Abstract
BACKGROUND
Ambulatory blood pressure (BP) monitoring (ABPM) is the preferred method to characterize BP status, and its use in kidney transplant recipients is increasing. Data on longitudinal ambulatory BP (ABP) trends in pediatric and young adult kidney transplant recipients are limited.
METHODS
Retrospective review of a large cohort of children and young adults following kidney transplantation and evaluation of their ABP status over time and its associations with any patient and clinical characteristics.
RESULTS
Two hundred and two patients had baseline ABPM available for analysis, and 123 of them had a follow up (median time 2.3 years) ABPM. At the time of follow up, more patients were treated for hypertension (80% vs. 72%, P = 0.02), and less patients had ambulatory hypertension (36% vs. 54%, P = 0.005), uncontrolled or untreated, compared with baseline, with 45% of all patients classified as having controlled hypertension (compared to 26% at baseline, P = 0.002). Prevalence of ambulatory hypertension decreased only in patients who were less than 18 years old at baseline. High baseline mean 24-hour systolic BP was independently associated with persistent hypertension.
CONCLUSIONS
In young kidney transplant recipients followed by ABPM, the prevalence of ambulatory hypertension decreases over time, mainly due to the increased number of patients with controlled hypertension.
Keywords: ABPM, blood pressure, hypertension, kidney transplant, children
Studies in pediatric kidney transplant recipients over the last 2 decades have indicated a high prevalence of abnormal ambulatory blood pressure (ABP).1–11 We recently evaluated BP by ABP monitoring (ABPM) in a large cohort of kidney transplant recipients from the Midwest Pediatric Nephrology Consortium (MWPNC). Masked hypertension was present in one third of patients, confirming poor BP control in these patients outside the medical office.12 While few studies have analyzed longitudinal ABP trends in pediatric kidney transplantation, some small studies suggest that ABPM can lead to improved BP control in these patients.13,14
The aim of the current study was to longitudinally evaluate ABP in pediatric kidney transplant recipients. We also sought to identify factors associated with improved or worsened BP control over time. To accomplish this, we utilized data from patients in our original MWPNC cohort who underwent repeated ABPM evaluations.
METHODS
A retrospective analysis of children and young adult kidney transplant recipients who had an ABPM study conducted between January 2010 and December 2015 was carried out at 3 centers from the MWPNC, which perform ABPM as part of the routine posttransplantation follow up. The study was reviewed and approved by the Institutional Review Boards of all participating centers.
Inclusion criteria were a functioning kidney transplant and age <23 years at the time of initial ABPM. Charts were reviewed for demographic information (age, gender, race), etiology of end-stage renal disease (glomerular vs. structural/congenital), medications, history of prior transplants, dialysis prior to transplant, prior episode of rejection, anthropometric parameters (height, weight), and laboratory data (serum creatinine and hemoglobin) at the time of the first ABPM after transplantation and at follow up ABPM, if conducted ≥3 months after the initial one. Medication information collected specifically included all immunosuppressive and antihypertensive agents. Allograft function was determined based on the bedside Schwartz formula.15
Casual BP (CBP) was defined as the mean of 2 manual clinic measurements on the day of ABPM and was classified as follows16: “Normal”: systolic/diastolic BP (SBP/DBP) <90th percentile and <120/80 mm Hg; “Prehypertension”: SBP/DBP at the 90th–95th percentile or 120–140/80–90 mm Hg; “Hypertension”: SBP/DBP ≥95th percentile or ≥140/90 mm Hg.
The SpaceLabs 90217 monitor (SpaceLabs Healthcare, Issaquah, WA) was used for all ABPM studies. All centers utilized standard methodology to measure BP: for wake hours—every 20 minutes; for sleep hours, measurements were performed either every 20 or 30 minutes.
The ABP parameters of interest included mean SBP and DBP, ABP index, nocturnal dip, and BP load. ABP index was calculated as the mean ABP divided by the corresponding 95th percentile. Thus, an index of 1 indicates ABP equal to the threshold value for a clinical diagnosis of hypertension, and an index of 1.1 is 10% above that threshold.17 Since the 95th percentile is gender and height specific, this measure allows for comparison of BP across a wide range of pediatric normal values.
For patients under 18 years of age, wake and sleep BP loads were calculated as the percent of readings at or above the 95th percentile based on published normative data.18 ABP status for patients under 18 years of age was estimated based on mean 24-hour ABP and 24-hour BP load as recommended by the American Heart Association (AHA).19 However, considering the high cardiovascular risk nature of the kidney transplant recipient population, for BP status classification using both CBP and ABP readings, BP load >25% (even if mean BP was <95th percentile) was used as the cutoff for abnormal ABP, as previously described12: “Normal”: CBP <95th percentile and 24-hour BP load <25%; “White coat hypertension (WCH)”: CBP ≥95th percentile, 24-hour BP load <25%; “Masked hypertension”: CBP <95th percentile and 24-hour BP load ≥25%; “Sustained hypertension”: CBP ≥95th percentile and 24-hour BP load ≥25%.
For patients over 18 years of age, the abnormal cutoffs were based on adult criteria utilizing mean BP only20: “Normal”: CBP <140/90 mm Hg, mean wake ABP <135/85 mm Hg, and mean sleep ABP <120/70 mm Hg; “WCH”: CBP ≥140/90 mm Hg, mean wake ABP <135/85 mm Hg, and mean sleep ABP <120/70 mm Hg; “Masked hypertension”: CBP <140/90 mm Hg, mean wake ABP ≥135/85 mm Hg, and/or mean sleep ABP ≥120/70 mm Hg; “Sustained hypertension”: CBP ≥140/90 mm Hg, mean wake ABP ≥135/85 mm Hg, and/or mean sleep ABP ≥120/70 mm Hg.
Since pediatric and adult cutoffs for normal and abnormal ABP are different, the cohort was divided into 3 different age groups: “adult-adult”: subjects ≥18 years at baseline; “pediatric-pediatric”: subjects <18 years at baseline and follow up; “pediatric-adult”: subjects <18 years at baseline, but ≥18 years at follow up. In order to better compare the BP status between these groups, and the baseline and follow up status of the pediatric-adults group, we applied the formal definitions of the pediatric AHA classification, i.e., using a mean day/night BP >95th percentile per height as the cutoff for abnormal ABP on all the patients.
Controlled hypertension was defined as normal BP as a result of treatment with antihypertensive medications. Uncontrolled hypertension (masked and sustained) was defined as hypertension while on antihypertensive treatment. For the purpose of this study, in addition to the standard definition of WCH, patients on BP medications and BP measurements consistent with WCH were also classified as having WCH.
Statistical analysis
For descriptive statistics, categorical variables were reported as percentages, and continuous variables reported as median and interquartile ranges. For univariate analyses, demographic and clinical characteristics were compared between BP categories using chi-square testing for categorical variables and the Wilcoxon rank–sum test for continuous variables. Comparison of patients’ status at baseline and last follow up was done using McNamer’s test for categorical variables and paired T-test for continuous ones. Logistic regression was used to investigate the association of baseline characteristics with normalization ABP or development of abnormal ABP (masked + sustained hypertension). All baseline variables associated with improving or worsening BP status in univariate analyses (P < 0.15) were initially included in the model. These included sex, race, weight status, treatment with calcium channel blockers, elevated 24-hour SBP, and elevated wake SBP for improving BP status; and sex, steroid treatment, hemoglobin, and estimated glomerular filtration rate for worsening BP status. Backward elimination was performed to determine variables included in the final model, with an inclusion criterion of P <0.05. Odds ratios were reported for each independent predictor along with Wald 95% confidence intervals. All statistical analyses were performed using SAS 9.3 statistical software.
RESULTS
Two hundred and two pediatric and young adult kidney transplant recipients had at least one ABPM. Among them, 123 had a repeated ABPM (98 with 1 follow up ABPM and 25 with more) with a median time between the first and last ABPM of 2.3 years. Baseline characteristics of patients with and without a repeated ABPM are shown in Table 1. Compared to those who did not receive a repeat ABPM, patients with a follow-up ABPM had more prevalent baseline masked hypertension (37% vs. 20%), less prevalent baseline normal BP (40% vs. 65%), and were more likely to be treated with more than one antihypertensive medication at baseline.
Table 1.
Comparison of baseline characteristics of patients with and without follow-up ABPM
| Baseline cohort (n = 202) | Patients with no follow up (n = 79) | Patients with follow up (n = 123) | P value | |
|---|---|---|---|---|
| Age, yearsa | 16.7 (13.5–19.1) | 16.1 (13.3–19.1) | 16.9 (13.7–19.8) | 0.63 |
| Age ≥18 years, n (%) | 76 (38) | 29 (37) | 47 (38) | 0.83 |
| Male gender, n (%) | 129 (64) | 46 (58) | 83 (67) | 0.18 |
| Race, n (%) | ||||
| White | 143 (71) | 54 (68) | 89 (72) | 0.88 |
| African American/Biracial | 30 (15) | 12 (15) | 18 (15) | |
| Hispanic | 16 (8) | 7 (9) | 9 (7) | |
| Others | 13 (6) | 6 (8) | 7 (6) | |
| Age at transplantation, yearsa | 11.1 (6.6–16.3) | 12.0 (8.2–17.7) | 11.0 (5.6–14.0) | 0.11 |
| Time posttransplantation, yearsa | 2.4 (1.0–6.1) | 2.8 (1.0–5.2) | 2.3 (1.0–6.7) | 0.86 |
| Primary acquired glomerular disease, n (%) | 44 (22) | 14 (18) | 30 (24) | 0.47 |
| First transplant, n (%) | 191 (95) | 73 (92) | 119 (96) | 0.27 |
| Living donor, n (%) | 122 (61) | 44 (56) | 78 (64) | 0.24 |
| Prior dialysis, n (%) | 131 (66) | 52 (66) | 79 (66) | 0.99 |
| History of rejection, n (%) | 54 (27) | 24 (30) | 30 (24) | 0.35 |
| Immunosuppression, n (%) | ||||
| Calcineurin inhibitors | 161 (80) | 65 (82) | 96 (79) | 0.53 |
| Steroids | 93 (46) | 31 (39) | 62 (51) | 0.11 |
| Mycophenolate mofetil | 154 (77) | 62 (78) | 92 (75) | 0.62 |
| Height (cm)a | 159 (141–169) | 160 (142–172) | 158 (139–168) | 0.29 |
| Overweight/obese, n (%) | 68 (34) | 26 (33) | 42 (35) | 0.79 |
| Hemoglobin, g/dla | 12.3 (11.3–13.3) | 12.3 (11.3–13.3) | 12.3 (11.2–13.4) | 0.66 |
| Anemia, n (%) | 41 (20) | 12 (15) | 29 (24) | 0.15 |
| Treated for hypertension, n (%) | 138 (68) | 49 (62) | 89 (72) | 0.12 |
| Treated with ≥1 drug | 39 (19) | 8 (10) | 31 (25) | 0.008 |
| ACEI/ARB | 44 (22) | 16 (20) | 28 (23) | 0.65 |
| BB | 36 (18) | 12 (15) | 24 (20) | 0.42 |
| CCB | 89 (44) | 28 (35) | 61 (50) | 0.04 |
| Diuretics | 5 (2) | 2 (3) | 3 (2) | 0.97 |
| eGFR, (ml/min/1.73 m2)a | 65 (51–80) | 67 (52–82) | 62 (49–77) | 0.09 |
| Routine initial ABPM, n (%) | 151 (75) | 56 (71) | 95 (77) | 0.31 |
| Casual BP status, n (%) | ||||
| Normal | 96 (48) | 41 (52) | 55 (45) | 0.33 |
| Prehypertension | 65 (32) | 26 (33) | 39 (32) | |
| Hypertension | 41 (20) | 12 (15) | 29 (23) | |
| ABP classification, n (%) | ||||
| Normal | 100 (50) | 51 (65) | 49 (40) | 0.002 |
| White coat hypertension | 8 (4) | 0 (0) | 8 (7) | |
| Masked hypertension | 61 (30) | 16 (20) | 45 (37) | |
| Sustained hypertension | 33 (16) | 12 (15) | 21 (17) | |
Abbreviations: ABP, ambulatory blood pressure; ABPM, ambulatory blood pressure monitoring; ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers; BB beta blockers; CCB, calcium channel blockers; eGFR, estimated glomerular filtration rate.
aData are presented as median [interquartile range (IQR)].
Comparison of BP at baseline vs. follow up is shown in Table 2. Overall, BP control improved over time: there were more patients with normal CBP (54% vs. 40%) and less patients with casual hypertension (11% vs. 23%, P = 0.01) at follow up compared to baseline. Improvements in many ABP parameters were noticed at follow-up, including mean 24-hour SBP and DBP indices, mean wake SBP index, and mean sleep SBP and DBP indices. During the follow up period, antihypertensive medications were initiated in 12 patients, their number was increased in 20, decreased in 7, and discontinued in 3 patients. At the time of follow-up ABPM, more patients were taking antihypertensive medications compared to baseline (80% vs. 72%, P = 0.02) and more patients were taking more than 1 antihypertensive medication (37% vs. 25%, P = 0.004). The number of antihypertensive medications was increased in 35% of patients with baseline ambulatory hypertension, compared with 16% of those with normal ABP at baseline (P = 0.02).
Table 2.
BP characteristics at baseline and last follow up (n = 123)
| Baseline | Follow up | P value | |
|---|---|---|---|
| Casual BP | |||
| Casual SBP, mm Hg | 118 (110–126) | 118 (110–124) | NS |
| Casual SBP index | 0.90 (0.84–0.97) | 0.87 (0.82–0.94) | 0.001 |
| Casual DBP, mm Hg | 68 (62–77) | 68 (62–76) | NS |
| Casual DBP index | 0.79 (0.73–0.91) | 0.79 (0.72–0.89) | NS |
| Casual BP status, n (%) | |||
| Normal | 55 (45%) | 66 (54%) | 0.01a |
| Prehypertension | 39 (32%) | 43 (35%) | |
| Hypertension | 29 (23%) | 14 (11%) | |
| 24-hour BP parameters | |||
| Mean 24-hour SBP, mm Hg | 117 (112–124) | 116 (110–123) | NS |
| Mean 24-hour SBP index | 0.95 (0.88–0.99) | 0.91 (0.88–0.96) | 0.01 |
| Abnormal mean 24-hour SBP , n (%) | 28 (23%) | 19 (15%) | 0.09 |
| Mean 24-hour DBP, mm Hg | 71 (67–74) | 69 (65–74) | NS |
| Mean 24-hour DBP index | 0.91 (0.86–0.96) | 0.89 (0.83–0.94) | 0.02 |
| Abnormal mean 24-hour DBP , n (%) | 19 (15%) | 15 (12%) | NS |
| Wake BP parameters | |||
| Mean wake SBP, mm Hg | 122 (117–127) | 121 (114–128) | NS |
| Mean wake SBP index | 0.93 (0.88–0.98) | 0.91 (0.87–0.96) | 0.02 |
| Abnormal mean wake SBP , n (%) | 25 (20%) | 16 (13%) | 0.07 |
| Mean wake DBP, mm Hg | 75 (71–79) | 73 (70–78) | NS |
| Mean wake DBP index | 0.91 (0.84–0.95) | 0.88 (0.82–0.93) | 0.08 |
| Abnormal mean wake DBP , n (%) | 14 (11%) | 10 (8%) | NS |
| Sleep BP parameters | |||
| Mean sleep SBP, mm Hg | 109 (103–119) | 108 (100–117) | NS |
| Mean sleep SBP index | 0.94 (0.89–1.01) | 0.91 (0.86–0.98) | 0.05 |
| Abnormal mean sleep SBP , n (%) | 39 (32%) | 22 (18%) | 0.008 |
| Mean sleep DBP, mm Hg | 63 (59–68) | 61 (56–68) | NS |
| Mean sleep DBP index | 0.94 (0.86–1.01) | 0.90 (0.83–0.99) | 0.01 |
| Abnormal mean sleep DBP , n (%) | 38 (31%) | 27 (22%) | 0.08 |
Data is presented as median (IQR). Abbreviations: BP, blood pressure; DBP, diastolic blood pressure; IQR, interquartile range; NS, nonsignificant; SBP, systolic blood pressure.
aPrevalence of hypertension.
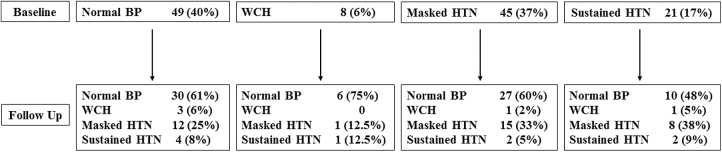
Change in BP category according to baseline status is shown in Figure 1. Among patients with normal BP at baseline, whereas the majority (61%) remained normotensive, 33% were subsequently found to have either masked (25%) or sustained (8%) hypertension; among patients with WCH at baseline, 75% had normal BP at follow up; among patients with masked hypertension at baseline, 60% had normal BP at follow up, 33% continued to have masked hypertension, and 5% developed sustained hypertension; among patients with sustained hypertension at baseline, over half (53%) had controlled hypertension at follow-up, 38% had masked hypertension, and 9% continued to have sustained hypertension.
Figure 1.
Change in BP status at follow from baseline status. Abbreviations: BP, blood pressure; HTN, hypertension; WCH, White coat hypertension.
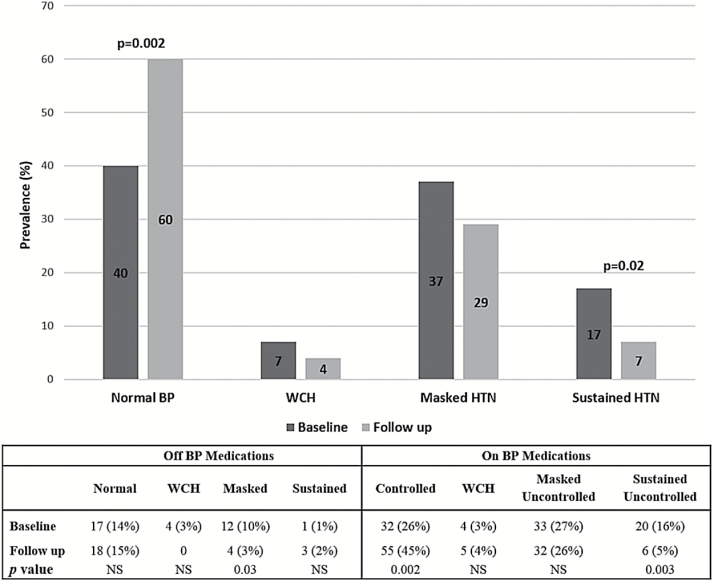
Overall, ambulatory hypertension (masked + sustained) was present in 54% at baseline and in 36% at follow-up (P = 0.005): the percentage of patients with masked hypertension decreased from 37% to 29%, and those with sustained hypertension decreased from 17% to 7%. The majority of improvement was attributable to: (i) an increased number of transplant recipients with controlled hypertension (26% at baseline and 45% at follow-up, P = 0.002), while the number of patients with normal BP (not on antihypertensive treatment) did not change; (ii) a decrease in the number of patients with masked hypertension (not on antihypertensive treatment) and sustained uncontrolled hypertension (Figure 2). Nevertheless, 39% of patients on antihypertensive medications still had uncontrolled hypertension, mainly related to a stable number of patients with masked uncontrolled hypertension overtime (27% and 26% at baseline and follow up, respectively).
Figure 2.
Prevalence of ambulatory hypertension at baseline and follow up.
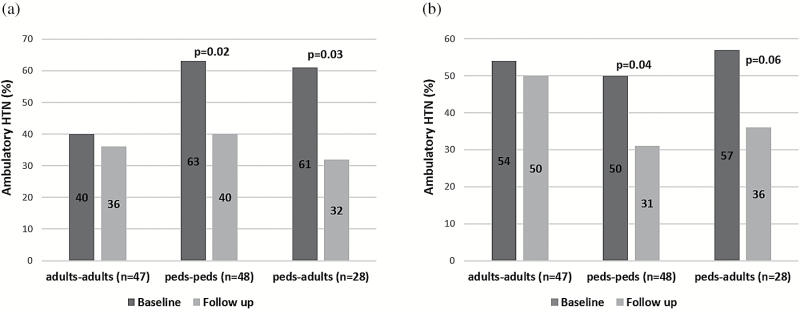
We then compared BP control according to age at baseline and follow up (Figure 3). Using standard pediatric and adult definitions (Figure 3a), the prevalence of abnormal (masked +sustained) ABP was significantly lower in young adults than in children at baseline (40% vs. 62%, P = 0.02). In the adult-adult subgroup, no significant difference in BP control was found between baseline and follow up. In contrast, the prevalence of ambulatory hypertension in the pediatric-pediatric (63% at baseline and 40% at follow up, P = 0.02) and pediatric-adult (61% at baseline and 32% at follow up, P = 0.03) subgroups significantly decreased over time.
Figure 3.
Prevalence of ambulatory hypertension at baseline and last follow up based on age group. Ambulatory hypertension is defined: (a) standard adult and pediatric definitions and (b) pediatric definition as ABP ≥95th percentile per height. “Adults-adults”: subjects ≥18 years at baseline; “peds-peds”: subjects <18 years at baseline and follow up; “peds-adults”: subjects <18 years at baseline, but ≥18 years at follow up. Abbreviation: ABP, ambulatory blood pressure.
When a comparable definition of abnormal ABP (≥95th percentile per height) was used in both children and adults (Figure 3b), no significant difference in the prevalence of ambulatory hypertension at baseline between young adults (54%) and children (53%) was found. As with the standard definition, no improvement in BP control was seen in the adult-adult subgroup, but ABP has improved in pediatric-pediatric and pediatric-adult subgroups.
In order to evaluate factors associated with improvement or worsening in ABP status over time, we analyzed separately patients with abnormal and normal ABP at baseline. Among patients with abnormal baseline ABP, those whose ABP improved to normal during follow up were more likely to be male (82% vs. 59%, P = 0.04) and to be treated with calcium channel blockers at baseline (50% vs. 35%, P = 0.04), but less likely to be overweight (24% vs. 46%, P = 0.06) and to have elevated baseline mean 24-hour SBP (28% vs. 63%, P = 0.005) or mean wake SBP (28% vs. 52%, P = 0.05). In a multivariate analysis, elevated baseline mean 24-hour SBP was the only independent predictor of lack of improvement in ABP status (Table 3).
Table 3.
Logistic regression analysis of factors associated with improvement/worsening ABP status during follow-up
| Parameter | OR | 95% CI | P value |
|---|---|---|---|
| Improvement | |||
| Males | 2.55 | 0.73–8.92 | 0.14 |
| Calcium channel blockade | 1.50 | 0.48–4.70 | 0.49 |
| Overweight | 0.45 | 0.14–1.51 | 0.20 |
| Mean 24-hour SBP, mm Hg | 0.21 | 0.07–0.67 | 0.008 |
| Worsening | |||
| eGFR (for each 10 ml/min/1.73 m2) | 0.77 | 0.55–1.06 | 0.11 |
| Females | 4.5 | 1.06–19.05 | 0.04 |
| Steroid treatment | 4.18 | 1.01–17.32 | 0.048 |
Abbreviations: ABP, ambulatory blood pressure; CI, confidence interval; eGFR, estimated glomerular filtration rate; OR, odds ratio; SBP, systolic blood pressure.
Patients with normal baseline ABP but ambulatory hypertension at follow up were more likely to be females (56%, vs. 31%, P = 0.07), be treated with steroids (78% vs. 36%, P = 0.003), have lower hemoglobin (11.4 mg/dl vs. 12.7, P = 0.007) and lower estimated glomerular filtration rate (49 vs. 65 ml/min/1.73 m2, P = 0.046). In a multivariate analysis, being female and steroids treatment were independently associated with development of ambulatory hypertension (Table 3).
DISCUSSION
This is the largest study to evaluate trends in ABP control over time in the pediatric and young adult kidney transplant recipient population. Our results demonstrate that overall, BP control improved overtime. These findings confirm the results of 2 previous smaller pediatric kidney transplant studies13,14 which also showed improved ABP control. Similar to the study by Seeman et al.,14 in which significant improvement was observed only for nighttime BP, our results showed more improvement in sleep BP status. Of note, absolute BP levels did not change over time, and the improvement in BP indices reflects the growth of the patients during the study period leading to higher cutoffs for abnormal BP at follow up. CBP status also improved over time in our study population, similar to the findings of 2 other recent studies which followed pediatric kidney transplant recipients.21,22
We observed an increased number of patients taking antihypertensive medications and more patients taking more than 1 antihypertensive medication at follow up, especially in those with baseline ambulatory hypertension at baseline. However, despite more patients taking BP medications and more patients with controlled hypertension at last follow up, we could not find an association between starting antihypertensive treatment/increase in number of BP medications and improvement in BP status. Of note, one third of our patients still had uncontrolled ambulatory hypertension, many of whom were under treated: at follow-up, less than two thirds (61%) of the patients receiving antihypertensive medications had normal ABP.
In the general pediatric population, a higher risk of sustained hypertension was observed in patients with baseline masked or WCH.23–25 While in our study, patients with a higher baseline mean 24-hour SBP were at increased risk for persistent hypertension at follow up, the presence of a particular BP phenotype (e.g., masked or sustained hypertension) at baseline did not statistically predict follow up hypertension, probably reflecting the relatively low number of patients with sustained hypertension in our cohort, and the relatively large proportion (33%) of patients with normal baseline BP who had abnormal ABP at follow up. Female sex and steroid treatment were also significantly associated with the development of ambulatory hypertension over time. While steroid treatment is a well-known risk factor for hypertension, we cannot explain the worsening BP status in females. Contrary to our findings, a study conducted in the general pediatric population showed that females had lower rates of “progression” from masked to sustained hypertension.23 Thus, our finding might reflect other aspects effecting BP status, not captured in our study.
Our results showed that at baseline, young adult patients had a lower prevalence of ambulatory hypertension compared to pediatric patients when standard adult and pediatric definitions of ambulatory hypertension were used. However, when height-based pediatric cutoffs for hypertension were used in young adults, we found no significant difference in the rates of ambulatory hypertension among children and adults at baseline. These findings highlight the problematic nature of defining ABP status during the transition period from childhood to adulthood. Importantly, regardless of the classification (pediatric or adult), BP status of pediatric patients improved whether they reached adulthood or not.
Our study has several limitations. First, this was a retrospective chart review with almost one third of our baseline cohort not having a follow-up ABPM. As all the 3 centers who participated in the study perform routine ABPM in kidney transplant recipients, it is reasonable to believe that some of these patients simply transitioned/were lost to follow up before their second ABPM or did not adhere to the follow up plan. The reason for the relatively low number of patients with initial normal BP and follow up ABPM may be explained by lower tendency to perform repeat ABPM in patients with “proved normotension.” Whatever the reason for the lack of follow up, our results likely reflect the status of a group of patients with a higher prevalence of ambulatory hypertension and not of the general pediatric kidney transplant recipient population. Second, we did not have information on the dose and frequency of changes in antihypertensive medications or the adherence to the antihypertensive regimen. Third, ABPM frequency was not standardized across centers making it difficult to assess ABP trends systematically.
In summary, this longitudinal study of ABP in pediatric and young adult kidney transplant recipients demonstrated improved control of BP over time in patients followed by ABPM. However, the prevalence of masked uncontrolled hypertension remained relatively high, and a third of the baseline normotensives had ambulatory hypertension at follow up. Our findings, underscore the important role of routine ABPM, even in normotensive patients, and the need for aggressive BP management in this population. Further research is needed to assess ABP status over longer follow up period and to examine the association of ABP control with target organ damage such as allograft dysfunction and left ventricular hypertrophy.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
Data in this manuscript were collected by the members of Midwest Pediatric Nephrology Consortium (MWPNC). Dr. Mitsnefes and this research were supported by NIH grant K24DK090070.
REFERENCES
- 1. Calzolari A, Giordano U, Matteucci MC, Pastore E, Turchetta A, Rizzoni G, Alpert B. Hypertension in young patients after renal transplantation: ambulatory blood pressure monitoring versus casual blood pressure. Am J Hypertens 1998; 11:497–501. [DOI] [PubMed] [Google Scholar]
- 2. Giordano U, Matteucci MC, Calzolari A, Turchetta A, Rizzoni G, Alpert BS. Ambulatory blood pressure monitoring in children with aortic coarctation and kidney transplantation. J Pediatr 2000; 136:520–523. [DOI] [PubMed] [Google Scholar]
- 3. Seeman T, Dusek J, Vondrak K, Simkova E, Kreisinger J, Feber J, Janda J. Ambulatory blood pressure monitoring in children after renal transplantation. Transplant Proc 2004; 36:1355–1356. [DOI] [PubMed] [Google Scholar]
- 4. Serdaroglu E, Mir S, Berdeli A. Hypertension and ace gene insertion/deletion polymorphism in pediatric renal transplant patients. Pediatr Transplant 2005; 9:612–617. [DOI] [PubMed] [Google Scholar]
- 5. McGlothan KR, Wyatt RJ, Ault BH, Hastings MC, Rogers T, DiSessa T, Jones DP. Predominance of nocturnal hypertension in pediatric renal allograft recipients. Pediatr Transplant 2006; 10:558–564. [DOI] [PubMed] [Google Scholar]
- 6. Ferraris JR, Ghezzi L, Waisman G, Krmar RT. ABPM vs office blood pressure to define blood pressure control in treated hypertensive paediatric renal transplant recipients. Pediatr Transplant 2007; 11:24–30. [DOI] [PubMed] [Google Scholar]
- 7. Paripovic D, Kostic M, Spasojevic B, Kruscic D, Peco-Antic A. Masked hypertension and hidden uncontrolled hypertension after renal transplantation. Pediatr Nephrol 2010; 25:1719–1724. [DOI] [PubMed] [Google Scholar]
- 8. Basiratnia M, Esteghamati M, Ajami GH, Amoozgar H, Cheriki C, Soltani M, Derakhshan A, Fallahzadeh MH. Blood pressure profile in renal transplant recipients and its relation to diastolic function: tissue Doppler echocardiographic study. Pediatr Nephrol 2011; 26:449–457. [DOI] [PubMed] [Google Scholar]
- 9. Gülhan B, Topaloğlu R, Karabulut E, Ozaltın F, Aki FT, Bilginer Y, Beşbaş N. Post-transplant hypertension in pediatric kidney transplant recipients. Pediatr Nephrol 2014; 29:1075–1080. [DOI] [PubMed] [Google Scholar]
- 10. Cameron C, Vavilis G, Kowalski J, Tydén G, Berg UB, Krmar RT. An observational cohort study of the effect of hypertension on the loss of renal function in pediatric kidney recipients. Am J Hypertens 2014; 27:579–585. [DOI] [PubMed] [Google Scholar]
- 11. Tainio J, Qvist E, Miettinen J, Hölttä T, Pakarinen M, Jahnukainen T, Jalanko H. Blood pressure profiles 5 to 10 years after transplant in pediatric solid organ recipients. J Clin Hypertens (Greenwich) 2015; 17:154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hamdani G, Nehus EJ, Hanevold CD, Sebestyen Van Sickle J, Woroniecki R, Wenderfer SE, Hooper DK, Blowey D, Wilson A, Warady BA, Mitsnefes MM. Ambulatory blood pressure, left ventricular hypertrophy, and allograft function in children and young adults after kidney transplantation. Transplantation 2017; 101:150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krmar RT, Berg UB. Blood pressure control in hypertensive pediatric renal transplants: role of repeated ABPM following transplantation. Am J Hypertens 2008; 21:1093–1099. [DOI] [PubMed] [Google Scholar]
- 14. Seeman T, Simková E, Kreisinger J, Vondrák K, Dusek J, Gilík J, Dvorák P, Janda J. Improved control of hypertension in children after renal transplantation: results of a two-yr interventional trial. Pediatr Transplant 2007; 11:491–497. [DOI] [PubMed] [Google Scholar]
- 15. Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol 2009; 20:629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004; 114(Suppl 2):555–576. [PubMed] [Google Scholar]
- 17. Sorof JM, Cardwell G, Franco K, Portman RJ. Ambulatory blood pressure and left ventricular mass index in hypertensive children. Hypertension 2002; 39:903–908. [DOI] [PubMed] [Google Scholar]
- 18. Wühl E, Witte K, Soergel M, Mehls O, Schaefer F; German Working Group on Pediatric hypertension. . Distribution of 24-h ambulatory blood pressure in children: normalized reference values and role of body diementions. J Hypertens 2002; 20:1995–2007. Erratum in: J hypertens 2003; 21:2205–2206. [DOI] [PubMed] [Google Scholar]
- 19. Flynn JT, Daniels SR, Hayman LL, Maahs DM, McCrindle BW, Mitsnefes M, Zachariah JP, Urbina EM; American Heart Association Atherosclerosis, Hypertension and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young Update: ambulatory blood pressure monitoring in children and adolescents: a scientific statement from the American Heart Association. Hypertension 2014; 63:1116–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005; 111:697–716. [DOI] [PubMed] [Google Scholar]
- 21. Kaidar M, Berant M, Krauze I, Cleper R, Mor E, Bar-Nathan N, Davidovits M. Cardiovascular risk factors in children after kidney transplantation–from short-term to long-term follow-up. Pediatr Transplant 2014; 18:23–28. [DOI] [PubMed] [Google Scholar]
- 22. Stabouli S, Printza N, Dotis J, Gkogka C, Kollios K, Kotsis V, Papachristou F. Long-term changes in blood pressure after pediatric kidney transplantation. Am J Hypertens 2016; 29:860–865. [DOI] [PubMed] [Google Scholar]
- 23. Lurbe E, Thijs L, Torro MI, Alvarez J, Staessen JA, Redon J. Sexual dimorphism in the transition from masked to sustained hypertension in healthy youths. Hypertension 2013; 62:410–414. [DOI] [PubMed] [Google Scholar]
- 24. Lurbe E, Torro I, Alvarez V, Nawrot T, Paya R, Redon J, Staessen JA. Prevalence, persistence, and clinical significance of masked hypertension in youth. Hypertension 2005; 45:493–498. [DOI] [PubMed] [Google Scholar]
- 25. Mancia G, Bombelli M, Facchetti R, Madotto F, Quarti-Trevano F, Polo Friz H, Grassi G, Sega R. Long-term risk of sustained hypertension in white-coat or masked hypertension. Hypertension 2009; 54:226–232. [DOI] [PubMed] [Google Scholar]