Abstract
Background and Objectives
The purpose of the present study was to investigate the advantages and disadvantages of verifying genetic abnormalities using array comparative genomic hybridization (a-CGH) immediately after diagnosis of congenital heart disease (CHD).
Methods
Among neonates under the age of 28 days who underwent echocardiography from January 1, 2014 to April 30, 2016, neonates whose chromosomal and genomic abnormalities were tested using a-CGH in cases of an abnormal finding on echocardiography were enrolled.
Results
Of the 166 patients diagnosed with CHD, 81 underwent a-CGH and 11 patients (11/81, 13.5%) had abnormal findings on a-CGH. 22q11.2 deletion syndrome was the most common (4/11, 36.4%). On the first a-CGH, 4 patients were negative (4/81, 5%). Three of them were finally diagnosed with Williams syndrome using fluorescent in situ hybridization (FISH), 1 patient was diagnosed with Noonan syndrome through exome sequencing. All of them exhibited diffuse pulmonary artery branch hypoplasia, as well as increased velocity of blood flow, on repeated echocardiography. Five patients started rehabilitation therapy at mean 6 months old age in outpatient clinics and epilepsy was diagnosed in 2 patients. Parents of 2 patients (22q11.2 deletion syndrome and Patau syndrome) refused treatment due to the anticipated prognosis.
Conclusions
Screening tests for genetic abnormalities using a-CGH in neonates with CHD has the advantage of early diagnosis of genetic abnormality during the neonatal period in which there is no obvious symptom of genetic abnormality. However, there are disadvantages that some genetic abnormalities cannot be identified on a-CGH.
Keywords: Array comparative genomic hybridization; Heart defects, congenital; Neonate
INTRODUCTION
Congenital heart disease (CHD) affects about 8 in 1,000 live births and is known to be a multifactorial disease, with both genetic and environmental factors having a significant influence.1) Genetic abnormalities are present in about 7% of people without CHD and 30% of patients with CHD, and they can manifest as various genetic syndromes.2) Therefore, it is essential to verify the absence or presence of genetic abnormalities in neonates with CHD. The early verification of genetic defects in CHD patients through screening tests can enable us to predict neurological problems and extracardiac malformations early, thereby helping to prevent irreversible damage by facilitating proper early intervention and the provision of appropriate advice to the patient's family.3)
Because of the flourishing molecular genetics, screening for genetic abnormalities in neonates can be performed early through a blood test and the results can be rapidly obtained. Karyotyping is a technique in which the chromosomes are visualized under a microscope to identify aberrations in chromosomal number and structure. Because the overall resolution of karyotyping is about 5 megabases, there is a risk of missing subtle abnormalities. Array comparative genomic hybridization (a-CGH) has become widely used and can detect a copy number change in the genome at a higher resolution than karyotyping.
A recent study using a-CGH for neonatal screening demonstrated that chromosomal imbalances could be readily and rapidly verified from a small amount of blood, even if a neonate did not present any obvious clinical manifestations to raise suspicion of a genetic abnormality.4) However, although a-CGH has been widely used to detect various chromosomal aberrations, there have been no reports regarding advantages and disadvantages of using a-CGH to screen neonates with CHD.
The purpose of the present study was to analyze the results of a-CGH screening in CHD neonates in a tertiary hospital and investigate the advantages and disadvantages of verifying genetic abnormalities by a-CGH immediately after diagnosing CHD in neonates.
METHODS
Subjects
Neonates under the age of 28 days who underwent echocardiography in an outpatient clinic or a neonatal care unit in Kyungpook National University Hospital from January 1, 2014 to April 30, 2016 were enrolled. Indications of echocardiography were cyanosis, tachypnea, heart murmur, abnormal morphology/activity, or needing preoperative cardiac evaluation for an operation to correct an extracardiac abnormality. According to the results obtained by echocardiography, patients with small atrial septal defect (ASD) <5 mm and/or nearly closed patent ductus arteriosus were excluded. In cases with an abnormal finding on echocardiography, genetic abnormalities were tested using the Array Analysis software program (MGMED Inc., Seoul, Korea) after obtaining consent from the patient's parents.
The parameters of sex, gestational age, birth weight, delivery method, family history, current deformity, name of the CHD and its respective treatment, neurological development, and treatment for the current deformity during the follow-up period were investigated retrospectively by reviewing the medical records for all patients.
a-CGH
Blood draws for a-CGH were conducted on the same day as echocardiography or with a blood test during hospitalization. After blood was withdrawn from a vein, placed in a bottle containing ethylenediaminetetraacetic acid, and stored in a refrigerator, it was transported to the laboratories of MGMED Inc. and a-CGH was performed. In cases with an abnormal finding following a-CGH, blood was drawn again by the same method, retested, and the findings were confirmed for diagnosis.
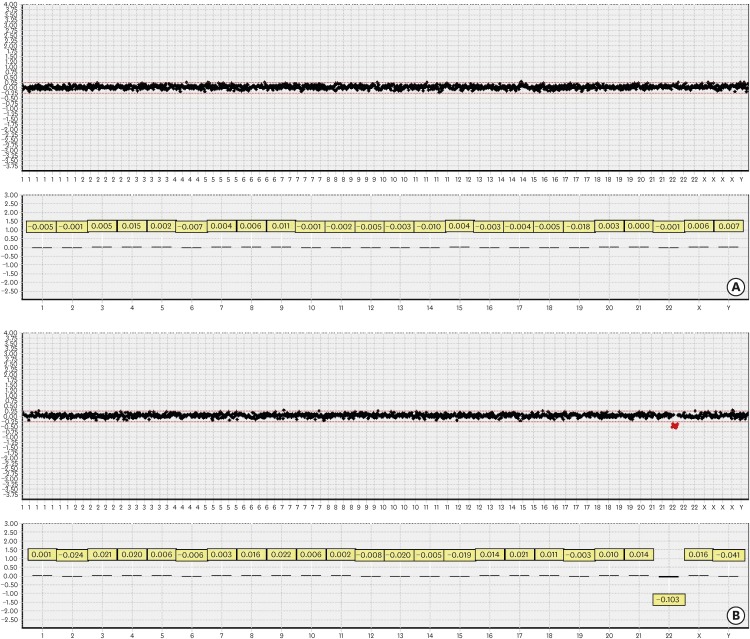
The a- CGH chip data were analyzed using the chromofluor image analysis system (Array Analysis; MGMED Inc.). The slides contained 1,440 human bacterial artificial chromosome (BAC) clones including the specific loci of more than 200 chromosomal disorders and 356 cancer-related genes from BAC libraries at an average resolution of 0.1–2.3 Mb for the entire genome. Each BAC clone was represented on an array as triplicate spots, and each array was scanned using a GenePix® 4000B scanner (Axon Instruments, Foster City, CA, USA) and analyzed with the Array Analysis software (MG VIEWER; MGMED Inc.). Green (test) to red (reference) (G/R) ratios were automatically determined for each sample, and the normalized G/R ratio represented the relative average number of copies of the sequence for those spots that were selected as controls. Spots with G/R ratios of the mean plus 0.25 standard deviations or more (i.e., G/R ≥1.25) were considered to show an amplification or gain of the indicated copy number; while those with G/R ratios of the mean minus 0.25 standard deviations or less (i.e., G/R ≤0.75) were considered to show a loss of the copy number (Figure 1).
Figure 1.
a-CGH copy number profiles of constitutional DNA samples from the normal control and syndromic DNA samples from the patient with DiGeorge syndrome. a-CGH results from normal control (A) and DiGeorge syndrome (B) DNA analysis. The X-axis represents the chromosomes, while the Y-axis represents the log2 patient/healthy control fluorescence intensity ratios (thresholds −1 [loss] and +1 [gain]) for each chromosome, respectively. a-CGH results from constitutional DNAs (A) revealed gains and losses of small chromosomal regions. a-CGH results from DiGeorge syndrome DNAs (B) revealed significant losses of chromosome 22.
a-CGH = array comparative genomic hybridization.
Ethics statement
This study was approved by the Institutional Review Board of the Kyungpook National University Hospital (IRB No. 2016-02-013). Written informed consent was obtained from all patients.
RESULTS
The characteristics of patients
Two hundred and forty-seven neonates underwent echocardiography during the research period. Of them, 166 patients demonstrated abnormal findings. Among the patients diagnosed with CHD, 81 underwent a-CGH following parental consent and 15 neonates (15/81, 18.5%) had genetic abnormalities. Of 15 neonates, 13 (86.7%) showed extra-cardiac symptoms on face, digits, genitourinary and/or gastrointestinal tracts.
Patients showing positive results on a-CGH screening
Eleven (11/81, 13.6%) patients had abnormal findings on a-CGH (Table 1). None of those 11 patients had a family history of genetic diseases. 22q11.2 deletion syndrome was the most common test result (3/11 patients, 27.3%). 22q11.2 duplication syndrome was found in 2 patients, and 6p22.3p25.3 duplication + 18q21.3q23 deletion, 9p24.3 deletion + 9q34.3 deletion, and 1p36 deletion syndrome were found in 1 patient each. Among the numerical chromosome abnormalities, Down syndrome and Patau syndrome were found in 2 patients and one patient, respectively.
Table 1. Positive a-CGH patients in whom gene abnormalities were detected.
| Patient's number | Chromosome locus | CHD type | Heart surgery | Other anomalies | Prognosis |
|---|---|---|---|---|---|
| 1 | 22q11.2 deletion | TOF | Yes | - | - |
| 2 | 22q11.2 deletion | COA VSD | No | Imperforate anus, cryptorchidism | Expire |
| 3 | 22q11.2 deletion | PM VSD 3 mm, ASD 6 mm | No | Imperforate anus, inguinal hernia | - |
| 4 | 22q11.2 duplication | COA | Yes | Congenital ptosis | Developmental delay |
| 5 | 22q11.2 duplication | Muscular VSD 3 mm | No | Ambiguous genitalia | - |
| 6 | Trisomy 21 | TOF | Yes | Dysmorphic face | - |
| 7 | Trisomy 21 | PM VSD 7 mm | Yes | Dysmorphic face | Developmental delay |
| 8 | Trisomy 13 | HLHS | No | Polydactily | Expire |
| 9 | 6p22.3p25.3 duplication + 18q21.3q23 deletion | PA IVS | Yes | - | Seizure, developmental delay |
| 10 | 9p24.3 + 9q34.3 deletion | Muscular VSD 2 mm | No | Inguinal hernia | Developmental delay |
| 11 | 1p36 deletion | COA VSD | Yes | Dysmorphic face | Seizure, developmental delay |
a-CGH = array comparative genomic hybridization; ASD = atrial septal defect; CHD = congenital heart disease; COA = coarctation of aorta; HLHS = hypoplastic left heart syndrome; IVS = intact ventricular septum; PA = pulmonary atresia; PM = perimembranous; TOF = tetralogy of Fallot; VSD = ventricular septal defect.
The CHDs that were found included a ventricular septal defect (VSD) in 4 patients, aortic coarctation with VSD in 2 patients, aortic coarctation alone in 1 patient, tetralogy of Fallot in 2 patients, pulmonary atresia with intact ventricular septum in 1 patient, and hypoplastic left heart syndrome (HLHS) in 1 patient. Six patients (6/11, 54.5%) underwent open heart surgery, and among them, 4 patients (4/6, 66.7%) underwent surgery in the neonatal period.
Three patients were found to have extracardiac deformities requiring repair. One patient with 9p24.3 deletion + 9q34.3 deletion had an inguinal hernia, which was treated with inguinal herniorrhaphy 3 months after birth. Two patients had 22q11.2 deletion syndrome with an imperforate anus; one patient underwent anoplasty 3 days after birth and the parents of the other patient refused surgery due to the prognosis of the genetic abnormality and the current deformity.
Patients showing negative results on a-CGH screening
On the initial a-CGH screening analysis, 4 patients (4/81, 4.9%) were negative (Table 2). Three of them were finally diagnosed with Williams syndrome using fluorescent in situ hybridization (FISH). Two of those 3 patients received open heart surgery, 1 for an interrupted aortic arch and VSD and the other due to total anomalous pulmonary venous return. The remaining patient was being followed-up for an ASD. All 3 patients exhibited diffuse pulmonary artery branch hypoplasia and an increased velocity of blood flow on repeated echocardiography.
Table 2. Negative a-CGH patients in whom gene abnormalities were detected by other tools.
| Patient's number | Chromosome locus | CHD type | Heart surgery | Other anomalies |
|---|---|---|---|---|
| 1 | 7q11.23 deletion | IAA, VSD, PPBS | Yes | Dysmorphic face, inguinal hernia |
| 2 | 7q11.23 deletion | PPBS, ASD | No | Dysmorphic face |
| 3 | 7q11.23 deletion | TAPVR, PPBS | Yes | Dysmorphic face |
| 4 | PTPN11 mutation | VSD, valvular PS | Yes | Webbed neck, short stature, Ptosis |
a-CGH = array comparative genomic hybridization; ASD = atrial septal defect; CHD = congenital heart disease; IAA = interrupted aortic arch; PPBS = pheripheral pulmonary artery branch stenosis; PS = pulmonary stenosis; TAPVR = total anomalous pulmonary venous return; VSD = ventricular septal defect.
One patient who had a negative result on the initial a-CGH screening analysis also demonstrated a negative result on the repeated a-CGH analysis and on FISH, and was diagnosed with Noonan syndrome through exome sequencing. This patient received open heart surgery for VSD and pulmonary valve stenosis. Exome sequencing was performed because Noonan syndrome was suspected based on persisting pulmonary artery stenosis after surgery, developmental delay, and external abnormalities, such as webbing of the neck, short stature, and ptosis.
Clinical progress of patients
The development of the patients was evaluated monthly in outpatient clinics, and 5 patients had developmental delay and started rehabilitation therapy after a mean 6 months of age. Epilepsy was diagnosed in 2 patients (Table 1).
There were 2 deaths. In a patient who was transferred from another hospital after birth due to an imperforate anus and undescended testes, cardiac deformities including severe aortic coarctation and VSD were found on the echocardiography performed as a pre-operative evaluation, and bilateral kidney hypoplasia was also found. The patient expired after the parents refused surgery, despite strong persuasion, because of the genetic abnormality and current deformity after a diagnosis of 22q11.2 deletion syndrome by a-CGH. Another patient who had HLHS, polydactyly, and microtia received cardiopulmonary resuscitation due to bradycardia, as well as reduced oxygen saturation, on the 12th day after birth. Afterwards, Patau syndrome was confirmed on a genetic test, and the parents refused surgery for the cardiac malformations although its necessity for survival was explained.
DISCUSSION
The results of the present study showed that the identification of genetic abnormalities in CHD neonates by performing a-CGH enabled their treatment direction to be determined earlier. However, some genetic abnormalities could not be verified, and some parents refused treatment due to the anticipated prognosis.
Typical genetic tests include karyotyping, FISH, and a-CGH. Karyotyping is widely used to detect abnormalities in chromosomal number or structure. FISH is a method to identify genetic defects or replication using a DNA probe and can be used to diagnose disorders with copy number variations such as Williams syndrome, DiGeorge syndrome, and Alagille syndrome. a-CGH is a test to examine not only the presence of abnormalities in chromosomal number or structure, but also genetic abnormalities, and can analyze the entire genome by comparing with those of normal control.3) Notably, a-CGH can enable a diagnosis to be made even in cases where a genetic syndrome is suspected but karyotyping shows no abnormality,5) and FISH is negative despite the presence of 22q11.2 deletion syndrome.6) Furthermore, a-CGH can be readily performed in neonates as it is feasible with a small amount of blood.
In a previous report, extra-cardiac congenital anomalies were shown to often be present in cases of CHD. The rate of non-syndromic congenital anomaly has been reported as 11.4%, that of genetic syndrome as 2.2%, and that of overall extra-cardiac congenital anomaly as 13.6% among all patients with CHD. Furthermore, it has been reported that extra-cardiac congenital anomalies have a great influence on the progress and prognosis of CHD.12) Whereas, in the present study using a-CGH, a genetic syndrome was found in 18.5%, extra-cardiac symptoms on face, digits, genitourinary and/or gastrointestinal tracts in 86.7%, and extra-cardiac anomalies requiring repair in 26.7%. The differences in the patients enrolled in each study, their ethnic background, and the test methods used should be taken into consideration as possible reasons for this discrepancy. Nevertheless, from initial application of a-CGH for neonates with CHD, we could detect more genetic abnormalities earlier in comparison with the previous report. We supposed that the difference in the incidence of extra-cardiac symptoms between the present study and the previous study may have been because extra-cardiac congenital anomalies or symptoms may not be expressed during neonatal period. Thus, an earlier genetic study using a-CGH should be considered in neonates with CHD even though extra-cardiac symptoms or clinical manifestations are not evident.
In the present study, some genetic abnormalities were not diagnosed in the initial screening test using a-CGH, but were diagnosed by repeated a-CGH screening or other tests. These abnormalities included the Williams syndrome and Noonan syndrome. Williams syndrome, which is caused by 7q11.23 deletion, is characterized by specific facial characteristics, CHD (mainly supravalvular aortic stenosis and peripheral pulmonary artery stenosis), mild mental retardation, developmental delay, and hypercalcemia.7) 7q11.23 deletion can be positive or negative according to the location of the deletion and the resolution and coverage of a-CGH. Considering that most diagnoses of Williams syndrome can be confirmed by FISH, it is essential to use FISH for confirmation if there is possibility of Williams syndrome. Noonan syndrome, which is an autosomal dominant disorder, has various phenotypes and can present with short stature, CHD, characteristic facial features (e.g., ocular hypertelorism with downslanting palpebral fissures, ptosis, and low-set ears), webbed neck, and chest deformities. Mild developmental delay can occur, and undescended testicles can be found in male neonates.8) Noonan syndrome can be caused by various gene mutations such as PTPN11, KRAS, SOS1, RAF1, NRAS, BRAF, SHOC2, CBL, and RIT1. These genetic mutations cannot be verified by a-CGH9),10) and should be diagnosed by performing DNA sequencing or whole exome sequencing.11)
From these results, we can recommend for neonates with CHD to be screened for genetic abnormalities using a-CGH whenever they are accompanying any extra-cardiac symptoms. However, the disadvantages of neonatal screening by a-CGH should be considered that some genetic abnormalities cannot be identified by a-CGH. FISH should be added for neonates or infants with clinical features suggesting Williams syndrome or syndromes related with known genetic causes. If we cannot have any positive results from a-CGH and FISH despite patients' CHD and abnormal clinical features, whole exome sequencing should be considered.
In the present study, all 15 patients with genetic syndrome have possibilities of developmental delay and epilepsy. Early diagnosis of genetic syndrome using a-CGH allowed us to predict developmental delay and epilepsy, and to confirm the occurrence of those phenomena. We performed surgery for the current deformities of the patients whose genetic abnormality was confirmed at an early stage through consultation with neonatologists, pediatric neurologists, pediatric surgeons, and pediatric rehabilitation therapists. Rehabilitation therapy was performed to prevent the developmental delay that can develop in the future even if there are no obvious signs during the neonatal and infancy period.
The American Heart Association and the American Academy of Pediatrics recommend that disease recurrence can be explained to families by identifying the genetic cause(s) of CHD and can be prevented by testing family members for the genetic abnormality.3) In the present study, parental counseling was performed for all patients who had abnormal results on a-CGH. The disease and prognosis were explained and a genetic test was recommended to the parents, if needed. However, none of the parents chose to undergo genetic testing. In the 2 cases of death, the parents refused surgery after genetic counseling. Consequentially, although there are many advantages in performing early genetic diagnosis in patients with CHD, some parents may assume a poor prognosis upon the diagnosis of a genetic abnormality, leading to them refusing treatment. Early adoption can raise issues of ethics, patient selection, analysis technique and communication of results. To overcome these disadvantages, it would be essential to further develop techniques for parental counseling, and the clinicians have to challenge ethical, legal and economic regulation in clinical genetics.
The limitations of the present study include its retrospective design and the small number of included patients with genetic diseases. Creating a family tree was not feasible as most of the parents of the patients were reluctant to talk about their family history of genetic diseases in detail. Furthermore, objective gene flows could not be found because the parents refused genetic testing for various reasons.
In conclusion, we recommend for neonates with CHD to be screened for genetic abnormalities using a-CGH whenever they are accompanying any extra-cardiac symptoms. However, because some genetic abnormalities cannot be identified by a-CGH, FISH, or whole exome sequencing should be also considered when we get the negative result from a-CGH. To prevent the adverse effects of early diagnosis, it would be essential to further develop techniques for parental counseling.
Footnotes
Funding: This work was supported by Biomedical Research Institute grant, Kyungpook National University Hospital (2016).
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Kim YH.
- Data curation: Choi BG, Kwon JE.
- Validation: Kim YH.
- Writing - original draft: Choi BG, Hwang SK.
References
- 1.Nora JJ. Multifactorial inheritance hypothesis for the etiology of congenital heart diseases. The genetic-environmental interaction. Circulation. 1968;38:604–617. doi: 10.1161/01.cir.38.3.604. [DOI] [PubMed] [Google Scholar]
- 2.Pediatric Cardiac Genomics Consortium. The Congenital Heart Disease Genetic Network Study: rationale, design, and early results. Circ Res. 2013;112:698–706. doi: 10.1161/CIRCRESAHA.111.300297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pierpont ME, Basson CT, Benson DW, Jr, et al. Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115:3015–3038. doi: 10.1161/CIRCULATIONAHA.106.183056. [DOI] [PubMed] [Google Scholar]
- 4.Park SJ, Jung EH, Ryu RS, Kang HW, Chung HD, Kang HY. The clinical application of array CGH for the detection of chromosomal defects in 20,126 unselected newborns. Mol Cytogenet. 2013;6:21. doi: 10.1186/1755-8166-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Trier DC, Feenstra I, Bot P, de Leeuw N, Draaisma JM. Cardiac anomalies in individuals with the 18q deletion syndrome; report of a child with Ebstein anomaly and review of the literature. Eur J Med Genet. 2013;56:426–431. doi: 10.1016/j.ejmg.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Anderlid BM, Schoumans J, Annerén G, et al. Subtelomeric rearrangements detected in patients with idiopathic mental retardation. Am J Med Genet. 2002;107:275–284. doi: 10.1002/ajmg.10029. [DOI] [PubMed] [Google Scholar]
- 7.Committee on Genetics. American Academy of Pediatrics: health care supervision for children with Williams syndrome. Pediatrics. 2001;107:1192–1204. [PubMed] [Google Scholar]
- 8.van der Burgt I. Noonan syndrome. Orphanet J Rare Dis. 2007;2:4. doi: 10.1186/1750-1172-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts AE, Allanson JE, Tartaglia M, Gelb BD. Noonan syndrome. Lancet. 2013;381:333–342. doi: 10.1016/S0140-6736(12)61023-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee BH, Kim JM, Jin HY, Kim GH, Choi JH, Yoo HW. Spectrum of mutations in Noonan syndrome and their correlation with phenotypes. J Pediatr. 2011;159:1029–1035. doi: 10.1016/j.jpeds.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 11.Ko JM, Kim JM, Kim GH, Yoo HW. PTPN11, SOS1, KRAS, and RAF1 gene analysis, and genotype-phenotype correlation in Korean patients with Noonan syndrome. J Hum Genet. 2008;53:999–1006. doi: 10.1007/s10038-008-0343-6. [DOI] [PubMed] [Google Scholar]
- 12.Egbe A, Lee S, Ho D, Uppu S, Srivastava S. Prevalence of congenital anomalies in newborns with congenital heart disease diagnosis. Ann Pediatr Cardiol. 2014;7:86–91. doi: 10.4103/0974-2069.132474. [DOI] [PMC free article] [PubMed] [Google Scholar]