Abstract
BACKGROUND
The Systolic Blood Pressure Intervention Trial (SPRINT) showed that targeting a systolic blood pressure (SBP) of ≤ 120 mm Hg (intensive treatment) reduced cardiovascular disease (CVD) events compared to SBP of ≤ 140 mm Hg (standard treatment); however, it is unclear if this effect is similar in all racial/ethnic groups.
METHODS
We analyzed SPRINT data within non-Hispanic White (NHW), non-Hispanic Black (NHB), and Hispanic subgroups to address this question. High-risk nondiabetic hypertensive patients (N = 9,361; 30% NHB; 11% Hispanic) 50 years and older were randomly assigned to intensive or standard treatment. Primary outcome was a composite of the first occurrence of a myocardial infarction, acute coronary syndrome, stroke, decompensated heart failure, or CVD death.
RESULTS
Average postbaseline SBP was similar among NHW, NHB, and Hispanics in both treatment arms. Hazard ratios (HRs) (95% confidence interval) (intensive vs. standard treatment groups) for primary outcome were 0.70 (0.57–0.86), 0.71 (0.51–0.98), 0.62 (0.33–1.15) (interaction P value = 0.85) in NHW, NHB, and Hispanics. CVD mortality HRs were 0.49 (0.29–0.81), 0.77 (0.37–1.57), and 0.17 (0.01–1.08). All-cause mortality HRs were 0.61 (0.47–0.80), 0.92 (0.63–1.35), and 1.58 (0.73–3.62), respectively. A test for differences among racial/ethnic groups in the effect of treatment assignment on all-cause mortality was not significant (Hommel-adjusted P value = 0.062) after adjustment for multiple comparisons.
CONCLUSION
Targeting a SBP goal of ≤ 120 mm Hg compared to ≤ 140 mm Hg led to similar SBP control and was associated with similar benefits and risks among all racial ethnic groups, though NHBs required an average of ~0.3 more medications.
CLINICAL TRIALS REGISTRATION
Trial Number NCT01206062, ClinicalTrials.gov Identifier at https://clinicaltrials.gov/ct2/show/NCT01206062.
Keywords: African Americans, blood pressure, clinical outcomes, clinical trials, Hispanics, hypertension, race and ethnicity
Racial and ethnic differences in cardiovascular disease (CVD) remain a major public health challenge in the United States.1–3 Hypertension is one of the most important, modifiable CVD risk factors leading to coronary heart disease, stroke, end-stage renal disease, and overall mortality.4–6 Non-Hispanic Black (NHB) and Hispanic adults (compared to non-Hispanic Whites [NHWs]) have higher rates of uncontrolled blood pressure (BP) (50%, 54%, and 46%, respectively), and NHBs are at greater risk of hypertension-related CVD morbidity and mortality.4 CVD age-adjusted death rates are 33% higher among NHBs when compared to the overall US population.4,5 Hypertension-related age-adjusted mortality rates of adults aged 25 years and older are 127.2 vs. 135.9 per 100,000 populations for Hispanics vs. NHWs, respectively, though considerable heterogeneity in CVD risk is seen in Hispanics based on country of origin.7,8 These disparities cost the US health care system an estimated $49 billion per year.2,5,9 Therefore, BP control interventions to reduce CVD morbidity and mortality in underrepresented racial and ethnic groups are important at both the individual and population levels.
Over the past 2 decades, studies have demonstrated that lowering BP with antihypertensive medications reduces the risk of CVD morbidity and mortality, including in NHB and Hispanic populations.10–14 Until recently, there was insufficient data to determine optimal BP targets for the treatment of hypertension in these populations.2,7 Lowering systolic BP (SBP) with antihypertensive medications significantly reduced CVD morbidity and mortality in the Systolic Hypertension in the Elderly Program (SHEP), Systolic Hypertension in Europe (Syst-Eur) Trial, and the Hypertension in the Very Elderly Trial (HYVET).15–17 However, SBP treatment goals in the more intensive treatment arms of these trials were between 150 and 160 mm Hg and only SHEP included African American (AA) participants (14%).15,17 Two small trials (with limited statistical power) that compared a SBP target of <140 mm Hg to <160 mm Hg found no significant difference in CVD outcomes.18,19 The Action to Control Cardiovascular Risk in Diabetes BP trial (ACCORD), which included a racially diverse population (24% NHBs and 7% Hispanics) of 4,733 high-risk hypertensives with type 2 diabetes mellitus treated to a SBP target <120 mm Hg (compared to SBP <140 mm Hg), identified no difference in the primary CVD composite outcome but a significant reduction in stroke risk in the <120 mm Hg group.20
More recently, random assignment to a SBP target <120 mm Hg compared to <140 mm Hg among people at high CVD risk (without diabetes or history of stroke) resulted in a 25% reduction in the primary outcome (a CVD composite) and a 27% reduction in all-cause mortality in the National Institutes of Health (NIH) sponsored Systolic Blood Pressure Intervention Trial (SPRINT).13,21 Of the 9,361 SPRINT participants, 30% were NHBs and 11% were Hispanic.13,22,23 Exploration of the trial results in non-Black compared to Black participants was a prespecified analysis. Thus, SPRINT provides a large database for the study of BP treatment targets in these diverse populations.21 This report describes the major outcomes in SPRINT by race and Hispanic ethnicity.
METHODS
Patient selection
Details of the SPRINT study design and rationale have been previously reported and more extensive details of the study protocol and procedures are publicly available.13,21,24 All participants gave written informed consent and the trial protocol was approved by the institutional review board at each participating site. The SPRINT cohort consisted of men and women, aged 50 years or older with a SBP between 130–180 mmHg on 0–4 antihypertensive medications and at high CVD risk. High CVD risk was defined by the presence of one or more of the following at study entry: clinical or subclinical CVD (other than stroke); chronic kidney disease (CKD) defined as an estimated glomerular filtration rate calculated with the 4-variable Modification of Diet in Renal Disease (MDRD) equation of 20–59 ml/min/1.73 m2 using the most recent serum creatinine drawn within the preceding 6 months; a Framingham Risk Score for 10-year CVD risk ≥15%; or age ≥75 years. Individuals with history of diabetes, stroke, polycystic kidney disease, or heart failure were excluded.21
Race was self-reported by participants as Black (or AA), White, Native American, Asian, Native Hawaiian or other Pacific Islander, and other; and ethnicity was based upon independent self-identification as Hispanic or non-Hispanic. In this report, the 984 participants who self-identified as Hispanic were considered part of that group (regardless of self-identified race). Non-Hispanic participants who self-identified as AA alone were grouped as NHB (n = 2802). Non-Hispanic participants who self-identified as White alone were grouped as NHW (n = 5399). Participants who self-identified as belonging to other race categories, selected more than one race category or did not specify a race were excluded from this analysis (n = 176).
Intervention
SPRINT participants were randomly assigned to 1 of 2 SBP targets standard (SBP <140 mm Hg) or intensive (SBP <120 mm Hg) treatment. SPRINT investigators initiated and adjusted antihypertensive medications to achieve the assigned SBP targets according to the SPRINT step-care treatment algorithm using US Food and Drug Administration approved antihypertensive drugs provided by the study. At each visit, trained clinical staff measured BPs with an automated BP device (Omron-HEM-907 XL) using standardized procedures.21,24 BP measurement requirements included measuring BP early in the visit and not following stressful exam components such as blood draws, proper positioning of the participant in a chair with back support, and proper cuff size determination. The SPRINT Manual of Procedures (MOP) stated that participants should be resting, not completing questionnaires, and not speaking with study staff during the 5-minute rest period or while BP measurements were being taken. The MOP also stated that staff should leave the room during the 5-minute rest period, and provided a script that staff could use to explain that they would be absent during the 5-minute rest period and would then enter the room and obtain the measurements without speaking to the participant.24
Study measures and outcomes
At baseline, information on sociodemographics, cardiovascular risk factors, concomitant medications, social and medical history, anthropometrics, dementia screening, and quality of life measurements were collected on all eligible patients. Routine follow-up visits were conducted at 1, 2, 3, and every 3 months thereafter during the trial. Specific laboratory data (e.g., serum creatinine, fasting serum glucose) were collected at baseline and every 3 months. Additional visits were scheduled as needed for management of adverse effects or SBP goal attainment.
The primary outcome was a composite of the first occurrence of a myocardial infarction, acute coronary syndrome not leading to a myocardial infarction, stroke, decompensated heart failure, or cardiovascular death. Major secondary outcomes included CVD mortality, all-cause mortality, and a composite of total mortality and the primary outcome. Other prespecified secondary outcomes analyzed in this report included decline in renal function or development of end-stage renal disease. Definitions of these outcomes were prospectively defined in the SPRINT protocol.24 Self-reported study outcomes were ascertained quarterly by clinical site staff using structured interviews for both treatment groups. Medical records and other corroborating data were collected for each potential outcome; all study outcomes were reviewed and adjudicated by the trial’s outcome committee using a prespecified protocol and blinded to treatment assignment.21,24
In contrast to study outcomes, adverse events, including serious adverse events (SAEs), could be reported at any visit. SAEs were defined as an adverse experience judged by an investigator to be life threatening and/or resulting in death, permanent disability, or hospitalization or prolongation of hospitalization, whether or not the event was thought to be related to study intervention. SPRINT considered any emergency visit for heart failure, bradycardia, stroke, transient ischemic attack, or electrolyte abnormalities, and any syncope or injurious falls as a reportable SAE. Clinical and laboratory variables (serum potassium, creatinine levels, and estimated glomerular filtration rate) were also examined as potential adverse effects. An independent Data and Safety Monitoring Board (DSMB) monitored unblinded study data and provided oversight of participant safety.
Statistical analysis
Descriptive statistics (means and SD for continuous variables; frequencies and percentages for categorical variables) of baseline characteristics were computed by race/ethnicity and by treatment group within each stratum of race/ethnicity. Pairwise comparisons (i.e., each race/ethnicity compared to the other) were made using 1-way analysis of variance with pairwise contrasts for continuous variables and separate chi-square tests for categorical variables. Mean and SE of follow-up SBP was estimated by race/ethnicity and treatment group using mixed linear models with unstructured variance-covariance to control for within-subject correlation. Effect of treatment arm assignment on time to the first event within race/ethnicity stratum (i.e., CVD outcomes, mortality, CKD outcomes, and SAEs) was analyzed based on the intention-to-treat approach using univariable Cox proportional-hazards regression models for treatment arm assignment with 2-sided tests at the 5% level of significance and stratification by clinical site. Two-way interactions between treatment effect and race/ethnicity group were assessed using likelihood-ratio tests and Hommel’s technique to adjust for multiple comparisons. Since the subgroup definitions in this report differ from the prespecified race subgroup of Blacks vs. non-Blacks (in the prespecified comparison, the Black group included both Hispanic and non-Hispanic ethnicities who self-identified as Black while the non-Black group included both Hispanics and non-Hispanics who self-identified as White), outcome data for the prespecified race subgroups are presented in the Appendix (Supplementary Table S2). All analyses were performed using SAS version 9.4 software (SAS Institute, Cary, NC).
Following a recommendation by the trial’s independent DSMB, the SPRINT BP intervention was halted on 20 August 2015 by the National Heart, Lung, and Blood Institute (NHLBI) Director after a mean follow-up of 3.26 years. Follow-up was censored at the date of last assessment for a study event in each participant prior to 21 August 2015. This publication is based on a database that was frozen on 16 September 2016 and includes outcome events from baseline until the termination of the trial intervention on 21 August 2015.
RESULTS
Study participants
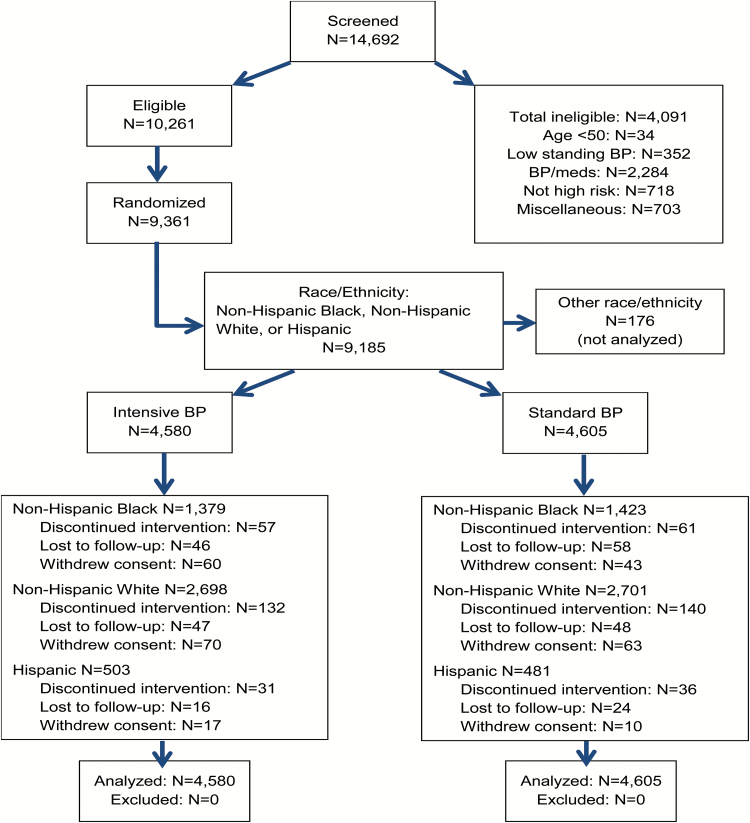
Figure 1 displays the CONSORT diagram for SPRINT by race/ethnicity group. Baseline characteristics by race/ethnicity and treatment assignment are provided in Table 1 and by race/ethnicity, pooled across treatment (Supplementary Table S1). After stratifying by race/Hispanic ethnicity, few significant differences in baseline characteristics and risk profile were noted between treatment groups. Significant differences in weight between randomized treatment groups were seen in the Hispanic subgroup, statin use in NHBs, and aspirin use in NHWs (Table 1).
Figure 1.
Consort diagram. Race/ethnicity was self-reported and participants were classified as: Hispanic regardless of self-identified race; non-Hispanic Black if self-identified as African American alone; and non-Hispanic White if self-identified as White alone.
Table 1.
Baseline clinical characteristics of SPRINT participants stratified by race/ethnicity and randomized group
| Non-Hispanic White (n = 5,399)a | Non-Hispanic Black (n = 2,802)a | Hispanic (n = 984)a | ||||
|---|---|---|---|---|---|---|
| Characteristics | Intensive | Standard | Intensive | Standard | Intensive | Standard |
| No. randomized | 2,698 | 2,701 | 1,379 | 1,423 | 503 | 481 |
| Age | ||||||
| 50–64 | 782 (29.0) | 813 (30.1) | 824 (59.8) | 831 (58.4) | 267 (53.1) | 247 (51.4) |
| 65–74 | 939 (34.8) | 901 (33.4) | 329 (23.9) | 366 (25.7) | 147 (29.2) | 149 (31.0) |
| ≥75 | 977 (36.2) | 987 (36.5) | 225 (16.3) | 226 (15.9) | 89 (17.7) | 85 (17.7) |
| Female gender | 795 (29.5) | 757 (28.0) | 630 (45.7) | 641 (45.1) | 225 (44.7) | 229 (47.6) |
| Chronic kidney diseaseb | 885 (32.8) | 893 (33.1) | 325 (23.6) | 312 (21.9) | 94 (18.7) | 96 (20.0) |
| Cardiovascular disease | ||||||
| Clinical | 526 (19.5) | 507 (18.8) | 135 (9.8) | 144 (10.1) | 65 (12.9) | 63 (13.1) |
| Subclinical | 207 (7.7) | 222 (8.2) | 121 (8.8) | 120 (8.4) | 47 (9.3) | 48 (10.0) |
| Framingham 10-year CVD risk score (%) | 26.8 ± 12.8 | 26.8 ± 12.6 | 21.7 ± 11.7 | 21.9 ± 11.8 | 23.0 ± 12.3 | 22.4 ± 11.8 |
| Baseline blood pressure | ||||||
| SBP, mm Hg | 139.6 ± 15.5 | 139.2 ± 15.2 | 139.5 ± 16.7 | 140.0 ± 15.8 | 140.2 ± 14.5 | 141.2 ± 15.1 |
| DBP, mm Hg | 76.8 ± 11.4 | 76.5 ± 11.6 | 81.2 ± 12.5 | 81.3 ± 12.3 | 77.6 ± 11.2 | 77.3 ± 11.2 |
| Pulse, bpm | 65.2 ± 11.3 | 65.2 ± 11.5 | 68.7 ± 11.9 | 68.4 ± 11.9 | 65.4 ± 10.3 | 66.1 ± 10.7 |
| # of BP Medications | 1.78 ± 1.03 | 1.78 ± 1.05 | 1.99 ± 1.06 | 1.96 ± 1.05 | 1.79 ± 0.97 | 1.71 ± 0.93 |
| Weight, lbs. | 190.0 ± 40.9 | 191.2 ± 41.4 | 196.7 ± 42.7 | 195.7 ± 42.5 | 180.1 ± 36.3* | 175.6 ± 35.3* |
| BMI, kg/m2 | 29.4 ± 5.5 | 29.4 ± 5.4 | 31.0 ± 6.4 | 30.8 ± 6.3 | 29.8 ± 5.3 | 29.3 ± 5.0 |
| # of chronic diseases | 3.02 ± 1.70 | 3.02 ± 1.74 | 2.18 ± 1.56 | 2.20 ± 1.53 | 2.24 ± 1.64 | 2.16 ± 1.47 |
| Smoking status | ||||||
| Current | 238 (8.8) | 230 (8.5) | 324 (23.6) | 315 (22.2) | 68 (13.6) | 45 (9.4) |
| Past | 1,340 (49.8) | 1,340 (50.0) | 453 (33.0) | 484 (34.2) | 157 (31.3) | 146 (30.5) |
| Statin use | 1,311 (48.9) | 1,338 (50.0) | 430 (31.4)† | 513 (36.3)† | 193 (38.6) | 198 (41.3) |
| Aspirin use | 906 (33.8)† | 805 (30.1)† | 365 (26.7) | 364 (25.8) | 120 (24.0) | 117 (24.4) |
| Baseline laboratory values | ||||||
| Fasting glucose (mg/dl) | 99.3 ± 13.2 | 99.5 ± 11.9 | 98.0 ± 15.3 | 97.6 ± 16.1 | 98.3 ± 11.6 | 98.5 ± 12.2 |
| Cholesterol, total (mg/dl) | 186.4 ± 40.9 | 186.3 ± 41.1 | 196.6 ± 42.2 | 195.2 ± 39.7 | 192.8 ± 40.3 | 194.9 ± 40.3 |
| Cholesterol, LDL (mg/dl) | 108.8 ± 34.5 | 108.2 ± 34.4 | 119.7 ± 36.4 | 118.5 ± 34.3 | 114.1 ± 35.2 | 115.0 ± 34.2 |
| Cholesterol, HDL (mg/dl) | 52.3 ± 14.1 | 41.9 ± 14.3 | 54.9 ± 15.2 | 55.3 ± 14.9 | 50.7 ± 12.3 | 50.6 ± 14.1 |
| Triglycerides (mg/dl) | 127.7 ± 79.3 | 131.7 ± 83.1 | 113.1 ± 102.1 | 110.0 ± 110.3 | 140.6 ± 68.6 | 149.8 ± 97.9 |
| Potassium (mmol/l) | 4.28 ± 0.43 | 4.27 ± 0.42 | 4.07 ± 0.41 | 4.08 ± 0.49 | 4.19 ± 0.43 | 4.21 ± 0.40 |
| Sodium (mmol/l) | 139.9 ± 2.6 | 140.0 ± 2.5 | 140.5 ± 2.3 | 140.4 ± 2.2 | 140.3 ± 2.2 | 140.2 ± 2.3 |
| eGFR (ml/min/1.73 m2) | 68.3 ± 18.1 | 68.0 ± 18.3 | 76.3 ± 23.4 | 76.7 ± 22.3 | 78.0 ± 22.3 | 77.2 ± 22.3 |
| Creatinine (mg/dl) | 1.06 ± 0.31 | 1.07 ± 0.31 | 1.14 ± 0.40 | 1.13 ± 0.38 | 0.96 ± 0.33 | 0.96 ± 0.34 |
Plus–minus values are means ± SD: *P < 0.05, †P < 0.01 Standard vs. intensive arms. SI conversion factors: to convert the values for creatinine to micromoles per liter, multiply by 88.4. To convert the values for cholesterol to millimoles per liter, multiply by 0.0259. To convert the values for triglycerides to millimoles per liter, multiply by 0.0113. To convert the values for glucose to millimoles per liter, multiply by 0.0555. Abbreviations: BMI, Body Mass Index; BP, blood pressure; CVD, cardiovascular disease primary outcome; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure; SPRINT, Systolic Blood Pressure Intervention Trial.
aRace/ethnicity was self-reported and participants were classified as: Hispanic regardless of self-identified race; non-Hispanic Black if self-identified as African American alone; and non-Hispanic White if self-identified as white alone.
bChronic kidney disease was defined as an eGFR of less than 60 ml/min/1.73 m2.
Blood pressure
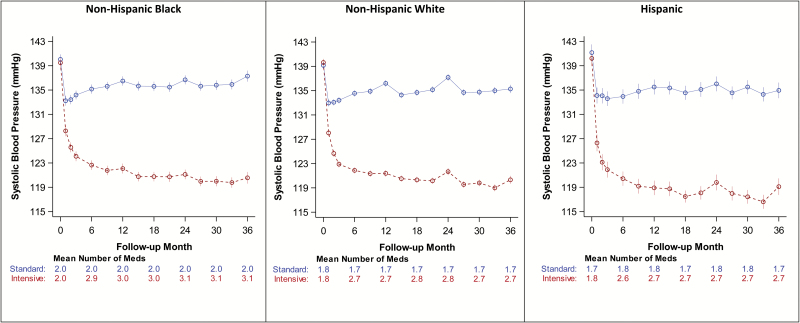
SBP levels were similar by race/ethnicity stratum and between treatment groups at baseline (Table 1). SBP over time is shown in Figure 2. Overall, SBP decreased substantially during the first year of the study in all race/ethnicity groups and showed only modest differences between racial/ethnic groups for both SBP. Average postbaseline mean ± SE follow-up SBP in the standard arm was 134.7 ± 0.1 mm Hg in NHW, 135.5 ± 0.2 mm Hg in NHB, and 134.8 ± 0.3 mm Hg in Hispanic participants; compared to intensive arm values of 121.8 ± 0.2, 122.6 ± 0.2, and 119.9 ± 0.4 in NHW, NHB, and Hispanic participants, respectively. The mean number of antihypertensive medications prescribed were significantly higher in NHB (mean ± SE intensive arm 3.01 ± 0.03, standard arm 1.99 ± 0.03) compared to in NHW (intensive arm 2.74 ± 0.02, standard arm 1.75 ± 0.02; P value <0.0001 vs. NHB, both arms), and in Hispanics (intensive arm 2.70 ± 0.05, standard arm 1.77 ± 0.05; P value <0.0001 vs. NHB, both arms) (Figure 2).
Figure 2.
Follow-up SBP and mean number of antihypertensive meds by treatment arm and race/ethnicity. The SBP separation between treatment groups at year 12, 24, and 36 months was 14.4, 15.6, and 16.7 mm Hg, respectively in NHBs; 14.8, 15.5, and 15.0.
CVD outcomes
The HRs (95% confidence interval) (intensive vs. standard treatment groups) for the primary composite outcome were 0.70 (0.57–0.86), 0.71 (0.51–0.98), 0.62 (0.33–1.15) in NHWs, NHBs, and Hispanics, respectively (Table 2). For CV mortality, HRs were 0.49 (0.29–0.81) in NHW, 0.77 (0.37–1.57) in NHB, and 0.17 (0.01–1.08) in Hispanics, though for all-cause mortality they were 0.61 (0.47–0.80) in NHW, 0.92 (0.63–1.35) in NHB, 1.58 (0.73–3.62) in Hispanics. The effect of treatment arm assignment was homogenous with all interaction P values >0.05 across racial/ethnic groups for the primary CVD outcome, as well as the secondary outcomes of myocardial infarction, acute coronary syndrome, stroke, heart failure, CVD death, and primary outcome.
Table 2.
Primary and secondary outcomes stratified by treatment group and race/ethnicity
| Outcome | Race/ethnicity | Intensive arm | Standard arm | Intensive vs. standard hazard ratio | Interaction P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Events | % per Year | N | Events | % per Year | HR | Lower 95% CI | Upper 95% CI | |||
| Primary outcomea | (Non-Hispanic) White | 2,698 | 167 | 1.9 | 2,701 | 229 | 2.7 | 0.70 | 0.57 | 0.86 | 0.85 |
| (Non-Hispanic) Black | 1,379 | 64 | 1.5 | 1,423 | 93 | 2.1 | 0.71 | 0.51 | 0.98 | ||
| Hispanic | 503 | 20 | 1.2 | 481 | 26 | 1.7 | 0.62 | 0.33 | 1.15 | ||
| Myocardial infarction | (Non-Hispanic) White | 2,698 | 69 | 0.8 | 2,701 | 88 | 1.0 | 0.77 | 0.56 | 1.05 | 0.63 |
| (Non-Hispanic) Black | 1,379 | 22 | 0.5 | 1,423 | 35 | 0.8 | 0.64 | 0.36 | 1.09 | ||
| Hispanic | 503 | 9 | 0.6 | 481 | 13 | 0.8 | 0.48 | 0.18 | 1.18 | ||
| Acute coronary syndrome | (Non-Hispanic) White | 2,698 | 30 | 0.3 | 2,701 | 26 | 0.3 | 1.15 | 0.68 | 1.95 | 0.35 |
| (Non-Hispanic) Black | 1,379 | 7 | 0.2 | 1,423 | 9 | 0.2 | 0.86 | 0.30 | 2.33 | ||
| Hispanic | 503 | 3 | 0.2 | 481 | 5 | 0.3 | 0.57 | 0.11 | 2.34 | ||
| Stroke | (Non-Hispanic) White | 2,698 | 44 | 0.5 | 2,701 | 49 | 0.6 | 0.87 | 0.58 | 1.32 | 0.38 |
| (Non-Hispanic) Black | 1,379 | 14 | 0.3 | 1,423 | 22 | 0.5 | 0.66 | 0.33 | 1.28 | ||
| Hispanic | 503 | 6 | 0.4 | 481 | 5 | 0.3 | 1.05 | 0.31 | 3.69 | ||
| Heart failure | (Non-Hispanic) White | 2,698 | 40 | 0.5 | 2,701 | 70 | 0.8 | 0.55 | 0.37 | 0.81 | 0.27 |
| (Non-Hispanic) Black | 1,379 | 22 | 0.5 | 1,423 | 29 | 0.6 | 0.80 | 0.45 | 1.40 | ||
| Hispanic | 503 | 4 | 0.2 | 481 | 5 | 0.3 | 0.61 | 0.15 | 2.35 | ||
| Cardiovascular death | (Non-Hispanic) White | 2,698 | 23 | 0.3 | 2,701 | 45 | 0.5 | 0.49 | 0.29 | 0.81 | 0.098 |
| (Non-Hispanic) Black | 1,379 | 13 | 0.3 | 1,423 | 18 | 0.4 | 0.77 | 0.37 | 1.57 | ||
| Hispanic | 503 | 1 | 0.1 | 481 | 6 | 0.4 | 0.17 | 0.01 | 1.08 | ||
| Non-CVD death | (Non-Hispanic) White | 2,698 | 57 | 0.6 | 2,701 | 82 | 0.9 | 0.69 | 0.49 | 0.97 | 0.006 |
| (Non-Hispanic) Black | 1,379 | 30 | 0.7 | 1,423 | 26 | 0.6 | 1.16 | 0.68 | 1.98 | ||
| Hispanic | 503 | 12 | 0.7 | 481 | 4 | 0.3 | 3.28 | 0.98 | 14.77 | ||
| All-cause mortality | (Non-Hispanic) White | 2,698 | 89 | 1.0 | 2,701 | 144 | 1.6 | 0.61 | 0.47 | 0.80 | 0.008 |
| (Non-Hispanic) Black | 1,379 | 51 | 1.2 | 1,423 | 56 | 1.2 | 0.92 | 0.63 | 1.35 | ||
| Hispanic | 503 | 19 | 1.1 | 481 | 12 | 0.8 | 1.58 | 0.73 | 3.62 | ||
| Primary outcome or death | (Non-Hispanic) White | 2,698 | 222 | 2.6 | 2,701 | 310 | 3.6 | 0.70 | 0.59 | 0.83 | 0.082 |
| (Non-Hispanic) Black | 1,379 | 94 | 2.2 | 1,423 | 122 | 2.7 | 0.78 | 0.59 | 1.030 | ||
| Hispanic | 503 | 35 | 2.1 | 481 | 31 | 2.0 | 1.00 | 0.60 | 1.67 | ||
Abbreviations: CI, confidence interval, CVD, cardiovascular disease primary outcome; HR, hazard ratio.
aThe primary outcome includes the first occurrence of myocardial infarction, acute coronary syndrome, stroke, heart failure, or death from cardiovascular causes. Median follow-up of 3.26 years.
There appeared to be heterogeneity of effect for all-cause mortality and for non-CVD death (interaction P value = 0.008 and 0.006, respectively) (Table 2). However, after adjusting for multiple comparisons, the treatment by race/ethnicity interaction approached significance only for all-cause death (Hommel-adjusted P value = 0.062). CVD mortality was similarly reduced in all race/ethnic groups (treatment by race/ethnic interaction P value = 0.098), including in treatment comparisons by Blacks vs. non-Blacks (Supplementary Tables S2 and S3), or whether Hispanics resided in Puerto Rico (n = 437) or the US mainland (n = 550) (data not shown). Treatment by residence interaction P values were 0.40, 0.68, and 0.43 for primary outcome, CVD mortality, and all-cause mortality, respectively.
CKD outcomes
The effect of treatment arm assignment on CKD outcomes (stratified by baseline CKD vs. non-CKD subgroup) by race/ethnicity is shown in Table 3 and Supplementary Table S2. The numbers of events in the CKD subgroup were small, particularly for the primary composite renal outcome of ≥50% reduction in estimated glomerular filtration rate or end-stage renal disease; treatment effects were similar across race/ethnicity groups for all 4 outcomes.
Table 3.
CKD outcomes in the CKD and non-CKD subgroups, stratified by treatment group and race/ethnicity
| Outcome | Subgroup | Intensive arm | Standard arm | Intensive vs. standard hazard ratio | Interaction P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Events | % per Year | N | Events | % per Year | HR | Lower 95% CI | Upper 95% CI | |||
| CKD subgroup: composite renal outcomea | (Non-Hispanic) White | 885 | 8 | 0.3 | 893 | 7 | 0.24 | 1.14 | 0.38 | 3.41 | 0.76 |
| (Non-Hispanic) Black | 325 | 9 | 0.9 | 312 | 8 | 0.79 | 1.09 | 0.40 | 3.14 | ||
| Hispanic | 94 | 0 | 0.0 | 96 | 0 | 0.00 | – | – | – | ||
| CKD subgroup: incident albuminuriab | (Non-Hispanic) White | 365 | 49 | 4.3 | 346 | 60 | 5.7 | 0.79 | 0.53 | 1.18 | 0.17 |
| (Non-Hispanic) Black | 118 | 11 | 3.0 | 106 | 18 | 5.7 | 0.26 | 0.08 | 0.69 | ||
| Hispanic | 35 | 4 | 3.7 | 41 | 7 | 5.8 | 1.39 | 0.28 | 7.17 | ||
| Non-CKD subgroup: ≥30% eGFR reduction to CKDc | (Non-Hispanic) White | 1,808 | 88 | 1.5 | 1,798 | 21 | 0.4 | 4.38 | 2.77 | 7.24 | 0.22 |
| (Non-Hispanic) Black | 1,046 | 42 | 1.3 | 1,103 | 16 | 0.5 | 2.61 | 1.47 | 4.83 | ||
| Hispanic | 406 | 17 | 1.3 | 383 | 4 | 0.3 | 4.02 | 1.44 | 14.3 | ||
| Non-CKD subgroup: incident albuminuria | (Non-Hispanic) White | 965 | 81 | 2.7 | 955 | 101 | 3.4 | 0.77 | 0.57 | 1.03 | 0.51 |
| (Non-Hispanic) Black | 576 | 45 | 2.5 | 642 | 65 | 3.4 | 0.73 | 0.49 | 1.08 | ||
| Hispanic | 219 | 15 | 2.1 | 213 | 15 | 2.2 | 0.80 | 0.36 | 1.75 | ||
Abbreviations: CI, confidence interval, CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate.
aFor participants with CKD at baseline, composite renal outcome was the first occurrence of a reduction in eGFR by 50% (measure twice at least 90 days apart) or long-term dialysis or kidney transplant.
bIncident albuminuria denotes a urinary albumin to creatinine ratio of less than 10 mg/g at baseline and doubled to a creatinine ratio from less than 10 mg/g to 10 mg/g or greater (measured twice at least 90 days apart).
cIncludes a 30% reduction in eGFR (measured twice at least 90 days apart) to an eGFR of less than 60 ml/min/1.73 m2, dialysis, or a kidney transplant in participants without CKD at baseline.
Serious adverse events
Results for SAEs are displayed in Table 4. Between the 2 treatment groups, no heterogeneity of effect was noted between the 3 racial ethnic groups in terms of overall SAEs or the 6 select SAEs: hypotension, syncope, bradycardia, electrolyte abnormality, injurious falls, or acute kidney injury.
Table 4.
Selected serious adverse events stratified by treatment group and by race/ethnicity
| Outcome | Race/ethnicity | Intensive arm | Standard arm | Intensive vs. standard hazard ratio | Interaction P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Events | % per Year | N | Events | % per Year | HR | Lower 95% CI | Upper 95% CI | |||
| Any SAEa | (Non-Hispanic) White | 2,698 | 430 | 5.5 | 2,701 | 5.9 | 17.0 | 0.93 | 0.82 | 1.06 | 0.24 |
| (Non-Hispanic) Black | 1,379 | 146 | 3.6 | 1,379 | 147 | 3.6 | 1.02 | 0.81 | 1.29 | ||
| Hispanic | 503 | 42 | 2.7 | 481 | 29 | 1.9 | 1.40 | 0.87 | 2.24 | ||
| Hypotension | (Non-Hispanic) White | 2,698 | 38 | 0.4 | 2,701 | 27 | 0.3 | 1.40 | 0.86 | 2.30 | 0.28 |
| (Non-Hispanic) Black | 1,379 | 11 | 0.3 | 1,379 | 11 | 0.3 | 1.03 | 0.45 | 2.38 | ||
| Hispanic | 503 | 2 | 0.1 | 481 | 0 | 0.0 | – | – | – | ||
| Syncope | (Non-Hispanic) White | 2,698 | 39 | 0.5 | 2,701 | 31 | 0.4 | 1.24 | 0.77 | 1.99 | 0.94 |
| (Non-Hispanic) Black | 1,379 | 9 | 0.2 | 1,379 | 7 | 0.2 | 1.32 | 0.49 | 3.56 | ||
| Hispanic | 503 | 5 | 0.3 | 481 | 3 | 0.2 | 1.59 | 0.38 | 6.66 | ||
| Bradycardia | (Non-Hispanic) White | 2,698 | 32 | 0.4 | 2,701 | 29 | 0.3 | 1.10 | 0.66 | 1.81 | 0.30 |
| (Non-Hispanic) Black | 1,379 | 4 | 0.1 | 1,379 | 8 | 0.2 | 0.51 | 0.15 | 1.70 | ||
| Hispanic | 503 | 1 | 0.1 | 481 | 3 | 0.2 | 0.32 | 0.03 | 3.05 | ||
| Electrolyte abnormalityb | (Non-Hispanic) White | 2,698 | 48 | 0.6 | 2,701 | 37 | 0.4 | 1.29 | 0.84 | 1.98 | 0.11 |
| (Non-Hispanic) Black | 1,379 | 16 | 0.4 | 1,379 | 13 | 0.3 | 1.27 | 0.61 | 2.64 | ||
| Hispanic | 503 | 4 | 0.3 | 481 | 0 | 0.0 | – | – | – | ||
| Injurious fallc | (Non-Hispanic) White | 2,698 | 93 | 1.1 | 2,701 | 115 | 1.4 | 0.80 | 0.61 | 1.05 | 0.11 |
| (Non-Hispanic) Black | 1,379 | 26 | 0.6 | 1,379 | 21 | 0.4 | 1.28 | 0.72 | 2.27 | ||
| Hispanic | 503 | 6 | 0.4 | 481 | 2 | 0.1 | 2.88 | 0.58 | 14.3 | ||
| Acute kidney injuryd | (Non-Hispanic) White | 2,698 | 66 | 0.8 | 2,701 | 49 | 0.6 | 1.34 | 0.93 | 1.94 | 0.46 |
| (Non-Hispanic) Black | 1,379 | 43 | 1.0 | 1,379 | 23 | 0.5 | 1.95 | 1.17 | 3.23 | ||
| Hispanic | 503 | 6 | 0.4 | 481 | 5 | 0.3 | 1.15 | 0.35 | 3.78 | ||
Abbreviation: CI, confidence interval, SAE, serious adverse event.
aSAEs were defined as an adverse experience judged by an investigator to be life threatening and/or resulting in death, permanent disability, hospitalization, or prolongation of hospitalization, whether or not the event was thought to be related to the study intervention.
bElectrolyte abnormality were adverse laboratory measures detected on routine or unscheduled tests; routine laboratory tests were performed at 1 month, then quarterly during the first year, then every 6 months.
cAn injurious fall was defined as a fall that resulted in evaluation in an emergency department or that resulted in hospitalization.
dAcute kidney injury or acute renal failure were based on a primary or secondary diagnosis listed in the hospital discharge summary and was believed by the safety officer to be 1 of the top 3 reasons for admission or continue.
DISCUSSION
We found that targeting a SBP <120 mm Hg compared to the currently recommended <140 mm Hg led to similar reductions in the relative risk for the primary outcome across major racial/ethnic groups—NHB (29%), NHW (30%), and Hispanics (38%). We also found that although NHBs required slightly more antihypertensive therapy to achieve this lower target, there was no difference in achieved SBP by race in the intensive arm.
Moreover, while statistically significant differences in baseline characteristics including cardiovascular risk profile (e.g., age, gender, cigarette smoking, prevalent CKD/CVD) were seen between the race/ethnic subgroups (Tables 1 and Supplementary Table S1), these differences were small. This suggests the benefit was unaffected by these population differences (though we had limited statistical power to detect such an effect). The population impact of implementing SPRINT may be even greater among NHBs given the higher prevalence of hypertension in this population and the fact that hypertension accounts for a greater proportion of CVD events among NHB and Hispanics.9
These findings are consistent with those in previous SPRINT publications13,25 and extends the findings of previous studies.26,27 In the Hypertension Detection Follow-up trial (HDFP) trial, Blacks (Hispanic ethnicity was not reported), who made up 44% of study participants, had a significant reduction in all-cause mortality with more intensive “stepped” care compared to less intensive “referred” care.28 While the BP target in HDFP was based on a DBP goal of <90 mm Hg, SBP decreased from a baseline of 159 mm Hg to 130 mm Hg by years 4 and 5.29 In the ACCORD trial, the mean on-treatment intensive arm SBPs were 119.2 ± 0.2, 122.7 ± 0.4, and 121.7 ± 0.7 in the NHW, NHB, and Hispanic groups, respectively (written communication from P Byrington, November 2016). However, no benefit on the composite CVD outcome was seen with the <120 mm Hg target, either overall or in subgroups defined by race.
No racial or ethnic differences by treatment assignment were seen in renal outcomes or in SAEs in SPRINT. The AASK trial is the only other renal outcome trial with significant numbers of AAs and showed no benefit of more intensive therapy in this population with hypertensive renal disease except in participants with proteinuria (protein-to-creatinine ratio of more than 0.22).30,31 The small number of renal events in SPRINT make it unable to evaluate this finding.
Literature assessing incident CVD mortality in Hispanics and NHW found a statistically significant association between Hispanic ethnicity and lower CV and all-cause mortality despite having a worse CV risk profile when compared to NHW.32 This has been referred to as the Hispanic paradox.33 However, the risk profile of Hispanics in SPRINT was not greater than that seen in the other subgroups and did not result in an all-cause and cardiovascular-specific mortality advantage among Hispanics in SPRINT. Instead, we note a similar effect size on CV protection across NHWs, NHBs, and Hispanics. Though the sample sizes were small, SPRINT outcomes also did not differ between Hispanics residing on the US mainland vs. those in Puerto Rico.
The strengths of SPRINT include its large sample size, a diverse patient population, and its success in both implementing the protocol and achieving the SBP targets and difference in SBP between the 2 interventional groups throughout the trial, including in NHBs and Hispanics. While by design SPRINT was not powered to specifically examine treatment effects of the lower SBP goal in these subgroups, this analysis supports the benefits of intensive BP lowering on the primary composite endpoint which was similar across these race/ethnicity groups. A somewhat surprising limitation was the baseline characteristics and CVD risk profile of the NHB and Hispanic populations in SPRINT was not significantly higher than that of NHWs in SPRINT, which is not a representation of that found in the overall community. However, SPRINT assessment of race and ethnicity was through self-designation which also has its limitations.34 NHBs and Hispanics are admixed populations, and the US concept of race being only White or Black is seen as confusing35 and may have caused significant misclassification when trying to dichotomize White Hispanics and Black Hispanics.36 Finally, the small sample size of Hispanics limited meaningful comparisons making the analysis of Hispanics underpowered and potentially unstable.
The clinical implications of the SPRINT results are substantial. Considering the high prevalence of hypertension and uncontrolled hypertension among NHBs and Hispanics, intensive SBP lowering is bound to have a greater public health impact among these populations.4 Importantly, achieving lower SBP targets in NHB will require more antihypertensive therapy to achieve this goal. Our results indicate that most individuals above age 50 years with higher than average cardiovascular risk profile and SBP ≥130 mm Hg, including many above age 75 years old regardless of racial/ethnic origin benefit from treating to a SBP target of <120 mm Hg.
Overall, SPRINT findings showed benefit of a <120 mm Hg target (compared to one <140 mm Hg) in the NHB, Hispanic, and NHW populations and provide no evidence for heterogeneity of effect by race or ethnicity.
SUPPLEMENTARY MATERIAL
Supplementary data are available at American Journal of Hypertension online.
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGEMENTS
The Systolic Blood Pressure Intervention Trial is funded with Federal funds from the National Institutes of Health (NIH), including the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute on Aging (NIA), and the National Institute of Neurological Disorders and Stroke (NINDS), under Contract Numbers HHSN268 200900040C, HHSN268200900046C, HHSN268200 900047C, HHSN268200900048C, HHSN268200900049C, and Inter-Agency Agreement Number A-HL-13-002-001. It was also supported in part with resources and use of facilities through the Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International, Inc. All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the coauthors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the US Department of Veterans Affairs, or the United States Government. We also acknowledge the support from the following CTSAs funded by NCATS: CWRU: UL1TR000439, OSU: UL1RR025755, U Penn: UL1RR024134 and UL1TR000003, Boston: UL1RR025771, Stanford: UL1TR000093, Tufts: UL1RR025752, UL1TR000073 and UL1TR001064, University of Illinois: UL1TR000050, University of Pittsburgh: UL1TR000005, UT Southwestern: 9U54TR000017-06, University of Utah: UL1TR000105- 05, Vanderbilt University: UL1 TR000445, George Washington University: UL1TR000075, University of CA, Davis: UL1 TR000002, University of Florida: UL1 TR000064, University of Michigan: UL1TR000433, Tulane University: P30GM103337 COBRE Award NIGMS.
REFERENCE
- 1. Whelton PK, Einhorn PT, Muntner P, Appel LJ, Cushman WC, Diez Roux AV, Ferdinand KC, Rahman M, Taylor HA, Ard J, Arnett DK, Carter BL, Davis BR, Freedman BI, Cooper LA, Cooper R, Desvigne-Nickens P, Gavini N, Go AS, Hyman DJ, Kimmel PL, Margolis KL, Miller ER, 3rd, Mills KT, Mensah GA, Navar AM, Ogedegbe G, Rakotz MK, Thomas G, Tobin JN, Wright JT, Yoon SS, Cutler JA; National Heart, Lung, and Blood Institute Working Group on Research Needs to Improve Hypertension Treatment and Control in African Americans Research needs to improve hypertension treatment and control in African Americans. Hypertension 2016; 68:1066–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borrell LN, Crawford ND. Disparities in self-reported hypertension in Hispanic subgroups, non-Hispanic Black and non-Hispanic White adults: the National Health Interview Survey. Ann Epidemiol 2008; 18:803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carson AP, Howard G, Burke GL, Shea S, Levitan EB, Muntner P. Ethnic differences in hypertension incidence among middle-aged and older adults: the multi-ethnic study of atherosclerosis. Hypertension 2011; 57:1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee; Stroke Statistics Subcommittee Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 2016; 133:e38–360. [DOI] [PubMed] [Google Scholar]
- 5. Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the united states: National Health and Nutrition Examination Survey, 2011–2012. NCHS Data Brief 2013; 1–8. [PubMed] [Google Scholar]
- 6. Gu A, Yue Y, Desai RP, Argulian E. Racial and ethnic differences in antihypertensive medication use and blood pressure control among US adults with hypertension: the National Health and Nutrition Examination Survey, 2003 to 2012. Circulation: Cardiovascular Quality and Outcomes 2016; 10:e003166. [DOI] [PubMed] [Google Scholar]
- 7. Balfour PC, Jr, Ruiz JM, Talavera GA, Allison MA, Rodriguez CJ. Cardiovascular disease in Hispanics/Latinos in the United States. J Lat Psychol 2016; 4:98–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sorlie PD, Allison MA, Avilés-Santa ML, Cai J, Daviglus ML, Howard AG, Kaplan R, Lavange LM, Raij L, Schneiderman N, Wassertheil-Smoller S, Talavera GA. Prevalence of hypertension, awareness, treatment, and control in the Hispanic Community Health Study/Study of Latinos. Am J Hypertens 2014; 27:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Balfour PC, Jr, Rodriguez CJ, Ferdinand KC. The role of hypertension in race-ethnic disparities in cardiovascular disease. Curr Cardiovasc Risk Rep 2015; 9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr, Narva AS, Ortiz E. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520. [DOI] [PubMed] [Google Scholar]
- 11. Guzman NJ. Epidemiology and management of hypertension in the Hispanic population: a review of the available literature. Am J Cardiovasc Drugs 2012; 12:165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Margolis KL, Piller LB, Ford CE, Henriquez MA, Cushman WC, Einhorn PT, Colon PJ, Sr, Vidt DG, Christian R, Wong ND, Wright JT, Jr, Goff DC, Jr; Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group Blood pressure control in Hispanics in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Hypertension 2007; 50:854–861. [DOI] [PubMed] [Google Scholar]
- 13. Wright JT, Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC, Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT; SPRINT Research Group A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wright JT, Jr, Dunn JK, Cutler JA, Davis BR, Cushman WC, Ford CE, Haywood LJ, Leenen FH, Margolis KL, Papademetriou V, Probstfield JL, Whelton PK, Habib GB; ALLHAT Collaborative Research Group Outcomes in hypertensive Black and nonblack patients treated with chlorthalidone, amlodipine, and lisinopril. JAMA 2005; 293:1595–1608. [DOI] [PubMed] [Google Scholar]
- 15. SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the systolic hypertension in the elderly program (SHEP). SHEP Cooperative Research Group. JAMA 1991; 265:3255–3264. [PubMed] [Google Scholar]
- 16. Still CH, Ferdinand KC, Ogedegbe G, Wright JT., Jr Recognition and management of hypertension in older persons: focus on African Americans. J Am Geriatr Soc 2015; 63:2130–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ; HYVET Study Group Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898. [DOI] [PubMed] [Google Scholar]
- 18. JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31:2115–2127. [DOI] [PubMed] [Google Scholar]
- 19. Ogihara T, Saruta T, Rakugi H, Matsuoka H, Shimamoto K, Shimada K, Imai Y, Kikuchi K, Ito S, Eto T, Kimura G, Imaizumi T, Takishita S, Ueshima H; Valsartan in Elderly Isolated Systolic Hypertension Study Group Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension 2010; 56:196–202. [DOI] [PubMed] [Google Scholar]
- 20. Cushman WC, Evans GW, Byington RP, Goff DC, Jr, Grimm RH, Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F; ACCORD Study Group Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. SPRINT Research Group. Systolic blood pressure intervention trial (SPRINT) protocol <https://www.sprinttrial.org/public/Protocol_Current.pdf> Updated 2012. Accessed 1 November 2016.
- 22. Still CH, Craven TE, Freedman BI, Van Buren PN, Sink KM, Killeen AA, Bates JT, Bee A, Contreras G, Oparil S, Pedley CM, Wall BM, White S, Woods DM, Rodriguez CJ, Wright JT, Jr; SPRINT Study Research Group Baseline characteristics of African Americans in the Systolic Blood Pressure Intervention Trial. J Am Soc Hypertens 2015; 9:670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rodriguez CJ, Still CH, Garcia KR, Wagenknecht L, White S, Bates JT, Del Cid MV, Lioudis M, Lopez Barrera N, Moreyra A, Punzi H, Ringer RJ, Cushman WC, Contreras G, Servilla K, Rocco M; SPRINT Research Group Baseline blood pressure control in Hispanics: characteristics of Hispanics in the Systolic Blood Pressure Intervention Trial. J Clin Hypertens (Greenwich) 2017; 19:116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, Fine LJ, Goff DC, Jr, Johnson KC, Killeen AA, Lewis CE, Oparil S, Reboussin DM, Rocco MV, Snyder JK, Williamson JD, Wright JT, Jr, Whelton PK; SPRINT Study Research Group The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials 2014; 11:532–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH, Kitzman DW, Kostis JB, Krousel-Wood MA, Launer LJ, Oparil S, Rodriguez CJ, Roumie CL, Shorr RI, Sink KM, Wadley VG, Whelton PK, Whittle J, Woolard NF, Wright JT, Jr, Pajewski NM; SPRINT Research Group Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA 2016; 315:2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA 2002; 288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 27. Julius S, Alderman MH, Beevers G, Dahlöf B, Devereux RB, Douglas JG, Edelman JM, Harris KE, Kjeldsen SE, Nesbitt S, Randall OS, Wright JT., Jr Cardiovascular risk reduction in hypertensive black patients with left ventricular hypertrophy: the LIFE study. J Am Coll Cardiol 2004; 43:1047–1055. [DOI] [PubMed] [Google Scholar]
- 28. Hypertension Detection and Follow-up Program Cooperative Group. Five-year findings of the hypertension detection and follow-up program. I. reduction in mortality of persons with high blood pressure, including mild hypertension. hypertension detection and follow-up program cooperative group. JAMA 1979; 242:2562–2571. [PubMed] [Google Scholar]
- 29. Abernethy J, Borhani NO, Hawkins CM, Crow R, Entwisle G, Jones JW, Maxwell MH, Langford H, Pressel S. Systolic blood pressure as an independent predictor of mortality in the Hypertension Detection and Follow-up Program. Am J Prev Med 1986; 2:123–132. [PubMed] [Google Scholar]
- 30. Wright JT, Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG; African American Study of Kidney Disease and Hypertension Study Group Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 2002; 288:2421–2431. [DOI] [PubMed] [Google Scholar]
- 31. Appel LJ, Wright JT, Jr, Greene T, Agodoa LY, Astor BC, Bakris GL, Cleveland WH, Charleston J, Contreras G, Faulkner ML, Gabbai FB, Gassman JJ, Hebert LA, Jamerson KA, Kopple JD, Kusek JW, Lash JP, Lea JP, Lewis JB, Lipkowitz MS, Massry SG, Miller ER, Norris K, Phillips RA, Pogue VA, Randall OS, Rostand SG, Smogorzewski MJ, Toto RD, Wang X; AASK Collaborative Research Group Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 2010; 363:918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu J, Murphy SL, Kochanek KD, Bastian BA. Deaths: final data for 2013. Natl Vital Stat Rep 2016; 64:1–119. [PubMed] [Google Scholar]
- 33. Cortes-Bergoderi M, Goel K, Murad MH, Allison T, Somers VK, Erwin PJ, Sochor O, Lopez-Jimenez F. Cardiovascular mortality in Hispanics compared to non-Hispanic Whites: a systematic review and meta-analysis of the Hispanic paradox. Eur J Intern Med 2013; 24:791–799. [DOI] [PubMed] [Google Scholar]
- 34. Kaufman JS, Cooper RS. Commentary: considerations for use of racial/ethnic classification in etiologic research. Am J Epidemiol 2001; 154:291–298. [DOI] [PubMed] [Google Scholar]
- 35. Rodriguez CJ, Allison M, Daviglus ML, Isasi CR, Keller C, Leira EC, Palaniappan L, Piña IL, Ramirez SM, Rodriguez B, Sims M; American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Cardiovascular and Stroke Nursing Status of cardiovascular disease and stroke in Hispanics/Latinos in the United States: a science advisory from the American Heart Association. Circulation 2014; 130:593–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bryc K, Durand EY, Macpherson JM, Reich D, Mountain JL. The genetic ancestry of African Americans, Latinos, and European Americans across the United States. Am J Hum Genet 2015; 96:37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.