Abstract
This review focuses on indeterminate lesions on prostate magnetic resonance imaging (MRI), assigned as PI-RADS category 3. The prevalence of PI-RADS 3 index lesion in the diagnostic work-up is significant, varying between one in three (32%) to one in five (22%) men, depending on patient cohort of first biopsies, previously negative biopsies, and active surveillance biopsies. A management strategy must be developed for this group of men with an indeterminate suspicion of having clinically significant prostate cancer (csPCa). Currently available data show that the actual prevalence of csPCa after targeted biopsy in PI-RADS 3 lesions vary between patients groups from one in five (21%) to one in six (16%), depending on previous biopsy status. Although this prevalence is lower in comparison to PI-RADS 4 and PI-RADS 5 lesions, still a considerable proportion of men harbor significant disease. Men with such a PI-RADS 3 lesion should therefore be adequately managed. In general, the clinical approach of using a threshold of PI-RADS ≥4 instead of PI-RADS ≥3 to select MRI for targeted biopsies is not supported by data from our explorative literature search using current definitions of csPCa. A possible adaptation to the threshold of PI-RADS ≥4 in combination with other clinical markers could be considered within an active surveillance protocol, where the balance between the individual risk of missing csPCa and the constant process of repeating prostate biopsies is crucial. In the future, improvements in MR imaging and interpretation, combined with molecular biomarkers and multivariate risk models will all be employed in prostate cancer detection and monitoring. These combinations will aid decision-making in challenging circumstances, such as unclear and diagnostic equivocal results for csPCa at early detection.
Keywords: Prostate cancer, biopsy, magnetic resonance imaging (MRI), MRI-guided targeted biopsy, PSA density, PI-RADS, PI-RADS 3, risk stratification, indeterminate, equivocal
Introduction
Over the last decades significant advances have been made in the acquisition, interpretation, and reporting of MR images of the prostate. Pre-biopsy magnetic resonance imaging (MRI) can now be considered as an additional diagnostic test to serum prostate-specific antigen (PSA) and transrectal ultrasound (TRUS)-guided biopsies (1). A high negative predictive value is the underlying premise for the use of MR imaging (2). In other words, even when MR imaging does not provide a specific answer, it may be used to exclude malignancy in many circumstances. Guidelines with recommendations on prostate MR imaging have been published and are further implemented in clinical routine (3-5).
The PI-RADS (Prostate Imaging-Reporting and Data System) standardized image acquisition and reporting. It was designed to be used by medical professionals in the initial evaluation of patients to assess the risk of clinically significant prostate cancer (csPCa) that may require biopsy and treatment (4). PI-RADS v2 assessment uses a 5-point scale based on the likelihood (probability) that a combination of multiparametric MRI findings on T2w, diffusion weighted imaging (DWI), and dynamic contrast enhanced (DCE) imaging correlates with the presence of a clinically significant cancer for each lesion in the prostate gland.
However, PI-RADS v2 guidelines do not say how to deal with imaging findings that are indeterminate. Lesions on prostate MRI that are termed as ‘intermediate’ or ‘equivocal on the presence of clinically significant cancer’ are scored as PI-RADS category 3 lesions. A targeted biopsy may appear to be the first approach, but monitoring lesion characteristics with follow-up MRI seems to be a pragmatic and acceptable alternative in these men, reducing the burden and the risk of additional biopsies, especially when other markers such as digital rectal examination and PSA-density are stable. Directions for the further management of PI-RADS category 3 lesions that were negative for (clinically significant) prostate cancer on targeted biopsies, is currently also lacking. Follow-up MRI might be useful to monitor such lesions; however, no clear management recommendations for monitoring or repeat biopsy in these intermediate or undetermined lesions have yet been defined.
This review focuses on indeterminate lesions on prostate MRI (assigned as PI-RADS category 3), evaluates the prevalence in different patient groups, and investigates the prevalence of csPCa within this category 3. In addition, this review explores what could be a feasible PI-RADS threshold for monitoring or biopsying, taking into account the balance between harm (too much testing) and benefit (detection of csPCa).
What is a PI-RADS 3 lesion?
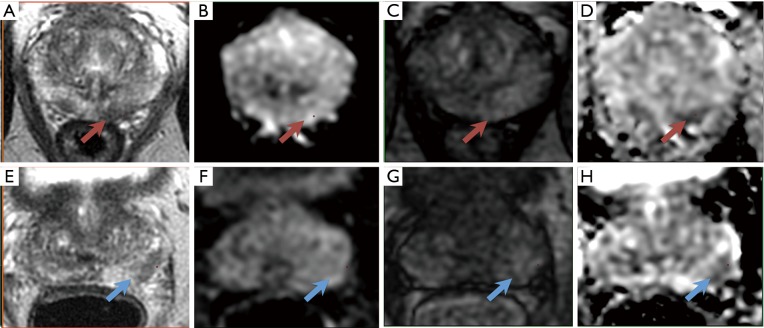
In a PI-RADS category 3 lesion, the presence of csPCa is considered to be equivocal, as defined by the PI-RADS v2 guidelines (4). For lesions located in the peripheral zone of the prostate, the dominant MRI sequence in PI-RADS v2 is DWI and reconstructed apparent diffusion coefficient (ADC). Lesions are characterized as focal mildly to moderately hypointense on ADC, and isointense to mildly hyperintense on high b-value DWI. This is in combination with heterogeneous signal intensity or non-circumscribed, rounded, and moderate hypointensity on T2w images, and specifically excludes lesions with characteristics that qualify as PI-RADS 2, 4, or 5. No focal enhancement on DCE MRI should be visualized (Figure 1).
Figure 1.
A left peripheral zone lesion (red arrows) on (A) axial T2-weighted image, (B) high-b-value DWI, (C) early DCE time-point, and (D) ADC map. The overall PI-RADS assessment category 3 was assigned (T2w 3, DWI/ADC 3, DCE −). On subsequent targeted MRI/US fusion biopsy, the lesion exhibited Gleason score 3+4 tumor (red arrows). PI-RADS category 3 lesions are characterized as focal mildly to moderately hypointense on ADC, and isointense to mildly hyperintense on high b-value DWI. This is in combination with heterogeneous signal intensity or non-circumscribed, rounded, and moderate hypointensity on T2w images. No focal enhancement on DCE MRI should be visualized. The dominant MRI sequence in PI-RADS v2 is DWI/ADC. (E-H) A left peripheral zone lesion (blue arrows). The overall PI-RADS assessment category 3 was assigned (T2w 3, DWI/ADC 3, DCE −). On subsequent targeted MRI/US fusion biopsy, the lesion exhibited benign prostatic tissue (blue arrows).
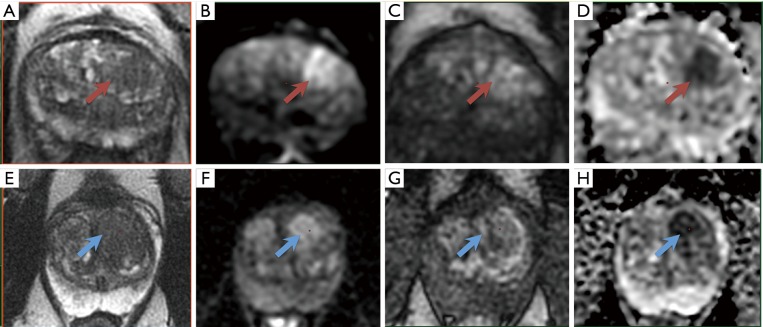
For lesions located in the transition zone, the dominant sequence in PI-RADS v2 is T2w sequence (4). Signal intensity in a lesion should be visually compared to the average signal of “normal” transition zone. Lesions are characterized as heterogeneous signal intensity with obscured margins (and include characteristics that do not qualify as PI-RADS 2, 4, or 5), together with focal mildly to moderately hypointense on ADC, and isointense to mildly hyperintense on high b-value DWI. Focal enhancement on DCE MRI may be present (Figure 2).
Figure 2.
A left transition zone lesion (arrow) on (A) axial T2-weighted image, (B) high-b-value DWI, (C) early DCE time-point, and (D) ADC map. The overall PI-RADS assessment category 3 was assigned (T2w 3, DWI/ADC 4, DCE +). On subsequent targeted MRI/US fusion biopsy, the lesion exhibited Gleason score 3+4 tumor (red arrows). PI-RADS category 3 lesions in the transition zone are characterized as heterogeneous signal intensity with obscured margins, together with focal mildly to moderately hypointense on ADC, and isointense to mildly hyperintense on high b-value DWI. Focal enhancement on DCE MRI may be present. The dominant sequence in PI-RADS v2 is T2w. (E-H) A left transition zone lesion (blue arrows). The overall PI-RADS assessment category 3 was assigned (T2w 3, DWI/ADC 4, DCE −). On subsequent targeted MRI/US fusion biopsy, the lesion exhibited no prostate cancer (blue arrows).
The interpretation of the transition zone is considered to provide a greater challenge than the peripheral zone. While a normal peripheral zone is brightly hyperintense on T2w images, and therefore hypointense abnormalities can be easily identified, the transition zone shows heterogeneous signal intensities related to the presence of nodules of benign prostatic hyperplasia, and therefore the identification of suspicious abnormalities can be more difficult (Figure 2).
What is the prevalence of PI-RADS 3 lesions?
The prevalence of prostate cancer and csPCa in published reports varies greatly (2). Several factors may influence the calculated prevalence, such as patient population, recruitment, definition of csPCa, and the diagnostic procedures. In MRI studies on prostate cancer, the prevalence of positive MRI (assigned as PI-RADS category 3 to 5) varies comparably (2).
However, the prevalence of the maximal PI-RADS 3 score for the whole prostate (further mentioned as the PI-RADS 3 index lesion) is not clearly studied in the literature. For this review we initiated an explorative search to get insight into the prevalence of PI-RADS category 3 lesions. We identified relevant manuscripts published in the period 2014 to 2017. We summarized the results of each study and categorized the multiparametric MRI data into PI-RADS 1–2, 3, 4 and 5, and separately into a PI-RADS 4–5 group (Table 1). In addition a sub classification was made within the patient groups of (I) first biopsies, (II) previously negative biopsies, and (III) active surveillance biopsies for the PI-RADS 3 lesions.
Table 1. Prevalence of PI-RADS assessment categories in men with first biopsies, previously negative biopsies, and active surveillance biopsies.
| Population/Group | Year | Center | Patients (n) | PI-RADS 1–2 | PI-RADS 3 | PI-RADS 4 | PI-RADS 5 | PI-RADS 4–5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | ||||||||
| First biopsy | |||||||||||||||||
| Borkowetz (6) | 2017 | Dresden, Heidelberg, Germany | 214 | 66 | 31 | 39 | 18 | 71 | 33 | 39 | 18 | 110 | 51 | ||||
| Filson (7) | 2016 | Los Angeles, CA, USA | 333 | 60 | 18 | 129 | 39 | 109 | 33 | 35 | 11 | 144 | 43 | ||||
| Hansen (8) | 2016 | Cambridge, United Kingdom | 107 | 22 | 21 | 21 | 20 | 23 | 21 | 41 | 38 | 64 | 60 | ||||
| Hansen (9) | 2017 | Cambridge, Heidelberg, Melbourne | 807 | 236 | 29 | 153 | 19 | – | – | – | – | 418 | 52 | ||||
| Cambridge, United Kingdom | 163 | 30 | 18 | 34 | 21 | – | – | – | – | 99 | 61 | ||||||
| Heidelberg, Germany | 402 | 140 | 35 | 91 | 23 | – | – | – | – | 171 | 43 | ||||||
| Melbourne, Australia | 242 | 66 | 27 | 28 | 12 | – | – | – | – | 148 | 61 | ||||||
| Jambor (10) | 2017 | Turku, Finland | 161 | 38 | 24 | 24 | 15 | 21 | 13 | 78 | 48 | 99 | 61 | ||||
| Pokorny (11) | 2014 | Brisbane, Australia | 233 | 81 | 35 | 33 | 14 | – | – | – | – | 109 | 47 | ||||
| Venderink (12) | 2017 | Nijmegen, The Netherlands | 184 | – | – | 17 | n.a. | 67 | n.a. | 100 | n.a. | 167 | n.a. | ||||
| Total | – | – | 1,855 | 503 | 27 | 399 | 22 | 224 | 27 | 193 | 24 | 944 | 51 | ||||
| Previous negative biopsy | |||||||||||||||||
| Filson (7) | 2016 | Los Angeles, CA, USA | 324 | 59 | 18 | 148 | 46 | 87 | 27 | 30 | 9 | 117 | 36 | ||||
| Hansen (8) | 2016 | Cambridge, United Kingdom | 295 | 91 | 31 | 75 | 25 | 70 | 24 | 59 | 20 | 129 | 44 | ||||
| Hansen (13) | 2016 | Cologne/Aachen, Heidelberg, Germany | 487 | 144 | 30 | 128 | 26 | 100 | 21 | 115 | 24 | 215 | 44 | ||||
| Simmons (14) | 2017 | London, United Kingdom | 249 | 35 | 14 | 85 | 34 | 55 | 22 | 74 | 30 | 129 | 52 | ||||
| Venderink (12) | 2017 | Nijmegen, The Netherlands | 649 | – | – | 106 | n.a. | 228 | n.a. | 315 | n.a. | 543 | n.a. | ||||
| Total | – | – | 1,355 | 329 | 24 | 436 | 32 | 312 | 23 | 278 | 21 | 590 | 44 | ||||
| Mixed (first + previous negative biopsy) | |||||||||||||||||
| Borkowetz (15) | 2017 | Dresden, Germany | 625 | 65 | 10 | 196 | 31 | 247 | 40 | 117 | 19 | 364 | 58 | ||||
| Radtke (16) | 2017 | Heidelberg, Germany | 1,159 | 225 | 19 | 367 | 32 | 346 | 30 | 221 | 19 | 567 | 49 | ||||
| Siddiqui (17) | 2016 | Bethesda, MD, USA | 1,003 | 176* | 18 | 718 | 72 | 109 | 11 | 827 | 82 | ||||||
| Active surveillance biopsy | |||||||||||||||||
| Filson (7) | 2016 | Los Angeles, CA, USA | 389 | 102 | 26 | 158 | 41 | 105 | 27 | 24 | 6 | 129 | 33 | ||||
| Hansen (8) | 2016 | Cambridge, United Kingdom | 132 | 43 | 33 | 21 | 16 | 29 | 22 | 39 | 30 | 68 | 52 | ||||
| Pessoa (18) | 2017 | Sao Paulo, Brazil | 95 | 31 | 33 | 11 | 12 | 37 | 39 | 16 | 17 | 53 | 56 | ||||
| Radtke (19) | 2017 | Heidelberg, Germany | 45 | 21 | 47 | 9 | 20 | 12 | 27 | 3 | 7 | 15 | 33 | ||||
| Recabal (20) | 2016 | New York, NY, USA | 206 | 71 | 34 | 18 | 9 | 107 | 52 | 10 | 5 | 117 | 57 | ||||
| Schoots (21) | 2018 | Rotterdam, The Netherlands | 331 | 133 | 40 | 50 | 15 | 101 | 31 | 47 | 14 | 148 | 45 | ||||
| Venderink (12) | 2017 | Nijmegen, The Netherlands | 224 | – | – | 33 | n.a. | 78 | n.a. | 113 | n.a. | 191 | n.a. | ||||
| Total | – | – | 1,198 | 401 | 33 | 267 | 22 | 391 | 33 | 139 | 12 | 530 | 44 | ||||
*, no discrimination between PI-RADS 1–3. Italic numbers are not included in the calculated totals. n.a., not applicable.
On the basis of the studies reviewed, comprising 8,252 men (6-21), PI-RADS category 1–2, 3, 4 and 5 appear to be equally distributed in all suspicious or positive MRIs, represented by approximately one fourth for each category (Table 1). Furthermore, this distribution is comparable for men with first biopsies (total n=1,855), previously negative biopsies (total n=1,355), and men with active surveillance biopsies (total n=1,198). As expected, less maximal PI-RADS 5 lesions and more PI-RADS 4 lesions were observed in men on active surveillance, reflecting smaller lesions in men already diagnosed with low-risk disease. Most likely, although the far majority of these men were diagnosed on the basis of traditional systematic biopsy sampling, this technique apparently identifies some of the larger lesions.
In men with respectively first biopsies, previously negative biopsies, and active surveillance biopsies, prostate MRIs were classified as PI-RADS 3 in 22% (range, 14–39%), 32% (range, 25–46%) and 22% (range, 9–41%). In two large cohorts of men with mixed first and previously negative biopsies, the prevalence of maximal PI-RADS 3 score was 31% (196/625) (15) and 32% (367/1,159) (16) (Table 1).
These percentages are indicative as we observed some overlap between published results. However, we may conclude that the number of men classified with a PI-RADS 3 index lesion in the diagnostic work-up is significant, varying between one in three to one in five men. These numbers represent a significant group of men with equivocal suspicion of csPCa, and adequate management strategies should be developed.
PI-RADS 3 index lesions appear to be equally distributed between the groups, however the percentages calculated within each group exhibit great variation. Data show a trend towards increased PI-RADS 3 scores in men with previously negative biopsies, as compared to men with first biopsies and active surveillance biopsies. This increase might be related to the decrease of PI-RADS 4–5 lesions. We may argue that some part of csPCa that would have been identified on MRI as PI-RADS 4–5, have already been detected by previous systematic biopsies. Still, the prevalence of the PI-RADS category 4–5 is remarkably large, varying from 44% to 51% (Table 1).
As one fourth of all MRIs are assigned to the PI-RADS 1–2 category, three fourth are assigned to PI-RADS 3–5, which shows that the majority of men suspected of having prostate cancer turned out to have an abnormal MRI. This MRI testing will all result in subsequently MRI-targeted biopsies. This invasive diagnostic procedure may contribute to harm, and should be critically evaluated. In fact, the purpose of additional biopsy testing should result in an additional diagnostic yield, but this yield should be balanced against the harms.
What is the prevalence of csPCa in PI-RADS 3 lesions?
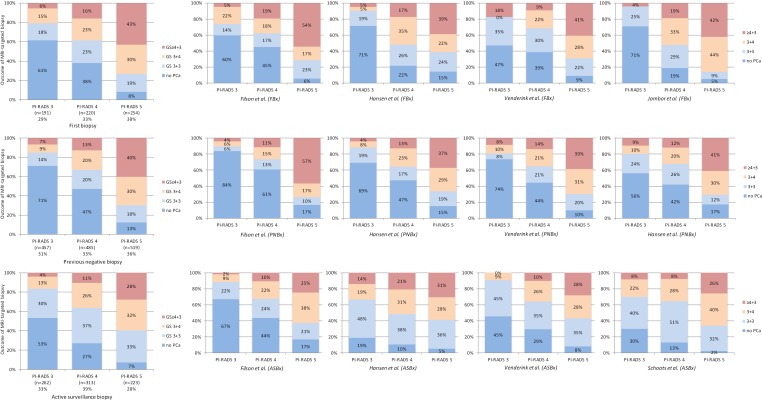
The prevalence of csPCa in PI-RADS 3 lesions has not been studied directly and therefore can only be interpreted from validation studies such as in Table 1. Some of these studies reported their results according to the Standards of reporting for MRI-targeted biopsy studies (START) of the prostate (22), and Gleason grading was correlated to the PI-RADS assessment score 1 to 5. Summary results of these studies (7,8,10,12,21,23) are shown in Figures 3 and S1.
Figure 3.
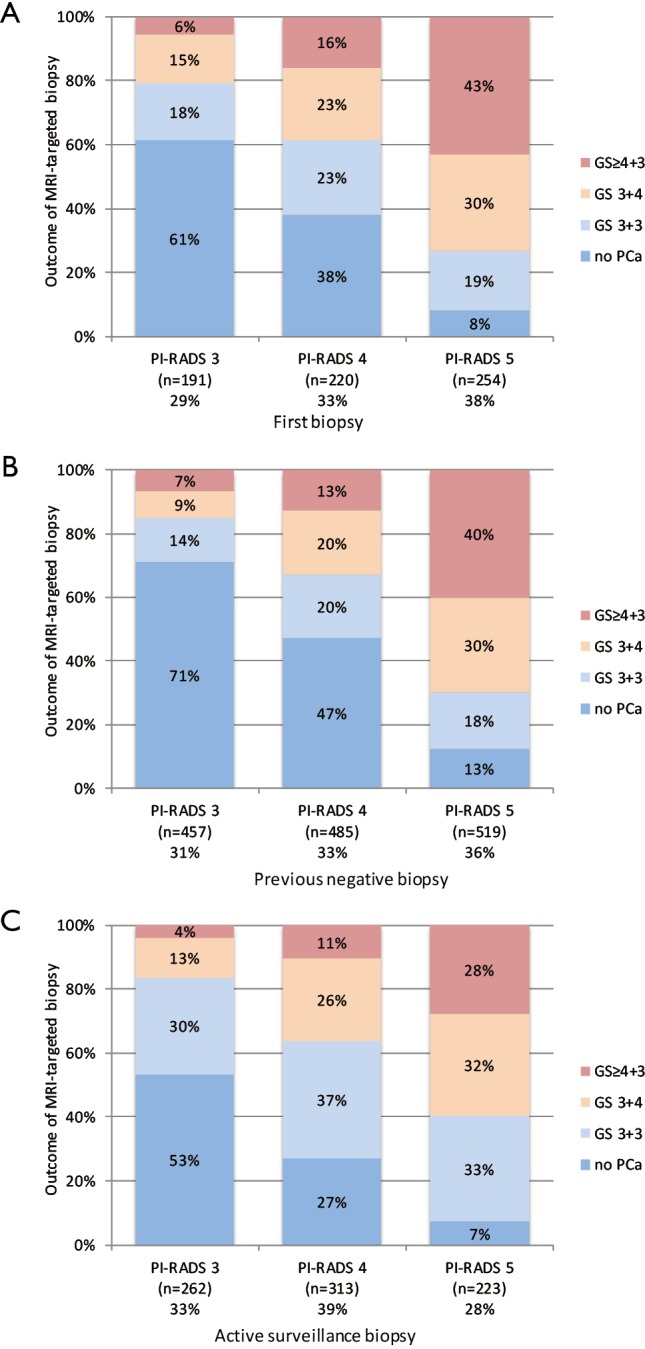
Histology outcome by Gleason score (GS) of PI-RADS assessment categories 3, 4 and 5, with subsequently MRI-targeted biopsies, in men with first biopsies (A), previous negative biopsies (B), and active surveillance biopsies (C). Cumulated data, generated by individual reports (7,8,10,12,13,21) (see Figure S1).
PI-RADS categories 3, 4 and 5 appear to be equally distributed in all suspicious MRIs (Figure 3), similar to the numbers in Table 1. Furthermore, this distribution is similar for men with first biopsies, previous negative biopsies, and active surveillance biopsies. The prevalence of csPCa in each PI-RADS category is indicative; our search and further analyses are explorative and have not been performed in an adequate systematic approach. The definition of csPCa to determine the prevalence in this analysis is based on Gleason score (GS) only, as recommended by the Standards of reporting for MRI-targeted biopsy studies (START) (22). In Figure 3, csPCa is defined as GS ≥3+4.
The prevalence of csPCa in PI-RADS 3 lesions with subsequently targeted biopsies was 21% (4–27%) in men with first biopsies, 16% (10–19%) in men with previously negative biopsies, and 17% (9–30%) in men with active surveillance biopsies (Figures 3 and S1). In PI-RADS 4 lesions, the prevalence of csPCa in these patient cohorts was 39% (31–52%), 33% (26–36%), and 37% (32–52%), respectively; in PI-RADS 5 lesions the prevalence of csPCa was 73% (61–86%), 70% (66–74%), and 60% (56–66%).
There is a structural increase of detected csPCa from PI-RADS 3 to PI-RADS 4 to PI-RADS 5 index lesions. For example, in men with first biopsies, the rates of identified csPCa are 21%, 39%, and 73%, respectively. The rates for men with previous negative biopsies are similar, represented by 16%, 33%, and 70%, respectively. Again, these rates are indicative, but the trends within this PI-RADSv2 assessment of prostate MRI in identifying csPCa appears to be repeatedly consistent in all groups, proving the additional value of this classification system.
If we use the threshold of GS ≥4+3 instead of GS ≥3+4 for csPCa, the prevalence of csPCa in PI-RADS 3 lesions with subsequently targeted biopsies was 6% (4–18%) in men with first biopsies, 7% (4–9%) in men with previously negative biopsies, and 4% (0–14%) in men with active surveillance biopsies (Figure 3). As a result of the so-called risk inflation by MRI targeting biopsies (24), we may argue shifting towards this threshold in some circumstances (21).
The prevalence of csPCa (GS ≥3+4) in PI-RADS category 3 lesions vary between patients groups from 1 in 5 (21%) to 1 in 6 (16%). Although this prevalence is lower than that found in PI-RADS 4 and 5, it is a considerable proportion and definitely not negligible. Ideally, the criteria for PI-RADS category 3 should be redefined to reduce the amount of csPCa in this category and the related uncertainty of the presence of csPCa in the diagnostic work-up and follow-up.
Should we redefine PI-RADS 3 lesion criteria to reduce csPCa in this category?
PI-RADS v2 seeks to define the five assessment categories in a way that maintains a balance between achieving high sensitivity for GS ≥7 tumors and avoiding an excessive number of biopsies that are benign or harbor low-grade tumor. Criteria for upgrading a lesion’s assigned category, based on combinations of multiple suspicious findings, are intended to help improve the sensitivity of individual PI-RADS assessment categories for csPCa. As such, for a lesion located in the peripheral zone, the assessment category matches the score assigned based on DWI, regardless of the assessment based on other pulse sequences. However, if the lesion is assigned with PI-RADS category 3 and the DCE score is also positive, than the overall category should be upgraded from 3 to 4 (Table 2).
Table 2. Proposed decision rules for PI-RADS v2 by Rosenkrantz et al. (25).
| Decision rules for PI-RADS v2 assessment category 3 to be upgraded |
| Existing PI-RADS v2 |
| ❖ In PZ, upgrade 3 to 4 if DCE score of positive |
| ❖ In TZ, upgrade 3 to 4 if DWI score of 5 |
| Proposed PI-RADS v2 |
| ❖ In PZ, upgrade 3 to 4 if T2w imaging score of 4 |
| ❖ In PZ, upgrade 3 to 4 if T2w imaging score of 5 |
| ❖ In PZ, upgrade 3 to 4 if size ≥10 mm |
| ❖ In TZ, upgrade 3 to 4 if DWI score of 4 |
| ❖ In TZ, upgrade 3 to 4 if DCE imaging score of positive |
| ❖ In TZ, upgrade 3 to 4 if size ≥10 mm |
PZ, peripheral zone; TZ, transition zone; DCE, dynamic contrast enhanced; DWI, diffusion weighted imaging.
The details of the current PI-RADS v2 decision rules are largely based on collective experience. Since PI-RADS v2 is considered to be in evolution, warranting further optimization based on continued experience and objective data, scientific investigations are needed to validate the system and help to guide potential future revisions. With the intent of further improving the sensitivity for GS ≥7 tumors at a given PI-RADS threshold, Rosenkrantz et al. proposed adjustments (additional criteria) to the current PI-RADS v2 decision rules for increasing a lesion’s final PI-RADS category (25), based on either lesion size or suspicious findings across pulse sequences of T2w, ADC and DCE (Table 2). In fact, the proposed refinements almost exclusively address upgrade from a PI-RADS category 3 to a 4, so as to potentially impact clinical decisions regarding patient selection for targeted biopsy, using the threshold of PI-RADS 4 instead of PI-RADS 3 (Table 2). Redefining criteria in this equivocal or indeterminate category of PI-RADS 3 is the way forward, and should be further investigated and confirmed by others.
Why are PI-RADS 3 lesions challenging?
Relationships between MRI signal intensities and the underlying architectural prostatic tissue are complex. Langer and colleagues showed the association between specific alterations in tissue composition and MR imaging measurements (26). They have shown that the increased cellular texture (nuclei and cytoplasm, with decreased luminal space), correspond to a decrease in T2-weighted and ADC signal intensities. T2-weighted imaging is sensitive to extracellular water, and ADC is sensitive to diffusion in the luminal space. This increased cellular texture is significantly different between malignant and benign peripheral zone tissue. Thus these mechanisms influence prostate cancer detection with MR imaging.
Overlap with benign conditions
MRI derived parameters are reflective of pathologically determined characteristics of prostate cancer, however, there is great overlap with benign conditions, such as benign prostate hyperplasia, inflammation or fibrosis. These benign abnormalities have been implicated as sources of false-positive MR imaging findings (27-30) or poor radiologic-pathologic volumetric correspondence (31). These issues are at most applicable to PI-RADS 3 lesions, as shown by the high rate of benign outcomes of targeted biopsies: prostate cancer was not detected in respectively 61% and 71% in men with first biopsies and previous negative biopsies (Figure 3).
Sparse malignant tissue
In Gleason grade 3 tumors, the glands are usually small and infiltrative, but the degree of intervening stroma can vary widely, giving either a sparse or more densely packed tumor. Within Gleason grade 4, there is marked heterogeneity with respect to the tumor architecture. Gleason grade 4 now encompasses various sub-patterns, including large dilated glands filled with abundant epithelium (large cribriform), small infiltrative poorly formed glands, glandular fusion, and mucinous tumors. Given the variety of histologic patterns, differing MRI characteristics may be observed on T2-weighted imaging (32) and other sequences. Knowledge of the relationship between MRI signal and Gleason grade sub-pattern could facilitate accurate contouring of heterogeneous tumors on MRI, facilitating targeted biopsy or lesion monitoring.
Langer and colleagues showed that no significant differences in ADC or quantitative T2-weighted values were present between the surrounding normal peripheral zone tissue and the “sparse” prostate tumors, which contain a high percentage of normal peripheral zone tissue, intermixed with prostate cancer (33). The prostate tumors that are less visible by using T2-weighted and ADC-based tissue contrast, may limit accurate determination, and might be classified as PI-RADS category 3, despite Gleason 4 patterns.
Small lesion size
Tumor size next to tumor aggressiveness may have serious impact on tumor visibility, detection and interpretation on MRI (34). Vargas and colleagues found that the integrated PI-RADS v2 scores resulted in the correct classification of 94% peripheral zone tumors and 95% transition zone tumors with ≥0.5 cc on pathology with any Gleason grading. However, the majority of GS ≥4+3 tumors with volumes <0.5 cc on pathology were not detectable on MRI. In PI-RADS v2, no lower limits of size on MRI for csPCa have been defined. In small lesions, the MRI derived parameters are less reflective of pathologically determined characteristics, and therefore the reading confidence is decreased. Such examples might be classified in PI-RADS category 3.
Interobserver agreement
Interobserver agreement among six experienced prostate radiologists by using PI-RADS v2 for assessment category PI-RADS ≥4 was moderate (k=0.552 in both zones combined) (35). Others found slightly lower interobserver agreement (k=0.46) (36). The transition zone traditionally is considered to provide a greater challenge than the peripheral zone. This is largely related to the presence of nodules of benign prostatic hyperplasia throughout the transition zone. Agreement appears to be higher in the peripheral zone than in the transition zone (35,37). Agreement, as indicated by k coefficients, appears to be better at an overall PI-RADS assessment category of ≥4 than of ≥3, regardless of zone assessment (35,37). This observation is of particular relevance for deciding the further management of these patients which lesions on MRI should be targeted by biopsies.
Should we biopsy PI-RADS 3 lesions?
PI-RADS suspicion levels of 3 or 4 may serve as thresholds for performing targeted biopsy. In order for a given threshold to be widely accepted and integrated into daily clinical practice, radiologists must be able to evaluate MRI examinations at that threshold in a reproducible fashion.
Although using a threshold of PI-RADS ≥4 achieves greater reproducibility based on these interobserver studies, our data presented in Figure 3 however, show that in this scenario we miss a substantial proportion of csPCa, 24%, 16% and 17% in men with first biopsies, previous negative biopsies, and active surveillance biopsies, respectively. Based on the current definitions of csPCa (GS ≥3+4), data from our explorative literature search do not irrefutably support clinical biopsy management that use a threshold of PI-RADS ≥4 instead of ≥3 to select MRI lesions for targeted biopsy.
If we use the threshold of GS ≥4+3 instead of GS ≥3+4 for csPCa, this substantial proportion of csPCa is reduced to 6%, 7% and 4% in men with first biopsies, previous negative biopsies, and active surveillance biopsies, respectively (Figure 3). Whether this threshold of csPCa in combination with a PI-RADS 3 lesion is acceptable should be discussed on an individual patient basis.
The decision to perform targeted biopsy of MRI lesions will continue to be influenced by a range of clinical factors including PSA kinetics, previous biopsy results, and patient preference (38). The risks of missing intermediate- or high-grade cancer must be balanced against saving biopsies and reducing harm on an individual basis. The clinical approach of using a threshold of PI-RADS ≥4 instead of PI-RADS ≥3 for selecting targeted biopsies could therefore be better supported within an active surveillance protocol, than at first biopsies.
Should we monitor PI-RADS 3 lesions?
Small index lesions on prostate MRI may correspond to benign lesions or indolent cancers based on grade and size, as shown by Rais-Bahrami et al. (39). This cohort consists patients with either no index lesion or one or more index lesions measuring ≤7 mm in greatest dimension on the initial MRI. In this study cohort, all index lesions were classified as low to moderate (maximal PI-RADS 3). The National Institute of Health (NIH) ‘lesion MRI suspicion scores’ of low, moderate and high are analogous to PI-RADS 1–2, 3 and 4–5, respectively (36). Lesions measuring ≤7 mm did not harbor Gleason 7 prostate cancer, and the vast majority of patients had benign prostatic tissue on targeted biopsies. Slow growth rate of these small index lesions on serial prostate MRI suggests that the interval-imaging follow-up can span a minimum of two years. If this is confirmed, an MRI with small index tumor and PI-RADS 3 may allow a reduction in the frequency of monitoring biopsies. In addition, changes in size or appearance of the MRI lesion may predict upgrading and trigger biopsy. However, data on monitoring PI-RADS 3 lesions are very sparse.
In a cohort of men with low-risk disease (i.e., GS 3+3) on active surveillance with at least 2 serial MRIs (interval 1 year), Frye et al. investigated men whose prostates were classified on initial MRI as low (PI-RADS 1–2) and moderate risk (PI-RADS 3) for csPCa (40). Twenty-nine percent (37/128) of these men showed pathological progression (to GS ≥3+4 and higher), based on combined targeted and systematic biopsies, 20% (25/128) showed pathological progression on targeted biopsies only. Although not mentioned in the report, we may assume that the index lesions were larger than in the previously described cohort of Rais-Bahrami et al. MRI progression was observed in 78% (29/37) of low risk patients who had pathological progression. Of patients with initial MRI suspicion scores indicating low and moderate risk, pathological progression was identified in 25% and 33%, respectively. These results plead for a stricter monitoring of PI-RADS 3 lesions, in combination with additional targeted biopsies.
From the perspective that size matters, a further subcategorization of PI-RADS category 3 has been proposed (41); (3a) indolent or low-risk lesions with volume <0.5 mL, and (3b) significant or high-risk lesions with volume ≥0.5 mL. Implication for clinical management is that subgroup 3a (low-risk lesion) may undergo clinical surveillance (periodic monitoring of PSA value and repeated MRI 1 year later) and subgroup 3b (high-risk lesion) may undergo targeted biopsy. This categorization should be further investigated before clinical introduction.
Are men with a PI-RADS 3 lesion at initial diagnostic work-up willing to forego immediate biopsy for a strategy of monitoring involving PSA measurement and/or mp-MRI repeated at intervals? van der Sar et al. investigated in retrospect the results of such a strategy in which 57% (n=95) of these men preferred the strategy of initial surveillance (42). The risk profile of the cancers identified by both strategies appeared similar, but many men in the surveillance group avoided the risks, complications, and costs of biopsy. Long-term results are awaited.
What combined strategies in PI-RADS 3 lesions have been investigated?
In the setting of suspicious imaging findings, it is accepted that MRI cannot negate the need for biopsy. Histopathological proof by targeted biopsies is necessary due to the high false-positive rate of MRI (43). If additional information can help to clarify further risk of suspicious lesions on MRI, the number of biopsies and false positive results can be reduced. Several strategies of combining additional information (i.e., PSA, PSA-density or molecular/genetic markers) to MR imaging are under investigation. They may demonstrate a benefit in making a decision about which patient needs a biopsy and concurrently help avoid unnecessary biopsies. Studies on the added value in classifying further risk of PI-RADS category 3 lesions are limited.
Prostate specific antigen (PSA)
Shakir et al. showed that most upgrading to csPCa occurred in men with a suspicious MRI and subsequently targeted biopsies, when the PSA was above 5.2 ng/mL (44). In this mixed population, csPCa was defined as GS 4+3 and higher, and further subdivision into PI-RADS 3 to 5 was not reported. This threshold of 5.2 ng/mL corresponded to potentially sparing biopsy in 36% of patients who underwent MRI. Hansen et al. recently investigated a further subdivision in PI-RADS category 3 lesions in men with first biopsies (9). PSA serum levels were categorized into <4, 4–10, and >10. The detection of GS ≥3+4 and GS ≥4+3 by targeted biopsies was 15%, 22%, 41%, and 0%, 7%, 12% respectively. These results suggest a PSA threshold of 4–5 ng/mL for improved detection of GS 4+3 and higher in PI-RADS 3 lesions.
PSA-density
In this recent study of Hansen et al. in men with first biopsies, also the PSA-density in PI-RADS 3 lesions was investigated and further categorized into <0.10, 0.10–0.20, and >0.20. The detection of GS ≥3+4 and GS ≥4+3 was 18%, 31%, 46%, and 3%, 9%, 4%, respectively.
Felker et al. investigated the yield of targeted biopsies in men with transition zone PI-RADS category 3 lesions for GS ≥3+4 (45). Among men with PSA-density of ≥0.15 ng/mL2 and lesion ADC value of <1,000 mm2/s, the detection of csPCa significantly improved from 15% to 60% (AUC >0.9). If biopsy had been restricted to these criteria, a reduction of 89% biopsies would have been obtained, with only 9% missing GS 7 tumors.
Alberts et al. used a PSA-density cut-off of 0.15 ng/mL2 in men on active surveillance (46), and was recently updated (21). In this study the detection of GS ≥3+4 and GS ≥4+3 in PI-RADS 3 lesions in combination with PSA-density cut-off of ≥0.15 ng/mL2 was 47% and 13%, without missing any upgrade to GS 3+4 or higher. Which implies that men on active surveillance with a PI-RADS 3 index lesion and a PSA-density of <0.15 ng/mL2 may not benefit from a follow-up biopsy. The use of this threshold in PI-RADS 3 lesions would result in a MRI-targeted biopsy reduction of 36% in this category. In a receiver operating characteristic (ROC) analysis by Lai et al., the optimal PSA-density cut-off point in men with low-risk disease on active surveillance was 0.18 ng/mL2 with an AUC of 0.77 (47). The optimal cut-off in men on active surveillance should be further determined in larger cohorts.
Other molecular biomarkers
Studies into the combination of PI-RADS 3 lesions and molecular markers other than PSA are lacking. The additional value of prostate cancer gene 3 (PCA3) was investigated in a small cohort of 45 PI-RADS 3 lesions in men with previously negative biopsies (48). PCA3 >35 had no additional prognostic value in the whole cohort. However, the diagnostic uncertainty in the PI-RADS 3 lesions could be ameliorated by the addition of PCA3 cut-off of 35 to avoid potential unnecessary biopsies.
Multivariate risk modeling
Combined risk models including clinical and imaging parameters may predict csPCa better than clinical risk calculators and MRI alone. Benefits of these combined risk models may exceed those of original risk calculators and PI-RADS alone in the selection of patients who should receive biopsy (16,49,50).
Conclusions
This review focuses on indeterminate lesions on prostate MRI, assigned as PI-RADS category 3. We may conclude that the prevalence of PI-RADS 3 index lesion in the diagnostic work-up is significant, varying between one in three (32%) to one in five (22%) men, depending on patient cohort of first biopsies, previously negative biopsies, and active surveillance biopsies. Management strategies should be developed for this group of men with an indeterminate suspicion of having csPCa. Currently available data show that the actual prevalence of csPCa after targeted biopsy in PI-RADS 3 lesions varies between patients groups from one in five (21%) to one in six (16%), depending on previous biopsy status. Although this prevalence is lower in comparison to PI-RADS 4 and PI-RADS 5 lesions, still a considerable proportion of men harbor significant disease. Men with such a PI-RADS 3 lesion must therefore be adequately managed.
In general, the clinical approach of using a threshold of PI-RADS ≥4 instead of PI-RADS ≥3 to select MRI for targeted biopsies cannot be supported by data from our explorative literature search using the current definitions of csPCa. A possible adaptation to the threshold of PI-RADS ≥4 in combination with other clinical markers could be considered within an active surveillance protocol, where the balance between the individual risk of missing csPCa and the constant process of repeating prostate biopsies is crucial.
In addition to improvements in MR imaging, combinations with molecular biomarkers and multivariate risk models should be employed in prostate cancer detection and monitoring. These combinations will aid decision-making in challenging circumstances, such as unclear and diagnostic equivocal results for csPCa at early detection.
Figure S1.
Histology outcome by Gleason score (GS) of PI-RADS assessment categories 3, 4 and 5, with subsequently MRI-targeted biopsies, in men with first biopsies, previous negative biopsies, and active surveillance biopsies. Cumulated data (left), generated by individual reports (middle to right) (7,8,10,12,13,21).
Acknowledgements
None.
Footnotes
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- 1.Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol 2017;71:618-29. 10.1016/j.eururo.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 2.Moldovan PC, Van den Broeck T, Sylvester R, et al. What Is the Negative Predictive Value of Multiparametric Magnetic Resonance Imaging in Excluding Prostate Cancer at Biopsy? A Systematic Review and Meta-analysis from the European Association of Urology Prostate Cancer Guidelines Panel. Eur Urol 2017;72:250-66. 10.1016/j.eururo.2017.02.026 [DOI] [PubMed] [Google Scholar]
- 3.Dickinson L, Ahmed HU, Allen C, et al. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur Urol 2011;59:477-94. 10.1016/j.eururo.2010.12.009 [DOI] [PubMed] [Google Scholar]
- 4.Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol 2012;22:746-57. 10.1007/s00330-011-2377-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol 2016;69:16-40. 10.1016/j.eururo.2015.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borkowetz A, Hadaschik B, Platzek I, et al. Prospective comparison of transperineal magnetic resonance imaging/ultrasonography fusion biopsy and transrectal systematic biopsy in biopsy-naïve patients. BJU Int 2018;121:53-60. 10.1111/bju.14017 [DOI] [PubMed] [Google Scholar]
- 7.Filson CP, Natarajan S, Margolis DJ, et al. Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: The role of systematic and targeted biopsies. Cancer 2016;122:884-92. 10.1002/cncr.29874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen N, Patruno G, Wadhwa K, et al. Magnetic Resonance and Ultrasound Image Fusion Supported Transperineal Prostate Biopsy Using the Ginsburg Protocol: Technique, Learning Points, and Biopsy Results. Eur Urol 2016;70:332-40. 10.1016/j.eururo.2016.02.064 [DOI] [PubMed] [Google Scholar]
- 9.Hansen NL, Barrett T, Kesch C, et al. Multicentre evaluation of magnetic resonance imaging supported transperineal prostate biopsy in biopsy-naive men with suspicion of prostate cancer. BJU Int 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 10.Jambor I, Bostrom PJ, Taimen P, et al. Novel biparametric MRI and targeted biopsy improves risk stratification in men with a clinical suspicion of prostate cancer (IMPROD Trial). J Magn Reson Imaging 2017;46:1089-95. 10.1002/jmri.25641 [DOI] [PubMed] [Google Scholar]
- 11.Pokorny MR, de Rooij M, Duncan E, et al. Prospective study of diagnostic accuracy comparing prostate cancer detection by transrectal ultrasound-guided biopsy versus magnetic resonance (MR) imaging with subsequent MR-guided biopsy in men without previous prostate biopsies. Eur Urol 2014;66:22-9. 10.1016/j.eururo.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 12.Venderink W, van Luijtelaar A, Bomers JG, et al. Results of Targeted Biopsy in Men with Magnetic Resonance Imaging Lesions Classified Equivocal, Likely or Highly Likely to Be Clinically Significant Prostate Cancer. Eur Urol 2017. [Epub ahead of print]. [DOI] [PubMed]
- 13.Hansen NL, Kesch C, Barrett T, et al. Multicentre evaluation of targeted and systematic biopsies using magnetic resonance and ultrasound image-fusion guided transperineal prostate biopsy in patients with a previous negative biopsy. BJU Int 2017;120:631-8. 10.1111/bju.13711 [DOI] [PubMed] [Google Scholar]
- 14.Simmons LAM, Kanthabalan A, Arya M, et al. The PICTURE study: diagnostic accuracy of multiparametric MRI in men requiring a repeat prostate biopsy. Br J Cancer 2017;116:1159-65. 10.1038/bjc.2017.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borkowetz A, Platzek I, Toma M, et al. Evaluation of Prostate Imaging Reporting and Data System Classification in the Prediction of Tumor Aggressiveness in Targeted Magnetic Resonance Imaging/Ultrasound-Fusion Biopsy. Urol Int 2017;99:177-85. 10.1159/000477263 [DOI] [PubMed] [Google Scholar]
- 16.Radtke JP, Wiesenfarth M, Kesch C, et al. Combined Clinical Parameters and Multiparametric Magnetic Resonance Imaging for Advanced Risk Modeling of Prostate Cancer-Patient-tailored Risk Stratification Can Reduce Unnecessary Biopsies. Eur Urol 2017;72:888-96. 10.1016/j.eururo.2017.03.039 [DOI] [PubMed] [Google Scholar]
- 17.Siddiqui MM, George AK, Rubin R, et al. Efficiency of Prostate Cancer Diagnosis by MR/Ultrasound Fusion-Guided Biopsy vs Standard Extended-Sextant Biopsy for MR-Visible Lesions. J Natl Cancer Inst 2016;108. pii: djw039. 10.1093/jnci/djw039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pessoa RR, Viana PC, Mattedi RL, et al. Value of 3-Tesla multiparametric magnetic resonance imaging and targeted biopsy for improved risk stratification in patients considered for active surveillance. BJU Int 2017;119:535-42. 10.1111/bju.13624 [DOI] [PubMed] [Google Scholar]
- 19.Radtke JP, Kuru TH, Bonekamp D, et al. Further reduction of disqualification rates by additional MRI-targeted biopsy with transperineal saturation biopsy compared with standard 12-core systematic biopsies for the selection of prostate cancer patients for active surveillance. Prostate Cancer Prostatic Dis 2016;19:283-91. 10.1038/pcan.2016.16 [DOI] [PubMed] [Google Scholar]
- 20.Recabal P, Assel M, Sjoberg DD, et al. The Efficacy of Multiparametric Magnetic Resonance Imaging and Magnetic Resonance Imaging Targeted Biopsy in Risk Classification for Patients with Prostate Cancer on Active Surveillance. J Urol 2016;196:374-81. 10.1016/j.juro.2016.02.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schoots IG, Osses DF, Drost FH, et al. Safe reduction of MRI-targeted biopsies in men with low-risk prostate cancer on active surveillance by stratifying to PI-RADS and PSA-density, with different thresholds for significant disease. Transl Androl Urol 2018. doi: 10.21037/tau.2017.12.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore CM, Kasivisvanathan V, Eggener S, et al. Standards of reporting for MRI-targeted biopsy studies (START) of the prostate: recommendations from an International Working Group. Eur Urol 2013;64:544-52. 10.1016/j.eururo.2013.03.030 [DOI] [PubMed] [Google Scholar]
- 23.Hansen NL, Caglic I, Berman LH, et al. Multiparametric Prostate Magnetic Resonance Imaging and Cognitively Targeted Transperineal Biopsy in Patients With Previous Abdominoperineal Resection and Suspicion of Prostate Cancer. Urology 2016;96:8-14. 10.1016/j.urology.2016.04.037 [DOI] [PubMed] [Google Scholar]
- 24.Robertson NL, Hu Y, Ahmed HU, et al. Prostate cancer risk inflation as a consequence of image-targeted biopsy of the prostate: a computer simulation study. Eur Urol 2014;65:628-34. 10.1016/j.eururo.2012.12.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenkrantz AB, Babb JS, Taneja SS, et al. Proposed Adjustments to PI-RADS Version 2 Decision Rules: Impact on Prostate Cancer Detection. Radiology 2017;283:119-29. 10.1148/radiol.2016161124 [DOI] [PubMed] [Google Scholar]
- 26.Langer DL, van der Kwast TH, Evans AJ, et al. Prostate tissue composition and MR measurements: investigating the relationships between ADC, T2, K(trans), v(e), and corresponding histologic features. Radiology 2010;255:485-94. 10.1148/radiol.10091343 [DOI] [PubMed] [Google Scholar]
- 27.Quint LE, Van Erp JS, Bland PH, et al. Prostate cancer: correlation of MR images with tissue optical density at pathologic examination. Radiology 1991;179:837-42. 10.1148/radiology.179.3.2028002 [DOI] [PubMed] [Google Scholar]
- 28.Jager GJ, Ruijter ET, van de Kaa CA, et al. Local staging of prostate cancer with endorectal MR imaging: correlation with histopathology. AJR Am J Roentgenol 1996;166:845-52. 10.2214/ajr.166.4.8610561 [DOI] [PubMed] [Google Scholar]
- 29.Cheikh AB, Girouin N, Colombel M, et al. Evaluation of T2-weighted and dynamic contrast-enhanced MRI in localizing prostate cancer before repeat biopsy. Eur Radiol 2009;19:770-8. 10.1007/s00330-008-1190-8 [DOI] [PubMed] [Google Scholar]
- 30.Shukla-Dave A, Hricak H, Eberhardt SC, et al. Chronic prostatitis: MR imaging and 1H MR spectroscopic imaging findings--initial observations. Radiology 2004;231:717-24. 10.1148/radiol.2313031391 [DOI] [PubMed] [Google Scholar]
- 31.Sommer FG, Nghiem HV, Herfkens R, et al. Determining the volume of prostatic carcinoma: value of MR imaging with an external-array coil. AJR Am J Roentgenol 1993;161:81-6. 10.2214/ajr.161.1.8517328 [DOI] [PubMed] [Google Scholar]
- 32.Downes MR, Gibson E, Sykes J, et al. Determination of the Association Between T2-weighted MRI and Gleason Sub-pattern: A Proof of Principle Study. Acad Radiol 2016;23:1412-21. 10.1016/j.acra.2016.07.013 [DOI] [PubMed] [Google Scholar]
- 33.Langer DL, Van Der Kwast TH, Evans AJ, et al. Intermixed normal tissue within prostate cancer: Effect on MR imaging measurements of apparent diffusion coefficient and T2-sparse versus dense cancers. Radiology 2008;249:900-8. 10.1148/radiol.2493080236 [DOI] [PubMed] [Google Scholar]
- 34.Vargas HA, Hötker AM, Goldman DA, et al. Updated prostate imaging reporting and data system (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: critical evaluation using whole-mount pathology as standard of reference. Eur Radiol 2016;26:1606-12. 10.1007/s00330-015-4015-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenkrantz AB, Ginocchio LA, Cornfeld D, et al. Interobserver Reproducibility of the PI-RADS Version 2 Lexicon: A Multicenter Study of Six Experienced Prostate Radiologists. Radiology 2016;280:793-804. 10.1148/radiol.2016152542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller BG, Shih JH, Sankineni S, et al. Prostate Cancer: Interobserver Agreement and Accuracy with the Revised Prostate Imaging Reporting and Data System at Multiparametric MR Imaging. Radiology 2015;277:741-50. 10.1148/radiol.2015142818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Purysko AS, Bittencourt LK, Bullen JA, et al. Accuracy and Interobserver Agreement for Prostate Imaging Reporting and Data System, Version 2, for the Characterization of Lesions Identified on Multiparametric MRI of the Prostate. AJR Am J Roentgenol 2017;209:339-49. 10.2214/AJR.16.17289 [DOI] [PubMed] [Google Scholar]
- 38.Mendhiratta N, Meng X, Taneja SS. Using multiparametric MRI to 'personalize' biopsy for men. Curr Opin Urol 2015;25:498-503. 10.1097/MOU.0000000000000216 [DOI] [PubMed] [Google Scholar]
- 39.Rais-Bahrami S, Turkbey B, Rastinehad AR, et al. Natural history of small index lesions suspicious for prostate cancer on multiparametric MRI: recommendations for interval imaging follow-up. Diagn Interv Radiol 2014;20:293-8. 10.5152/dir.2014.13319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frye TP, George AK, Kilchevsky A, et al. Magnetic Resonance Imaging-Transrectal Ultrasound Guided Fusion Biopsy to Detect Progression in Patients with Existing Lesions on Active Surveillance for Low and Intermediate Risk Prostate Cancer. J Urol 2017;197:640-6. 10.1016/j.juro.2016.08.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scialpi M, Martorana E, Aisa MC, et al. Score 3 prostate lesions: a gray zone for PI-RADS v2. Turk J Urol 2017;43:237-40. 10.5152/tud.2017.01058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Sar ECA, Kasivisvanathan V, Brizmohun M, et al. Management of Radiologically Indeterminate Magnetic Resonance Imaging Signals in Men at Risk of Prostate Cancer. Eur Urol Focus 2017. [Epub ahead of print]. 10.1016/j.euf.2017.03.016 [DOI] [PubMed] [Google Scholar]
- 43.Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017;389:815-22. 10.1016/S0140-6736(16)32401-1 [DOI] [PubMed] [Google Scholar]
- 44.Shakir NA, George AK, Siddiqui MM, et al. Identification of threshold prostate specific antigen levels to optimize the detection of clinically significant prostate cancer by magnetic resonance imaging/ultrasound fusion guided biopsy. J Urol 2014;192:1642-8. 10.1016/j.juro.2014.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Felker ER, Raman SS, Margolis DJ, et al. Risk Stratification Among Men With Prostate Imaging Reporting and Data System version 2 Category 3 Transition Zone Lesions: Is Biopsy Always Necessary? AJR Am J Roentgenol 2017;209:1272-7. 10.2214/AJR.17.18008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alberts AR, Roobol MJ, Drost FH, et al. Risk-stratification based on magnetic resonance imaging and prostate-specific antigen density may reduce unnecessary follow-up biopsy procedures in men on active surveillance for low-risk prostate cancer. BJU Int 2017;120:511-9. 10.1111/bju.13836 [DOI] [PubMed] [Google Scholar]
- 47.Lai WS, Gordetsky JB, Thomas JV, et al. Factors predicting prostate cancer upgrading on magnetic resonance imaging-targeted biopsy in an active surveillance population. Cancer 2017;123:1941-8. 10.1002/cncr.30548 [DOI] [PubMed] [Google Scholar]
- 48.Kaufmann S, Bedke J, Gatidis S, et al. Prostate cancer gene 3 (PCA3) is of additional predictive value in patients with PI-RADS grade III (intermediate) lesions in the MR-guided re-biopsy setting for prostate cancer. World J Urol 2016;34:509-15. 10.1007/s00345-015-1655-8 [DOI] [PubMed] [Google Scholar]
- 49.Satasivam P, Poon BY, Ehdaie B, et al. Can Confirmatory Biopsy be Omitted in Patients with Prostate Cancer Favorable Diagnostic Features on Active Surveillance? J Urol 2016;195:74-9. 10.1016/j.juro.2015.07.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alberts AR, Schoots IG, Bokhorst LP, et al. Risk-based Patient Selection for Magnetic Resonance Imaging-targeted Prostate Biopsy after Negative Transrectal Ultrasound-guided Random Biopsy Avoids Unnecessary Magnetic Resonance Imaging Scans. Eur Urol 2016;69:1129-34. 10.1016/j.eururo.2015.11.018 [DOI] [PubMed] [Google Scholar]