Abstract
BACKGROUND
Electrocardiographic (ECG) left ventricular hypertrophy (LVH) is a strong predictor of cardiovascular (CV) morbidity and mortality. However, the predictive value of ECG LVH in treated hypertensive patients remains unclear.
METHODS
A total of 33,357 patients (aged ≥ 55 years) with hypertension and at least 1 other coronary heart disease (CHD) risk factor were randomized to chlorthalidone, amlodipine, or lisinopril. The outcome of the present study was all-cause mortality; and secondary endpoints were CHD, nonfatal myocardial infarction (MI), stroke, angina, heart failure (HF), and peripheral arterial disease. Cornell voltage criteria (S in V3 + R in aVL > 28 [men] or >22 mm [women]) defined ECG LVH.
RESULTS
ECGs were available at baseline in 26,384 patients. Baseline Cornell voltage LVH was present in 1,741 (7%) patients, who were older (67.4 vs. 66.6 years, P < 0.001), more likely to be female (74 vs. 44%, P < 0001) with a higher systolic blood pressure (151 vs. 146 mm Hg, P < 0.001) than patients without ECG LVH. During 5.0 ± 1.4 years mean follow-up, baseline and in-study ECG LVH was significantly associated with 29 to 98% increased risks of all-cause mortality, MI, CHD, stroke, and HF in multivariable Cox analyses.
CONCLUSIONS
Baseline Cornell voltage LVH is associated with increased CV morbidity and all-cause mortality in treated hypertensive patients independent of treatment modality and other CV risk factors.
CLINICAL TRIALS REGISTRATION
Trial Number NCT00000542.
Keywords: blood pressure, Cornell voltage, electrocardiographic left ventricular hypertrophy, hypertension
Several studies have shown that left ventricular hypertrophy (LVH), detected by echocardiography1 or the 12-lead electrocardiogram (ECG),2 is not only a cardinal adaptation to increased hemodynamic load in hypertension, but also a common manifestation of preclinical cardiovascular (CV) disease that strongly predicts CV morbidity and mortality.
The Losartan Intervention For Endpoint reduction in hypertension (LIFE) study3,4 has previously shown that higher values of ECG LVH by Cornell product and/or Sokolow-Lyon voltage criteria during antihypertensive therapy were associated with higher rates of CV morbidity and mortality, independent of treatment modality and of decreases in blood pressure (BP) in a prospectively studied population of patients with hypertension selected to be at increased risk of CV events based on the presence of LVH on a screening ECG.5 However, the relation of baseline and in-study ECG LVH to death and CV events in less-selected, lower risk hypertensive patients with average or low prevalence of ECG LVH has not yet been evaluated. Accordingly, the present study was undertaken to determine the predictive value of baseline or development of ECG LVH in hypertensive patients enrolled in the Antihypertensive and Lipid-Lowering Treatment to prevent Heart Attack Study (ALLHAT).
METHODS
Study design
The ALLHAT rationale and design have been reported previously.6,7 Briefly, participants were men and women aged ≥55 years who had stage 1 or 2 hypertension plus an additional risk factor for coronary heart disease (CHD). Individuals with a history of hospitalized or treated symptomatic heart failure (HF) or known left ventricular ejection fraction <35% were excluded. Participants (n = 33,357) were randomly assigned to chlorthalidone, amlodipine, or lisinopril and pairwise comparisons of the latter 2 agents to the diuretic were to be undertaken to assess the incidence of outcomes.
All participants gave written informed consent, and all centers obtained institutional review board approval. Follow-up visits were at 1, 3, 6, 9, and 12 months and quarterly thereafter. Goal BP of <140/90 mm Hg was to be achieved by titration of the assigned study drug (step 1) and addition of open-label agents (step 2 or 3) when necessary. Step 1 drugs were encapsulated and identical in appearance. Dosages were 12.5–25 mg/day for chlorthalidone, 2.5–10 mg/day for amlodipine, and 10–40 mg/day for lisinopril. The choice of step 2 drugs was at the clinician’s discretion. Study-supplied open-label drugs were atenolol, reserpine, and clonidine for step 2 and hydralazine for step 3. Other open-label drugs were permitted if clinically indicated.
For the present post-hoc analysis, patients with pacemaker or left or right ventricular branch block were excluded.
Electrocardiography
ECGs were recorded at clinical sites using standardized procedures at baseline and biannually thereafter until study termination or patient death. Individual ECG tracings were forwarded to the core ECG Reading Center (University of Minnesota, Minneapolis), where cross-sectional and serial coding of multiple variables was performed manually by reviewers blinded to treatment assignment. These readings were obtained from 1994 to 2002. Patients were excluded if they had baseline complete left bundle branch block, complete right bundle branch block, nonspecific intraventricular conduction delay QRS ≥120 ms or pacemaker.8 Cornell voltage, the sum of R wave amplitude in aVL and S wave amplitude in V3, >22 mm in women and >28 mm in men was used to identify LVH.9
Endpoints
Follow-up procedures, study endpoints, and ascertainment of events have been described previously.7,10 The primary endpoint of the present study was all-cause mortality; and secondary endpoints were CHD, nonfatal myocardial infarction (NFMI), stroke, angina, HF, and peripheral arterial disease.
Statistical analyses
Data are expressed as mean (SD) or as proportions. Differences between groups with and without LVH were assessed by independent samples t tests and contingency tables. To test the hypothesis that LVH during antihypertensive therapy results in more clinical events, independent of antihypertensive treatment type and degree of BP lowering, the effect of baseline ECG LVH on risk of clinical endpoints was analyzed, following all randomized patients with baseline ECG LVH values for endpoints for the entire duration of the study, regardless of protocol violations or discontinuation of study medication. The effect of baseline ECG LVH on the risk of clinical endpoints, expressed as the hazard ratio and its 95% confidence interval was analyzed using multivariable Cox regression models. In these models, Cornell voltage was examined using four separate approaches: as a dichotomous variable for the presence or absence of LVH; as a continuous variable; as quartiles of increasing voltage; and as a dichotomous variable comparing the upper quartile with the lower 3 quartiles. The relationship of clinical outcomes to changing levels of ECG LVH during treatment was assessed using Cox models in which ECG LVH measured during the study was entered as a time-varying covariate. The multivariable models were adjusted to standard baseline covariates of age, treatment group, race, ethnicity; history of diabetes, CHD, smoking, and aspirin use; and measured values of heart rate, body mass index, systolic and diastolic BP, potassium, glucose, (estimated) glomerular filtration rate, total cholesterol, low-density lipoprotein and high-density lipoprotein cholesterol, and triglycerides in a stepwise fashion. Data management and analyses were performed by the ALLHAT study group.
RESULTS
Patient characteristics
Detailed baseline characteristics of ALLHAT participants have been reported previously. Briefly, mean age was 67 years, 53% were male, 25% had a history of CHD, and 36% had a history of diabetes mellitus.
Baseline characteristics of patients with and without baseline ECG LVH are shown in Table 1. Patients with ECG LVH were older; were more likely to be female, Black, nonsmoker; had a higher systolic and diastolic BP, higher heart rate, higher glucose, higher total, low-density lipoprotein and high-density lipoprotein cholesterol; but had less aspirin use, slightly lower kidney function, potassium, and triglyceride levels.
Table 1.
Baseline characteristics by Cornell voltage left ventricular hypertrophy at baseline for subjects with baseline electrocardiogram
| Total | Cornell voltage LVH No | Cornell voltage LVH Yes | P | |
|---|---|---|---|---|
| N a | 26,384 | 24,643 | 1,741 | |
| Age—mean (SD) years | 66.7 (7.6) | 66.6 (7.5) | 67.4 (8.4) | <0.001 |
| Female—n (%) | 12,207 (46.3) | 10,920 (44.3) | 1,287 (73.9) | <0.001 |
| Black—n (%) | 9,020 (34.2) | 8,020 (32.5) | 1,000 (57.4) | <0.001 |
| Hispanic—n (%) | 4,515 (17.1) | 4,218 (17.1) | 297 (17.1) | 0.951 |
| Systolic blood pressure—mean (SD) mm Hg | 146 (16) | 146 (16) | 151 (15) | <0.001 |
| Diastolic blood pressure—mean (SD) mm Hg | 84 (10) | 84 (10) | 86 (11) | <0.001 |
| Heart rate—mean (SD) beats/min | 73 (11) | 73 (11) | 74 (11) | <0.001 |
| Body mass index | 29.7 (6.1) | 29.7 (6.1) | 29.9 (6.3) | 0.232 |
| Current smoker—n (%) | 5,868 (22.2) | 5,534 (22.5) | 334 (19.2) | 0.002 |
| Atherosclerotic coronary vascular disease—n (%) | 13,483 (51.1) | 12,617 (51.2) | 866 (49.7) | 0.240 |
| Type II diabetes—n (%) | 9,257 (35.1) | 8,627 (35.0) | 630 (36.2) | 0.319 |
| History of coronary heart disease—n (%) | 6,571 (25.1) | 6,198 (25.4) | 373 (21.6) | 0.001 |
| Aspirin use—n (%) | 9,623 (36.5) | 9,122 (37.0) | 501 (28.8) | <0.001 |
| Assigned antihypertensive treatment group | 0.750 | |||
| Chlorthalidone—n (%) | 12,106 (45.9) | 11,322 (45.9) | 784 (45.0) | |
| Amlodipine—n (%) | 7,152 (27.1) | 6,670 (27.1) | 482 (27.7) | |
| Lisinopril—n (%) | 7,126 (27.0) | 6,651 (27.0) | 475 (27.3) | |
| Potassium—mean (SD) mEq/l | 4.3 (0.5) | 4.3 (0.5) | 4.2 (0.5) | <0.001 |
| Glucose—mean (SD) mg/dl | 123.5 (59.6) | 123.3 (59.3) | 126.4 (63.6) | 0.041 |
| Estimated glomerular filtration rate—mean (SD) ml/min per 1.73 m2†,b | 77.8 (19.4) | 77.9 (19.3) | 76.2 (21.5) | 0.001 |
| Cholesterol—mean (SD) mg/dl | 216.3 (43.2) | 215.7 (43.0) | 223.7 (45.9) | <0.001 |
| LDL—mean (SD) mg/dl | 136.0 (36.9) | 135.6 (36.8) | 141.2 (38.6) | <0.001 |
| HDL—mean (SD) mg/dl | 46.8 (14.8) | 46.5 (14.7) | 50.8 (16.0) | <0.001 |
| Triglycerides mean (SD) mg/dl | 176.8 (136.6) | 177.6 (135.1) | 165.4 (155.6) | <0.001 |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; LVH, left ventricular hypertrophy.
aYears to death, mean (SD): Total = 5.0 (1.4); Cornell voltage LVH No = 5.0 (1.3), Yes = 4.9 (1.5); P < 0.001.
bSimplified 4-variable Modification of Diet in Renal Disease Study Formula.
Changes in ECG LVH
Number of individuals with consecutive ECGs at follow-up is shown in Table 2. Cornell voltage characteristics at baseline and years 2, 4, and 6 of the study are presented in Table 3. At baseline, 1,741 patients had ECG LVH. During the study, there was a progression of Cornell voltage in patients with Cornell voltage LVH and a small decrease in Cornell voltage in patients without Cornell voltage LVH (Table 3).
Table 2.
Number of individuals with consecutive electrocardiograms at follow-up (of 31,189 baseline electrocardiograms for 33,357 randomized subjects)
| N | % | |
|---|---|---|
| Baseline | 26,384 | 84.6 |
| Baseline and one other electrocardiogram (any year) | 20,676 | 66.3 |
| Baseline and year 2 | 18,751 | 60.1 |
| Baseline and years 2 and 4 | 14,227 | 45.6 |
| Baseline and years 2, 4, and 6 | 4,069 | 13.1 |
Table 3.
Cornell voltage characteristics
| Total | |||
|---|---|---|---|
| N | Mean (SD) | Range | |
| Baseline | 26,384 | 14.74 (6.53) | |
| Q1 | 7,165 | 7.51 (2.23) | 0–10 |
| Q2 | 6,838 | 12.51 (1.11) | 11–14 |
| Q3 | 5,819 | 16.39 (1.11) | 15–18 |
| Q4 | 6,562 | 23.48 (4.76) | 19–64 |
| Year 2 | 18,762 | 14.39 (6.43) | |
| Q1 | 5,370 | 7.37 (2.27) | 0–10 |
| Q2 | 4,935 | 12.49 (1.11) | 11–14 |
| Q3 | 4,107 | 16.39 (1.12) | 15–18 |
| Q4 | 4,350 | 23.30 (4.65) | 19–63 |
| Difference from baseline | 18,751 | −0.15 (4.84) | |
| Cornell voltage Yes | 1,080 | 4.67 (7.64) | |
| Cornell voltage No | 17,671 | −0.44 (4.44) | |
| Year 4 | 15,913 | 14.51 (6.63) | |
| Q1 | 4,646 | 7.38 (2.33) | 0–10 |
| Q2 | 4,019 | 12.51 (1.10) | 11–14 |
| Q3 | 3,366 | 16.40 (1.12) | 15–18 |
| Q4 | 3,882 | 23.47 (4.81) | 19–61 |
| Difference from baseline | 15,903 | −0.02 (5.28) | |
| Cornell voltage Yes | 1,023 | 6.05 (7.76) | |
| Cornell voltage No | 14,880 | −0.44 (4.79) | |
| Year 6 | 5,324 | 14.67 (6.92) | |
| Q1 | 1,560 | 7.31 (2.34) | 0–10 |
| Q2 | 1,308 | 12.53 (1.11) | 11–14 |
| Q3 | 1,132 | 16.44 (1.12) | 15–18 |
| Q4 | 1,324 | 25.93 (5.20) | 19–51 |
| Difference from baseline | 5,319 | −0.04 (5.78) | |
| Cornell voltage Yes | 375 | 7.03 (8.40) | |
| Cornell voltage No | 4,944 | −0.57 (5.16) | |
Clinical end points
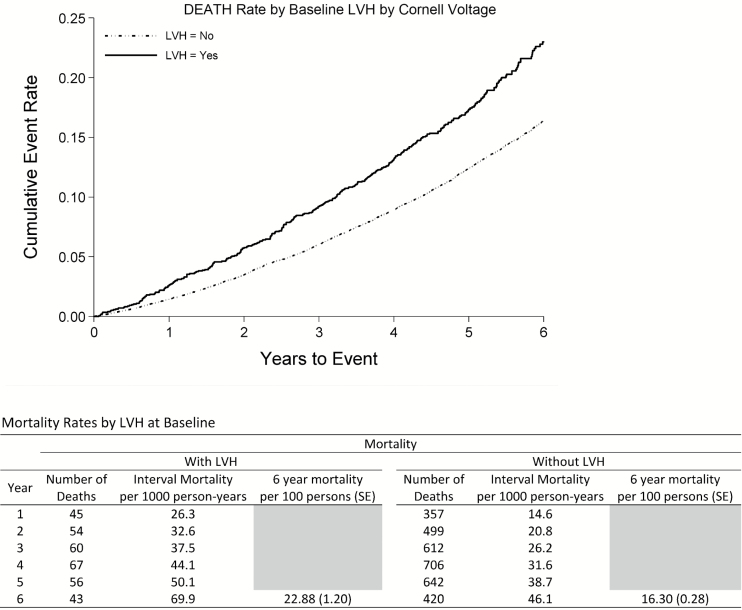
The primary outcome of all-cause mortality occurred in 3,561 patients during 6 years of follow-up; 69.9/1,000 patient-years in patients with LVH and 46.1/1,000 patient-years in patients without LVH. CHD occurred in 2,290 patients, NFMI in 1,084, stroke in 1,168, angina in 2,780, HF in 1,575, and peripheral arterial disease in 841 patients. No outcomes had significant study treatment and Cornell voltage interactions; the test for interaction between study treatment and Cornell voltage on the primary endpoint of mortality was amlodipine/chlorthalidone P = 0.189, lisinopril/chlorthalidone = 0.754.
All-cause mortality.
The results of multivariable Cox proportional hazards analyses considering baseline Cornell voltage are summarized in Table 4. In multivariable analysis adjusting for baseline characteristics, higher baseline Cornell voltage examined as a continuous variable and ECG LVH presence were strongly associated with higher risk of death (Figure 1). Furthermore, having a Cornell voltage in the 4th quartile or the 75th percentile were associated with higher risk of death compared to the 1st quartile and the lower than 75th percentile, respectively. Similar relationships to all-cause mortality were seen when baseline ECG LVH presence and Cornell voltage were replaced with on-treatment measures of Cornell voltage treated as time-dependent variables, with statistically significant associations with all-cause mortality (Table 5).
Table 4.
Multivariable Cox hazard ratios (95% confidence intervals) for baseline Cornell voltage left ventricular hypertrophy
| Deatha | P | Coronary heart diseaseb | P | Nonfatal myocardial infarctionc | P | Stroked | P | Anginae | P | Heart failuref | P | Peripheral arterial diseaseg | P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cornell voltage (per mm) | ||||||||||||||
| Total | 1.02 (1.01–1.02) | <0.001 | 1.02 (1.01–1.02) | <0.001 | 1.01 (1.00–1.02) | 0.042 | 1.03 (1.02–1.04) | <0.001 | 1.01 (1.00–1.02) | 0.005 | 1.03 (1.02–1.04) | <0.001 | 1.01 (1.00–1.02) | 0.038 |
| LVH by Cornell voltage | ||||||||||||||
| Total | 1.30 (1.15–1.47) | <0.001 | 1.29 (1.09–1.51) | 0.002 | 1.28 (1.00–1.64) | 0.049 | 1.71 (1.41–2.07) | <0.001 | 1.18 (1.01–1.39) | 0.041 | 1.92 (1.63–2.27) | <0.001 | 0.97 (0.71–1.33) | 0.859 |
| Cornell voltage quartile | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||
| Q2 | ||||||||||||||
| Total | 1.07 (0.97–1.18) | 0.176 | 1.04 (0.92–1.18) | 0.505 | 0.93 (0.78–1.11) | 0.426 | 1.09 (0.91–1.31) | 0.345 | 1.01 (0.91–1.13) | 0.858 | 0.97 (0.84–1.14) | 0.740 | 1.05 (0.86–1.29) | 0.618 |
| Q3 | ||||||||||||||
| Total | 1.10 (0.99–1.21) | 0.074 | 1.13 (1.00–1.29) | 0.049 | 1.10 (0.93–1.32) | 0.271 | 1.25 (1.04–1.50) | 0.018 | 1.04 (0.93–1.17) | 0.487 | 0.97 (0.83–1.14) | 0.736 | 1.16 (0.94–1.42) | 0.177 |
| Q4 | ||||||||||||||
| Total | 1.28 (1.16–1.41) | <0.001 | 1.28 (1.13–1.44) | <0.001 | 1.10 (0.93–1.32) | 0.268 | 1.60 (1.35–1.90) | <0.001 | 1.14 (1.03–1.28) | 0.016 | 1.58 (1.38–1.82) | <0.001 | 1.26 (1.03–1.55) | 0.025 |
| Cornell voltage 75th percentile | ||||||||||||||
| Total | 1.21 (1.13–1.31) | <0.001 | 1.21 (1.10–1.33) | <0.001 | 1.10 (0.95–1.27) | 0.213 | 1.45 (1.27–1.65) | <0.001 | 1.13 (1.03–1.23) | 0.010 | 1.61 (1.44–1.80) | <0.001 | 1.19 (1.00–1.40) | 0.046 |
Abbreviations: BMI, body mass index; CHD, coronary heart disease; DBP, diastolic blood pressure; GFR, glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LVH, left ventricular hypertrophy; SBP, systolic blood pressure.
Adjusted for baseline variables of:
aTotal = age, treatment group, race, ethnicity, history of diabetes, history of CHD, current or former smoker, heart rate, SBP, DBP, BMI, potassium, glucose, GFR, HDL, and triglycerides; females-age, treatment group, ethnicity, history of diabetes, history of CHD, current or former smoker, heart rate, SBP, BMI, glucose, GFR, HDL, and triglycerides; males-age, treatment group, race, history of diabetes, history of CHD, aspirin use, current or former smoker, heart rate, SBP, DBP, BMI, potassium, glucose, GFR, and triglycerides.
bTotal = age, treatment group, race, ethnicity, history of diabetes, history of CHD, aspirin use, current or former smoker, SBP, DBP, BMI, potassium, glucose, GFR, LDL, and HDL; females-age, treatment group, ethnicity, history of diabetes, history of CHD, current or former smoker, SBP, BMI, potassium, glucose, GFR, total cholesterol, and HDL; males-age, treatment group, race, history of diabetes, history of CHD, aspirin use, current or former smoker, SBP, DBP, glucose, GFR, and total cholesterol.
cTotal = age, treatment group, race, ethnicity, history of diabetes, history of CHD, aspirin use, current or former smoker, heart rate, BMI, glucose, GFR, HDL, and total cholesterol; females-age, treatment group, ethnicity, history of diabetes, history of CHD, aspirin use, current or former smoker, BMI, potassium, glucose, total cholesterol, LDL, and triglycerides; males-age, treatment group, race, ethnicity, history of diabetes, history of CHD, aspirin use, current or former smoker, GFR, LDL, and HDL.
dTotal = age, treatment group, race, ethnicity, history of diabetes, history of CHD, aspirin use, current or former smoker, SBP, BMI, glucose, GFR, LDL, and HDL; females-age, treatment group, ethnicity, history of diabetes, current or former smoker, SBP, glucose, GFR, and HDL; males - age, treatment group, race, ethnicity, history of diabetes, history of CHD, current or former smoker, heart rate, SBP, BMI, glucose, GFR, LDL, and HDL.
eTotal = age, treatment group, race, ethnicity, history of diabetes, history of CHD, aspirin use, heart rate, DBP, BMI, LDL, HDL, and triglycerides; females-age, treatment group, ethnicity, history of CHD, aspirin use, DBP, BMI, LDL, and triglycerides; males-age, treatment group, race, ethnicity, history of diabetes, history of CHD, aspirin use, heart rate, BMI, total cholesterol, HDL, and triglycerides.
fTotal = age, treatment group, ethnicity, history of diabetes, history of CHD, aspirin use, current or former smoker, heart rate, SBP, DBP, BMI, glucose, GFR, and HDL; females-age, treatment group, ethnicity, history of diabetes, history of CHD, aspirin use, current or former smoker, heart rate, SBP, DBP, BMI, glucose, and GFR; males-age, treatment group, ethnicity, history of diabetes, history of CHD, current or former smoker, heart rate, SBP, DBP, BMI, glucose, GFR, LDL, and HDL.
gTotal = age, treatment group, ethnicity, history of diabetes, history of CHD, aspirin use, current or former smoker, heart rate, SBP, DBP, BMI, potassium, glucose, GFR, total cholesterol, HDL, and triglycerides; females-age, treatment group, ethnicity, history of diabetes, history of CHD, aspirin use, current or former smoker, SBP, DBP, BMI, potassium, and HDL; males-age, treatment group, ethnicity, history of diabetes, history of CHD, aspirin use, current or former smoker, SBP, DBP, BMI, potassium, glucose, GFR, HDL, total cholesterol.
Figure 1.
Death rates by baseline electrocardiographic left ventricular hypertrophy. Abbreviation: LVH, left ventricular hypertrophy.
Table 5.
Multivariable Cox hazard ratios (95% confidence intervals) for time-varying Cornell voltage left ventricular hypertrophy
| Deatha | P | Coronary heart diseaseb | P | Nonfatal myocardial infarctionc | P | Stroked | P | Anginae | P | Heart failuref | P | Peripheral arterial diseaseg | P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cornell voltage (per mm) | ||||||||||||||
| Total | 1.02 (1.02–1.02) | <0.001 | 1.03 (1.02–1.03) | <0.001 | 1.02 (1.01–1.03) | <0.001 | 1.03 (1.03–1.04) | <0.001 | 1.01 (1.01–1.02) | <0.001 | 1.04 (1.03–1.05) | <0.001 | 1.01 (1.00–1.02) | 0.265 |
| LVH by Cornell voltage | ||||||||||||||
| Total | 1.41 (1.26–1.59) | <0.001 | 1.63 (1.41–1.88) | <0.001 | 1.64 (1.31–2.04) | <0.001 | 1.75 (1.45–2.12) | <0.001 | 1.26 (1.08–1.47) | 0.004 | 1.98 (1.69–2.32) | <0.001 | 0.99 (0.73–1.35) | 0.966 |
| Cornell voltage quartile (Q) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||
| Q2 | ||||||||||||||
| Total | 1.07 (0.97–1.18) | 0.156 | 1.07 (0.95–1.22) | 0.262 | 0.93 (0.77–1.11) | 0.424 | 1.10 (0.91–1.32) | 0.330 | 1.07 (0.96–1.19) | 0.244 | 1.11 (0.95–1.30) | 0.183 | 0.95 (0.77–1.17) | 0.629 |
| Q3 | ||||||||||||||
| Total | 1.05 (0.95–1.16) | 0.358 | 1.23 (1.08–1.39) | 0.002 | 1.29 (1.08–1.54) | 0.005 | 1.34 (1.12–1.62) | 0.002 | 1.14 (1.01–1.28) | 0.027 | 1.20 (1.03–1.41) | 0.023 | 1.18 (0.96–1.45) | 0.120 |
| Q4 | ||||||||||||||
| Total | 1.34 (1.22–1.47) | <0.001 | 1.50 (1.33–1.69) | <0.001 | 1.32 (1.11–1.57) | 0.002 | 1.76 (1.49–2.09) | <0.001 | 1.22 (1.10–1.37) | <0.001 | 1.83 (1.58–2.11) | <0.001 | 1.14 (0.93–1.40) | 0.194 |
| Cornell voltage 75th percentile | ||||||||||||||
| Total | 1.29 (1.20–1.39) | <0.001 | 1.37 (1.25–1.50) | <0.001 | 1.25 ( 1.08–1.44) | 0.002 | 1.55 (1.36–1.76) | <0.001 | 1.15 (1.05–1.26) | 0.003 | 1.66 (1.49–1.86) | <0.001 | 1.11 (0.93–1.31) | 0.240 |
Abbreviations: BMI, body mass index; CHD, coronary heart disease; DBP, diastolic blood pressure; GFR, glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LVH, left ventricular hypertrophy; SBP, systolic blood pressure.
Adjusted for baseline variables of:
aTotal = age, treatment group, race, ethnicity, history of diabetes, history of CHD, current or former smoker, heart rate, SBP, DBP, BMI, potassium, glucose, GFR, HDL, and triglycerides; females-age, treatment group, ethnicity, history of diabetes, history of CHD, current or former smoker, heart rate, SBP, BMI, glucose, GFR, HDL, and triglycerides; males-age, treatment group, race, history of diabetes, history of CHD, aspirin use, current or former smoker, heart rate, SBP, DBP, BMI, potassium, glucose, GFR, and triglycerides.
bTotal = age, treatment group, race, ethnicity, history of diabetes, history of CHD, aspirin use, current or former smoker, BMI, potassium, glucose, GFR, LDL, and HDL; females-age, treatment group, ethnicity, history of diabetes, history of CHD, current or former smoker, SBP, BMI, potassium, glucose, GFR, LDL, and HDL; males-age, treatment group, race, history of diabetes, history of CHD, aspirin use, current or former smoker, SBP, DBP, glucose, GFR, and total cholesterol.
cTotal = age, treatment group, race, ethnicity, history of diabetes, history of CHD, aspirin use, current or former smoker, heart rate, BMI, potassium, glucose, GFR, total cholesterol, and HDL; females-age, treatment group, ethnicity, history of diabetes, history of CHD, aspirin use, current or former smoker, potassium, glucose, total cholesterol, LDL, and triglycerides; males-age, treatment group, race, ethnicity, history of diabetes, history of CHD, aspirin use, current or former smoker, GFR, LDL, and HDL.
dTotal = age, treatment group, race, ethnicity, history of diabetes, history of CHD, aspirin use, current or former smoker, SBP, BMI, glucose, GFR, LDL, and HDL; females-age, treatment group, race, ethnicity, history of diabetes, current or former smoker, SBP, glucose, GFR, and HDL; males-age, treatment group, race, history of diabetes, history of CHD, aspirin use, current or former smoker, heart rate, SBP, BMI, GFR, LDL, and HDL.
eTotal = age, treatment group, race, ethnicity, history of diabetes, history of CHD, aspirin use, heart rate, DBP, BMI, LDL, HDL, and triglycerides; females-age, treatment group, ethnicity, history of CHD, aspirin use, DBP, BMI, LDL, and triglycerides; males-age, treatment group, race, ethnicity, history of diabetes, history of CHD, aspirin use, heart rate, BMI, total cholesterol, HDL, and triglycerides.
fTotal = age, treatment group, ethnicity, history of diabetes, history of CHD, aspirin use, current or former smoker, heart rate, SBP, DBP, BMI, glucose, GFR, and HDL; females-age, treatment group, ethnicity, history of diabetes, history of CHD, aspirin use, current or former smoker, heart rate, SBP, DBP, BMI, glucose, and GFR; males-age, treatment group, ethnicity, history of diabetes, history of CHD, current or former smoker, heart rate, SBP, DBP, BMI, glucose, GFR, LDL, and HDL.
gTotal = age, treatment group, ethnicity, history of diabetes, history of CHD, aspirin use, current or former smoker, heart rate, SBP, DBP, BMI, potassium, glucose, GFR, HDL, total cholesterol; females-age, treatment group, ethnicity, history of diabetes, history of CHD, aspirin use, current or former smoker, SBP, DBP, BMI, potassium, and HDL; males-age, treatment group, ethnicity, history of diabetes, history of CHD, aspirin use, current or former smoker, SBP, DBP, BMI, potassium, glucose, GFR, and total cholesterol.
Coronary heart disease.
In multivariable Cox analyses (Table 4), higher baseline Cornell voltage, baseline ECG LVH presence, Cornell voltage in the 4th quartile and the 75th percentile were associated with higher risk of CHD (Table 4). Similar relationships to CHD were seen when baseline ECG LVH presence and Cornell voltage were replaced with on-treatment measures of Cornell voltage treated as time-dependent variables; higher baseline Cornell voltage, baseline ECG LVH presence, Cornell voltage in the 4th quartile and the 75th percentile were associated with higher risk of CHD (Table 5).
Nonfatal Myocardial Infarction.
In multivariable (Table 4) Cox analyses, higher baseline Cornell voltage and baseline ECG LVH presence were associated with higher risk of NFMI. Similar relationships to NFMI were seen when baseline ECG LVH presence and Cornell voltage were replaced with on-treatment measures of Cornell voltage treated as time-dependent variables, with statistically significant associations with NFMI for higher baseline Cornell voltage, baseline ECG LVH presence, Cornell voltage in the 4th quartile or the 75th percentile in multivariable analyses (Table 5).
Stroke.
In multivariable (Table 4) Cox analysis, higher baseline Cornell voltage examined as a continuous variable and ECG LVH presence were strongly associated with higher risk of stroke. Furthermore, having a Cornell voltage in the 3rd or 4th quartile or the 75th percentile were associated with higher risk of stroke compared to the 1st quartile and the lower than 75th percentile, respectively. Similar relationships to stroke were seen when baseline ECG LVH presence and Cornell voltage were replaced with on-treatment measures of Cornell voltage treated as time-dependent variables, with statistically significant associations with stroke for all analyses (Table 5).
Angina.
In multivariable Cox analyses higher baseline Cornell voltage, baseline ECG LVH presence, as well as Cornell voltage in the 4th quartile or the 75th percentile, were associated with angina.
When baseline ECG LVH presence and Cornell voltage were replaced with on-treatment measures of Cornell voltage treated as time-dependent variables (Table 5), higher baseline Cornell voltage examined as a continuous variable and ECG LVH presence were associated with higher risk of angina. Furthermore, having a Cornell voltage in the 3rd or the 4th quartile or the 75th percentile were associated with higher risk of angina compared to the 1st quartile and the lower than 75th percentile, respectively (Table 5).
Heart failure.
In multivariable analysis (Table 4), higher baseline Cornell voltage examined as a continuous variable and ECG LVH presence were strongly associated with higher risk of HF. Furthermore, having a Cornell voltage in the 4th quartile or the 75th percentile were associated with higher risk of HF compared to the 1st quartile and the lower than 75th percentile, respectively. Similar relationships to HF were seen when baseline ECG LVH presence and Cornell voltage were replaced with on-treatment measures of Cornell voltage treated as time-dependent variables, with statistically significant associations with HF for all analyses (Table 5).
Peripheral arterial disease.
In multivariable analyses, higher baseline Cornell voltage, along with a Cornell voltage in the 4th quartile or the 75th percentile, were associated with higher risk of PAD (Table 4). However, there was no association of baseline Cornell voltage or ECG LVH presence to PAD when baseline ECG LVH presence and Cornell voltage were replaced with on-treatment measures of Cornell voltage treated as time-dependent variables, with no statistically significant associations with PAD for any analyses (Table 5).
DISCUSSION
This study provides the first evidence; from a large population of hypertensive patients not selected to have LVH at baseline, that both baseline and time-varying ECG LVH as well as higher baseline and time-varying Cornell voltage as a continuing variable are associated with higher rates of morbidity and mortality in a middle aged population of hypertensive patients with stage 1 or 2 hypertension plus an additional risk factor for CHD.
In an echocardiographic LIFE substudy,11 the presence of ECG LVH by Cornell product and/or Sokolow-Lyon voltage criteria identified patients with hypertension having a greater than 70% likelihood of having echocardiographic LVH as well as those not fulfilling the strict cutoff criteria for echocardiographic LVH but with high normal values of indexed LV mass. Moreover, regression of ECG LVH by Cornell product criteria was associated with greater reductions in LV mass and a higher likelihood of regression of echocardiographic LVH in the LIFE study,12 suggesting that changes in ECG LVH and echocardiographic LVH are linked.
Previous studies show that ECG LVH regression is associated with lower rates of CV events and mortality.2,5,13,14 The Framingham Heart Study showed in an observational study with ECG LVH by various criteria2 that a significant decline in Cornell voltage was associated with lower risk of CV disease, whereas a significant increase in Cornel voltage identified individuals at increased risk of CV disease. As a proof of concept, the LIFE study demonstrated that regression of ECG LVH improved prognosis, independent of improvements in BP during antihypertensive therapy.5,15–17
The LIFE study5 included hypertensive patients with ECG LVH as a surrogate of increased risk of CV events, whereas the present study included hypertensive patients with an additional risk factor for CHD including; previous (6 > months) myocardial infarction or stroke, LVH demonstrated by ECG or echocardiography, history of type 2 diabetes, current cigarette smoking, low high-density lipoprotein cholesterol or other documented other atherosclerotic CV disease. The use of Cornell voltage and Sokolow-Lyon voltage to select patients in the LIFE study5 limits the generalizability of these findings which may not be representative of other hypertensive populations. In contrast, only 8% of patients had ECG LVH at baseline in the present study. Despite the different population, the present study detected significant increased rates of CV events and all-cause mortality in patients with baseline ECG LVH and higher Cornell voltage independent of antihypertensive treatment and co-morbidity. Moreover, during antihypertensive treatment persistence or development of ECG LVH was significantly associated with increased rates of CV events and all-cause mortality independent of BP.
In a study of 126 never-treated subjects with essential hypertension, echocardiographic LV hypertrophy predicted complex ventricular arrhythmias independently of age and high nocturnal BP.18 In the LIFE study, lower in-treatment Cornell voltage-duration product was associated with a lower risk of SCD, death resulting from HF, CV death after 24 hours, and death resulting from other CV causes but not death resulting from non-CV death.17 Other studies have shown that ECG LVH can lead to scars in the myocardium and thereby create a substrate of electrophysiology disorders and fatal arrhythmias irreversible to antihypertensive treatment.19 This may in part explain not only why patients with LVH have increased rates of CV mortality and SCD, but also explain why some patients with LVH are less responsive to antihypertensive treatment.
Moderate LVH and some geometrical hypertrophic patterns might be an adaption to the moderate higher afterload and angiotensin concentration in hypertensive patients. However, severe LVH can result in some irreversible inappropriate geometrical hypertrophic pattern of LV which has shown to be associated with increased risk of CV events and all-cause mortality independent of antihypertensive treatment.20 Compared to the present study, the Heart Outcomes Prevention Evaluation (HOPE) trial had similar prevalence (8.2%) of baseline LVH,13 and showed in accordance with our findings, that persistence or development of LVH was associated with higher risk of death; and regression of LVH was associated with better outcome. A subanalysis of patients without baseline LVH in the HOPE trial showed that these patients had a lower risk of developing LVH if they received antihypertensive treatment with angiotensin-converting enzyme inhibitor compared to other subgroups in their study. These findings suggest that hypertensive patients with severe LVH at baseline may have too great a burden of myocardial scar or fibrosis and/or developed inappropriate geometrical hypertrophic pattern with little or no effect of antihypertensive treatment on myocardial regression and thereby no improvement of survival.
Limitations of the study
The present study was undertaken in patients selected for the combination of mild to moderate hypertension and an additional risk factor for CHD but without HF and thereby may not be directly applicable to patients with isolated hypertension or patients with more severe hypertension or HF. Also, the absence of information on QRS duration precluded calculation of Cornell voltage-duration product criteria that have been utilized in analyses from the LIFE study.21
As analyses was only adjusted to baseline and not time-varying BP, we cannot exclude that lower drop in BP caused by treatment resistance or low compliance could have contributed to the increased risk of mortality and morbidity associated with baseline LVH.
In conclusion, the present study extends findings from previous studies showing that higher Cornell voltage, as well as ECG LVH, is an independent risk factor of higher rates of CV events and all-cause mortality, not only in patients know with LVH but also in patients with only mild to moderate hypertension and low prevalence of LVH at baseline.
DISCLOSURE
Drs Okin and Devereux received grant support from Merck & Co. Dr Devereux consults for Merck & Co, Inc and General Electric Medical Systems. Dr Davis has worked as a consultant for Takeda, Merck, and Glaxo Smith Kline. The other authors declared no conflict of interest.
ACKNOWLEDGMENTS
This study was supported by a contract with the National Heart, Lung, and Blood Institute (US NIH Grant Number: P20RR011104). The ALLHAT investigators acknowledge contributions of study medications supplied by Pfizer, Inc (amlodipine and doxazosin), AstraZeneca (atenolol and lisinopril), and Bristol-Myers Squibb (pravastatin) and financial support provided by Pfizer, Inc.
REFERENCES
- 1. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990; 322:1561–1566. [DOI] [PubMed] [Google Scholar]
- 2. Levy D, Salomon M, D’Agostino RB, Belanger AJ, Kannel WB. Prognostic implications of baseline electrocardiographic features and their serial changes in subjects with left ventricular hypertrophy. Circulation 1994; 90:1786–1793. [DOI] [PubMed] [Google Scholar]
- 3. Dahlöf B, Devereux R, de Faire U, Fyhrquist F, Hedner T, Ibsen H, Julius S, Kjeldsen S, Kristianson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H. The Losartan Intervention For Endpoint reduction (LIFE) in Hypertension study: rationale, design, and methods. The LIFE Study Group. Am J Hypertens 1997; 10:705–713. [PubMed] [Google Scholar]
- 4. Dahlöf B, Devereux RB, Julius S, Kjeldsen SE, Beevers G, de Faire U, Fyhrquist F, Hedner T, Ibsen H, Kristianson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H. Characteristics of 9194 patients with left ventricular hypertrophy: the LIFE study. Losartan Intervention For Endpoint Reduction in Hypertension. Hypertension 1998; 32:989–997. [DOI] [PubMed] [Google Scholar]
- 5. Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Nieminen MS, Snapinn S, Harris KE, Aurup P, Edelman JM, Wedel H, Lindholm LH, Dahlof B. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA 2004; 292:2343–2349. [DOI] [PubMed] [Google Scholar]
- 6. ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002; 288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 7. Davis BR, Cutler JA, Gordon DJ, Furberg CD, Wright JT Jr, Cushman WC, Grimm RH, LaRosa J, Whelton PK, Perry HM, Alderman MH, Ford CE, Oparil S, Francis C, Proschan M, Pressel S, Black HR, Hawkins CM. Rationale and design for the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). ALLHAT Research Group. Am J Hypertens 1996; 9(4 pt 1):342–360. [DOI] [PubMed] [Google Scholar]
- 8. Ernst ME, Davis BR, Soliman EZ, Prineas RJ, Okin PM, Ghosh A, Cushman WC, Einhorn PT, Oparil S, Grimm RH Jr. Electrocardiographic measures of left ventricular hypertrophy in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. J Am Soc Hypertens 2016; 10:930–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med 1991; 114:345–352. [DOI] [PubMed] [Google Scholar]
- 10. ALLHAT Collaborative Research Group. Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA 2000; 283:1967–1975. [PubMed] [Google Scholar]
- 11. Devereux RB, Bella J, Boman K, Gerdts E, Nieminen MS, Rokkedal J, Papademetriou V, Wachtell K, Wright J, Paranicas M, Okin PM, Roman MJ, Smith G, Dahlöf B. Echocardiographic left ventricular geometry in hypertensive patients with electrocardiographic left ventricular hypertrophy: the LIFE Study. Blood Press 2001; 10:74–82. [DOI] [PubMed] [Google Scholar]
- 12. Okin PM, Devereux RB, Liu JE, Oikarinen L, Jern S, Kjeldsen SE, Julius S, Wachtell K, Nieminen MS, Dahlöf B. Regression of electrocardiographic left ventricular hypertrophy predicts regression of echocardiographic left ventricular mass: the LIFE study. J Hum Hypertens 2004; 18:403–409. [DOI] [PubMed] [Google Scholar]
- 13. Mathew J, Sleight P, Lonn E, Johnstone D, Pogue J, Yi Q, Bosch J, Sussex B, Probstfield J, Yusuf S. Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation 2001; 104:1615–1621. [DOI] [PubMed] [Google Scholar]
- 14. Verdecchia P, Reboldi G, Angeli F, Avanzini F, De Simone G, Pede S, Perticone F, Schillaci G, Vanuzzo D, Maggioni AP. Prognostic value of serial electrocardiographic voltage and repolarization changes in essential hypertension: the HEART Survey study. Am J Hypertens 2007; 20:997–1004. [DOI] [PubMed] [Google Scholar]
- 15. Okin PM, Devereux RB, Harris KE, Jern S, Kjeldsen SE, Julius S, Edelman JM, Dahlof B. Regression of electrocardiographic left ventricular hypertrophy is associated with less hospitalization for heart failure in hypertensive patients. Ann Intern Med 2007; 147:311–319. [DOI] [PubMed] [Google Scholar]
- 16. Okin PM, Wachtell K, Devereux RB, Harris KE, Jern S, Kjeldsen SE, Julius S, Lindholm LH, Nieminen MS, Edelman JM, Hille DA, Dahlof B. Regression of electrocardiographic left ventricular hypertrophy and decreased incidence of new-onset atrial fibrillation in patients with hypertension. JAMA 2006; 296:1242–1248. [DOI] [PubMed] [Google Scholar]
- 17. Wachtell K, Okin PM, Olsen MH, Dahlöf B, Devereux RB, Ibsen H, Kjeldsen SE, Lindholm LH, Nieminen MS, Thygesen K. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive therapy and reduction in sudden cardiac death: the LIFE Study. Circulation 2007; 116:700–705. [DOI] [PubMed] [Google Scholar]
- 18. Schillaci G, Verdecchia P, Borgioni C, Ciucci A, Zampi I, Battistelli M, Gattobigio R, Sacchi N, Porcellati C. Association between persistent pressure overload and ventricular arrhythmias in essential hypertension. Hypertension 1996; 28:284–289. [DOI] [PubMed] [Google Scholar]
- 19. Koyanagi S, Eastham C, Marcus ML. Effects of chronic hypertension and left ventricular hypertrophy on the incidence of sudden cardiac death after coronary artery occlusion in conscious dogs. Circulation 1982; 65:1192–1197. [DOI] [PubMed] [Google Scholar]
- 20. Bang CN, Gerdts E, Aurigemma GP, Boman K, De Simone G, Dahlof B, Kober L, Wachtell K, Devereux RB. Four group classification of left ventricular hypertrophy based on ventricular concentricity and dilatation identifies a low-risk subset of eccentric hypertrophy in hypertensive patients. Circ Cardiovasc Imaging 2014; 7:422–429. [DOI] [PubMed] [Google Scholar]
- 21. Bang CN, Devereux RB, Okin PM. Regression of electrocardiographic left ventricular hypertrophy or strain is associated with lower incidence of cardiovascular morbidity and mortality in hypertensive patients independent of blood pressure reduction—a LIFE review. J Electrocardiol 2014; 47:630–635. [DOI] [PubMed] [Google Scholar]