Abstract
Background
We evaluated the effectiveness and cost-effectiveness of interventions targeting hepatitis C virus (HCV) and HIV infections among people who inject drugs (PWID) in Eastern Europe/Central Asia. We specifically considered the needle-syringe program (NSP), opioid substitution therapy (OST), HCV and HIV diagnosis, antiretroviral therapy (ART), and/or new HCV treatment (direct acting antiviral [DAA]) in Belarus, Georgia, Kazakhstan, Republic of Moldova, and Tajikistan.
Methods
We developed a deterministic dynamic compartmental model and evaluated the number of infections averted, costs, and incremental cost-effectiveness ratios (ICERs) of interventions. OST decreased frequencies of injecting by 85% and NSP needle sharing rates by 57%; ART was introduced at CD4 <350 and DAA at fibrosis stage ≥F2 at a $2370 to $23 280 cost.
Results
Increasing NSP+OST had a high impact on transmissions (infections averted in PWID: 42% in Tajikistan to 55% in Republic of Moldova for HCV; 30% in Belarus to 61% in Kazakhstan for HIV over 20 years). Increasing NSP+OST+ART was very cost-effective in Georgia (ICER = $910/year of life saved [YLS]), and was cost-saving in Kazakhstan and Republic of Moldova. NSP+OST+ART and HIV diagnosis was very cost-effective in Tajikistan (ICER = $210/YLS). Increasing the coverage of all interventions was always the most effective strategy and was cost-effective in Belarus and Kazakhstan (ICER = $12 960 and $21 850/YLS); it became cost-effective/cost-saving in all countries when we decreased DAA costs.
Conclusion
Increasing NSP+OST coverage, in addition to ART and HIV diagnosis, had a high impact on both epidemics and was very cost-effective and even cost-saving. When HCV diagnosis was improved, increased DAA averted a high number of new infections if associated with NSP+OST.
Keywords: cost-effectiveness, Eastern Europe & Central Asia, hepatitis C, HIV, people who inject drugs
Eastern Europe and Central Asia (EECA) is a region where epidemics of HIV/AIDS and chronic hepatitis C have grown rapidly in recent years. In 2013, 1.1 million HIV-infected people were estimated to live in EECA, with 110 000 new annual infections overall [1]. This accounted for an increase of almost 100% new cases per year since 2003. In these countries, a high proportion of those infected with HIV and hepatitis C virus (HCV) are people who inject drugs (PWID), because of needle sharing in particular. In 2013, 9 of the 16 countries of EECA had an HIV prevalence among PWID above 10%, such in Estonia, Ukraine, and Belarus In Russian Federation, it was estimated that 16% (320000) of PWID were infected by HIV in 2009 [2]. Regarding HCV, it was estimated that 2.3 million PWID were infected in Eastern Europe in 2013, with a prevalence higher than 50% in this population in countries such as Georgia, Kazakhstan, Russian Federation, and Ukraine [3, 4].
Because of this high prevalence among PWID, HIV and HCV prevention strategies must target this population to contain the epidemics in the whole country. Programs have been initiated to reduce the incidence of these infections among PWID. Needle-syringe programs (NSPs) are one of the most frequent and efficacious strategies, and they consist of distributing sterile injecting equipment among PWID, but coverage remains low in EECA [5]. Another strategy consists in providing PWID with opioid substitution therapy (OST) to reduce the risk of infected needle sharing. Although this strategy combined with other interventions has proved to be effective and cost-effective in Western countries [6–8], its use remains very limited in EECA [1]. In some countries of EECA, there is still no OST program in place (Uzbekistan), and such programs are even legally prohibited (Russian Federation, Turkmenistan) [1].
Few analyses on the cost-effectiveness of these interventions among PWID in EECA countries have been reported. In addition, they have only concerned HIV infection in specific country settings [8–12]. Most studies have evaluated the impact of individual strategies and not the simultaneous implementation of strategies such as NSP, OST, HCV/HIV screening, access to anti-HCV treatment, and antiviral therapy (ART), specifically in the enlarged EECA region [10–13].
The objective of this study was to evaluate the comparative costs, effectiveness, and cost-effectiveness of interventions among PWID in averting new HCV and HIV infections and HCV- and AIDS-related deaths in 5 countries in Eastern Europe and Central Asia: Belarus, Georgia, Kazakhstan, Republic of Moldova, and Tajikistan.
METHODS
Analytic Overview
We developed a dynamic compartmental model to simulate the probability of HIV and HCV transmission and the natural history of these diseases in PWID and non-PWID (NPWID) in the 5 countries studied. We estimated the impact of different strategies from 2013 to 2033. The strategies differed according to the level of single and combined interventions: the increase of NSP and OST coverage, HIV and HCV diagnosis, and access to HIV and HCV treatment. An additional scenario was simulated to evaluate a “what if” situation when the coverage of interventions would have been very limited or fallen back to very low levels. Supplementary Table 1 details the set of strategies considered and their direct impact on the outcomes.
Outcomes included projected cumulative number of new HCV and HIV infections among PWID and NPWID, total costs related to interventions and HCV and HIV care, years of life saved (YLS), and the incremental cost-effectiveness ratio (ICER) measured in 2013 USD per years of life saved ($/YLS). A strategy was labeled “cost-effective” if its ICER was less than 3 times the nation’s gross domestic product (GDP) per capita, as recommended by World Health Organization (WHO) [14]; “very cost-effective” if less than 1x GDP/capita; “cost-saving” if less expensive and more effective than the reference; and “dominated” if more expensive and less effective than the reference. Costs and YLS were discounted using a 0.03 per annum rate [14].
The Model Structure
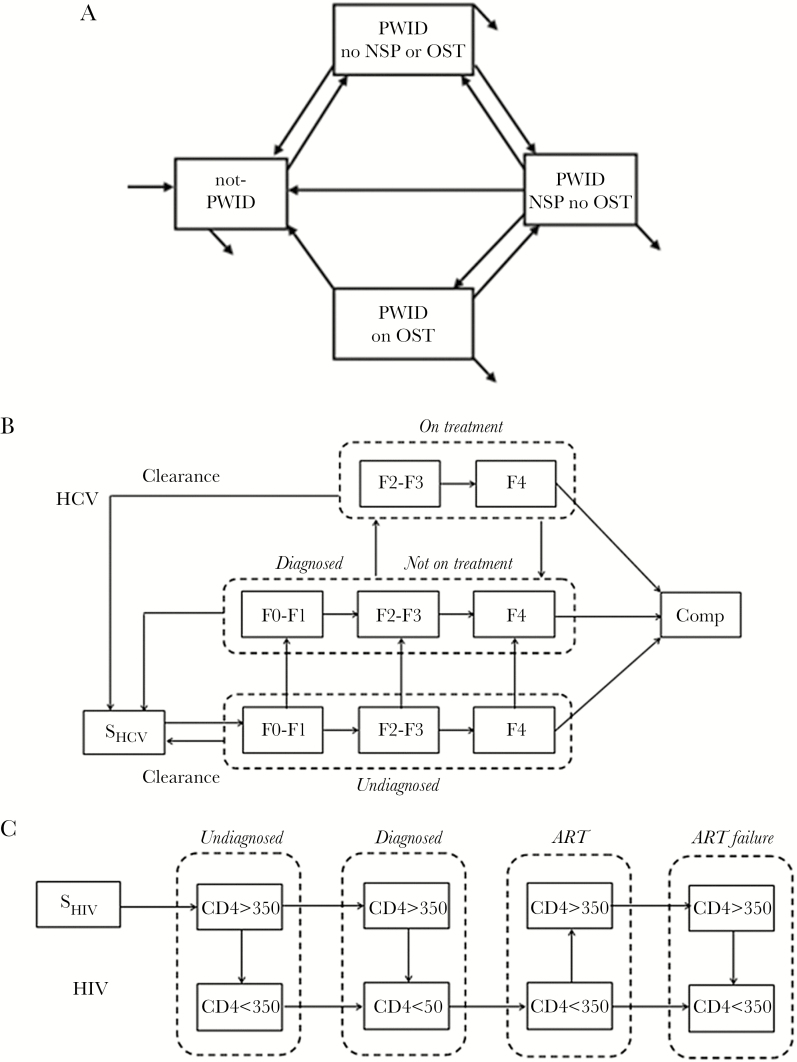
We simulated the population moving between injecting statuses: non-PWID; PWID using neither NSP nor OST; PWID using NSP but not OST; and PWID using OST. PWID moved to the NSP compartment if they received more than 100% of their injections (Figure 1) [15]. Individuals entered the model at age 15 years [16] and were neither PWID nor infected with HIV and HCV; they exited when they died or passed age 65 years.
Figure 1.
Compartmental model used for the analysis. The population was divided according to (A) the injecting status considering nonusers (NPWID), people who inject drugs (PWID) reached by needle-syringe programs (NSPs; no opioid substitution therapy [OST]), opioid substitution therapy (on OST), or none of them (no NSP or OST); (B) hepatitis C virus (HCV) status considering uninfected people (SHCV), the natural history of HCV infection (with fibrosis stages F0-F1, F2-F3, F4, and complications [Comp]), and the cascade of care (undiagnosed, diagnosed not in treatment, and in treatment); (C) HIV status considering uninfected people (SHIV), the natural history of HIV infection (according to the CD4 cell count: CD4 > 350 or CD4 < 350), and the cascade of care (undiagnosed, diagnosed, on antiretroviral therapy [ART], and ART failure). Every possible trajectory considered in the modeling is represented by the arrows between each compartment. Respective probabilities of death were also attributed to each compartment.
Both natural histories of HIV and HCV infection included “undiagnosed,” “diagnosed,” and “in treatment” states. Hepatitis C progressed through fibrosis stages aggregated into 3 compartments: F0-F1, F2-F3, and F4. When diagnosed, HCV treatment was initiated at F2 considering new direct acting antiviral (DAA) regimens. Patients could clear the virus spontaneously in stage F0-F1 or when treated, based on HCV treatment efficacy. Patients in stage F4 could develop complications such decompensated cirrhosis or hepatocellular carcinoma. For HIV, firstline treatments were initiated for diagnosed patients with a CD4 count below 350 cells/mm3, because in 2013 ART was still initiated according to this threshold. On efficacious treatment, patients moved into the CD4>350 category. Patients moved to the ART failure category when all available ART regimens failed considering a probability based on treatment regimens available in each country. The model allowed HIV/HCV co-infections.
Transmission
HIV and HCV transmission occurred though syringe sharing but differed according to NSP status, estimated to decrease by 50% the risk of transmission in PWID receiving 100% of their injection [15], and OST status, considered to reduce by 83% the number of injections per year [6]. The forces of infection were calculated according to baseline risks of transmission per contaminated syringe for HIV [17] and HCV [6], the annual number of injections per PWID, the total number of syringes distributed by NSP, the number of PWID reached by NSP and OST, and the number of PWID on ART and/or DAA.
HIV transmission also occurred through sexual contact, as well as HCV transmission, but at a very low risk. The forces of infection were calculated according to baseline risks of transmission per sexual act for HIV [17, 18] and HCV [19], the mean number of sexual intercourse acts per person, the condom use rate and its efficacy [12, 20], and the number of injecting drug partners, considering the affinity rate among PWID [9, 21]. We also considered the relative risk reduction (RRR) of sexual transmission for HIV-infected patients on ART [9, 12] and/or HCV-infected patients on DAA. HCV-infected patients with sustained viral response had a zero risk of HCV transmission.
Input Parameters
Input parameters were derived from the international literature and data collections initiated by teams in each country who provided country-specific parameters: HIV and HCV prevalence, injecting and sexual behavior, and intervention coverage, as well as costs of interventions and costs of HIV and HCV care per patient per year (Table 1). Because new DAAs were not yet available in the countries, we hypothesized that costs were equal to 2013 treatment costs.
Table 1.
Summary of Specific Parameters by Countries
| Parameters (2012–13) | Belarus | Georgia | Kazakhstan | Moldova | Tajikistan | Sourcesa |
|---|---|---|---|---|---|---|
| Population | ||||||
| Population aged 15–65 y | 6739080 | 3120600 | 11504430 | 2628642 | 4927304 | World Bank |
| Number of PWID | 75000 | 45000 | 116840 | 19400 | 25000 | UNAIDS |
| Number on OST | 908 | 2250 | 184 | 310 | 300 | UNAIDS |
| Number of syringes distributed | 2400000 | 1021870 | 23000000 | 1887578 | 4981270 | UNAIDS |
| HCV prevalence in general population | 1.5% | 6.7% | 3.1% | 4.9% | 4.0% | UNAIDS |
| HCV prevalence in PWID | 39.0% (9–39) | 51.0% | 56.6% | 47.2% | 25.0% | UNAIDS |
| % diagnosed | 14% (13.5–27) | 27% (13.5–27) | 14% (13.5–27) | 14% (13.5–27) | 14% (13.5–27) | UNAIDS |
| % on DAA | 0.001% | 0.001% | 0.001% | 0.001% | 0.001% | UNAIDS |
| HIV prevalence in general population | 0.35% | 0.30% | 0.20% | 0.30% | 0.52% | UNAIDS |
| HIV prevalence in PWID | 13.7% | 3.0% (3.0–3.45) | 8.0% | 14.7% | 13.5% | UNAIDS |
| % diagnosed | 66% (33–66) | 90% | 90% | 66% | 63% | UNAIDS |
| % of ART-eligible treated among HIV-diagnosed | 66% | 88% | 38% | 46% | 69% | UNAIDS |
| CD4 count at diagnosis in PWID | 361 | 212 | 415 | 254 | 231 | UNAIDS |
| % of HCV-infected among HIV-infected in PWID | 40% | 74% | 93% | 88% | 40% | UNAIDS |
| Behavior | ||||||
| Baseline risk of HIV transmission/contaminated syringe | 0.63% | 0.63% | 0.63% | 0.63% | 0.63% | [17] |
| Baseline risk of HCV transmission/contaminated syringe | 4.0% | 4.0% | 4.0% | 4.0% | 4.0% | [6] |
| Number of injections per y per PWID | 200 | 180 | 268 | 142 | 400 | UNAIDS |
| Sharing rate (baseline - without NSP) | 4.9% (4.9–9.8) | 6.7% (6.7–13>4) | 6.7% (6.7–13.4) | 8.1% (8.1–16.2) | 7.4% (7.4–14.8) | UNAIDS |
| RR of transmission if 100% NSP | 50% | 50% | 50% | 50% | 50% | [15] |
| RR of injections if OST | 17% (15–85) | 17% (15–85) | 17% (15–85) | 17% (15–85) | 17% (15–85) | Expert opinion [6] |
| Sexual behavior | ||||||
| Baseline risk of HIV transmission/sexual intercourse | 0.08% | 0.08% | 0.08% | 0.08% | 0.08% | [17, 18] |
| Baseline risk of HCV transmission/sexual intercourse | 0.01% | 0.01% | 0.01% | 0.01% | 0.01% | Assumption [19] |
| Affinity rate (among PWID) | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | [9, 21] |
| Costs (2013 USD)b | ||||||
| HIV-diagnosed with CD4 >350/y | 365 | 565 | 302 | 659 | 96 | UNAIDS |
| HIV-diagnosed with CD4 <350/y | 365 | 829 | 297 | 659 | 99 | UNAIDS |
| Average cost of ART/patient/y (1st line) | 192 | 324 | 1064 | 316 | 166 | UNAIDS |
| HCV-diagnosed with F2-F3/y | 55 | 127 | 179 | 0 | 14 | UNAIDS |
| HCV-diagnosed with F4/y | 62 | 498 | 1078 | 537 | 85 | UNAIDS |
| Average costs of complications (decomp. cirrhosis and HCC) | 3106 | 1628 | 5401 | 764 | 425 | UNAIDS |
| Average cost of DAA/patient/y (based on Peg+Riba) | 11424 (900–11424) | 23280 (900–23280) | 13105 (900–13105) | 12986 (900–12986) | 2372 (900–2372) | UNAIDS |
| Total cost of OST/patient/y | 594 | 1290 | 1216 | 879 | 540 | UNAIDS |
| Total cost of NSP/PWID/y | 114 | 79 | 59 | 73 | 110 | UNAIDS |
| GDP per capita (2013) | $7575 | $3602 | $13172 | $2230 | $1037 | World Bank |
Abbreviations: 100% NSP, at least 100% of the injections are covered by NSP; ART, antiretroviral therapy; DAA, direct active agent; F0-F4, fibrosis stages 0 to 4; GDP, gross domestic product; HCC, hepatocellular carcinoma; NSP, needle-syringe programs; OST, opioid substitution therapy; PWID, people who inject drugs; RR, relative risk.
aAll country-specific data were collected and validated by UNAIDS partners in each country.
bWhen data were missing or incomplete, we estimated total costs on the basis of data collected by other countries according to respective GDP per capita.
Sensitivity Analyses
Extensive sensitivity analyses were conducted to evaluate the impact of uncertainty surrounding key parameters to validate the robustness of results. We also evaluated the impact in terms of cost-effectiveness of “what if” scenarios regarding the cost of new DAAs and treatment initiation criteria.
An extended summary of the input parameters and more details on the model structure in costs are presented in the Supplementary Material.
RESULTS
Effectiveness
The impact on the number of new HCV infections averted (Figure 2A, B) compared with the current strategy was the highest, with the strategy increasing NSP, OST, HCV screening, and access to new DAAs (infections averted in PWID: 50% in Tajikistan to 70% in Republic of Moldova; NPWID: 3% in Tajikistan to 28% in Belarus). When considering strategies that increase intervention individually, access to new DAAs had a limited impact (infections averted in PWID: 1% in Kazakhstan to 15% in Republic of Moldova; in NPWID: 1% in Tajikistan to 8% in Belarus). The increase of NSP+OST had a high impact in particular in PWID (infections averted: 42% in Tajikistan to 55% in Republic of Moldova; in NPWID: from –3% in Tajikistan to 9% in Belarus).
Figure 2.
Percentage of new infections averted compared with the baseline over 20 years. A and B, The percentage of new HCV infections averted among people who inject drugs (PWID) and the general population (NPWID), respectively, when the coverage of interventions against hepatitis C virus (HCV) transmission is increased among PWID (including needle-syringe programs [NSPs], opioid substitution therapies [OSTs], access to new direct active agents [DAAs], and/or HCV screening). C and D, The percentage of new HIV infections averted among PWID and NPWID, respectively, when the coverage of interventions against HIV transmission is increased (including NSP, OST, access to antiretroviral therapy [ART], and/or HIV screening). aIn Georgia and Kazakhstan, HIV screening currently being ≥90% among PWID, the increase of the coverage was not included in the strategies tested.
The impact on the number of new HIV infections averted (Figure 2C, D) was the highest, with the strategy increasing NSP, OST, access to ART and HIV screening (infections averted in PWID: 35% in Georgia to 75% in Kazakhstan; NPWID: 1% in Georgia to 39% in Kazakhstan). Increasing NSP and OST alone had again a high impact on the reduction of new HIV infections in PWID (infections averted: 32% in Georgia to 62% in Kazakhstan; in NPWID: from 1% in Georgia to 20% in Kazakhstan). Strategies increasing access to ART and HIV screening in PWID also had an impact in NPWID (infections averted in PWID: 2% in Georgia to 40% in Republic of Moldova; in NPWID: from 0.5% in Georgia to 32% in Kazakhstan).
Cost-effectiveness
The most effective strategy over 20 years was to increase the coverage of all interventions, including NSP, OST, HCV, and HIV screening, and access to new DAAs and ARTs: Strategy 9 for HCV, from 26 000 YLS in Georgia to 210 090 in Kazakhstan; Strategy 11 for HIV, from 63 830 YLS in Belarus to 15 470 in Republic of Moldova and 33 080 in Tajikistan (Table 2). This full combined strategy was also the most expensive ($466 850 000 and $179 090 000 in Belarus and Tajikistan, respectively, over 20 years).
Table 2.
Cost-effectiveness Results Over 20 Years in Each Country
| Strategiesb | Undiscounted | 3% Discounted | ICERc ($/YLS) | ||||
|---|---|---|---|---|---|---|---|
| Total Costs (% Increase), $ | YLS (‰ Increase) | Total Costs, $ |
YLS | ||||
| Belarus (GDP per capita = $7575) | |||||||
| 5) ART, DIAG HIV (90% vs 66%) | 331820 k | (-5.0) | 12650 | (+0.1) | 249570 k | 7890 | Cost-savingd |
| 4) ART (90% vs 67%) | 340050 k | (-2.6) | 4440 | (+0.0) | 253730 k | 2770 | Cost-saving |
| 1) Baseline | 349220 k | (ref) | -- | (ref) | 259700 k | -- | -- |
| 0) Alternative scenario with 2005 coverages | 368880 k | (+5.6) | -21080 | (-0.2) | 269600 k | -13220 | Dominatede |
| 8) NSP, OST, ART, DIAG HIV | 371300 k | (+6.3) | 53560 | (+0.4) | 279810 k | 34900 | 210 |
| 7) NSP, OST, ART | 379000 k | (+8.5) | 45710 | (+0.3) | 283670 k | 30000 | Dominated |
| 3) NSP, OST (20% vs 1.2%) | 387500 k | (+11.0) | 41500 | (+0.3) | 289210 k | 27370 | Dominated |
| 2) NSP (40% vs 8% at baseline) | 390560 k | (+11.8) | 1600 | (+0.0) | 291220 k | 980 | Dominated |
| 6) ART, DIAG HIV, anti-HCV (50% vs 0.001%) | 383640 k | (+9.9) | 17350 | (+0.1) | 293640 k | 10910 | Dominated |
| 9) NSP, OST, ART, DIAG HIV, anti-HCV | 416790 k | (+19.3) | 58250 | (+0.4) | 319580 k | 37920 | Ext. dominf |
| 10) ART, DIAG HIV, anti-HCV, DIAG HCV (75% vs 27%) | 444900 k | (+27.4) | 23100 | (+0.2) | 348050 k | 14620 | Dominated |
| 11) NSP, OST, ART, DIAG HIV, anti-HCV, DIAG HCV | 466850 k | (+33.7) | 63830 | (+0.5) | 365790 k | 41540 | 12960 |
| Georgia (GDP per capita = $3602) | |||||||
| 5) ART (90% vs 88%), DIAG HIVa | 468600 k | (-0.1) | 160 | (+0.0) | 350180 k | 100 | Cost-saving |
| 1) Baseline | 469080 k | (ref) | -- | (ref) | 350490 k | -- | -- |
| 0) Alternative scenario with 2005 coverages | 484390 k | (+3.2) | -15730 | (-0.3) | 359000 k | -10080 | Dominated |
| 8) NSP, OST, ART, DIAG HIVa | 482520 k | (+2.8) | 17530 | (+0.3) | 361110 k | 11620 | 910 |
| 3) NSP, OST (20% vs 5%) | 482940 k | (+2.9) | 17390 | (+0.3) | 361380 k | 11530 | Dominated |
| 2) NSP (40% vs 6% at baseline) | 483920 k | (+3.1) | 630 | (+0.0) | 362020 k | 380 | Dominated |
| 6) ART, anti-HCV (50% vs 0.001%) | 553000 k | (+15.2) | 4020 | (+0.1) | 421710 k | 2580 | Dominated |
| 9) NSP, OST, ART, DIAG HIV,a anti-HCV | 557650 k | (+15.9) | 21400 | (+0.3) | 426360 k | 14100 | 26230 |
| 11) NSP, OST, ART, DIAG HIV,a anti-HCV, DIAG HCV | 647810 k | (+27.6) | 26000 | (+0.4) | 507910 k | 17080 | 27460 |
| 10) ART, DIAG HIV,a anti-HCV, DIAG HCV (75% vs 27%) | 663000 k | (+29.2) | 8740 | (+0.1) | 517430 k | 5620 | Dominated |
| Kazakhstan (GDP per capita = $13 172) | |||||||
| 8) NSP, OST, ART, DIAG HIVa | 1661940 k | (-6.9) | 185280 | (+0.8) | 1237040 k | 118680 | Cost-saving |
| 3) NSP, OST (20% vs 0.2%) | 1710180 k | (-3.9) | 160720 | (+0.7) | 1265370 k | 103610 | Cost-saving |
| 5) ART (90% vs 38%), DIAG HIVa | 1713180 k | (-3.7) | 32750 | (+0.1) | 1267570 k | 19940 | Cost-saving |
| 0) Alternative scenario with 2005 coverages | 1784870 k | (+0.4) | -31500 | (-0.1) | 1301450 k | -19320 | Cost-saving |
| 1) Baseline | 1777110 k | (ref) | -- | (ref) | 1304890 k | -- | -- |
| 2) NSP (60% vs 37% at baseline) | 1776030 k | (-0.1) | 7840 | (+0.0) | 1307680 k | 4720 | Ext. domin |
| 9) NSP, OST, ART, DIAG HIV,a anti-HCV | 1775600 k | (-0.1) | 196170 | (+0.9) | 1338410 k | 125680 | 270 |
| 6) ART, anti-HCV (50% vs 0.001%) | 1888810 k | (+5.9) | 43910 | (+0.2) | 1411650 k | 27110 | Dominated |
| 11) NSP, OST, ART, DIAG HIV,a anti-HCV, DIAG HCV | 2002450 k | (+11.3) | 210090 | (+0.9) | 1534280 k | 134640 | 21850 |
| 10) ART, DIAG HIV,a anti-HCV, DIAG HCV (75% vs 27%) | 2258080 k | (+21.3) | 58580 | (+0.3) | 1706340 k | 36520 | Dominated |
| Republic of Moldova (GDP per capita = $2230) | |||||||
| 5) ART, DIAG HIV (90% vs 66%) | 349310 k | (-8.0) | 7670 | (+0.1) | 256900 k | 4730 | Cost-saving |
| 8) NSP, OST, ART, DIAG HIV | 353240 k | (-6.8) | 21050 | (+0.4) | 260180 k | 13510 | Cost-saving |
| 4) ART (90% vs 46%) | 359910 k | (-4.8) | 4240 | (+0.1) | 263000 k | 2620 | Cost-saving |
| 7) NSP, OST, ART | 363160 k | (-3.9) | 17800 | (+0.3) | 265870 k | 11500 | Cost-saving |
| 6) ART, DIAG HIV, anti-HCV (50% vs 0.001%) | 367310 k | (-2.7) | 9050 | (+0.2) | 272220 k | 5630 | Cost-saving |
| 1) Baseline | 377210 k | (ref) | -- | (ref) | 273800 k | -- | -- |
| 9) NSP, OST, ART, DIAG HIV, anti-HCV | 369030 k | (-2.2) | 22430 | (+0.4) | 274000 k | 14400 | 10 |
| 3) NSP, OST (20% vs 1.6%) | 379220 k | (+0.5) | 13910 | (+0.3) | 275970 k | 9090 | Dominated |
| 2) NSP (40% vs 34% at baseline) | 382290 k | (+1.3) | 580 | (+0.0) | 277850 k | 350 | Dominated |
| 0) Alternative scenario with 2005 coverages | 398860 k | (+5.4) | -6220 | (-0.1) | 286920 k | -3860 | Dominated |
| 11) NSP, OST, ART, DIAG HIV, anti-HCV, DIAG HCV | 388590 k | (+2.9) | 24080 | (+0.5) | 291740 k | 15470 | 16600 |
| 10) ART, DIAG HIV, anti-HCV, DIAG HCV (75% vs 27%) | 390700 k | (+3.5) | 10740 | (+0.2) | 292760 k | 6720 | Dominated |
| Tajikistan (GDP per capita = $1037) | |||||||
| 0) Alternative scenario with 2005 coverages | 137130 k | (-16.1) | -12540 | (-0.1) | 96770 k | -7760 | Cost-saving |
| 4) ART (90% vs 69%) | 158660 k | (-0.4) | 2100 | (+0.0) | 113370 k | 1290 | Cost-saving |
| 5) ART, DIAG HIV (90% vs 63%) | 158700 k | (-0.3) | 6050 | (+0.1) | 113610 k | 3710 | Cost-saving |
| 1) Baseline | 159250 k | (ref) | -- | (ref) | 113680 k | -- | -- |
| 6) ART, DIAG HIV, anti-HCV (50% vs 0.001%) | 163790 k | (+2.8) | 7040 | (+0.1) | 117290 k | 4340 | Ext. domin |
| 7) NSP, OST, ART | 168020 k | (+5.2) | 26630 | (+0.3) | 120460 k | 17180 | Ext. domin |
| 8) NSP, OST, ART, DIAG HIV | 168110 k | (+5.3) | 30200 | (+0.3) | 120720 k | 19380 | 360 |
| 3) NSP, OST (20% vs 1.2%) | 168560 k | (+5.5) | 24900 | (+0.3) | 120760 k | 16120 | Dominated |
| 2) NSP (40% vs 25% at baseline) | 170490 k | (+6.6) | 1260 | (+0.0) | 122010 k | 760 | Dominated |
| 9) NSP, OST, ART, HIV DIAG, NEW DAA | 171660 k | (+7.2) | 31130 | (+0.3) | 123400 k | 19970 | 4550 |
| 10) ART, DIAG HIV, anti-HCV, DIAG HCV (75% vs 27%) | 175960 k | (+9.5) | 9220 | (+0.1) | 126300 k | 5710 | Dominated |
| 11) NSP, OST, ART, DIAG HIV, anti-HCV, DIAG HCV | 179090 k | (+11.1) | 33080 | (+0.3) | 129220 k | 21210 | 4690 |
Abbreviations: ART, antiretroviral therapy; DIAG, diagnosis; GDP, gross domestic product; NEW DAA, new direct active agent; NSP, needle-syringe program; OST, opioid substitution therapy; YLS, years of life saved.
aIn Georgia and Kazakhstan, HIV screening currently being ≥90% among PWID, the increase of the coverage was not included in these strategies.
bBaseline: current coverages, NSP, OST, ART, DIAG, NEW DAA, YLS, GDP.
cIncremental cost-effectiveness ratios in US dollar per years of life saved ($/YLS). Calculated from the 3% discounted outcomes; the comparator strategy is always the next smallest, not dominated, alternative.
dCost-saving: less expensive and more effective than the current strategy (baseline).
eDominated: less effective and more costly than some alternative strategies.
fExtendedly dominated: has an incremental cost-effectiveness ratio that is greater than that of a more effective strategy.
In all countries, increasing either ART alone (Strategy 4) or HIV screening and ART (Strategy 5) was cost-saving compared with the baseline strategy over 20 years. In Belarus and Tajikistan, in addition to increasing ART and HIV screening, NSP and OST coverage increase (Strategy 8) was very cost-effective over 20 years (ICER = $210 and $360/YLS). In Georgia, in addition to increasing ART, the increase of NSP and OST was very cost-effective compared with the baseline (Strategy 8: ICER = $910/YLS). In Kazakhstan and Republic of Moldova, increasing ART and HIV screening NSP and OST was cost-saving.
When considering the increase of all interventions, the combined strategy was cost-effective in Belarus (ICER = $12 960/YLS). The increase of NSP, OST, access to ART, HCV screening, and access to new DAAs was cost-effective in Kazakhstan (ICER = $21 850/YLS). The full combined strategies were not cost-effective in Republic of Moldova, Georgia, and Tajikistan.
Sensitivity Analyses
Regarding uncertainties concerning key parameters, results were robust to sensitivity analyses in each country when we varied inputs at baseline such as needle sharing rates, the proportion of HIV and HCV–diagnosed PWID, the efficacy of OST in terms of reduction of injections, and the percentage of PWID reached by 100% NSP.
For alternative scenarios, when considering the decrease of cost of new DAAs to $900 per therapy, the strategies combining the increase of all interventions including HCV screening and access to new DAAs (Strategy 11) became cost-effective in Tajikistan ($1890/YLS), very cost-effective in Belarus and Georgia ($540 and $2060/YLS), and cost-saving in Kazakhstan and Republic of Moldova compared with the baseline. Initiating treatment at fibrosis stage ≥F0 made the increase of all interventions (Strategy 11) cost-effective if costs of new DAAs remained below $7030 in Belarus; $1750 in Georgia; $7440 in Kazakhstan; $2820 in Republic of Moldova; and $300 in Tajikistan (Supplementary Figure 1), and increased the number of new HCV infections averted by 14% in Belarus to 38% in Tajikistan overall. When considering the eligibility of ART for everyone, whatever the CD4 at diagnosis, strategies increasing NSP, OST, HIV screening, and access to ART (Strategy 8) remained very cost-effective in Belarus, Georgia, Republic of Moldova, and Tajikistan ($1120, $1930, $360, and $660/YLS), and cost-saving in Kazakhstan, and they increased the number of new HIV infections averted by 8% in Kazakhstan to 51% in Georgia overall.
All results on sensitivity analyses are presented in Supplementary Tables 3–7.
DISCUSSION
This study illustrates that a drastic reduction in the incidence and prevalence of HCV and HIV epidemics in Georgia and Kazakhstan in the next 20 years cannot be achieved without an increase of combined intervention coverages among PWID, such as NSP, OST, HCV screening, and access to ART and new DAAs, as well as HIV screening in addition in Belarus, Republic of Moldova, and Tajikistan. However, these strategies were associated with high costs and unfavorable cost-effectiveness ratios in most of the countries studied, in particular Georgia, Republic of Moldova, and Tajikistan, because of the high cost of new DAAs. When considering a lower cost (based on the cost of new DAAs in Egypt in 2013), the full combined strategy would save the largest number of life years and would become very cost-effective in Belarus, Georgia, and Tajikistan, and even cost-saving in Kazakhstan and Republic of Moldova over 20 years compared with current coverages in 2012/13, although the overall costs of this strategy remained high. Moreover, it suggested that initiating new DAAs regardless of fibrosis stages would also avoid even larger numbers of transmission and large numbers of life-years and remain cost-effective in Belarus, Georgia, Kazakhstan, and Republic of Moldova by 2033. However, in all scenarios, the impact of increasing access to new DAAs would be significant only if associated with the increase of the percentage of diagnosed PWID.
Increases of NSP and OST in particular would have a high impact on HCV and HIV transmission, and their use is fundamental to the fight against the HCV and HIV epidemics. It would have been cost-effective if we did not consider other combinations of interventions. However, adding more interventions, although associated with higher intervention costs, was even more effective by decreasing the number of infections, and therefore decreased the overall costs. Consequently, when other interventions were added to the picture, NSP+OST alone, being less effective, was associated with higher overall costs and was dominated.
Considering the UNAIDS Fast Track 2020 objectives [22], we show that access to ART to 90% of diagnosed PWID and HIV screening increase to 90% of infected PWID would have a high impact on HIV transmissions and would save costs when considering a 20-year horizon compared with the current coverage of these strategies in 2012/13. Consequently, combined increases of NSP, OST, access to ART, and HIV screening among PWID would be effective, very favorable in terms of cost-effectiveness ratios, and would even save costs in Georgia and Kazakhstan compared with coverages in 2012/13. These findings corroborate results of previous studies performed in EECA countries, such as that OST and ART to high levels among PWID were effective and very cost-effective [9] and that strategies expanding NSP and HIV testing in addition were also cost-effective [8]. In 2012, a modeling study on HCV/HIV infection conducted in 8 EECA countries by Wilson DP et al. also demonstrated that the increase of NSP among PWID was very cost-effective [13].
In 2013, HIV-screened patients in the countries studied initiated ART when the CD4 count dropped below 350 cells/mm3, but new 2015 WHO guidelines now recommend that ART should be initiated regardless the CD4 cell count [23]. We have illustrated that it would avert HIV infections, save more years of life, and remain cost-effective when considering the increased access to ART combined with NSP, OST, and HIV screening compared with current strategies in Belarus, Georgia, Kazakhstan, and Republic of Moldova.
With regards to the general population, the strategies increasing interventions in PWID had a low impact on HCV transmission in non-PWID because of the very low risk of sexual transmission. However, the impact on HIV infection was more significant among non-PWID because the increased interventions among PWID reduced the risk of infection through unprotected sexual intercourse that PWID may have had with non-PWID.
Our study presents several limitations regarding uncertainty surrounding input data. First, regarding NSP, our model only considered PWID who had a full needle-syringe exchange; we assumed that this program has a significant impact on transmission only when it reaches high distribution levels [15]. Consequently, we needed to estimate the proportion of PWID reached by 100% NSP from the total proportion of PWID reached by NSP in different countries. We varied the value of this key parameter, and the results remained robust to this variation. Second, there was uncertainty regarding OST, and particularly its effectiveness in terms of reduction in the frequency of injections, but, again, the change in the effectiveness of OST did not affect the results. Moreover, we remained conservative because we could also consider that OST has an additional favorable impact on coverage of ART among HIV-infected PWID, as assessed in a recent meta-analysis by Low et al. [24]. Third, cost estimates were not fully available, in particular the costs of new DAAs. In the base case analysis, we used costs of past anti-HCV treatments available in countries at the time of our analysis. We decreased these costs in sensitivity analyses to evaluate the impact on the results of introducing DAAs at drastically lower cost. In addition to uncertainties concerning variables in this analysis, we used a simulation model that relies on multiple assumptions. However, extensive sensitivity analyses were conducted, and the results were robust to alternative assumptions. The infectivity of those treated for HCV infection was considered uniform across fibrosis stages and age. Such considerations would have made the model much more complex, and consequently would have had a negative impact on the validity of the model given that the population would have been even more compartmentalized, leading to more assumptions on parameters that are not really reported in the literature. We also assumed that disease progression did not change if an individual was HIV/HCV co-infected. This assumption may be correct only in treated HIV-infected patients with a high CD4 count, which was not always the case in the model. We didn’t account for specific reinfection rates after spontaneous clearance; individuals returned to susceptible states with the same probabilities of HCV infection and clearance [25]. Finally, this study was performed in 5 countries from a region where HIV and HCV epidemics are specifically important, in particular among PWID, and conclusions on cost-effectiveness of such combined interventions may not be extrapolated to other regions of the world.
This study illustrates that OST and NSP combined with other interventions, such as access to treatments and screening, would have a high impact in terms of survival benefits while containing costs, not only from the perspective of PWID, but the overall population in 5 representative countries of Eastern Europe and Central Asia.
Supplementary Material
Acknowledgments
We would like to thank in particular all partners from countries who worked on the data collection and provided a huge amount of information needed for the modeling study.
Financial support. This work was supported by the French National Agency for Research on AIDS and Viral Hepatitis (ANRS) and the Joint United Nations Programme on HIV/AIDS (UNAIDS) Regional Support Team for Eastern Europe and Central Asia.
Potential conflicts of interest. Y.Y. is a board member and reports receiving consultancy honoraria from Abbott, BMS, Gilead, MSD, Roche, Tibotec, and ViiV Healthcare and honoraria for development of educational presentations from Abbott, BMS, Gilead, Tibotec, and ViiV Healthcare. All other authors declare no competing interests.
References
- 1. UNAIDS. Gap report. 2014. Available at: http://www.unaids.org/en/resources/campaigns/2014/2014gapreport/gapreport. Accessed 14 March 2018. [Google Scholar]
- 2. UNAIDS. AIDSinfo Available at: http://aidsinfo.unaids.org/. Accessed 1 August 2017.
- 3. Global Commission on Drug Policy. The negative impact of the war on drugs on public health: The hidden hepatitis C epidemic. 2013. Available at: http://www.globalcommissionondrugs.org/reports/the-negative-impact-of-the-war-on-drugs-on-public-health-the-hidden-hepatitis-c-epidemic/. Accessed 14 March 2018. [Google Scholar]
- 4. Bouscaillou J, Champagnat J, Luhmann N et al. Hepatitis C among people who inject drugs in Tbilisi, Georgia: an urgent need for prevention and treatment. Int J Drug Policy 2014; 25:871–8. [DOI] [PubMed] [Google Scholar]
- 5. Eurasian Harm Reduction Network (EHRN). Quitting while not ahead: The Global Fund’s retrenchment and the looming crisis for harm reduction in Eastern Europe and Central Asia 2012. Available at: http://www.harm-reduction.org/sites/default/files/pdf/ehrn_quitting_while_not_ahead_final_july_12.pdf. Accessed 14 March 2018.
- 6. Bayoumi AM, Zaric GS. The cost-effectiveness of Vancouver’s supervised injection facility. CMAJ 2008; 179:1143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cipriano LE, Zaric GS, Holodniy M et al. Cost effectiveness of screening strategies for early identification of HIV and HCV infection in injection drug users. PLoS One 2012; 7:e45176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dutta A, Wirtz A, Stanciole A et al. The Global HIV Epidemics Among People Who Inject Drugs. Washington, DC: World Bank; 2013. [Google Scholar]
- 9. Alistar SS, Owens DK, Brandeau ML. Effectiveness and cost effectiveness of expanding harm reduction and antiretroviral therapy in a mixed HIV epidemic: a modeling analysis for Ukraine. PLoS Med 2011; 8:e1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vickerman P, Kumaranayake L, Balakireva O et al. The cost-effectiveness of expanding harm reduction activities for injecting drug users in Odessa, Ukraine. Sex Transm Dis 2006; 33:S89–102. [DOI] [PubMed] [Google Scholar]
- 11. Kumaranayake L, Vickerman P, Walker D et al. The cost-effectiveness of HIV preventive measures among injecting drug users in Svetlogorsk, Belarus. Addiction 2004; 99:1565–76. [DOI] [PubMed] [Google Scholar]
- 12. Long EF, Brandeau ML, Galvin CM et al. Effectiveness and cost-effectiveness of strategies to expand antiretroviral therapy in St. Petersburg, Russia. AIDS 2006; 20:2207–15. [DOI] [PubMed] [Google Scholar]
- 13. Wilson DP, Zhang L, Kerr CC et al. Needle-syringe programs are cost-effective in Eastern Europe and Central Asia: costing, data synthesis, modeling and economics for eight case study countries. UNAIDS 2012. Available at: http:// aids.md/aids/files/1258/Cost-effectiveness%20NSP%20manuscript%20(submission).docx. Accessed 14 March 2018. [Google Scholar]
- 14. World Health Organization. Making choices in health: WHO guide to cost-effectiveness analysis. World Health Organization; 2003. Available at: http://www.who.int/choice/publications/p_2003_generalised_cea.pdf. Accessed 14 March 2018. [Google Scholar]
- 15. Turner KM, Hutchinson S, Vickerman P et al. The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction 2011; 106:1978–88. [DOI] [PubMed] [Google Scholar]
- 16. Human Mortality Database Available at: http://www.mortality.org/. Accessed 13 August 2017.
- 17. Patel P, Borkowf CB, Brooks JT et al. Estimating per-act HIV transmission risk: a systematic review. AIDS 2014; 28:1509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lasry A, Sansom SL, Wolitski RJ et al. HIV sexual transmission risk among serodiscordant couples: assessing the effects of combining prevention strategies. AIDS 2014; 28:1521–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Terrault NA. Sexual activity as a risk factor for hepatitis C. Hepatology 2002; 36:S99–105. [DOI] [PubMed] [Google Scholar]
- 20. Pinkerton SD, Abramson PR. Effectiveness of condoms in preventing HIV transmission. Soc Sci Med 1997; 44:1303–12. [DOI] [PubMed] [Google Scholar]
- 21. Barcal K, Schumacher JE, Dumchev K, Moroz LV. A situational picture of HIV/AIDS and injection drug use in Vinnitsya, Ukraine. Harm Reduct J 2005; 2:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. UNAIDS. 90-90-90 - An ambitious treatment target to help end the AIDS epidemic. 2014. Available at: http://www.unaids.org/en/resources/ documents/2017/90-90-90. Accessed 14 March 2018. [Google Scholar]
- 23. World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV 2015: 78 Available at: http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/. Accessed 14 March 2018.
- 24. Low AJ, Mburu G, Welton NJ et al. Impact of opioid substitution therapy on antiretroviral therapy outcomes: a systematic review and meta-analysis. Clin Infect Dis 2016; 63:1094–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rapid Response Service. Hepatitis C Virus (HCV) Re-infection Rates Among People Who Use Drug. Toronto, ON, Canada: Ontario HIV Treatment Networ; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.