Abstract
BACKGROUND
Indirect evidence suggests that angiotensin 1-7 (Ang1-7) may counterbalance prohypertensive actions of angiotensin II (AngII), via activation of vascular and/or renal tubular receptors to cause vasodilation and natriuresis/diuresis. We examined if Ang1-7 would attenuate the development of hypertension, renal vasoconstriction, and decreased natriuresis in AngII-infused rats and evaluated the mechanisms involved.
METHODS
AngII, alone or with Ang1-7, was infused to conscious Sprague-Dawley rats for 13 days and systolic blood pressure (SBP) and renal excretion were repeatedly determined. In anesthetized rats, acute actions of Ang1-7 and effects of blockade of angiotensin AT1 or Mas receptors (candesartan or A-779) were studied.
RESULTS
Chronic AngII infusion increased SBP from 143 ± 4 to 195 ± 6 mm Hg. With Ang1-7 co-infused, SBP increased from 133 ± 5 to 161 ± 5 mm Hg (increase reduced, P < 0.002); concurrent increases in urine flow (V) and sodium excretion (UNaV) were greater. In anesthetized normotensive or AngII-induced hypertensive rats, Ang1-7 infusion transiently increased mean arterial pressure (MABP), transiently decreased renal blood flow (RBF), and caused increases in UNaV and V. In normotensive rats, candesartan prevented the Ang1-7-induced increases in MABP and UNaV and the decrease in RBF. In anesthetized normotensive, rats intravenous A-779 increased MABP (114 ± 5 to 120 ± 5 mm Hg, P < 0.03) and urine flow. Surprisingly, these changes were not observed with A-779 applied during background Ang1-7 infusion.
CONCLUSIONS
The results suggest that in AngII-dependent hypertension, Ang1-7 deficit contributes to sodium and fluid retention and thereby to BP elevation; a correction by Ang1-7 infusion seems mediated by AT1 and not Mas receptors.
Keywords: angiotensin 1-7, AngII-dependent hypertension, blood pressure, hypertension, renal blood flow, sodium excretion
The renin–angiotensin system comprises a complex enzymatic cascade producing several peptides having diverse actions that influence cardiovascular and renal control of blood pressure (BP) and the volume and composition of the extracellular fluid.1,2 Angiotensin-converting enzyme 2 (ACE2), a more recently recognized member of the ACE family, can cleave the decapeptide AngI to generate an inactive Ang1-9 peptide which can be converted by ACE or other peptidases to Ang1-7. ACE2 has a greater affinity for angiotensin II (AngII) than for AngI and directly metabolizes AngII to generate Ang1-7. Accumulating evidence suggests that Ang1-7 may counterbalance both vascular and tubular actions of AngII,3 it activates the Mas receptor which can elicit vasodilator, natriuretic, and diuretic effects.4 There is evidence that alamandine, a heptapeptide with the structure similar to Ang1-7, an agonist of MrgD, a G-protein coupled receptor, displays actions similar to Ang1-7.5–7
The data on the role of Ang1-7 in regulating renal hemodynamics are contradictory. Ang1-7 has been reported to induce vasodilation or vasoconstriction, and also to antagonize pressor responses to AngII.8–11 Assessment of Ang1-7 effects on rat renal vasculature showed that while Ang1-7 per se had no vasoactive actions, it prevented AngII-induced constriction of isolated renal arteries in vitro.12 Reports on Ang1-7 effects on renal glomerular arterioles are conflicting. Ren et al.13 showed that Ang1-7 caused afferent arteriolar dilatation via stimulation of NO, whereas in other studies Ang1-7 did not affect afferent or efferent arterioles preconstricted by AngII in anesthetized rats, nor did it alter total renal blood flow (RBF) initially reduced by AngII in conscious rats.12 Modification of body electrolyte balance and cardiovascular function appears to be the most important action of Ang1-714: it exerts natriuretic actions countering the well-known AngII-dependent antinatriuresis, an action which may be direct or mediated by increased release of atrial natriuretic peptide.15–17
On the whole, Ang1-7 appears to be a counterbalancing factor to limit the effects of AngII. There seems to be a close interaction between the 2 renin–angiotensin system enzyme/peptide axes: AngII was shown to downregulate ACE2 expression in astrocytes, the heart, and kidneys,18,19 which suggests that during high-AngII states the generation of Ang1-7 might be reduced. Alternatively, ACE2 expression might be suppressed by the increased arterial pressure, as observed in many animal models of hypertension. In AngII-induced hypertension, increased circulating AngII levels lead to progressive augmentation of intrarenal AngII content and reduced levels of Ang1-7 associated with decreased expression of ACE2, thus limiting the Ang1-7 available to counterbalance the effects of increased AngII.19
In a previous study, increases in collecting duct renin activity coupled with decreases of Ang1-7 content and ACE2 activity, and increases in AngII and ACE, were observed both in Goldblatt 2-kidney, 1-clip rats and in AngII-infused rats.19 These changes may contribute to the progressively increasing BP during AngII-induced hypertension. However, the consequences of reduced Ang1-7 levels in AngII-dependent hypertension have not been established. In the present study, we aimed to determine if either acute or chronic increases in Ang1-7 levels or activity affect BP and renal hemodynamics and sodium excretion (UNaV) in AngII-induced hypertension. Given that the expected natriuretic effects of Ang1-7 could in some part be secondary to changes in intrarenal circulation, in addition to total RBF the perfusion of the renal medulla was also measured.
We hypothesized that the suppression of renal ACE2 in AngII-infused rats might lead to decreased intrarenal synthesis of Ang1-7. If so, maintaining Ang1-7 levels by co-infusing it, would attenuate the development of hypertension, renal vasoconstriction, and reduction of UNaV. Acute Ang1-7 infusions tested the direct pressor, constrictor, and UNaV responses. To explore whether Mas or other Ang receptors are involved in this process, further acute experiments examined the effects of Ang1-7 infusion as related to AT1 or Mas receptor blockade.
MATERIALS AND METHODS
All the protocols were approved by the Institutional Animal Care and Use Committee of Tulane University Health Sciences Center, New Orleans, Louisiana, United States, and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male Sprague-Dawley rats, normotensive or with angiotensin-induced hypertension were used. They were fed a standard diet (TD 90229, Harlan-Tekland), and had free access to water.
Chronic studies
The effects of chronic infusion of AngII alone or together with angiotensin 1-7 (Ang1-7) on systolic BP (SBP) and renal excretion were examined. Rats weighing 195 ± 2 g were anesthetized with isoflurane and osmotic minipumps (model 2002; Alzet Corp., Palo Alto, CA) were implanted subcutaneously at the nape, as previously described.18 The rats received AngII (Calbiochem-Novabiochem, La Jolla, CA), at 400 ng/kg/min, alone (n = 8) or together with Ang1-7 (Phoenix Pharmaceuticals, CA) at 400 ng/kg/min for 13 days (n = 6). On the day before implantation (day 0) and days 3, 7, and 11 of infusion, SBP was measured in conscious rats, using tail-cuff plethysmography (model 52-0338; Harvard Apparatus, Holliston, MS). Over at least 7 preceding days the animals were being accustomed to the restraint tubes and the measurement procedure itself. Twenty-four hour urine collections for determination of urine flow (V) and UNaV were made.
Acute studies with anesthetized rats
Experiments were conducted in normotensive Sprague-Dawley rats and rats with hypertension induced by 13 days’ infusion of AngII as described above.
On the day of acute experiment, rats were anesthetized with Inactin (thiobutabarbital sodium, Sigma, Saint Louis, MO) at 100 mg/kg i.p. a heated surgery table ensured constant temperature (37 °C). A tracheal cannula ensured free airways. For fluid infusions and mean arterial BP (MABP) measurement, the femoral vein and artery were used, respectively. The left kidney was exposed from a subcostal flank incision and immobilized in a plastic cup. The ureter was cannulated for timed urine collection. Total RBF was measured using a cuff probe on the renal artery, connected to a Transonic flow meter (type T106, Transonic System, Ithaca, NY).
Regional renal perfusion was recorded using laser-Doppler Periflux 4001 system (Perimed AB, Jarfalla, Sweden). The cortical blood flow (CBF) was measured using a PF 408 probe placed on the kidney surface, and the medullary blood flow (MBF) with a needle probe (PF 411) inserted 4–5 mm deep to reach the outer–inner medullary border.
To compensate for fluid losses during the surgery, 6% bovine albumin in isotonic saline solution was infused i.v. at 1.2 ml/h. After placement of the renal artery and laser-Doppler probes, infusate albumin concentration was reduced to 2%. The rats were then allowed to stabilize during 1 hour as described previously.20
Protocols
Acute Ang1-7 infusions.
In normotensive Sprague-Dawley rats, (n = 6) or angiotensin-induced hypertensive rats (n = 8), two 30-minute control measurements and urine collections were made, followed by 6 periods of intravenous Ang1-7 infusion at 110 fmol/min. In preliminary experiments, the dose-dependency of Ang1-7 effects on MABP and renal excretion was established in normotensive rats. The ultimately selected dose of 110 fmol/min increased MABP from 114 ± 3 to 123 ± 5 mm Hg, and UNaV 0.06 ± 0.03 to 0.96 ± 0.76 µmol/min, respectively (P < 0.05 for both), and this dose was further used.
A saline time-control group was also studied in normotensive rats (n = 4). In each group MABP, RBF, CBF, and MBF were recorded continuously, and urine flow (V) and UNaV were determined for each urine collection period.
Mas receptor blockade.
In normotensive rats (n = 6), after two 30-minute control periods, the Mas receptor blocker (A-779, Bachem AG, Bubendorf, Switzerland) was infused intravenously at 10 ng/min for 30 minutes. Thereafter, MABP measurements and urine collections were conducted over 5 additional 30-minute periods. In another group (n = 6), Ang1-7 was first infused at the rate as stated previously and A-779 infusion was superimposed 60 minute after the start of Ang1-7.
Effects of Ang1-7 after AT1 receptor blockade.
In normotensive rats (n = 8), an angiotensin AT1 receptor blocker (candesartan, courtesy of Dr. P. Morsing, Astra Hassle, Gothenburg, Sweden) was given as a bolus at 0.1 mg/kg. This dose was previously established to completely block AT1 receptors.21,22 Subsequently, Ang1-7 infusion was initiated with the timing and dosage as described above.
Analytical procedures
Urine volumes were determined gravimetrically and sodium concentrations by flame photometry (Flame Photometer IL 973; Instrumentation Laboratory, Lexington, MA).
Statistical analysis
The significance of changes within 1 group over time was evaluated by repeated measures analysis of variance, followed by Student’s test for dependent variables. Differences between groups were first analyzed by 1-way analysis of variance followed by modified Student’s t-test for independent variables. The SEM was used as the measure of data dispersion. Statistical significance is defined at a value of P <0.05.
RESULTS
Chronic AngII and Ang1-7 infusions
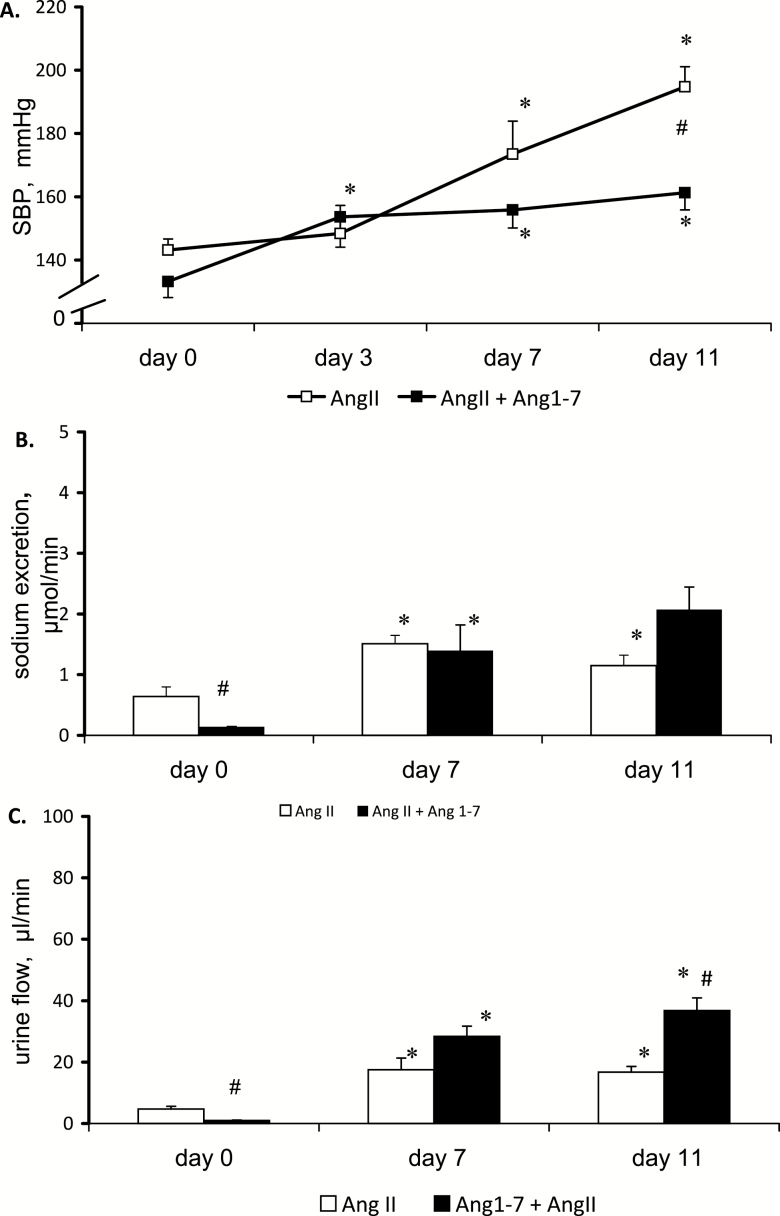
During chronic infusion of AngII alone, no change in SBP was visible until day 7, thereafter it increased progressively (Figure 1a). Co-infusion with Ang1-7 attenuated the magnitude of the BP changes: following an early increase (P < 0.02) SBP stabilized at a level significantly above control (days 7 and 11). By day 11, SBP in rats infused with both peptides was significantly lower than in rats infused with AngII alone (161 ± 5 vs. 195 ± 6 mm Hg, P < 0.05). Thus, Ang1-7 significantly attenuated the pressure increase elicited by AngII.
Figure 1.
Effects of chronic subcutaneous infusion of angiotensin II (400 ng/kg/min), alone or together with angiotensin 1-7 (400 ng/kg/min) on (a) systolic blood pressure (SBP), (b) sodium excretion, and (c) urine flow. Means ± SEM. *Significantly different from control (day 0), #Significantly different from AngII alone for the same time point; n = 8 for AngII and n = 6 for AngII + Ang1-7 group.
The baseline UNaV and V (day 0) differed markedly between the 2 groups (Figure 1b and c): the result of high variability common in outbred Sprague-Dawley rats. Nevertheless, it was clear that in the group receiving AngII alone the renal excretion significantly increased between days 0 and 7 but remained stable thereafter; in contrast, rats infused with both peptides showed a progressive increase in renal excretion.
Acute Ang1-7 infusions
Acute effects of Ang1-7 in normotensive and hypertensive rats
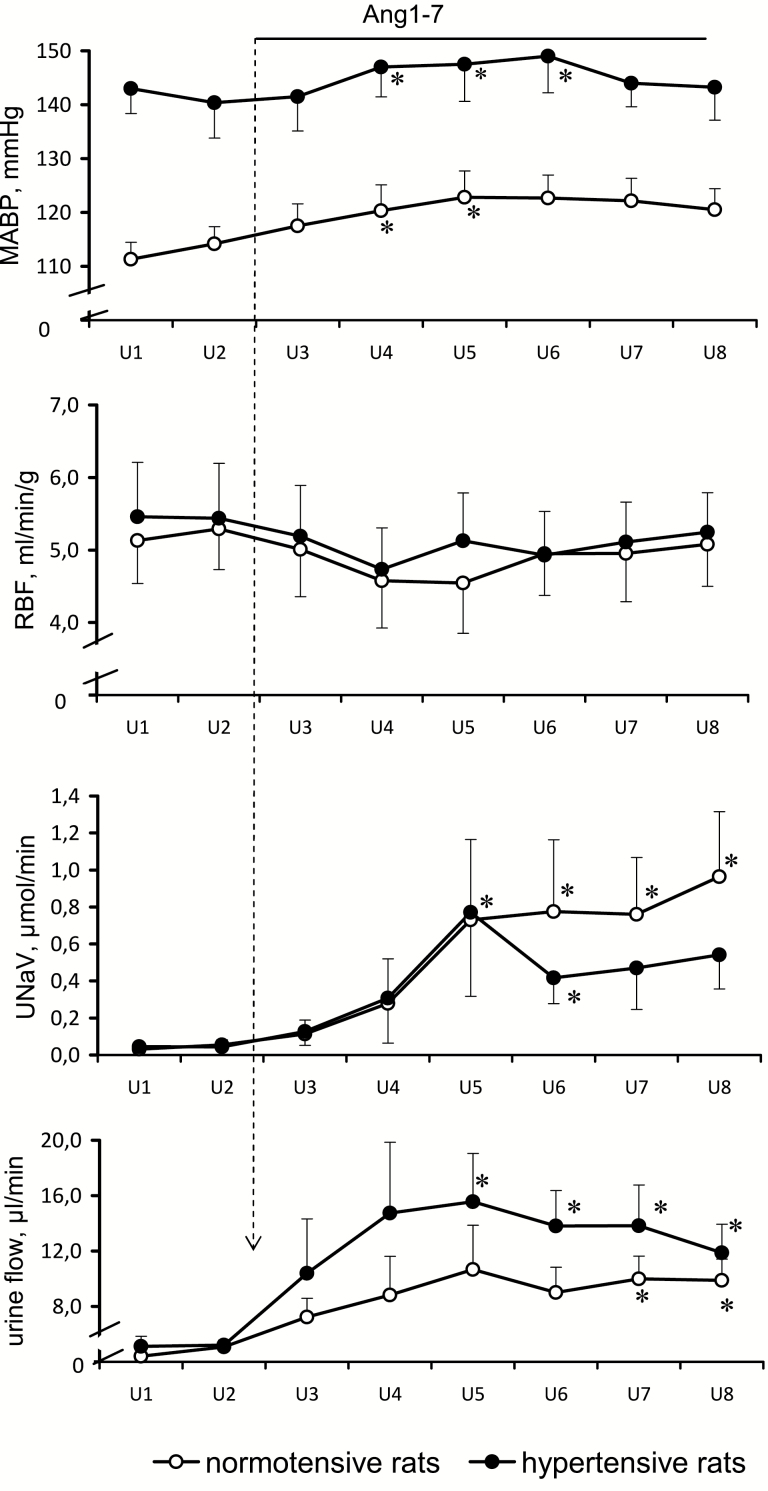
The responses to intravenous Ang1-7 of anesthetized rats, normotensive, or rendered hypertensive by chronic AngII infusion, are shown in Figure 2. While the baseline preinfusion MABP was distinctly higher in hypertensive rats, RBF values were not significantly different in the 2 groups; as a consequence, the renal vascular resistance calculated as MABP-to-RBF ratio was about 25% higher in hypertensive rats.
Figure 2.
Effects of intravenous Ang1-7 infusion (99 pg/min i.e., 110 fmol/min) on mean arterial blood pressure (MABP), total renal blood flow (RBF), sodium excretion (UNaV), and urine flow in normotensive (n = 6) and in AngII-infused hypertensive rats (n = 8). Means ± SEM. *Significantly different from the u2 control period, P < 0.05.
During Ang1-7 infusion, both normotensive and hypertensive rats showed quite similar increases in MABP, by 8 and 6%, respectively (P < 0.05 for both); however, the increases were only transient (notably, in our additional studies with conscious normotensive rats Ang1-7 infusion did not cause significant changes in arterial pressure). RBF transiently decreased by about 15% in either group (P < 0.05 for both). There was also a transient 23% decrease in MBF in the normotensive group (P < 0.05); in the hypertensive group, the change in MBF from 182 ± 34 to 163 ± 28 PU was not significant. Simultaneously, CBF remained stable in both groups (611 ± 38 vs. 584 ± 31 and 543 ± 58 vs. 537 ± 46 PU in normotensive and hypertensive rats, respectively).
Baseline renal excretion values were comparable in normotensive and hypertensive rats. During Ang1-7 infusion UNaV increased progressively during 3 periods (90 minutes), which in hypertensive rats was followed by a significant decrease (P < 0.05). In the hypertensive group, the increase in V was significant beginning from 150 minutes after the start of infusion, whereas in the normotensive group the increase was less steep and was not significant until period 7 (after 210 min of infusion) (Figure 2).
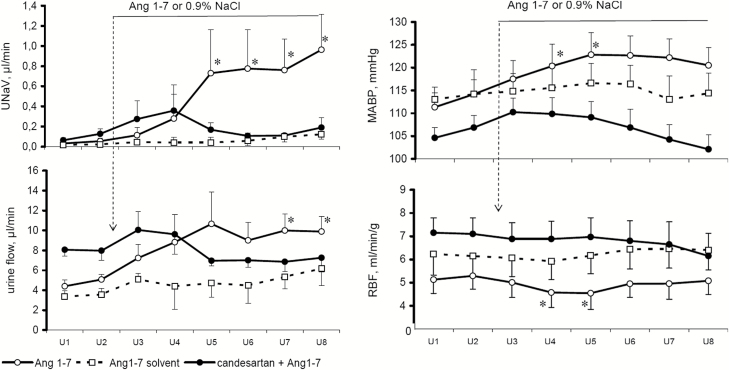
Effects of Mas receptor blockade.
In normotensive rats, an intravenous infusion of A-779, a Mas receptor blocker modestly increased MABP (P < 0.003) (Figure 3). In a parallel experiment, an initial infusion of Ang1-7 significantly increased MABP, from 105 ± 7 to 114 ± 7 mm Hg (P < 0.01). Thereafter, a superimposed infusion of A-779 did not significantly alter MABP. Thus, surprisingly, under baseline conditions blockade of Mas receptor increased MABP and so did the presumable stimulation of this receptor with Ang1-7. However, under conditions of such stimulation, Mas receptor blockade was without effect.
Figure 3.
Effects of Mas receptor blockade by intravenous infusion of A-779 (10 ng/min), applied under baseline conditions (left side) and during background i.v. infusion of Angiotensin 1-7 (right hand side), on mean arterial blood pressure (MABP), urine flow and sodium excretion (UNaV); n = 6 in either group. Means ± SEM. *Significantly different from control, P < 0.05.
Without background infusion of Ang1-7, Mas receptor blockade did not increase RBF significantly and did not change MBF (124 ± 53 compared to 122 ± 49 PU). When applied during background Ang1-7 infusion, A-779 significantly increased RBF (+8%, P < 0.01), whereas MBF remained unchanged (120 ± 23 compared to 124 ± 22 PU; RBF and MBF data not shown in the figure).
Inhibition of Mas receptor under control conditions slightly increased V (P < 0.04) (Figure 3). Ang1-7 infusion modestly but significantly increased V; when applied during this infusion, A-779 increased it further. The corresponding parallel changes in UNaV were not significant, because of a large scatter of the values.
Effects of Ang1-7 under conditions of AT1 receptor blockade.
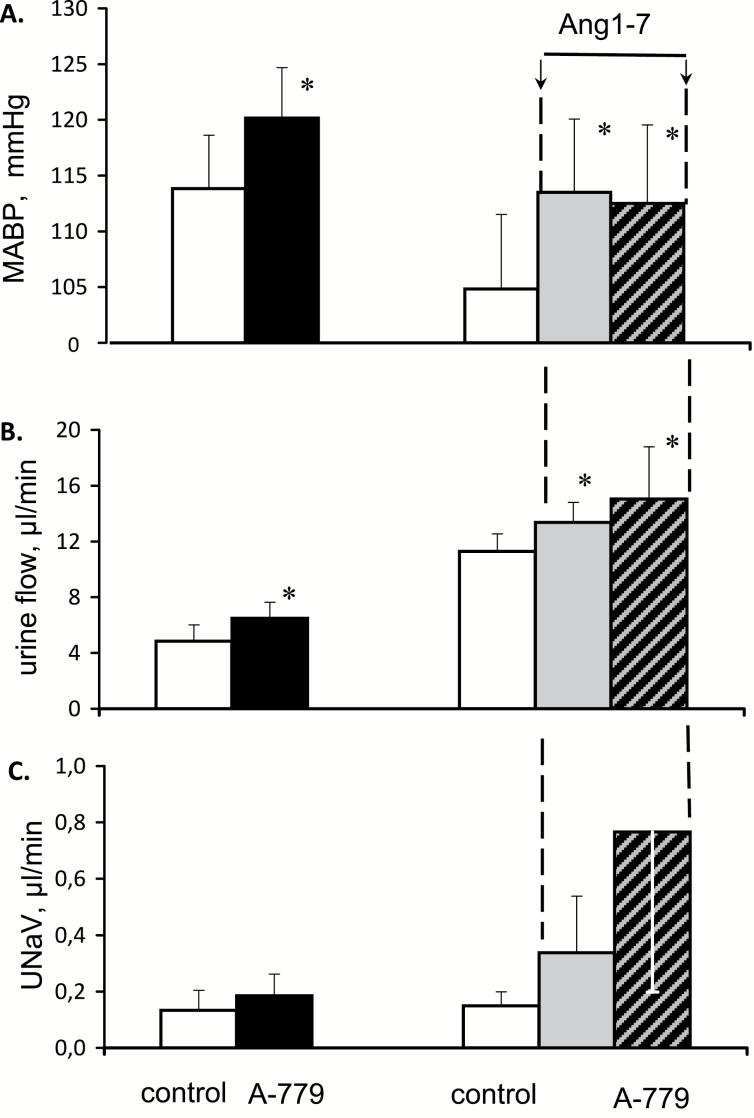
Effects of Ang1-7 on MABP, RBF, UNaV, and V in normotensive rats untreated or pretreated with candesartan are shown in Figure 4. After blockade of angiotensin AT1 receptor with candesartan, baseline MABP of normotensive rats was lower than in the untreated group. Ang1-7 caused a progressive increase in MABP which was only transiently significant and tended to subside. AT1 receptor blockade largely prevented this increase which was followed by a clear decrease.
Figure 4.
Effects of Ang1-7 infusion, 99 pg/min i.v., on mean arterial blood pressure (MABP), total renal blood flow (RBF), sodium excretion (UNaV), and urine flow in normotensive Sprague-Dawley rats, untreated (n = 8) or pretreated with candesartan, 0.1 g/kg i.v. (n = 6). Effects of Ang1-7 saline solvent infusion (n = 4) are also shown. Means ± SEM. *Significantly different from the u2 control, P < 0.05.
In the candesartan treated group, baseline RBF was distinctly higher than in the noninhibited rats. Ang1-7 infusion did not substantially alter RBF profile in untreated or candesartan-pretreated rats. However, Ang1-7 infusion decreased CBF significantly only in the latter group from 592 ± 53 to 503 ± 57 PU (P < 0.01). This was accompanied by a decrease in MBF from 198 ± 14 to 177 ± 17 PU (P < 0.002).
Without candesartan pretreatment, Ang1-7 infusion significantly increased UNaV and, to a lesser degree, V. Candesartan pretreatment clearly prevented these increases (Figure 4).
DISCUSSION
Ang1-7 modulates the AngII-induced increase in BP
Chronic infusion of AngII, at doses that are acutely subpressor, elicited a progressive increase in BP which became significant by day 7. In Goldblatt 2-kidney, 1-clip rats such an increase was previously shown to be associated with increasing plasma AngII but also with decreasing ACE2 activity and Ang1-7 levels.19 The ACE2 deficiency would both decrease Ang1-7 levels and further increase AngII levels, a consequence of reduced degradation of AngII by ACE2. Another consequence of ACE2 deficiency would be reduced generation of alamandine; this would reduce its vasodilator effect mediated by MrgD receptor.5 To determine if such changes and the consequent increase in BP could be altered by supplementation of Ang1-7, we co-infused this peptide with AngII. Indeed, we found that supplying the presumably deficient Ang1-7 distinctly attenuated the AngII-mediated increase in BP, particularly during the second week of AngII infusion. Most probably, the known vascular effects of Ang 1-7, such as vasodilation and antagonization of the pressor responses to AngII,8–11,13 were responsible for the attenuation of BP increase in our experiments. Notably, there were no simultaneous persistent and consistent changes in RBF or perfusion of the renal medulla.
Of interest was the time pattern of association of the BP increases with AngII or/and Ang1-7 infusions with urine flow and UNaV. With AngII alone, an increase in excretion did not extend beyond day 7 of infusion; thereafter, BP continued to increase while the excretion was stable. The first-phase increase in urine flow and UNaV might reflect a prevalence of the pressure-natriuresis effect over the well-known direct sodium retaining action of AngII.23 In the second phase, the increasing concentration of the peptide in renal tubules may have further enhanced reabsorption and thereby limited the pressure effects on tubular sodium transport, resulting in no further increase in renal UNaV.
Importantly, addition of chronic Ang1-7 infusion to AngII shifted the overall balance toward greater UNaV. This finding agrees well with the diuretic and natriuretic action mediated by activation of Mas receptors, as reported from a rat study.24,25 Inhibition of the increase in BP concurrent with an increase in UNaV suggests that in rats receiving Ang1-7 together with AngII, the latter increase was partially responsible for the attenuation of the hypertensive response.
Acute responses to Ang1-7 and A-779
To examine in more detail the role of Ang1-7 dependent diuresis and natriuresis, in addition to a direct vascular effect, as a possible mechanism of BP reduction, we infused Ang1-7 to anesthetized Sprague-Dawley rats that were either normotensive or with AngII-dependent hypertension (Figures 2 and 4). We found the expected increase in UNaV and urine flow; there was also an intriguing transitory mild increase in MABP concurrent with some decrease in RBF; these responses were seen in both normotensive and hypertensive groups.
The transitory Ang1-7-induced increase in BP appears to contradict the evidence on the peptide’s vasodilator activity reported from in vitro studies.24,25 However, Handa et al.23 reported that Ang1-7 exerts weak vasoconstrictor effects mediated by the AT1 receptor and at the same time directly inhibits tubular Na reabsorption. It should also be considered that Ang1-7 may act as a biased agonist of AT1 receptor and thereby antagonize the usual action of AngII.26,27 The data on the effects of Ang1-7 in experimental models of hypertension and/or renal disease are relatively scanty and often contradictory. Ang1-7 had no effect on renal function or BP in Goldblatt hypertension,28 and in Sprague-Dawley rats after subtotal nephrectomy Ang1-7 infusion induced an increase of MABP.29
One reason why the effects of Ang1-7 on BP may be difficult to predict is that beside activation of Mas receptor the peptide binds also to AT1 and other angiotensin receptors.27,30 In disagreement with earlier indirect evidence, a most recent study showed that AT2 is not a functional receptor for Ang1-7, on the other hand, after its conversion to alamandine, the peptide binds to a newly characterized G-protein–coupled MrgD receptor.5,7 Indeed, an interaction between different angiotensin receptor species was proposed.1 Second, considering the poor stability of Ang1-7 in plasma, one should be aware that effects of its intravenous infusion could have not only on Ang1-7 per se but represent a balance of action of this peptide and its metabolites: a recently discovered vasodilator alamandine.5 If the contribution of alamandine was dominating, the ineffectiveness of Mas receptor blockade would be understandable.6 Still other peptides might influence the final vasomotor effect: Ang3-7 was found to elevate BP when microinjected into the rostral ventrolateral medulla.31
Mas receptors do not mediate post-Ang1-7 BP elevation and increased renal excretion
Since acute effects of Ang1-7 were similar in normotensive and hypertensive rats (Figure 2), we examined the role of Mas receptor by its selective inhibition in normotensive rats only. As expected, intravenous A-779 increased MABP, but when superimposed on Ang1-7 infusion, the effect on BP was abolished. Surprisingly, inhibition of Mas receptor per se increased urine flow but, as was the case with MABP, the inhibitor given during Ang1-7 infusion did not further increase urine flow. Therefore, it is unlikely that Ang1-7 increased water and UNaV by activation of Mas receptors. Admittedly, there is also evidence that Ang1-7, via Mas receptor activation, can exert an inhibitory action on AT1 receptor-mediated cell signaling thereby blunting the action of AngII.32
Regarding the lack of A-779 effect on BP when the blockade was superimposed on Ang1-7 infusion it can also be speculated that raising Ang1-7 levels enhanced its transformation to alamandine whose influence started to prevail. If so, blockade of Mas receptors would be without effect because alamandine’s action is mediated by MrgD and not Mas receptors5; in this case a blocker of both receptor species, such as D-Pro7 Ang1-7 would be needed. It is still counterintuitive and unclear why MABP and renal excretion were similarly increased by exogenous Ang1-7 and by Mas receptor blockade.
Lara and coworkers showed that, if Ang1-7 is applied alone, it binds to the losartan-sensitive AT1 receptor, whereas if AT1 receptors are activated by Ang II, Ang1-7 binds to the A779-sensitive Mas receptor.32 This indicates that in proximal tubule Ang1-7 interacts with Mas receptor only in the situation when AT1 receptor is activated.
Thus, the transitory increase in BP observed in acute experiments after Ang1-7 infusion was most likely due to activation of AT1 receptors rather than Mas receptors. To explore this possibility, we performed experiments in which the application of Ang1-7 was preceded by AT1 receptor blockade with candesartan. The increases in MABP and in urine flow and UNaV were prevented, which implicates the predominant role of AT1 receptor in these complex actions of Ang1-7. While it is plausible that Ang1-7 dependent vasoconstriction and BP elevation is mediated by AT1 receptor, its simultaneous role in mediating diuretic and natriuretic responses is puzzling. Possibly, increased excretion is partially secondary to BP elevation, however, other mechanisms should also be considered.
O’Neill and coworkers showed that when AngII generation is reduced, the natriuretic response to intrarenal Ang1-7 is blunted and, conversely, with elevated AngII levels, the excretory effect is enhanced.33 This is consistent with the evidence on counterregulatory action Ang1-7 under conditions of renin–angiotensin system activation, and accords with our observation that Ang1-7 induced increase in renal excretion was more pronounced in AngII-infused rats (Figure 2).
Intrarenal infusion of Ang1-7 in dogs was reported to increase water and UNaV, an effect that was partially blocked by EXP3174, an AT1 antagonist, but not by blocking AT2 receptors with PD123319.34 Notably, the selectivity of PD123319 was recently questioned.6,7 In isolated rat proximal straight tubules, Ang1-7 actions mediated through AT1 receptors was shown to be dose-dependent: at low concentrations (10−12 M) Ang1-7 increased, whereas at high concentrations (10−8 M) it inhibited fluid reabsorption.35 It is possible that Ang1-7 may serve both as a weak antagonist as well as an agonist of the AT1 receptor, particularly at lower concentrations.27 Accordingly, Ang1-7 could cause natriuresis and diuresis via blockade of tubular AT1 receptors. Another possibility is that the natriuretic/diuretic actions are mediated by atrial natriuretic peptide; indeed, Ang1-7 was reported to induce atrial natriuretic peptide release.14 It could be speculated that even a mild increase in BP mediated via AT1 receptor would induce an increase in venous return, which would trigger atrial natriuretic peptide release followed by natriuresis and diuresis. An appropriately focused study would be needed to examine this hypothesis.
In summary, our results suggest that in AngII-dependent hypertension a deficit of Ang1-7, possibly most pronounced at the kidney tissue level, could contribute to sodium and water retention and thereby lead to further BP elevation. Concurrent Ang1-7 infusion exerted effects primarily by increasing sodium and water excretion, perhaps by Ang1-7 dependent inhibition of tubular transport rather than through direct vascular effects. These actions most probably involve activation of angiotensin AT1 and not Mas receptors.
DISCLOSURE
This work was partially presented at FASEB Experimental Biology Congress and American Heart Association congress. The authors declared no conflict of interest.
ACKNOWLEDGMENTS
Dr. Marta Kuczeriszka was supported by Program Mobility Plus (646/MOB/2011/0), Ministry of Science and Higher Education, Republic of Poland. The experimental studies were supported by NIH grants from NHLBI (RO1-HL-26371) and by NIGMS IDeA Program (CoBRE, P30-GM-103337) to L.G.N.
REFERENCES
- 1. Castrop H, Höcherl K, Kurtz A, Schweda F, Todorov V, Wagner C. Physiology of kidney renin. Physiol Rev 2010; 90:607–673. [DOI] [PubMed] [Google Scholar]
- 2. Navar LG, Prieto-Carrasquero MC, Kobori H. Molecular aspects of the renal renin-angiotensin system. In R., Re, D. J., DiPette, E. L., Schiffrin, J. R., Sowers (ed), Molecular Mechanisms in Hypertension, Taylor & Francis Group: London, UK, 2006, pp. 3–14 [Google Scholar]
- 3. Chappell MC, Diz DI, Yunis C, Ferrario CM. Differential actions of angiotensin-(1-7) in the kidney. Kidney Int Suppl 1998; 68:S3–S6. [DOI] [PubMed] [Google Scholar]
- 4. Liu GC, Oudit GY, Fang F, Zhou J, Scholey JW. Angiotensin-(1-7)-induced activation of ERK1/2 is cAMP/protein kinase A-dependent in glomerular mesangial cells. Am J Physiol Renal Physiol 2012; 302:F784–F790. [DOI] [PubMed] [Google Scholar]
- 5. Lautner RQ, Villela DC, Fraga-Silva RA, Silva N, Verano-Braga T, Costa-Fraga F, Jankowski J, Jankowski V, Sousa F, Alzamora A, Soares E, Barbosa C, Kjeldsen F, Oliveira A, Braga J, Savergnini S, Maia G, Peluso AB, Passos-Silva D, Ferreira A, Alves F, Martins A, Raizada M, Paula R, Motta-Santos D, Klempin F, Kemplin F, Pimenta A, Alenina N, Sinisterra R, Bader M, Campagnole-Santos MJ, Santos RA. Discovery and characterization of alamandine: a novel component of the renin-angiotensin system. Circ Res 2013; 112:1104–1111. [DOI] [PubMed] [Google Scholar]
- 6. Soares ER, Barbosa CM, Campagnole-Santos MJ, Santos RAS, Alzamora AC. Hypotensive effect induced by microinjection of Alamandine, a derivative of angiotensin-(1-7), into caudal ventrolateral medulla of 2K1C hypertensive rats. Peptides 2017; 96:67–75. [DOI] [PubMed] [Google Scholar]
- 7. Tetzner A, Gebolys K, Meinert C, Klein S, Uhlich A, Trebicka J, Villacañas Ó, Walther T. G-protein-coupled receptor MrgD is a receptor for angiotensin-(1-7) involving adenylyl cyclase, cAMP, and phosphokinase A. Hypertension 2016; 68:185–194. [DOI] [PubMed] [Google Scholar]
- 8. Ferrario CM, Chappell MC, Tallant EA, Brosnihan KB, Diz DI. Counterregulatory actions of angiotensin-(1-7). Review. Hypertension 1997; 30:535–541 [DOI] [PubMed] [Google Scholar]
- 9. Iyer SN, Ferrario CM, Chappell MC. Angiotensin-(1-7) contributes to the antihypertensive effects of blockade of the renin-angiotensin system. Hypertension 1998; 31:356–361. [DOI] [PubMed] [Google Scholar]
- 10. Roks AJ, van Geel PP, Pinto YM, Buikema H, Henning RH, de Zeeuw D, van Gilst WH. Angiotensin-(1-7) is a modulator of the human renin-angiotensin system. Hypertension 1999; 34:296–301. [DOI] [PubMed] [Google Scholar]
- 11. Stegbauer J, Vonend O, Oberhauser V, Rump LC. Effects of angiotensin-(1-7) and other bioactive components of the renin-angiotensin system on vascular resistance and noradrenaline release in rat kidney. J Hypertens 2003; 21:1391–1399. [DOI] [PubMed] [Google Scholar]
- 12. van der Wouden EA, Ochodnický P, van Dokkum RP, Roks AJ, Deelman LE, de Zeeuw D, Henning RH. The role of angiotensin(1-7) in renal vasculature of the rat. J Hypertens 2006; 24:1971–1978. [DOI] [PubMed] [Google Scholar]
- 13. Ren Y, Garvin JL, Carretero OA. Vasodilator action of angiotensin-(1-7) on isolated rabbit afferent arterioles. Hypertension 2002; 39:799–802. [DOI] [PubMed] [Google Scholar]
- 14. Faria-Silva R, Duarte FV, Santos RA. Short-term angiotensin(1-7) receptor MAS stimulation improves endothelial function in normotensive rats. Hypertension 2005; 46:948–952. [DOI] [PubMed] [Google Scholar]
- 15. Bernardi S, Burns WC, Toffoli B, Pickering R, Sakoda M, Tsorotes D, Grixti E, Velkoska E, Burrell LM, Johnston C, Thomas MC, Fabris B, Tikellis C. Angiotensin-converting enzyme 2 regulates renal atrial natriuretic peptide through angiotensin-(1-7). Clin Sci (Lond) 2012; 123:29–37. [DOI] [PubMed] [Google Scholar]
- 16. Bernardi S, Zennaro C, Palmisano S, Velkoska E, Sabato N, Toffoli B, Giacomel G, Buri L, Zanconati F, Bellini G, Burrell LM, De Manzini N, Fabris B. Characterization and significance of ACE2 and Mas receptor in human colon adenocarcinoma. J Renin Angiotensin Aldosterone Syst 2012; 13:202–209. [DOI] [PubMed] [Google Scholar]
- 17. Shah A, Oh YB, Shan G, Song CH, Park BH, Kim SH. Angiotensin-(1-7) attenuates hyposmolarity-induced ANP secretion via the Na+-K+ pump. Peptides 2010; 31:1779–1785. [DOI] [PubMed] [Google Scholar]
- 18. Gonzalez-Villalobos RA, Satou R, Ohashi N, Semprun-Prieto LC, Katsurada A, Kim C, Upchurch GM, Prieto MC, Kobori H, Navar LG. Intrarenal mouse renin-angiotensin system during ANG II-induced hypertension and ACE inhibition. Am J Physiol Renal Physiol 2010; 298:F150–F157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prieto MC, González-Villalobos RA, Botros FT, Martin VL, Pagán J, Satou R, Lara LS, Feng Y, Fernandes FB, Kobori H, Casarini DE, Navar LG. Reciprocal changes in renal ACE/ANG II and ACE2/ANG 1-7 are associated with enhanced collecting duct renin in Goldblatt hypertensive rats. Am J Physiol Renal Physiol 2011; 300:F749–F755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dobrowolski L, Kuczeriszka M, Castillo A, Majid DS, Navar LG. Role of atrial natriuretic peptide in mediating the blood pressure-independent natriuresis elicited by systemic inhibition of nitric oxide. Pflugers Arch 2015; 467:833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cervenka L, Wang CT, Navar LG. Effects of acute AT1 receptor blockade by candesartan on arterial pressure and renal function in rats. Am J Physiol Renal Physiol 1998; 274:F940–F945 [DOI] [PubMed] [Google Scholar]
- 22. Navar LG, Harrison-Bernard LM, Imig JD, Cervenka L, Mitchell KD. Renal responses to AT1 receptor blockade. Am J Hypertens 2000; 13:45S–54S. [DOI] [PubMed] [Google Scholar]
- 23. Handa RK, Ferrario CM, Strandhoy JW. Renal actions of angiotensin-(1-7): in vivo and in vitro studies. Am J Physiol 1996; 270:F141–F147. [DOI] [PubMed] [Google Scholar]
- 24. Santos RA, Campagnole-Santos MJ, Andrade SP. Angiotensin-(1-7): an update. Regulatory Peptides 2000; 91:45–62 [DOI] [PubMed] [Google Scholar]
- 25. Santos RA, Ferreira AJ, Verano-Braga T, Bader M. Angiotensin-converting enzyme2, angiotensin-(1-7) and Mas: new players of the renin-angiotensin system. J. Endocrinol 2013; 216:R1–R17. [DOI] [PubMed] [Google Scholar]
- 26. Handa RK. Metabolism alters the selectivity of angiotensin-(1-7) receptor ligands for angiotensin receptors. J Am Soc Nephrol 2000; 11:1377–1386. [DOI] [PubMed] [Google Scholar]
- 27. Galandrin S, Denis C, Boularan C, Marie J, M’Kadmi C, Pilette C, Dubroca C, Nicaise Y, Seguelas MH, N’Guyen D, Banères JL, Pathak A, Sénard JM, Galés C. Cardioprotective angiotensin-(1-7) peptide acts as a natural-biased ligand at the angiotensin II type 1 receptor. Hypertension 2016; 68:1365–1374. [DOI] [PubMed] [Google Scholar]
- 28. Bürgelová M, Vanourková Z, Thumová M, Dvorák P, Opocenský M, Kramer HJ, Zelízko M, Malý J, Bader M, Cervenka L. Impairment of the angiotensin-converting enzyme 2-angiotensin-(1-7)-Mas axis contributes to the acceleration of two-kidney, one-clip Goldblatt hypertension. J Hypertens 2009; 27:1988–2000. [DOI] [PubMed] [Google Scholar]
- 29. Velkoska E, Dean RG, Griggs K, Burchill L, Burrell LM. Angiotensin-(1-7) infusion is associated with increased blood pressure and adverse cardiac remodelling in rats with subtotal nephrectomy. Clin Sci (Lond) 2011; 120:335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dilauro M, Burns K. Angiotensin-(1-7) and its effects in the kidney. Review. Sci. World J 2009; 9:522–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ferreira PM, Souza Dos Santos RA, Campagnole-Santos MJ. Angiotensin-(3-7) pressor effect at the rostral ventrolateral medulla. Regul Pept 2007; 141:168–174. [DOI] [PubMed] [Google Scholar]
- 32. Lara LS, Vives D, Correa JS, Cardozo FP, Marques-Fernades MF, Lopes AG, Caruso-Neves C. PKA-mediated effect of MAS receptor in counteracting angiotensin II-stimulated renal Na+-ATPase. Arch Biochem Biophys 2010; 496:117–122. [DOI] [PubMed] [Google Scholar]
- 33. O’Neill J, Corbett A, Johns EJ. Dietary sodium intake modulates renal excretory responses to intrarenal angiotensin (1-7) administration in anesthetized rats. Am J Physiol Regul Integr Comp Physiol 2013; 304:R260–R266. [DOI] [PubMed] [Google Scholar]
- 34. Heller J, Kramer HJ, Malý J, Cervenka L, Horácek V. Effect of intrarenal infusion of angiotensin-(1-7) in the dog. Kidney Blood Press Res 2000; 23:89–94. [DOI] [PubMed] [Google Scholar]
- 35. Garcia NH, Garvin JL. Angiotensin 1-7 has a biphasic effect on fluid absorption in the proximal straight tubule. J Am Soc Nephrol 1994; 5:1133–1138. [DOI] [PubMed] [Google Scholar]