Abstract
Background
Penicillin allergy frequently impacts antibiotic choice. As beta-lactams are superior to vancomycin in treating methicillin-susceptible Staphylococcus aureus (MSSA) bacteremia, we examined the effect of reported penicillin allergy on clinical outcomes in patients with MSSA bacteremia.
Methods
In this retrospective cohort study of adults with MSSA bacteremia admitted to a large tertiary care hospital, outcomes were examined according to reported penicillin allergy. Primary outcomes included 30-day and 90-day mortality rates. Multivariable regression models were developed to quantify the effect of reported penicillin allergy on mortality while adjusting for potential confounders.
Results
From 2010 to 2015, 318 patients with MSSA bacteremia were identified. Reported penicillin allergy had no significant effect on adjusted 30-day mortality (odds ratio [OR], 0.73; 95% confidence interval [CI], 0.29–1.84; P = .51). Patients with reported penicillin allergy were more likely to receive vancomycin (38% vs 11%, P < .01), but a large number received cefazolin regardless of reported allergy (29 of 66, 44%). Mortality rates were highest among nonallergic patients receiving vancomycin (22.6% vs 7.4% for those receiving beta-lactams regardless of reported allergy, P < .01). In multivariable analysis, beta-lactam receipt was most strongly associated with survival (OR, 0.26; 95% CI, 0.12–0.54).
Conclusions
Reported penicillin allergy had no significant effect on 30- or 90-day mortality. Non-penicillin-allergic patients receiving vancomycin for treatment of MSSA bacteremia had the highest mortality rates overall. Receipt of a beta-lactam was the strongest predictor of survival. These results underscore the importance of correct classification of patients with penicillin allergy and appropriate treatment with a beta-lactam when tolerated.
Keywords: bacteremia, methicillin-susceptible Staphylococcus aureus, penicillin allergy
Staphylococcus aureus is a leading cause of bacteremia, infective endocarditis, and device-related infection [1]. Mortality rates for S. aureus bacteremia range from 10% to 50%, influenced in part by site of infection and choice of treatment [2, 3]. While vancomycin remains the preferred therapy for methicillin-resistant S. aureus (MRSA), beta-lactams are strongly associated with superior outcomes in methicillin-sensitive S. aureus (MSSA) bacteremia [4].
Up to 10% of inpatients report beta-lactam allergies, frequently limiting appropriate treatment options for MSSA bacteremia [5, 6]. While prior studies have evaluated the treatment of patients with a reported penicillin allergy and outcomes of various antibiotic regimens, to our knowledge none have examined the influence of a reported penicillin allergy on MSSA bacteremia outcomes directly. We hypothesized that reported penicillin allergy might adversely impact survival in MSSA bacteremia by influencing treatment. Therefore, we performed this retrospective cohort study to quantify the effect of reported penicillin allergy on relevant clinical outcomes in adults with MSSA bacteremia.
METHODS
We retrospectively identified all adults with MSSA bacteremia admitted to Duke University Hospital (Durham, NC) from January 1, 2010, to December 31, 2015. MSSA bacteremia was defined as 1 or more blood cultures positive for S. aureus that was susceptible to oxacillin per Clinical Laboratory and Standards Institute guidelines (minimum inhibitory concentration ≤ 2 µg/mL). We excluded patients with polymicrobial bacteremia, those who died before final culture and susceptibility results were available, and those who elected not to pursue treatment (eg, pursued hospice care or left against medical advice). Our study was approved by the Institutional Review Board of the Duke University Health System.
Data collected retrospectively included each patient’s demographic characteristics, sequential organ failure assessment (SOFA) score on the first day positive blood cultures were obtained, comorbid conditions, Charlson comorbidity index, reported drug allergies as of the day of admission, antibiotics received, duration of therapy, and serum vancomycin trough concentrations when available. Reported drug allergies were obtained directly from the electronic medical record, as recorded by the admitting physician, along with a description of the drug reaction when available. The source of infection was recorded from either an infectious disease consult note or the relevant discharge summary. Of note, our hospital implemented a policy for mandatory infectious diseases consultation for S. aureus bacteremia during the latter half of the study period, 2013–2016.
We analyzed 2 factors for association with clinical outcomes. Reported penicillin allergy was obtained from each patient’s list of drug allergies documented within their admission history and physical. Definitive antibiotic choice was defined as the antibiotic prescribed once susceptibility results were known. Patients who expired before antibiotic susceptibility results were available were excluded from analysis. This was done to minimize the risk of bias toward worse outcomes with vancomycin given that nearly all patients received vancomycin as part of initial empiric therapy. Sensitivity analysis showed no significant effect upon including or excluding these patients (data not shown).
The primary end points included death at 30 days and death at 90 days. Secondary outcomes included time to clearance of blood cultures (defined as the number of days from first positive to first confirmed negative blood cultures), recurrence of bacteremia by 90 days, and incidence of adverse drug events. We used standard descriptive statistics. Comparisons between categorical variables were conducted using either the Fisher exact or χ2 where appropriate. Comparisons between continuous variables were conducted using t tests. All tests were 2-sided; P values were considered significant if <.05. Outcomes of interest were examined first according to reported penicillin allergy, as this was our primary question. We next analyzed the data according to treatment choice as we hypothesized that reported penicillin allergy might influence outcomes indirectly by altering treatment choice.
Mortality outcomes were also evaluated using multivariable logistic regression. We constructed a stepwise regression model to adjust for confounders. All variables with P < .2 were included initially, with backwards elimination to remove confounders with a <10% effect on mortality. We did not include receipt of a beta-lactam in the multivariate model estimating the effect of reported penicillin allergy, as we considered this factor a potential mediator of the effect of penicillin allergy on mortality. We used the statistical platform R version 3.4.1 (available at https://www.r-project.org/) for all analyses.
RESULTS
Six hundred eighty-seven patients had S. aureus bacteremia during the study period. Exclusion of those with methicillin-resistant Staphylococcus aureus (MRSA) infection, polymicrobial infection, or those choosing not to pursue treatment left 335 patients. Of these 335 patients with MSSA bacteremia, 17 expired before susceptibility results were known and before definitive therapy could be chosen. These 17 were excluded from analysis, leaving 318 subjects with MSSA bacteremia in our final cohort. The majority of patients were male (n = 203, 64%), and the mean age was 58 (±16) years. The majority of patients received a beta-lactam antibiotic (n = 243, 76%). A large number of patients reported a penicillin allergy (n = 66, 21%). Among patients reporting penicillin allergy, the most common reactions reported were rash (n = 19, 27%) and hives (n = 15, 21%). A complete description of the reaction was not available for 15 (21%) cases. Reported penicillin reactions are summarized in Table 1. Twenty-nine (44%) reportedly penicillin-allergic patients still received cefazolin. Adverse drug events were too infrequent for analysis. Only a single incidence of suspected drug rash was noted in a patient with reported penicillin allergy who received a beta-lactam, along with 2 episodes of acute renal injury attributed to antibiotics. No episodes of anaphylaxis were noted.
Table 1.
Reported Reactions to Penicillin
| Reported Reaction to Penicillin | No. (%) |
|---|---|
| Rash | 19 (27) |
| Hives | 15 (21) |
| Swelling/angioedema | 8 (11) |
| Anaphylaxis | 5 (7) |
| Interstitial nephritis | 5 (7) |
| Dyspnea | 3 (4) |
| Gastro-intestinal upset | 1 (1) |
| Unspecified | 15 (21) |
The group with reported penicillin allergy was different than the nonallergic group by sex and some comorbidities (Table 2), but the 2 groups were similar in terms of overall Charlson comorbidity index and sequential organ failure assessment (SOFA) scores. There was a trend toward higher frequency of catheter infections in non-penicillin-allergic patients and a higher frequency of endovascular or soft tissue infection in penicillin-allergic patients (Table 3), though only the latter 2 reached statistical significance. Mortality rates were not significantly different between penicillin-allergic and non-penicillin-allergic patients at 30 days (10.6% vs 11.9%, P = .99) or 90 days (13.6% vs 18.3%, P = .47) in univariate analysis. Penicillin-allergic patients were slower to clear blood cultures (6.2 days vs 4.1 days, P < .01) and more likely to suffer recurrence within 90 days (7% vs 1.5%, P = .02).
Table 2.
Demographics and Comorbidities for 318 Patients With MSSA Bacteremia According to Penicillin Allergy
| Characteristics | Allergy History | P | |
|---|---|---|---|
| Nonallergic (n = 252), No. (%) |
Penicillin-Allergic (n = 66), No. (%) |
||
| Demographics | |||
| Age, mean (SD), y | 56.6 (15) | 60.9 (18) | .05 |
| Male | 169 (67) | 34 (51) | .02 |
| Comorbidities | |||
| Myocardial infarction | 56 (22) | 24 (36) | .03 |
| Heart failure | 101 (40) | 38 (58) | .01 |
| Vascular disease | 51 (20) | 27 (41) | <.01 |
| Cerebrovascular disease | 80 (32) | 34 (52) | <.01 |
| Dementia | 9 (3) | 2 (3) | .99 |
| Pulmonary | 90 (36) | 27 (41) | .47 |
| Connective tissue disease | 30 (12) | 9 (14) | .68 |
| Peptic ulcer disease | 27 (11) | 10 (15) | .38 |
| Liver disease | 25 (10) | 5 (8) | .64 |
| Diabetes | 128 (51) | 40 (60) | .17 |
| Diabetes with complications | 71 (28) | 24 (36) | .22 |
| Paraplegia | 15 (6) | 2 (3) | .54 |
| Renal disease | 133 (53) | 46 (69) | .02 |
| Cancer | 77 (31) | 12 (18) | .05 |
| Metastatic cancer | 39 (16) | 4 (6) | .07 |
| Severe liver disease | 24 (10) | 2 (3) | .13 |
| HIV | 7 (3) | 1 (2) | .99 |
| Charlson comorbidity index, mean (SD) | 5.6 (3) | 6.2 (3) | .23 |
| SOFA score, mean (SD) | 3.1 (3) | 3.3 (3) | .58 |
Abbreviation: MSSA, methicillin-susceptible Staphylococcus aureus.
Table 3.
Site of Infection, Treatment, and Outcomes for 318 Patients With MSSA Bacteremia According to Penicillin Allergy
| Characteristics | Allergy History | P | |
|---|---|---|---|
| Nonallergic (n = 252), No. (%) |
Penicillin-Allergic (n = 66), No. (%) |
||
| Site of infection | |||
| Catheter | 41 (16) | 5 (8) | .08 |
| Pneumonia | 18 (7) | 4 (6) | .99 |
| Endovascular | 47 (19) | 21 (32) | .03 |
| Osteoarticular | 43 (17) | 11 (17) | .99 |
| Soft tissue | 50 (20) | 21 (31) | .05 |
| Unknown | 63 (25) | 14 (21) | .62 |
| Treatment | |||
| Vancomycin | 28 (11) | 25 (38) | <.01 |
| Cefazolin | 98 (39) | 29 (44) | .58 |
| Nafcillin | 113 (45) | 3 (5) | <.01 |
| Other | 13 (5) | 9 (14) | .04 |
| Vancomycin trough, mean (SD) | 16.1 (7) | 16 (6) | .97 |
| Duration, mean (SD), wk | 4.3 (2) | 4.7 (2) | .25 |
| ID consultation | 146 (58) | 49 (74) | .02 |
| Outcomes | |||
| Time to clearance, mean (SD), d | 4.1 (4) | 6.2 (6) | <0.01 |
| 30-d mortality (%) | 30 (11.9) | 7 (10.6) | .99 |
| 90-d mortality (%) | 46 (18.3) | 9 (13.6) | .47 |
| 90-d recurrence (%) | 4 (1.5) | 5 (7.0) | .02 |
Abbreviation: MSSA, methicillin-susceptible Staphylococcus aureus.
When analyzed according to choice of definitive treatment, patients receiving vancomycin were more likely to have penicillin allergy, underlying diabetes, or renal disease, and they carried slightly higher mean SOFA scores (3.7 vs 2.9, P = .04) and Charlson comorbidity indices (6.6 vs 5.5, P = .03) (Table 4). Compared with patients treated with beta-lactam antibiotics, patients treated with vancomycin had higher mortality rates at 30 days (22.6% vs 7.4%, P = .002) and 90 days (26.4% vs 13.6%, P = .036) on univariate analysis. Patients who received beta-lactam antibiotics were more likely to have had an infectious diseases (ID) consultation than those receiving vancomycin, though the difference was not statistically significant (65% vs 53%, P = .12). Mortality rates were lower overall for patients receiving an ID consultation (8.7% vs 16.3% at 30 days, P = .04; 13.9% vs 22.8% at 90 days, P = .05). The initiation of mandatory ID consultation did not impact this finding (data not shown).
Table 4.
Demographics, Clinical Features, Site of Infection, and Outcomes for 296 Patients With MSSA Bacteremia Treated With Either Vancomycin or a Beta-Lactam
| Characteristics | Definitive Antibiotic Choice | P | |
|---|---|---|---|
| Vancomycin (n = 53), No. (%) |
Beta-lactam (n = 243), No. (%) |
||
| Demographics | |||
| Age, mean (SD), y | 55.5 (17) | 58.0 (16) | .35 |
| Male | 31 (58) | 161 (66) | .34 |
| SOFA, mean (SD) | 3.7 (3.1) | 2.9 (2.5) | .04 |
| CCmI, mean (SD) | 6.6 (3) | 5.5 (3) | .03 |
| Penicillin allergic | 25 (47) | 32 (13) | <.01 |
| Site of infection | |||
| Catheter | 9 (17) | 35 (14) | .67 |
| Pneumonia | 6 (11) | 15 (6) | .23 |
| Endovascular | 12 (23) | 53 (22) | .86 |
| Osteoarticular | 4 (8) | 49 (20) | .03 |
| Soft tissue | 11 (21) | 55 (23) | .86 |
| Unknown | 14 (26) | 53 (22) | .47 |
| Treatment | |||
| Vancomycin trough, mean (SD) | 18.0 (6.1) | 15.3 (6.6) | .10 |
| Duration, mean (SD), wk | 4.3 (1.8) | 4.5 (1.8) | .57 |
| ID consultation | 28 (53) | 159 (65) | .12 |
| Outcomes | |||
| Time to clearance, mean (SD), d | 5.0 (5.3) | 4.4 (4.2) | .47 |
| 30-d mortality (%) | 12 (22.6) | 18 (7.4) | <.01 |
| 90-d mortality (%) | 14 (26.4) | 33 (13.6) | .04 |
| 90-d recurrence (%) | 5 (9.4) | 3 (1.2) | <.01 |
Abbreviation: CCmI, Charlson co-morbidity index; MSSA, methicillin-susceptible Staphylococcus aureus; SOFA, sequential organ failure assessment.
We next created a multivariable logistic regression model to quantify the effect of reported penicillin allergy on mortality outcomes while accounting for potential confounders. Our initial models included age >65 years, sex, SOFA score, cancer, heart failure, kidney disease, skin/soft tissue source of infection, osteoarticular source of infection, penicillin allergy, and ID consultation. Both the 30- and 90-day models achieved c-statistics >0.8. Severity of illness represented by the SOFA score was the strongest independent predictor of 30-day mortality in our adjusted model (Table 5). The following factors were independently associated with 90-day mortality in our adjusted model: age >65 years, SOFA score, and congestive heart failure. Reported penicillin allergy had no significant effect on mortality at 30 or 90 days.
Table 5.
Logistic Regression Model to Quantify Effect of Penicillin Allergy on 30-Day and 90-Day Mortality Rates in Patients With MSSA Bacteremia While Adjusting for Confounders
| Variable | Odds Ratio (95% CI) | P Value |
|---|---|---|
| 30-d mortality model | ||
| Penicillin allergy | 0.73 (0.29–1.84) | .51 |
| SOFA score | 1.32 (1.17–1.49) | <.01 |
| Age > 65 y | 1.91 (0.91–3.98) | .09 |
| 90-d mortality model | ||
| Penicillin allergy | 0.60 (0.15–1.40) | .23 |
| SOFA score | 1.32 (1.17–1.48) | <.01 |
| Age > 65 y | 1.94 (1.01–3.78) | .05 |
| CHF | 1.96 (1.03–3.75) | .04 |
| Cancer | 2.28 (1.15–4.50) | .02 |
Abbreviations: CHF, congestive heart failure; CI, confidence interval; MSSA, methicillin-susceptible Staphylococcus aureus; SOFA, sequential organ failure assessment.
Considering that a significant proportion of patients with reported penicillin allergy still received cefazolin (44%, n = 29), we recreated the multivariable models to assess the effect of receiving a beta-lactam antibiotic on 30- and 90-day mortality (Table 6). Receipt of a beta-lactam was strongly protective against mortality at 30 and 90 days.
Table 6.
Logistic Regression Model to Quantify Effect of Beta-Lactam Receipt on 30-Day and 90-Day Mortality Rates in Patients With MSSA Bacteremia While Adjusting for Confounders
| Variable | Odds Ratio (95% CI) | P Value |
|---|---|---|
| 30-d mortality model | ||
| Beta-lactam | 0.26 (0.12–0.54) | <.01 |
| SOFA score | 1.30 (1.14–1.47) | <.01 |
| Age > 65 y | 2.03 (0.96–4.30) | .06 |
| 90-d mortality model | ||
| Beta-lactam | 0.41 (0.21–0.81) | .01 |
| SOFA score | 1.30 (1.16–1.46) | <.01 |
| Age > 65 y | 1.88 (0.97–3.64) | .06 |
| CHF | 1.94 (1.01–3.72) | .05 |
| Cancer | 2.48 (1.26–4.90) | .01 |
Abbreviations: CHF, congestive heart failure; CI, confidence interval; MSSA, methicillin-susceptible Staphylococcus aureus; SOFA, sequential organ failure assessment.
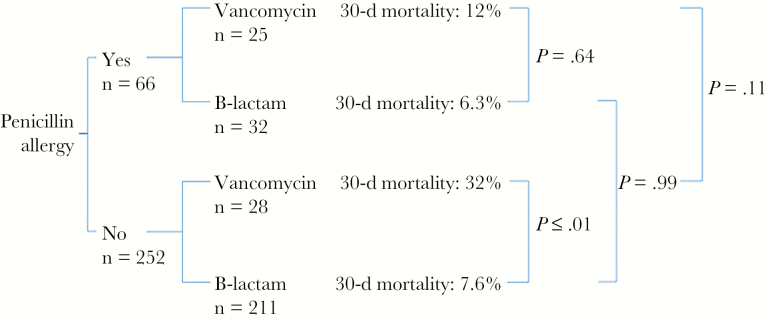
Thirty-day mortality outcomes were stratified according to both penicillin allergy and definitive antibiotic choice in Figure 1. No significant difference was noted in univariate analysis comparing mortality rates for penicillin-allergic or non-penicillin-allergic patients based on the definitive treatment received. We conducted additional analysis of these subgroups to test for possible interaction between reported penicillin allergy and receipt of a beta-lactam (Table 7). As predicted in our model, receipt of a beta-lactam was protective irrespective of penicillin allergy whereas vancomycin use was associated with higher rates of 30-day mortality. An interaction term was not significant in our final model but allowed us to calculate adjusted odds ratios for these subgroups. The single largest predictor of mortality was receipt of vancomycin among non-penicillin-allergic patients (odds ratio, 4.6; 95% confidence interval, 2.16–9.84; P < .001 compared with the subgroup who received a beta-lactam and had no penicillin allergy).
Figure 1.
Comparison of 30-day mortality rates for patients with methicillin-susceptible Staphylococcus aureus bacteremia according to penicillin allergy and definitive treatment. Note that some patients were treated with neither vancomycin nor a beta-lactam (eg, some received daptomycin, ceftaroline, linezolid and are not shown).
Table 7.
Assessment for Interaction Between Penicillin Allergy and Receipt of a Beta-Lactam With Regards to 30-Day Mortality
| Subgroups | Adjusted Odds Ratio (95% CI) | P Value |
|---|---|---|
| Nonallergic, treated with beta-lactam | 1.0 | NA |
| Allergic, treated with beta-lactam | 0.36 (0.09–1.41) | .09 |
| Nonallergic, treated with vancomycin | 4.61 (2.16–9.84) | <.01 |
| Allergic, treated with vancomycin | 2.19 (0.88–5.46) | .14 |
Abbreviation: CI, confidence interval.
Recognizing the known limitations of patient-reported drug allergy and the high prevalence of reported penicillin allergy (21%) in our cohort, we conducted a sensitivity analysis using a more stringent definition of penicillin allergy to assure against misclassification bias. According to the more stringent definition, only patients reporting anaphylaxis, hives, dyspnea, or angioedema/swelling were considered allergic. With the more stringent definition, incidence of penicillin allergy in our cohort fell to 9.2%—in agreement with previously reported prevalence rates [5, 6]. On sensitivity analysis, our modeled effect measures remained unchanged (data not shown).
We conducted a post hoc comparison between nafcillin and cefazolin given prior hypothetical concerns for inferior outcomes with cefazolin in deep-seated infections (Table 8) [7, 8]. Despite patients receiving cefazolin having higher SOFA scores (3.5 vs 2.2, P ≤ .01) and higher Charlson comorbidity indices (6.5 vs 4.5, P ≤ .01), we found no significant difference in 30-day (8.6% for nafcillin vs 6.3% for cefazolin, P = .63) or 90-day (12.9% vs 14.2%, P = .85) mortality rates. A higher incidence of renal disease in patients receiving cefazolin (65% vs 42%, P < .01) may explain the trend toward higher SOFA and Charlson comorbidity indices, as renal failure is a component of both scores.
Table 8.
Demographics, Clinical Features, and Outcomes for 243 Patients With MSSA Bacteremia Receiving Either Cefazolin or Nafcillin
| Characteristics | Definitive Antibiotic Choice | P Value | |
|---|---|---|---|
| Cefazolin (n = 127), No. (%) |
Nafcillin (n = 116), No. (%) |
||
| Demographics | |||
| Age, mean (SD), y | 59.3 (15) | 56.5 (16) | .18 |
| Male | 82 (65) | 79 (68) | .59 |
| SOFA, mean (SD) | 3.5 (2.6) | 2.2 (2.3) | <.01 |
| CCmI, mean (SD) | 6.5 (3) | 4.5 (3) | <.01 |
| Site of infection | |||
| Catheter | 24 (19) | 11 (10) | .04 |
| Pneumonia | 9 (7) | 6 (5) | .60 |
| Endovascular | 34 (27) | 19 (16) | .06 |
| Osteoarticular | 21 (17) | 28 (24) | .15 |
| Soft tissue | 24 (19) | 31 (27) | .17 |
| Unknown | 25 (20) | 28 (24) | .44 |
| Outcomes | |||
| Time to clearance, mean (SD) | 4.5 (5) | 4.2 (3) | .55 |
| 30-d mortality (%) | 8 (6.3) | 10 (8.6) | .63 |
| 90-d mortality (%) | 18 (14.2) | 15 (12.9) | .85 |
Abbreviations: CCmI, Charlson co-morbidity index; SOFA, sequential organ failure assessment.
DISCUSSION
While prior studies have assessed the effects of different antibiotics on outcomes in MSSA bacteremia, few have examined the effect of reported penicillin allergy [7]. In this retrospective cohort study, reported penicillin allergy did not significantly impact 30- or 90-day mortality rates. While the overall prevalence of reported penicillin allergy was higher than previously reported, many of these reactions were either undocumented or involved a nonspecific rash. Nearly half of penicillin-allergic patients were still safely treated with cefazolin—consistent with either over-reporting of allergy or the low incidence rate for cross-reactivity with cefazolin in particular [8]. Patients receiving beta-lactams consistently had the lowest mortality rates (7.4%), regardless of reported allergy history, even after we applied a more stringent allergy definition. This suggests that reported penicillin allergy was not an accurate marker for clinically relevant reactions concerning enough to avoid cephalosporins. The most striking mortality gap is the 4-fold greater mortality rate for nonallergic patients receiving vancomycin instead of a beta-lactam. Multivariable modeling also confirmed receipt of a beta-lactam as the single strongest predictor of survival, regardless of reported allergy history or adjustment for more stringent criteria defining penicillin allergy. These data highlight 2 crucial points for improving outcomes in MSSA bacteremia. First, a reported penicillin allergy only matters if it prevents a patient from receiving a beta-lactam. Second, while clearly over-reported, penicillin allergy is not the only reason that patients are missing out on optimal therapy.
The superiority of beta-lactams over vancomycin in treating MSSA bacteremia is consistent with several prior studies [9–19]. In our study, vancomycin use was associated with a 3-fold higher mortality rate at 30 days (P = .002) and a 2-fold higher mortality rate at 90 days (P = .036). Others have separately examined the influence of reported penicillin allergy on treatment choice—concluding that penicillin allergy was associated with an increased likelihood of receiving vancomycin by nearly 3-fold, in close agreement with the 3.3-fold increase we found [7].
With such a stark difference in mortality rates, and clear evidence that patients with a reported penicillin allergy can still achieve superior outcomes if they receive another beta-lactam, our data suggest that every effort should be made to ensure that patients with MSSA bacteremia receive a beta-lactam. These efforts take several forms. Simple interventions such as an enhanced drug allergy history may reveal that patients can safely tolerate cephalosporins, as was the case for 44% of our penicillin-allergic cohort. In prior studies of patients with reported allergies, nearly 50% of patients could be transitioned to a beta-lactam based on more detailed drug history alone [20, 21]. When uncertainty persists, penicillin skin testing can be used to determine whether penicillin antibiotics can be given safely [22–28]. Formal testing confirms 5% or less of reported penicillin allergies—permitting still more patients to receive preferred therapy [5]. While all of these interventions are being actively applied and studied presently (including at our own institution), the question of how to manage the remaining patients with true penicillin allergy remains. A 2- to 3-fold increase in mortality seems unacceptable for second-line therapy of a treatable disease (in this case, use of vancomycin). It may be time to consider desensitization as a preferred next step in the management of MSSA bacteremia for the truly allergic patients. Certainly the precedent exists when other medications are felt to be crucial to outcomes (eg, penicillin desensitization for neuro-syphilis or aspirin desensitization for coronary artery disease). As far as we are aware, no studies have examined this strategy to date.
Until desensitization can be fully evaluated as a treatment for MSSA bacteremia in penicillin-allergic patients, cefazolin often becomes the default beta-lactam in the subset of patients allergic to penicillin but tolerant of cephalosporins. The use of cefazolin carries with it some concerns based on a few case reports describing treatment failures, particularly in deep-seated MSSA infections, theoretically attributed to an inoculum effect [29, 30]. To add to the body of evidence addressing these concerns, we also conducted a post hoc subanalysis comparing outcomes between nafcillin and cefazolin and found no significant difference in 30- or 90-day mortality rates, despite patients receiving cefazolin having higher mean SOFA and Charlson comorbidity scores. Cefazolin’s ability to achieve similar outcomes despite predictors usually associated with higher mortality is both reassuring and consistent with prior evidence [13, 14]. Indeed, the recently published literature suggests that outcomes with cefazolin are comparable to outcomes with nafcillin but with fewer adverse effects [31].
Outside of the issues surrounding treatment of penicillin-allergic patients, the remaining nonallergic patients who received vancomycin (nearly 10% in our study) pose a distinct challenge worthy of further study as well. Vancomycin might be chosen for a variety of other reasons—including persistent knowledge gaps among providers regarding vancomycin’s efficacy in MSSA bacteremia, concern for alternate sources of infection or other pathogens, or the impression of convenient dosing (eg, infrequent dosing or dosing with dialysis). Investigating the reasons for vancomycin use other than true drug allergy might help target quality improvement or antimicrobial stewardship efforts and prevent the use of a less efficacious drug in this population as well.
Our study carries several limitations. First, penicillin allergy was determined based on documentation in the history and physical, which has known problems with measurement error [32, 33]. While the prevalence of penicillin allergy was high, a significant proportion of reactions were either unspecified or nonspecific—consistent with likely over-reporting of penicillin allergy. We conducted a sensitivity analysis using a more stringent allergy definition to assess for misclassification bias, with no alteration in any of our primary effect measures. Irrespective of accuracy, reported drug allergy remains clinically relevant given that treatment decisions are typically made based on the treating physician’s assessment. Second, we did not have sufficient numbers to adequately assess adverse drug events. Third, any comparisons with vancomycin must be considered with the caveat that vancomycin troughs were not available for many patients, particularly after discharge, and thus we are unable to assure therapeutic levels for the duration of treatment. Finally, given the diversity of infections included, we were unable to include an assessment of source control as another factor potentially influencing mortality. We hope to incorporate these additional factors in our ongoing and future research.
In summary, reported penicillin drug allergy did not equate with increased mortality in MSSA bacteremia in our cohort. We believe this is because nearly half of the labeled “allergic” group still received a beta-lactam. Receipt of a beta-lactam, including the use of cefazolin in many patients with reported penicillin allergy, was the single strongest predictor of survival. Conversely, patients receiving vancomycin—whether due to a drug allergy history or other reasons—suffered 2- to 3-fold higher mortality rates. In particular, patients who received vancomycin without a drug allergy had the highest mortality risk. Further investigation is needed to understand why some nonallergic patients still receive vancomycin. These data highlight 2 crucial points for improving outcomes in MSSA bacteremia. First, penicillin allergy only matters if it prevents a patient from receiving a beta-lactam. Second, penicillin allergy is not the only reason that patients are missing out on optimal therapy. Future efforts should include improvement of allergy assessment and documentation, de-labeling patients based on reactions unconcerning for true allergy, and perhaps even desensitization for truly penicillin-allergic patients.
Acknowledgments
Financial support. This work was supported by the Duke University Stead Research Grant. Nicholas Turner, MD, is supported by a fellowship grant from the Antibiotic Resistance Leadership Group.
Potential conflicts of interest. Dr. Drew reports royalties from UpToDate, honorarium from the American Society of Microbiology, honorarium from Cidara, and honorarium from CustomID outside the submitted work. Dr. Fowler reports grants from National Institutes of Health (NIH), Centers for Disease Control and Prevention (CDC), MedImmune, Cerexa/Forest/Actavis/Allergan, Pfizer, Advanced Liquid Logics, Theravance, Novartis, Cubist/Merck, Medical Biosurfaces, Locus, Affinergy, Contrafect, Karius, Genentech; consultancies with Pfizer, Novartis, Galderma, Novadigm, Durata, Debiopharm, Genentech, Achaogen, Affinium, Medicines Co., Cerexa, Tetraphase, Trius, MedImmune, Bayer, Theravance, Cubist, Basilea, Affinergy, Janssen, xBiotech; contrafect personal fees from Green Cross, Cubist, Cerexa, Durata, Theravance, Debiopharm; and royalties from UpToDate; and has a patent Sepsis Diagnostics pending. Dr. Anderson reports grants from the CDC during the conduct of the study; grants from NIH/NIAID Antimicrobial Resistance Leadership Group and personal fees from UpToDate outside the submitted work. Dr. Moehring reports grants or grants pending from the CDC and AHRQ and royalties from UpToDate outside the submitted work. Drs Cunningham Sarubbi and Wrenn report no conflicts of interest. Dr. Turner reports no additional conflicts of interest beyond the Antibiotic Resistance Leadership Group and internal grants disclosed in the funding above. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Tong SY, Davis JS, Eichenberger E et al. . Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015; 28:603–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Rosa FG, Corcione S, Motta I et al. . Risk factors for mortality in patients with Staphylococcus aureus bloodstream infection. J Chemother 2016; 28:187–90. [DOI] [PubMed] [Google Scholar]
- 3. Braquet P, Alla F, Cornu C et al. ; VIRSTA-AEPEI study group Factors associated with 12 week case-fatality in Staphylococcus aureus bacteraemia: a prospective cohort study. Clin Microbiol Infect 2016; 22:948.e1–7. [DOI] [PubMed] [Google Scholar]
- 4. Wong D, Wong T, Romney M, Leung V. Comparative effectiveness of β-lactam versus vancomycin empiric therapy in patients with methicillin-susceptible Staphylococcus aureus (MSSA) bacteremia. Ann Clin Microbiol Antimicrob 2016; 15:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Macy E. Penicillin and beta-lactam allergy: epidemiology and diagnosis. Curr Allergy Asthma Rep 2014; 14:476. [DOI] [PubMed] [Google Scholar]
- 6. Solensky R. Hypersensitivity reactions to beta-lactam antibiotics. Clin Rev Allergy Immunol 2003; 24:201–20. [DOI] [PubMed] [Google Scholar]
- 7. Blumenthal KG, Shenoy ES, Huang M et al. . The impact of reporting a prior penicillin allergy on the treatment of methicillin-sensitive Staphylococcus aureus bacteremia. PLoS One 2016; 11:e0159406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zagursky RJ, Pichichero ME. Cross-reactivity in β-lactam allergy. J Allergy Clin Immunol Pract 2018; 6:72–81.e1. [DOI] [PubMed] [Google Scholar]
- 9. Stryjewski ME, Szczech LA, Benjamin DK Jr et al. . Use of vancomycin or first-generation cephalosporins for the treatment of hemodialysis-dependent patients with methicillin-susceptible Staphylococcus aureus bacteremia. Clin Infect Dis 2007; 44:190–6. [DOI] [PubMed] [Google Scholar]
- 10. Small PM, Chambers HF. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob Agents Chemother 1990; 34:1227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. González C, Rubio M, Romero-Vivas J et al. . Bacteremic pneumonia due to Staphylococcus aureus: a comparison of disease caused by methicillin-resistant and methicillin-susceptible organisms. Clin Infect Dis 1999; 29:1171–7. [DOI] [PubMed] [Google Scholar]
- 12. Loubet P, Burdet C, Vindrios W et al. . Cefazolin versus anti-Staphylococcal penicillins for treatment of methicillin-susceptible Staphylococcus aureus bacteremia: a narrative review. Clin Micro Infect 2018; 24:125–32. [DOI] [PubMed] [Google Scholar]
- 13. Li J, Echevarria KL, Hughes DW et al. . Comparison of cefazolin versus oxacillin for treatment of complicated bacteremia caused by methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother 2014; 58:5117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schweizer ML, Furuno JP, Harris AD et al. . Comparative effectiveness of nafcillin or cefazolin versus vancomycin in methicillin-susceptible Staphylococcus aureus bacteremia. BMC Infect Dis 2011; 11:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wong D, Wong T, Romney M, Leung V. Comparison of outcomes in patients with methicillin-susceptible Staphylococcus aureus (MSSA) bacteremia who are treated with β-lactam vs vancomycin empiric therapy: a retrospective cohort study. BMC Infect Dis 2016; 16:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paul M, Zemer-Wassercug N, Talker O et al. . Are all beta-lactams similarly effective in the treatment of methicillin-sensitive Staphylococcus aureus bacteraemia?Clin Microbiol Infect 2011; 17:1581–6. [DOI] [PubMed] [Google Scholar]
- 17. Kim SH, Kim KH, Kim HB et al. . Outcome of vancomycin treatment in patients with methicillin-susceptible Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2008; 52:192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chang FY, Peacock JE Jr, Musher DM et al. . Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore) 2003; 82:333–9. [DOI] [PubMed] [Google Scholar]
- 19. McDanel JS, Roghmann MC, Perencevich EN et al. . Comparative effectiveness of cefazolin versus nafcillin or oxacillin for treatment of methicillin-susceptible Staphylococcus aureus infections complicated by bacteremia: a nationwide cohort study. Clin Infect Dis 2017; 65:100–6. [DOI] [PubMed] [Google Scholar]
- 20. Leis JA, Palmay L, Ho G et al. . Point-of-care beta-lactam allergy skin testing by antimicrobial stewardship programs: a pragmatic multicenter prospective evaluation. Clin Inf Dis 2017; doi: 10.1093/cid/cix512. [DOI] [PubMed] [Google Scholar]
- 21. Bourke J, Pavlos R, James I, Phillips E. Improving the effectiveness of penicillin allergy de-labeling. J Allergy Clin Immunol Pract 2015; 3:365–34.e1. [DOI] [PubMed] [Google Scholar]
- 22. Trubiano JA, Adkinson NF, Phillips EJ. Penicillin allergy is not necessarily forever. JAMA 2017; 318:82–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rimawi RH, Cook PP, Gooch M et al. . The impact of penicillin skin testing on clinical practice and antimicrobial stewardship. J Hosp Med 2013; 8:341–5. [DOI] [PubMed] [Google Scholar]
- 24. King EA, Challa S, Curtin P, Bielory L. Penicillin skin testing in hospitalized patients with β-lactam allergies: effect on antibiotic selection and cost. Ann Allergy Asthma Immunol 2016; 117:67–71. [DOI] [PubMed] [Google Scholar]
- 25. del Real GA, Rose ME, Ramirez-Atamoros MT et al. . Penicillin skin testing in patients with a history of beta-lactam allergy. Ann Allergy Asthma Immunol 2007; 98:355–9. [DOI] [PubMed] [Google Scholar]
- 26. Arroliga ME, Radojicic C, Gordon SM et al. . A prospective observational study of the effect of penicillin skin testing on antibiotic use in the intensive care unit. Infect Control Hosp Epidemiol 2003; 24:347–50. [DOI] [PubMed] [Google Scholar]
- 27. Sacco KA, Bates A, Brigham TJ et al. . Clinical outcomes following inpatient penicillin allergy testing: a systematic review and meta-analysis. Allergy 2017; 72:1288–96. [DOI] [PubMed] [Google Scholar]
- 28. Trubiano JA, Beekmann SE, Worth LJ et al. . Improving antimicrobial stewardship by antibiotic allergy delabeling: evaluation of knowledge, attitude, and practices throughout the emerging infections network. Open Forum Infect Dis 2016; 3:ofw153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Quinn EL, Pohlod D, Madhavan T et al. . Clinical experiences with cefazolin and other cephalosporins in bacterial endocarditis. J Infect Dis 1973; 128:S386–9. [DOI] [PubMed] [Google Scholar]
- 30. Nannini EC, Singh KV, Murray BE. Relapse of type A beta-lactamase-producing Staphylococcus aureus native valve endocarditis during cefazolin therapy: revisiting the issue. Clin Infect Dis 2003; 37:1194–8. [DOI] [PubMed] [Google Scholar]
- 31. Lee S, Song KH, Jung SI et al. . Comparative outcomes of cefazolin versus nafcillin for methicillin-susceptible Staphylococcus aureus bacteremia: a prospective multicenter cohort study in Korea. Clin Microbiol Infect 2017; doi:10.1016/j.cmi.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 32. Preston SL, Briceland LL, Lesar TS. Accuracy of penicillin allergy reporting. Am J Hosp Pharm 1994; 51:79–84. [PubMed] [Google Scholar]
- 33. Miller LE, Knoderer CA, Cox EG, Kleiman MB. Assessment of the validity of reported antibiotic allergic reactions in pediatric patients. Pharmacotherapy 2011; 31:736–41. [DOI] [PubMed] [Google Scholar]