Abstract
Several studies have been conducted on the quality of life (QoL) in men with low risk prostate cancer (PCa) who choose active surveillance (AS). While recent reviews have shown a lack of consistency among the available QoL-studies, a few key points have been identified, including decision-making (DM)-related issues and their potential effect on QoL. The importance of this theme has also been recently highlighted by the international task force of the European School of Oncology. However, to our knowledge, there are no studies that have specifically marshalled scientific knowledge on the association between DM and QoL among men with low-risk PCa undergoing AS. We performed a literature review to fill this gap, taking a systematic approach to retrieving and selecting articles that included both DM and QoL measures. Among the 272 articles retrieved, we selected nine observational, quantitative articles with both DM and QoL measures. The most considered DM aspects within these studies were decisional conflict and preference for the patient’s role in the DM process, as well as health-related QoL aspects. The studies included 42 assessments of the relationship between an empirical measure of DM and an empirical measure of QoL. Among these assessments, 23 (55%) were both positive and significant. They mostly concerned the relationship between patient-related (decisional self-efficacy, decisional control and knowledge) and external (presence of social support, collaborative role within the DM process, and influence of different physicians) DM aspects, as well as the QoL after choice. The findings of these studies revealed key challenges to research and clinical practice related to DM and QoL in AS. These include adopting a person-centred perspective where clinicians, caregivers and their interactions are also included in evaluations and where the psychosocial existential experience of individuals within the DM and AS journey is considered. Much more attention needs to be paid to the DM process after diagnosis, as well as to all the other moments where patients may have to or want to review their decision. Healthcare professionals play a key role in enabling men to make informed decisions and to take care of their health and well-being during AS. There is still work that needs to be done in training healthcare professionals from different disciplines to work together in a model of shared DM and AS tailored to the needs of low-risk PCa patients and their family members.
Keywords: Prostate cancer (PCa), active surveillance (AS), quality of life (QoL), decision-making (DM)
Introduction
Patients with low-risk prostate cancer (PCa) have the option to choose among surgery, radiotherapy (either brachytherapy or external beam), and active surveillance (AS). All of these options are internationally accepted and equally effective from a medical point of view (1-3). While the oncologic outcomes are equal, the options differ in terms of side effects. Urinary and sexual complaints have been reported for surgery, and sexual and rectal dysfunctions have been reported for radiotherapy (4). In general, AS presents no threats for men in terms of their health-related quality of life (QoL) (5). However, AS can increase anxiety symptoms due to the postponement of treatment and living with untreated cancer (5,6). Patients undergoing AS may also experience the fear of cancer progression and the burden of both frequent examinations and periodic biopsies (7). It is an uncertain road, and patients may feel distressed (8). Even if their health-related QoL may be preserved, their overall QoL in terms of general subjective well-being may be impaired.
As QoL considerations largely rely on an individual’s values and preferences, it may be that the different interventions are not equally acceptable from a personal point of view. Therefore, given the number of choices available and their potential side effects, newly diagnosed PCa patients may experience difficulty in deciding which treatment is best suited for them, for their health, and for their home support environment (9). The decision-making (DM) process for low-risk PCa patients becomes a complex scenario, influenced by the different actors who are part of such a process: the patient, the patient’s family, and the clinician(s). Knowledge, preferences, and exchanges among these actors may influence treatment choice and satisfaction, as well as QoL (9). Furthermore, having AS in the range of avenues available can make the DM process even more demanding and conflictual. Indeed, it challenges patients with the opportunity and burden of not treating their cancer until it becomes necessary from a clinical perspective or a personal point of view.
Not surprisingly, considering these premises, DM and QoL issues have been frequently explored among patients undergoing AS. Different literature reviews have been conducted to organize this knowledge. However, they have focused on DM and QoL issues separately or, when DM and QoL were considered together, their relationship has been approached marginally. This is the case of some of the reviews on QoL in AS where DM-related aspects were highlighted as a key aspect to be considered in QoL research for AS patients (5,7,8). Other reviews have highlighted how QoL is also relevant leverage for low-risk PCa patients in making a choice (9,10).
Despite the suggested importance of QoL for the DM process and of the DM process for QoL, as was also recently highlighted by the international task force on QoL in AS conducted by the European School of Oncology (11), to our knowledge, there are no studies that have specifically or systematically marshalled the scientifically reported associations between DM and QoL among men with low-risk PCa undergoing AS. This study aimed to systematically address knowledge of the association between DM and QoL.
Methods
A literature review was conducted that took a systematic approach to retrieving and selecting articles that included both DM and QoL measures.
Search methods
On the 20th October, 2017, a search was launched on each of the main scientific databases (Scopus, PubMed, ISI Web of Science) using the following terms: “prostate cancer” and “quality of life” and “Active Surveillance” and “low risk”. The term “decision-making” was not included in the search strategy and was used instead as a screening criterion to allow us not to lose relevant articles focused on DM but that used synonyms or paraphrases to express this concept.
The databases were searched without year or language restrictions. The initial electronic search strategy was supplemented by screening the reference lists from eligible included studies.
Selection of studies
The search results were uploaded to an MS Excel file, and duplicates were removed. In the first round of screening, abstracts and titles were screened for inclusion. Following the abstract screening, eligibility was assessed by screening the full-texts of the articles. One researcher (Julia Menichetti) performed the screening, and doubts were solved by consensus with the other authors.
In particular, articles were screened for the following:
Clear mentions of DM issues in the abstract or title;
Reports of observational quantitative studies.
The exclusion criteria during the screening process included the following:
Articles not mentioning QoL issues (a generic definition of QoL as general well-being was used, i.e., articles on psycho-social aspects were included);
Articles reporting studies that did not include patients with low-risk PCa;
Articles not including the option of AS specifically and/or separately from other monitoring strategies (articles focusing on watchful waiting/other monitoring strategies or treating these patients together with patients on AS were excluded);
Articles where the full-text was not retrievable.
Data collection
In each study, we extracted the following: study year, country where the study was conducted, number of participating sites, number of participants, target of the study (patient/clinician/both/other), study design (qualitative/observational/experimental/review/other), main result, and main focus.
Results
The search yielded 266 articles, and six more were included by searching the references of the included articles. After the screening process, nine articles matched the inclusion criteria (Table 1). Figure 1 reports the flow chart of the identification and screening of the articles.
Table 1. Characteristics of the studies included.
| Reference | Time points | N | % AS | DM measures | QoL measures | Main finding for DM-QoL | N DM-QoL evaluations* [N significant] |
|---|---|---|---|---|---|---|---|
| Hurwitz et al. 2016 (12) | Before/after DM | 925 | 11 | Control Preferences Scale; Satisfaction with Decision Scale; Decision Regret Scale | EPIC, SF-36 | Patients attending a multidisciplinary counselling for DM are satisfied about their choice and report low regret | 0 |
| van den Bergh et al. 2010 (13) | Just after entrance in AS; 9 months after entrance | 150 | 100 | Decisional Conflict Scale; ad hoc question for physician involvement in the DM process | CES-D, STAI-6, MAX-PC, SF-12, PCS | Decisional conflict (linked to a shared physician role in treatment DM) impacts on anxiety and distress at 9 months | 9 [3] |
| Davison and Goldenberg 2011 (14) | After choice | 73 | 100 | Control Preferences Scale; ad hoc questions on role men assumed with their physician in treatment DM, factors influencing decision to go on AS, and resources required while on AS | ad hoc questions on satisfaction and anxiety | Men are influenced by the treating specialist in taking up AS | 0* |
| Bellardita et al. 2013 (15) | Just after entrance in AS; 10 months after entrance | 103 | 100 | Decisional Conflict Scale, ad hoc questions on n° physicians consulted and social support | FACT-P, SF-36 | High levels of HRQoL during AS are predicted by a patient having consulted several physicians about the choice of AS, by the presence of a partner, and the mental health of patients at baseline | 4 [3] |
| Hoffman et al. 2017 (16) | 6 months after choice and at 15 years | 934 | 10.2 | Ad hoc questions for decisional regret, perception of informed decision | SF-36, UCLA Prostate Cancer Index, EPIC; ad hoc questions for health worry, PSA concern, outlook on life | Low regret (8%) in AS; lack of informed decision making, PSA concern, and sexual and bowel function bother are associated with regret | 8 [4] |
| Taylor et al. 2016 (17) | Before choice | 1,140 | 39.3 | Decisional Conflict Scale; Control Preference Scale; ad hoc question for treatment preference | Cancer Control Subscale of the Health Worry Scale | Men preferring AS (vs. radical treatments), similar to those without a preference, are older, more educated, have greater PCa knowledge and greater awareness of PCa, are less certain about their treatment preference, have greater PCa anxiety, and prefer a shared treatment decision | 0* |
| Orom et al. 2016 (18) | Just after choice; 6 months after choice | 1,529 | 22.3 | Decisional Conflict Scale; Satisfaction with Decision Scale; ad hoc question on decisional control and DM difficulty | EPIC | PCa knowledge and control impact on DM outcomes (choice, satisfaction, conflict, difficulty), and QoL after choice | 15 [11] |
| Cuypers et al. 2017 (19) | Before/after choice | 377 | 34 | Decision self-efficacy scale | EORTC QLQ-C30, PR25, LOT-R | Optimism relates with health-related QoL before choice, decisional self-efficacy positively associates with health-related QoL after choice | 4 [2] |
| Repetto et al. 2016 (20) | Just after entrance in AS; after exit | 105 | 100 | Treatment regret scale | FACT-P, MINI-MAC | Low regret in AS, no impact from QoL aspects | 2 [0] |
*, the type of choice/preference for an option was not considered as DM evaluation. DM, decision-making; QoL, quality of life; AS, Active Surveillance; SF, Short Form Health Survey; EPIC, Expanded Prostate Cancer Index Composite; FACT-P, Functional Assessment of Cancer Therapy-Prostate; STAI, Spielberger State Trait Anxiety Inventory; MAX-PC, Memorial Anxiety Scale for Prostate Cancer; CES-D, Center for Epidemiological Studies Depression; LOT-R, Life Orientation Test Revised; PCS, Pain Catastrophizing Scale; MINI-MAC, Mental Adjustment to Cancer Scale.
Figure 1.
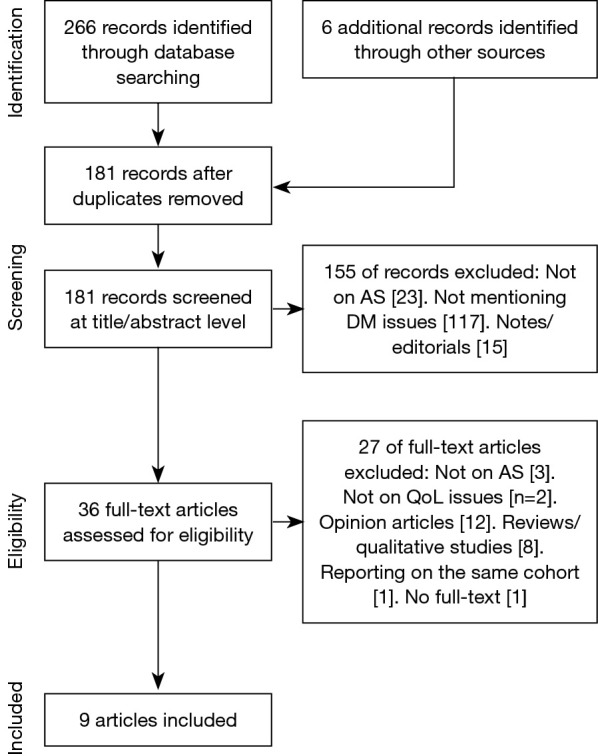
PRISMA flow chart.
Description of studies
The nine studies included were published after 2010, and most of them (n=6) had been done so in the prior 2 years.
Most of the nine observational studies were conducted in the United States (n=4), followed by European countries such as the Netherlands (n=2) and Italy (n=2). One article was from Canada.
The studies used self-reported questionnaires at one (n=2) or more (n=7) time points, generally (n=6) after the treatment decision had been made. Four studies focused on AS patients only. Among the studies that included radical prostatectomy and radical therapy as well, the average percentage of patients undergoing AS was 22.5 (range, 10.2–39.3).
On average, these studies included 593 participants (range, 73–1,529), adding up to a total of 5,336 patients.
How is DM measured and what are the key DM results?
The DM aspects that were considered within the nine selected studies were decisional conflict (n=4), preference for the patient’s role in the DM process (n=4), role of the physician in the DM process (n=2), decision regret (n=3), decision satisfaction (n=2), and decision self-efficacy (n=1). Several measures were used to assess these aspects, and most of them were patient-reported validated scales.
Among the adopted validated measures, the Decisional Conflict Scale (DCS) was the most commonly used (21). The DCS evaluates the overall decisional conflict of individuals and includes subscales for personal feelings of uncertainty, being informed, clarity of values, support, and effective decision (n=4 studies). In one study (17), this scale was used in an abbreviated version of four items (22).
The second most commonly included validated measure was the Control Preference Scale (CPS) (23), in which patients rate their perceptions about their role and their level of involvement (active, collaborative, passive) in DM (n=3 studies).
Other validated measures were the Decision Regret Scale (24) (n=2 studies), the Satisfaction with Decision Scale (25) (n=2 studies), and the Decision Self-Efficacy Scale (26) (n=1 study).
Furthermore, ad hoc questions were used to evaluate physician involvement in the DM process (n=2 studies), factors influencing the decision to undergo AS (n=1 study), decisional regret (n=1 study), perception of the informed decision (n=1 study), treatment preference (n=1 study), decisional control (n=1 study), and decision difficulty (n=1 study).
The main findings of the studies that used the DCS and the CPS were that 63% of the 925 low-risk PCa patients who may have chosen AS ideally preferred to play an active role in the DM process (12). When attention was paid to the actual patient’s role in DM after the treatment decision, the results were contradictory (14,18). On the one hand, Davison’s study reported that 41% of the 73 patients involved experienced a collaborative DM (“my doctor and I shared responsibility”) during the DM process with the urologist; almost one third of them declared that the urologist made the decision (passive DM) (14). On the other hand, Orom’s study evaluated 1,529 low-risk PCa patients who had the possibility to choose AS, and most of the men made the decision on their own or with their clinician’s input (actively; 66.8%) (18). In both these studies, the older the patients were, the more they played a passive role in the DM process.
Considering the decisional conflict of the patients who chose AS, a study reported favourable levels of decisional conflict, which were predicted by the perceived importance of the role of the physician in shared DM (13). Another study confirmed the low decisional conflict of low-risk PCa patients (18). It also reported how men with greater PCa knowledge and decisional control had less conflict but experienced greater difficulty with the treatment decision (18). In all these studies, the subscales of the DCS were not reported. Two other studies used the DCS but did not report the outcomes (15,17).
How is QoL measured and what are the key QoL results?
The general QoL aspects that were considered were health-related QoL (n=4 studies), anxiety (n=2 studies), health worry (n=2 studies), depression (n=1 study), optimism (n=1 study), pain catastrophizing (n=1 studies), coping (n=1 study), PSA concern (n=1 study), and outlook on life (n=1 study).
Studies adopted both ad hoc questions and/or validated scales. For health-related QoL, the most frequently adopted measures were the Short Form Health Survey (27) (n=3 studies) and the Expanded Prostate Cancer Index Composite (EPIC) (28) (n=3 studies). Other QoL measures were the Functional Assessment of Cancer Therapy-Prostate (29) (n=2 studies), the UCLA Prostate Cancer Index (30) (n=1 study), and the EORTC QLQ-C30 (31) (n=1 study).
Anxiety was assessed with the short-form of the Spielberger State-Trait Anxiety Inventory (32) and with the Memorial Anxiety Scale for Prostate Cancer (33) (n=1 per study), whereas depression was assessed with the Center For Epidemiological Studies Depression scale (34) (n=1). Other used measures were the Life Orientation Test-Revised (35), the Pain Catastrophizing Scale (36), the Mental Adjustment to Cancer scale (37), and the Cancer Control Subscale of the Health Worry Scale (38) (n=1 per study). Ad hoc questions were then used to assess satisfaction, anxiety, health worry, PSA concern, and outlook on life.
The main findings of the studies that used the Short Form Health Survey and the EPIC were that, overall, the choice of AS did not lower patients’ health-related QoL (13,15) compared to the choice of undergoing radical treatments (16). Studies using the EPIC also reported good pre-treatment health-related QoL scores of low-risk PCa patients, comparable to those of the general population (12), except in terms of the sexual QoL scores, which were under the “normal” threshold (18).
What are the reported associations between DM and QoL?
The studies included 42 assessments of the relationship between an empirical measure of DM and an empirical measure of QoL. Among these assessments, 23 (55%) were both positive and significant. In Table 1, details of the key results of the nine studies included are reported.
The first hinge: patient-related DM aspects—QoL after choice
The first group of findings concerned studies that reported associations between patient-related DM aspects and patients’ QoL after choice (n=2 studies). In one study where patients completed questionnaires before and after prostate biopsy and treatment DM, it was found that, even if QoL before diagnosis was associated with having an optimistic outlook, after DM QoL was associated with patients’ decisional self-efficacy (19). Orom’s study observed that higher decisional control and PCa knowledge positively impacted decisional conflict and satisfaction of low-risk PCa patients. PCa knowledge, in particular, had an impact on the QoL of patients six months after every choice (18).
The second hinge: external DM aspects—QoL after choice
Another group of findings reported associations between external (clinician-related, family-related, organization-related) DM aspects and the patients’ QoL after choice (n=4 studies). Bellardita’s study followed a cohort of patients undergoing AS and observed that both the influence of different physicians and the lack of a partner impacted the QoL of patients nine months after inclusion in AS (15). Then, another study demonstrated that clinician-related aspects impacted anxiety and distress levels of patients undergoing AS (13). In particular, the study found that a shared role taken by the clinician during the DM process impacted the patients’ decisional conflict, which, in turn, predicted anxiety and distress nine months after inclusion in AS (13). In another study, it was reported that reaching an informed treatment decision predicted low levels of treatment decision regret in the long-term (15 years), which was generally low for patients choosing AS (16). Then, another study explored the impact of clinician-related aspects on the anxiety and distress of patients undergoing AS (13). The study found that the greater the shared role taken by the clinician during the DM process, the higher the patients’ decisional conflict, but not their anxiety and distress, was nine months after inclusion in AS (13). Finally, one study observed treatment DM of patients attending a multidisciplinary clinic and observed that patients attending multidisciplinary counselling on treatment DM had generally low regret and high satisfaction with the decision (12). As the study was descriptive and lacked a control group, it was difficult to evaluate the findings obtained.
The third hinge: QoL within DM—DM outcomes
The third group of findings were concerned with the association between QoL before/during treatment DM and DM outcomes, with limited results from the two relevant studies. Davison’s study reported that QoL considerations of urinary function side effects, as well as age and the urologist’s opinion, had a role in influencing treatment decisions (14). A study focused on the regret of patients exiting AS reported the absence of regret in this population, with none of the QoL variables at baseline reaching significance in explaining regret (20).
The fourth hinge: DM/QoL aspects—AS choice
Finally, there were three studies that focused on the association between DM/QoL aspects and the AS choice. Taylor and colleagues accounted for 1,140 men waiting to make a decision. The study showed that men with a preference for AS had greater anxiety and uncertainty for their treatment preference at baseline and preferred a shared treatment decision, similar to those without a clear preference for a certain option. At the same time, these men were more aware and informed of their PCa (17). In the study by Davison, it was reported that most men were comfortable (82%) and satisfied (90%) with the AS choice; more than half (55%) reported not to be anxious about the cancer progression (14). The urologist’s opinion, current age, and the impact of treatment on urinary function were main factors influencing the treatment decisions (14). Then, another study found that the QoL scores before the diagnostic biopsy were unrelated to the selection of a particular treatment or to personality characteristics (19).
Discussion
In this study, we summarized the scientific knowledge about the association between DM and QoL aspects in low-risk PCa patients who chose to undergo AS. The overall finding was that, even if DM and QoL are key issues in this clinical setting, few studies have empirically measured their associations. We found a total of nine unique studies, which included 42 assessments of the relationship between an empirical measure of DM and an empirical measure of QoL. A high variability of measures, evaluation times, and type of associations was observed. This variability prevents us from making clear cut conclusions, even if we can soundly suggest that DM should receive further attention in AS literature on QoL.
Considering the measures adopted, the DCS and the CPS and the SF-36 and the EPIC scales were the most used measures for DM aspects and for QoL aspects, respectively, among the studies that included DM and QoL evaluations. All these measures are internationally accepted and adopted validated scales that deserve to be included in low-risk PCa patients’ evaluations, as they allow one to make comparisons with other group of patients. For example, previous literature on cancer patients has shown a mismatch between patients’ preferred role in DM (collaborative) and their experienced role (mostly passive, 36%) (39). In the present study, which focused on low-risk PCa patients undergoing AS, it was reported that patients prefer playing an active role during the DM, and they actually do play an active/collaborative role. Therefore, the adoption of the CPS in the literature allowed a revelation to be made of the difference between cancer patients and low-risk PCa patients in their desired/played role within the DM process. This may be explained by the high relevance of eliciting patients’ preferences and engaging them has in low-risk PCa DM (40). Since, in this review, no major associations were observed between the key DM and health-related QoL measures, other QoL outcomes closer to an individual’s well-being may be associated with the experience of DM. For example, in a review investigating the association between shared DM and patient outcomes, the relevance of affective-cognitive aspects, such as trust, confidence, empowerment, and satisfaction with care, was highlighted in relation to shared DM (41). Furthermore, we observed that all the measures included were patient-reported, and the studies lacked reports of the outcomes from the other relevant actors of DM (clinicians, caregivers). Therefore, one key challenge may be advisable when looking at the measures adopted to investigate the association between DM and QoL among low-risk PCa patients. There is a need to move from a classical perspective where only the patient and health-related QoL issues are considered, to a person-centred perspective where clinicians, caregivers and their interactions are also included in evaluations and where broader aspects of QoL are considered by giving relevance to the psychosocial existential experience of individuals within the DM journey (42,43).
Looking at the time points considered to assess patients, it was observed that DM and QoL aspects were usually assessed after the DM process, and most of the times, immediately after it. The reason may lie in the difficulty of reaching patients before the DM process and to assess them at multiple timepoints. At the same time, findings of studies conducted in this way not be able to precisely grasp the DM experience and reflect it at the time when patients have to manage it. Furthermore, it reveals a lack of attention on other critical points of the AS journey, where decisions have to be made. It is, for example, the case of patients exiting AS and needing to make a decision on how to treat their cancer, which was explored only by one study (20). Therefore, evaluation times in the future should include the DM process after diagnosis but also all the other moments where patients may have to or want to review their decision. Problems with biopsies and reported concerns may all be relevant potential decisional time points where patients could renegotiate/consolidate the DM process. Placing these moments in the spotlight of DM issues may also strengthen patients’ engagement towards their choice and care (44).
Finally, considering the DM-QoL associations observed, these mostly concerned DM aspects and patients’ QoL after their choice. In particular, patient-related (decisional self-efficacy, decisional control and knowledge) and clinician-related (collaborative role within the DM process, influence of different physicians) aspects played a role in patients’ QoL. The type of DM was also highlighted, with reaching a shared and informed treatment decision being associated with better well-being outcomes. In doing this, multidisciplinary care has been suggested to be a key strategy to improve DM and QoL outcomes. Overall, these findings may suggest that enabling patients to take power and control over the treatment decision can be a valuable road to enhancing their DM experience and well-being outcomes after choice. There is an increasing amount of literature that highly values making patients actors and leaders of their care for better care outcomes (45-47). Studies exploring the voices of clinicians and patients undergoing AS also maintained the relevance of providing patients with knowledge and resources to make informed decisions and to manage AS through a collaborative work during and after the choice (48,49). In such an effort, the key role of clinicians in giving patients the instruments, means, and power role to decide for themselves is highlighted both in our study and in the previous literature (48-50). Clinicians’ knowledge and attitudes, the quality of the doctor-patient relationship, shared DM, and the presence of different professionals may indeed affect decision regarding and adherence to AS, as well as reduce decisional regret and distress after choice (50). Furthermore, patient and clinician preferences, the healthcare setting, and family or spouse factors have the potential to influence professionals’ treatment recommendations for men with low-risk PCa (50,51). Therefore, training healthcare professionals from different disciplines to work together to support a shared DM process and to make patients and families feel engaged with DM and overall care can be a key effort that still needs to be approached and researched (52). Finally, we noticed that the DM-QoL evaluations lacked research that included QoL considerations within the DM process. Information on QoL outcomes associated with PCa options has been suggested to be included in pre-treatment DM counselling to guide patients (10). Further studies should seek to demonstrate that including a QoL discussion could impact both the DM process and choice and to examine how such a discussion should occur.
To conclude, key challenges for research and clinical practice related to DM and QoL issues in AS may be advisable. These include adopting and enacting a person-centred perspective wherein clinicians, caregivers and their interactions are also included in evaluations, and where the psychosocial existential experience of individuals within the DM and AS journey is considered. Much more attention needs to be paid to the DM process after diagnosis, as well as to the subsequent relevant DM moments where patients may have to or want to review their decision. At the same time, time constraints need also to be considered. Healthcare professionals play a key role in enabling men to make informed, deliberate decisions and to take care for their health and well-being. There is still room for improvement in the training of healthcare professionals from different disciplines to work together in a model of shared DM and AS tailored to the needs of low-risk PCa men and their family members.
Acknowledgements
We are grateful to the patients and the professionals who share the low-risk PCa journey in our Institution, for providing us with daily valuable insights that enrich our clinical and research activity on DM and QoL in AS.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Hamdy FC, Donovan JL, Lane JA, et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med 2016;375:1415-24. 10.1056/NEJMoa1606220 [DOI] [PubMed] [Google Scholar]
- 2.Bokhorst LP, Valdagni R, Rannikko A, et al. A Decade of Active Surveillance in the PRIAS Study: An Update and Evaluation of the Criteria Used to Recommend a Switch to Active Treatment. Eur Urol 2016;70:954-60. 10.1016/j.eururo.2016.06.007 [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part 1: Screening, diagnosis, and local treatment with curative intent - Update 2013. Eur Urol 2014;65:124-37. 10.1016/j.eururo.2013.09.046 [DOI] [PubMed] [Google Scholar]
- 4.Punnen S, Cowan JE, Chan JM, et al. Long-term health-related quality of life after primary treatment for localized prostate cancer: Results from the CaPSURE registry. Eur Urol 2015;68:600-8. 10.1016/j.eururo.2014.08.074 [DOI] [PubMed] [Google Scholar]
- 5.Bellardita L, Valdagni R, van den Bergh R, et al. How does active surveillance for prostate cancer affect quality of life? A systematic review. Eur Urol 2015;67:637-45. 10.1016/j.eururo.2014.10.028 [DOI] [PubMed] [Google Scholar]
- 6.Gilbourd D, Boehm-Wilcox C, Louie-Johnsun M. Quantifying anxiety and quality of life amongst patients on active surveillance for prostate cancer in an Australian setting. BJU Int 2013;111 Supplement 1:48. [DOI] [PubMed] [Google Scholar]
- 7.van den Bergh RCN, Korfage IJ, Bangma CH. Psychological aspects of active surveillance. Curr Opin Urol 2012;22:237-42. 10.1097/MOU.0b013e328351dcb1 [DOI] [PubMed] [Google Scholar]
- 8.Kazer MW, Psutka SP, Latini DM, Bailey DE. Psychosocial aspects of active surveillance. Curr Opin Urol 2013;23:273-7. [DOI] [PubMed] [Google Scholar]
- 9.Hall IJ, Lee Smith J. Evolution of a CDC Public Health Research Agenda for Low-Risk Prostate Cancer. Am J Prev Med 2015;49:S483-8. 10.1016/j.amepre.2015.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lardas M, Liew M, van den Bergh RC, et al. Quality of Life Outcomes after Primary Treatment for Clinically Localised Prostate Cancer: A Systematic Review. Eur Urol 2017;72:869-85. 10.1016/j.eururo.2017.06.035 [DOI] [PubMed] [Google Scholar]
- 11.Villa S, Kendel F, Venderbos L, et al. Setting an Agenda for Assessment of Health-related Quality of Life Among Men with Prostate Cancer on Active Surveillance: A Consensus Paper from a European School of Oncology Task Force. Eur Urol 2017;71:274-80. 10.1016/j.eururo.2016.09.041 [DOI] [PubMed] [Google Scholar]
- 12.Hurwitz LM, Cullen J, Elsamanoudi S, et al. A prospective cohort study of treatment decision-making for prostate cancer following participation in a multidisciplinary clinic. Urol Oncol 2016;34:233.e17-25. 10.1016/j.urolonc.2015.11.014 [DOI] [PubMed] [Google Scholar]
- 13.van den Bergh RC, Essink-Bot ML, Roobol MJ, et al. Do Anxiety and Distress Increase During Active Surveillance for Low Risk Prostate Cancer? J Urol 2010;183:1786-91. 10.1016/j.juro.2009.12.099 [DOI] [PubMed] [Google Scholar]
- 14.Davison BJ, Goldenberg SL. Patient acceptance of active surveillance as a treatment option for low-risk prostate cancer. BJU Int 2011;108:1787-93. 10.1111/j.1464-410X.2011.10200.x [DOI] [PubMed] [Google Scholar]
- 15.Bellardita L, Rancati T, Alvisi MF, et al. Predictors of health-related quality of life and adjustment to prostate cancer during active surveillance. Eur Urol 2013;64:30-6. 10.1016/j.eururo.2013.01.009 [DOI] [PubMed] [Google Scholar]
- 16.Hoffman RM, Lo M, Clark JA, et al. Treatment Decision Regret Among Long-Term Survivors of Localized Prostate Cancer: Results From the Prostate Cancer Outcomes Study. J Clin Oncol 2017;35:2306-14. 10.1200/JCO.2016.70.6317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor KL, Hoffman RM, Davis KM, et al. Treatment Preferences for Active Surveillance versus Active Treatment among Men with Low-Risk Prostate Cancer. Cancer Epidemiol Biomarkers Prev 2016;25:1240-50. 10.1158/1055-9965.EPI-15-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orom H, Biddle C, Underwood W, et al. What Is a “Good” Treatment Decision? Decisional Control, Knowledge, Treatment Decision Making, and Quality of Life in Men with Clinically Localized Prostate Cancer. Med Decis Making 2016;36:714-25. 10.1177/0272989X16635633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuypers M, Lamers RE, Cornel EB, Pet al. The impact of prostate cancer diagnosis and treatment decision-making on health-related quality of life before treatment onset. Support Care Cancer 2017. [Epub ahead of print] 10.1007/s00520-017-3953-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Repetto C, Rancati T, Magnani T, et al. "What if…": decisional regret in patients who discontinued active surveillance. Tumori 2016;102:562-8. 10.5301/tj.5000564 [DOI] [PubMed] [Google Scholar]
- 21.O’Connor AM. Validation of a Decisional Conflict Scale. Med Decis Making 1995;15:25-30. 10.1177/0272989X9501500105 [DOI] [PubMed] [Google Scholar]
- 22.Légaré F, Kearing S, Clay K, et al. Are you SURE? Assessing patient decisional conflict with a 4-item screening test. Can Fam Physician 2010;56:e308-14. [PMC free article] [PubMed] [Google Scholar]
- 23.Degner LF, Sloan JA, Venkatesh P. The Control Preferences Scale. Can J Nurs Res 1997;29:21-43. [PubMed] [Google Scholar]
- 24.Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a Decision Regret Scale. Med Decis Making 2003;23:281-92. 10.1177/0272989X03256005 [DOI] [PubMed] [Google Scholar]
- 25.Holmes-Rovner M, Kroll J, Schmitt N, et al. Patient Satisfaction with Health Care Decisions: The Satisfaction with Decision Scale. Med Decis Making 1996;16:58-64. 10.1177/0272989X9601600114 [DOI] [PubMed] [Google Scholar]
- 26.Betz NE, Luzzo DA. Career assessment and the Career Decision-Making Self-Efficacy Scale. J Career Assess 1996;4:413-28. 10.1177/106907279600400405 [DOI] [Google Scholar]
- 27.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey Manual and Interpretation Guide. The Health Institute, New England Medical Center 1993. [Google Scholar]
- 28.Dunn RL. Scoring Instructions for the Expanded Prostate cancer Index Composite (EPIC)*. The University of Michigan 2002:734. [Google Scholar]
- 29.Esper P, Mo F, Chodak G, et al. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology 1997;50:920-8. 10.1016/S0090-4295(97)00459-7 [DOI] [PubMed] [Google Scholar]
- 30.Litwin MS, Hays RD, Fink A, et al. The UCLA Prostate Cancer Index: development, reliability, and validity of a health-related quality of life measure. Med Care 1998;36:1002-12. 10.1097/00005650-199807000-00007 [DOI] [PubMed] [Google Scholar]
- 31.Fayers P, Bottomley A. Quality of life research within the EORTC—the EORTC QLQ-C30. Eur J Cancer 2002;38:S125-33. 10.1016/S0959-8049(01)00448-8 [DOI] [PubMed] [Google Scholar]
- 32.Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI). Br J Clin Psychol 1992;31:301-6. 10.1111/j.2044-8260.1992.tb00997.x [DOI] [PubMed] [Google Scholar]
- 33.Roth AJ, Rosenfeld B, Kornblith AB, et al. The memorial anxiety scale for prostate cancer: validation of a new scale to measure anxiety in men with with prostate cancer. Cancer 2003;97:2910-8. 10.1002/cncr.11386 [DOI] [PubMed] [Google Scholar]
- 34.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385-401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- 35.Scheier MF, Carver CS, Bridges MW. Revised Life Orientation Test (LOT-R). J Pers Soc Psychol 1994;67:1063-78. 10.1037/0022-3514.67.6.1063 [DOI] [PubMed] [Google Scholar]
- 36.Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychol Assess 1995;7:524-32. 10.1037/1040-3590.7.4.524 [DOI] [Google Scholar]
- 37.Watson M, Law MG, Santos M, dos, et al. The Mini-MAC: Further development of the Mental Adjustment to Cancer scale. J Psychosoc Oncol 1994;12:33-46. 10.1300/J077V12N03_03 [DOI] [Google Scholar]
- 38.Clark JA, Inui TS, Silliman RA, et al. Patients’ perceptions of quality of life after treatment for early prostate cancer. J Clin Oncol 2003;21:3777-84. 10.1200/JCO.2003.02.115 [DOI] [PubMed] [Google Scholar]
- 39.Singh JA, Sloan JA, Atherton PJ, et al. Preferred roles in treatment decision making among patients with cancer: a pooled analysis of studies using the Control Preferences Scale. Am J Manag Care 2010;16:688-96. [PMC free article] [PubMed] [Google Scholar]
- 40.Wang EH, Gross CP, Tilburt JC, et al. Shared Decision Making and Use of Decision Aids for Localized Prostate Cancer Perceptions From Radiation Oncologists and Urologists. Jama Intern Med 2015;175:792-9. 10.1001/jamainternmed.2015.63 [DOI] [PubMed] [Google Scholar]
- 41.Shay LA, Lafata JE. Where is the evidence? A systematic review of shared decision making and patient outcomes. Med Decis Making 2015;35:114-31. 10.1177/0272989X14551638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clayman ML, Gulbrandsen P, Morris MA. A patient in the clinic; a person in the world. Why shared decision making needs to center on the person rather than the medical encounter. Patient Educ Couns 2017;100:600-4. 10.1016/j.pec.2016.10.016 [DOI] [PubMed] [Google Scholar]
- 43.Gulbrandsen P, Clayman ML, Beach MC, et al. Shared decision-making as an existential journey: Aiming for restored autonomous capacity. Patient Educ Couns 2016;99:1505-10. 10.1016/j.pec.2016.07.014 [DOI] [PubMed] [Google Scholar]
- 44.Thompson L, McCabe R. The effect of clinician-patient alliance and communication on treatment adherence in mental health care: a systematic review. BMC Psychiatry 2012;12:87. 10.1186/1471-244X-12-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elwyn G, Frosch D, Thomson R, et al. Shared decision making: A model for clinical practice. J Gen Intern Med 2012;27:1361-7. 10.1007/s11606-012-2077-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hibbard JH, Mahoney E, Sonet E. Does patient activation level affect the cancer patient journey? Patient Educ Couns 2017;100:1276-9. 10.1016/j.pec.2017.03.019 [DOI] [PubMed] [Google Scholar]
- 47.Graffigna G, Barello S, Bonanomi A. The role of Patient Health Engagement Model (PHE-model) in affecting patient activation and medication adherence: A structural equation model. PLoS One 2017;12:e0179865. 10.1371/journal.pone.0179865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oliffe JL, Davison BJ, Pickles T, Mroz L. The Self-Management of Uncertainty Among Men Undertaking Active Surveillance for Low-Risk Prostate Cancer. Qual Health Res 2009;19:432-43. 10.1177/1049732309332692 [DOI] [PubMed] [Google Scholar]
- 49.Lyons KD, Li HH, Mader EM, et al. Cognitive and Affective Representations of Active Surveillance as a Treatment Option for Low-Risk Prostate Cancer. Am J Mens Health 2016. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dall’Era MA. Patient and disease factors affecting the choice and adherence to active surveillance. Curr Opin Urol 2015;25:272-6. 10.1097/MOU.0000000000000154 [DOI] [PubMed] [Google Scholar]
- 51.Davis K, Bellini P, Hagerman C, et al. Physicians’ Perceptions of Factors Influencing the Treatment Decision-making Process for Men With Low-risk Prostate Cancer. Urology 2017;107:86-95. 10.1016/j.urology.2017.02.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ehdaie B, Assel M, Benfante N, et al. A Systematic Approach to Discussing Active Surveillance with Patients with Low-risk Prostate Cancer. Eur Urol 2017;71:866-71. 10.1016/j.eururo.2016.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]


