Abstract
The diagnostic management of patients with angina pectoris typically centres on the detection of obstructive epicardial CAD, which aligns with evidence-based treatment options that include medical therapy and myocardial revascularisation. This clinical paradigm fails to account for the considerable proportion (approximately one-third) of patients with angina in whom obstructive CAD is excluded. This common scenario presents a diagnostic conundrum whereby angina occurs but there is no obstructive CAD (ischaemia and no obstructive coronary artery disease—INOCA). We review new insights into the pathophysiology of angina whereby myocardial ischaemia results from a deficient supply of oxygenated blood to the myocardium, due to various combinations of focal or diffuse epicardial disease (macrovascular), microvascular dysfunction or both. Macrovascular disease may be due to the presence of obstructive CAD secondary to atherosclerosis, or may be dynamic due to a functional disorder (eg, coronary artery spasm, myocardial bridging). Pathophysiology of coronary microvascular disease may involve anatomical abnormalities resulting in increased coronary resistance, or functional abnormalities resulting in abnormal vasomotor tone. We consider novel clinical diagnostic techniques enabling new insights into the causes of angina and appraise the need for improved therapeutic options for patients with INOCA. We conclude that the taxonomy of stable CAD could improve to better reflect the heterogeneous pathophysiology of the coronary circulation. We propose the term ‘stable coronary syndromes’ (SCS), which aligns with the well-established terminology for ‘acute coronary syndromes’. SCS subtends a clinically relevant classification that more fully encompasses the different diseases of the epicardial and microvascular coronary circulation.
Keywords: cardiac computer tomographic (ct) imaging, cardiac magnetic resonance (cmr) imaging, cardiac risk factors and prevention, chronic coronary disease, pharmacology
Introduction
Ischaemic heart disease (IHD) persists as the leading global cause of death and lost life years in adults.1 Reductions in morbidity and mortality are not consistent across subgroups, with mortality being persistently high in younger women.2 Overall, stable ischaemic heart disease (SIHD) remains a worldwide public health problem of unmet need.
Stable coronary artery disease (CAD), or SIHD, refers to the syndrome of recurrent, transient episodes of chest pain reflecting demand-supply mismatch, that is, angina pectoris. In this article, we reappraise the causes of angina based on new insights into coronary pathophysiology. We focus on disorders of coronary artery function and their clinical relevance.
Taxonomy
Given the unmet need of IHD, recent advances in diagnostics and the need for further improvements in primary and secondary prevention, we propose the term ‘stable coronary syndromes’ (SCS) to succinctly reflect the heterogeneous pathophysiology of epicardial, microvascular and endothelial abnormalities in patients with stable angina. SCS aligns with terminology for acute coronary syndromes, and helps to standardise the hierarchy of IHD endotypes, including ischaemia with no obstructive coronary artery disease (INOCA)3 and myocardial infarction with no obstructive CAD (figure 1).
Figure 1.
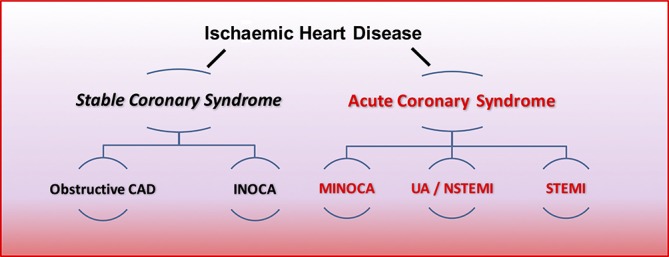
Hierarchical nomenclature of coronary artery disease endotypes that cause ischaemic heart disease. Modified with permission.2 CAD, coronary artery disease; INOCA, ischaemia and no obstructive coronary artery disease; MINOCA, myocardial infarction with no obstructive coronary artery disease.
The clinical conundrum of angina
Classically, angina is considered to be due to flow-limiting CAD,4 which by definition results in a supply-demand mismatch in myocardial perfusion. Anatomical thresholds for CAD severity vary. A widely used cut-off for obstructive CAD is taken as a stenosis of 70% in a main coronary artery (>2.5 mm) in one angiographic projection, or 50% in two projections, and 50% of the left main coronary artery.5 The management of patients with angina appropriately centres on the detection of obstructive epicardial CAD, which may be challenging to diagnose objectively (e.g.mild tandem lesions in series may cause flow-limiting disease). Systemic problems including anaemia and aortic stenosis also influence the propensity to angina and should be considered. In patients with obstructive epicardial CAD, the treatment involves optimal medical therapy and consideration of myocardial revascularisation with either percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG). However, this paradigm fails to account for one-third or more patients with angina in whom obstructive CAD is excluded. A US registry of 398 978 patients referred for coronary angiography demonstrated that 39.2% of patients had no evidence of epicardial CAD.6 Also, angina may persist following PCI and CABG. The reasons for ‘negative’ coronary angiography are multifactorial. However, a growing body of evidence supports the use of coronary function tests, especially since a disorder of coronary artery function may be the unifying diagnosis in a patient with symptoms not explained by anatomical imaging.7
Historically described as cardiac syndrome X, the term coronary microvascular dysfunction (CMD) is used to describe abnormalities that result in microvascular angina (MVA). CMD is classified into five groups (table 1).8 The pathophysiology of CMD involves functional and/or structural abnormalities in the coronary microcirculation. MVA is prognostically important, and given the challenges in diagnosing and treating this problem in daily clinical practice, it is a condition of unmet need.9
Table 1.
Classification of coronary microvascular dysfunction
| Coronary microvascular dysfunction (CMD) | |
| Type 1 | Primary CMD in the absence of underlying myocardial disease or obstructive epicardial CAD |
| Type 2 | CMD in the presence of myocardial disease (eg, hypertrophic cardiomyopathy, hypertensive heart disease) |
| Type 3 | CMD in the presence of obstructive CAD (either stable CAD or acute coronary syndrome) |
| Type 4 | Iatrogenic CMD secondary to myocardial revascularisation |
| Type 5 | CMD following cardiac transplantation |
CAD, coronary artery disease.
Pathophysiology of the coronary circulation
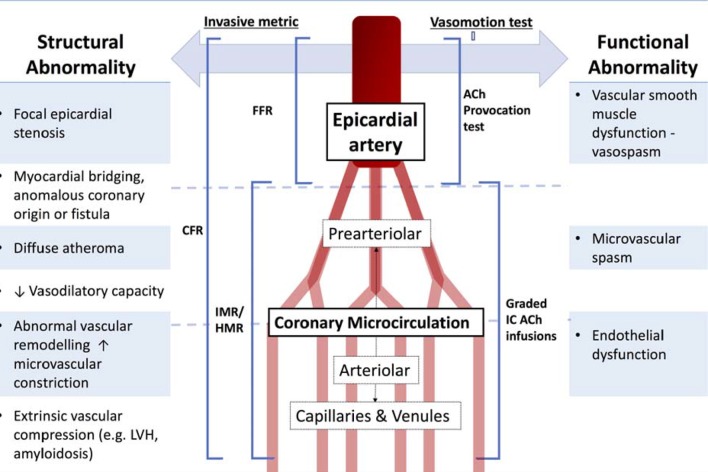
Epicardial arteries (diameter >500 µm) are predominantly capacitance vessels and offer little resistance to flow in the healthy state. The coronary microvasculature governs resistance to myocardial perfusion. Coronary prearterioles and arterioles (vessels <500 µm) contribute approximately 25% and 50% of coronary resistance, respectively, in response to flow, stretch and metabolic stimuli.10 Myocardial ischaemia may result from pathophysiological processes affecting the epicardial conduit artery, the microvasculature or both (figure 2).
Figure 2.
Structural and functional disorders of the coronary circulation. CFR, coronary flow reserve; FFR, fractional flow reserve; IMR, index of microcirculatory resistance; HMR, hyperaemic microvascular resistance; ACh, Acetycholine; LVH, left ventricular hypertrophy
Aetiology
Cardiovascular risk factors, notably hypertension, are prevalent in patients with INOCA. Hypertension is a cause and consequence of endothelial dysfunction, atherosclerosis, microvascular remodelling, rarefaction and interstitial fibrosis. Obesity and cigarette smoking may also be relevant. Importantly, many patients with INOCA do not have risk factors for vascular disease. In these patients, the aetiology may involve a genetic abnormality, perturbations in neuroendocrine function (e.g.dysregulation of the endothelin system), autonomic nervous system abnormalities, or natural changes, such as the menopause.3 Finally, since the natural history of disease is rarely static, the duration and evolution (progression or remission) of disease and ageing are also relevant considerations.
Anatomical abnormalities in the coronary circulation
In addition to evidence-based management of obstructive atherosclerotic CAD,4 other structural coronary problems (including anomalous coronary vessels, coronary artery fistula, certain coronary artery bridges or aneurysms) should be considered.
Coronary microvascular disease may reflect anatomical abnormalities including microvascular remodelling (ie, reductions in capillary luminal size) and number (ie, rarefaction), and therefore increased microvascular resistance to myocardial blood flow (Poiseuille’s law). Angina may result from systemic disorders, such as hypertension, or myocardial pathology such as hypertrophic cardiomyopathy, which involves remodelling of intramural coronary arterioles, vascular rarefaction and perivascular fibrosis.11
In vivo, the diagnosis of anatomical changes in coronary small vessels is challenging. Yamamoto et al 12 performed endomyocardial biopsy in 11 patients with angina and no angiographic obstructive CAD, and demonstrated cardiomyocyte hypertrophy and replacement fibrosis compared with a control population. Osamichi et al 13 undertook endomyocardial biopsy in 24 patients with MVA and demonstrated smooth muscle cell hypertrophy and narrowed microvasculature due to basement membrane thickening. In contrast, Richardson et al 14 performed endomyocardial biopsy in seven patients with invasively diagnosed MVA and found no significant morphological abnormality.
Functional microvascular abnormalities
Functional abnormalities of the epicardial arteries and microvessels relate to (1) enhanced vasoconstriction, (2) impaired vasodilation secondary to endothelium-independent or endothelium-dependent mechanisms, or (3) a combination of these problems. Disorders of coronary vasomotion include epicardial and/or microvascular coronary spasm, impaired coronary artery vasorelaxation and endothelial dysfunction-related reduced myocardial blood flow.15 Various vasoactive substances maybe implicated. For example, endothelin-1 (ET-1) concentrations are elevated in patients with primary CMD; in 1034 patients who underwent stress positron emission tomography (PET) imaging for the investigation of angina, Johnson et al 16 identified abnormal diffuse heterogeneous myocardial perfusion that was associated with CMD. In an animal model, this abnormal perfusion pattern was recreated using intracoronary infusions of ET-1, implying that ET-1 contributes to abnormal vasoconstriction in patients with CMD.17
The coronary endothelium regulates vascular tone and myocardial blood flow via nitric oxide (NO)-dependent mechanisms.10 Abnormal vasoconstrictive responses to acetylcholine infusion, consistent with impaired endothelial function, occur in patients with angina and non-obstructive epicardial CAD.15 Abnormal endothelium-independent vasodilator function may involve resistance to NO, adenosine and prostacyclin.18
Angina-myocardial ischaemia discordance and propensity to ischaemia
The ‘ischaemic threshold’ (the heart rate–blood pressure product at the onset of angina or ECG changes) differs between individuals.19 Innate variations in neurogenic vascular tone and endocrine changes (eg, menopause) dictate the propensity to vasospasm while environmental factors including cold temperature, exertion and mental stress are relevant.20
Silent ischaemia is common and prognostically important.21 Interestingly, the large US CLARIFY registry highlighted the importance of symptoms, showing that angina with or without concomitant ischaemia was more predictive of adverse cardiac events compared with silent ischaemia alone.22 Variations in pain thresholds and cardiac innervation and diabetic neuropathy are all potential mechanisms for the discordance between symptoms and ischaemia.23 Patients with MVA may have abnormal adrenergic function, increased painful sensitivity to innocuous cardiac stimuli (eg, radiographic contrast), and a lower pain threshold and tolerance to the algogenic effects of adenosine (thought to be the main effector of ischaemia-mediated chest pain).24 25
Advances in the diagnosis of disorders of coronary artery function
Coronary angiography has a resolution of 500 µm and the microvasculature is not visible on CT or invasive angiography. Myocardial biopsy is not a feasible diagnostic option. Therefore, currently, the diagnosis of CMD is empirical when specific tests of coronary function are not used (figure 3).
Figure 3.

Clinical case demonstrating the utility of non-invasive and invasive diagnostic tests for coronary artery function. A 73-year-old woman presented with a 2-year history of typical Canadian cardiovascular society (CCS) class 2 angina. The patient had type 2 diabetes mellitus, an elevated body mass index and had previously been documented to have a normal invasive coronary angiogram 8 years previously. Invasive coronary angiography (A,B) demonstrated unobstructed epicardial coronary arteries. In the left anterior descending artery, the fractional flow reserve (FFR) value was 0.95, consistent with no epicardial flow-limiting stenosis (C). The coronary flow reserve (CFR) was reduced (1.3, normal >2.0), and the index of microcirculatory resistance (IMR) was elevated (33 units, normal <25), indicative of impaired epicardial and microvascular vasodilation and increased microvascular resistance respectively (C). Coronary endothelial function assessment using graded intracoronary acetylcholine infusion revealed mild vasoconstriction (dashed line) consistent with endothelial dysfunction (D) compared with endothelial-independent function testing with intracoronary glyceryl trinitrate (E). There was inducible coronary vasospasm using 100 µg acetylcholine bolus over 20 s (not shown). The patient subsequently underwent adenosine stress perfusion CMR, which demonstrated an inducible circumferential subendocardial perfusion defect in the basal short axis slice (arrows) with adenosine stress (F), compared with the corresponding rest perfusion imaging (G). A pixel-wide fully quantitative myocardial blood flow analysis confirmed markedly reduced myocardial blood flow in the subendocardium with adenosine stress (H) compared with the corresponding rest perfusion image (I). A diagnosis of coronary microvascular dysfunction was made. The patient was symptomatically improved at 3-month follow-up after treatment with nebivolol, statin and ACE inhibitors was started. The CMR methods were provided by Andrew Arai and Li-Yueh Hsu, National Institutes of Health, MD.
Non-invasive assessment of coronary artery function
Microvascular disease may be a generalised process resulting in diffuse myocardial perfusion abnormalities. Therefore, traditional non-invasive ischaemia tests may be normal in patients with CMD due to the absence of regional perfusion abnormalities typically seen in obstructive CAD. Myocardial perfusion scintigraphy has comparatively low spatial resolution (~1×1 cm per pixel), and is thus relatively insensitive for detection of subtle perfusion abnormalities secondary to microvascular dysfunction. Stress transthoracic Doppler echocardiography (TTDE) is typically performed in the left anterior descending, and is a potentially feasible and cheap method of assessing flow velocity at rest and during maximal hyperaemia to estimate coronary flow reserve (CFR); however, TTDE lacks accuracy and does not interrogate all myocardial segments.26
The reference-standard non-invasive assessment of myocardial blood flow is stress PET imaging, which permits quantitative flow derivation in mL/g/min. Clinically, PET-derived quantification of myocardial blood flowcan assist in the diagnosis of diffuse and impaired CFR, which is associated with increased risk of major adverse cardiac events (MACE).27 In real-world practice, the use of PET is limited by its availability (including radioisotopes), cost and exposure to ionising radiation.
Cardiac magnetic resonance (CMR) imaging holds most promise as a preferred non-invasive imaging option. Although CMR is also comparatively expensive, it has clear benefits, including lack of ionising radiation, high spatial resolution (ie, ~2.5×2.5 mm at 1.5 Tesla, ~1 ×1 mm at 3.0 Tesla), high sensitivity and specificity for perfusion abnormalities, and multiparametric imaging techniques (reference-standard left ventricular volumes and function, myocardial tissue characterisation with late gadolinium enhancement imaging and parametric mapping). Panting et al 28 demonstrated the qualitative detection of inducible circumferential subendocardial perfusion defects in patients with syndrome X. Semiquantitative assessment of CFR (the myocardial perfusion reserve index, MPRi) from CMR has been shown to predict abnormal response to invasive coronary reactivity testing, and is an important prognosticator in a large natural history study of women with non-obstructive CAD.29 Novel pixel-wise absolute perfusion quantification of myocardial perfusion by CMR will likely improve the efficiency of absolute quantification of myocardial blood flow by CMR.30
Invasive guidewire-based techniques
Invasive tests of coronary artery function are the reference standard for the diagnosis of CMD.31 We contend that a complete diagnostic evaluation of the coronary circulation should assess structural and functional pathology. Pressure-derived indices, such as fractional flow reserve (FFR), contrast-enhanced FFR, instantaneous wave-free ratio (iFR) and resting Pd/Pa, are useful tests to guide revascularisation decisions.32 However, as is the case with coronary angiography, these indices do not inform the clinician about microvascular resistance and or vasodilator potential.
CFR reflects the ratio of hyperaemic flow to basal flow and was first described by Gould et al in 1974.33 Microvascular resistance may be measured by thermodilution (index of microcirculatory resistance, IMR)34 or Doppler (hyperaemic microvascular resistance, HMR).35 CFR and IMR/HMR reflect distinct properties of vascular (dys)function and discordance (normal/abnormal) is common.36 CFR reflects the combined vasodilator capacity of the epicardial coronary artery and its subtended microvasculature. There are some limitations to using invasively measured CFR in isolation due to its sensitivity to systemic haemodynamics, myocardial contractility and challenges with establishing true resting coronary blood flow during invasive coronary angiography. Specific measures of microvascular resistance (i.e., IMR and HMR) are more reproducible, specific and are directly informative about microvascular disease.37
Sezer et al 38 assessed coronary physiology in patients with diabetes with INOCA, showing that early reduction in CFR was driven by disturbed coronary regulation and high resting flow. In long-standing diabetes, elevated microvascular resistance may reflect structural remodelling of small vessels. This process parallels the paradox of microvascular disease in diabetic nephropathy where increased glomerular filtration rate (GFR) typifies the early stages of disease prior to later structural damage and reduction in GFR.
Functional disorders of coronary vasomotion
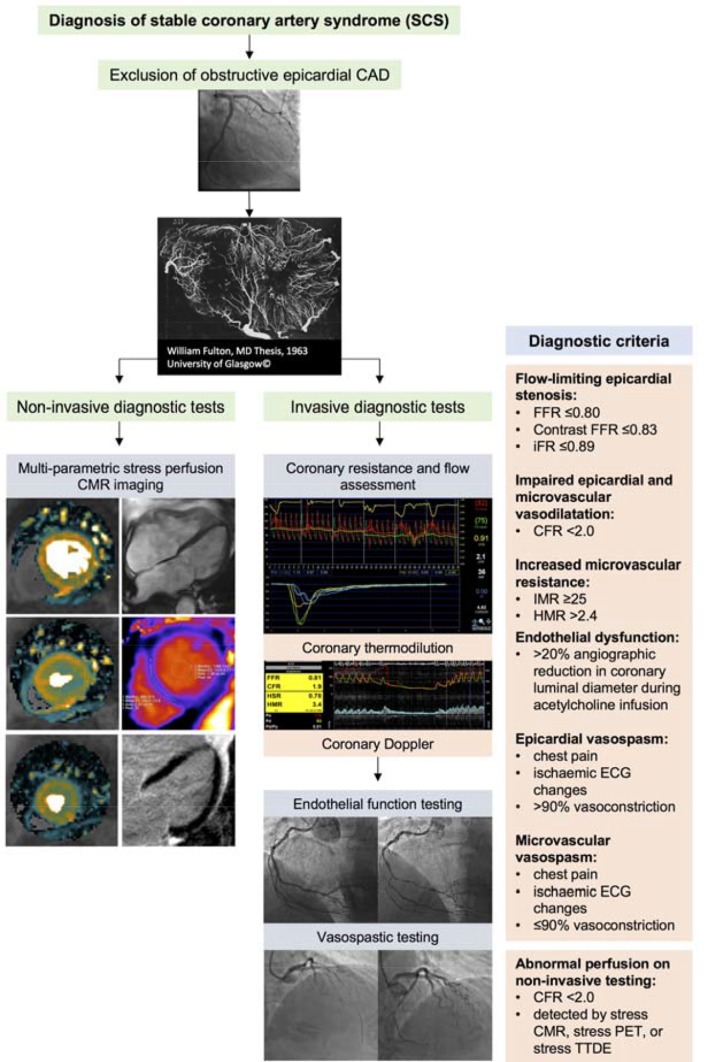
FFR, CFR and IMR are typically derived using intravenous adenosine, which is an endothelium-independent vasodilator. Assessment of coronary endothelial function has distinct prognostic utility.39 Abnormal flow response to endothelial agonist can be assessed using symptoms and angiography alone (ie, >20% angiographic reduction in coronary luminal diameter during acetylcholine infusion),31 intracoronary Doppler flow measurement or with thermodilution. Acetylcholine may be used at higher bolus doses (eg, 100–200 µg) in a provocation test to detect abnormal coronary vasoreactivity (ie, vasospasm). A consensus document by the Coronary Vasomotion Disorders International Study Group (COVADIS) defines the criteria for a positive provocative test as meeting the following criteria: (1) reproduction of the usual chest pain, (2) ischaemic ECG changes and (3) >90% vasoconstriction on angiography40 (figure 4).
Figure 4.
Schematic illustration of the diagnostic work-up for SCS following exclusion of obstructive epicardial CAD. (1) Non-invasive diagnostic testing with multiparametric stress perfusion CMR imaging assessment demonstrating pixel-wide fully quantitative myocardial blood flow analysis from cardiac base to apex, cine imaging, native T1 parametric mapping and late gadolinium enhancement imaging. (2) Invasive diagnostic testing with (A) dual pressure-sensitive and temperature-sensitive coronary wire or coronary Doppler and pressure-sensitive wire, and (B) endothelial and vasospastic testing with intracoronary acetylcholine. CAD, coronary artery disease; CFR, coronary flow reserve; CMR, cardiac magnetic resonance; FFR, fractional flow reserve; HMR, hyperaemic microvascular resistance; iFR, instantaneous wave-free ratio; IMR, index of microcirculatory resistance; PET, positron emission tomography; SCS, stable coronary artery syndrome; TTDE, transthoracic Doppler echocardiography.
Recent clinical evidence
Detection and incidence
Lee et al prospectively enrolled 139 consecutive patients in a single-centre study with angina and no obstructive CAD. During comprehensive invasive multimodality assessment at angiography, all patients had atherosclerosis on intravascular ultrasound, 21% had abnormal IMR, 44% had endothelial dysfunction and only 23% had no explanation for their symptoms.41 Coronary vasoreactivity testing with acetylcholine is generally safe and useful for the detection of epicardial and/or microvascular spasm.15 The prevalence of microvascular spasm and vasospastic angina (VSA) is not fully resolved, but these conditions may occur in up to two-thirds of patients with a ‘negative’ angiogram.42
Coronary atherosclerosis and abnormal vasomotion are inextricably linked. A Korean study of CFR and IMR in angiographically moderate epicardial lesions demonstrated around a quarter of 516 coronary arteries had an elevated IMR and a similar proportion had reduced CFR (<2.0).36 Both low CFR with elevated IMR were associated with poor prognosis.
Prognosis of patients and no obstructive CAD
The prognosis of SCS is linked with the underlying pathophysiological mechanism and varies depending on the population studied.9 Patients with angiographically normal coronaries and only exercise-induced symptoms may be in a better prognostic group.43 Data from the Women’s Ischemia Syndrome Evaluation (WISE) study suggests that there is a worse prognosis; the 5-year annualised risk of MACE was 16.0% in women with non-obstructive CAD, 7.9% in women with normal coronary arteries and 2.4% in an asymptomatic control group (p≤0.002 after adjustment for baseline cardiovascular risk).9 Similarly, a Danish cohort study of 11 223 patients with angina found an increased risk of MACE for patients with diffuse non-obstructive CAD and those with normal coronaries (adjusted HR of 1.85 and 1.52, respectively), compared with a reference population.
Therapy
Pharmacological symptomatic therapy
A detailed review of therapy for the different disorders of coronary artery function is beyond the scope of this review.44 A summary of currently available therapies aligning with the different SCS disease endotypes is shown in table 2 (see additional references in online supplementary file 1). Robust evidence for the treatment of SCS is lacking. The treatment effect in many studies is potentially diluted by enrolment of heterogeneous groups of patients with distinct pathophysiological mechanisms of CMD that may respond differently to specific treatment modalities. Current European Society of Cardiology (ESC) guidelines provide recommendations for patients with CMD suggesting ß-blockers as first-line therapy, with calcium antagonists recommended if the former is not tolerated or efficacious.4 Unlike in patients with angina and obstructive CAD, nitrates are not generally effective for treating SCS due to CMD.45 In a randomised, placebo-controlled clinical trial of ranolazine led by the WISE investigators, although there were no overall improvements in angina and MPRi with ranolazine, benefit was observed in the subgroup of patients with a reduced CFR (<2.5) at baseline.46 Patients with VSA may benefit symptomatically from treatment with both nitrate and calcium channel antagonists while the latter may have prognostic benefit.47 Rho-kinase inhibitors and endothelin-receptor antagonists represent potential future therapeutic options.
Table 2.
Treatment of SCS endotypes
| SCS endotype | Investigation | Pathophysiology | Treatment | Efficacy | Side effects |
| Microvascular angina secondary to impaired vasodilation | Reduced CFR and/or increased microvascular resistance | Anatomical remodelling, vascular rarefaction, disturbed coronary regulation | ß-blockers | Reduction in myocardial oxygen consumption | Fatigue, blurred vision, cold hands |
| ACE inhibitors | Improve CFR, reduce workload, may improve microvascular remodelling | Cough, renal impairment, hyperkalaemia | |||
| Ranolazine | Improves MPRi in patients with MVA and reduced CFR | Nausea, dizziness, headache | |||
| Phosphodiesterase inhibitors | ↓cGMP degradation, ↑vascular smooth muscle relaxation and ↑ CFR for those with baseline CFR <2.5 | Flushing, tinnitus, headache | |||
| Microvascular angina secondary to abnormal vasoconstriction | Hyper-reactivity to stimuli (eg, acetylcholine, exercise, stress) | Endothelial dysfunction, inappropriate prearteriolar vasoconstriction | ACE inhibitors | Improves endothelial vasomotor dysfunction | Cough, renal impairment, hyperkalaemia |
| Calcium antagonists | Vascular smooth muscle relaxation, reduction in myocardial oxygen consumption | Constipation, ankle swelling, flushing | |||
| Nicorandil | Potassium channel activator with coronary microvascular dilatory effect | Dizziness, flushing, weakness, nausea | |||
| Statins | Improved coronary endothelial function, pleiotropic effects including reduced vascular inflammation | Myalgia, headache, cramps | |||
| Exercise | Beneficial effect on endothelium, ↓ resting blood flow and ↑ vasodilatory capacity | Muscle fatigue, myalgia | |||
| Hormone replacement therapy | Oestrogen therapy improves endothelial function short term in CMD | ↑ Risk of breast cancer, marginally ↑ risk of CVD | |||
| Microvascular angina secondary to abnormal pain processing | Enhanced nociception | Dysfunctional cortical pain processing | Tricyclic antidepressants | Improved symptom burden potentially through reduced visceral pain | Blurred vision, dry mouth, drowsiness, impaired coordination |
| Xanthine derivatives | Antialgogenic effect (due to the direct involvement of adenosine in cardiac pain generation) | Nausea and vomiting, palpitations | |||
| Epicardial and/or microvascular coronary vasospasm | Propensity to coronary vasospasm | Vascular smooth muscle hyper-reactivity | Calcium channel antagonists | ↓ Spontaneous and inducible coronary spasm via vascular smooth muscle relaxation and ↓ oxygen demand | Constipation, ankle swelling, flushing |
| Nitrates | ↓ Spontaneous and inducible coronary spasm via large epicardial vasodilation, ↓ oxygen demand; lack of efficacy in microvascular angina with potentially deleterious effect | Headaches, dizziness, flushing | |||
| Rho-kinase inhibitors | ↓ Calcium sensitivity of smooth muscle by ↑ phosphatase activity reducing phosphorylated (active) myosin light chains | Rash, dizziness; not licensed for use in Europe or USA | |||
| Adjunctive non-pharmacological interventions | May be useful in all endotypes | Metabolic syndrome, endothelial dysfunction, cardiovascular risk factors, anxiety/depression | Smoking cessation, exercise, cardiac rehabilitation, Mediterranean diet, cognitive behavioural therapy | ||
CFR, coronary flow reserve; CMD, coronary microvascular dysfunction; MPRi, myocardial perfusion reserve index; MVA, microvascular angina; SCS, stable coronary syndrome; CVD, cardiovascular disease; cGMP, Cyclic guanosine monophosphate
heartjnl-2017-311446supp001.pdf (144.5KB, pdf)
Secondary prevention
ESC guidelines support the use of secondary prevention with aspirin and statin therapy.4 In contrast, the UK National Institute for Health and Care Excellence guideline-95 on management of stable angina cites the enigmatic term ‘Syndrome X’, but guidance on the diagnosis and management is limited, reflecting the limited evidence base.48 Contemporary guidelines should be followed for the management of vascular risk factors in patients with INOCA.3 41 In patients with CMD, statin therapy reduces ischaemia as revealed by reduced ST-segment deviation following exercise testing and improves exercise capacity and flow-mediated dilatation in the brachial artery in patients with CMD.49 ACE inhibitors (ACEi) improve endothelial dysfunction and vasoreactivity via NO stimulation helping to reverse vascular hypertrophy and improve vascular compliance.
Non-pharmacological therapy
Lifestyle measures promote well-being in patients with INOCA through smoking cessation, healthy eating (eg, Mediterranean diet) and physical activity.3 The Comprehensive Treatment of Angina in Women With Microvascular Dysfunction trial (NCT02910154) will randomise women with angina and CMD to comprehensive multimodality intervention (dietary, exercise, and statin and ACEi therapy) or control therapy to determine whether angina and microvascular dysfunction can be improved.
Future directions
Overall, there is a critical missing link between the use of relevant diagnostic tests of coronary artery function, therapeutic agents with proven efficacy and health outcomes of patients with angina without obstructive CAD. This gap in evidence is currently being addressed in the British Heart Foundation CORonary MICrovascular Angina randomised controlled trial (CorMicA NCT03193294). A personalised approach to therapy is desirable and it is reasonable to target those patients with impairment of microvascular dilation characterised by reduced CFR with anti-anginals that reduce heart rate and oxygen consumption (eg, ß-blockers), whereas vasodilators or ACEi would be more appropriate for patients with evidence of in microvascular constriction.50
‘Stable coronary syndrome’ reflects a disease-based, clinically relevant classification that reflects the interaction between structural and functional disorders throughout the coronary circulation. The paucity of pathway-specific therapy presents an opportunity for novel research using stratified medicine. Our vision is for a personalised medicine approach whereby SCS endotypes, defined by the results of coronary function tests, may benefit from targeted therapy. Further research is needed to determine whether this paradigm may lead to patient benefits (table 3).
Table 3.
Proposed comprehensive research strands for patients with ischaemia and no obstructive coronary artery disease (INOCA)
| Comprehensive INOCA research strands | |
| Stratified medicine trials |
|
| Vascular science |
|
| Imaging and modelling |
|
| Molecular pathology and vascular histopathology |
|
| Therapeutic trials |
|
| Health informatics and value assessments |
|
| Patient and public involvement |
|
Acknowledgments
We acknowledge the patients who have participated in research studies cited in this article.
Footnotes
TJF and DC contributed equally.
Twitter: @UofGICAMS
Contributors: CB conceived the article. TJF and DC provided the first draft, figures and revisions. All authors have approved the final manuscript.
Funding: The British Heart Foundation has supported DC (FS/14/15/30661), TJF (RE/13/5/30177) and CB (RE/13/5/30177; FS/14/15/30661; FS172632744; PG-17-25-32884).
Competing interests: CB is employed by the University of Glasgow, which holds consultancy and research agreements with companies that have commercial interests in the diagnosis and treatment of angina. The companies include Abbott Vascular, AstraZeneca, Boehringer Ingelheim, Menarini Pharmaceuticals and Siemens Healthcare. None of the other authors have any disclosures.
Ethics approval: Obtained.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1. Wang H, Naghavi M, Allen C, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the global burden of disease study 2015. Lancet 2016;388:1459–544. 10.1016/S0140-6736(16)31012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berry C. Stable coronary syndromes: the case for consolidating the nomenclature of stable ischemic heart disease. Circulation 2017;136:437 9. 10.1161/CIRCULATIONAHA.117.028991 [DOI] [PubMed] [Google Scholar]
- 3. Bairey Merz CN, Pepine CJ, Walsh MN, et al. Ischemia and No Obstructive Coronary Artery Disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation 2017;135:1075–92. 10.1161/CIRCULATIONAHA.116.024534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Montalescot G, Sechtem U, Achenbach S, et al. ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European society of cardiology. Eur Heart J 2013;34:2949–3003. 10.1093/eurheartj/eht296 [DOI] [PubMed] [Google Scholar]
- 5. Greenwood JP, Ripley DP, Berry C, et al. Effect of care guided by cardiovascular magnetic resonance, myocardial perfusion scintigraphy, or NICE guidelines on subsequent unnecessary angiography rates: the CE-MARC 2 randomized clinical trial. JAMA 2016;316:1051–60. 10.1001/jama.2016.12680 [DOI] [PubMed] [Google Scholar]
- 6. Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med 2010;362:886–95. 10.1056/NEJMoa0907272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Camici PG, d’Amati G, Rimoldi O. Coronary microvascular dysfunction: mechanisms and functional assessment. Nat Rev Cardiol 2015;12:48–62. 10.1038/nrcardio.2014.160 [DOI] [PubMed] [Google Scholar]
- 8. Herrmann J, Kaski JC, Lerman A. Coronary microvascular dysfunction in the clinical setting: from mystery to reality. Eur Heart J 2012;33:2771–82. 10.1093/eurheartj/ehs246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gulati M, Cooper-DeHoff RM, McClure C, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the women’s ischemia syndrome evaluation study and the St James women take heart project. Arch Intern Med 2009;169:843–50. 10.1001/archinternmed.2009.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duncker DJ, Koller A, Merkus D, et al. Regulation of coronary blood flow in health and ischemic heart disease. Prog Cardiovasc Dis 2015;57:409–22. 10.1016/j.pcad.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mancini M, Petretto E, Kleinert C, et al. Mapping genetic determinants of coronary microvascular remodeling in the spontaneously hypertensive rat. Basic Res Cardiol 2013;108:316 10.1007/s00395-012-0316-y [DOI] [PubMed] [Google Scholar]
- 12. Yamamoto S, James TN, Kawamura K, et al. Cardiocytic apoptosis and capillary endothelial swelling as morphological evidence of myocardial ischemia in ventricular biopsies from patients with angina and normal coronary arteriograms. Coron Artery Dis 2002;13:25–35. [DOI] [PubMed] [Google Scholar]
- 13. Osamichi S, Kouji K, Yoshimaro I, et al. Myocardial glucose metabolism assessed by positron emission tomography and the histopathologic findings of microvessels in syndrome X. Circ J 2004;68:220–6. [DOI] [PubMed] [Google Scholar]
- 14. Richardson PJ, Livesley B, Oram S, et al. Angina pectoris with normal coronary arteries. Transvenous myocardial biopsy in diagnosis. Lancet 1974;2:677–80. [DOI] [PubMed] [Google Scholar]
- 15. Ong P, Athanasiadis A, Borgulya G, et al. High prevalence of a pathological response to acetylcholine testing in patients with stable angina pectoris and unobstructed coronary arteries. The ACOVA study (Abnormal COronary VAsomotion in patients with stable angina and unobstructed coronary arteries). J Am Coll Cardiol 2012;59:655–62. 10.1016/j.jacc.2011.11.015 [DOI] [PubMed] [Google Scholar]
- 16. Johnson NP, Gould KL. Clinical evaluation of a new concept: resting myocardial perfusion heterogeneity quantified by markovian analysis of PET identifies coronary microvascular dysfunction and early atherosclerosis in 1,034 subjects. J Nucl Med 2005;46:1427–37. [PubMed] [Google Scholar]
- 17. Johnson NP, Gould KL. Physiology of endothelin in producing myocardial perfusion heterogeneity: a mechanistic study using darusentan and positron emission tomography. J Nucl Cardiol 2013;20:835–44. 10.1007/s12350-013-9756-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marcus ML, Chilian WM, Kanatsuka H, et al. Understanding the coronary circulation through studies at the microvascular level. Circulation 1990;82:1–7. [DOI] [PubMed] [Google Scholar]
- 19. Garber CE, Carleton RA, Camaione DN, et al. The threshold for myocardial ischemia varies in patients with coronary artery disease depending on the exercise protocol. J Am Coll Cardiol 1991;17:1256–62. [DOI] [PubMed] [Google Scholar]
- 20. Dubois-Randé JL, Dupouy P, Aptecar E, et al. Comparison of the effects of exercise and cold pressor test on the vasomotor response of normal and atherosclerotic coronary arteries and their relation to the flow-mediated mechanism. Am J Cardiol 1995;76:467–73. [DOI] [PubMed] [Google Scholar]
- 21. Davies RF, Goldberg AD, Forman S, et al. Asymptomatic Cardiac Ischemia Pilot (ACIP) study two-year follow-up: outcomes of patients randomized to initial strategies of medical therapy versus revascularization. Circulation 1997;95:2037–43. [DOI] [PubMed] [Google Scholar]
- 22. Steg PG, Greenlaw N, Tendera M, et al. Prevalence of anginal symptoms and myocardial ischemia and their effect on clinical outcomes in outpatients with stable coronary artery disease: data from the International Observational CLARIFY Registry. JAMA Intern Med 2014;174:1651–9. 10.1001/jamainternmed.2014.3773 [DOI] [PubMed] [Google Scholar]
- 23. Ambepityia G, Kopelman PG, Ingram D, et al. Exertional myocardial ischemia in diabetes: a quantitative analysis of anginal perceptual threshold and the influence of autonomic function. J Am Coll Cardiol 1990;15:72–7. [DOI] [PubMed] [Google Scholar]
- 24. Lanza GA, Giordano A, Pristipino C, et al. Abnormal cardiac adrenergic nerve function in patients with syndrome X detected by [123I]metaiodobenzylguanidine myocardial scintigraphy. Circulation 1997;96:821–6. [DOI] [PubMed] [Google Scholar]
- 25. Pasceri V, Lanza GA, Buffon A, et al. Role of abnormal pain sensitivity and behavioral factors in determining chest pain in syndrome X. J Am Coll Cardiol 1998;31:62–6. [DOI] [PubMed] [Google Scholar]
- 26. Rigo F, Richieri M, Pasanisi E, et al. Usefulness of coronary flow reserve over regional wall motion when added to dual-imaging dipyridamole echocardiography. Am J Cardiol 2003;91:269–73. [DOI] [PubMed] [Google Scholar]
- 27. Murthy VL, Naya M, Foster CR, et al. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation 2012;126:1858–68. 10.1161/CIRCULATIONAHA.112.120402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Panting JR, Gatehouse PD, Yang GZ, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med 2002;346:1948–53. 10.1056/NEJMoa012369 [DOI] [PubMed] [Google Scholar]
- 29. Doyle M, Weinberg N, Pohost GM, et al. Prognostic value of global MR myocardial perfusion imaging in women with suspected myocardial ischemia and no obstructive coronary disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. JACC Cardiovasc Imaging 2010;3:1030–6. 10.1016/j.jcmg.2010.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hsu LY, Groves DW, Aletras AH, et al. A quantitative pixel-wise measurement of myocardial blood flow by contrast-enhanced first-pass CMR perfusion imaging: microsphere validation in dogs and feasibility study in humans. JACC Cardiovasc Imaging 2012;5:154–66. 10.1016/j.jcmg.2011.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sheikh AR WJ, Bariey Merz N, Beltrame JF. The current state of invasive coronary evaluation and management of patients with angina and nonobstructive coronary arteries: American college of cardiology;2016 [Expert Analysis] . 2017. http://www.acc.org/latest-in-cardiology/articles/2016/05/26/08/31/the-current-state-of-invasive-coronary-evaluation-and-management-of-patients-with-angina-and-nonobstructive-coronary-arteries?w_nav=LC (accessed 20th July 2017).
- 32. Berry C, Corcoran D, Hennigan B, et al. Fractional flow reserve-guided management in stable coronary disease and acute myocardial infarction: recent developments. Eur Heart J 2015;36:3155–64. 10.1093/eurheartj/ehv206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gould KL, Lipscomb K, Hamilton GW. Physiologic basis for assessing critical coronary stenosis. Instantaneous flow response and regional distribution during coronary hyperemia as measures of coronary flow reserve. Am J Cardiol 1974;33:87–94. [DOI] [PubMed] [Google Scholar]
- 34. Fearon WF, Balsam LB, Farouque HM, et al. Novel index for invasively assessing the coronary microcirculation. Circulation 2003;107:3129–32. 10.1161/01.CIR.0000080700.98607.D1 [DOI] [PubMed] [Google Scholar]
- 35. Meuwissen M, Siebes M, Chamuleau SA, et al. Hyperemic stenosis resistance index for evaluation of functional coronary lesion severity. Circulation 2002;106:441–6. [DOI] [PubMed] [Google Scholar]
- 36. Lee JM, Jung JH, Hwang D, et al. Coronary flow reserve and microcirculatory resistance in patients with intermediate coronary stenosis. J Am Coll Cardiol 2016;67:1158–69. 10.1016/j.jacc.2015.12.053 [DOI] [PubMed] [Google Scholar]
- 37. Ford TJ, Corcoran D, Berry C. Coronary artery disease: physiology and prognosis. Eur Heart J 2017. (Epub ahead of print 27 May 2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sezer M, Kocaaga M, Aslanger E, et al. Bimodal pattern of coronary microvascular involvement in diabetes mellitus. J Am Heart Assoc 2016;5:e003995 10.1161/JAHA.116.003995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation 2002;106:653–8. [DOI] [PubMed] [Google Scholar]
- 40. Beltrame JF, Crea F, Kaski JC, et al. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J 2017;38:2565-2568 10.1093/eurheartj/ehv351 [DOI] [PubMed] [Google Scholar]
- 41. Lee BK, Lim HS, Fearon WF, et al. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation 2015;131:1054–60. 10.1161/CIRCULATIONAHA.114.012636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sara JD, Widmer RJ, Matsuzawa Y, et al. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv 2015;8:1445–53. 10.1016/j.jcin.2015.06.017 [DOI] [PubMed] [Google Scholar]
- 43. Lanza GA, Filice M, De Vita A, et al. Primary atable microvascular angina: a long-term clinical follow-up study. Circulation 2017;135:1982–4. 10.1161/CIRCULATIONAHA.117.027685 [DOI] [PubMed] [Google Scholar]
- 44. Ong P, Athanasiadis A, Sechtem U. Treatment of angina pectoris associated with coronary microvascular dysfunction. Cardiovasc Drugs Ther 2016;30:351–6. 10.1007/s10557-016-6676-z [DOI] [PubMed] [Google Scholar]
- 45. Russo G, Di Franco A, Lamendola P, et al. Lack of effect of nitrates on exercise stress test results in patients with microvascular angina. Cardiovasc Drugs Ther 2013;27:229–34. 10.1007/s10557-013-6439-z [DOI] [PubMed] [Google Scholar]
- 46. Bairey Merz CN, Handberg EM, Shufelt CL, et al. A randomized, placebo-controlled trial of late Na current inhibition (ranolazine) in coronary microvascular dysfunction (CMD): impact on angina and myocardial perfusion reserve. Eur Heart J 2016;37:1504–13. 10.1093/eurheartj/ehv647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nishigaki K, Inoue Y, Yamanouchi Y, et al. Prognostic effects of calcium channel blockers in patients with vasospastic angina--a meta-analysis. Circ J 2010;74:1943–50. 10.1253/circj.CJ-10-0292 [DOI] [PubMed] [Google Scholar]
- 48. (NICE) NIfHaCE. Stable angina: management. NICE guideline (CG126). London: NICE, 2011. [Google Scholar]
- 49. Kayikcioglu M, Payzin S, Yavuzgil O, et al. Benefits of statin treatment in cardiac syndrome-X1. Eur Heart J 2003;24:1999–2005. 10.1016/S0195-668X(03)00478-0 [DOI] [PubMed] [Google Scholar]
- 50. Crea F, Lanza GA. Treatment of microvascular angina: the need for precision medicine. Eur Heart J 2016;37:1514–6. 10.1093/eurheartj/ehw021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2017-311446supp001.pdf (144.5KB, pdf)