal RR, Riemann CD, Berrocal MH, Regillo CD, Ho AC. Outcomes of 27 gauge microincision vitrectomy surgery for posterior segment disease. Am J Ophthalmol. 2016;161:36–43. doi: 10.1016/j.ajo.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 10.Rizzo S, Barca F, Caporossi T, Mariotti C. Twenty-seven-gauge vitrectomy for various vitreoretinal diseases. Retina. 2015;35(6):1273–1278. doi: 10.1097/IAE.0000000000000545. [DOI] [PubMed] [Google Scholar]
- 11.Toygar O, Mi CW, Miller DM, Riemann CD. Outcomes of transconjunctival sutureless 27-gauge vitrectomy with siliconee oil infusion. Graefes Arch Clin Exp Ophthalmol. 2016;254(11):2111–2118. doi: 10.1007/s00417-016-3355-5. [DOI] [PubMed] [Google Scholar]
- 12.Romano MR, Cennamo G, Ferrara M, Cennamo M, Cennamo G. Twenty-seven-gauge versus 25-gauge vitrectomy for primary rhegmatogenous retinal detachment. Retina. 2017;37(4):637–642. doi: 10.1097/IAE.0000000000001215. [DOI] [PubMed] [Google Scholar]
- 13.Lange C, Feltgen N, Junker B, Schulze-Bonsel K, Bach M. Resolving the clinical acuity categories “hand motion” and “counting fingers” using the Freiburg visual acuity test (FrACT) Graefes Arch Clin Exp Ophthalmol. 2009;247(1):137–142. doi: 10.1007/s00417-008-0926-0. [DOI] [PubMed] [Google Scholar]
- 14.Fine HF, Iranmanesh R, Iturralde D, Spaide RF. Outcomes of 77 consecutive cases of 23-gauge transconjunctival vitrectomy surgery for posterior segment disease. Ophthalmology. 2007;114(6):1197–1200. doi: 10.1016/j.ophtha.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 15.Acar N, Kapran Z, Unver YB, Altan T, Ozdogan S. Early postoperative hypotony after 25-gauge sutureless vitrectomy with straight incisions. Retina. 2008;28(4):545–552. doi: 10.1097/IAE.0b013e318162b008. [DOI] [PubMed] [Google Scholar]
- 16.Yonekawa Y, Thanos A, Abbey AM, Thomas BJ, Todorich B, Faia LJ, Williams GA, Capone A, Jr, Wolfe JD, Hassan TS. Hybrid 25- and 27-gauge vitrectomy for complex vitreoretinal surgery. Ophthalmic Surg Lasers Imaging Retina. 2016;47(4):352–355. doi: 10.3928/23258160-20160324-08. [DOI] [PubMed] [Google Scholar]
- 17.Lakhanpal RR, Humayun MS, de Juan E, Jr, Lim JI, Chong LP, Chang TS, Javaheri M, Fujii GY, Barnes AC, Alexandrou TJ. Outcomes of 140 consecutive cases of 25-gauge transconjunctival surgery for posterior segment disease. Ophthalmology. 2005;112(5):817–824. doi: 10.1016/j.ophtha.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 18.Gupta N, Punjabi OS, Steinle NC, Singh RP. Rate of hypotony following 25-gauge pars plana vitrectomy. Ophthalmic Surg Lasers Imaging Retina. 2013;44(2):155–159. doi: 10.3928/23258160-20130215-01. [DOI] [PubMed] [Google Scholar]
- 19.Osawa S, Oshima Y. 27-Gauge vitrectomy. Dev Ophthalmol. 2014;54:54–62. doi: 10.1159/000360449. [DOI] [PubMed] [Google Scholar]
- 20.Khan MA, Kuley A, Riemann CD, Berrocal MH, Lakhanpal RR, Hsu J, Sivalingam A, Ho AC, Regillo CD. Long-term visual outcomes and safety profile of 27-Gauge pars plana vitrectomy for posterior segment disease. Ophthalmology. 2018;125(3):423–431. doi: 10.1016/j.ophtha.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Narayanan R, Taylor SC, Nayaka A, Deshpande R, St Aubin D, Hrisomalos FN, Hu J, Rajagopal R, Tewari A, Apte RS. Visual outcomes after vitrectomy for terson syndrome secondary to traumatic brain injury. Ophthalmology. 2017;124(1):118–122. doi: 10.1016/j.ophtha.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Figueroa MS, Contreras I, Noval S. Anatomic and visual outcomes of 23-G vitrectomy without scleral buckling for primary rhegmatogenous retinal detachment. Eur J Ophthalmol. 2013;23(3):417–422. doi: 10.5301/ejo.5000234. [DOI] [PubMed] [Google Scholar]
- 23.Sommerville DN. Vitrectomy for vitreous floaters: analysis of the benefits and risks. Curr Opin Ophthalmol. 2015;26(3):173–176. doi: 10.1097/ICU.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 24.Liu SM, Li J, Dong WT, Li F, Zhou CH, Tang XL, Zhao YY, Jiang WJ, Xu XD, Zhong J. Recurrent retinal detachment in silicone oil-filled eyes treated with two-port 27-gauge pars plana vitrectomy. Guoji Yanke Zazhi (Int Eye Sci) 2017;17(9):1620–1624. [Google Scholar]
- 25.Abulon DJ. Vitreous flow rates through dual pneumatic cutters: effects of duty cycle and cut rate. Clin Ophthalmol. 2015;9:253–261. doi: 10.2147/OPTH.S71387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abulon DJ, Buboltz DC. Porcine vitreous flow behavior during high-speed vitrectomy up to 7500 cuts per minute. Transl Vis Sci Technol. 2016;5(1):7. doi: 10.1167/tvst.5.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]