Abstract
Small RNAs are key components of complex regulatory networks. These molecules can integrate multiple cellular signals to control specific target mRNAs. The recent development of high-throughput methods tremendously helped to characterize the full targetome of sRNAs. Using MS2-affinity purification coupled with RNA sequencing (MAPS) technology, we reveal the targetomes of two sRNAs, CyaR and RprA. Interestingly, both CyaR and RprA interact with the 5′-UTR of hdeD mRNA, which encodes an acid-resistance membrane protein. We demonstrate that CyaR classically binds to the RBS of hdeD, interfering with translational initiation. We identified an A/U-rich motif on hdeD, which is bound by the RNA chaperone Hfq. Our results indicate that binding of this motif by Hfq is required for CyaR-induced degradation of hdeD mRNA. Additional data suggest that two molecules of RprA must bind the 5′-UTR of hdeD to block translation initiation. Surprisingly, while both CyaR and RprA sRNAs bind to the same motif on hdeD mRNA, RprA solely acts at the translational level, leaving the target RNA intact. By interchanging the seed region of CyaR and RprA sRNAs, we also swap their regulatory behavior. These results suggest that slight changes in the seed region could modulate the regulation of target mRNAs.
INTRODUCTION
From the human intestinal tract to plant roots, bacteria face a plethora of harmful environmental factors. The nature of encountered factors can be abiotic (e.g. temperature, pH) or biotic (e.g. antibiotics, host immune system). To survive and persist in their ecological niche, bacteria have developed a mighty arsenal of sensing systems called two-component systems (TCS). Generally, TCS are composed of a membrane-associated sensor histidine kinase (HK) and a response regulator (RR) (1). For example, Escherichia coli genome harbors 30 HK and 32 RR (2), enabling to integrate multiple signals and to efficiently respond to stressful conditions. Indeed, in response to specific stimuli, the HK autophosphorylates and then transfers the phosphoryl group to the cognate RR. This last step of phosphorylation activates the RR. In E. coli, the vast majority of RRs are DNA-binding transcription factors (2). A perfect example is the CpxAR two-component system that responds to envelope stress by controlling the transcription of dozens of genes including two small regulatory RNAs (sRNAs) called CyaR and RprA (3,4).
Since the discovery of sRNAs in the early 80’s, accumulated results showed the prime importance of these post-transcriptional regulators in every aspect of bacterial physiology (5). Generally, sRNAs regulate multiple target mRNAs through imperfect base-pairing in the vicinity of the ribosome binding site (RBS). By steric hindrance, sRNAs prevent the binding of the translational machinery and protein synthesis. This is generally followed by passive or active mRNA decay orchestrated by specific ribonucleases such as RNase E (6).
CyaR sRNA (cyclic AMP-activated RNA) was previously shown to negatively regulate a broad spectrum of targets such as ompX (7,8), yqaE, nadE, luxS (9) and yobF mRNAs (10). The expression of CyaR is quite complex as it is controlled by various effectors. Indeed, CyaR is regulated by cAMP-CRP and is subject to catabolite repression (11). Moreover, CyaR expression is also regulated by the CpxAR two-component system and presumably by the alternative sigma factor σE (3,7).
In concert with three other sRNAs, RprA positively regulates the general stress sigma factor σS (12). In addition, RprA negatively controls the transcriptional factor CsgD and the diguanylate cyclase YdaM, key factors in biofilm formation (13). In Salmonella, RprA inhibits the conjugation of pSLT, a virulence plasmid, via the activation of RicI translation (14). The expression of rprA is induced during the stationary phase of growth by the RcsCDB phosphorelay and the CpxAR two-component system (3,15). The transcription of RprA is also repressed by the global regulator of flagellar synthesis LrhA (16).
Both CyaR and RprA sRNAs are involved in complex regulatory networks. In 2015, our group used MS2-affinity purification coupled with RNA sequencing (MAPS) technology to characterize three sRNA targetomes (17,18). Here, we performed MAPS to reveal the targetomes of CyaR and RprA sRNAs. While our data expand the targetomes of these sRNAs, we noticed that an acid-resistance membrane protein called HdeD is under the control of both CyaR and RprA. To understand this apparent functional redundancy, we investigated the sRNA-dependent regulatory mechanism that occurs on hdeD mRNA. We provided evidence that, surprisingly, two molecules of RprA are required to block the translational initiation of hdeD. In stark contrast, CyaR classically binds to the RBS of hdeD mRNA to regulate hdeD at both post-transcriptional and translational levels. Our results suggest that Hfq binding on target mRNA may be a prerequisite for the formation of a sRNA/mRNA/Hfq/RNase E quaternary complex.
MATERIALS AND METHODS
Strains and growth conditions
All experiments used derivatives of E. coli MG1655 strain (Supplementary Table S1). Cells were grown in rich medium (LB). Ampicillin was used at a final concentration of 50 μg/ml and chloramphenicol at 30 μg/ml, as needed.
RNA extraction and northern blot analysis
Total RNA was extracted following the hot-phenol protocol described by Aiba et al. (19). 0.1% arabinose was added when indicated to induce gene expression from the pBAD vector.
For northern blot analysis, 5–10 μg of total RNA were loaded on a polyacrylamide gel (5–10% acrylamide 29:1, 8 M urea) or 20 μg on an agarose gel (1%, MOPS 1×). Then, RNA was electro-transferred to a Hybond-XL membrane (Amersham Bioscience) for a polyacrylamide gel or transferred by capillarity on a Biodyne B membrane (Pall) for an agarose gel. Cross-linking was performed by UV (1200 J). Prehybridization was performed in Church buffer (20). Radiolabeled DNA probes used in this study are described in Supplementary Table S2. Membranes were then exposed to phosphor storage screens and analyzed using a Typhoon Trio (GE Healthcare) instrument. Results reported here correspond to data from at least two independent experiments.
MS2-affinity purification coupled with RNAseq
The MS2 aptamer was fused to the 5′end of cyaR and rprA gene. To validate MS2-sRNA constructs, we first verified that they are expressed at a level similar to untagged sRNA (control). Then, we compared its activity with the control by northern blot analysis (Supplementary Figure S1). To purify the maximum of target mRNAs, we used a ΔsRNA rne131 (RNA degradosome assembly mutant) strain.
Affinity purification assays were performed as described in Lalaouna et al. (21). Here, cells were grown in LB supplemented with 50 μg/ml ampicillin (diluted 1/1000 from an overnight culture grown) and harvested (after induction with 0.1% arabinose for 10 min) in exponential (OD600 nm = 0.5; 100 ml) and stationary phase of growth (OD600 nm = 1.5; 100 ml) for MS2-CyaR and only in stationary phase of growth (OD600 nm = 1.5; 100 ml) for MS2-RprA.
cDNA libraries were prepared using ScriptSeq™ v2 RNA-Seq Library Preparation Kit (Illumina) and sequenced with Illumina MiSeq. Data processing was performed according to Lalaouna et al. (21). Most highly co-purified targets are presented in Tables 1 and 2. Reads alignment were visualized using Genome Browser (Supplementary Figures S2 and S3) (22). The whole list of genes enriched is also available in Supplementary Table S3.
Table 1. Characterization of CyaR targetome using MAPS technology.
| Gene | Ratio MS2-CyaR/Ctrl | Function/activity | Reference |
|---|---|---|---|
| yacL | 370 | Conserved protein | This study |
| mgrB | 339 | Regulator of the PhoQP system | This study |
| hdeD | 199 | Acid-resistance membrane protein | This study |
| nhaA | 160 | Na+:H+ antiporter | This study |
| luxS | 108 | S-ribosylhomocysteine lyase | De Lay, 2009 |
| yebO | 93 | Hypothetical protein | This study |
| yqaE | 70 | Putative membrane protein | De Lay, 2009 |
| yobF | 38 | Stress response protein | Wright, 2013 |
| nadE | 37 | NAD synthetase | De Lay, 2009 |
| ompX | 32 | Outer membrane protein | Johansen, 2008 |
| nhaR | 7 | Na+:H+ antiporter regulator | This study |
List of most significantly co-purified mRNAs using MS2-CyaR as bait in an rne131 ΔcyaR background (ratio MS2-CyaR/CyaR control). New putative target mRNAs are highlighted in gray. Only reads on gene sequences are reported here. The complete list of candidates is available in Supplementary Table S3.
Table 2. Identification of potential RprA-regulated mRNAs using MAPS technology.
| Gene | Ratio MS2-RprA/Ctrl | Function/Activity | Reference |
|---|---|---|---|
| nlpD | 2295 | Divisome associated factor | Majdalani, 2001 |
| grcA | 1803 | Pyruvate formate-lyase subunit | This study |
| hdeD | 1655 | Acid-resistance membrane protein | This study |
| rpoS | 1528 | σS factor (σ38) | Majdalani, 2001 |
| focA | 1101 | Formate transporter | This study |
| lrhA | 744 | Transcriptional regulator | This study |
| pal | 569 | Subunit of TolB-Pal complex | This study |
| tolB | 324 | Subunit of TolB-Pal complex | This study |
| cpoB | 103 | Cell division coordinator | This study |
| csgD | 23 | Transcriptional regulator | Mika, 2012 |
| ydaM | 21 | Diguanylate cyclase | Mika, 2012 |
List of most significantly co-purified mRNAs using MS2-RprA as bait in an rne131 ΔrprA background (ratio MS2-RprA/RprA control). New putative target mRNAs are highlighted in gray. Only reads on gene sequences are reported here. The complete list of candidates is available in Supplementary Table S3.
β-Galactosidase assays
β-Galactosidase assays were performed as previously described (23). When required, expression of respective sRNAs was induced by addition of 0.1% arabinose at an OD600 nm = 0.5 (LB medium 37°C). When the cells reached an OD600 nm of 2, specific β-galactosidase activity was calculated using the formula Vmax/OD600 nm. Data represent the mean of three independent experiments (± standard deviation, SD). See Supplementary Figure S4 and Supplementary Materials and Methods for details on the construction of lacZ fusions.
Probing experiments
Lead acetate degradation and In-line probing assays were performed as described by Lalaouna et al. (18). In brief, 0.2 μM of in vitro-generated hdeD+195 5′-end-labeled was incubated with or without 1 μM CyaR or RprA sRNA. Radiolabeled RNA was incubated 5 min at 90°C with alkaline buffer or 5 min at 37°C with ribonuclease T1 (0.1 U; Ambion) to generate the alkaline (OH) ladder and the T1 ladder, respectively. RNA was analyzed on an 8% acrylamide/7 M urea gel.
RESULTS
CyaR sRNA targetome revealed by MAPS
As previously performed for RyhB, RybB and DsrA sRNAs (17,18), we used the MAPS approach to uncover the targetome of CyaR sRNA. The MS2-CyaR construct was expressed from a pBAD promoter by addition of 0.1% arabinose in an rne131 ΔcyaR background. Then, we co-purified all interacting RNAs in both exponential (OD600 nm = 0.5) and stationary (OD600 nm = 1.5) phases of growth. Processed data are available in Supplementary Table S3. We summarize most enriched candidates in Table 1 and show reads alignment visualized using Genome Browser (22) in Supplementary Figure S2.
In addition to the five previously characterized targets of CyaR, we found four new candidates (i.e. yacL, mgrB-yebO, hdeD and nhaA-nhaR). Briefly, yacL mRNA encodes a conserved cytoplasmic protein of unknown function. Both mgrB and yebO mRNAs are transcribed as a single polycistronic transcript: while MgrB is described as a negative regulator of PhoQP two-component system (24), the function of YebO protein is still unknown. The hdeD mRNA encodes a membrane protein involved in high-density acid resistance (25). Finally, NhaA is an Na+:H+ antiporter involved in cellular salt and pH homeostasis and NhaR is its associated transcriptional regulator (26). Notably, those putative targets of CyaR were not previously predicted by in silico analysis or identified by microarray assays (9,10).
To validate these four potential targets of CyaR, we pulse-expressed CyaR in vivo, and used northern blot assays for detection (Supplementary Figures S5A and Figure 1A). We observed a rapid decrease for all target mRNAs, suggesting that CyaR negatively regulates these mRNAs at the post-transcriptional level. To further characterize CyaR-mediated regulation, we used the lacZ reporter gene fused in-frame with the coding sequence of yacL and hdeD. β-galactosidase assays with these translational lacZ fusions confirmed CyaR as an efficient repressor (Supplementary Figure S5B). Unfortunately, we were not able to obtain workable translational lacZ fusions for mgrB-yebO and nhaA-nhaR polycistrons.
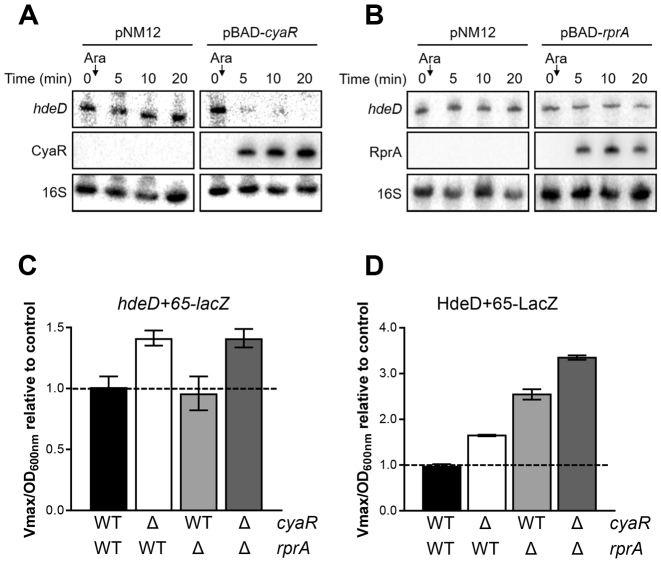
Figure 1.
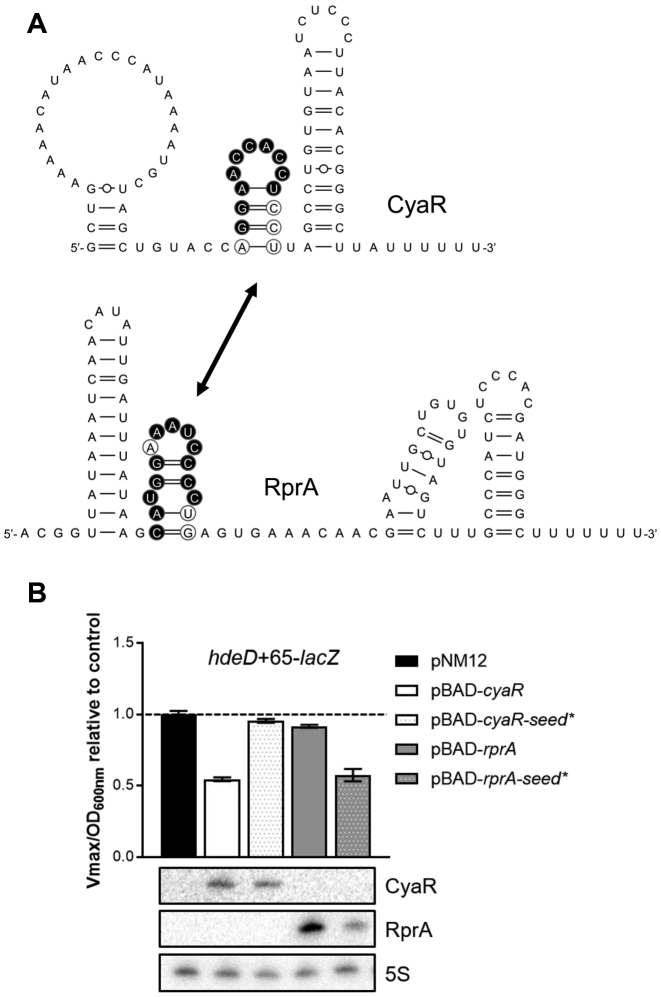
hdeD mRNA is part of two regulatory networks. Northern blot analysis of hdeD mRNA. The expression of (A) cyaR or (B) rprA from a pBAD promoter was induced by addition of 0.1% arabinose (Ara) when cells reached an OD600 nm = 1.5 (ΔcyaR or ΔrprA background). The empty vector pNM12 was used as a control. 16S rRNA was used as a loading control. Data are representative of two independent experiments. β-galactosidase assays using (C) hdeD+65-lacZ (transcriptional) and (D) HdeD+65-LacZ (translational) fusions in WT (black), ΔcyaR (white), ΔrprA (gray) and ΔcyaR ΔrprA (dark gray) backgrounds. Samples were taken at an OD600 nm of 2.0. Data represent the mean of three independent experiments ± SD.
Finding additional targets of RprA sRNA
Similar to CyaR, we performed MS2-RprA affinity purification and sequenced co-purified RNAs. Ratio and reads alignment of most significant candidates are presented in Table 2 and Supplementary Figure S3, respectively.
We were able to enrich previously known targets (i.e. rpoS (12), csgD (13) and ydaM (13)) as well as new putative targets (i.e. grcA, hdeD, lrhA, focA and tolB-pal-cpoB). Two of them play a role in formate metabolism: GrcA, also called YfiD, is part of a stress-induced alternate pyruvate formate-lyase (27) and FocA is a pH-dependent formate transporter (28). As described in the Introduction, LrhA is a transcriptional factor which represses RprA sRNA synthesis. The polycistronic transcript tolB-pal-cpoB encodes proteins involved in outer membrane invagination during cell division (29,30).
We visualized the effect of RprA overexpression on candidate targets using northern blot assays (Supplementary Figure S6A and Figure 1B). RprA sRNA induced only a slight or no decrease of grcA, hdeD and tolB-pal-cpoB mRNAs signal after 20 minutes when compared to lrhA and the previously described target, ydaM. Unfortunately, no clear results were obtained for focA mRNA because of its weak expression in aerobic conditions. Using translational lacZ fusions, we noticed a clear down-regulation of all targets (35–50%), suggesting that RprA mainly regulates these targets at the translational level (Supplementary Figure S6B). The only exception was lrhA due to non-workable translational fusion.
hdeD mRNA, a common but differently regulated target
Regulation of hdeD at the post-transcriptional level
Our MAPS data pointed out that both CyaR and RprA sRNAs bind the target mRNA hdeD (Tables 1 and 2). We further investigated the regulatory mechanism occurring on this specific mRNA. First, we used a transcriptional lacZ fusion (hdeD+65-lacZ) to monitor the influence of each sRNA deletion on hdeD mRNA level in the cell (Figure 1C). While the mutation of rprA has no effect, the activity of hdeD+65-lacZ transcriptional fusion increased in absence of CyaR (1.41-fold), suggesting that CyaR induces hdeD mRNA decay. We also observed that β-galactosidase activities in ΔcyaR and ΔcyaR ΔrprA mutants are similar. This confirms the inability of RprA to induce a significant cleavage of hdeD, such as previous northern blot analysis (Figure 1A and B). The same results were observed when we overproduced CyaR and RprA sRNAs (Supplementary Figure S7; hdeD+65-lacZ).
Previously, we demonstrated that RyhB sRNA induces sodB mRNA degradation through a distal cleavage site (352 nt downstream from the RBS) (31). To verify the presence of a remote cleavage site on hdeD transcript, we used a longer lacZ fusion (hdeD+602-lacZ) containing the whole ORF of hdeD (except the stop codon). Again, only CyaR can significantly induce mRNA decay (Supplementary Figure S8). These data suggest that RprA strictly regulates hdeD mRNA at the translational level.
We then explored CyaR-dependent mRNA degradation. As RNase E is commonly involved in sRNA-mediated decay, we compared the effect of CyaR in a WT and an RNase E-thermosensitive mutant (rne3071) (Supplementary Figure S9A). After inactivation of RNase E at 44°C, we induced cyaR expression from a pBAD promoter and monitored the cellular level of hdeD. We noticed that, under those conditions, CyaR becomes unable to destabilize hdeD mRNA, which indicates that RNase E is crucial for hdeD degradation. The same conclusion was reached when we used an hdeD+65-lacZ fusion in an rne3071 background (Supplementary Figure S9B).
Regulation of hdeD at the translational level
Then, using a translational fusion (HdeD+65-LacZ), we confirmed that both CyaR and RprA induce a translational block (Figure 1D). Indeed, we noticed an increase of the β-galactosidase activity in both ΔcyaR and ΔrprA backgrounds (1.7- and 2.6-fold respectively). Notably, RprA represses hdeD translation more efficiently than CyaR.
Because CyaR sRNA induces mRNA decay, we performed the same experiment in an rne3071 background to prevent degradation and focus on translation regulation (Supplementary Figure S9B). Even in absence of active degradation, CyaR repressed efficiently hdeD at the translational level (83%) showing that CyaR regulates hdeD at both the post-transcriptional and translational level.
The overproduction of CyaR or RprA also confirmed that these sRNAs repress translation of hdeD (Supplementary Figure S7; HdeD+65-LacZ). Remarkably, when we compared the effect of CyaR overexpression in ΔcyaR and ΔcyaR ΔrprA mutants, we noticed an increase of the post-transcriptional repression in absence of RprA (1.4-fold for hdeD+65 and 1.1-fold for HdeD+65) (Supplementary Figure S7). We observed the same result for RprA in absence of CyaR, but at the translational level (1.5-fold for HdeD+65). As a result, it seems that CyaR activity is hindered by the presence of RprA, and vice-versa. We can assume that CyaR and RprA share a common binding site on hdeD mRNA or at least that these binding sites overlap.
Both CyaR and RprA sRNAs bind to the 5′-UTR of hdeD mRNA
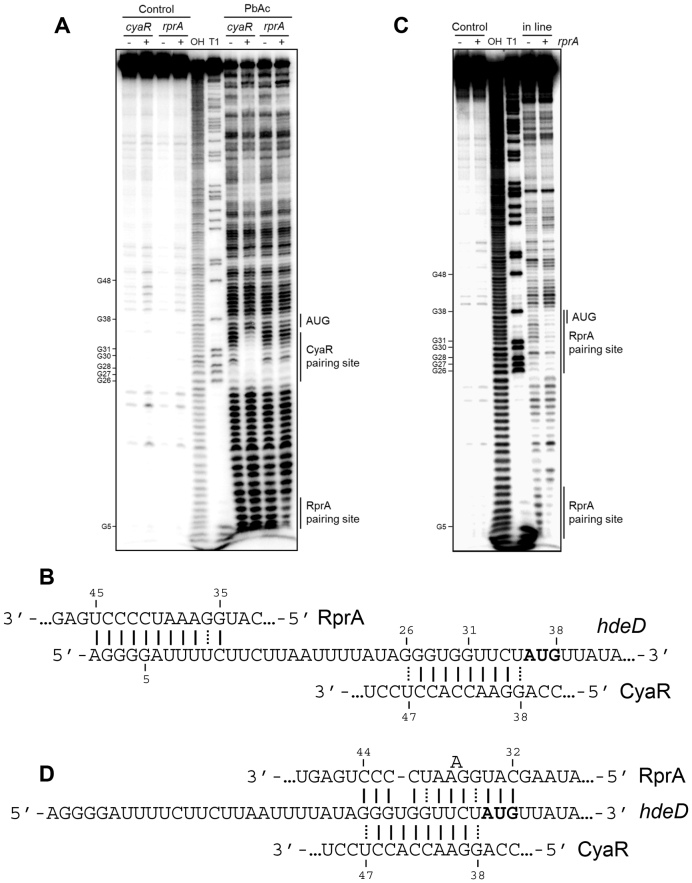
To identify both CyaR and RprA sRNAs binding sites on the hdeD sequence, we performed lead acetate (PbAc) probing assays using a 5′-radiolabeled hdeD+195 RNA fragment incubated with CyaR or RprA (Figure 2A). In the presence of CyaR, we observed a clear protection of nucleotides +26 to +35 containing the Shine-Dalgarno sequence (SD). In the case of RprA, an uncommon binding site is noticed, from +1 to +11 nucleotides. In silico predictions by IntaRNA software (32) suggest the same pairing sites on hdeD mRNA. Binding sites are represented in Figure 2B. Remarkably, an additional but slight protection is observed in the RBS. To verify this, we used in line probing assays in presence or absence of RprA (Figure 2C). In addition to the first protected region in the 5′end of hdeD, we noticed a second site from nucleotides +26 to +38, covering the SD and start codon of hdeD (Figure 2D). Interestingly, the same region of RprA (seed sequence) is used to base-pair with both putative pairing sites, suggesting that two RprA molecules could bind to the 5′-UTR of hdeD.
Figure 2.
The 5′-UTR of hdeD mRNA is targeted by both CyaR and RprA in vitro. (A) Lead acetate (PbAC) probing of 5′end-radiolabeled hdeD+195 incubated in presence or absence of CyaR and RprA. OH, alkaline ladder; T1, RNase T1 ladder. The numbers to the left indicate sequence positions with respect to the +1 of hdeD. (B) Validated pairing between hdeD and both sRNAs. (C) In-line (MgCl2) probing of 5′end-radiolabeled hdeD+195 in presence or absence of RprA. Samples were incubated with MgCl2 during 48 h. OH, alkaline ladder; T1, RNase T1 ladder. (D) Representation of the second validated pairing site of RprA on hdeD mRNA.
Repression of hdeD translation requires the binding of two RprA molecules
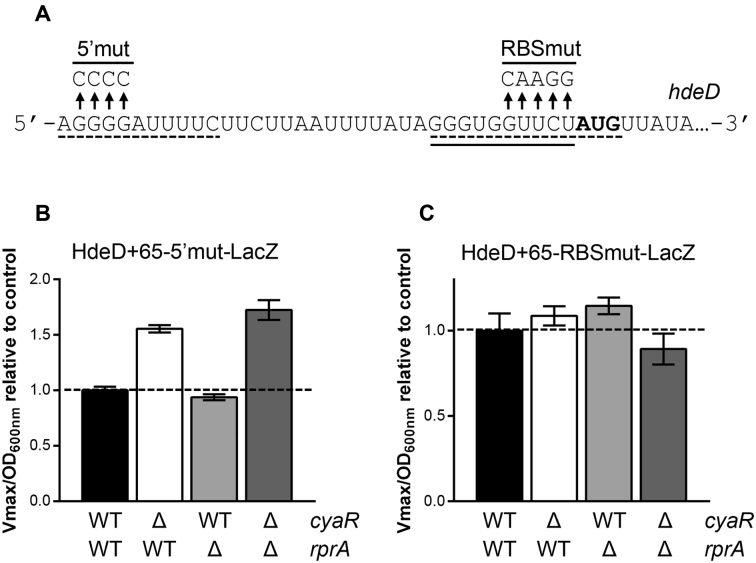
Our in vitro probing assays (Figure 2) suggested the presence of two putative RprA binding sites on hdeD. We hypothesized that RprA could target both the 5′end and the RBS of hdeD mRNA. To validate this, we individually mutated both potential binding sites as indicated in Figure 3A (5′mut and RBSmut). The effect of each mutation on the absolute β-galactosidase activity of HdeD+65-LacZ fusion is shown in Supplementary Figure S10. Even if the mutation of the spacer sequence between the SD and the initiator codon strongly reduces β-galactosidase activity, we were able to obtain a workable lacZ fusion.
Figure 3.
Two RprA binding sites are essential for hdeD regulation in vivo. (A) Mutation of the 5′end (5′mut) and RBS (RBSmut) of hdeD mRNA. Solid line and dashed lines indicate CyaR and RprA binding sites, respectively. The translation start codon is shown in bold. β-galactosidase assays using (B) HdeD+65–5′mut-LacZ and (C) HdeD+65-RBSmut-LacZ translational fusions in WT (black), ΔcyaR (white), ΔrprA (gray) and ΔcyaR ΔrprA (dark gray) backgrounds. Samples were taken at an OD600 nm of 2.0. Data represent the mean of three independent experiments ± SD.
We performed β-galactosidase assays using HdeD+65–5′mut-LacZ (Figure 3B) and HdeD+65-RBSmut-LacZ (Figure 3C) translational fusions. In Figure 3B, we showed that the 5′end mutation of hdeD fully negates RprA repression, but not CyaR repression. This confirms that the 5′end of hdeD is essential for RprA activity. The second pairing site located in the RBS is also crucial, as suggested by the complete loss of RprA effect on HdeD+65-RBSmut-LacZ (Figure 3C).
To prove a direct pairing between RprA and hdeD mRNA, we used compensatory mutations for each described mutant (Supplementary Figure S11). First, we confirmed that RprA failed to down-regulate HdeD+65–5′mut-LacZ translational fusion (Supplementary Figure S11A). Second, the same effect is observed by mutating the corresponding binding site on RprA (RprA-5′mut) with an HdeD+65-LacZ construct. Finally, overexpression of RprA compensatory mutant re-establishes RprA-mediated regulation of HdeD+65–5′mut. Hence, RprA directly binds to the 5′end of hdeD mRNA.
We then performed the same experiment with the second pairing site (RBSmut) (Supplementary Figure S11B). We noticed a strong loss of regulation by mutating either hdeD or RprA. However, we still observed a significant RprA-mediated regulation with HdeD+65-RBSmut (30% instead of 60% for the WT construct). Here, we preserved the SD sequence and only mutated the adjacent nucleotides. It seems that the binding site is partially disrupted but still provides enough complementarities, explaining the residual activity of RprA. Unfortunately, we were not able to restore the regulation using compensatory mutants (Supplementary Figure S11B). Nonetheless, accumulated results strongly argue that RprA regulates hdeD through dual binding of 5′ and RBS sites.
Hfq binding to the 5′-UTR of hdeD is necessary to induce mRNA decay
Using the HdeD+65-RBSmut-LacZ translational fusion (Figure 3A), we observed that the sequence between the SD sequence and the start codon is essential for CyaR-mediated regulation of hdeD mRNA (Figure 3C). As observed for RprA, the mutation of either hdeD or CyaR led to a significant derepression (Supplementary Figure S12). Again, we were not able to restore CyaR activity using compensatory mutations. However, evidence tends to validate a direct binding of CyaR to the RBS of hdeD.
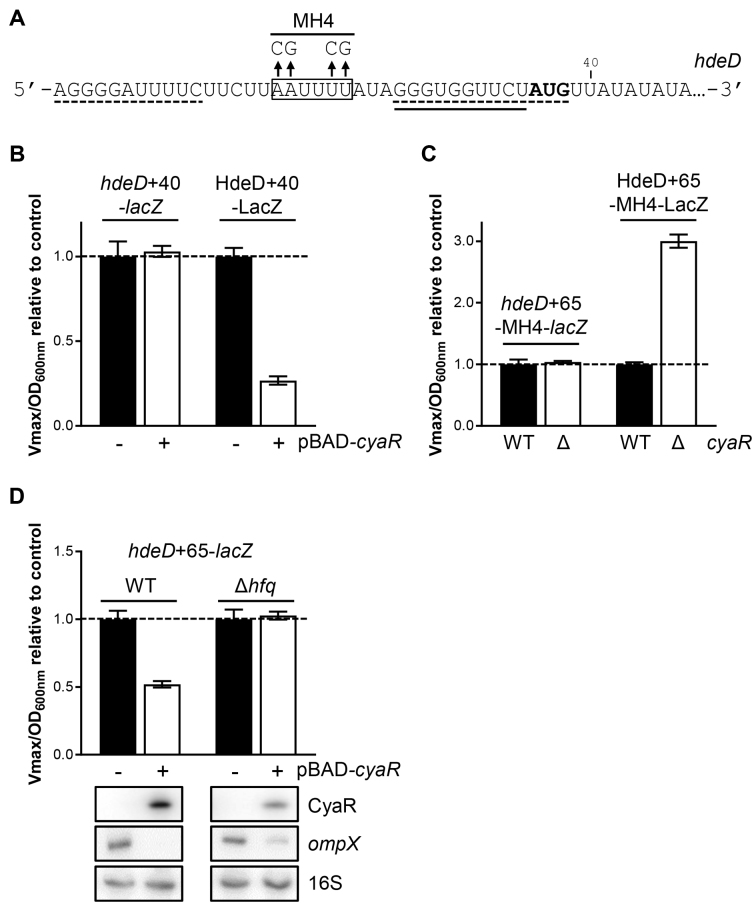
Although CyaR and RprA sRNAs bind to the RBS sequence of hdeD mRNA, only CyaR induces a strong and rapid mRNA decay (Figure 1A, Supplementary Figures S5A and S6A). Data presented in Supplementary Figure S9 strongly suggest that RNase E is actively recruited to induce the degradation of hdeD mRNA. Interestingly, we noticed the presence of two A/U-rich motif, upstream of the CyaR binding site (nucleotides +15 to +25) and just after the initiator codon (nucleotides +39 to +47) (Figure 4A). Both Hfq and RNase E are known to preferentially bind to this motif (33,34).
Figure 4.
Hfq binding to hdeD mRNA is required to induce mRNA decay. (A) Mutation of the A/U-rich region (MH4) localized between CyaR and RprA binding sites. Boxed text corresponds to the in vitro determined Hfq binding site. Solid line and dashed lines indicate CyaR and RprA binding sites, respectively. The translation start codon is shown in bold. (B) β-Galactosidase assays using hdeD+40-lacZ transcriptional fusion or HdeD+40-LacZ translational fusion in a ΔcyaR background. Strains carry either an empty vector (pNM12; black) or a pBAD-cyaR (white). The expression of cyaR was induced by addition of 0.1% arabinose when cells reached an OD600 nm = 0.5. Samples were taken at an OD600 nm of 1.8. Data represent the mean of three independent experiments ± SD. (C) β-Galactosidase assays using hdeD+65-MH4-lacZ transcriptional fusion or HdeD+65-MH4-LacZ translational fusion in WT (black) and ΔcyaR (white) backgrounds. Samples were taken at an OD600 nm of 2.0. (D) β-Galactosidase assays using hdeD+65-lacZ transcriptional fusions in ΔcyaR ΔrprA and ΔcyaR ΔrprA Δhfq backgrounds. Strains carry either an empty vector (pNM12; black) or a pBAD-cyaR (white). Samples were taken 2h after the induction of cyaR expression with 0.1% arabinose (at OD600 nm of 0.5). Northern blot assays were performed at the same time to monitor the level of CyaR sRNA and ompX mRNA. 16S rRNA was used as a loading control. Data are representative of two independent experiments.
To determine if the A/U rich sequence localized close to the AUG codon (nucleotides +39 to +47) is involved in hdeD decay, we removed it using a shorter lacZ fusion (hdeD+40-lacZ; Figure 4B). We previously used this approach to determine the RNAse E cleavage site on sodB (31), sdhC (35) and rbsD (17). Even if cyaR was overexpressed, no regulation at the post-transcriptional level was observed, hinting that the RNase E cleavage site is localized between nucleotides +40 and +65. As a control, we showed that CyaR is still able to pair with hdeD mRNA and block its translation (HdeD+40-LacZ; 73%).
Then, we monitored the effect of the mutation of the second A/U-rich sequence (MH4), localized upstream of CyaR binding site (Figure 4A). We verified that MH4 mutation does not affect the basal β-galactosidase activity of HdeD+65-LacZ fusion (Supplementary Figure S10). Using hdeD+65-MH4-lacZ transcriptional fusion, we observed a complete loss of regulation by CyaR (Figure 4C). Remarkably, the mutation MH4 does not prevent translational repression by CyaR as shown by HdeD+65-MH4-LacZ translational fusion (Figure 4C). In 2015, Schu et al. (34) have shown that target mRNAs regulated by Class II sRNAs (such as CyaR) contain an A/U-rich sequence allowing the binding of the RNA chaperone protein Hfq. We first validated that Hfq recognizes and binds this A/U-rich motif in the 5′-UTR of hdeD using in vitro probing (Supplementary Figure S13). The identified binding site is indicated in Figure 4A. Then, we performed β-galactosidase assays in a Δhfq background (Figure 4D). CyaR overexpression has no effect on an hdeD+65-lacZ transcriptional fusion, suggesting that Hfq is involved in hdeD mRNA decay. However, CyaR is described as an Hfq-dependent sRNA, notably for its stability (7,8). Thus, results obtained in Figure 4D could be also explained by the lack of stability or functionality of CyaR sRNA in a Δhfq background. To discard this hypothesis, we extracted total RNA of samples used in Figure 4D and observed a significant amount of CyaR after 2h. Moreover, we demonstrated that CyaR is still functional as CyaR induces the decay of ompX mRNA (61% in Δhfq strain compared to 88% in WT). Therefore, the binding of Hfq to the 5′-UTR of hdeD mRNA is critical to induce its decay.
Interchanging the seed region of sRNAs also interchanges the ability to degrade hdeD mRNA
To determine if the signal required to induce the degradation of hdeD mRNA is held in the pairing region of CyaR, we decided to switch seed regions of CyaR and RprA. As both sequences are part of a stem-loop structure, we simply exchanged them (CyaR-seed* and RprA-seed*; see Figure 5A). As shown in Figure 5B, the modified version of CyaR sRNA (carrying RprA seed) has lost the ability to induce hdeD mRNA decay (4% instead of 45% for WT CyaR). As a control, we verified that CyaR-seed* construct is stable in vivo using northern blot analysis (Figure 5B). Moreover, we observed that CyaR-seed* is still able to block the initiation of hdeD translation (Supplementary Figure S14). On the contrary, RprA-seed* construct (RprA carrying CyaR binding sequence) is now able to promote hdeD degradation (43% compared to 45% for WT CyaR). Thus, the seed region of CyaR is sufficient to induce hdeD mRNA decay, even within a sRNA known to be ineffective to degrade this target.
Figure 5.
Interchanging the seed sequence of CyaR and RprA also interchanges the ability to induce hdeD mRNA decay. (A) Secondary structures of CyaR and RprA sRNAs determined using Mfold software (35) and visualized with VARNA software (36). To construct CyaR-seed* and RprA-seed*, the hairpins bearing the seed region of CyaR and RprA were switched. Exchanged nucleotides are circled. Binding sites are indicated in black. (B) β-Galactosidase assays with hdeD+65 transcriptional lacZ fusion in a ΔcyaR ΔrprA background. Overexpression of cyaR, cyaR-seed*, rprA or rprA-seed* was induced by addition of 0.1% arabinose when cells reached an OD600 nm of 0.5. Samples were taken at an OD600 nm = 1.5. Data represent the mean of three independent experiments ± SD.
CyaR-dependent regulation of hdeD mRNA is hindered in presence of glucose or sodium pyruvate
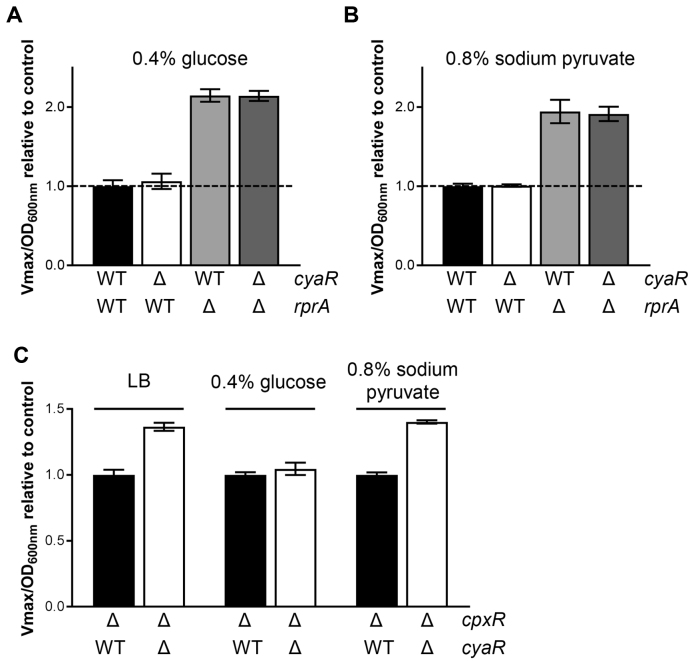
As described in Supplementary Figure S15, CyaR and RprA sRNAs are under the control of major transcriptional regulators, responding to specific stimuli. Notably, cyaR expression is repressed in presence of glucose (CRP-AMPc and CpxR) and pyruvate (CpxR) (7,38). Consequently, these growth conditions may favor the RprA-mediated regulation of hdeD mRNA. To validate this assumption, we monitored the β-galactosidase activity of an HdeD+65-LacZ translational fusion in presence of 0.4% glucose (Figure 6A) or 0.8% sodium pyruvate (Figure 6B). This led to the complete inhibition of CyaR synthesis as no difference was observed between WT and ΔcyaR strains. The same result is noticed when we compared the ΔrprA and ΔcyaR ΔrprA backgrounds. However, RprA still negatively regulates hdeD mRNA at the translational level.
Figure 6.
CyaR-dependent regulation of hdeD mRNA is hindered in presence of glucose or sodium pyruvate. β-galactosidase assays using HdeD+65-LacZ (translational) fusions in WT (black), ΔcyaR (white), ΔrprA (gray) and ΔcyaR ΔrprA (dark gray) backgrounds. When cells reached an OD600 nm = 0.5, (A) 0.4% glucose or (B) 0.8% sodium pyruvate was added. (C) β-Galactosidase assays using HdeD+65-LacZ (translational) fusions in ΔcpxR (black) and ΔcpxR ΔcyaR (white) in LB medium supplemented with 0.4% glucose or 0.8% sodium pyruvate. LB medium is used as a control. Samples were taken at an OD600 nm of 2.0. Data represent the mean of three independent experiments ± SD.
To confirm that the repression of CyaR activity in presence of glucose or pyruvate is notably due to the response regulator CpxR, we performed similar experiments in a ΔcpxR background (Figure 6C). In absence of CyaR, we observed a comparable derepression of HdeD+65-LacZ fusion when we compared cpxR+ (Figure 1D; 1.41-fold) and ΔcpxR backgrounds (Figure 6C; 1.37-fold). As in Figure 6A, the mutation of cyaR had no effect in presence of 0.4% glucose, certainly due to the inactivation of the cAMP-CRP system. On the contrary, CyaR sRNA still negatively regulates HdeD+65-LacZ fusion in presence of 0.8% sodium pyruvate (Figure 6C; 1.40-fold). This confirms that CpxR blocks cyaR transcription in response to pyruvate. Thus, depending on specific growth conditions, RprA-dependent regulation will be favored over that of CyaR and vice versa.
DISCUSSION
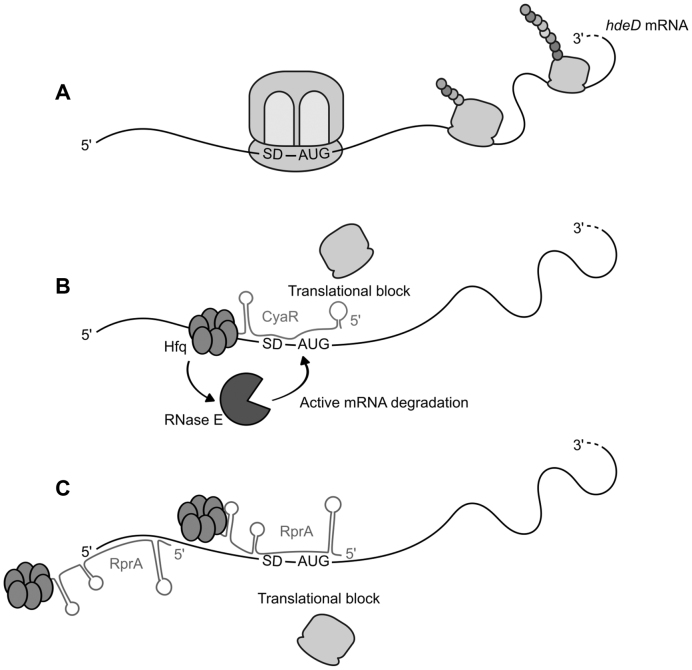
In this study, we demonstrated that both CyaR and RprA can block the translation initiation of hdeD but only CyaR induces mRNA decay. In bacteria, the sRNA-dependent translation repression is generally coupled with passive or active degradation of targeted mRNA (6). Upon translational inhibition, ‘naked’ mRNA becomes more sensitive to RNase E nucleolytic attacks (passive degradation). To speed up the process, sRNAs can actively recruit RNase E via the formation of a sRNA/Hfq/RNase E complex (active degradation), where Hfq directly binds the C-terminal region of RNase E (39). However, the mechanism of recruitment of RNase E is only partially understood. Our results indicated that both Hfq and RNase E are required for CyaR-dependent hdeD mRNA decay. Here, the role of Hfq is not limited to facilitating CyaR:hdeD interaction or stabilizing CyaR sRNA. Although nothing prevents the formation of the CyaR/Hfq/RNase E complex, our model suggests that Hfq should first recognize an A/U-rich motif in the 5′-UTR of hdeD. Therefore, a direct contact between Hfq and hdeD mRNA is essential to actively induce hdeD mRNA decay via RNase E (Figure 7).
Figure 7.
Descriptive model for CyaR and RprA-mediated hdeD regulation. (A) Under non-inducing conditions, hdeD mRNA is normally translated. Depending on environmental stimuli, CyaR and/or RprA are expressed within the cell, resulting in two distinct outcomes. (B) CyaR binds to the RBS of hdeD and interferes with translation initiation. In addition, CyaR induces an active degradation of hdeD mRNA. For this purpose, Hfq protein has to recognize and bind to both CyaR and the 5′-UTR of hdeD (A/U-rich motif). Through a direct protein:protein interaction, Hfq potentially recruits RNase E resulting in an active RNase E-dependent hdeD decay. (C) Two molecules of RprA are required to suppress hdeD translation. Indeed, RprA binds to two different sites, which are both essential for translational block. RprA has no effect on hdeD stability. The secondary structure of CyaR and RprA sRNAs was predicted in silico (Figure 5).
According to Schu et al. (34), sRNAs could be classified in function of their interaction with Hfq. Basically, an Hfq hexamer carries three RNA-binding elements: a proximal face, a rim and a distal face. Whereas class I sRNAs bind to Hfq proximal face and rim, class II sRNAs prefer its proximal and distal faces. The remaining binding surface interacts with the targeted mRNA. Each Hfq face recognizes a specific sequence: U-rich sequence for the proximal face, ARN motif for the distal face and A/U-rich sequence for the rim. CyaR is depicted as a class II sRNA and hdeD bears an A/U-rich sequence which is optimal for the formation of CyaR/hdeD/Hfq complex. Note that this supports a model where only one Hfq hexamer is required to bind simultaneously CyaR and hdeD. Interestingly, RprA is a non-exclusive class II sRNA (34), suggesting that Hfq should bind hdeD mRNA with its rim face as well.
Remarkably, by interchanging seed sequences of CyaR and RprA, we also interchanged the outcome at the post-transcriptional level. We previously used this approach with RyhB and Spot42 sRNAs (35). Both sRNAs were characterized as negative regulators of sdhC mRNA. While RyhB classically pairs with the SD sequence, Spot42 binds far upstream and must recruit Hfq close to the RBS to compete with initiating ribosomes. These distinct mechanisms of action were also interchanged by switching respective seed regions. Similarly, we confirmed that all the signal required to induce hdeD mRNA decay is contained in the short seed region of CyaR. In terms of similarities, both seeds encompass a short stem-loop structure located at nucleotides 36 and 31 from the 5′end of CyaR and RprA, respectively. As indicated in Figure 2D, CyaR base-pairs with hdeD mRNA via a short and perfect sequence (energy –8.84 kcal/mol, determined by IntaRNA (32)), while RprA binding site bears two non-interacting nucleotides (energy –7.71 kcal/mol) and is longer, overlapping the AUG initiator codon. However, the role of each single nucleotide constituting the seed sequence is still poorly understood (e.g. in mRNA target selection or in sRNA-mediated regulatory mechanism). Interestingly, recent studies have assessed the importance of single nucleotide changes, notably in the sRNA seed region (40–41). They determined that: (a) as little as one single nucleotide mutation can annihilate sRNA-dependent regulation, and (b) not all nucleotides of the seed are critical (nonessential bases). To complicate matters further, critical nucleotides can change in function of the targeted mRNA. As a conclusion, slight nucleotide substitutions could be accountable for the difference between CyaR and RprA regulatory mechanisms. Further work will be required to validate this assumption.
It is even more difficult to draw conclusions from seed characteristics as RprA is one of the rare examples of sRNAs that does not trigger mRNA decay upon binding. Indeed, only a few sRNAs were suggested to act primarily at the translational level in bacteria (42–44). This phenomenon seems target specific because RprA can induce the rapid degradation of other targets (i.e. csgD, ydaM and lrhA transcripts) (Supplementary Figure S6A and Table S4). Nevertheless, it remains difficult to compare those examples to highlight determinants or factors required to seal the fate of targeted mRNAs. Indeed, nothing is known about ydaM and lrhA decay. Moreover, RNase E has only a limited effect on csgD stability (13).
Consistent with the classical model of sRNA-dependent translational repression, the binding of a sRNA to the RBS is sufficient to interfere with ribosome assembly and consequently translation initiation. Although both CyaR and RprA target the RBS of hdeD, only CyaR meets the criteria to directly block translation without any additional requirements. The RprA-mediated mechanism of hdeD regulation remains unclear, but is quite uncommon (Figure 7). Indeed, we demonstrated that mutation of either site I (5′end) or site II (RBS) completely abolished RprA effect on hdeD translation (Figure 3). This differs from previously known examples where both binding sites act additively. For instance, two regions of base-pairing were identified on csgD mRNA, another target of RprA (13). Site I (close to the 5′end) and site II (RBS) seem functionally redundant as the mutation of both sites is required to affect RprA-mediated regulation of csgD mRNA. Binding two sites in the 5′UTR of a specific target as shown here for RprA is not a unique feature. For example, Bos et al. also suggested an additive effect of RyhB pairing with two distinct sites in the 5′-UTR of msrB mRNA (45). Interestingly, the second base-pairing site can be localized in the coding sequence (CDS) instead of in the 5′end. For example, SgrS base-pairs twice on asd mRNA (46). Each individual site is sufficient for translational regulation but pairing at both sites is required for optimal asd repression. Similarly, both MicF sRNA pairing sites on lpxR mRNA act additively in Salmonella Typhimurium, the second binding site (CDS) being essential to induce RNase E cleavage (47).
CyaR and RprA are under the control of multiple regulatory systems (i.e. sigma factors, two-component systems), which themselves respond to numerous and various stimuli (Supplementary Figure S15). Depending on stimuli detected by cells, one of these regulators will prevail, resulting in one of the situations presented in Figure 7. A perfect example is the CpxAR two-component system which has an antagonistic effect on CyaR and RprA. In presence of a preferred source of carbon (e.g. pyruvate), the response regulator CpxR is phosphorylated (38) and, therefore, CyaR synthesis is shut down. Inversely, CyaR should take over in presence of a non-preferred source of carbon and induce total degradation of hdeD mRNA. Since RprA and CyaR are both produced in stationary phase of growth, they could potentially compete for binding. However, we only observed a competition when CyaR or RprA were overexpressed (Supplementary Figure S7), suggesting that hdeD mRNA is not limiting in endogenous conditions (Figure 1D and Supplementary Figure S16).
Other sRNAs belong to the Cpx regulon. For instance, Chao and Vogel (48) demonstrated that CpxR directly triggers the transcription of cpxP gene. Afterward, the 3′UTR of cpxP mRNA is processed by RNase E leading to the release of CpxQ sRNA. Interestingly, both CpxP protein and CpxQ sRNA are involved in the inner membrane homeostasis. MicF, OmrA and OmrB sRNAs, three post-transcriptional regulators of outer membrane proteins, are also regulated by the response regulator CpxR, although indirectly (3,49). Through the induction of mzrA gene, CpxR indirectly activates the histidine kinase EnvZ, which is part of the EnvZ/OmpR two-component system (3). Indeed, MzrA protein is known to interact with EnvZ and to activate it. Then, EnvZ phosphorylates OmpR, enabling the transcription of micF, omrA and omrB genes (3,49). However, there is no evidence supporting the involvement of aforementioned sRNAs in hdeD mRNA regulation.
According to Mates et al., HdeD is involved in an acid-resistance mechanism exhibited only at high cellular density (25). As described in Supplementary Figure S16, hdeD mRNA is most exclusively expressed during the stationary phase of growth, which is consistent with a role of HdeD protein at high density (>2 of OD600 nm). Notably, hdeD gene is part of an acid fitness island and its expression is directly activated by GadX and GadE (also named YhiE), two regulators of the acid resistance system (25,50). Nonetheless, its role remains poorly understood. Indeed, studies failed to reach a consensus: the effect of HdeD depletion seems to vary upon the methodology used to test acid resistance (51,52).
The function of other putative targets of CyaR and RprA is also related to pH homeostasis (Supplementary Figure S15). Both GrcA and FocA reduce the accumulation of acidic metabolites within the cell, in a pH-dependent manner (28,53). MgrB influences the expression of multiple acid stress-associated genes by sequestering the histidine kinase of the PhoQP two-component system (24). On the contrary, the Na+:H+ antiporter NhaA and, by extent, its regulator NhaR are involved in alkaline pH homeostasis (54).
In E. coli, most 5′UTRs are between 20 and 40 nucleotides long (55). Within the 35 nt-long hdeD 5′-UTR, we discovered three sRNA binding sites as well as one Hfq recognition motif (Figure 4A). Other mRNAs such as rpoS and csgD are major hubs for signal integration. Both rpoS and csgD mRNAs bear relatively large 5′-UTR, around 570 and 140 nt respectively. The 5′-UTR of rpoS mRNA is the ‘runway’ for four sRNAs (DsrA, RprA, ArcZ and OxyS) and Hfq (56,57) and at least six sRNAs were shown to fine-tune CsgD translation (58,59). Our data suggest that a huge 5′-UTR is not required to integrate multiple signals.
The last decade has been characterized by conceptual and technical innovations enabling the development of high-throughput RNA sequencing methods with the aim of unraveling all sRNA:RNA interactions (60). These are based on RNA co-purification with either a specific protein (61–63) or a particular sRNA (65,66). For instance, MAPS technology, based on the use of MS2-tagged sRNA, has already demonstrated its efficiency by revealing RyhB, RybB and DsrA sRNA regulatory networks (17,18). In the current study, we explored two additional regulatory networks which are under the control of CyaR and RprA sRNAs. Recently, Melamed et al. (63) developed a new method called RIL-Seq (for RNA interaction by ligation and sequencing), based on the co-purification of sRNA:RNA complexes associated with the Hfq-tagged protein. RIL-Seq and MAPS were performed in similar conditions (LB medium, exponential and stationary phases). The putative CyaR-regulated mRNAs identified by MAPS were also revealed by RIL-Seq (among 313 potential CyaR:RNA interactions). However, only two of those transcripts were highly co-purified by RIL-Seq, mgrB-yebO and nhaA-nhaR. Surprisingly, hdeD and grcA, two strongly enriched mRNAs with MAPS, were not identified by RIL-Seq as potentially regulated by RprA. Conversely, MAPS failed to significantly capture top putative targets determined by RIL-Seq. Therefore, the information gained through distinct co-purification approaches seems not redundant but complementary.
AVAILABILITY
MAPS data have been deposited in GEO under accession GSE90128 (MS2-CyaR) and GSE80020 (MS2-RprA).
Supplementary Material
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Canadian Institutes of Health Research (CIHR) (to E.M.) MOP69005. Funding for open access charge: CIHR.
Conflict of interest statement. None declared.
REFERENCES
- 1. Jung K., Fried L., Behr S., Heermann R.. Histidine kinases and response regulators in networks. Curr. Opin. Microbiol. 2012; 15:118–124. [DOI] [PubMed] [Google Scholar]
- 2. Ortet P., Whitworth D.E., Santaella C., Achouak W., Barakat M.. P2CS: updates of the prokaryotic two-component systems database. Nucleic Acids Res. 2015; 43:D536–D541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vogt S.L., Evans A.D., Guest R.L., Raivio T.L.. The Cpx envelope stress response regulates and is regulated by small noncoding RNAs. J. Bacteriol. 2014; 196:4229–4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grabowicz M., Silhavy T.J.. Envelope stress responses: an interconnected safety net. Trends Biochem. Sci. 2017; 42:232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wagner E.G., Romby P.. Small RNAs in bacteria and archaea: who they are, what they do, and how they do it. Adv. Genet. 2015; 90:133–208. [DOI] [PubMed] [Google Scholar]
- 6. Lalaouna D., Simoneau-Roy M., Lafontaine D., Masse E.. Regulatory RNAs and target mRNA decay in prokaryotes. Biochim. Biophys. Acta. 2013; 1829:742–747. [DOI] [PubMed] [Google Scholar]
- 7. Johansen J., Eriksen M., Kallipolitis B., Valentin-Hansen P.. Down-regulation of outer membrane proteins by noncoding RNAs: unraveling the cAMP-CRP- and sigmaE-dependent CyaR-ompX regulatory case. J. Mol. Biol. 2008; 383:1–9. [DOI] [PubMed] [Google Scholar]
- 8. Papenfort K., Pfeiffer V., Lucchini S., Sonawane A., Hinton J.C., Vogel J.. Systematic deletion of Salmonella small RNA genes identifies CyaR, a conserved CRP-dependent riboregulator of OmpX synthesis. Mol. Microbiol. 2008; 68:890–906. [DOI] [PubMed] [Google Scholar]
- 9. De Lay N., Gottesman S.. The Crp-activated small noncoding regulatory RNA CyaR (RyeE) links nutritional status to group behavior. J. Bacteriol. 2009; 191:461–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wright P.R., Richter A.S., Papenfort K., Mann M., Vogel J., Hess W.R., Backofen R., Georg J.. Comparative genomics boosts target prediction for bacterial small RNAs. Proc. Natl. Acad. Sci. U.S.A. 2013; 110:E3487–E3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bruckner R., Titgemeyer F.. Carbon catabolite repression in bacteria: choice of the carbon source and autoregulatory limitation of sugar utilization. FEMS Microbiol. Lett. 2002; 209:141–148. [DOI] [PubMed] [Google Scholar]
- 12. Majdalani N., Chen S., Murrow J., St John K., Gottesman S.. Regulation of RpoS by a novel small RNA: the characterization of RprA. Mol. Microbiol. 2001; 39:1382–1394. [DOI] [PubMed] [Google Scholar]
- 13. Mika F., Busse S., Possling A., Berkholz J., Tschowri N., Sommerfeldt N., Pruteanu M., Hengge R.. Targeting of csgD by the small regulatory RNA RprA links stationary phase, biofilm formation and cell envelope stress in Escherichia coli. Mol. Microbiol. 2012; 84:51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Papenfort K., Espinosa E., Casadesus J., Vogel J.. Small RNA-based feedforward loop with AND-gate logic regulates extrachromosomal DNA transfer in Salmonella. Proc. Natl. Acad. Sci. U.S.A. 2015; 112:E4772–E4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Majdalani N., Hernandez D., Gottesman S.. Regulation and mode of action of the second small RNA activator of RpoS translation, RprA. Mol. Microbiol. 2002; 46:813–826. [DOI] [PubMed] [Google Scholar]
- 16. Peterson C.N., Carabetta V.J., Chowdhury T., Silhavy T.J.. LrhA regulates rpoS translation in response to the Rcs phosphorelay system in Escherichia coli. J. Bacteriol. 2006; 188:3175–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lalaouna D., Morissette A., Carrier M.C., Masse E.. DsrA regulatory RNA represses both hns and rbsD mRNAs through distinct mechanisms in Escherichia coli. Mol. Microbiol. 2015; 98:357–369. [DOI] [PubMed] [Google Scholar]
- 18. Lalaouna D., Carrier M.C., Semsey S., Brouard J.S., Wang J., Wade J.T., Masse E.. A 3′ external transcribed spacer in a tRNA transcript acts as a sponge for small RNAs to prevent transcriptional noise. Mol. Cell. 2015; 58:393–405. [DOI] [PubMed] [Google Scholar]
- 19. Aiba H., Adhya S., de Crombrugghe B.. Evidence for two functional gal promoters in intact Escherichia coli cells. J. Biol. Chem. 1981; 256:11905–11910. [PubMed] [Google Scholar]
- 20. Church G.M., Gilbert W.. Genomic sequencing. Proc. Natl. Acad. Sci. U.S.A. 1984; 81:1991–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lalaouna D., Prevost K., Eyraud A., Masse E.. Identification of unknown RNA partners using MAPS. Methods. 2017; 117:28–34. [DOI] [PubMed] [Google Scholar]
- 22. Schneider K.L., Pollard K.S., Baertsch R., Pohl A., Lowe T.M.. The UCSC archaeal genome browser. Nucleic Acids Res. 2006; 34:D407–D410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Majdalani N., Cunning C., Sledjeski D., Elliott T., Gottesman S.. DsrA RNA regulates translation of RpoS message by an anti-antisense mechanism, independent of its action as an antisilencer of transcription. Proc. Natl. Acad. Sci. U.S.A. 1998; 95:12462–12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lippa A.M., Goulian M.. Feedback inhibition in the PhoQ/PhoP signaling system by a membrane peptide. PLoS Genet. 2009; 5:e1000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mates A.K., Sayed A.K., Foster J.W.. Products of the Escherichia coli acid fitness island attenuate metabolite stress at extremely low pH and mediate a cell density-dependent acid resistance. J. Bacteriol. 2007; 189:2759–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dover N., Higgins C.F., Carmel O., Rimon A., Pinner E., Padan E.. Na+-induced transcription of nhaA, which encodes an Na+/H+ antiporter in Escherichia coli, is positively regulated by nhaR and affected by hns. J. Bacteriol. 1996; 178:6508–6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhu J., Shalel-Levanon S., Bennett G., San K.Y.. The YfiD protein contributes to the pyruvate formate-lyase flux in an Escherichia coli arcA mutant strain. Biotechnol. Bioeng. 2007; 97:138–143. [DOI] [PubMed] [Google Scholar]
- 28. Sawers R.G. Formate and its role in hydrogen production in Escherichia coli. Biochem. Soc. Trans. 2005; 33:42–46. [DOI] [PubMed] [Google Scholar]
- 29. Gray A.N., Egan A.J., Van’t Veer I.L., Verheul J., Colavin A., Koumoutsi A., Biboy J., Altelaar A.F., Damen M.J., Huang K.C. et al. Coordination of peptidoglycan synthesis and outer membrane constriction during Escherichia coli cell division. eLife. 2015; 4: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gerding M.A., Ogata Y., Pecora N.D., Niki H., de Boer P.A.. The trans-envelope Tol-Pal complex is part of the cell division machinery and required for proper outer-membrane invagination during cell constriction in E. coli. Mol. Microbiol. 2007; 63:1008–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Prevost K., Desnoyers G., Jacques J.F., Lavoie F., Masse E.. Small RNA-induced mRNA degradation achieved through both translation block and activated cleavage. Genes Dev. 2011; 25:385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wright P.R., Georg J., Mann M., Sorescu D.A., Richter A.S., Lott S., Kleinkauf R., Hess W.R., Backofen R.. CopraRNA and IntaRNA: predicting small RNA targets, networks and interaction domains. Nucleic Acids Res. 2014; 42:W119–W123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chao Y., Li L., Girodat D., Forstner K.U., Said N., Corcoran C., Smiga M., Papenfort K., Reinhardt R., Wieden H.J. et al. In vivo cleavage map illuminates the central role of RNase E in coding and non-coding RNA pathways. Mol. Cell. 2017; 65:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schu D.J., Zhang A., Gottesman S., Storz G.. Alternative Hfq-sRNA interaction modes dictate alternative mRNA recognition. EMBO J. 2015; 34:2557–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Desnoyers G., Masse E.. Noncanonical repression of translation initiation through small RNA recruitment of the RNA chaperone Hfq. Genes Dev. 2012; 26:726–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003; 31:3406–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Darty K., Denise A., Ponty Y.. VARNA: interactive drawing and editing of the RNA secondary structure. Bioinformatics. 2009; 25:1974–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wolfe A.J., Parikh N., Lima B.P., Zemaitaitis B.. Signal integration by the two-component signal transduction response regulator CpxR. J. Bacteriol. 2008; 190:2314–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ikeda Y., Yagi M., Morita T., Aiba H.. Hfq binding at RhlB-recognition region of RNase E is crucial for the rapid degradation of target mRNAs mediated by sRNAs in Escherichia coli. Mol. Microbiol. 2011; 79:419–432. [DOI] [PubMed] [Google Scholar]
- 40. Peterman N., Lavi-Itzkovitz A., Levine E.. Large-scale mapping of sequence-function relations in small regulatory RNAs reveals plasticity and modularity. Nucleic Acids Res. 2014; 42:12177–12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rutherford S.T., Valastyan J.S., Taillefumier T., Wingreen N.S., Bassler B.L.. Comprehensive analysis reveals how single nucleotides contribute to noncoding RNA function in bacterial quorum sensing. Proc. Natl. Acad. Sci. U.S.A. 2015; 112:E6038–E6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moller T., Franch T., Udesen C., Gerdes K., Valentin-Hansen P.. Spot 42 RNA mediates discoordinate expression of the E. coli galactose operon. Genes Dev. 2002; 16:1696–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chabelskaya S., Gaillot O., Felden B.. A Staphylococcus aureus small RNA is required for bacterial virulence and regulates the expression of an immune-evasion molecule. PLoS Pathog. 2010; 6:e1000927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Eyraud A., Tattevin P., Chabelskaya S., Felden B.. A small RNA controls a protein regulator involved in antibiotic resistance in Staphylococcus aureus. Nucleic Acids Res. 2014; 42:4892–4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bos J., Duverger Y., Thouvenot B., Chiaruttini C., Branlant C., Springer M., Charpentier B., Barras F.. The sRNA RyhB regulates the synthesis of the Escherichia coli methionine sulfoxide reductase MsrB but not MsrA. PLoS One. 2013; 8:e63647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bobrovskyy M., Vanderpool C.K.. Diverse mechanisms of post-transcriptional repression by the small RNA regulator of glucose-phosphate stress. Mol. Microbiol. 2016; 99:254–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Corcoran C.P., Podkaminski D., Papenfort K., Urban J.H., Hinton J.C., Vogel J.. Superfolder GFP reporters validate diverse new mRNA targets of the classic porin regulator, MicF RNA. Mol. Microbiol. 2012; 84:428–445. [DOI] [PubMed] [Google Scholar]
- 48. Chao Y., Vogel J.. A 3′ UTR-derived small RNA provides the regulatory noncoding arm of the inner membrane stress response. Mol. Cell. 2016; 61:352–363. [DOI] [PubMed] [Google Scholar]
- 49. Coyer J., Andersen J., Forst S.A., Inouye M., Delihas N.. micF RNA in ompB mutants of Escherichia coli: different pathways regulate micF RNA levels in response to osmolarity and temperature change. J. Bacteriol. 1990; 172:4143–4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hommais F., Krin E., Coppee J.Y., Lacroix C., Yeramian E., Danchin A., Bertin P.. GadE (YhiE): a novel activator involved in the response to acid environment in Escherichia coli. Microbiology. 2004; 150:61–72. [DOI] [PubMed] [Google Scholar]
- 51. Tucker D.L., Tucker N., Conway T.. Gene expression profiling of the pH response in Escherichia coli. J. Bacteriol. 2002; 184:6551–6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Peng C.A., Oliver M.J., Wood A.J.. Is the Rehydrin TrDr3 from Tortula ruralis associated with tolerance to cold, salinity, and reduced pH? Physiological evaluation of the TrDr3-orthologue, HdeD from Escherichia coli in response to abiotic stress. Plant Biol. (Stuttg.). 2005; 7:315–320. [DOI] [PubMed] [Google Scholar]
- 53. Wyborn N.R., Messenger S.L., Henderson R.A., Sawers G., Roberts R.E., Attwood M.M., Green J.. Expression of the Escherichia coli yfiD gene responds to intracellular pH and reduces the accumulation of acidic metabolic end products. Microbiology. 2002; 148:1015–1026. [DOI] [PubMed] [Google Scholar]
- 54. Padan E., Bibi E., Ito M., Krulwich T.A.. Alkaline pH homeostasis in bacteria: new insights. Biochim. Biophys. Acta. 2005; 1717:67–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kim D., Hong J.S., Qiu Y., Nagarajan H., Seo J.H., Cho B.K., Tsai S.F., Palsson B.O.. Comparative analysis of regulatory elements between Escherichia coli and Klebsiella pneumoniae by genome-wide transcription start site profiling. PLoS Genet. 2012; 8:e1002867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lalaouna D., Masse E.. The spectrum of activity of the small RNA DsrA: not so narrow after all. Curr. Genet. 2016; 62:261–264. [DOI] [PubMed] [Google Scholar]
- 57. Soper T.J., Woodson S.A.. The rpoS mRNA leader recruits Hfq to facilitate annealing with DsrA sRNA. RNA. 2008; 14:1907–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mika F., Hengge R.. Small RNAs in the control of RpoS, CsgD, and biofilm architecture of Escherichia coli. RNA Biol. 2014; 11:494–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bordeau V., Felden B.. Curli synthesis and biofilm formation in enteric bacteria are controlled by a dynamic small RNA module made up of a pseudoknot assisted by an RNA chaperone. Nucleic Acids Res. 2014; 42:4682–4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Saliba A.E., S C.S., Vogel J.. New RNA-seq approaches for the study of bacterial pathogens. Curr. Opin. Microbiol. 2017; 35:78–87. [DOI] [PubMed] [Google Scholar]
- 61. Holmqvist E., Wright P.R., Li L., Bischler T., Barquist L., Reinhardt R., Backofen R., Vogel J.. Global RNA recognition patterns of post-transcriptional regulators Hfq and CsrA revealed by UV crosslinking in vivo. EMBO J. 2016; 35:991–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chao Y., Papenfort K., Reinhardt R., Sharma C.M., Vogel J.. An atlas of Hfq-bound transcripts reveals 3′ UTRs as a genomic reservoir of regulatory small RNAs. EMBO J. 2012; 31:4005–4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Melamed S., Peer A., Faigenbaum-Romm R., Gatt Y.E., Reiss N., Bar A., Altuvia Y., Argaman L., Margalit H.. Global mapping of small RNA-target interactions in bacteria. Mol. Cell. 2016; 63:884–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Waters S.A., McAteer S.P., Kudla G., Pang I., Deshpande N.P., Amos T.G., Leong K.W., Wilkins M.R., Strugnell R., Gally D.L. et al. Small RNA interactome of pathogenic E. coli revealed through crosslinking of RNase E. EMBO J. 2017; 36:374–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lalaouna D., Masse E.. Identification of sRNA interacting with a transcript of interest using MS2-affinity purification coupled with RNA sequencing (MAPS) technology. Genomics Data. 2015; 5:136–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Han K., Tjaden B., Lory S.. GRIL-seq provides a method for identifying direct targets of bacterial small regulatory RNA by in vivo proximity ligation. Nat. Microbiol. 2016; 2:16239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.