Abstract
BACKGROUND
Physical activity (PA) has been associated with decreased left ventricular (LV) hypertrophy in previous studies. However, little is known about the relationship between PA and LV structure and factors which influence this relationship among African Americans.
METHODS
We evaluated 1,300 African Americans with preserved LV ejection fraction (EF > 50%) from the Genetic Epidemiology Network of Arteriopathy (GENOA) Study (mean age 62.4 years, 73% women). PA index was calculated as 3 * heavy activity hours + 2 * moderate activity hours + slight activity hours/day. The relationship between PA index and LV structure was evaluated using generalized estimating equation. The association between PA index and LV mass index by age group, sex, body mass index (BMI), history of hypertension, diabetes or coronary heart disease, estimated glomerular filtration rate, and current smoking status were plotted.
RESULTS
After adjustment for these factors, higher PA index was independently associated with lower LV mass index (P < 0.05). There were significant interactions between PA index and obesity (BMI ≥ 30) and history of hypertension on LV mass index (P for interaction <0.05, for both). Higher PA index was associated with lower LV mass index more in obese or hypertensive participants compared with nonobese or nonhypertensive participants.
CONCLUSIONS
Higher PA index was associated with reduced LV hypertrophy in obese and hypertensive African Americans. Prospective studies aimed at assessing whether increasing PA prevents LV hypertrophy and potentially reduces the risk of heart failure in these at risk groups are warranted.
Keywords: blood pressure, hypertension, LV hypertrophy, LV mass index, physical activity.
African Americans have a higher risk of cardiovascular disease including left ventricular (LV) hypertrophy and heart failure compared with Whites, Hispanics, or Asians.1,2 The increased cardiovascular risk has long been attributed to a higher prevalence of hypertension and obesity in African Americans.1,3 Furthermore, nocturnal and nondipping hypertension are more prevalent among African Americans than Whites, and this may be due to increased salt-sensitivity.4 Progression from prehypertension to hypertension is accelerated in African Americans and these factors may contribute to the higher cardiovascular risk among African Americans.5 The prevalence of diabetes is also much higher among African Americans than Whites and it may also contribute to increased cardiovascular risk among African Americans.6 Previous data suggest the racial disparity may be increasing.7 LV hypertrophy is clearly associated with an increased risk of heart failure and is frequently seen in patients with hypertension or obesity.8,9 Regression of LV mass after treatment of hypertension is associated with a favorable prognosis.10 Thus, prevention of LV hypertrophy may reduce the risk of heart failure events among African Americans.
Increased physical activity (PA) has been associated with reduced rates of incident heart failure.11,12 PA has also been associated with reduced LV mass or LV mass index.13,14 However, factors that influence this relationship have not been completely investigated. Since hypertension and obesity are closely associated with LV hypertrophy, the association between PA and LV mass may be different between those with or without hypertension and obesity.15,16 On the other hand, the association between PA and LV hypertrophy may be independent of changes in blood pressure or body mass even in patients with hypertension or obesity.17 Plasma N-terminal prohormone brain natriuretic peptide (NT pro-BNP) concentration level is well established marker of LV hypertrophy18 and plasma mid-regional prohormone atrial natriuretic peptide (MR pro-ANP) is also associated with LV mass index.19
We hypothesized that increased PA is associated with decreased LV mass and its biomarkers, and this relationship is more profound in those with hypertension or obesity. Therefore, the purpose of this study is to investigate (i) whether PA is associated with LV structure and function and NT pro-BNP or MR pro-ANP, (ii) whether these associations are independent of blood pressure or body mass, and (iii) whether hypertension and obesity moderate the relationship between PA and LV mass in the African American cohort of the well-characterized Genetic Epidemiology Network of Arteriopathy (GENOA).
METHODS
Study population
The present study population consisted of participants from the Jackson, MS cohort of the GENOA study. Study participants gave informed consent for this study. This study protocol conforms to the ethical guidelines of the Declaration of Helsinki and was approved by the Institutional Review Boards of the University of Mississippi Medical Center, Jackson, MS. The design and procedures for the GENOA study have been previously described.20 Briefly, GENOA cohorts were originally ascertained (1995–2000) through sibships in which at least 2 siblings had essential hypertension diagnosed prior to age 60 years. After identification of the initial pairs of hypertensive siblings, all siblings in the sibship were invited to participate regardless of hypertension status. GENOA cohorts included African Americans from Jackson, MS (N = 1,854 at the 1st exam), we included this cohort. From the GENOA population of African Americans who returned the second visit (n = 1,518), we excluded the following participants from this analysis: 85 participants who had LV ejection fraction (EF) less than 50%, 81 participants whose LV EF had not been measured, 38 participants who had an LV wall motion abnormality, and 14 participants who were without at least one of the covariates. Thus, the remaining 1,300 participants were included in this study.
Definition of PA
PA information was obtained from self-report on how many hours per day of heavy activity, moderate activity, slight activity, and sedentary participants usually engaged in. Various examples (eg, jogging is heavy exercise) of activities that comprise these intensity levels were provided. Each activity was defined as follows: Heavy activity—activity that is equal or greater than 6 metabolic equivalents intensity and includes profuse sweating for periods greater than 30 minutes; Moderate activity—activity that is greater than 3 metabolic equivalents and lower than 6 metabolic equivalents intensity and concludes around the onset of sweating; Slight activity—activity that is lower than 3 metabolic equivalents intensity and does not induce sweating. The activities response list (Supplementary Material) was read to participants to accurately assess the PA intensity if applicable. We semiquantitatively evaluated PA/day by calculating a PA index as 3 * heavy PA hours + 2 * moderate PA hours + 1 * light PA hours as a predictor variable.21
Laboratory measurements
Blood was collected by venipuncture after an overnight fast and processed using standardized protocols at each collection site. Plasma N-terminal prohormone brain natriuretic peptide (NT pro-BNP) concentration levels, plasma mid-regional prohormone atrial natriuretic peptide (MR pro-ANP) concentration levels, serum high-sensitivity C-reactive protein concentration levels were evaluated as previously described.22 Fasting insulin and glucose concentrations were measured by standard enzymatic methods and homeostasis model assessment-insulin resistance was calculated as (fasting insulin concentration * fasting glucose concentration)/405. Serum creatinine concentrations were measured by standard enzymatic methods, and estimated glomerular filtration rate (eGFR) was calculated from the serum creatinine using following equations23,24: eGFR = exp (1.911 + 5.249/SCr − 2.114/SCr2 − 0.00686 * Age − 0.205 (if female) (where SCr: serum creatinine, if SCr < 0.8 mg/dl, 0.8 was used for SCr).
Definition of comorbidities
Essential hypertension, was defined as: (i) average of the last 2 out of 3 systolic BP readings ≥140 mm Hg or (ii) an average of the last 2 out of 3 diastolic BP readings ≥90 mm Hg or (iii) previous diagnosis of hypertension and antihypertensive medication prescribed by a physician to be taken daily during the last month. Diabetes was defined as present if a participant was receiving treatment with insulin, oral agents, or had fasting serum glucose levels ≥126 mg/dl. Coronary heart disease was defined as present if a participant had undergone coronary arteriography before and had a narrowing of artery or obstruction, or had a past history of myocardial infarction. Body mass index (BMI) was calculated as body weight (kg)/(height (m)).2 Current smoking status was obtained by self-report.
Echocardiography
In the GENOA study, standardized echocardiography methods, along with training and certification, were used by field-center technicians to achieve high-quality recordings. Readings were performed at the New York Presbyterian Hospital–Weill Cornell Medical Center and verified by a single highly experienced investigator. Correct orientation of planes for imaging and Doppler recordings was verified using standardized protocols. Measurements were made using a computerized review station equipped with digitizing tablet and monitor screen overlay for calibration and performance of each measurement. LV internal dimension and interventricular septal and posterior wall thicknesses were measured at end diastole and end systole in 3 cardiac cycles according to the recommendations of the American Society of Echocardiography.25 Calculations of LV mass were made using the following equation as recommended by American Society of Echocardiography25: LV mass index (g/m2.7) = (0.8 * {1.04 * [(LVDd + IVSd + PWd)3 − (LVDd) 3]} + 0.6)/height2.7. Where LVDd is LV diastolic dimension, IVSd is interventricular septum thickness at end diastole, PWd is posterior wall thickness at end diastole. Relative wall thickness (RWT) was calculated as 2 * (PWd)/LVDd. LV EF was calculated by Teichholz method.
Statistical analysis
Results are expressed as median (25th − 75th percentile) and categorical variables as number (percentage) unless otherwise specified. The mean value and median value of PA index were both 10. To examine the dose dependent associations between PA index with several outcomes, participants were divided into following categories; PA index 0–5: C0, 6–10: C1, 11–15: C2, and 16~: C3. A nonparametric test for trend for continuous variables and the chi-squared test for categorical variables were used for comparison of variables between the PA index category groups. Generalized estimating equations (GEEs) with identity link were used to estimate the association of PA index category and cardiac structure and function while accounting for potential correlation among siblings in the study participants. Two models were included in the analyses: model 1, adjusted for age and sex, and model 2, adjusted for age, sex, education levels, BMI, systolic blood pressure, antihypertensive medication use, past history of diabetes and coronary heart disease, eGFR, and current smoking status. The relationship between PA index and LV mass index, RWT, NT pro-BNP levels, and MR pro-ANP levels were constructed using model 2 adjustments and GEE analysis,26 where we used the PA index as a continuous variable. Additionally, the marginal effects of PA index on LV mass index were examined, and interactions between total PA index and several categories including age (≥75, ≥50 to <75, <50), sex, BMI (>30, ≥25 to <30, <25), past history of hypertension, diabetes and coronary artery disease, current smoking status, and eGFR (≥60, <60) were assessed by creating a cross-product term, and including it along with the main effects and covariates in the model 2. A 2-sided significance level of 0.05 was used for each statistical test. All statistical analyses were performed with STATA version 14 (STATA, College Station, TX).
RESULTS
Baseline characteristics
Table 1 shows clinical and demographic characteristics of the study cohort classified into the PA index category. As expected, those with a higher PA index are younger, have a lower prevalence of hypertension and diabetes, have lower systolic blood pressure, higher diastolic blood pressure, and higher eGFR compared with those with lower PA index.
Table 1.
Baseline characteristics
| Variables | All participants (n = 1,300) | Physical activity index category | P for trend | |||
|---|---|---|---|---|---|---|
| 0~5 (n = 99) | 6~10 (n = 661) | 11~15 (n = 482) | 16~ (n = 58) | |||
| Age, years | 63 (57, 69) | 72 (66, 77) | 63 (57, 69) | 61 (55, 67) | 63.5 (58, 67) | <0.001 |
| Female, n (%) | 943 (73) | 63 (64) | 532 (80) | 317 (66) | 31 (53) | <0.001 |
| Education, years | 12 (10, 15) | 9 (7, 12) | 12 (10, 14) | 13 (12, 16) | 14 (12, 16) | <0.001 |
| BMI, kg/m2 | 30.6 (27.0, 35.1) | 31.8 (27.0, 36.2) | 31.3 (27.3, 36.0) | 29.4 (26.5, 34.0) | 29.9 (27.5, 33.0) | <0.001 |
| Hypertension, n (%) | 1,015 (78) | 86 (87) | 531 (80) | 360 (74) | 42 (72) | <0.01 |
| CHD, n (%) | 62 (5) | 11 (11) | 36 (5.4) | 14 (2.9) | 1 (1.7) | <0.01 |
| Diabetes, n (%) | 365 (28) | 45 (44) | 201 (30) | 102 (21) | 14 (24) | <0.001 |
| Fasting insulin, µU/ml | 6.7 (3.9, 11.6) | 8.1 (4.7, 14.6) | 6.7 (3.9, 11.6) | 6.6 (3.7, 11.1) | 5.6 (3.9, 11.6) | 0.03 |
| Fasting glucose, mg/dl | 109 (99, 128) | 118 (103, 139) | 110 (100, 129) | 109 (98, 123) | 108 (102, 121) | 0.01 |
| HOMA-IR | 1.9 (1.1, 3.6) | 2.5 (1.2, 4.7) | 1.9 (1.1, 3.7) | 1.8 (1.0, 3.3) | 1.5 (1.0, 3.3) | <0.01 |
| Hs-CRP, mg/l | 3.4 (1.7, 7.1) | 4.5 (2.0, 10.2) | 3.8 (1.9, 7.9) | 2.9 (1.3, 6.0) | 2.7 (1.5, 6.0) | <0.001 |
| LDL cholesterol, mg/dl | 120 (96, 147) | 135 (97, 156) | 117 (96, 146) | 121 (97, 146) | 137 (110, 162) | 0.51 |
| Current smoker, n (%) | 172 (13) | 15 (15) | 85 (13) | 67 (14) | 5 (9) | 0.65 |
| Systolic BP, mm Hg | 136 (124, 151) | 140 (127, 157) | 137 (124, 152) | 134 (123, 146) | 137 (125, 149) | <0.01 |
| Diastolic BP, mm Hg | 79 (72, 85) | 77 (72, 82) | 79 (73, 85) | 79 (73, 86) | 81 (72, 86) | 0.03 |
| Estimated GFR, ml/min | 92 (84, 99) | 83 (62, 91) | 92 (84, 98) | 94 (87, 101) | 93 (85, 100) | <0.001 |
| PA index | 10 (8, 12) | 4 (2, 4) | 9 (8, 9) | 12 (11, 13) | 18 (17, 20) | <0.001 |
| Total PA, hours | 9 (8, 10) | 4 (2, 4) | 9 (8, 9) | 10 (10, 11) | 13 (12, 13) | <0.001 |
Abbreviations: BMI, body mass index; BP, blood pressure; CHD, coronary heart disease; DWS, diastolic wall strain; GFR, glomerular filtration rate; HOMA-IR, homeostasis model assessment-insulin resistance; Hs-CRP, high-sensitivity c-reactive protein; LDL, low-density lipoprotein; PA, physical activity.
Effects of PA index on LV structure and function
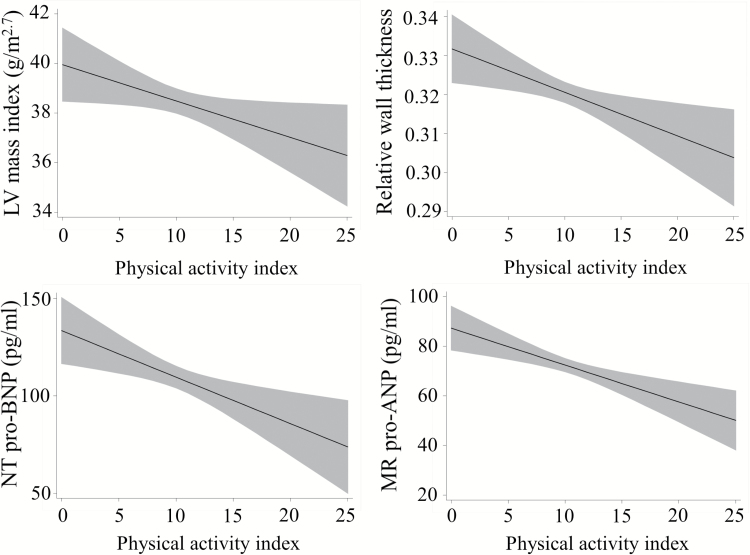
Table 2 shows the association between PA index category and LV structure and function, NT pro-BNP, and MR pro-ANP levels analyzed using GEE model. PA index was inversely associated with LV mass index, RWT, NT pro-BNP, and MR pro-ANP levels after adjustment for potential confounders. However, LV EF was not associated with PA index category after adjustment. Figure 1 shows the adjusted plot (GEE model 2, regression line and 95% confidence interval) between PA index and LV mass index, RWT, NT pro-BNP, and MR pro-ANP concentration levels. Those with higher PA index had a smaller LV mass index, lower RWT, lower NT pro-BNP, and MR pro-ANP levels.
Table 2.
Physical activity index was inversely associated with LVMI, RWT, and NT pro-BNP
| Variables | Model | Physical activity index category | |||
|---|---|---|---|---|---|
| 0~5 (n = 103) | 6~10 (n = 667) | 11~15 (n = 486) | 16~ (n = 58) | ||
| LVMI, g/m2.7 | 1 | Ref (0) | −4.49 (−6.87, −2.11)‡ | −5.26 (−7.59, −2.94)‡ | −6.39 (−9.15, −3.64)‡ |
| 2 | Ref (0) | −3.48 (−5.59, −1.37)‡ | −2.97 (−5.07, −0.87)† | −3.84 (−6.38, −1.31)‡ | |
| RWT (for 0.01 unit increase) | 1 | Ref (0) | −2.12 (−3.22, −1.01)‡ | −2.37 (−3.49, −1.25)‡ | −2.48 (−3.98, −0.98)† |
| 2 | Ref (0) | −1.91 (−3.03, −0.78)‡ | −2.03 (−3.18, −0.88)‡ | −2.09 (−3.59, −0.59)† | |
| LVEF, % | 1 | Ref (0) | −0.60 (−1.77, 0.57) | 0.03 (−1.18, 1.23) | −0.57 (−2.32, 1.17) |
| 2 | Ref (0) | −0.80 (−1.99, 0.38) | −0.46 (−1.68, 0.76) | −1.04 (−2.79, 0.71) | |
| NT pro-BNP, pg/ml | 1 | Ref (0) | −22.9 (−55.0, 9.1) | −37.4 (−68.6, −6.3)* | −54.6 (−87.3, −22.0)† |
| 2 | Ref (0) | −15.0 (−42.5, 12.6) | −23.8 (−50.3, 2.75) | −38.9 (−67.4, −10.5)† | |
| MR pro-ANP, pg/ml | 1 | Ref (0) | −14.2 (−27.6, −0.77)* | −19.3 (−32.6, −5.9)* | −29.9 (−44.3, −15.5)‡ |
| 2 | Ref (0) | −11.6 (−24.7, 1.5) | −14.5 (−27.4, −1.5)* | −22.4 (−36.6, −8.1)‡ | |
*Represents 1 unit change in physical activity index and the unit change in the corresponding outcome unless otherwise specified. All models account for correlation among family members; model 1: adjusted for age and sex; model 2: adjusted for age, sex, education, body mass index, systolic blood pressure, antihypertension medication use, diabetes, coronary heart disease, estimated glomerular filtration rate, and current smoking status. Abbreviations: LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; RWT, relative wall thickness; MR pro-ANP, mid-regional prohormone atrial natriuretic peptide; NT pro-BNP, N-terminal prohormone brain natriuretic peptide. *P < 0.05, †P < 0.01, ‡P < 0.001.
Figure 1.
PA index and LV structure and function. Marginal effect of PA index on LV mass index, RWT, NT pro-BNP concentration levels, and MR pro-ANP concentration levels with a pointwise 95% confidence interval, from a linear regression model 2. Abbreviations: LV, left ventricular; MR pro-ANP, mid-regional prohormone atrial natriuretic peptide; NT pro-BNP, N-terminal prohormone brain natriuretic peptide; RWT, relative wall thickness.
BMI and history of hypertension moderate the relationship between TPA hours and LV mass index
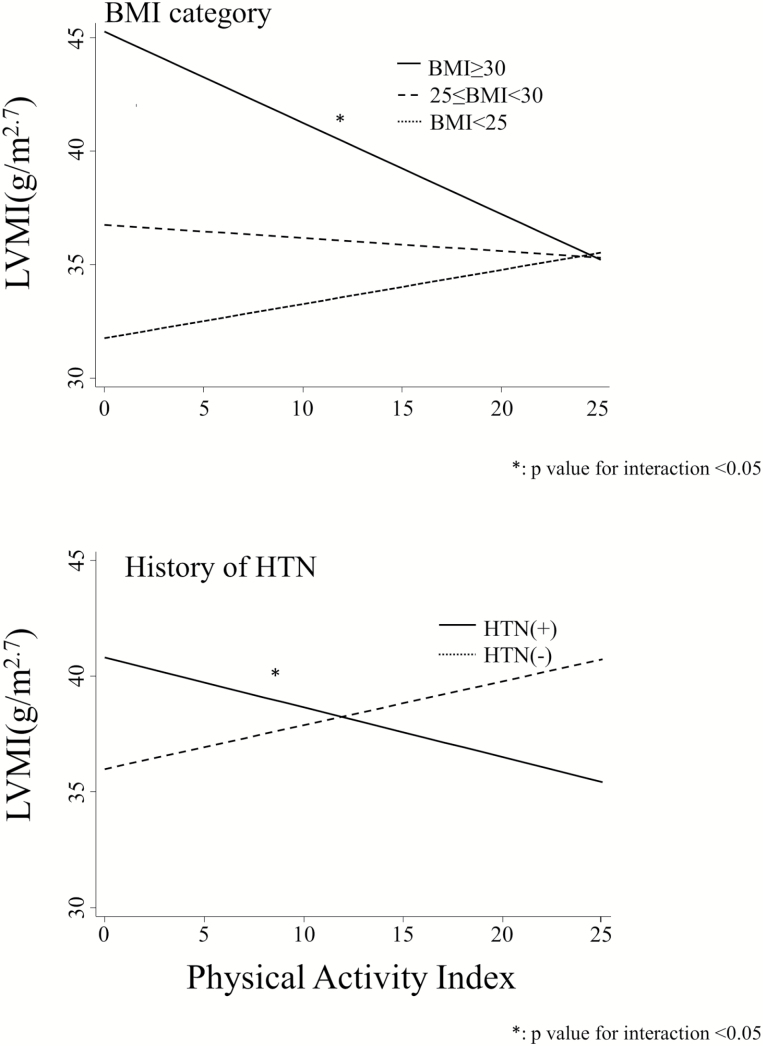
The effects of obesity and hypertension on the relationships between PA index and LV mass index are shown in Figure 2. There is a strong effect of BMI (obese vs. over weight vs. nonobese) at all PA index levels. The coefficient for the interaction between PA index and BMI ≥30 is −0.57 (95% confidence interval: −1.00 to −0.13, P = 0.01). There is also a strong effect of having a history of hypertension at all PA index levels. The coefficient for the interaction between PA index and history of hypertension is −0.41 (95% confidence interval: −0.65 to −0.16, P < 0.01). There were not any interactions between PA index and other categories on LV mass index.
Figure 2.
Marginal plots of the effect of PA index on LV mass index at each subgroup. The model 2 was used here. Abbreviations: BMI, body mass index; CHD, coronary heart disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HTN, hypertension; LVMI, left ventricular mass index.
DISCUSSION
The main findings of this study were that higher PA index is associated with decreased LV mass index, lower RWT, lower NT pro-BNP levels, and lower MR pro-ANP levels after adjustment for confounding factors in African Americans. These findings are all consistent with beneficial effects of PA on cardiac structure and function. The effects of PA on LV mass index were not different between age categories, sexes, presence or absence of diabetes, eGFR categories, or smoking status. However, PA index was significantly negatively associated with LV mass index in obese and hypertensive participants but not nonobese or nonhypertensive African American participants.
Several previous studies have investigated the relationship between PA and LV mass, including prospective studies in patients with hypertension and have shown favorable effects of PA on LV structure and function.13,14,27–30 In the current study, higher PA index was associated with smaller LV mass index and lower RWT, in line with these previous reports. Furthermore, our study indicated that the relationship between PA and LV mass index may be more significant in those with hypertension and obesity. To our knowledge, there are not any other studies which have investigated specific factors which influence the relationship between PA and LV mass. In this study, age, sex, presence of diabetes, chronic kidney disease assessed by eGFR, and current smoking status did not have an interaction with PA on LV mass index. However, there were statistically significant interactions between PA index and obesity or having a past history of hypertension on LV mass index, suggesting that PA may be effective at preventing an increase in LV mass especially in patients with hypertension or obesity.
Habitual aerobic exercise has been shown to lower blood pressure.31–33 In this study, those with higher PA index had lower systolic pressure and higher diastolic pressure. This may be attributed to reductions in circulating noradrenaline and its receptors, reduced angiotensin II, and increased nitric oxide bioavailability.34 In the current study, PA index was associated with LV mass index independent of systolic blood pressure and antihypertensive medication use. Thus, our findings suggest the negative association of PA with LV mass index might be at least partially independent of the effects of lowering blood pressure.
In this study, PA index was not associated with LV systolic function (LV EF) after adjustment. Although some animal studies have reported that PA may be associated with improved LV contractility, some clinical studies suggest no association between PA and LV systolic function.35,36 Other measures such as myocardial strain or assessment of systolic function with cardiac magnetic resonance imaging, may be more sensitive in assessing systolic function; therefore, these techniques may be useful to evaluate the relationship between PA and LV systolic function in future studies.
As others have noted, we observed that those who were spending many hours for PA spent shorter times for sedentary activity (data not shown), and those who were spending many hours for sedentary activity spent shorter time for PA. Sedentary activity has been associated with increased LV mass, therefore, there is a possibility that the increase in LV mass in those with less PA in this study was attributed to the increased sedentary activity.37 Further investigation is warranted to clarify the effects of sedentariness vs. PA on LV mass.
Some previous reports suggest that although there could be differences in myocardial remodeling (eccentric vs. concentric remodeling), both isotonic and isometric exercise may lead to increased LV mass index.38 Although these previous reports were predominantly performed in young athletes and the type of PA may be different, it may be of value to examine in which population and what kind of PA benefits LV structure and function.
This study has a few limitations. Our study is a cross-sectional analysis of the baseline data from a cohort study. Therefore, we cannot determine cause-and-effect relationships to determine if PA has beneficial effects on LV structure. Second, PA was assessed via self-report, which is prone to measurement error, including biases associated with recall and social desirability. Third, this study was conducted among African Americans, so the results might not be generalizable to other ethnic groups. However, African Americans have high rates of obesity and hypertension and are at increased risk of heart failure compared to other ethnic groups. Therefore, our findings provide further rationale for implementing increased PA to prevent important risk factors for heart failure and mortality, particularly in African Americans who are disproportionately affected and understudied.
In conclusions, in a community-based cohort of African Americans, higher PA index was independently associated with smaller LV mass index, lower RWT, lower NT pro-BNP levels, and lower MR pro-ANP levels—all markers of improved cardiac structure and function. Furthermore, higher PA index was associated with decreased LV mass index in those with obesity and hypertension. Therefore, further prospective studies to determine if increased PA prevents LV hypertrophy in obese and hypertensive African Americans are warranted.
SUPPLEMENTARY MATERIAL
Supplementary data are available at American Journal of Hypertension online.
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by grants U01-HL054463 from the National Institutes of Health. MEH is supported by 1K08DK099415 from the National Institutes of Health.
REFERENCES
- 1. Wali RK, Weir MR. Hypertensive cardiovascular disease in African Americans. Curr Hypertens Rep 1999; 1:521–528. [DOI] [PubMed] [Google Scholar]
- 2. Gidding SS, Liu K, Colangelo LA, Cook NL, Goff DC, Glasser SP, Gardin JM, Lima JA. Longitudinal determinants of left ventricular mass and geometry: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Circ Cardiovasc Imaging 2013; 6:769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jones DW. What is the role of obesity in hypertension and target organ injury in African Americans? Am J Med Sci 1999; 317:147–151. [DOI] [PubMed] [Google Scholar]
- 4. Muntner P, Lewis CE, Diaz KM, Carson AP, Kim Y, Calhoun D, Yano Y, Viera AJ, Shimbo D. Racial differences in abnormal ambulatory blood pressure monitoring measures: results from the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Hypertens 2015; 28:640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Selassie A, Wagner CS, Laken ML, Ferguson ML, Ferdinand KC, Egan BM. Progression is accelerated from prehypertension to hypertension in Blacks. Hypertension 2011; 58:579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988-1994 and 1999-2010. Ann Intern Med 2014; 160:517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gorelick PB. Cerebrovascular disease in African Americans. Stroke 1998; 29:2656–2664. [DOI] [PubMed] [Google Scholar]
- 8. Gupta S, Berry JD, Ayers CR, Matulevicius SA, Peshock RM, Patel PC, Markham DW, Drazner MH. Association of Health Aging and Body Composition (ABC) Heart Failure score with cardiac structural and functional abnormalities in young individuals. Am Heart J 2010; 159:817–824. [DOI] [PubMed] [Google Scholar]
- 9. Schnabel RB, Rienstra M, Sullivan LM, Sun JX, Moser CB, Levy D, Pencina MJ, Fontes JD, Magnani JW, McManus DD, Lubitz SA, Tadros TM, Wang TJ, Ellinor PT, Vasan RS, Benjamin EJ. Risk assessment for incident heart failure in individuals with atrial fibrillation. Eur J Heart Fail 2013; 15:843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Gattobigio R, Zampi I, Reboldi G, Porcellati C. Prognostic significance of serial changes in left ventricular mass in essential hypertension. Circulation 1998; 97:48–54. [DOI] [PubMed] [Google Scholar]
- 11. Echouffo-Tcheugui JB, Butler J, Yancy CW, Fonarow GC. Association of physical activity or fitness with incident heart failure: a systematic review and meta-analysis. Circ Heart Fail 2015; 8:853–861. [DOI] [PubMed] [Google Scholar]
- 12. Pandey A, Garg S, Khunger M, Darden D, Ayers C, Kumbhani DJ, Mayo HG, de Lemos JA, Berry JD. Dose-response relationship between physical activity and risk of heart failure: a meta-analysis. Circulation 2015; 132:1786–1794. [DOI] [PubMed] [Google Scholar]
- 13. Kokkinos PF, Narayan P, Colleran JA, Pittaras A, Notargiacomo A, Reda D, Papademetriou V. Effects of regular exercise on blood pressure and left ventricular hypertrophy in African-American men with severe hypertension. N Engl J Med 1995; 333:1462–1467. [DOI] [PubMed] [Google Scholar]
- 14. Turner MJ, Spina RJ, Kohrt WM, Ehsani AA. Effect of endurance exercise training on left ventricular size and remodeling in older adults with hypertension. J Gerontol A Biol Sci Med Sci 2000; 55:M245–M251. [DOI] [PubMed] [Google Scholar]
- 15. Frohlich EDApstein CChobanian AVDevereux RBDustan HPDzau VFauad-Tarazi FHoran MJMarcus MMassie B, Pfeffer MA, Re RN, Roccella EJ, Savage D, Shub C. . The heart in hypertension. N Engl J Med. 1992;327:998–1008. [DOI] [PubMed] [Google Scholar]
- 16. Lauer MS, Anderson KM, Kannel WB, Levy D. The impact of obesity on left ventricular mass and geometry. The Framingham Heart Study. JAMA 1991; 266:231–236. [PubMed] [Google Scholar]
- 17. Hegde SM, Solomon SD. Influence of physical activity on hypertension and cardiac structure and function. Curr Hypertens Rep 2015; 17:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goetze JP, Mogelvang R, Maage L, Scharling H, Schnohr P, Sogaard P, Rehfeld JF, Jensen JS. Plasma pro-B-type natriuretic peptide in the general population: screening for left ventricular hypertrophy and systolic dysfunction. Eur Heart J 2006; 27:3004–3010. [DOI] [PubMed] [Google Scholar]
- 19. Coutinho T, Al-Omari M, Mosley TH Jr, Kullo IJ. Biomarkers of left ventricular hypertrophy and remodeling in Blacks. Hypertension 2011; 58:920–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O’Meara JG, Kardia SL, Armon JJ, Brown CA, Boerwinkle E, Turner ST. Ethnic and sex differences in the prevalence, treatment, and control of dyslipidemia among hypertensive adults in the GENOA study. Arch Intern Med 2004; 164:1313–1318. [DOI] [PubMed] [Google Scholar]
- 21. Kullo IJ, Jan MF, Bailey KR, Mosley TH, Turner ST. Ethnic differences in low-density lipoprotein particle size in hypertensive adults. J Clin Lipidol 2007; 1:218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim CX, Bailey KR, Klee GG, Ellington AA, Liu G, Mosley TH Jr, Rehman H, Kullo IJ. Sex and ethnic differences in 47 candidate proteomic markers of cardiovascular disease: the Mayo Clinic proteomic markers of arteriosclerosis study. PLoS One 2010; 5:e9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med 2004; 141:929–937. [DOI] [PubMed] [Google Scholar]
- 24. Rigalleau V, Lasseur C, Raffaitin C, Perlemoine C, Barthe N, Chauveau P, Combe C, Gin H. The Mayo Clinic quadratic equation improves the prediction of glomerular filtration rate in diabetic subjects. Nephrol Dial Transplant 2007; 22:813–818. [DOI] [PubMed] [Google Scholar]
- 25. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ; Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography . Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005; 18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 26. Royston P. marginscontplot: Plotting the marginal effects of continuous predictors. Stata J 2013; 13:510–527. [Google Scholar]
- 27. Pitsavos C, Chrysohoou C, Koutroumbi M, Aggeli C, Kourlaba G, Panagiotakos D, Michaelides A, Stefanadis C. The impact of moderate aerobic physical training on left ventricular mass, exercise capacity and blood pressure response during treadmill testing in borderline and mildly hypertensive males. Hellenic J Cardiol 2011; 52:6–14. [PubMed] [Google Scholar]
- 28. Palatini P, Visentin P, Dorigatti F, Guarnieri C, Santonastaso M, Cozzio S, Pegoraro F, Bortolazzi A, Vriz O, Mos L; HARVEST Study Group . Regular physical activity prevents development of left ventricular hypertrophy in hypertension. Eur Heart J 2009; 30:225–232. [DOI] [PubMed] [Google Scholar]
- 29. Boman K, Olofsson M, Dahlöf B, Gerdts E, Nieminen MS, Papademetriou V, Wachtell K, Devereux RB. Left ventricular structure and function in sedentary and physically active subjects with left ventricular hypertrophy (the LIFE Study). Am J Cardiol 2005; 95:280–283. [DOI] [PubMed] [Google Scholar]
- 30. Hinderliter A, Sherwood A, Gullette EC, Babyak M, Waugh R, Georgiades A, Blumenthal JA. Reduction of left ventricular hypertrophy after exercise and weight loss in overweight patients with mild hypertension. Arch Intern Med 2002; 162:1333–1339. [DOI] [PubMed] [Google Scholar]
- 31. Blair SN, Goodyear NN, Gibbons LW, Cooper KH. Physical fitness and incidence of hypertension in healthy normotensive men and women. JAMA 1984; 252:487–490. [PubMed] [Google Scholar]
- 32. Duncan JJ, Farr JE, Upton SJ, Hagan RD, Oglesby ME, Blair SN. The effects of aerobic exercise on plasma catecholamines and blood pressure in patients with mild essential hypertension. JAMA 1985; 254:2609–2613. [PubMed] [Google Scholar]
- 33. Nelson L, Jennings GL, Esler MD, Korner PI. Effect of changing levels of physical activity on blood-pressure and haemodynamics in essential hypertension. Lancet 1986; 2:473–476. [DOI] [PubMed] [Google Scholar]
- 34. Yung LM, Laher I, Yao X, Chen ZY, Huang Y, Leung FP. Exercise, vascular wall and cardiovascular diseases: an update (part 2). Sports Med 2009; 39:45–63. [DOI] [PubMed] [Google Scholar]
- 35. Kemi OJ, Wisløff U. Mechanisms of exercise-induced improvements in the contractile apparatus of the mammalian myocardium. Acta Physiol (Oxf) 2010; 199:425–439. [DOI] [PubMed] [Google Scholar]
- 36. Guirado GN, Damatto RL, Matsubara BB, Roscani MG, Fusco DR, Cicchetto LA, Seki MM, Teixeira AS, Valle AP, Okoshi K, Okoshi MP. Combined exercise training in asymptomatic elderly with controlled hypertension: effects on functional capacity and cardiac diastolic function. Med Sci Monit 2012; 18:CR461–CR465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gibbs BB, Reis JP, Schelbert EB, Craft LL, Sidney S, Lima J, Lewis CE. Sedentary screen time and left ventricular structure and function: the CARDIA study. Med Sci Sports Exerc 2014; 46:276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weiner RB, Wang F, Isaacs SK, Malhotra R, Berkstresser B, Kim JH, Hutter AM Jr, Picard MH, Wang TJ, Baggish AL. Blood pressure and left ventricular hypertrophy during American-style football participation. Circulation 2013; 128:524–531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.