Abstract
Purpose
To characterize a new species of parasitic nematode that triggers uveitis.
Observations
Three previously healthy, relatively young people each contracted a corneal stromal nematode that, upon surgical removal and examination, did not match any known nematodes. Clinical ocular findings included corneal opacification, visible corneal worms, conjunctival injection, and uveitis.
Conclusions and Importance
The three cases presented here represent a previously undescribed parasitic infection of the cornea by an unidentified nematode. These findings may represent a previously unrecognized zoonotic infection from wildlife sources and potentially a newly documented nematode requiring description. Future clinical findings regarding this newly described nematode are needed to further develop our understanding of the disease.
Keywords: Parasite, Nematode, Ocular, Uveitis, Cornea, Stroma
1. Introduction
Ocular parasites—including protozoa, nematodes, cestodes, and trematodes—are well-documented, and ocular parasitosis has been found to be significantly more common in regions with favorable environmental factors and poor sanitary conditions.1, 2, 3 In these regions, ocular parasitosis can be endemic in the canine and feline populations, as well as in a range of wildlife species including other mammals or birds, providing a breeding ground from which arthropod vectors can transmit parasites to humans.2 However, it is unusual to find a live worm in intraocular structures. Nematode parasites do not usually proliferate within their definitive hosts, but rather grow, molt, mature as dioecious adults in specific anatomical sites, mate, and then produce eggs, larvae or microfilariae.4 During this life cycle, worms can migrate to different locations within the body, including the eye1, 2, 3, 4; migration takes place via blood borne carriage or through tissue to the eye or adjacent structures.1, 2, 3, 5,5 The eye's immune privilege may allow further growth and development relative to other tissues,6, 7, 8, 9, 10, 11 and helminth parasites can infect the conjunctiva, eyelid, and intraocular cavities.1, 2, 3 A diverse assemblage of zoo parasitic nematodes have been documented in ocular infections in people, and involve both fully developed nematodes or larval stages: for example, zoonotic species of Onchocerca have been documented to involve the cornea (probably Onchocerca cervicalis)2,12,14 and the anterior chamber.2,13,14 The following is a report of three patients from the southwestern Pacific island of Saipan, in the Mariana Islands, who presented with corneal stromal nematodes between 1997 and 2009. We believe these nematodes to be of a previously undescribed species.
2. Findings
2.1. Case 1
Two weeks prior to presenting to an ophthalmologist in March 1997 on Saipan, the patient, a healthy 29-year-old Chamorro male without prior ocular, medical, or surgical history, had seen an optometrist for photophobia of 2 weeks duration in his left eye. He reported having traveled to Honolulu, Hawaii two weeks prior to development of his symptoms, but had not been outside either the Hawaiian Islands or the Mariana Islands recently. He was placed on prednisolone acetate 1% q 1 hour, and homatropine 5% TID.
On examination by the ophthalmologist, his best corrected visual acuity with plano lenses was RE 20/20, LE 20/25+. External exam, pupils, and motility were normal, and intraocular pressure was 10 mmHg in both eyes. Slit lamp examination of the right eye was unremarkable.
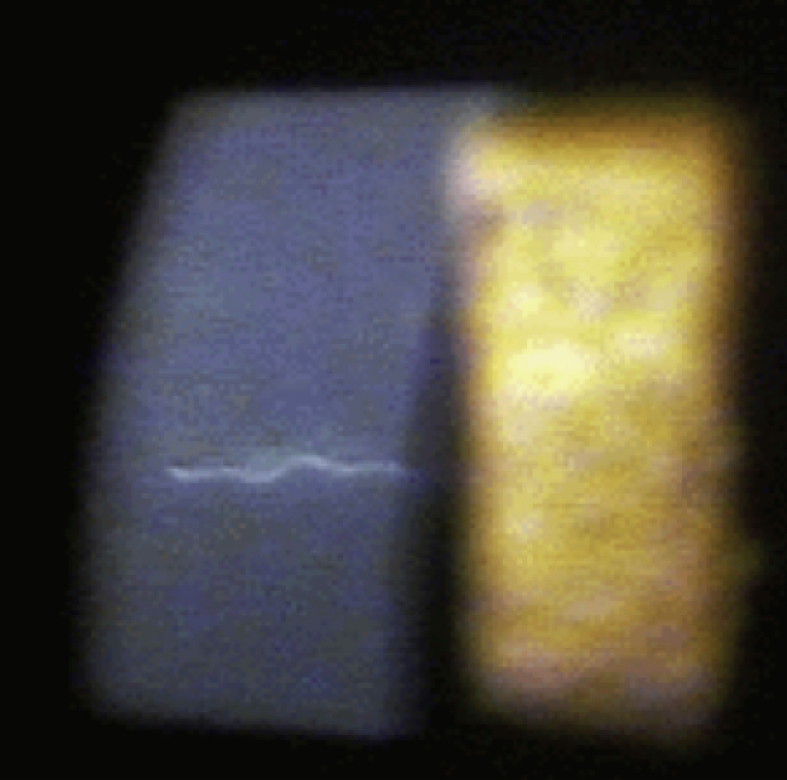
The left eye showed areas of peripheral and mid-peripheral corneal opacification. There was no injection. There was a 1.5 mm long translucent motile worm located approximately ⅔ depth within the mid-periphery of the corneal stroma (Supplemental Fig. 1). The movement of the worm was primarily undulating, and its speed through the cornea was not fast enough to note any forward or backward movement. The worm appeared to be photophobic, contracting to light from the slit lamp beam.
The anterior chamber showed rare cells and no flare. Dilated fundus exam was normal for both eyes, without evidence of vitreous cell, posterior segment parasites, or chorio-retinal tracks. The prednisolone dose was decreased to every 2 hours, and the homatropine to once daily. Complete blood count was normal with no eosinophilia. Liver function tests were also normal. Stool examination showed no mature or larval parasites or eggs.
Over the ensuing weeks, the worm was noted to traverse the mid-peripheral cornea, moving as far as 2–3 mm per day, but often remaining in the same area of the cornea: 3–4 mm from the limbus. There was no change during this period in the visual acuity, corneal opacity, or anterior inflammation. It had been hoped that the worm would move far enough to the periphery to allow a direct cut-down over the worm, in order to remove the worm with minimal refractive effect of the incision.
After 2 weeks of observation, surgical intervention was considered. Removal of the worm was attempted with a slit lamp, as visualization under the operating microscope was impaired. A 22-degree SuperBlade™ was used to place a 2 mm horizontal incision ⅔ depth into the cornea directly over the worm, at the 5:00 mid-periphery. As the incision was made, it was noted that the intracorneal worm was moving vigorously away from the incision site. Viscous lidocaine was placed into the incision, and the worm stopped moving. However, the light reflexes from the corneal stroma and the incision made it difficult to distinguish the worm from the corneal stroma. Fluorescein dye was placed, which did not significantly highlight the worm. After multiple attempts to grasp and remove what may have been the worm through the incision, the decision was made to cease further manipulations. The cornea and anterior chamber were carefully examined, and it was noted that the parasite was not visible in either, so it was presumed to be present in the area of the incision. The partial thickness incision was left unsutured. Antibiotic ointment and a patch were placed, and the patient discharged.
On postoperative day 1, visual acuity decreased to 20/40. There was 3 + conjunctival injection. The horizontal incision site at 5:00 was well approximated, and at 3:00 in the mid-periphery, the live worm was visible. The patient and his wife were highly distressed. The decision was made to attempt to kill the worm.
An argon laser was used through an Abraham lens, at a spot size of 100 μm, and duration of 0.1 msec. Beginning at 80 mW, the power was titrated up to 400 mW, focusing treatment on the ends of the worm. A total of 35 spots were placed along the length of the worm, and at the end of the treatment, the worm had ceased moving. The following day, the worm had moved to the 2:00 mid-periphery. It was motile and continued to contract when exposed to the slit lamp beam.
The patient was referred to a corneal specialist for further evaluation and management. The worm was measured at the slit lamp to be approximately 1500 μm long, at ⅔ depth into the paracentral cornea.
It was considered that destroying the worm by cryoablation or photoablation might incite a severe immunologic reaction, and for this reason, the decision was made to proceed with surgical removal. Because topography showed that the previous vertical incision had resulted in astigmatism, it was elected to use an astigmatically neutral lamellar surgical approach and “bring the organism to the surface”.
Six weeks after presentation, the patient was brought to the operating room, and under local anesthesia, a disposable Katena™ Barron-Hessburg suction trephine was used. The trephine was centered to include the worm, and trephination done to approximately 300 μm. The parasite was removed intact and passed directly to the parasitologist. The specimen, however, was lost during processing.
Prednisolone and homatropine were gradually tapered. Four months after surgery, there were no signs of active inflammation. Visual acuity was 20/50, correctable to 20/20. The patient showed no signs of systemic or further ocular parasitic infection.
2.2. Case 2
In November 2005, a healthy 8-year-old Chamorro male presented on Saipan with a 3 week history of redness, itching, glare sensitivity and blurred vision OD. He had no past ocular or medical history. He had traveled one month prior to Houston, Texas, and 10 months prior to that to southern California.
His visual acuity was RE 20/30, correctable to 20/25 (−0.50 + 1.00 × 065), LE 20/20. External exam, pupils and motility were normal. Slit lamp exam of the right eye showed diffuse +1 conjunctival injection. The cornea showed diffuse subepithelial opacities from the 11:00 limbus to 2:30, with surrounding cell (Supplemental Fig. 2). The corneal epithelium showed no staining or ulceration. Within the corneal stroma, at the 2:30 o'clock position mid-periphery, a 1 mm long motile worm was visible. It was translucent with tapered ends and seemed to have a visible cavity through the center along the longitudinal axis. This translucency was presumed to be an intestine. The worm contracted to light. The anterior chamber showed no cell or flare. The iris and lens were normal. The slit lamp exam of the left eye was unremarkable. Intraocular pressure was normal in both eyes. Dilated retinal exam was normal in both eyes, with no vitreous cell, and no signs of retinal or choroidal tracks.
Systemic evaluation by a pediatrician was normal, as were laboratory examination of stool for parasites, complete blood count including eosinophils, abdominal ultrasound, and serum chemistries.
The patient was referred for removal of the corneal worm. Exams during the ensuing 2 weeks showed that the worm remained in the same area of the cornea, though it rotated its orientation relative to the limbus, from 90° to the limbus to 60° to the limbus. The worm was extracted in December 2005 through a freehand lamellar dissection with a limbal incision into the cornea. The worm, however, was not removed intact, and there was no specimen available for pathological or parasitological examination.
Postoperatively, the patient did well, with visual acuity returning to 20/20 within one week. Slit lamp exam showed no visible worm remnants. The patient has been followed for 10 years postoperatively, and his vision has remained 20/20, with slight corneal scarring superiomedially. There have been no signs of recurrence of the ocular worm during this period.
2.3. Case 3
In December 2008, a generally healthy, 34-year-old Chamorro woman with a history of soft contact lens wear for myopia presented to an optometrist with blurred vision in her right eye for one week. Her travel history was negative except for a one week trip to San Diego, California, 3 weeks prior.
Her visual acuity with soft contact lenses was 20/30 OD, 20/20 OS. Slit lamp exam OD noted no injection, +2 diffuse superficial punctate keratitis with staining, and +1 diffuse central stromal haze. There were no anterior chamber cells or flare. The anterior segment of the left eye was normal. It was assumed that the signs were related to the contact lens, and the contact lenses were discontinued, with instructions to return in 3 days. No improvement was noted, and the patient was referred to an ophthalmologist.
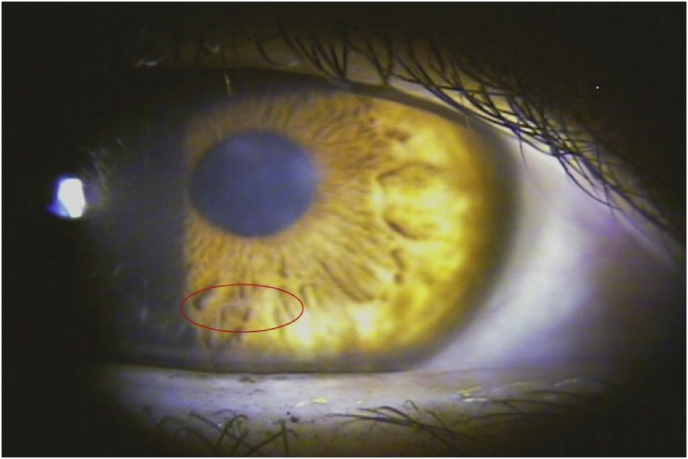
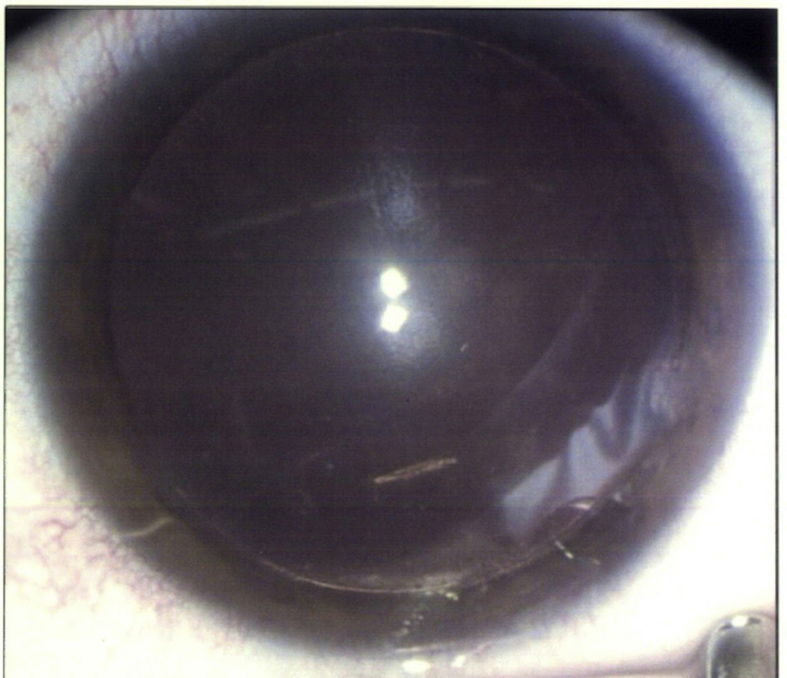
On examination, her visual acuity with glasses was 20/50–2 OD (−2.75 sph), and 20/20 OS (−3.00 sph). Slit lamp exam OD showed trace conjunctival injection. The cornea showed diffuse epithelial haze extending to the anterior stroma with 2–3+ central endothelial keratic precipitates (Fig. 1). Inferiorly in the mid-peripheral stroma, a 1 mm long and very thin translucent motile worm was noted (Fig. 1, Supplemental Fig. 3). The anterior chamber was clear with no cell or flare. The iris and lens were normal. The slit lamp exam of the left eye was unremarkable. Intraocular pressure was normal in both eyes. Dilated retinal exam was normal in both eyes, with no vitreous cell, and no signs of retinal or choroidal tracks.
Fig. 1.
Intrastromal haze and poorly visible worm inferiorly (circled) in Case 3.
There is diffuse epithelial haze extending to the anterior stroma with 2–3+ central endothelial keratic precipitates. Inferiorly in the mid-peripheral stroma, a 1 mm long and very thin translucent motile worm is poorly visible.
Because of the inflammation, she was placed on topical prednisolone acetate OD every 2 hours, and was followed closely while awaiting referral to Hawaii for surgical removal of the worm. Within 10 days, she had only trace keratic precipitates, and her prednisolone acetate had been tapered to 3 times a day.
During the ensuing month, logistical arrangements were made for her transfer to Hawaii for surgery. By the date of her transfer in February, 2009, her vision had improved to 20/30, the keratic precipitates were noted to be “trace”, while the prednisolone acetate had been tapered to one drop daily. The worm remained alive, and in the same region of the cornea with +1 epithelial haze. The patient had a negative systemic workup, including blood and stool cultures for parasitic infection.
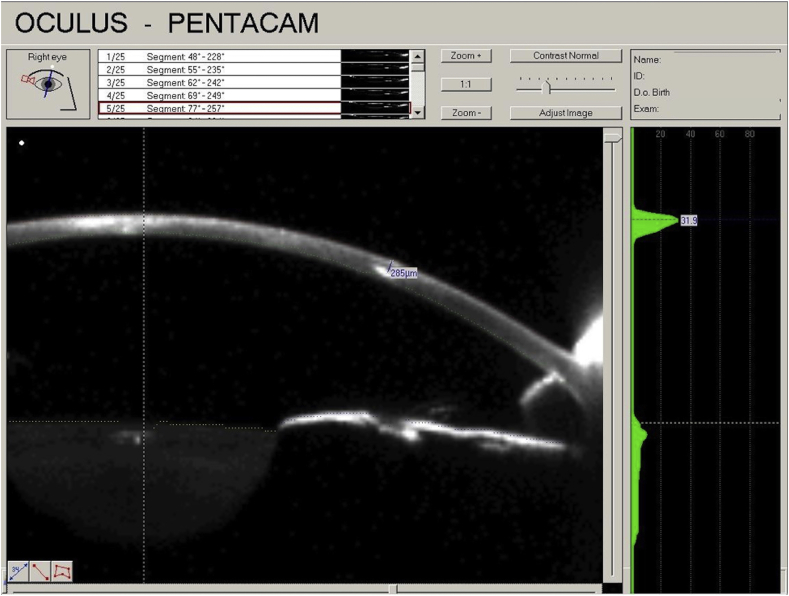
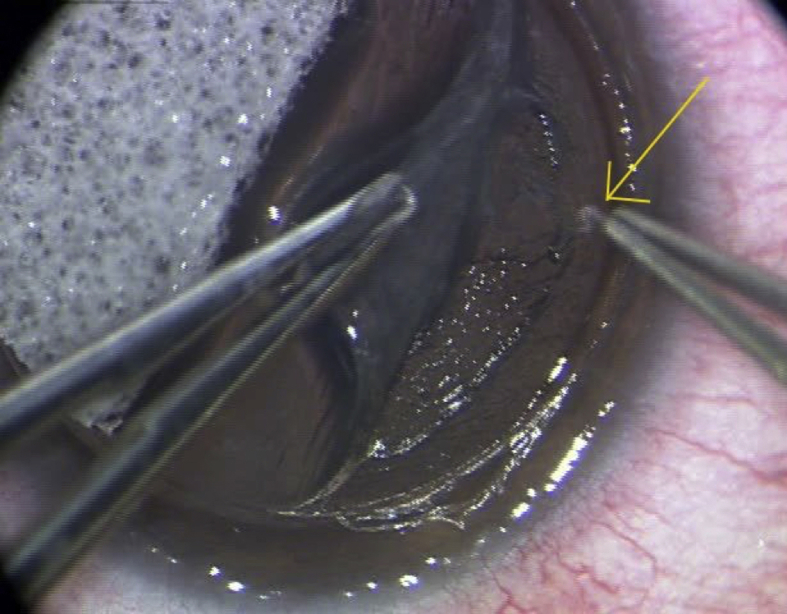
Upon arriving in Hawaii, the patient was examined and noted to have a live worm in the cornea. Diagnostic examination by a Pentacam™ was performed and a Scheimpflug image was obtained (Fig. 2). Given the success of using a lamellar approach in the prior cases, the patient was taken to surgery. The patient was dilated preoperatively in order to improve visualization against a red reflex (Supplemental Fig. 4). The Intralase™ femtolaser used to perform a precise trephination and partial lamellar dissection above the depth of the worm (Supplemental Fig. 5).
Fig. 2.
Scheimpflug showing the depth of the intrastromal worm in Case 3.
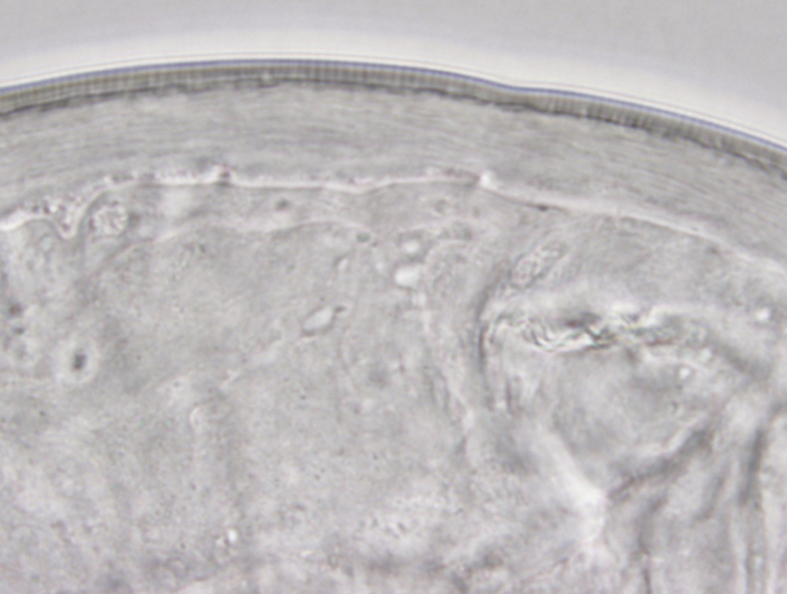
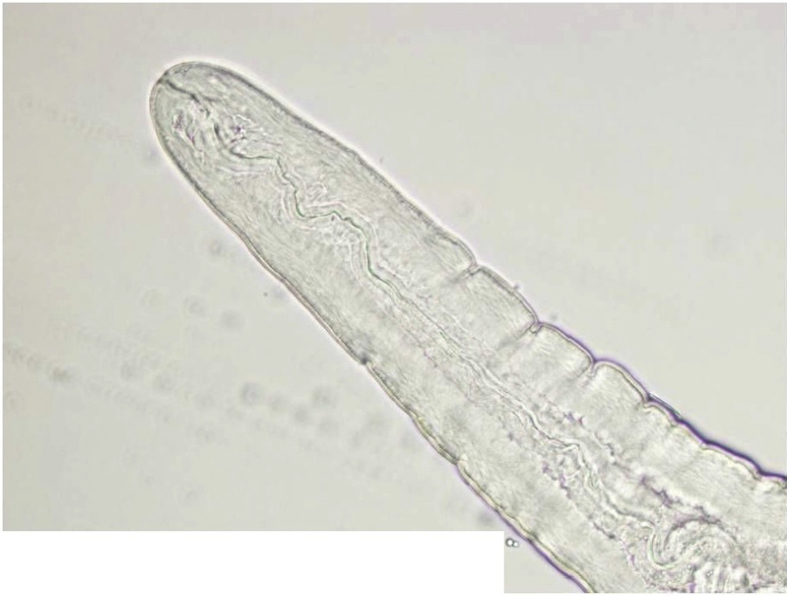
Because of the small size and translucent organism, the worm was placed in a single drop of balanced salt solution in a clear petri dish and directly handed off to the parasitologist for microscopy and processing. Microscopic photos were taken (Fig. 3 and Supplemental Figs. 6–11). The specimen was sent to the Armed Forces Institute of Pathology (AFIP), Washington, DC.
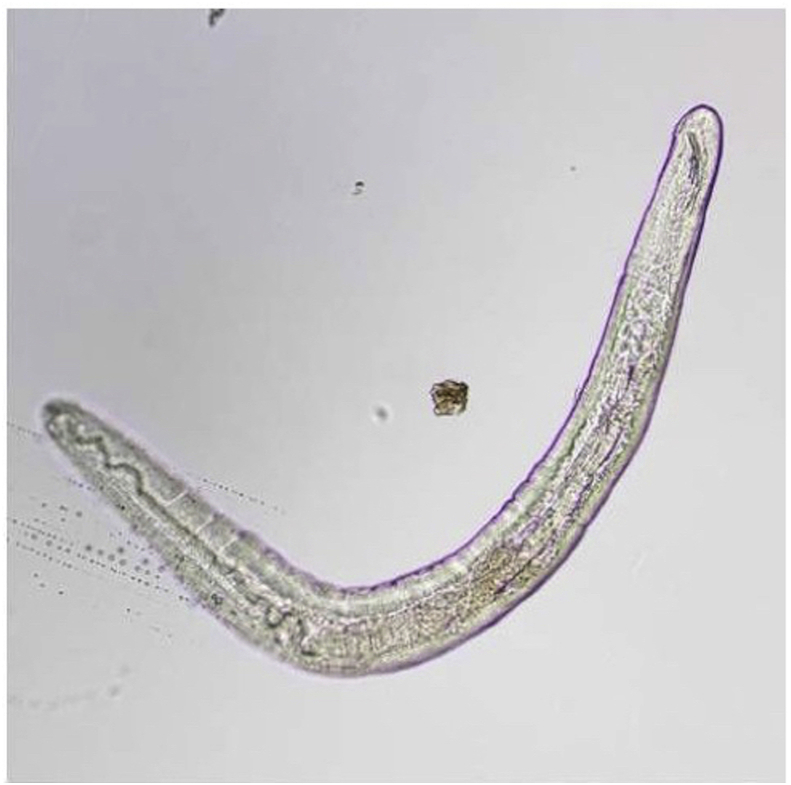
Fig. 3.
Entire worm in Case 3.
An intact white translucent adult male nematode measuring 1,050 μm in length and 90 μm in diameter with slightly tapered ends, visualized by microscope.
Postoperative visual acuity improved to 20/20, with mild corneal haze. Over the ensuing 3 years, vision gradually decreased to 20/80, with opacification of the cornea which was felt to be the result of epithelial downgrowth. There was no recurrence of a corneal worm. The patient was lost to follow-up after 4 years.
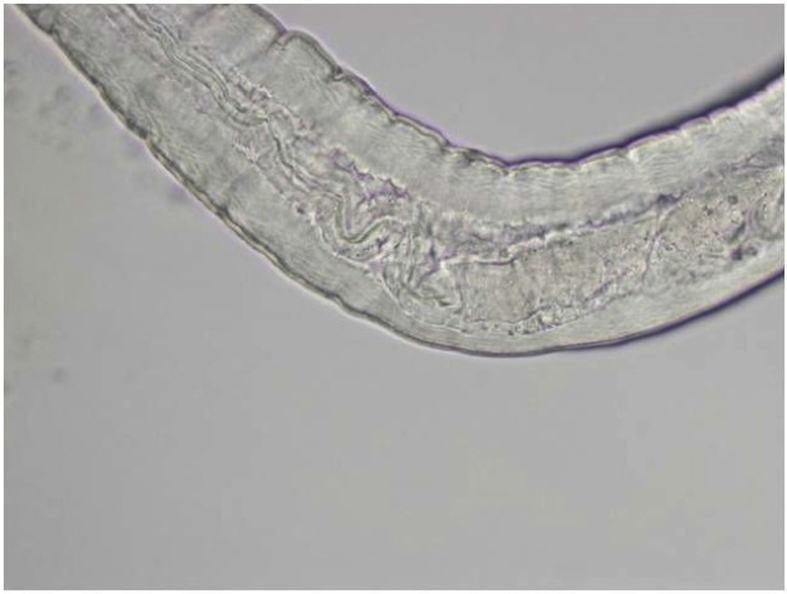
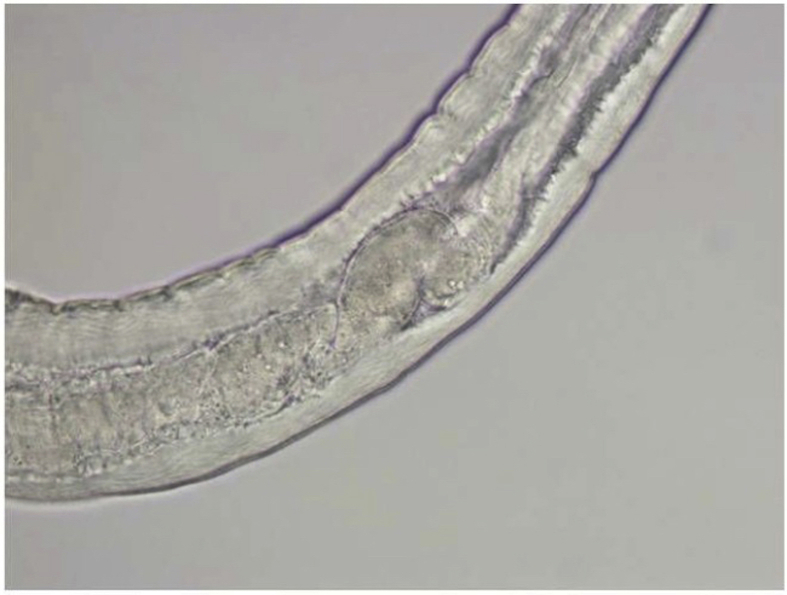
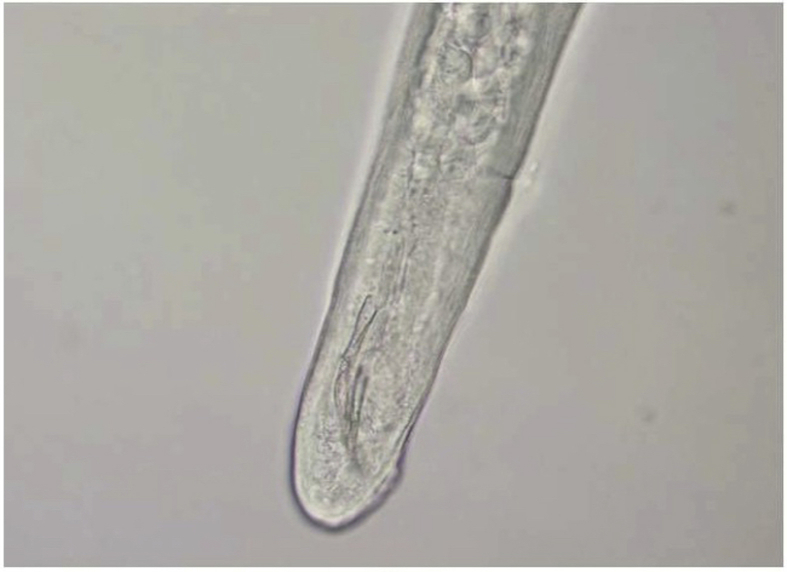
Gross microscopic examination at the AFIP revealed an intact white translucent adult male nematode which was 1050 μm in length and 90 μm in diameter (Fig. 3). It was uniform in diameter with slight tapering at both ends, and bluntly rounded cephalic and caudal extremities. The cuticle was 2 μm thick and finely striated (Supplemental Fig. 6). The mouth or stoma leads to a cuticular-lined esophagus that was about 350 μm long and occupied about 1/3 of the length of the worm (Supplemental Figs. 7–9). The base of the esophagus revealed no glandular or muscular modifications. The intestine was a thick tube about 35 μm in diameter (Supplemental Fig. 10). The anus was about 30 μm from the posterior tip of the worm. The posterior end of the worm had 2 spicules: a long, jointed spicule measuring 65 × 17 μm, and a short simple spicule measuring 40 × 12 μm (Supplemental Fig. 11). It was attempted to perform histological examination; unfortunately, however, the specimen was lost in processing.
3. Discussion
Although a specimen for pathological examination was obtained from only one of the patients, it is presumed that all 3 were infected with the same genus and species of nematode, based on the clinical presentation, course, and morphology on slit lamp examination. All 3 patients were otherwise healthy, relatively young and living on the island of Saipan in the Mariana Islands when infected with an ocular worm. Each patient presented with mild ocular symptoms (such as mild blurred vision, redness or photophobia) and was found to have only corneal stromal inflammation. None of the patients had signs of posterior segment or systemic infection.
In each case, there was a single motile translucent intrastromal corneal nematode, with a relatively robust or stout body, not tapering strongly in the caudal and cephalic extremities, and ranging in length from approximately 1 to 1.5 mm. The worms had features common to all nematodes: specimens are round with bilateral symmetry of the body and radial symmetry for the cephalic region, and possess a complete digestive tract, separate sexes and a protective nonliving cuticle. The base of the esophagus is not modified, and accessory esophageal glands were not evident. Phylum Nematoda and particularly the monophyletic Class Chromadorea (formerly within the paraphyletic Secernentea) and the Class Dorylaimea contain the most parasitic worms infecting humans and otherwise is a very large group of megadiversity.15 The only specimen available for gross examination was a completely developed adult male nematode with fully cuticularized spicules, which are accessory copulatory organs. The length of the morphologically dissimilar spicules (65 μm and 40 μm) is the most specific feature apparent that can be used in identification.
We considered the nematodes that have been reported to infect the cornea, anterior segment or bulbar conjunctiva of humans and primates, and a range of other mammals and birds. Among these, all of those that occur as adults in mammalian hosts can be excluded because of the length or morphology of their spicules and site of localization in the definitive host (Table 1). The dorylaim nematodes and especially the Muspiceoidea (miniscule parasites occurring in tissues of bats and mice) due to the presence of esophageal glands in the basal region of the esophagus, which are lacking in the specimen from Saipan.16
Table 1.
Characteristics of nematodes reported to infect the eye as adults.
| Species | Usual site in definitive host | Range of length of spicules (μm) or distinctive morphology |
|
|---|---|---|---|
| Right | Left | ||
| Ancylostoma caninum17 | Intestine in dog and cat | Copulatory bursa | |
| Angiostrongylus cantonensis2,18,19 | Lung in rats | 1200 | 1200 |
| Copulatory bursa | |||
| aBrugia malayi2,18,20 | Soft tissue in human | 100–120 | 290–365 |
| aDipetalonema arbuta21, 22, 23 | Peritoneal cavity in porcupine | 112 | 220 |
|
aZoonotic Dirofilaria species2,11,20,24: D.immitis D. repens D. tenuis |
Pulmonary artery in dog (D. immitis) Subcutaneous in dog (D. repens) Subcutaneous in raccoon (D. tenuis) |
100–229 | 210–590 |
| Dracunculus medinensis18,25 | Skin in human | 490–730 | 490–730 |
| aDunnifilaria ramachandrani26,27 | Heart in rats | 65–80 | 70–90 |
| aLoa loa18,23,28 | Subcutaneous in human | 123 | 88 |
| aLoaina sp probably similar to Loaina (Dirofilaria) uniformis2,23,29,30 | Soft tissue in rabbits | 131 | 94 |
| aMacacanema formosana31,32 | Soft tissue in monkeys | 120 | 512–590 |
| aMansonella perstans2,3 | Subcutaneous in human | 287–360 | 125 |
| aMolinema (Dipetalonema, Acanthocheilonema) reconditum2,33 | Soft tissue in dog | 92–104 | 220–300 |
| aMolinema (Dipetalonema) sprenti2,21,34 | Peritoneal cavity in beaver | 170 | 270 |
|
aZoonotic Onchocerca species2,12,18,35, 36, 37, 38: O. cervicalis O. dewittei japonica O. gutturosa O. jakutensis O. lupi O. reticulata |
Ligaments and tendons in horse (O. cervicalis) Subcutaneous in boar (O. dewittei japonica) Ligament in cattle (O. gutturosa) Subcutaneous in deer (O. jakutensis) Sclera in dog (O. lupi) Ligament in horse (O. reticulate) |
64–295 | 116–360 |
|
Oxyspirura species18,39,40: O. mansoni O. youngi |
Conjunctival sac, nasolacrimal duct, nictitating membrane in birds and primates |
145–220 | 1125–3500 |
| aParafilaria bovicola18,41 | Subcutaneous in cattle | Single spicule | |
| aPelecitus sp.42, 43, 44 | Soft tissue in birds | 62–66 | 81–82 |
| aSetaria labiatopapillosa18,45 | Peritoneal cavity in cattle and buffalo | 100–160 | 250–280 |
| Thelazia californiensis2,18,46 | Conjunctiva and nictitating membrane in cat, dog, rabbits, carnivores | 1500–1700 | 150–187 |
| aWuchereria bancrofti18,23 | Lymphatics in human | 190–250 | 490–650 |
Species belonging to Family Onchocercidae.
We considered the possibility that some nematodes that could infect the eye in the juvenile stage might under unusual circumstances mature to the adult stage in this aberrant anatomic location. Most of these worms can also be excluded because of the morphology or considerably greater length of spicules in adults (Table 2). Other potential Rhabditida or Pangrolaimida including some genera and species in the families Rhabditidae and Cephalobidae can also be excluded based on distinct morphological attributes. Males remain unknown among some cephalobes such as Halicephalobus.52
Table 2.
Length of spicules of nematodes reported to infect the eye as larvae.
| Species | Usual site in definitive host | Range of length of spicules (μm) |
|
|---|---|---|---|
| Right | Left | ||
| Baylisascaris procyonis2,47,48 | Raccoon | 380–620 | 380–620 |
| Gnathostoma species2,18,49 | Carnivores | 400–800 | 1100–2600 |
| Toxocara canis2,18 | Dog | 750–1300 | 750–1300 |
| Toxocara cati2,50 | Cat | 1700–1900 | 1700–1900 |
| Trichinella spiralis51 | Human, swine, bears, raccoon, foxes | No spicules | |
We suspect that, although the specimen examined was an adult having completed the 4th molt, the worm had not reached its complete length perhaps because it had recently molted or because it was in an unusual anatomic location and host. Identification of the worm would require collection of another specimen in order to develop comparative DNA sequence data which could be diagnostic, and examination of the distribution of caudal papillae in male specimens, an important morphologic feature in classification. Although we are unable to identify the genus and species of this worm, it has morphological features that are consistent with some genera and species referred to the Superfamily Filarioidea (order Spirurida) especially within the Family Onchocercidae. Specific attributes consistent with such a provisional identification include a strongly rounded head, lack of cephalic development or expansion, a cuticular-lined, undivided, esophagus that occupies one-third of the worm's length, absence of modification in the basal region of the esophagus, dissimilar spicules of different lengths (left longer than right, and with a joint separating the shaft from the lamina), and a very short, blunt and rounded tail lacking apparent alae or ventral genital papillae. Furthermore, among many Onchocercinae (including some number of genera typical of bats, such as species of Litomosa, and species of Dipetalonema and Onchocerca in mammalian hosts of diverse orders), the left spicule is considerably longer than the right and possesses a characteristic joint separating the shaft and lamina. Most of the species in Table 1, Table 2, encompassing those reported in ocular infections, belong to the Family Onchocercidae.
There are many vertebrate parasites in the Onchocercidae that have not yet been shown to cause zoonotic eye infections in humans, but which may have the potential to do so. Overall there are about 70–80 genera of onchocercids partitioned across 8 subfamilies including the Onchocercinae which contains the greatest taxonomic diversity for species circulating in birds or mammals. For example, species of at least 16 genera of filarioid nematodes, in addition to those of Pelecitus, have been described as parasites of wild birds alone.44
Because of the rarity of this infection and the fact that it has not been described previously, it is likely a zoonotic infection. The mode of transmission is unknown. Parasites can reach the cornea either by migration from the anterior chamber or by direct inoculation from the external environment. It is most likely that the worm penetrated the cornea from the anterior chamber, which would imply that the worm was inoculated into the patient as a larval form by an arthropod vector and then developed into an adult worm somewhere in the body before migrating to the anterior chamber and finally the cornea (Cameron J, personal communication, 2016).
Most zoonotic nematodes that have a tropism for the eye reach the eye by migration through the host tissue (possibly through the optic nerve) or through the circulation while in the larval stage.2 It is not known which route is more common or which are the preferred or aberrant routes. The eye is an immune privileged site that may allow for growth and development of a parasite beyond what could occur in other tissues.5, 6, 7 Among filarioids, the transition from microfilaria to larva occurs in an arthropod host; therefore, if this worm is a filarioid, it did not migrate to the eye as a microfilaria.
Larval stage filarial nematodes such as those of Onchocerca spp., Loa loa, and Dirofilaria spp., are inoculated by arthropod bite into the definitive host.1, 2, 3, 53,53 The development of microfilaremia requires copulation of mature adult male and female worms. None of the 3 patients reported here was found to have microfilaremia or peripheral eosinophilia suggesting that fully developed adult nematodes were not present in other anatomical sites. Some other nematodes, such as Gnathostoma spp. and Toxocara canis, are acquired through the ingestion of eggs or larvae, sources of which include contaminated water, soil and undercooked meat and vegetables.1,2
It is rare for nematodes to infect the eye by direct inoculation, such as Thelazia sp.46 Because the human eye has a number of defense mechanisms in place to protect against microorganism breach, trauma is usually required for parasites to enter through this route.1,2 Though this may have been a mechanism in one patient who was a contact lens wearer (Case 3), there was no source of trauma or microtrauma in the other two cases. Rare corneal involvement has been documented with onchocerciasis2,12 and gnathostomiasis.49
In brief, all three patients presented with mild anterior segment inflammation, and were found to have a motile translucent corneal intrastromal nematode 1–1.5 mm in length. The one specimen that was successfully retrieved was a completely developed male nematode. Because the specimen was lost in processing prior to histological examination, only its morphological features are known. In this specimen, the spicules, a form of accessory copulatory organs, are the most specific feature that can be used for identification. Based on this and other morphological features, all nematodes known to infect the anterior segment in humans, primates, and a range of other mammals and birds, either as adult nematodes, or in a juvenile state, can be excluded. This nematode's morphological features are consistent with some genera and species in the Superfamily Filariodra, especially the Family Onchoceridae. Based upon the rarity of infection, it is likely a zoonotic nematode, however the mode of transmission is unknown. The most likely route of transmission is inoculation of the patient with the larval state by an arthropod vector, with maturation into an adult somewhere in the body, before migration to the anterior chamber and penetration into the cornea.
4. Conclusions
The 3 cases presented here represent a previously undescribed parasitic infection of the cornea by an unidentified nematode. The gross description of the worm does not match those of previously reported ocular parasites. Infections represented in these cases are likely attributable to a single species, which may represent a previously unrecognized zoonotic infection from wildlife sources and potentially a newly documented nematode requiring description. Resolution of the source and identity of these nematodes can be based on early recognition of infection, and collection of archival specimens from the Southwest Pacific suitable for detailed comparative morphological study and comparative molecular-based analyses.
Patient consent
Informed consent was obtained from the patients for the use of their health information.
No IRB approval was obtained for this case series.
Funding
No funding or grant support.
Conflicts of interest
None.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
None.
The views expressed in this article are those of the authors and do not reflect the official policy of the Department of the Army, Navy or Air Force, Department of Defense, or US Government.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ajoc.2018.01.013.
Contributor Information
Shan McBurney-Lin, Email: shan.mcburney.lin@duke.edu.
David Khorram, Email: david@marianaseye.com.
Stephen Gee, Email: stephen2gee@yahoo.com.
Eric P. Hoberg, Email: geocolonizer@gmail.com.
Mary K. Klassen-Fischer, Email: mary.k.klassen-fischer.civ@mail.mil.
Ronald C. Neafie, Email: rfneafie1519@gmail.com.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Supplemental Fig. 1.
Intrastromal corneal worm in Case 1. A 1.5 mm long translucent motile worm located approximately ⅔ depth within the mid-periphery of the corneal stroma is visible.
Supplemental Fig. 2.
Evidence of intrastromal inflammation in Case 2. The cornea shows diffuse subepithelial opacities from the 11:00 limbus to 2:30, with surrounding cell.
Supplemental Fig. 3.
Slit lamp exam showing worm in Case 3. A clearer image of the 1 mm long, thin translucent motile worm upon slit lamp exam.
Supplemental Fig. 4.
Surgical view of worm visible on retro-illumination in Case 3. The patient was dilated preoperatively in order to improve visualization against a red reflex.
Supplemental Fig. 5.
Worm on the forceps in Case 3. The worm was removed after a precise trephination and partial lamellar dissection above the depth of the worm.
Supplemental Fig. 6.
Striated cuticle of worm in Case 3. The worm was uniform in diameter, and had a thick, finely striated cuticle measuring 2 μm in width.
Supplemental Fig. 7.
Anterior tip of worm in Case 3 demonstrating mouth and cuticular-lined esophagus.
Supplemental Fig. 8.
Anterior of worm in Case 3. A cuticular-lined esophagus occupies about 1/3 of the worm's length in Case 3.
Supplemental Fig. 9.
Esophageal-intestinal junction of worm in Case 3.
Supplemental Fig. 10.
Intestine of worm in Case 3 measuring 35 μm in diameter.
Supplemental Fig. 11.
Two unequal dissimilar spicules at posterior end of worm in Case 3.
References
- 1.Klotz S., Penn C., Negvesky G., Butrus S. Fungal and parasitic infections of the eye. Clin Microbiol Rev. 2000;13(4):662–685. doi: 10.1128/cmr.13.4.662-685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otranto D., Eberhard M. Zoonotic helminths affecting the human eye. Parasites Vectors. 2011;4:41. doi: 10.1186/1756-3305-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nimir A.R., Saliem A., Ibrahim I.A.A. Ophthalmic parasitosis: a review article. Interdiscip Perspect Infect Dis. 2012;2012:587402. doi: 10.1155/2012/587402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magill A.J., Strickland G.T., Maguire J.H., Ryan E.T., Solomon T. Elsevier; China: 2000. Helminthic Infections. Hunter's Tropical Medicine and Emerging Infectious Disease. [Google Scholar]
- 5.Streilein J. Karger; Basel, Switzerland: 1999. Regional Immunity and Ocular Immune Privilege. Immune Response and the Eye. [DOI] [PubMed] [Google Scholar]
- 6.Taylor A.W. Ocular immune privilege. Eye. 2009;23(10):1885–1889. doi: 10.1038/eye.2008.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou R., Caspi R.R. Ocular immune privilege. F1000 Biol Rep. 2010;2:3. doi: 10.3410/B2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hori J., Vega J.L., Masli S. Review of ocular immune privilege in the year 2010: modifying the immune privilege of the eye. Ocul Immunol Inflamm. 2010;18(5):325–333. doi: 10.3109/09273948.2010.512696. [DOI] [PubMed] [Google Scholar]
- 9.Chandler J.W., Gillette T.E. Immunologic defense mechanisms of the ocular surface. Ophthalmology. 1983;90(6):583–591. doi: 10.1016/s0161-6420(83)34510-3. [DOI] [PubMed] [Google Scholar]
- 10.Mannis M.J., Smolin G. Mosby; St. Louis, MO: 1996. Natural Defense Mechanism of the Ocular Surface. Ocular Infection and Immunity. [Google Scholar]
- 11.Mittal M., Sathish K.R., Bhatia P.G., Chidamber B.S. Ocular dirofilariasis in dubai, UAE. Indian J Ophthalmol. 2008;56(4):325–326. [PMC free article] [PubMed] [Google Scholar]
- 12.Burr W.E., Jr., Brown M.F., Eberhard M.L. Zoonotic onchocerca (Nematoda:Filarioidea) in the cornea of a Colorado resident. Ophthalmology. 1998;105(8):1494–1497. doi: 10.1016/S0161-6420(98)98035-6. [DOI] [PubMed] [Google Scholar]
- 13.Eberhard M.L., Sims A.C., Bishop H.S., Mathison B.A., Hoffman R.S. Ocular zoonotic onchocerca infection in a resident of Oregon. Am J Trop Med Hyg. 2012;87(6):1073–1075. doi: 10.4269/ajtmh.2012.12-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sallo F., Eberhard M.L., Fok E., Baska F., Hatvani I. Zoonotic intravitreal onchocerca in Hungary. Ophthalmology. 2005;112(3):502–504. doi: 10.1016/j.ophtha.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 15.Hodda M. Phyum nematoda. Zootaxa. 2007;1668:265–293. [Google Scholar]
- 16.Anderson R.C., Bain O. Keys to the genera of the superfamilies rhabditoidea, dioctophymatoidea. Trichinelloidea and Muspiceoidea. In: Chabaud A.G., Wilmott S., editors. CIH Keys to the Nematode Parasites of Vertebrates No. 9. RC Anderson. Commonwealth Agricultural Bureaux; Farnham Royal, UK: 1982. [Google Scholar]
- 17.Kwiatkowska-Kawecka Z. Ancyclostoma caninum (Ercolani) larva in eye's anterior segment. Klin Oczna. 1973;43(3):329–330. [PubMed] [Google Scholar]
- 18.Levine N.D. second ed. Burgess Publishing; Minneapolis, MN: 1980. Nematode Parasites of Domestic Animals and Man. [Google Scholar]
- 19.Sinawat S., Sanguansak T., Angkawinijwong T. Ocular angiostrongyliasis: clinical study of three cases. Eye. 2008;22(11):1446–1448. doi: 10.1038/eye.2008.135. [DOI] [PubMed] [Google Scholar]
- 20.Mohan A., Verghese A., Raman M., Biswas J. Live Brugia malayi in the anterior chamber: a case report from India. Eye. 2014;28(8):1038. doi: 10.1038/eye.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beaver P.C., Meyer E.A., Jarroll E.L., Rosenquist R.C. Dipetalonema from the eye of a man in Oregon, U.S.A. A case report. Am J Trop Med Hyg. 1980;29(3):369–372. doi: 10.4269/ajtmh.1980.29.369. [DOI] [PubMed] [Google Scholar]
- 22.Highby P.R. Dipetalonema arbuta n. sp. (Nematoda) from the porcupine, Erethizon dorsatum (L.) J Parasitol. 1943;29(4):239–242. [Google Scholar]
- 23.Beaver P.C. Intraocular filariasis: a brief review. Am J Trop Med Hyg. 1989;40:40–45. doi: 10.4269/ajtmh.1989.40.40. [DOI] [PubMed] [Google Scholar]
- 24.Ermakova L.A., Nagorny S.A., Krivorotova E.Y., Pshenichnaya N.Y., Matina O.N. Dirofilaria repens in the Russian Federation: current epidemiology, diagnosis, and treatment from a federal reference center perspective. Int J Infect Dis. 2014;23:47–52. doi: 10.1016/j.ijid.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Burnier M., Jr., Hidayat A.A., Neafie R. Dracunculiasisis of the orbit and eyelid: light and electron microscopic observations of two cases. Ophthalmology. 1991;98:919–924. doi: 10.1016/s0161-6420(91)32191-2. [DOI] [PubMed] [Google Scholar]
- 26.Lam H.H., Subrayan V., Khaw G. Intraocular filarial infection. Eye. 2010;24(12):1825–1826. doi: 10.1038/eye.2010.141. [DOI] [PubMed] [Google Scholar]
- 27.Mullin S.W., Balasingham S. Dunnifilaria ramachandrani gen. n., sp. n. (Nematoda: Filarioidea) from the long-tailed giant rat (Rattus sabanus) in Malaysia. Proc Helminthol Soc Wash. 1973;40:47–49. [Google Scholar]
- 28.Hassan S., Isyaku M., Yayo A. Adult Loa loa filarial worm in the anterior chamber of the eye: A first report from savanna belt of Northern Nigeria. PLoS Neglected Trop Dis. 2016;10(4):e0004436. doi: 10.1371/journal.pntd.0004436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baker D.G. John Wiley & Sons; Hoboken, NJ: 2008. Flynn's Parasites of Laboratory Animals. [Google Scholar]
- 30.Botero D., Aguledo L.M., Uribe F.J., Esslinger J.H., Beaver P.C. Intraocular filaria, a Loaina species, from man in Colombia. Am J Trop Med Hyg. 1984;33(4):578–582. doi: 10.4269/ajtmh.1984.33.578. [DOI] [PubMed] [Google Scholar]
- 31.Lau L.I., Lee F.L., Hsu W.M., Pampiglione S., Fioravanti M.L., Orihel T.C. Human subconjunctival infection of Macacanema formosana: The first case of human infection reported worldwide. Arch Ophthalmol. 2002;120(5):643–647. [PubMed] [Google Scholar]
- 32.Natarajan R. Another case of human subconjunctival infection by Macacanema formosana. Arch Ophthalmol. 2003;121(4):584–585. doi: 10.1001/archopht.121.4.584-b. [DOI] [PubMed] [Google Scholar]
- 33.Uni S., Bain O., Suzuki K. Acanthocheilonema delicata n. sp. (Nematoda: Filarioidea) from Japanese badgers (Meles anakuma): description, molecular identification, and Wolbachia screening. Parasitol Int. 2013;62(1):14–23. doi: 10.1016/j.parint.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Anderson R.C. Dipetalonema sprenti n.sp. from Castor canadensis Kuhl. Parasitology. 1953;43(3-4):215–221. doi: 10.1017/s0031182000018576. [DOI] [PubMed] [Google Scholar]
- 35.Uni S., Boda T., Daisaku K. Zoonotic filariasis caused by Onchocerca dewittei japonica in a resident of Hiroshima Prefecture, Honshu, Japan. Parasitol Int. 2010;59(3):477–480. doi: 10.1016/j.parint.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Uni S., Bain O., Takaoka H., Miyashita M., Suzuki Y. Onchocerca dewittei japonica n. subsp., a common parasite from wild boar in Kyushu Island, Japan. Parasite. 2001;8(3):215–222. doi: 10.1051/parasite/2001083215. [DOI] [PubMed] [Google Scholar]
- 37.Koehsler M., Soleiman A., Aspöck H., Auer H., Walochnik J. Onchocerca jakutensis filariasis in humans. Emerg Infect Dis. 2007;13(11):1749–1752. doi: 10.3201/eid1311.070017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mutafchiev Y., Dantas-Torres F., Giannelli A. Redescription of Onchocerca lupi (Spirurida: Onchocercidae) with histopathological observations. Parasites Vectors. 2013;6(1):309. doi: 10.1186/1756-3305-6-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ivanova E., Spiridonov S., Bain O. Ocular oxyspirurosis of primates in zoos: intermediate host, worm morphology, and probable origin of the infection in the Moscow zoo. Parasite. 2007;14(4):287–298. doi: 10.1051/parasite/2007144287. [DOI] [PubMed] [Google Scholar]
- 40.Addison E.M., Forrester D.J., Whitely R.D., Curtis M.M. Oxyspirura youngi sp. n. (Nematoda: Thelaziidae) from the patas monkey, Erythrocebus patas. Proc Helminthol Soc Wash. 1986;53(1):89–93. [Google Scholar]
- 41.Bhaibulaya M., Yoolek A., Kobkijcharoen M. Parafilaria bovicola Tubangui 1934 from a human eye in Thailand. Southeast Asian J Trop Med Publ Health. 2004;35(4):817–819. [PubMed] [Google Scholar]
- 42.Botero D., Aguledo L.M., Uribe F.J., Esslinger J.H., Beaver P.C. Intraocular filaria, a Loaina species, from Man in Columbia. Am J Trop Med Hyg. 1984;33(4):578–582. doi: 10.4269/ajtmh.1984.33.578. [DOI] [PubMed] [Google Scholar]
- 43.Bain O., Otranto D., Diniz D.G. Human intraocular filariasis caused by Pelecitus sp. nematode. Brazil. Emerg Infect Dis. 2011;17(5):867–869. doi: 10.3201/eid1705.101309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bartlett C.M. Filarioid Nematodes. In: Atkinson C., Thomas N., Hunter D., editors. Parasitic Diseases of Wild Birds. Blackwell; Hoboken, NJ: 2008. http://www.integrativescience.ca/uploads/activities/avian-filarioid-nematode-parasites-of-the-world-binder.pdf [Google Scholar]
- 45.Panaitescu D., Preda A., Bain O., Vasile-Bugarin A.C. Four cases of human filariosis due to Setaria labiatopapillosa found in Bucharest, Romania. Roum Arch Microbiol Immunol. 1999;58(2):203–207. [PubMed] [Google Scholar]
- 46.Otranto D., Dutto M. Human thelaziasis, Europe. Emerg Infect Dis. 2008;14(4):647–649. doi: 10.3201/eid1404.071205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kazacos K.R., Vestre W.A., Kazacos E.A. Raccoon ascarid larvae (Baylisascaris procyonis) as a cause of ocular larva migrans. Invest Ophthalmol Vis Sci. 1984;25(10):1177–1183. [PubMed] [Google Scholar]
- 48.Overstreet R.M. Baylisascaris procyonis (Stefanski and Zarnowski, 1951) from the kinkajou, Potos flavus, in Columbia. Proc Helminthol Soc Wash. 1970;37(2):192–195. [Google Scholar]
- 49.Bhattacharjee H., Das D., Medhi J. Intravitreal gnathostomiasis and review of literature. Retina. 2007;27(1):67–73. doi: 10.1097/01.iae.0000224943.98423.e3. [DOI] [PubMed] [Google Scholar]
- 50.Bowman D.D., Hendrix C.M., Lindsay D.S., Barr S.C. John Wiley & Sons; Hoboken, NJ: 2008. Feline Clinical Parasitology. [Google Scholar]
- 51.Kocięcki J., Czaplicka E., Kocięcka W. Ocular system involvement in the course of human trichinellosis: Pathological and diagnostic aspects. Acta Parasitol. 2014;59(3):493–501. doi: 10.2478/s11686-014-0272-9. [DOI] [PubMed] [Google Scholar]
- 52.Rames D.S., Miller D.K., Barthel R. Ocular Halicephalobus (syn. Micronema) deletrix in a horse. Vet Pathol. 1995;32(5):540–542. doi: 10.1177/030098589503200514. [DOI] [PubMed] [Google Scholar]
- 53.Farrar J., Hotez P., Junghanss T., Kang G., Lalloo D., White N. Elsevier; China: 2014. Helminthic Infections. Manson's Tropical Diseases. [Google Scholar]