Abstract
BACKGROUND
Sedentary behavior and obesity are major risk factors for cardiovascular disease. Regular physical activity has independent protective effects on the cardiovascular system, but the mechanisms responsible remain elusive. Recent studies suggest that the protein peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) participates in the response to exercise training. We hypothesized that the arterioles of athletes maintain dilation to flow despite combined inhibition of multiple vasodilators, but loss of PGC-1α renders these vessels susceptible to inhibition of a single vasodilator pathway. In addition, arterioles from overweight and obese individuals will display an an exercise-like phenotype when PGC-1α is activated.
METHODS
Isolated arterioles from exercise-trained (ET) and from mildly overweight or obese subjects (body mass index >25) were cannulated, and changes in lumen diameter in response to graded increases in flow were recorded in the absence and presence of compounds that inhibit various endothelium-dependent vasodilators.
RESULTS
Microvessels of ET subjects displayed robust dilation that could not be inhibited through targeting the combination of nitric oxide, prostaglandins, and hydrogen peroxide, but were inhibited via interference with membrane hyperpolarization. Loss of PGC-1α (siRNA) in the microcirculation of ET subjects eliminates this vasodilatory robustness rendering vessels susceptible to blockade of H2O2 alone. Pharmacological activation of PGC-1α with alpha-lipoic acid in isolated microvessels from sedentary, overweight, and obese subjects increases arteriolar resistance to vasodilator blockade and protects against acute increases in intraluminal pressure.
CONCLUSIONS
These findings suggest that the microvascular adaptations to exercise training, and the exercise-induced protection against acute vascular stress in overweight/obese subjects, are mediated by PGC-1α.
Keywords: blood pressure, exercise, flow-mediated dilation, hypertension, microcirculation, PGC-1 alpha
Cardiovascular disease is a top cause of mortality worldwide.1 Sedentary behavior, and an associated increase in obesity, is a major contributor to global cardiovascular disease burden and development of endothelial cell dysfunction.2,3 In contrast, regular exercise is known to directly combat development of cardiovascular disease, independent of its effects on traditional risk factor pathways.4 Although numerous studies have reported a beneficial effect of exercise in various animal and human models, the underlying cardiovascular mechanisms are only now being explored.5 Some experts have suggested the possibility of an “athlete artery”—similar to the well-characterized “athlete heart”—phenotype, but only indirect, descriptive evidence and scarce mechanistic and molecular characterization currently exist, particularly in the small resistance vessels where blood flow and peripheral resistance are predominantly regulated.6,7 Moreover, these resistance vessels are increasingly recognized as contributors to cardiovascular disease development,8,9 so an understanding of the impact of exercise on microcirculatory function is needed.
Endothelium-dependent dilation in the microcirculation is largely mediated by release of vasoactive substances such as nitric oxide (NO), prostaglandins (PG), and endothelium-derived hyperpolarizing factors that subsequently relax nearby smooth muscle cells in paracrine fashion. The mechanism and resilience of microvascular vasodilation are distinctly different in exercise-trained (ET) vs. sedentary adults. In sedentary individuals, microvascular vasodilation is primarily mediated by NO (inhibited by Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME), and acute vascular stress severely impairs vasodilation in both conduit and resistance vessels of these subjects.10 In contrast, the ET microcirculation is associated with relatively-maintained vasodilation during blockade of nitric oxide synthase (NOS) at baseline,10 and following exposure to acute vascular stress induced by weight lifting or elevated intraluminal pressure,10,11 indicating increased vasodilatory robustness. Recent evidence suggests that regular exercise training in obese adults can recapitulate this protective athlete microvascular phenotype.12
The endothelial and intracellular mechanisms that produce these distinct, exercise-associated microvascular phenotypes and afford protection against acute vascular stress have not been explored in detail. Previous studies have described a central role for the transcriptional coactivator Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) in promoting the adaptations to exercise in skeletal muscle and other nonvascular systems, despite PGC-1α’s ability to impact vascular function.13–16 One study in wheel-running mice reported an association between recurrent bouts of exercise and increased PGC-1α in the vascular endothelium.17 Unfortunately, this study did not demonstrate the functional or phenotypic significance of this increased PGC-1α on the vasculature, nor did the investigators determine whether PGC-1α is itself directly responsible for the vascular effects of exercise. A recent study from our lab illustrated that forced PGC-1α upregulation maintains flow-mediated dilation (FMD) after blockade of a single vasodilator and affords protection against acute increases in intraluminal pressure (IILP) in arterioles from subjects with coronary artery disease.18 Taken together, these findings suggest an overlap between exercise, PGC-1α upregulation, and microvascular protection against acute stress. Therefore, we sought to further examine the “athlete arteriole” during FMD and determine whether PGC-1α contributes to this phenotype. We hypothesized that resistance to blockade of the production of NO and other endothelial-released vasodilators in ET individuals is PGC-1α-dependent, such that loss of PGC-1α removes this ET phenotype. In addition, we predicted that PGC-1α overexpression in arterioles from overweight and obese adults would establish a microvascular phenotype corresponding to that observed after 8 weeks of exercise training in this population.
MATERIALS AND METHODS
Materials
Endothelin-1 was prepared in 1% bovine serum albumin (BSA). Lentiviral constructs (PGC-1α siRNA) were created by the Blood Center of Wisconsin Hybridoma Core Laboratory. Lentiviral siRNA was diluted in distilled water prior to treatment of isolated arterioles to achieve an approximate titer of 1 × 105 transfection units/ml. Bio-Enhanced Na-Rala (GeroNova Research), l-NAME (Sigma), and polyethylene (PEG)-catalase (Quanta BioDesign) were dissolved in distilled water. Bio-Enhanced Na-Rala (GeroNova Research) was dissolved in distilled water.
Cell culture
Cultured umbilical vein endothelial cells were raised in endothelial cell growth medium (EGM-2-MV; Lonza) containing 5% fetal calf serum. Cells were then transferred to 100 mm plates for subculturing in EGM-2 containing growth factors, cytokines, and supplements and fetal bovine serum of 5%. Cells at passages 3–5 with >85% viability were treated with 250 µM alpha-lipoic acid (ALA) (16–24 hours) to overexpress PGC-1α18 and harvested prior to evaluation of PGC-1α mRNA expression.
Quantitative real-time polymerase chain reaction
Total mRNA was isolated from cultured endothelial cells that were either untreated or treated with 250 µM ALA using an Ambion PureLink RNA Kit. Approximately 1.5 µg of RNA was used to synthesize cDNA using the Applied Biosystems High Capacity cDNA Reverse Transcription Kit. Gene expression was quantified by real-time-quantitative polymerase chain reaction using primers for PGC-1α (QT00095578) and SYBR green from Qiagen in a BioRad CFX96 Touch Real-Time PCR Detection System. PGC-1α mRNA expression was normalized to 18S (QT00199367).
Tissue acquisition and study population
Fresh human subcutaneous adipose arterioles were obtained from gluteal biopsies (ET subjects) or from surgical discard specimens (overweight subjects). Gluteal biopsy was performed by a trained surgeon. This procedure involves local anesthesia with lidocaine, making an incision in the superolateral quadrant of the buttocks, and removing a small piece of subcutaneous fat (~3 cc) using sterile technique. Samples were immediately placed in cold HEPES buffer. Arterioles were isolated from adipose tissue and cannulated within 24 hours. Arterioles treated with siRNA were placed into EGM-2-MV media for 48 hours prior to cannulation. All protocols were approved by the local Institutional Review Board.
ET subjects were defined as those who participated in a consistent exercise routine (>30 minutes/day, ≥3 ×/week; self-reported) for greater than the previous 6 months. Subjects were identified through local advertisements, and eligibility was confirmed by a preliminary phone screen as well as an in-person screening visit. Subjects fasted for 12 hours prior to the study visit and withheld from exercise for 48 hours before the gluteal biopsy.10 All ET subjects provided written informed consent. Overweight/obese was defined as those subjects with body mass index >25.
Microcirculatory FMD and videomicroscopy
Arterioles were cannulated on glass pipettes in an organ chamber containing physiological salt solution. The organ chamber was transferred to an inverted microscope, and vessels equilibrated at 30 mm Hg for 30 minutes, then pressure was raised to 60 mm Hg and vessel re-equilibrated for 30 minutes (37 °C) prior to preconstriction with endothelin until vessels stabilized at 30–60% of passive internal diameter. Following this, vessels were exposed to progressive increases in shear stress (5 to 100 cmH2O) reflecting physiological flow rates of 5–20 µl/min) at constant intraluminal pressure (i.e., moving the reservoirs in equal and opposite directions). Internal diameters were recorded after 5 minutes of flow exposure, at which point diameters achieve steady state. Two flow-response curves were obtained. Specific inhibitors to NOS [Nω-nitro-l-arginine methyl ester (l-NAME), 100 µmol/l], prostaglandins [Indomethacin (INDO), 100 µmol/l], scavengers of H2O2 [PEG-catalase, 500 µ/ml], MSPPOH [CYP450 epoxygenase inhibitor, 100 µmol/l], or hyperpolarization [KCl, 60 µmol/l] were added to the organ chamber bath for 30 minutes prior to the second curve to evaluate which vasoactive agent is responsible for FMD. Criteria for acceptable experiments include a maximal diameter of <300 microns, verification of >80% maximal (passive) dilation in response to an endothelium-independent dilator, papaverine (100 µM), at the end of the experiment, and application of reverse flow to verify matched impedance between pipettes.
Statistical analysis
Data are expressed as mean ± SEM. FMD is shown as a percentage of maximal dilation following preconstriction with endothelin-1. Two-way analysis of variance was used to determine differences in the magnitude of dilation between flow curves. When a significant difference was identified by analysis of variance, responses at individual concentrations were assessed using a Holm–Sidak multiple comparison test. Analyses were performed using SigmaPlot or GraphPad. Statistical significance was defined as P <0.05.
RESULTS
Subject demographics
Tissue was provided by a total of 39 subjects. Subject demographics are detailed in Table 1.
Table 1.
Patient demographics
| Overweight/obese | Exercise-trained | |
|---|---|---|
| Characteristics | ||
| Sex, M/F | 5/24 | 8/2 |
| Age, year (mean ± SD)a | 51.2 ± 13.1 | 25.1 ± 2.3 |
| BMI (mean ± SD)*a | 33.7 ± 7.3 | 24.9 ± 1.6 |
| Underlying conditions | ||
| CAD | 0 | 0 |
| DMII | 4 | 0 |
| HTN | 1 | 0 |
| HC | 4 | 0 |
| MI | 0 | 0 |
| CHF | 0 | 0 |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; DMII, diabetes mellitus type II; HTN, hypertension; HC, hypercholesterolemia; MI, myocardial infarction; CHF, congestive heart failure.
aSignificant difference between groups (P < 0.05).
ET subjects display arteriolar plasticity in the mechanism of FMD
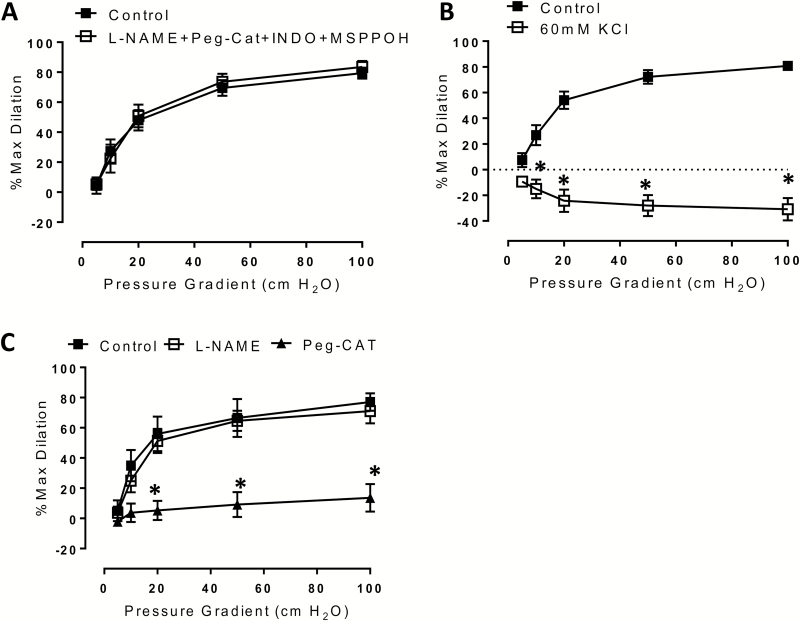
Vessels from ET subjects dilated to approximately 80% maximal dilation in the absence of any inhibitors. Addition of individual dilator inhibitors did not reduce overall dilation (data not shown). To exclude a possible compensatory action of these vasodilators, we used combined treatment with l-NAME (NOS inhibitor), Indomethacin (COX inhibitor), PEG-catalase (H2O2 scavenger), and MSPPOH (CYP450 epoxygenase inhibitor). Simultaneous treatment with this combination of inhibitors did not reduce dilation compared to control (Figure 1a) [% max diameter at 100 cm H2O: control 80.7 ± 3.7, l-NAME + PEG-cat + INDO + MSPPOH 83.5 ± 4.0]. However, interfering with membrane hyperpolarization using 60 mM KCl did fully abolish microvascular FMD (Figure 1b) [% max diameter at 100 cm H2O: control 80.7 ± 3.7, 60 mM KCl −30.8 ± 8.8; P < 0.001].
Figure 1.
Mechanism of flow-mediated dilation in exercise-trained subjects. (a) FMD is not reduced in arterioles from ET subjects following combined treatment with PEG-catalase (500 U/ml; H2O2 scavenger), l-NAME (10–4 M; eNOS inhibitor), MSPPOH (12 µM; CYP450 epoxygenase inhibitor), and Indomethacin (10–4 M; prostaglandin antagonist); (b) FMD is abolished in the presence of 60 mM KCl; (c) 48-hour incubation with lentiviral siRNA to PGC-1α renders ET arterioles susceptible to inhibition by PEG-catalase. N = 5–7 per treatment condition. *P < 0.05 vs. control curves at specific pressure gradients. Abbreviations: eNOS, endothelial nitric oxide synthase; ET, exercise-trained; FMD, flow-mediated dilation; l-NAME, Nω-nitro-l-arginine methyl ester hydrochloride; PEG, polyethylene; PGC, peroxisome proliferator-activated receptor gamma coactivator.
Loss of PGC-1α removes vasodilatory plasticity in arterioles of ET subjects
To assess whether conserved PGC-1α levels are responsible for resistance to blockade of multiple dilator pathways in arterioles from ET subjects, we treated these vessels with lentiviral siRNA to PGC-1α for 48 hours. We have recently confirmed that this approach reduces PGC-1α levels in the microcirculation.18 Following treatment with the lentivirus, arteriolar FMD was inhibited by PEG-catalase alone (Figure 1c) [% max diameter at 100 cm H2O: control 77.1 ± 5.8, PEG-catalase 13.6 ± 9.1; P < 0.001]. Thus, PGC-1α is necessary for maintaining FMD in the presence of inhibitors of NO, H2O2, and prostaglandin production.
Pharmacological overexpression of PGC-1α confers maintained FMD in arterioles from overweight and obese subjects treated with l-NAME
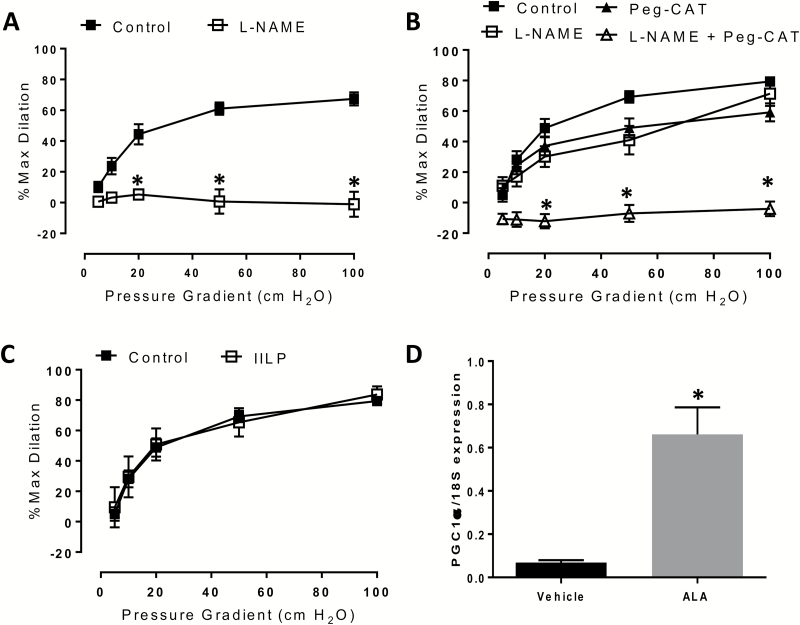
We next questioned whether PGC-1α overexpression in vessels from obese or overweight subjects without coronary artery disease would result in maintained dilation after incubation with l-NAME. Our lab has consistently demonstrated that l-NAME inhibits dilation in vessels from adults without cardiovascular disease,19–21 indicating exclusive reliance on NO-mediated dilation in the microcirculation of these subjects. We confirmed that l-NAME fully blocks dilation in untreated arterioles from obese and overweight subjects (Figure 2a) [% max diameter at 100 cm H2O: control 67.4 ± 4.2, l-NAME -1.1 ± 8.1; P < 0.001] However, following PGC-1α upregulation in vessels from overweight and obese patients, FMD was no longer inhibited by l-NAME alone, instead requiring addition of both l-NAME and PEG-catalase to block dilation (Figure 2b) [% max diameter at 100 cm H2O: control 79.6 ± 3.6, l-NAME + PEG-catalase −4.3 ± 5.7; P < 0.001]. This observation implies dual or compensatory release of NO and H2O2.
Figure 2.
Effect of pharmacological PGC-1α overexpression on FMD in microvessels from overweight and obese individuals. At baseline, (a) FMD is blocked by l-NAME alone. Following 250 µM ALA treatment, (b) inhibition of dilation is achieved only in the presence of both l-NAME and PEG-catalase; (c) FMD is preserved following exposure to IILP (150 mm Hg, 30 min); D) Effect of 250 µM ALA on PGC-1α mRNA expression, as determined by qPCR in cultured endothelial cells. Vessel studies: n = 4–7 per treatment condition. *P < 0.05 PEG-catalase + l-NAME vs. control at specific pressure gradients. Abbreviations: ALA, alpha-lipoic acid; FMD, flow-mediated dilation; IILP, increases in intraluminal pressure; l-NAME, Nω-nitro-l-arginine methyl ester hydrochloride; qPCR, quantitative polymerase chain reaction; PEG, polyethylene; PGC, peroxisome proliferator-activated receptor gamma coactivator.
Pharmacological PGC-1α overexpression protects against acute vascular stress in arterioles from overweight and obese subjects
We have previously observed reduced arteriolar FMD following acute IILP in subjects with18 and without21 cardiovascular disease (i.e., those relying exclusively on H2O2- or NO-dependent dilation, respectively). In contrast, ET subjects who rely on more than a single vasodilator mechanism have an associated protection against this pressure-induced vascular dysfunction.10,22 Therefore, we hypothesized that involvement of >1 vasodilator after PGC-1α overexpression in microvessels from overweight subjects might confer the same protection that is observed in arterioles from lean athletes, as well as obese subjects who participated in a prolonged exercise regimen. Indeed, the magnitude of FMD in PGC-1α-overexpressing arterioles was not reduced following IILP (Figure 2c) [% max diameter at 100 cm H2O: control 79.6 ± 3.6, IILP 84.8 ± 7.8], in contrast to what has been observed in subjects with and without cardiovascular disease who rely on a single vasodilator where an approximately 20–50% reduction in dilation was observed.18,21 Confirmation of ALA-induced PGC-1α overexpression in cultured endothelial cells via quantitative polymerase chain reaction (qPCR) is provided in Figure 2d, consistent with previous findings from our lab.18
DISCUSSION
There are 4 major findings of this study. First, increasing PGC-1α in overweight and obese individuals confers a microvascular phenotype similar to what is observed in this same cohort after 8 weeks of exercise training (recruitment of vasodilatory H2O2). Second, we demonstrate an association between reliance on >1 vasodilator and protection against acute IILP in overweight and obese subjects. Third, we confirm that FMD in the microvessels of athletes cannot be inhibited by interfering with NO production, in contrast to what has been previously observed in sedentary subjects.10 This phenotype is characterized by an ability to recruit more than 1 vasodilator as the mediator of FMD. Fourth, loss of PGC-1α removes this athlete arteriolar phenotype. Overall, these findings highlight the mechanistic participation of PGC-1α in the microvascular adaptations to exercise at rest and the exercise-induced protection against acute barostress in obese individuals.
Microvascular FMD
Athletes display robust FMD at baseline that is resistant to blockade of several well-known vasodilator pathways. Our data confirm and extend previous findings by demonstrating this response during dilation to flow and implicating PGC-1α as a key endogenous regulator of this microvascular phenotype. In contrast, FMD can be significantly reduced by inhibiting only NOS in overweight and obese subjects without coronary artery disease. However, after genetic upregulation of PGC-1α blockade of NOS no longer inhibits FMD. Instead, the microvascular phenotype of these overweight and obese subjects shifts closer to that of the ET subjects in that multiple pathways of dilation must be blocked to reduce dilation. While PGC-1α may recruit H2O2 in arterioles from obese subjects through several possible mechanisms, we have previously suggested18 that modulation of caveolin-1—recently shown by the Shimokawa laboratory to be responsible for establishing a balance between NO and H2O2 in resistance vessels23—may underlie this effect. This possibility is strengthened by the known association between caveolin-1 and PGC-1α expression.24 Others have also shown that caveolin-1 controls H2O2 release in arterioles and have even suggested that reduced caveolin-1 allows for compensatory dilation in obese mice by allowing for greater contribution of EDHF to vasodilation.25
Of note, PGC-1α overexpression did not fully recapitulate the “athlete arteriole” phenotype, but it did confer resistance to blockade of a single vasodilator. It is possible that PGC-1α upregulation is only partly responsible for the vasodilatory robustness of the athlete, and perhaps more prolonged PGC-1α overexpression, or even the influence of other factors such as shear stress or neurohumoral agents, is required to achieve the microvascular adaptions to exercise. It is also possible that obesity/overweight prevent full manifestation of the PGC-1α phenotype.
Microvascular response to acute IILP
Both acute and chronic elevations in pressure are known to precipitate adverse cardiovascular events. Altered vasodilator mechanisms following exposure to high pressure may play a role. Both exercise and forced PGC-1α overexpression can prevent pressure-induced microvascular dysfunction in subjects with cardiovascular risk factors. Intriguingly, in sedentary subjects who underwent an 8-week exercise training program, the newly conferred ability to maintain dilation after acute exertion was associated with an increased reliance on H2O2 as a vasodilator, and increased H2O2 production, with no increase in NO bioavailability.22 That PGC-1α overexpression in mildly overweight individuals similarly introduces H2O2 as a major vasodilator and protects against acute IILP reinforces the concept that PGC-1α can recruit >1 vasodilator and mediates the redundancy observed with exercise training. In addition, several lines of evidence now suggest an association between reliance on >1 vasodilator and protection against acute IILP.10,18 Moreover, a study by Thenchaisri et al. described the role for exercise-induced H2O2 release, in conjunction with NO-dependent mechanisms, in improving coronary arteriolar dilation,26 positioning this phenotype of >1 vasodilator—especially dual release of NO and H2O2—as a key mechanism underlying the adaptations to exercise in various settings. Perhaps the protection against acute IILP is related to the nature and not the number of dilators invoked by shear. For example, one can speculate that protection afforded by H2O2 in disease is more robust than dilation mediated by NO since H2O2 is more stable in an oxidative milieu. PGC-1α may be the master regulator of this arteriolar vasodilatory adaptation.
Continued exploration of the physiological stimulus responsible for this microvascular phenotype and further analysis of the underlying mechanism is warranted. Preliminary evidence suggests that prolonged shear stress, which has previously been suggested as a key stimulus governing the vascular dilatory response to exercise,27–30 recruits multiple vasodilators in human microvessels (Kadlec, unpublished data). In a cultured endothelial cell model, prolonged shear is known to increase PGC-1α,31,32 supporting a link between exercise, prolonged and elevated shear stress, and increased endothelial PGC-1α levels.
Study limitations
Due to an inability to obtain full clinical information from subjects whose discard tissue was obtained (de-identified information database), we cannot quantify levels of physical activity. However, we selected those with body mass indices in the overweight and obese range to comprise the “nonathletic” group since these subjects tend to be more sedentary, providing a proper context for studying the effect of ex vivo PGC-1α overexpression. Robinson et al. have reported that, in the overweight and obese cohort with known sedentary behavior, l-NAME significantly reduced dilation (25), consistent with the baseline FMD response (NO-dependent) of the arterioles from overweight/obese subjects that were used in our study.
We do not demonstrate that PGC-1α is upregulated in the endothelium of athletes vs. obese individuals. Although we did attempt to evaluate PGC-1α protein levels in these vessels via immunohistochemistry, the obtained images were equivocal. Since PGC-1α responds to exercise in diverse ways (e.g., upregulation,17 subcellular translocation33 and differential activation of splice variants34–36), increased protein expression is not necessary to activate PGC-1α. Given the substantial evidence demonstrating either increased or activated PGC-1α in the endothelium17 and other nonvascular tissues in response to exercise, we believe the available literature strongly characterizes a role for PGC-1α in exercise-induced adaptations.
One limitation is failure to identify the mediator of FMD in the athlete. Preliminary data suggest that gap junctions contribute to the capacity for microvessels from ET subjects to compensate during blockade of endothelium-derived vasodilators (Kadlec, unpublished data). Gap junctions may participate via direct hyperpolarization or may mediate paracrine transfer of vasodilators like NO and H2O2. Given that gap junctions are perceived to play a role in compensatory vasodilation,37 and are often responsible for the remaining endothelium-dependent dilation following inhibition of known vasodilators (reviewed in38), this is a particularly exciting next step. Future studies should clarify whether this mechanism exists in the ET microcirculation.
Differences exist in age and sex of the participants. We have previously reported that NO-mediated dilation predominates in subjects without coronary artery disease, regardless of age, so we do not believe that this factor confounds our results. Although we cannot determine the effects of sex on our findings in this study, we have not observed sex-based differences in the mediator of dilation. It is also possible that different forms of exercise produce different microvascular phenotypes in athletes, but previous studies from our lab observed similar microvessel responses in participants who perform either resistance or aerobic exercise.10
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
We thank the staff in the Adult TRU and MCW CTSI for their expertise in conducting human subjects research. We also thank the following locations for their assistance in tissue acquisition: Wisconsin Donor Network, St Joseph’s Hospital, Froedtert Memorial Lutheran Hospital, Aurora St Luke’s Medical Center, and Wheaton Franciscan Healthcare’s Elmbrook Memorial Hospital. A.O.K., M.J.D., and D.D.G. were responsible for study design. A.O.K and C.B. performed study procedures. A.O.K., C.B, M.J.D., and D.D.G. wrote and critically revised the manuscript. This work was supported by the Northwestern Mutual Endowed Professorship (to D.D. Gutterman). Andrew Kadlec is a member of the Medical Scientist Training Program at MCW, which is partially supported by a training grant from NIGMS T32-GM-080202.
REFERENCES
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, MohlerER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 2016; 133:e38–e360. [DOI] [PubMed] [Google Scholar]
- 2. Ford ES, Caspersen CJ. Sedentary behaviour and cardiovascular disease: a review of prospective studies. Int J Epidemiol 2012; 41:1338–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Warren TY, Barry V, Hooker SP, Sui X, Church TS, Blair SN. Sedentary behaviors increase risk of cardiovascular disease mortality in men. Med Sci Sports Exerc 2010; 42:879–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Joyner MJ, Green DJ. Exercise protects the cardiovascular system: effects beyond traditional risk factors. J Physiol 2009; 587:5551–5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature 2008; 454:463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Green DJ, Spence A, Rowley N, Thijssen DH, Naylor LH. Vascular adaptation in athletes: is there an ‘athlete’s artery’?Exp Physiol 2012; 97:295–304. [DOI] [PubMed] [Google Scholar]
- 7. Pedersen BK, Saltin B. Exercise as medicine–evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports 2015; 25:1–72. [DOI] [PubMed] [Google Scholar]
- 8. Gutterman DD, Chabowski DS, Kadlec AO, Durand MJ, Freed JK, Ait-Aissa K, Beyer AM. The human microcirculation: regulation of flow and beyond. Circ Res 2016; 118:157–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation 2002; 106:653–658. [DOI] [PubMed] [Google Scholar]
- 10. Durand MJ, Dharmashankar K, Bian JT, Das E, Vidovich M, Gutterman DD, Phillips SA. Acute exertion elicits a H2O2-dependent vasodilator mechanism in the microvasculature of exercise-trained but not sedentary adults. Hypertension 2015; 65:140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Phillips SA, Das E, Wang J, Pritchard K, Gutterman DD. Resistance and aerobic exercise protects against acute endothelial impairment induced by a single exposure to hypertension during exertion. J Appl Physiol (1985) 2011; 110:1013–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Robinson AT, Fancher IS, Sudhahar V, Bian JT, Cook MD, Mahmoud AM, Ali MM, Ushio-Fukai M, Brown MD, Fukai T, Phillips SA. Short-term regular aerobic exercise reduces oxidative stress produced by acute in the adipose microvasculature. Am J Physiol Heart Circ Physiol 2017; 312:H896–H906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Furrer R, Eisele PS, Schmidt A, Beer M, Handschin C. Paracrine cross-talk between skeletal muscle and macrophages in exercise by PGC-1alpha-controlled BNP. Sci Rep 2017;7:40789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, Lebrasseur NK, Yan Z, Spiegelman BM. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J Biol Chem 2007; 282:30014–30021. [DOI] [PubMed] [Google Scholar]
- 15. Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab 2013; 18:649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kadlec AO, Chabowski DS, Ait-Aissa K, Gutterman DD. Role of PGC-1alpha in vascular regulation: implications for atherosclerosis. Arterioscler Thromb Vasc Biol 2016;36:1467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim B, Lee H, Kawata K, Park JY. Exercise-mediated wall shear stress increases mitochondrial biogenesis in vascular endothelium. PLoS One 2014; 9:e111409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kadlec AO, Chabowski DS, Ait-Aissa K, Hockenberry JC, Otterson MF, Durand MJ, Freed JK, Beyer AM, Gutterman DD. PGC-1alpha (peroxisome proliferator-activated receptor gamma coactivator 1-alpha) overexpression in coronary artery disease recruits NO and hydrogen peroxide during flow-mediated dilation and protects against increased intraluminal pressure. Hypertension 2017;70:166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Freed JK, Beyer AM, LoGiudice JA, Hockenberry JC, Gutterman DD. Ceramide changes the mediator of flow-induced vasodilation from nitric oxide to hydrogen peroxide in the human microcirculation. Circ Res 2014; 115:525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beyer AM, Freed JK, Durand MJ, Riedel M, Ait-Aissa K, Green P, Hockenberry JC, Morgan RG, Donato AJ, Peleg R, Gasparri M, Rokkas CK, Santos JH, Priel E, Gutterman DD. Critical role for telomerase in the mechanism of flow-mediated dilation in the human microcirculation. Circ Res 2016; 118:856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beyer AM, Durand MJ, Hockenberry J, Gamblin TC, Phillips SA, Gutterman DD. An acute rise in intraluminal pressure shifts the mediator of flow-mediated dilation from nitric oxide to hydrogen peroxide in human arterioles. Am J Physiol Heart Circ Physiol 2014; 307:H1587–H1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Robinson AT, Franklin NC, Norkeviciute E, Bian JT, Babana JC, Szczurek MR, Phillips SA. Improved arterial flow-mediated dilation after exertion involves hydrogen peroxide in overweight and obese adults following aerobic exercise training. J Hypertens 2016; 34:1309–1316. [DOI] [PubMed] [Google Scholar]
- 23. Godo S, Sawada A, Saito H, Ikeda S, Enkhjargal B, Suzuki K, Tanaka S, Shimokawa H. Disruption of physiological balance between nitric oxide and endothelium-dependent hyperpolarization impairs cardiovascular homeostasis in mice. Arterioscler Thromb Vasc Biol 2016; 36:97–107. [DOI] [PubMed] [Google Scholar]
- 24. Fernández-Real JM, Catalán V, Moreno-Navarrete JM, Gómez-Ambrosi J, Ortega FJ, Rodriguez-Hermosa JI, Ricart W, Frühbeck G. Study of caveolin-1 gene expression in whole adipose tissue and its subfractions and during differentiation of human adipocytes. Nutr Metab (Lond) 2010; 7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feher A, Rutkai I, Beleznai T, Ungvari Z, Csiszar A, Edes I, Bagi Z. Caveolin-1 limits the contribution of BK(Ca) channel to EDHF-mediated arteriolar dilation: implications in diet-induced obesity. Cardiovasc Res 2010; 87:732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thengchaisri N, Shipley R, Ren Y, Parker J, Kuo L. Exercise training restores coronary arteriolar dilation to NOS activation distal to coronary artery occlusion: role of hydrogen peroxide. Arterioscler Thromb Vasc Biol 2007; 27:791–798. [DOI] [PubMed] [Google Scholar]
- 27. Tinken TM, Thijssen DH, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension 2010; 55:312–318. [DOI] [PubMed] [Google Scholar]
- 28. Gielen S, Schuler G, Adams V. Cardiovascular effects of exercise training: molecular mechanisms. Circulation 2010; 122:1221–1238. [DOI] [PubMed] [Google Scholar]
- 29. Padilla J, Simmons GH, Bender SB, Arce-Esquivel AA, Whyte JJ, Laughlin MH. Vascular effects of exercise: endothelial adaptations beyond active muscle beds. Physiology (Bethesda) 2011; 26:132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Green DJ. Exercise training as vascular medicine: direct impacts on the vasculature in humans. Exerc Sport Sci Rev 2009; 37:196–202. [DOI] [PubMed] [Google Scholar]
- 31. Chen Z, Peng IC, Cui X, Li YS, Chien S, Shyy JY. Shear stress, SIRT1, and vascular homeostasis. Proc Natl Acad Sci USA 2010; 107:10268–10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim JS, Kim B, Lee H, Thakkar S, Babbitt DM, Eguchi S, Brown MD, Park JY. Shear stress-induced mitochondrial biogenesis decreases the release of microparticles from endothelial cells. Am J Physiol Heart Circ Physiol 2015; 309:H425–H433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Safdar A, Little JP, Stokl AJ, Hettinga BP, Akhtar M, Tarnopolsky MA. Exercise increases mitochondrial PGC-1alpha content and promotes nuclear-mitochondrial cross-talk to coordinate mitochondrial biogenesis. J Biol Chem 2011; 286:10605–10617. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34. Norrbom J, Sällstedt EK, Fischer H, Sundberg CJ, Rundqvist H, Gustafsson T. Alternative splice variant PGC-1α-b is strongly induced by exercise in human skeletal muscle. Am J Physiol Endocrinol Metab 2011; 301:E1092–E1098. [DOI] [PubMed] [Google Scholar]
- 35. Ydfors M, Fischer H, Mascher H, Blomstrand E, Norrbom J, Gustafsson T. The truncated splice variants, NT-PGC-1alpha and PGC-1alpha4, increase with both endurance and resistance exercise in human skeletal muscle. Physiol Rep 2013;1:e00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Silvennoinen M, Ahtiainen JP, Hulmi JJ, Pekkala S, Taipale RS, Nindl BC, Laine T, Hakkinen K, Selanne H, Kyrolainen H, Kainulainen H. PGC-1 isoforms and their target genes are expressed differently in human skeletal muscle following resistance and endurance exercise. Physiol Rep 2015;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morton JS, Rueda-Clausen CF, Davidge ST. Mechanisms of endothelium-dependent vasodilation in male and female, young and aged offspring born growth restricted. Am J Physiol Regul Integr Comp Physiol 2010; 298:R930–R938. [DOI] [PubMed] [Google Scholar]
- 38. Griffith TM. Endothelium-dependent smooth muscle hyperpolarization: do gap junctions provide a unifying hypothesis?Br J Pharmacol 2004; 141:881–903. [DOI] [PMC free article] [PubMed] [Google Scholar]