Abstract
Early esophageal cancer is confined to the mucosa or submucosa of the esophagus. While most esophageal cancer is detected at an advanced stage (requiring surgical resection, chemotherapy, and radiation), early-stage mucosal lesions may be detected through Barrett’s surveillance programs or incidentally on diagnostic upper endoscopies performed for other reasons. These early-stage cancers are often amenable to endoscopic therapies, including mucosal resection, ablation, and cryotherapy. Studies suggest equivalent survival rates and reduced morbidity but higher recurrence rates with endoscopic removal of early-stage cancers compared to surgical resection. There is emerging data regarding the efficacy and long-term outcomes of endoscopic therapy for early esophageal cancer that is promising, and further research is needed to better define the role of endoscopic therapy in the management of early esophageal cancer.
Keywords: Esophageal cancer, Barrett’s esophagus, Endoscopic mucosal resection, Ablation, Cryotherapy, Esophagectomy
Introduction
Esophageal cancer is the 18th leading cause of cancer in the USA. While it is less common than other cancers, it carries a worse prognosis, resulting in death in a larger proportion of patients compared to many other cancers. The estimated prevalence in 2014 by the Surveillance, Epidemiology, and End Results (SEER) Cancer Database is that 18,170 people will be diagnosed, with an estimated 15,450 deaths [1]. Unfortunately, the majority of esophageal cancers are diagnosed when symptoms develop and are therefore usually at least locally advanced at the time of diagnosis. As a result, treatment is challenging and the disease generally carries a poor prognosis. It is imperative, therefore, to identify patients with early esophageal cancer and implement aggressive treatment plans. Early-stage cancers may be diagnosed through Barrett’s esophagus surveillance programs or found incidentally during diagnostic endoscopies performed for other reasons. The development of techniques including chromoendoscopy, narrow-band imaging, magnification endoscopy, confocal microscopy, and spectroscopy has further increased the sensitivity of endoscopic detection of early-stage carcinoma [2••]. Endoscopic therapy can be curative for patients with early mucosal cancers.
Endoscopic Staging of Early Esophageal Cancer
Early-stage esophageal cancer refers to lesions confined to the mucosa (lamina propria and muscularis mucosa), as opposed to lesions that invade through the submucosa into the muscularis propria. Stage T1a malignancies are confined to the mucosa, with associated subcategorization: M1 (intraepithelial), M2 (lamina propria), or M3 (muscularis mucosa). These mucosal lesions have a very low risk of local lymph node invasion compared to submucosal (T1b) lesions [3]. Evidence of lymphovascular invasion or poor differentiation generally portends a high risk for metastasis. Distinguishing the level of invasion is a key factor in determining the success of curative endoscopic interventions. Accurate staging of the mucosa with endoscopic ultrasound (EUS) and lymph node fine-needle aspiration is therefore imperative to determine the appropriate treatment strategy. EUS was found to be accurate for staging T1a and T1b tumors in a large meta-analysis [4], with an area under the receiver operating characteristic curve of 0.93 to 0.96, resulting in a sensitivity of 85 % and specificity of 87 % for the diagnosis of T1a tumors (sensitivity and specificity both 86 % for T1b tumors). EUS, computed tomography (CT), and FDG-positron emission tomography (FDG-PET) can be used to assess for lymph node involvement. The sensitivity and specificity for detecting celiac lymph node metastases with EUS are 85 % (95 % CI 72–99 %) and 96 % (95 % CI 92–100 %), respectively [5], compared to that of the sensitivity and specificity for regional lymph node detection for CT (50 and 83 %, respectively) and FDG-PET scan (57 and 85 %, respectively). CT and FDG-PET scans are generally used to evaluate for distant metastatic disease.
Endoscopes operating at frequencies of 7.5 and 12 MHz can visualize the five layers of the esophageal wall to determine level of tumor infiltration: superficial mucosa (hyperechoic), deep mucosa (hypoechoic), submucosa (hyperechoic), muscularis propria (hypoechoic), and adventitia (hyperechoic). High frequency mini-probes operating at 20 MHz allow for further differentiation of the lamina propria (between the superficial mucosa and muscularis mucosa) and the inner circular and outer longitudinal muscles of the muscularis propria. These catheters have accuracy as high as 84 % [6] and improve the accuracy of staging T1 lesions with EUS to 92 % [7]. Surgical therapy is often recommended if tumor invasion into the muscularis propria or lymph node invasion is seen. Endoscopic resection can be considered for mucosal disease. Depth of invasion can also be further defined by pathological examination of the resected specimen to determine if endoscopic resection alone is sufficient or if surgery is needed.
Curative Endoscopic Therapies for Early Esophageal Cancer
Curative endoscopic therapies for early-stage esophageal cancer include methods of mucosal resection or ablation. Resection encompasses endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). Both of these allow for the removal and pathological analysis of a targeted piece of tissue, which also aids accurate staging. Lesions larger than 2 cm usually require ESD, since they would otherwise require piecemeal resection (which increases the chance of complication and incomplete resection at the margins) and a higher level of endoscopic expertise.
Current Techniques for Endoscopic Resection
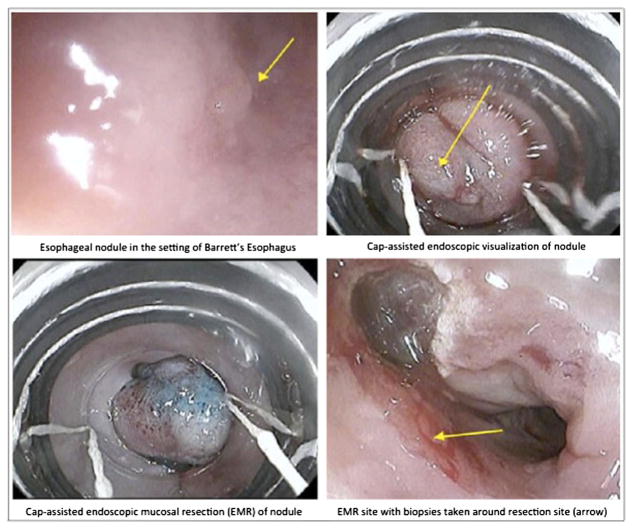
EMR is the primary method of resection for T1a lesions and nodular Barrett’s esophagus [8–11]. The most commonly used methods of endoscopic mucosal resection are snare resection and cap-assisted endoscopic resection. The lesion is usually first injected with saline or diluted epinephrine to allow for submucosal lifting. A snare can then be used to capture the tissue with a rim of normal mucosa and resected with electrocoagulation. In cap-assisted resection, a transparent cap with a resection snare is attached to the endoscope. Suction is applied over the injected lesion, allowing for the lifted submucosa to be sucked into the cap. The tissue is then captured in the snare and removed by electrocoagulation (Fig. 1). The large 18-mm flexible cap can allow for en bloc resection of lesions less than 2 cm in size. There are also variant band ligation devices that allow for the lesion to be sucked into the ligation cap and captured with a rubber band to create a pseudopolyp. This causes the esophageal muscle layer to retract, and therefore, submucosal lifting is not required prior to banding. The endoscope is removed from the patient, the ligation device is removed, and the endoscope is then reinserted into the esophagus to remove the polyp with a standard polypectomy snare. There are no significant differences in size of lesion resected or rate of complications between these methods. Overall, EMR is relatively safe, fast, and has low rates of complications.
Fig. 1.
Endoscopic mucosal resection of nodular Barrett’s esophagus
ESD has recently emerged as a prominent technique for resection in Europe and Japan but is not yet widely performed in the USA. It is a variant of EMR in which a specialized endoscopic knife is used to dissect targeted areas en bloc from the submucosa. The margins of the lesion are marked by electrocautery, and the submucosa is lifted with injection of saline or epinephrine. A circumferential incision around the lesion is made with a specialized electrocautery knife [12]. Further dissection from the deep layers is completed with the knife for complete resection. Various cutting devices have been developed, such as the needle knife (small contact area with high cutting power), the hook knife (right angle bed of the needle tip that can pull dissected tissue), the flex knife (soft cutting tip to prevent perforations), the insulated [13] tip knife (ceramic ball on top of needle knife to prevent perforation), the flush knife (allows washout of blood for visualization), and the triangle tip knife (can be used for any step of the procedure). Esophageal ESD is more challenging compared to gastric ESD because the esophagus is smaller in diameter and there is more movement from the adjacent heartbeat. In addition, the esophageal wall is thinner, which increases the risk of perforation of the muscularis propria and potentially life-threatening mediastinitis. The addition of a transparent hood can improve visualization by pushing tissues away from the endoscope. There have not been any studies evaluating the regeneration of mucosa after endoscopic resection, but the wounds generally heal in 3 to 6 weeks depending on the size of resection, and patients should be prescribed adequate acid suppression therapy post-resection to allow healing and reduce local scarring.
Future Techniques for Endoscopic Resection
New methods of endoscopic resection continue to be explored. Studies from Japan have described the use of needle-knives for ESD en bloc resection of large lesions after prolonged submucosal lifting with injection of viscous substances such as hyaluronidate or hydroxypropyl methylcellulose [14, 15]. A high level of endoscopic expertise is needed for these methods, and experience in their use is currently very limited. The future of endoscopic resection of these lesions may employ a combination of endoscopic resection techniques to remove lesions en bloc versus removal of residual neoplastic tissue.
Endoscopic Ablative Therapy
There are several ablative techniques for the treatment of both Barrett’s esophagus with dysplasia and intramucosal carcinoma. These include photodynamic therapy (PDT), cryotherapy, argon plasma coagulation (APC), heater probe treatment, and radiofrequency ablation (RFA). These are used either alone or as adjunctive therapy to mucosal resection. PDT is used most successfully for the ablation of high-grade dysplasia in Barrett’s esophagus [16, 17] in combination with resection or a second ablative modality. Strictures can form in up to one third of patients. However, robust data for the use of PDT in the management of early esophageal cancer is lacking and consists mostly of case reports and series. One series of 38 patients with T1 squamous cell carcinoma demonstrated an 87 % complete eradication rate, coupled with an 18 % recurrence rate [18]. Cryotherapy has also been used in combination with resection to treat high-grade dysplasia and intramucosal carcinoma in 27 patients, with a 90 % rate of elimination of the lesion or downgrading of disease stage [19]. Cryotherapy is reported to cause chest pain, dysphagia, and perforation in rare cases [20]. There is limited data to support the use of the other ablative modalities of APC, RFA, and heater probe as monotherapy for cure of intramucosal carcinoma. This may be due to the fact that these modalities are superficial in their effect and, therefore, may not provide curative therapy.
Outcomes of Endoscopic Treatments
The understanding of the efficacy and long-term outcomes for endoscopic management of superficial esophageal cancer is still in development, as data comes primarily from observational series with small sample sizes from a few centers of expertise, with limited duration of follow-up. Overall, EMR can successfully eradicate 91 to 98 % of T1a cancers [21–26]. One of the largest series [27] of 349 patients with Barrett’s esophagus and either high-grade dysplasia or intramucosal adenocarcinoma treated with endoscopic resection, photodynamic therapy, or both found a complete response rate in 96.6 % in patients. There was a 21.5 % recurrence rate, but no mortality secondary to esophageal cancer. Surgery was required in 3.7 % of patients after failure of endoscopic therapy. ESD is best used for areas of dysplasia >2 cm or T1b submucosal lesions, and data from Japan suggests en bloc resection rates of 100 % with 80 % rates of cure [28, 29]. A recent systematic review and meta-analysis [30] of 21 studies (1152 patients and 1240 lesions) found that the rate of en bloc resection with margins histologically free of disease (R0 resection) was 90 % (95 % CI 87–93 %). Comparisons of ESD and EMR have demonstrated higher en bloc resection rates for early squamous cell carcinoma (100 versus 53.3 %, p<0.05), as well as lower local recurrence rates (0.9 versus 9.8 %, s< 0.05) with ESD [29]. One study [31] found complete endoluminal remission rates of 87 % when endoscopic resection was paired with ablation therapy for tumors with shallow penetration into the submucosa, with a long-term remission rate of 90 %. Complete remission was higher for lesions smaller than 2 cm (97 versus 77 % for large or multifocal lesions).
Estimates for 5-year recurrence rates range from 3 to 32 % of patients depending on the method of endoscopic treatment used and duration of follow-up [32•, 33–35]. One of the largest studies with 1000 patients evaluating the efficacy of endoscopic resection in patients with early esophageal adenocarcinoma demonstrated a long-term complete remission rate of 96 % over 57 months [36•]. Of the patients, 3.7 % had surgery when endoscopic therapy failed. Patients of 140 (14.5 %) had recurrence of cancer or metachronous lesions, which were treated successfully with endoscopic re-treatment in 115 patients. Major complications that were managed conservatively occurred in 1.5 % of patients. The calculated 10-year survival rate of patients who underwent endoscopic resection was 75 %. Risk factors associated with recurrence [37] include piecemeal resection, multifocal or large lesions, long-segment Barrett’s esophagus, lack of adjuvant mucosal ablative therapies (such as APC) of Barrett’s esophagus after complete remission [38], and greater than 10 months’ time to achieve complete remission. Incomplete or unsuccessful lifting of the tumor with submucosal injection is a predictor of deep invasion and that the lesion is likely not amenable to endoscopic removal. Although recurrence rates are generally reported to be low, it is recommended that patients remain in a scheduled surveillance program. Five-year survival rates range from 76 to 100 %. Survival is lower in patients with multiple or circumferential lesions or with lesions that extend beyond the lamina propria. Retrospective series [39] have shown that in patients who undergo complete (R0) resection, 5-year use-specific survival was 100 % with no recurrences or metastases.
Complications of EMR include bleeding, perforation, and fibrosis and stricture formation at the site of resection. The most common of these is stricture formation, which is reported to occur in up to 37 % of cases, compared to post-resection bleeding in about 10 % of patients and perforation rates of less than 3 %. When they occur, strictures can be managed with endoscopic dilation.
The most common complications for ESD include stenosis or stricture formation (5 to 17 %), perforation (1 to 5 %), bleeding, and mediastinal emphysema [24, 28–30, 40].
The decision for whether a patient should undergo endoscopic therapy for early esophageal cancer is a multifaceted one, depending not only on lesion-specific factors but also on patient-specific factors such as age and comorbidities contributing to surgical risk. When compared to surgical resection for the treatment of early-stage esophageal cancer, endoscopic therapy has comparable cancer-free survival and lower morbidity [41]. Specifically, analyses of 742 patients in the SEER database demonstrated equivalent long-term survival (56 versus 59 months, p=0.41) between endoscopic treatment and surgery [42]. Though this study did not differentiate between modalities of endoscopic therapies, a single-center report by Zehetner et al. found similar survival at 3 years and significantly lower morbidity (0 versus 39 %, p<0.0001) in patients treated with the combination of endoscopic resection and ablation compared to surgical resection [43]. Larger and more recent studies have corroborated equivalent esophageal cancer-specific survival outcomes between endoscopic resection and esophagectomy at years 2 and 5 of follow-up [44•, 45]. Overall, endoscopic therapy for early esophageal adenocarcinoma associated with Barrett’s esophagus is also more cost-effective compared to that of surgical resection, with less expense and comparable quality-adjusted life years [46]. These studies suggest that lesions meeting criteria for early-stage esophageal cancer can be treated with endoscopic resection and ablative methods with curative intent and incur less morbidity and equivalent long-term survival compared to that of surgery.
Conclusion
Esophageal cancer carries a poor prognosis, and therefore, aggressive treatment of early-stage esophageal cancer confined to the mucosa is imperative. EMR and ablation offer possibilities for cost-effective, curative treatment of early-stage esophageal cancer. Accurate staging with EUS and FNA (when indicated) is necessary to determine whether a lesion can be considered for endoscopic treatment. EMR can be used for curative treatment of lesions confined to the mucosa; lesions invading the submucosa may require ESD or more radical resection. Ablative methods such as APC, heater probe, cryotherapy, or RFA are generally not effective for cure when used as monotherapy; however, they may have a role in ablation of residual high-risk tissue when combined with mucosal resection. Further research exploring specific combinations of ablative modalities with mucosal resection may shed light on the most effective treatment plan.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest Vaishali Patel and Rebecca A. Burbridge declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Vaishali Patel, Division of Gastroenterology, Duke University Medical Center, 190 Grey Elm Trail, Durham, NC 27713, USA.
Rebecca A. Burbridge, Division of Gastroenterology, Duke University Medical Center, Box 3662, Durham, NC 27710, USA
References
Papers of particular interest, published recently have been highlighted as:
• Of importance
•• Of major importance
- 1.Surveillance, Epidemiology, and End Results (SEER) Program. Surveillance Research Program. National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services; [Accessed May 18th, 2014]. http://seer.cancer.gov/statfacts/html/esoph.html. [Google Scholar]
- 2••.Evans, et al. ASGE Standards of Practice Committee. The role of endoscopy in the assessment and treatment of esophageal cancer. Gastrointest Endosc. 2013;77(3):328–34. doi: 10.1016/j.gie.2012.10.001. This is a comprehensive review of endoscopic therapy for esophageal cancer by the American Society of Gastrointestinal Endoscopy and provides guidelines for practice. [DOI] [PubMed] [Google Scholar]
- 3.Sepesi B, Watson TJ, Zhou D, et al. Are endoscopic therapies appropriate for superficial submucosal esophageal adenocarcinoma? An analysis of esophagectomy specimens. J Am Coll Surg. 2010;210:418–27. doi: 10.1016/j.jamcollsurg.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Thosani N, Singh H, Kapadia A, et al. Diagnostic accuracy of EUS in differentiating mucosal versus submucosal invasion of superficial esophageal cancers: a systematic review and meta-analysis. Gastrointest Endosc. 2012;75(2):242. doi: 10.1016/j.gie.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 5.van Vliet EP, Heijenbrok-Kal MH, Hunink MG, et al. Staging investigations for oesophageal cancer: a meta-analysis. Br J Cancer. 2008;98(3):547. doi: 10.1038/sj.bjc.6604200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murata Y, Suzuki S, Ohta M, et al. Small ultrasonic probes for determination of the depth of superficial esophageal cancer. Gastrointest Endosc. 1996;44(1):23. doi: 10.1016/s0016-5107(96)70224-9. [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa N, Niwa Y, Arisawa T, et al. Preoperative staging of superficial esophageal carcinoma: comparison of an ultrasound probe and standard endoscopic ultrasonography. Gastrointest Endosc. 1996;44(4):388. doi: 10.1016/s0016-5107(96)70086-x. [DOI] [PubMed] [Google Scholar]
- 8.Inoue H, Fukami N, Yoshida T, Kudo SE. Endoscopic mucosal resection for esophageal and gastric cancers. J Gastroenterol Hepatol. 2002;17:382. doi: 10.1046/j.1440-1746.2002.02732.x. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez Herrero L, Pouw RE, van Vilsteren FG, et al. Safety and efficacy of multiband mucosectomy in 1060 resections in Barrett’s esophagus. Endoscopy. 2011;43:177. doi: 10.1055/s-0030-1256095. [DOI] [PubMed] [Google Scholar]
- 10.May A, Gossner L, Behrens A, et al. A prospective randomized trial of two different endoscopic resection techniques for early stage cancer of the esophagus. Gastrointest Endosc. 2003;58:167. doi: 10.1067/mge.2003.339. [DOI] [PubMed] [Google Scholar]
- 11.Pouw RE, van Vilsteren FG, Peters FP, et al. Randomized trial on endoscopic resection-cap versus multiband mucosectomy for piecemeal endoscopic resection of early Barrett’s neoplasia. Gastrointest Endosc. 2011;74:35. doi: 10.1016/j.gie.2011.03.1243. [DOI] [PubMed] [Google Scholar]
- 12.Kantsevoy SV, Adler DG, Conway JD, et al. Endoscopic mucosal resection and endoscopic submucosal dissection. Technology status evaluation report. ASGE Technology Committee. Gastrointest Endosc. 2008;68(1):11–8. doi: 10.1016/j.gie.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 13.Miyamoto S, Muto M, Hamamoto Y, et al. A new technique for endoscopic mucosal resection with an insulated-tip electrosurgical knife improves the completeness of resection of intramucosal gastric neoplasms. Gastrointest Endosc. 2002;55:576. doi: 10.1067/mge.2002.122579. [DOI] [PubMed] [Google Scholar]
- 14.Higuchi K, Tanabe S, Azuma M, et al. A phase II study of endoscopic submucosal dissection for superficial esophageal neoplasms. Gastrointest Endosc. 2013;78(5):704–10. doi: 10.1016/j.gie.2013.04.182. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto H, Sekine Y, Higashizawa T, et al. Successful en bloc resection of a large superficial gastric cancer by using sodium hyaluronate and electrocautery incision forceps. Gastrointest Endosc. 2001;54:629. doi: 10.1067/mge.2001.118643. [DOI] [PubMed] [Google Scholar]
- 16.Overholt BF, Wang KK, Burdick JS, et al. Five-year efficacy and safety of photodynamic therapy with Photofrin in Barrett’s high-grade dysplasia. Gastrointest Endosc. 2007;66:460–8. doi: 10.1016/j.gie.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 17.Pacifico RJ, Wang KK, Wongkeesong LM, et al. Combined endoscopic mucosal resection and photodynamic therapy versus esophagectomy for management of early adenocarcinoma in Barrett’s esophagus. Clin Gastroenterol Hepatol. 2003;1(4):252. [PubMed] [Google Scholar]
- 18.Tanaka T, Matono S, Nagano T, et al. Photodynamic therapy for large superficial squamous cell carcinoma of the esophagus. Gastrointest Endosc. 2011;73:1–6. doi: 10.1016/j.gie.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 19.Dumot JA, Vargo JJ, 2nd, Falk GW, et al. An open-label, prospective trial of cryospray ablation for Barrett’s esophagus high-grade dysplasia and early esophageal cancer in high-risk patients. Gastrointest Endosc. 2009;70:635–44. doi: 10.1016/j.gie.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Greenwald BD, Dumot JA, Abrams JA, et al. Endoscopic spray cryotherapy for esophageal cancer: safety and efficacy. Gastrointest Endosc. 2010;71:686–93. doi: 10.1016/j.gie.2010.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirasawa K, Kokawa A, Oka H, et al. Superficial adenocarcinoma of the esophagogastric junction: long-term results of endoscopic submucosal dissection. Gastrointest Endosc. 2010;72:960. doi: 10.1016/j.gie.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 22.Ciocirlan M, Lapalus MG, Hervieu V, et al. Endoscopic mucosal resection for squamous premalignant and early malignant lesions of the esophagus. Endoscopy. 2007;39:24–9. doi: 10.1055/s-2006-945182. [DOI] [PubMed] [Google Scholar]
- 23.Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut. 2008;57:1200–6. doi: 10.1136/gut.2007.142539. [DOI] [PubMed] [Google Scholar]
- 24.Chennat J, Konda VJ, Ross AS, et al. Complete Barrett’s eradication endoscopic mucosal resection: an effective treatment modality for high-grade dysplasia and intramucosal carcinoma—an American single-center experience. Am J Gastroenterol. 2009;104:2684–92. doi: 10.1038/ajg.2009.465. [DOI] [PubMed] [Google Scholar]
- 25.Moss A, Bourke MK, Hourigan LF, et al. Endoscopic resection for Barrett’s high-grade dysplasia and early esophageal adenocarcinoma: an essential staging procedure with long-term therapeutic benefit. Am J Gastroenterol. 2010;105:1276–83. doi: 10.1038/ajg.2010.1. [DOI] [PubMed] [Google Scholar]
- 26.Ell C, May A, Pech O, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett’s cancer) Gastrointest Endosc. 2007;65:3–10. doi: 10.1016/j.gie.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 27.Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut. 2008;57(9):1200. doi: 10.1136/gut.2007.142539. [DOI] [PubMed] [Google Scholar]
- 28.Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc. 2009;70:860–6. doi: 10.1016/j.gie.2009.04.044. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi H, Arimura Y, Masao H, et al. Endoscopic submucosal dissection is superior to conventional endoscopic resection as a curative treatment for early squamous cell carcinoma of the esophagus (with video) Gastrointest Endosc. 2010;72:255–64. doi: 10.1016/j.gie.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 30.Sun F, Yuan P, Chen T, et al. Efficacy and complication of endoscopic submucosal dissection for superficial esophageal carcinoma: a systematic review and meta-analysis. J Cardiothorac Surg. 2014;9:78. doi: 10.1186/1749-8090-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manner H, Pech O, Heldmann Y, et al. Efficacy, safety, and long-term results of endoscopic treatment for early stage adenocarcinoma of the esophagus with low-risk sm1 invasion. Clin Gastroenterol Hepatol. 2013;11(6):630. doi: 10.1016/j.cgh.2012.12.040. [DOI] [PubMed] [Google Scholar]
- 32•.Yamashina T, Ishihara R, Nagai K, et al. Long-term outcome and metastatic risk after endoscopic resection of superficial esophageal squamous cell carcinoma. Am J Gastroenterol. 2013;108(4):544. doi: 10.1038/ajg.2013.8. This study provides data regarding the long-term outcomes following endoscopic therapy for early esophageal cancer. [DOI] [PubMed] [Google Scholar]
- 33.Saligram S, Chennat J, Hu H, et al. Endotherapy for superficial adenocarcinoma of the esophagus: an American experience. Gastrointest Endosc. 2013;77(6):872. doi: 10.1016/j.gie.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Yamada M, Oda I, Nonaka S, et al. Long-term outcome of endoscopic resection of superficial adenocarcinoma of the esophagogastric junction. Endoscopy. 2013;45(12):992–6. doi: 10.1055/s-0033-1344862. [DOI] [PubMed] [Google Scholar]
- 35.Nakagawa K, Koike T, Iijima K, et al. Comparison of the long-term outcomes of endoscopic resection for superficial squamous cell carcinoma and adenocarcinoma of the esophagus in Japan. Am J Gastroenterol. 2014;109(3):348–56. doi: 10.1038/ajg.2013.450. [DOI] [PubMed] [Google Scholar]
- 36•.Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology. 2014;146(3):652. doi: 10.1053/j.gastro.2013.11.006. This study provides data regarding the long-term efficacy and complications following endoscopic therapy for early esophageal cancer. [DOI] [PubMed] [Google Scholar]
- 37.Esaki M, Matsumoto T, Hirakawa K, et al. Risk factors for local recurrence of superficial esophageal cancer after treatment by endoscopic mucosal resection. Endoscopy. 2007;39:41–5. doi: 10.1055/s-2006-945143. [DOI] [PubMed] [Google Scholar]
- 38.Manner H, Rabenstein T, Pech O, et al. Ablation of residual Barrett’s epithelium after endoscopic resection: a randomized long-term follow-up study of argon plasma coagulation vs. surveillance (APE study) Endoscopy. 2014;46(1):6–12. doi: 10.1055/s-0033-1358813. [DOI] [PubMed] [Google Scholar]
- 39.Yamada M, Oda I, Nonaka S, et al. Long-term outcome of endoscopic resection of superficial adenocarcinoma of the esophagogastric junction. Endoscopy. 2013;45(12):992–6. doi: 10.1055/s-0033-1344862. [DOI] [PubMed] [Google Scholar]
- 40.Repici A, Hassan C, Carlino A, et al. Endoscopic submucosal dissection in patients with early esophageal squamous cell carcinoma: results from a prospective Western series. Gastrointest Endosc. 2010;71:715–21. doi: 10.1016/j.gie.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 41.Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology. 2009;137:815–23. doi: 10.1053/j.gastro.2009.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Das A, Singh V, Fleisher DE, et al. A comparison of endoscopic treatment and surgery in early esophageal cancer: an analysis of surveillance epidemiology and end results data. Am J Gastroenterol. 2008;103:1340–5. doi: 10.1111/j.1572-0241.2008.01889.x. [DOI] [PubMed] [Google Scholar]
- 43.Zehetner J, DeMeester SR, Hagen JA, et al. Endoscopic resection and ablation versus esophagectomy for high-grade dysplasia and intramucosal adenocarcinoma. J Thorac Cardiovasc Surg. 2011;141:39–47. doi: 10.1016/j.jtcvs.2010.08.058. [DOI] [PubMed] [Google Scholar]
- 44•.Wani S, Drahos J, Cook MB, et al. Comparison of endoscopic therapies and surgical resection in patients with early esophageal cancer: a population-based study. Gastrointest Endosc. 2014;79:224–32. doi: 10.1016/j.gie.2013.08.002. This study compares endoscopic therapy to surgical therapy for early esophageal cancer and is one of the few landmark studies investigating this comparison. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ngamuengphong S, Wolfsen HC, Wallace MB. Survival of patients with superficial esophageal adenocarcinoma after endoscopic treatment vs surgery. Clin Gastroenterol Hepatol. 2013;11:1424–9. doi: 10.1016/j.cgh.2013.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pohl H, Sonnenberg A, Strobel S, et al. Endoscopic versus surgical therapy for early cancer in Barrett’s esophagus: a decision analysis. Gastrointest Endosc. 2009;70:623–31. doi: 10.1016/j.gie.2008.11.047. [DOI] [PubMed] [Google Scholar]