Abstract
BACKGROUND
A high 24-hour ambulatory diastolic (DBP) but not systolic (SBP) blood pressure variability (BPV) is significantly predictive of long-term cardiovascular mortality in untreated hypertensive subjects, independent of office or 24-hour SBP. The present study was aimed to investigate hemodynamic factors that are independently associated with systolic and diastolic BPV from the 24-hour ambulatory blood pressure monitoring (ABPM).
METHODS
A cohort of 624 normotensive and 633 untreated hypertensive participants with baseline ABPM was drawn from a community-based survey. BPV was assessed by the read-to-read average real variability of the 24-hour SBP and DBP (ARVs and ARVd, respectively). Hemodynamic variables including total peripheral resistance (TPR), carotid-femoral pulse wave velocity (cf-PWV), and amplitudes of the decomposed forward (Pf) and backward (Pb) carotid pressure waves were analyzed.
RESULTS
In multivariable analyses, hemodynamic variables independently associated with 24-hour SBP were 24-hour heart rate (HR), TPR, cf-PWV, Pf, and Pb (model r2 = 0.535). Hemodynamic factors independently associated with ARV were 24-hour HR, Pf, and Pb for ARVs, and 24-hour HR, cf-PWV, Pf, and Pb for ARVd (model R2 = 0.345 and 0.220, respectively). Addition of 24-hour SBP to the ARV models only slightly improved variance explained by the models (R2 = 0.383 and 0.224, respectively). Pb accounted for >50% of total variance of ARVs and ARVd, whereas cf-PWV was a minor determinant of ARVd (<5% of total variance).
CONCLUSIONS
ARVd was associated with fewer hemodynamic variables than to 24-hour SBP. Among those hemodynamic variables wave reflection but not arterial stiffness had the dominant independent association with ARV.
Keywords: ambulatory blood pressure, blood pressure variability, hemodynamics; hypertension.
Evidence is accumulating that fluctuation of blood pressure, or blood pressure variability (BPV), may predict risks of end organ damage and cardiovascular events beyond the average of blood pressure levels.1–3 BPV is complex and manifests variously in conditions related to time or situations, namely, orthostatic, beat-to-beat, psychological or physical stress-induced, diurnal, day-to-day, office or clinic visit-to-visit, seasonal, and yearly.4 In particular, BPV occurring over 24 hours (24-h) ambulatory blood pressure monitoring (ABPM),1,3,5 and long-term visit-to-visit BPV2 have been associated with prognostic relevance independent of the average blood pressure. In fact, short-term BPV from the 24-h ABPM has been considered as an important contributory factor for cardiac and vascular alterations, subclinical organ damage, progression of microalbuminuria/proteinuria and renal dysfunction, and cardiovascular events and mortality.4 More recently, a new concept of “perfect 24-hour blood pressure control” accounting for the 24-h blood pressure level, circadian rhythm, and BPV has been proposed.6 Therefore, BPV may become an important target for perfect blood pressure treatment.6
Because blood pressure is a highly variable physiological trait, BPV is likely an inherent component of blood pressure. Thus, it is likely that the same hemodynamic parameters govern both blood pressure and BPV. Arterial compliance is a major determinant of blood pressure7 and may moderate the excess BPV induced by the neurohumeral, rheological, emotional, or behavioral factors.8,9 Indeed, increased short-term BPV has been associated with increased arterial stiffness (decreased arterial compliance) indexed by carotid-femoral pulse wave velocity (cf-PWV).10,11–14 However, the roles of other hemodynamic determinants of blood pressure, including arterial wave reflection, total peripheral resistance (TPR), and cardiac output, in modulating BPV remain unclear. In particular, the wave reflection phenomenon is omnipresent in the arterial tree throughout life and is a major determinant of the central blood pressure waveform and values,15,16 whereas large arteries gradually stiffen during the process of aging. It remains unknown whether arterial stiffness or wave reflection is more important in determining BPV.
We have previously demonstrated that a high BPV from the 24-h ABPM diastolic (DBP) but not systolic blood pressure (SBP) is significantly independently associated with predictive of long-term cardiovascular mortality in untreated hypertensive subjects, independent of office or 24-h SBP.3 The purpose of the present study was to investigate the hemodynamic associations of the 24-h ABPM BPV, focusing on the relative contribution of wave reflection versus arterial stiffness.
METHODS
Study population
We have reported previously the community-based study cohort of 1257 subjects (669 men, mean age 53.2 ± 13.1 years, range 30–79 years).3 Subjects with a previous history of diabetes mellitus, angina pectoris, peripheral vascular diseases, or with clinical or echocardiographic evidence of other significant cardiac diseases were excluded. Every participant received a baseline comprehensive cardiovascular evaluation, including complete medical history and physical examination, arterial tonometry, nondirectional Doppler flow velocimetry, echocardiography, and 24-h ABPM.17,18 All participants gave informed consent, and the study was approved by the institutional review board at Johns Hopkins University, United States.
Office and 24-hour ambulatory blood pressures and BPV
Office SBP and DBP were measured manually using a mercury sphygmomanometer by experienced cardiologists. Two or more measurements separated by at least 5 minutes were taken from the right arm of participants after they had been seated for at least 5 minutes.
Twenty-four hour ABPM (Model 90217, Spacelabs Redmond, WA) was performed on a usual working day.19 The recorder was programmed to measure blood pressure at 20-minute intervals during the daytime (7:00 am to 10:00 pm) and at 60-minute intervals during the nighttime (11:00 pm to 6:00 am). The 24-hour readings were not edited manually, and only records with 80% successful measurements were included in the analysis. BPV of SBP and DBP over 24 hours20,21 was evaluated by the read-to-read average real variability (ARV).22 ARV is calculated as22:
Where BP = blood pressure, k ranges from 1 to N, N is the number of blood pressure readings in 24 hours. ARVd and ARVs denote ARV of 24-h DBP and 24-h SBP, respectively.
Arterial stiffness
Carotid-femoral pulse wave velocity (cf-PWV) was measured using sequential nondirectional Doppler (Parks model 802; Parks Medical Electronics, Aloha, OR) flow velocity at the right carotid and femoral arteries and a simultaneous ECG.17
Arterial wave reflection
Right carotid artery pressure waveforms were registered noninvasively with a tonometer and the ensemble averaged common carotid artery pressure waveform was calibrated to brachial mean blood pressure and DBP.23 The calibrated carotid pressure waveform was analyzed to obtain augmented pressure (Pa), the pressure amplitude above the inflection point resulting from wave reflection, and augmentation index (AI), calculated as Pa divided by the carotid pulse pressure.23 The carotid pressure waveform was also separated into its forward and reflected components to calculate the transit time-independent parameter of wave reflection intensity using the triangulation method which we have previously validated.23,24 The forward and backward components of the pressure wave can be constructed using a triangular flow wave and the following equations:
| (1) |
| (2) |
Where Zc is the characteristic impedance, Pm(t) is the carotid pressure wave, F(t) is the approximated triangular-shaped flow wave, Pf(t) is the forward pressure component, and Pb(t) is the backward pressure component. Pf and Pb are the amplitudes of Pf (t) and Pb (t), respectively, with the latter being a transit-time independent index of wave reflections.23,25 Reflection magnitude (RM) is calculated as the ratio of Pb/Pf.24
Other hemodynamic parameters
Stroke volume was calculated from left ventricular volumes derived from end-diastolic and end-systolic left ventricular internal diameters by 2D-guided M-mode echocardiography using the Teichholz method.17 Cardiac output was calculated as stroke volume multiplied by heart rate (HR). Total peripheral vascular resistance (TPR) was calculated as the ratio of brachial mean blood pressure to cardiac output.17
Statistical analysis
Statistical analysis was performed using SPSS software (version 17.0, SPSS, Chicago, IL). All data were expressed as proportions or means and SD. Between-group differences for continuous variables were assessed by t-test. Pearson correlation coefficients of 24-h blood pressure and BPV vs. other variables were calculated. Multiple linear regression analyses were performed to identify the hemodynamic factors associated with ARVs, ARVd, 24-h SBP, and 24-h DBP. The independent hemodynamics associations were subjected to further forward stepwise multiple regression analysis. By calculating the ratios of individual partial R2 to the full model R2, the relative importance of each independent variable in the full model was determined. The statistical significance was established at 2-tailed P <0.05.
RESULTS
The characteristics of hypertensive and normotensive subjects in the present study population have been reported3 and the basic characteristics are summarized in Table 1. The hypertensive subjects have significantly higher cardiac output, TPR, cf-PWV, AI, Pa, Pb, Pf, and RM than the normotensive subjects (Table 2).
Table 1.
Characteristics of the study population (n = 1257)3
| Variable | Mean ± SD or counts |
|---|---|
| Age, years | 53.1 ± 13.1 |
| Waist circumference, cm | 85.3 ± 9.2 |
| Height, cm | 159.3 ± 8.7 |
| Body mass index, kg/m2 | 24.7 ± 3.7 |
| Cholesterol, mg/dl | 198.7 ± 38.7 |
| HDL-C, mg/dl | 50.8 ± 12.8 |
| Fasting glucose, mg/dl | 101.1 ± 27.2 |
| eGFR (ml/min/1.732) | 92.0 ± 23.2 |
| Smoking (yes/no) | 317/940 |
| Office blood pressure | |
| SBP, mm Hg | 136.1 ± 24.4 |
| DBP, mm Hg | 83.6 ± 13.9 |
| PP, mm Hg | 52.5 ± 17.4 |
| Ambulatory blood pressure | |
| 24-h SBP, mm Hg | 126.9 ± 17.5 |
| 24-h DBP, mm Hg | 80.9 ± 11.7 |
| 24-h PP, mm Hg | 46.0 ± 9.4 |
| 24-h HR, bpm | 77.4 ± 8.7 |
| Daytime SBP, mm Hg | 128.6 ± 17.9 |
| Daytime DBP, mm Hg | 82.4 ± 12.0 |
| Daytime PP, mm Hg | 46.3 ± 9.6 |
| Daytime HR, bpm | 80.3 ± 9.3 |
| Nighttime SBP, mm Hg | 119.8 ± 17.9 |
| Nighttime DBP, mm Hg | 74.6 ± 11.8 |
| Nighttime PP, mm Hg | 45.2 ± 9.9 |
| Nighttime HR, bpm | 67.1 ± 8.4 |
| Blood pressure variability | |
| ARVd, mm Hg | 8.8 ± 2.8 |
| ARV, mm Hg | 9.7 ± 2.7 |
Abbreviations: ARVs, average real variability of 24-h SBP; ARVd, average real variability of 24-h DBP; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL-C, high density lipoprotein; HR, Heart rate; 24-h, ambulatory 24 hours; PP, pulse pressure; SBP, systolic blood pressure.
Table 2.
Hemodynamics variables of the study population stratified by office hypertension status
| Variable | All, n = 1257 | Hypertension, n = 633 | Normotension, n = 624 | P value |
|---|---|---|---|---|
| Cardiac output, l/min | 6.1 ± 1.7 | 6.3 ± 1.7 | 5.8 ± 1.6 | <0.001 |
| TPR, mm Hg/l | 17.9 ± 6.2 | 19.3 ± 6.1 | 16.4 ± 6.1 | <0.001 |
| cf-PWV, m/s | 9.4 ± 2.4 | 10.3 ± 2.5 | 8.6 ± 1.9 | <0.001 |
| AI, % | 15.0 ± 17.1 | 20.4 ± 16.0 | 9.6 ± 16.5 | <0.001 |
| Pa, mm Hg | 7.6 ± 8.4 | 11.2 ± 9.5 | 4.0 ± 5.1 | <0.001 |
| Pb, mm Hg | 15.6 ± 6.6 | 19.1 ± 7.1 | 12.0 ± 3.5 | <0.001 |
| Pf, mm Hg | 33.6 ± 12.0 | 38.4 ± 13.1 | 28.7 ± 8.2 | <0.001 |
| RM, % | 47.2 ± 12.2 | 51.0 ± 11.7 | 43.6 ± 11.5 | <0.001 |
Abbreviations: AI, carotid augmentation index; cf-PWV, carotid-femoral pulse wave velocity; Pa, augmented pressure; Pb, amplitude of the decomposed backward pressure; Pf, amplitude of the decomposed forward pressure; RM, reflection magnitude; TPR, total peripheral resistance.
Correlation coefficients of ARVs, ARVd, 24-h SBP, and 24-h DBP with other variables are shown in Table 3. ARVs, ARVd, 24-h SBP, but not 24-h DBP significantly correlated with age. All 4 variables significantly correlated with waist circumference, body mass index, 24-h PP, 24-h HR, cardiac output, TRP, cf-PWV, AI, Pa, Pb, Pf, and RM. Both ARVs and ARVd significantly correlated with 24-h SBP and 24-h DBP. Correlation coefficients of ARVs were numerically greater than those of ARVd, except for 24-h HR (Table 3).
Table 3.
Correlates of 24-h ambulatory blood pressure and blood pressure variability
| Variable | ARVs | ARVd | 24-h SBP | 24-h DBP |
|---|---|---|---|---|
| Age | 0.395** | 0.276** | 0.265** | 0.055 |
| WC | 0.315** | 0.271** | 0.464** | 0.428** |
| BMI | 0.251** | 0.238** | 0.370** | 0.351** |
| 24-h SBP | 0.527** | 0.421** | 1 | 0.868** |
| 24-h DBP | 0.405** | 0.356** | 0.868** | 1 |
| 24-h PP | 0.480** | 0.342** | 0.786** | 0.375** |
| 24-h HR | 0.084** | 0.112** | 0.077** | 0.212** |
| Cardiac output | 0.117** | 0.065* | 0.240** | 0.229** |
| TPR | 0.099** | 0.099** | 0.160** | 0.171** |
| cf-PWV | 0.310** | 0.277** | 0.416** | 0.309** |
| AI | 0.268** | 0.196** | 0.324** | 0.237** |
| Pa | 0.396** | 0.297** | 0.474** | 0.293** |
| Pb | 0.484** | 0.372** | 0.598** | 0.341** |
| Pf | 0.394** | 0.305** | 0.490** | 0.211** |
| RM | 0.259** | 0.169** | 0.280** | 0.225** |
Abbreviations: ARVd, average real variability of 24-h DBP; ARVs, average real variability of 24-h SBP; BMI, body mass index; cf-PWV, carotid -femoral pulse wave velocity; DBP, diastolic blood pressure; HR, heart rate; Pa, augmented pressure; Pb, amplitude of the decomposed backward pressure; Pf, amplitude of the decomposed forward pressure; PP, pulse pressure; RM, reflection magnitude; SBP, systolic blood pressure; TPR, total peripheral resistance; WC, waist circumference.
*P < 0.05; **P < 0.01.
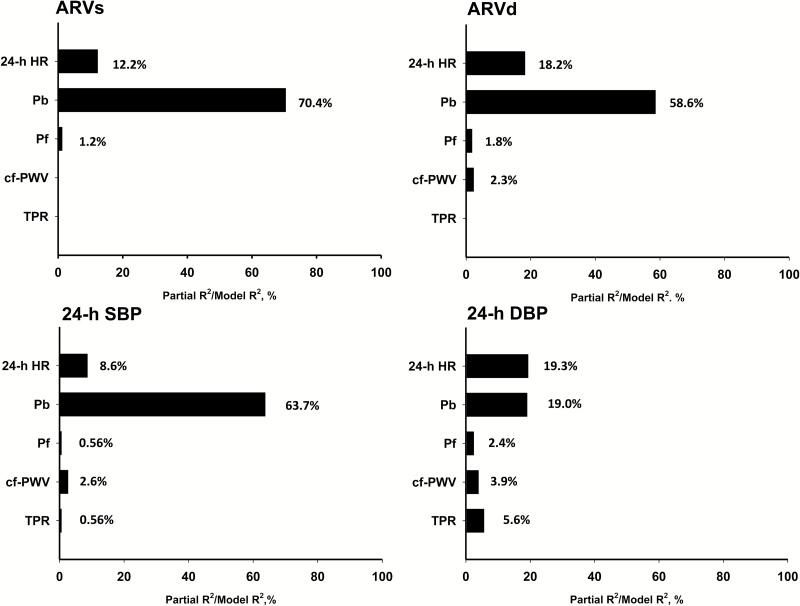
In multivariable analyses (Table 4), all major hemodynamics variables, including 24-h HR, TPR, cf-PWV, Pf, and Pb, had significant independent associations with 24-h SBP and 24-h DBP (model R2 = 0.535 for 24-h SBP and 0.410 for 24-h DBP, respectively). In contrast, the numbers of significant independent hemodynamic associations were fewer and the model R2 were smaller for ARVs (24-h HR, Pf, and Pb; R2 = 0.345) and ARVd (24-h HR, cf-PWV, Pf, and Pb; R2 = 0.220). Inclusion of 24-h SBP only slightly increased the model R2 0.383 for ARVs and 0.224 for ARVd, respectively). Similar results were obtained when Pb was substituted for RM (Supplementary Table S1).
Table 4.
Hemodynamic determinants of 24-h ambulatory blood pressure and blood pressure variability by multivariable stepwise regression analysis (n = 1257) variables
| ARVs | ARVd | 24-h SBP | 24-h DBP | |||||
|---|---|---|---|---|---|---|---|---|
| β | P value | β | P value | β | P value | β | P value | |
| Model 1 | R 2 = 0.345 | R 2 = 0.220 | R 2 = 0.535 | R 2 = 0.410 | ||||
| 24-h HR | 0.199 | <0.001 | 0.180 | <0.001 | 0.207 | <0.001 | 0.273 | <0.001 |
| TPR | NS | NS | NS | NS | 0.065 | 0.004 | 0.134 | <0.001 |
| cf-PWV | NS | NS | 0.076 | 0.020 | 0.147 | <0.001 | 0.157 | <0.001 |
| Pf | 0.094 | 0.017 | 0.097 | 0.025 | 0.093 | 0.036 | -0.156 | <0.001 |
| Pb | 0.342 | <0.001 | 0.223 | <0.001 | 0.511 | <0.001 | 0.507 | <0.001 |
| Model 2 | R 2 = 0.383 | R 2 = 0.224 | ||||||
| 24-h HR | 0.135 | <0.001 | 0.156 | <0.001 | ||||
| TPR | NS | NS | NS | NS | ||||
| cf-PWV | NS | NS | NS | NS | ||||
| Pf | NS | NS | NS | NS | ||||
| Pb | 0.243 | <0.001 | 0.221 | <0.001 | ||||
| 24-h SBP | 0.281 | <0.001 | 0.154 | <0.001 | – | – | ||
All models were also adjusted for age, sex, and body mass index.
Abbreviations: ARVd, average real variability of 24-h DBP; ARVs, average real variability of 24-h SBP; β, standardized regression coefficient; cf-PWV, carotid-femoral pulse wave velocity; DBP, diastolic blood pressure; HR, heart rate; NS, nonsignificant; Pb, amplitude of the decomposed backward pressure wave; Pf, amplitude of the decomposed forward pressure wave; SBP, systolic blood pressure; TPR, total peripheral resistance.
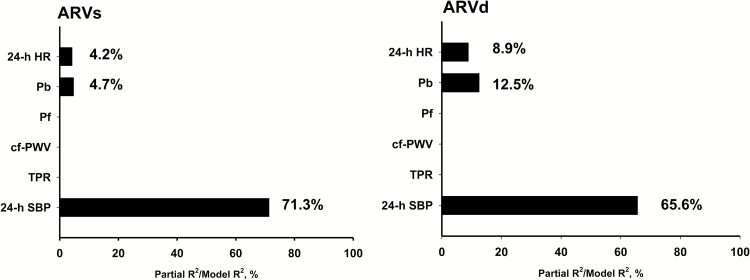
Among the major hemodynamic variables, Pb had the dominant independent association with ARVs, ARVd, 24-h SBP, and 24-h DBP (Figure 1). Pb explained more than 50% of total variance of ARVs and ARVd, whereas cf-PWV explained <5% of total variance of ARVd. When 24-h SBP was included as a hemodynamic variable, it had 24-h SBP became the dominant hemodynamic factor associated with both ARVs (Figure 2, left panel) and ARVd (Figure 2, right panel).
Figure 1.
Major hemodynamic associated determinants for ARVs, ARVd, 24-h SBP, and 24-h DBP. All models were also adjusted for age, sex, and body mass index. Abbreviations: ARVd, average real variability of 24-h DBP; ARVs, average real variability of 24-h SBP; cf-PWV, carotid-femoral pulse wave velocity; DBP, diastolic blood pressure; HR, heart rate; Pb, amplitude of the decomposed backward pressure wave; Pf, amplitude of the decomposed forward pressure wave; SBP, systolic blood pressure; TPR, total peripheral resistance.
Figure 2.
Major hemodynamic associated determinants for ARVs, ARVd, with inclusion of 24-h SBP in the multivariable models. All models were also adjusted for age, sex, and body mass index. Abbreviations: ARVd, average real variability of 24-h DBP; ARVs, average real variability of 24-h SBP; cf-PWV, carotid-femoral pulse wave velocity; DBP, diastolic blood pressure; HR, heart rate; Pb, amplitude of the decomposed backward pressure wave; Pf, amplitude of the decomposed forward pressure wave; SBP, systolic blood pressure; TPR, total peripheral resistance.
DISCUSSION
The present study, for the first time, provides comprehensive analyses on the hemodynamic determinants of short-term BPV. Our major findings are: (i) 24-h SBP and 24-h DBP are mainly determined by the major hemodynamics variables, namely, HR, total resistance from peripheral arterioles (TPR), large artery stiffness (cf-PWV), wave reflection (Pb), and left ventricular forward pressure (Pf); (ii) BPV is significantly associated with average blood pressure, and wave reflection but not arterial stiffness is the dominant hemodynamics determinant of both 24-h SBP and BPV; (iii) hemodynamic associations only explain part of the BPV variance, even when 24-h SBP is included in the model. These findings explain why BPV is an independent risk factor for cardiovascular events in addition to 24-h SBP. Perspectives gleaned regarding the major hemodynamic factors with BPV provide a Segway to interventional studies to identify the optimal targets for the management of BPV.
Wave reflection and BPV
Wave reflection, Pb was consistently best correlated with the measures of BPV as ARVs, ARVd, followed by Pa, Pf, cf-PWV, and AI (Table 3). Pb is independent of the reflected wave transit time, has a linear relationship with age,23 and is a better measure of wave reflections than AI or Pa.25 In the present study, the association of Pb with measures of the short-term BPV was stronger than cf-PWV, cardiac output, or total peripheral resistance. Because Pb increased linearly with age,23 the observed strong association between Pb and BPV was not likely due to the relatively young age of the study population. Furthermore, Pf, the forward component of the decomposed carotid pressure wave, was not associated with either ARVs or ARVd after adjustment for 24-h SBP; this implies that only the backward component of the blood pressure wave is relevant to the BPV. Thus, our results strongly indicate that, in addition to 24-h SBP, the magnitude of wave reflections but not arterial stiffness is the most important hemodynamic determinant of the short-term BPV.
Arterial wave reflections arise mainly from distal arterial tree and represent a major portion of left ventricular afterload during mid to late systole.26 It may be intriguing how wave reflection influences DBP27 and ARVd. Indeed, the concurrent reflected wave only augments on the late-systolic pressure of the same beat. However, according to the wave transmission theory,24 the reflected wave contributes 50% of the diastolic pressure throughout the diastole and thus influence substantially on DBP of the next beat.
In the general population, an increased arterial wave RM has been recognized as a risk factor for cardiovascular mortality,23 all cardiovascular events,26 hard cardiovascular events,26 and a novel strong risk factor for heart failure.26 Since our study shows that Pb is the major hemodynamic factor independently associated with the short-term BPV, increased short-term BPV may also impact adversely on cardiovascular morbidity and mortality via an increase of left ventricular afterload.
Arterial stiffness and BPV
Reduced arterial compliance has been identified as a significant determinant of increased BPV.4 In a population of 911 untreated, nondiabetic patients with uncomplicated hypertension and in another population of 2089 mostly treated hypertensive patients, measures of the short-term BPV, along with age, 24-h SBP, and other factors independently predicted cf-PWV.11 Similar associations between BPV and arterial stiffness have also been demonstrated in Asian populations.28 In our present study, cf-PWV was independently associated with ARVd but not ARVs (Table 4, model 1). Conversely, ARVd but not ARVs were significantly associated with cf-PWV independent of 24-h SBP in hypertensive subjects (Supplementary Table S2). The results may partly explain why ARVd, but not ARVs significantly associated with cardiovascular mortality independently of 24-h SBP in the hypertensives.3
Nonhemodynamics factors and BPV
In normal subjects, short-term rhythmic BPV is mainly determined by the sympathovagal balance.29 BPV may be considered as a sensor of cardiovascular dysregulation that is affected by an individual’s characteristics, medications, psychobehavioral factors, and environmental conditions.30,31 Kario et al. has also proposed the “resonance hypothesis” of BPV.32 Each phenotype of BPV has different time-phase variability from the short beat-by-beat to the long seasonal and yearly changes, and the synergistic resonance of each type of BPV would produce a great dynamic blood pressure surge which triggers a cardiovascular disease event.32
Drug treatment for BPV control
There are important differences between the various classes of antihypertensive drugs regarding their effects on arterial stiffness and wave reflections.33 Among the 5 classes of antihypertensive agents (nitroprusside, propranolol, phentolamine, captopril, and nifedipine), catheterization studies in patients with essential hypertension discovered that only nitroprusside and nifedipine could completely normalize arterial compliance and wave reflections.34 The superiority of calcium channel blockers than other class of antihypertensive medications in reducing the short-term BPV has been recently confirmed in a multicenter, multinational, randomized, double-blind, placebo-controlled study.35 In untreated hypertensive patients, amlodipine reduced both arterial stiffness and 24-h ABPM BPV, while valsartan reduced arterial stiffness to the same degree with amlodipine without reducing BPV.14 Thus, BPV might be a cause instead of a result of arterial stiffness. Recently, visit-to-visit BPV was identified as an independent and strong predictor of cardiovascular events, and patients receiving amlodipine had lower visit-to-visit as well as short-term BPV than patients receiving atenolol.36,37 Thus, reducing wave reflections with a calcium channel blocker may be a reasonable strategy to reduce the short-term BPV and improve the cardiovascular outcomes.
Limitations of the present study
In the present study, we only measured one set of the steady-state hemodynamic parameters during the single visit to the office for the survey. We were not able to investigate the contributions of the variability of the various hemodynamic determinants to the BPV. Although we have clearly identified wave reflection as the most important hemodynamic determinant for the short-term BPV, the hemodynamic parameters in total only explained <40% of the total variance of ARVs or ARVd (Table 4). This indicates that the modulation of BPV is complex and involves multiple neurohumoral, hemodynamic, emotional, behavioral, environmental, genetic, and other unidentified factors, many of which were not explored in the present study. Our interpretations were based on correlations of multiple measures from a cross-sectional study. Future interventional studies are required to directly prove that changes of wave reflection will cause changes of BPV.
In conclusion, in contrast to 24-hour SBP, BPV, ARVd in particular, was only partially determined by the hemodynamic variables. Among the hemodynamic variables, wave reflection but not arterial stiffness was the dominant determinant of BPV. Increased wave reflections may be a relevant target for BPV control since some classes of antihypertension medication, such as calcium channel blockers, have been shown to effectively reduce wave reflections and BPV.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by grants from the National Science Council (NSC 99-2314-B-010 -034 -MY3, MOST 103-2314-B-010 -050 -MY2, and MOST 104-2314-B-010-060), an intramural grant from the Taipei Veterans General Hospital (grant V104C-140), Research and Development contract NO1-AG-1-2118, grants from the Ministry of Health and Welfare (MOHW104-TDU-B-211-113-003), and the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
REFERENCES
- 1. Mancia G, Bombelli M, Facchetti R, Madotto F, Corrao G, Trevano FQ, Grassi G, Sega R. Long-term prognostic value of blood pressure variability in the general population: results of the Pressioni Arteriose Monitorate e Loro Associazioni Study. Hypertension 2007; 49:1265–1270. [DOI] [PubMed] [Google Scholar]
- 2. Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: findings from NHANES III, 1988 to 1994. Hypertension 2011; 57:160–166. [DOI] [PubMed] [Google Scholar]
- 3. Hsu PF, Cheng HM, Wu CH, Sung SH, Chuang SY, Lakatta EG, Yin FC, Chou P, Chen CH. High short-term blood pressure variability predicts long-term cardiovascular mortality in untreated hypertensives but not in normotensives. Am J Hypertens 2016; 29:806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood-pressure variability. Nat Rev Cardiol 2013; 10:143–155. [DOI] [PubMed] [Google Scholar]
- 5. Hansen TW, Thijs L, Li Y, Boggia J, Kikuya M, Björklund-Bodegård K, Richart T, Ohkubo T, Jeppesen J, Torp-Pedersen C, Dolan E, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Kawecka-Jaszcz K, Imai Y, Wang J, Ibsen H, O’Brien E, Staessen JA; International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes Investigators . Prognostic value of reading-to-reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension 2010; 55:1049–1057. [DOI] [PubMed] [Google Scholar]
- 6. Kario K. Essential Manual of 24 Hour Blood Pressure Management: From morning to nocturnal hypertension. Wiley-Blackwell: London, 2015. [Google Scholar]
- 7. Namasivayam M, McDonnell BJ, McEniery CM, O’Rourke MF; Anglo-Cardiff Collaborative Trial Study Investigators . Does wave reflection dominate age-related change in aortic blood pressure across the human life span? Hypertension 2009; 53:979–985. [DOI] [PubMed] [Google Scholar]
- 8. Parati G, Faini A, Valentini M. Blood pressure variability: its measurement and significance in hypertension. Curr Hypertens Rep 2006; 8:199–204. [DOI] [PubMed] [Google Scholar]
- 9. Avolio AP, Xu K, Butlin M. Application of cardiovascular models in comparative physiology and blood pressure variability. Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference 2013. Osaka, Japan, 3–7 July2013:217–220. [DOI] [PubMed] [Google Scholar]
- 10. Kotsis V, Stabouli S, Karafillis I, Papakatsika S, Rizos Z, Miyakis S, Goulopoulou S, Parati G, Nilsson P. Arterial stiffness and 24 h ambulatory blood pressure monitoring in young healthy volunteers: the early vascular ageing Aristotle University Thessaloniki Study (EVA-ARIS Study). Atherosclerosis 2011; 219:194–199. [DOI] [PubMed] [Google Scholar]
- 11. Schillaci G, Bilo G, Pucci G, Laurent S, Macquin-Mavier I, Boutouyrie P, Battista F, Settimi L, Desamericq G, Dolbeau G, Faini A, Salvi P, Mannarino E, Parati G. Relationship between short-term blood pressure variability and large-artery stiffness in human hypertension: findings from 2 large databases. Hypertension 2012; 60:369–377. [DOI] [PubMed] [Google Scholar]
- 12. Stabouli S, Papakatsika S, Kotronis G, Papadopoulou-Legbelou K, Rizos Z, Kotsis V. Arterial stiffness and SBP variability in children and adolescents. J Hypertens 2015; 33:88–95. [DOI] [PubMed] [Google Scholar]
- 13. García-García Á, García-Ortiz L, Recio-Rodríguez JI, Patino-Alonso MC, Agudo-Conde C, Rodriguez-Sanchez E, Gómez-Marcos MA. Relationship of 24-h blood pressure variability with vascular structure and function in hypertensive patients. Blood Press Monit 2013; 18:101–106. [DOI] [PubMed] [Google Scholar]
- 14. Ichihara A, Kaneshiro Y, Takemitsu T, Sakoda M. Effects of amlodipine and valsartan on vascular damage and ambulatory blood pressure in untreated hypertensive patients. J Hum Hypertens 2006; 20:787–794. [DOI] [PubMed] [Google Scholar]
- 15. Takazawa K, Kobayashi H, Shindo N, Tanaka N, Yamashina A. Relationship between radial and central arterial pulse wave and evaluation of central aortic pressure using the radial arterial pulse wave. Hypertens Res 2007; 30:219–228. [DOI] [PubMed] [Google Scholar]
- 16. Cheng HM, Wang KL, Chen YH, Lin SJ, Chen LC, Sung SH, Ding PY, Yu WC, Chen JW, Chen CH. Estimation of central systolic blood pressure using an oscillometric blood pressure monitor. Hypertens Res 2010; 33:592–599. [DOI] [PubMed] [Google Scholar]
- 17. Chen CH, Ting CT, Lin SJ, Hsu TL, Ho SJ, Chou P, Chang MS, O’Connor F, Spurgeon H, Lakatta E, Yin FC. Which arterial and cardiac parameters best predict left ventricular mass? Circulation 1998; 98:422–428. [DOI] [PubMed] [Google Scholar]
- 18. Sung SH, Cheng HM, Wang KL, Yu WC, Chuang SY, Ting CT, Lakatta EG, Yin FC, Chou P, Chen CH. White coat hypertension is more risky than prehypertension: important role of arterial wave reflections. Hypertension 2013; 61:1346–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen CH, Ting CT, Lin SJ, Hsu TL, Chou P, Kuo HS, Wang SP, Yin FC, Chang MS. Relation between diurnal variation of blood pressure and left ventricular mass in a Chinese population. Am J Cardiol 1995; 75:1239–1243. [PubMed] [Google Scholar]
- 20. Bilo G, Giglio A, Styczkiewicz K, Caldara G, Maronati A, Kawecka-Jaszcz K, Mancia G, Parati G. A new method for assessing 24-h blood pressure variability after excluding the contribution of nocturnal blood pressure fall. J Hypertens 2007; 25:2058–2066. [DOI] [PubMed] [Google Scholar]
- 21. Parati G, Bilo G, Vettorello M, Groppelli A, Maronati A, Tortorici E, Caldara G, Mancia G. Assessment of overall blood pressure variability and its different components. Blood Press Monit 2003; 8:155–159. [DOI] [PubMed] [Google Scholar]
- 22. Mena L, Pintos S, Queipo NV, Aizpúrua JA, Maestre G, Sulbarán T. A reliable index for the prognostic significance of blood pressure variability. J Hypertens 2005; 23:505–511. [DOI] [PubMed] [Google Scholar]
- 23. Wang KL, Cheng HM, Sung SH, Chuang SY, Li CH, Spurgeon HA, Ting CT, Najjar SS, Lakatta EG, Yin FC, Chou P, Chen CH. Wave reflection and arterial stiffness in the prediction of 15-year all-cause and cardiovascular mortalities: a community-based study. Hypertension 2010; 55:799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Westerhof BE, Guelen I, Westerhof N, Karemaker JM, Avolio A. Quantification of wave reflection in the human aorta from pressure alone: a proof of principle. Hypertension 2006; 48:595–601. [DOI] [PubMed] [Google Scholar]
- 25. Liao CF, Cheng HM, Sung SH, Yu WC, Chen CH. Determinants of pressure wave reflection: characterization by the transit time-independent reflected wave amplitude. J Hum Hypertens 2011; 25:665–671. [DOI] [PubMed] [Google Scholar]
- 26. Chirinos JA, Kips JG, Jacobs DR Jr, Brumback L, Duprez DA, Kronmal R, Bluemke DA, Townsend RR, Vermeersch S, Segers P. Arterial wave reflections and incident cardiovascular events and heart failure: MESA (Multiethnic Study of Atherosclerosis). J Am Coll Cardiol 2012; 60:2170–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nürnberger J, Dammer S, Opazo Saez A, Philipp T, Schäfers RF. Diastolic blood pressure is an important determinant of augmentation index and pulse wave velocity in young, healthy males. J Hum Hypertens 2003; 17:153–158. [DOI] [PubMed] [Google Scholar]
- 28. Lee HT, Lim YH, Kim BK, Lee KW, Lee JU, Kim KS, Kim SG, Kim JH, Lim HK, Shin J, Kim YM. The relationship between ambulatory arterial stiffness index and blood pressure variability in hypertensive patients. Korean Circ J 2011; 41:235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Laitinen T, Hartikainen J, Niskanen L, Geelen G, Länsimies E. Sympathovagal balance is major determinant of short-term blood pressure variability in healthy subjects. Am J Physiol 1999; 276:H1245–H1252. [DOI] [PubMed] [Google Scholar]
- 30. Imaizumi Y, Eguchi K, Taketomi A, Tsuchihashi T, Kario K. Exaggerated blood pressure variability in patients with pneumoconiosis: a pilot study. Am J Hypertens 2014; 27:1456–1463. [DOI] [PubMed] [Google Scholar]
- 31. Kayano H, Koba S, Matsui T, Fukuoka H, Kaneko K, Shoji M, Toshida T, Watanabe N, Geshi E, Kobayashi Y. Impact of depression on masked hypertension and variability in home blood pressure in treated hypertensive patients. Hypertens Res 2015; 38:751–757. [DOI] [PubMed] [Google Scholar]
- 32. Kario K. New insight of morning blood pressure surge into the triggers of cardiovascular disease-synergistic resonance of blood pressure variability. Am J Hypertens 2016; 29:14–16. [DOI] [PubMed] [Google Scholar]
- 33. Avolio AP, Van Bortel LM, Boutouyrie P, Cockcroft JR, McEniery CM, Protogerou AD, Roman MJ, Safar ME, Segers P, Smulyan H. Role of pulse pressure amplification in arterial hypertension: experts’ opinion and review of the data. Hypertension 2009; 54:375–383. [DOI] [PubMed] [Google Scholar]
- 34. Ting CT, Chen CH, Chang MS, Yin FC. Short- and long-term effects of antihypertensive drugs on arterial reflections, compliance, and impedance. Hypertension 1995; 26:524–530. [DOI] [PubMed] [Google Scholar]
- 35. Zhang Y, Agnoletti D, Safar ME, Blacher J. Effect of antihypertensive agents on blood pressure variability: the Natrilix SR versus candesartan and amlodipine in the reduction of systolic blood pressure in hypertensive patients (X-CELLENT) study. Hypertension 2011; 58:155–160. [DOI] [PubMed] [Google Scholar]
- 36. Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, Sever PS, Poulter NR. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet 2010; 375:895–905. [DOI] [PubMed] [Google Scholar]
- 37. Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, Poulter NR, Sever PS; ASCOT-BPLA and MRC Trial Investigators . Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol 2010; 9:469–480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.