Abstract
BACKGROUND
The prevalence of childhood elevated blood pressure (BP) has increased in the United States, particularly among African Americans. The influence of maternal plasma folate levels, alone or in combination with maternal cardiometabolic risk factors (hypertensive disorders, diabetes, and prepregnancy obesity), on child systolic BP (SBP) has not been examined in a prospective birth cohort. We hypothesize that adequate maternal folate levels can reduce the risk of elevated SBP in children born to mothers with cardiometabolic risk factors.
METHODS
This study included 1,290 mother–child dyads (875 African Americans (67.8%)) recruited at birth and followed prospectively up to age 9 years from 2003 to 2014 at the Boston Medical Center. Child SBP percentile was calculated according to US reference data and elevated SBP was defined as SBP ≥75th percentile.
RESULTS
Maternal folate levels, overall, were not associated with child SBP. However, we found a significant multiplicative interaction between maternal cardiometabolic risk factors and maternal folate levels (Pinteraction = 0.015) on childhood elevated SBP. Among children born to mothers with any cardiometabolic risk factors, those whose mothers had folate levels above (vs. below) the median had 40% lower odds of elevated childhood SBP (odds ratio = 0.60, 95% confidence interval: 0.40–0.90). The associations did not differ appreciably in analyses restricted to African Americans, and they were not explained by gestational age, size at birth, prenatal folate intake, or breastfeeding.
CONCLUSIONS
Findings from our urban minority birth cohort suggest that higher levels of maternal folate may help counteract the adverse associations of maternal cardiometabolic risk factors on child SBP.
Keywords: blood pressure, child, hypertension, interaction, maternal cardiometabolic risk factors, maternal folate
Since the late 1980s, the prevalence of childhood elevated blood pressure (BP) has increased in the United States, in particular among African Americans.1–3 This is of public health concern as childhood BP can predict BP values later in life, and individuals with higher BP are at greater risk of developing subclinical cardiovascular and metabolic disease.4 Because controlling hypertension and cardiovascular disease in adults is difficult and expensive, identifying early-life factors for the prevention of elevated childhood BP is an important public health objective.
There is growing evidence that maternal nutrition during pregnancy, through its impact on the fetal intrauterine environment, may influence offspring cardiometabolic health.5 Folate, which is involved in nucleic acid synthesis, DNA methylation, and cellular growth is particularly important.6 Folate may have beneficial effects on BP by increasing nitric oxide synthesis in endothelial cells,7 or by reducing plasma homocysteine, which itself can cause endothelial cell injury.8 In young adults, higher folate intake has been associated with a lower incidence of hypertension later in life.9 Although studies in ewes have shown that a low dietary intake of folate and vitamin B12 around conception leads to offspring with altered DNA methylation and higher BP later in life,10 human observational studies focused on the association between maternal folate and offspring BP have been inconsistent.11,12 The reason for this may be due to differences in the method and period in which folate was assessed. Furthermore, no previous studies have examined the association of maternal folate status and child BP among mothers with cardiometabolic risk factors in pregnancy.
Maternal cardiometabolic risk factors during pregnancy, including hypertensive disorders, diabetes, and obesity have been associated with higher offspring systolic blood pressure (SBP).13–15 Abnormal maternal metabolic status may influence the cardiometabolic health of offspring through fetal programming.14 Maternal metabolic risk factors have also been linked to low blood folate levels.16 From this perspective, high maternal folate may confer greater protection for offspring development of elevated BP among mothers with cardiometabolic risk factors.
In the current study, we used data on plasma folate levels from a prospective US urban birth cohort, enriched by low-income racial-and-ethnic minorities at high risk for elevated BP, to examine how maternal folate levels and cardiometabolic risk factors individually and jointly affect offspring BP measured between 3 and 9 years of age.
METHODS
Study participants
This analysis included mother–infant pairs from the Boston Birth Cohort (BBC). The BBC was initiated in 1998 with a rolling enrollment of mothers from the Boston Medical Center (BMC). This cohort comprises predominantly urban, low-income racial, and ethnic minority population that has been described previously.17 At enrollment, within 2 to 3 days of delivery, a standardized questionnaire was used to assess maternal demographic and environmental information, including prepregnancy weight, height, race/ethnicity, education, smoking status, parity, perceived stress during pregnancy, and prenatal multivitamin intake. The research team also drew random blood from all mothers at enrollment.
Since 2003, all children who were enrolled in the BBC and planned to receive primary care at BMC were eligible for postnatal follow-up. A standardized questionnaire was used to assess postnatal demographic and environmental information. The research team drew random blood from children during first postnatal follow-up visit (the mean age at blood draw was 2.5 (SD = 2.1) years). The study protocol was approved by the Institutional Review Boards of BMC, Ann & Robert H. Lurie Children’s Hospital of Chicago, and Johns Hopkins Bloomberg School of Public Health. Written informed consent was obtained from all of the study mothers.
In Supplementary eFigure 1, we demonstrate how participants were selected for this analysis. Of the 7,939 mother–child pairs enrolled in the BBC, 1,877 were followed from 2003 to 2014 and completed at least 1 postnatal well-child visit with BP measurements at age 3 to 9 years at the BMC. The current analysis was further restricted to 1,290 mother–child pairs who had complete data on prepregnancy body mass index (BMI), diabetes, hypertensive disorders in pregnancy, gestational age, birthweight, and maternal plasma folate levels. African Americans and mothers who did not smoke during pregnancy were more likely to be included, while other maternal demographic characteristics, birth outcomes, and maternal folate levels (included: (geometric mean = 29.5 nmol/l; 95% confidence interval: 28.6, 30.4) vs. excluded (geometric mean = 28.9 nmol/l; 95% confidence interval: 28.0, 29.9) (P = 0.41).) were comparable between participants included and excluded from the study (Supplementary eTable 1).
Perinatal variables
Maternal prepregnancy weight and height were ascertained by questionnaire within 2–3 days of delivery. Maternal BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2), and then categorized into 3 groups: normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), and obesity (≥30 kg/m2). Underweight mothers (n = 44) were removed from the analysis due to a small sample size.
Maternal educational attainment was classified into high school and below vs. college and above. Maternal smoking during pregnancy was classified into 3 groups: never smoker, intermittent, or continuous smoker.18 Maternal race/ethnicity was classified as Black, Hispanic, or other (which included White, Asian, Pacific Islander, and mothers who reported more than one race). Perceived stress during pregnancy was grouped into low vs. high.19 The frequency of maternal prenatal vitamin intake in the third trimester was classified as none or seldom (0–2/week) vs. often (≥3/week). Information on infant feeding was obtained using a standardized postnatal follow-up questionnaire, and categorized into 3 groups: (i) exclusively formula fed, (ii) exclusively breast fed, or (iii) breast and formula fed.20 Maternal diabetes status was classified as nondiabetic or diabetic (either pre-existing or gestational diabetes).17 Maternal hypertensive disorders in pregnancy21 included pregnancy-induced hypertension (i.e., gestational hypertension, preeclampsia, eclampsia, and Hemolysis, Elevated Liver enzymes, Low Platelet count (HELLP) syndrome) or hypertension that existed prior to pregnancy (referred to here as existing hypertension). We classified women as having no condition if they had no maternal cardiometabolic risk factors (i.e. hypertensive disorders of pregnancy, maternal diabetes, and/or prepregnancy obesity).
Gestational age was determined by the first day of the last menstrual period and early prenatal ultrasonographic results and categorized into term (≥37 weeks) and preterm (<37 weeks).17 Birthweight was abstracted from the electronic medical record. Birthweight for gestational age was categorized into 3 groups: small for gestational age (<10th percentile), large for gestational age (>90th percentile), and appropriate for gestational age (10th–90th percentile) according to an established local gender- and race-specific reference population.22
Anthropometric outcomes and BP
Child weight and height were measured by medical staff during well-child visits as documented in the electronic medical record. Height and weight were converted to age- and sex-specific height z-scores and weight z-scores using US reference data.23 For our primary outcome variable, we used SBP data as measured by medical staff in seated children at age 3–9 years. We focused on childhood SBP rather than diastolic BP because it is a better predictor of later outcomes and is more accurately measured.24,25 BP was measured in a quiet room, using an appropriate size cuff, measured at the right brachial artery using the validated automatic sphygmomanometer Masimo Set (2003–2008: the Welch Allyn 420 Spot Vital Signs monitor; 2008–2014: the Welch Allyn 45MT0 Spot Vital Signs LXi monitor). BP was measured twice, after a test measurement, and was considered valid if the difference between the 2 measurements was less than 10 mm Hg, otherwise a third measurement was taken. SBP was calculated by taking the mean value of the systolic measurements. SBP percentile was calculated using a US national for age-, sex-, and height-specific reference.26 In line with previous definitions used among pediatric populations,27 we defined elevated SBP as SBP ≥75th percentile.
Ascertainment of plasma folate and vitamin B12 levels
Plasma folate and vitamin B12 levels were measured by a commercial laboratory via chemiluminescent immunoassay using a MAGLUMI 2000 Analyzer (Snibe) with an interassay coefficient of variation of less than 4%.28
Statistical analysis
Our primary outcome of interest was SBP measured at the last well-child visit. We modeled SBP percentile (continuous variable) and elevated SBP (binary). Our primary exposure variable was maternal folate level, assessed overall, and per maternal cardiometabolic risk factor status.
We first examined the linear association of maternal plasma folate and child SBP percentile using smoothing plots (PROCLOESS). After determining that the association was nonlinear, we modeled folate in quartiles. We then examined quartiles of folate levels with child SBP percentile and elevated SBP. To assure statistical power for the tests, we also categorized folate into above the median (range: 30.33–185.51 nmol/l) and below the median (range: 6.64–30.31 nmol/l).
As a next step, we estimated the association between maternal folate and child SBP (linear regression) and child-elevated SBP (logistic regression) in different strata of maternal risk factors. We evaluated whether maternal cardiometabolic risk factors modified (on the multiplicative scale) the association of maternal folate status with child SBP percentiles or odds of elevated SBP by including, in our multivariable models, a cross-product term for any maternal cardiometabolic risk factors (yes vs. no) with maternal folate status.
We adjusted multivariable regression models for maternal age, race, education, smoking, alcohol intake, parity, perceived stress during pregnancy, and plasma vitamin B12 level during pregnancy. Covariates were selected based on previous literature documenting their association with the exposure and outcome of interest in our study. We further adjusted models for child plasma folate levels. Child age, sex, and height were not included in the regression models because they were already accounted for when we defined the outcome variables. In additional models we included: (i) gestational age and birth size in the regression models to assess mediation by these factors and (ii) prenatal vitamin intake and breastfeeding in the regression models to assess whether maternal folate was independent of these variables. To further assess the robustness of the findings, we conducted analyses restricted to participants who were Black race, and children ages 6–9 years at BP measurement. All statistical tests were 2-sided and significance was defined at P <0.05. All analyses were performed using SAS (SAS Institute), version 9.4.
RESULTS
We included 1,290 mother–child pairs, 875 of which were Black (67.8%) and 247 Hispanic (19.2%). Of the mothers, 492 (38.2%) had one or more cardiometabolic risk factors; 188 (14.6%) had hypertensive disorders, 143 (11.1%) had diabetes, and 324 (25.1%) had prepregnancy obesity. The median (interquartile range) for maternal plasma folate level was 30.32 nmol/l (interquartile range: 19.71–44.16 nmol/l). A total of 370 (28.7%) children had elevated SBP at age 3–9 years. Children with higher SBP were more likely to have mothers with prepregnancy obesity, hypertensive disorders, and diabetes. Children with elevated SBP were also more likely to have lower birthweight, lower gestational age, and higher current BMI (Table 1).
Table 1.
Characteristics of mother–child dyads in the Boston Birth Cohort, overall and stratified by elevated childhood SBP (elevated SBP, SBP ≥75th percentile)
| Variables | Total sample | Children without elevated SBP | Children with elevated SBP | P value |
|---|---|---|---|---|
| n | 1,290 | 920 | 370 | |
| Maternal characteristics | ||||
| Maternal age, years | 28.8 (6.6) | 28.8 (6.5) | 28.7 (6.9) | 0.822 |
| Race, No. (%) | 0.700 | |||
| Black | 875 (67.8) | 623 (67.7) | 252 (68.1) | |
| Hispanic | 247 (19.2) | 173 (18.8) | 74 (20.0) | |
| Other | 168 (13.0) | 124 (13.5) | 44 (11.9) | |
| Education, No. (%) | 0.838 | |||
| High school and lower | 840 (65.1) | 602 (65.4) | 238 (64.3) | |
| College and higher | 450 (34.9) | 318 (34.6) | 132 (35.7) | |
| Parity, No. (%) | 0.928 | |||
| Nulliparous | 510 (39.5) | 363 (39.5) | 147 (39.7) | |
| Multiparous | 780 (60.5) | 557 (60.5) | 223 (60.3) | |
| Prenatal vitamins intake in third trimester (n = 1,172) | 0.523 | |||
| None or seldom | 142 (12.1) | 106 (12.5) | 36 (11.0) | |
| Often | 1,030 (87.9) | 740 (87.5) | 290 (89.0) | |
| Smoking, No. (%) | 0.041 | |||
| Never | 1,068 (82.8) | 777 (84.5) | 291 (78.7) | |
| Intermittent | 99 (7.7) | 65 (7.1) | 34 (9.2) | |
| Continuous | 123 (9.5) | 78 (8.5) | 45 (12.2) | |
| Alcohol intake, No. (%) | 100 (7.8) | 71 (7.7) | 29 (7.8) | 0.942 |
| Perceived stress during pregnancy, No. (%) | 0.557 | |||
| Low | 1,053 (81.8) | 743 (80.8) | 310 (84.0) | |
| Severe | 237 (18.4) | 177 (19.2) | 60 (16.2) | |
| Pre- or gestational hypertension, No. (%) | 0.035 | |||
| No | 1,102 (85.4) | 798 (86.7) | 304 (82.2) | |
| Yes | 188 (14.6) | 122 (13.3) | 66 (17.8) | |
| Pre- or gestational diabetes, No. (%) | 0.418 | |||
| No | 1,147 (88.9) | 824 (89.6) | 323 (87.3) | |
| Yes | 143 (11.1) | 96 (10.4) | 47 (12.67) | |
| Prepregnancy BMI, kg/m2 | 27.2 (6.5) | 26.8 (6.1) | 28.2 (7.4) | 0.002 |
| Prepregnancy BMI category, No. (%) | 0.004 | |||
| 18.5–24.9kg/m2 | 572 (44.3) | 418 (45.4) | 154 (41.6) | |
| 25–29.9 kg/m2 | 394 (30.5) | 294 (32.0) | 100 (27.0) | |
| ≥30 kg/m2 | 324 (25.1) | 208 (22.6) | 116 (31.4) | |
| Maternal cardiometabolic risk factors, No. (%) | 492 (38.2) | 332 (36.1) | 160 (43.2) | 0.017 |
| Child characteristics | ||||
| Boy, No. (%) | 631 (48.9) | 473 (51.4) | 158 (42.7) | 0.005 |
| Age, year | 6.7 (2.1) | 6.7 (2.1) | 6.8 (2.2) | 0.569 |
| Birthweight (g) | 2,987.4 (797.3) | 3,048.8 (750.3) | 2,838.8 (886.3) | <0.001 |
| Gestational age (week) | 37.9 (3.3) | 38.2 (3.0) | 37.2 (3.9) | <0.001 |
| Preterm birth, No. (%) | 299 (23.2) | 189 (20.5) | 110 (29.7) | <0.001 |
| Birthweight for gestational age, No. (%) | 0.461 | |||
| SGA | 153 (11.8) | 104 (11.3) | 48 (13.0) | |
| AGA | 992 (77.0) | 708 (77.0) | 284 (77.0) | |
| LGA | 145 (11.2) | 108 (11.7) | 37 (10.0) | |
| Weight, Kg | 28.1 (11.6) | 27.5 (10.6) | 29.5 (13.6) | 0.009 |
| BMI, kg/m2 | 18.3 (4.1) | 17.9 (3.6) | 19.2 (4.8) | <0.001 |
| Breastfeeding (n=1247) | 0.276 | |||
| Exclusively formula | 321 (25.7) | 220 (24.6) | 101 (28.5) | |
| Exclusively breastfed | 84 (6.7) | 64 (7.2) | 20 (5.7) | |
| Both | 842 (67.5) | 609 (68.2) | 233 (65.8) | |
Data are shown as mean (SD) or No. (%). P value is for test of significance (either t-test or chi-squared test) between normal childhood blood pressure and elevated blood pressure group. Abbreviations: AGA, appropriate for gestational age; BMI, body mass index; LGA, large for gestational age; SBP, systolic blood pressure; SGA, small for gestational age.
MATERNAL FOLATE LEVEL, MATERNAL CARDIOMETABOLIC RISK FACTORS, AND CHILD SBP
Children born to mothers with the presence of hypertensive disorders, diabetes, prepregnancy obesity, or any cardiometabolic risk factors had higher SBP percentiles and higher odds of elevated SBP, before and after adjusting for covariates. Overall, maternal folate levels were not associated with child SBP percentile or elevated SBP (Table 2). However, the association of maternal folate levels and child SBP was modified by maternal cardiometabolic risk factors.
Table 2.
Associations of maternal folate levels and maternal cardiometabolic risk factors with childhood SBP percentiles and elevated SBP (SBP ≥75th percentile) in children aged 3–9 years from the Boston Birth Cohort (n = 1,290)
| N | Child SBP percentile | Child-elevated SBP | |||
|---|---|---|---|---|---|
| Mean (SD) | β (95% CI) | Case, no. (%) | OR (95% CI) | ||
| Maternal folatea | |||||
| Q4 | 322 | 56.0 (24.6) | 0 | 86 (26.7) | 1 |
| Q3 | 323 | 57.1 (24.7) | 0.57 (−3.35, 4.48) | 86 (26.6) | 0.96 (0.67, 1.36) |
| Q2 | 323 | 56.7 (26.7) | 0.16 (−3.74, 4.07) | 101 (31.3) | 1.21 (0.86, 1.71) |
| Q1 | 322 | 56.9 (25.6) | −0.07 (−4.02, 3.88) | 97 (30.1) | 1.10 (0.77, 1.56) |
| High (Q3–Q4) | 645 | 56.6 (24.6) | 0 | 172 (26.7) | 1 |
| Low (Q1–Q2) | 645 | 56.8 (26.1) | −0.24 (−3.01, 2.54) | 198 (30.7) | 1.18 (0.92, 1.51) |
| Maternal cardiometabolic risk factors | |||||
| No conditionb | 798 | 54.8 (25.6) | 0 | 210 (26.3) | 1 |
| Hypertensive disorders | 188 | 60.6 (24.4) | 6.08 (1.95, 10.21) | 66 (35.1) | 1.51 (1.07, 2.15) |
| Diabetes | 143 | 60.5 (24.0) | 5.71 (1.11, 10.31) | 47 (32.9) | 1.37 (0.92, 2.03) |
| Prepregnancy obesity | 324 | 60.8 (25.3) | 5.98 (2.66, 9.31) | 116 (35.8) | 1.56 (1.18, 2.07) |
| Any cardiometabolic risk factors | 492 | 59.7 (24.8) | 5.14 (2.26, 8.02) | 160 (32.5) | 1.37 (1.06, 1.76) |
Adjusted for maternal age, race, education, smoking, alcohol intake, parity, perceived stress during pregnancy, and plasma vitamin B12 concentration during pregnancy.
Additional adjustment for maternal cardiometabolic risk factors.
Comparison group: no condition means no maternal cardiometabolic risk factors; maternal folate was grouped into low folate (quartile (Q) Q1–Q2): median: 19.71 nmol/l (interquartile range [IQR]: 14.08–25.35 nmol/l) and high folate (Q3–Q4): median: 44.16 nmol/l (IQR: 36.17–56.72 nmol/l); OR: odds ratio; 95% CI: 95% confidence interval.
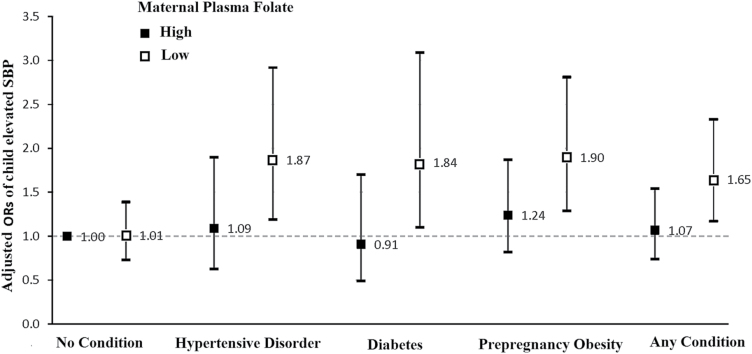
As shown in Figure 1, there was a synergistic joint association of maternal folate levels and cardiometabolic risk factors with odds of offspring having elevated SBP. Children born to mothers with cardiometabolic risk factors (hypertensive disorders, diabetes, prepregnancy obesity, and any cardiometabolic risk factors) and folate levels below the median had a 1.65- to 1.90-fold higher odds of elevated SBP, compared to children whose mothers had high median folate levels with no maternal cardiometabolic risk factors (no condition).
Figure 1.
Joint associations of maternal plasma folate and maternal cardiometabolic risk factors with odds of elevated SBP in children from the Boston Birth Cohort (BBC). The y-axis presents adjusted odds ratios (ORs) and 95% confidence intervals (CIs) of child-elevated SBP estimated from a logistic regression model with adjustment for maternal age, race, education, smoking, alcohol intake, parity, perceived stress during pregnancy, and plasma vitamin B12 concentration during pregnancy. There was significant interaction between maternal folate status and presence of any maternal cardiometabolic risk factors (Pinteraction = 0.015) on odds of elevated childhood SBP. White squares: Low folate (quartile (Q) Q1–Q2): median: 19.71 nmol/l; Black squares: High folate (Q3–Q4): median: 44.16 nmol/l. Abbreviations: CI, confidence interval; SBP, systolic blood pressure.
To assure statistical power for the test of multiplicative interaction, we focused on mothers with any cardiometabolic risk factors vs. no cardiometabolic risk factors. We found a statistically significant interaction between any maternal risk factors and folate on the odds of elevated SBP (Pinteraction = 0.015) (Table 3). Among children of mothers with risk factors, those who were born to mothers in the lowest quartile of folate (compared to those born to mothers in the highest quartile of folate) had higher odds of child-elevated SBP (odds ratio = 1.99; 95% confidence interval: 1.11–3.56). Similar associations were found when we examined associations above vs. below the median value for folate. Among the group with any maternal cardiometabolic risk factors, children born to mothers with high median folate levels were at 40% lower odds of having elevated SBP compared with children of mothers with low median folate levels (odds ratio = 0.60, 95% confidence interval: 0.40–0.90, P = 0.01). There was no such association in children born to mothers without the presence of cardiometabolic risk factors.
Table 3.
Associations of maternal folate levels with child SBP percentile and elevated SBP (SBP ≥ 75th percentile) according to maternal cardiometabolic risk factor status in the Boston Birth Cohort
| Maternal risk factors | Folate | N | Child SBP percentile | Child-elevated SBP | P interaction | |||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | β (95% CI) | P interaction | Case, no. (%) | OR (95% CI) | ||||
| No Conditiona | Q4 | 218 | 55.3 (25.3) | 0 | 59 (27.1) | 1 | ||
| Q3 | 192 | 55.8 (24.4) | 0.33 (−4.66, 5.32) | 48 (25.0) | 0.89 (0.57, 1.40) | |||
| Q2 | 202 | 55.1 (26.4) | −0.48 (−5.41, 4.44) | 60 (29.7) | 1.17 (0.76, 1.79) | |||
| Q1 | 186 | 52.9 (26.4) | −2.91 (−7.99, 2.16) | 43 (23.1) | 0.77 (0.48, 1.22) | |||
| High | 410 | 55.5 (24.8) | 0 | 107 (26.1) | 1 | |||
| Low | 388 | 54.1 (26.4) | −1.77 (−5.38, 1.83) | 103 (26.6) | 1.02 (0.74, 1.40) | |||
| Any maternal cardiometabolic risk factors | Q4 | 104 | 57.3 (23.2) | 0 | 0.046 | 27 (26.0) | 1 | 0.015 |
| Q3 | 131 | 59.1 (25.1) | 1.63 (−4.65, 7.92) | 38 (29.0) | 1.14 (0.63, 2.08) | |||
| Q2 | 121 | 59.4 (27.0) | 2.20 (−4.24, 8.63) | 41 (33.9) | 1.59 (0.87, 2.90) | |||
| Q1 | 136 | 62.4 (23.6) | 5.17 (−1.15, 11.49) | 54 (39.7) | 1.99 (1.11, 3.56) | |||
| High | 235 | 58.3 (24.2) | 0 | 65 (27.7) | 1 | |||
| Low | 257 | 61.0 (25.3) | 2.84 (−1.54, 7.21) | 95 (37.0) | 1.66 (1.11, 2.48) | |||
Adjusted for maternal age, race, education, smoking, alcohol intake, parity, perceived stress during pregnancy, plasma vitamin B12 concentration during pregnancy.
No condition means no maternal cardiometabolic risk factors; maternal folate was grouped into low folate (quartile (Q) Q1–Q2): median: 19.71 nmol/l (interquartile range [IQR]: 14.08–25.35 nmol/l) and high folate (Q3–Q4): median: 44.16 nmol/l (IQR: 36.17–56.72 nmol/l); OR: odds ratio; 95% CI: 95% confidence interval.
SENSITIVITY ANALYSES TO ASSESS THE ROBUSTNESS OF THE FINDINGS
Further adjustment for preterm birth, size at birth (data not shown), prenatal vitamin intake in the third trimester, and breastfeeding status (Supplementary eTable 2) did not materially alter our findings. The associations described above also did not differ appreciably when we restricted analyses to Black mother–child pairs (Supplementary eTables 3 and 4) or to children aged 6–9 years at follow up (Supplementary eTables 5 and 6). Furthermore, there was no correlation between the mother’s and the child’s folate levels (P = 0.66) and the strata-specific associations of maternal folate with offspring BP outcomes remained after adjustment for child folate status (Supplementary eTable 7).
DISCUSSION
To our knowledge, this was the first prospective birth cohort study to evaluate the association of maternal plasma folate level with childhood SBP across strata of maternal cardiometabolic risk factors. Overall, maternal folate during pregnancy had little or no impact on child SBP. However, among children from mothers with cardiometabolic risk factors, low maternal folate was associated with higher odds of elevated childhood SBP.
There is growing evidence that, through their impact on the fetal intrauterine environment, maternal cardiometabolic risk factors during pregnancy may influence development of hypertension in offpsring. Consistent with previous studies,13–15 we found that children born to mothers with the presence of hypertensive disorders, diabetes, and prepregnancy obesity had higher SBP.
Although folate is known to be a critical nutrient for reducing risk of birth defects,6 its potential role in altering offspring risk of high BP has been little explored. Outside of pregnancy, in adults and adolescents, several studies have shown that folate is associated with BP9 and related cardiovascular phenotypes like carotid artery stenosis29 and stroke.28 One study found that maternal folate and vitamin B12 in early pregnancy may be involved in cardiometabolic health of the offspring at age 5–6 years.30 Our findings show that higher maternal folate in pregnancy may help mitigate the risk of elevated offspring BP associated with children born to mothers who have cardiometabolic risk. Future studies are needed to confirm our findings, and if replicated, determine what level of folate and through which sources, confers protection.
McNulty31 reported on a pilot randomized controlled trial in pregnant women that continued supplementation with 400 µg FA/d in the second and third trimesters of pregnancy can increase maternal and cord blood folate status in late pregnancy. However, there is little clinical evidence to show how much maternal folate is needed to reach a protective level to prevent adverse childhood outcomes. In the BBC, about 90% of women took prenatal vitamins ≥3/week during their third trimester of pregnancy. The median maternal blood folate level for women in our cohort was 30.32 nmol/l (13.4 ng/ml), which is comparable with the US general population (median folate from NHANES = 10.6–12.6 ng/ml).32 Maternal prenatal vitamin use did not appear to differ across maternal blood folate levels. Thus, differences in blood folate may derive from differences in dietary folate or to variation in folate absorption. Unfortunately, we do not have data on dietary intake to directly test these hypotheses.
The mechanisms underlying the potentially beneficial influence of maternal folate on child SBP among mothers with risk factors are not clear. Maternal cardiometabolic disorders have been shown to lead to placental and systemic inflammation and oxidative stress,33,34 and folate has been shown to alleviate oxidative stress.35,36 Although higher inflammatory status and homocysteine level have been linked with low blood folate levels, in our study the association of maternal folate with child-elevated SBP was independent of maternal inflammatory and homocysteine status (data not shown), suggesting these factors do not explain the observed findings. As such, future studies are needed to elucidate the potential underlying mechanisms.
Our study had several limitations. We used maternal plasma folate levels taken 2–3 days after delivery, which is at best a proxy of folate nutrition during the third trimester of pregnancy. Although we did not measure fetal folate levels, a previous study has suggested that there is a high degree of transplacental passage of maternal folate to the fetus.37 Maternal prepregnancy BMI was based on self-reported height and weight, thus it may be subject to reporting bias. Nevertheless, in a subset of the study population (N = 672), self-reported BMIs compared to those taken from medical records showed a high degree of agreement (r = 0.89, P < 0.001). In addition, our exclusion of 587 children for a variety of reasons may have resulted in selection bias, though their demographic characteristics were comparable with those of the included participants. Furthermore, the BBC is an urban, predominantly low-income birth cohort that is enriched by preterm, low birthweight babies.17 Because of these design features, the BBC has a larger proportion of African Americans and ethnic minorities than the Boston metropolitan area general population. Future studies of the maternal folate–child BP relationship need to examine if the associations observed in our unique, high risk population generalize to other races, ethnicities, or cohorts with different clinical characteristics. Another limitation of our study is that it is observational, and thus we cannot rule out the possibility of residual confounding by measured covariates or confounding by factors not measured in our study, such as genetic polymorphisms or maternal uric acid levels, which could have influenced maternal levels of folic acid and childhood SBP.
Perspectives
In this urban and predominantly low-income, racial-and-ethnic minority, prospective birth cohort, mothers with cardiometabolic risk factors who had adequate-to-high plasma folate at the end of pregnancy had offspring with lower SBP and lower odds of elevated SBP during childhood, suggesting that high folate levels during pregnancy may counteract the adverse impacts of maternal cardiometabolic risk factors on offspring SBP. Interventions focused on increasing maternal folate intake among mothers with metabolic risk factors may help mitigate the transgenerational association of cardiometabolic diseases.
SUPPLEMENTARY MATERIAL
Supplementary data are available at American Journal of Hypertension online.
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
The Boston Birth Cohort (the parent study) was supported in part by the March of Dimes PERI grants (20-FY02-56, #21-FY07-605), and the National Institutes of Health (NIH) grants (R21ES011666, 2R01HD041702, R21HD066471). The follow-up study is supported in part by the NIH grants (U01AI090727, R21AI079872, R01HD086013); and the Maternal and Child Health Bureau (R40MC27443). Dr Hongjian Wang is supported by a Chinese Scholarships Council scholarship, grants from the National Natural Science Foundation of China (81300156) and the PUMC Youth Fund/the Fundamental Research Funds for the Central Universities (3332015103). The sponsors had no role in the design and/or conduct of the study; in the collection, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript.
REFERENCES
- 1. Sorof JM, Lai D, Turner J, Poffenbarger T, Portman RJ. Overweight, ethnicity, and the prevalence of hypertension in school-aged children. Pediatrics 2004; 113:475–482. [DOI] [PubMed] [Google Scholar]
- 2. Din-Dzietham R, Liu Y, Bielo MV, Shamsa F. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation 2007; 116:1488–1496. [DOI] [PubMed] [Google Scholar]
- 3. Manatunga AK, Jones JJ, Pratt JH. Longitudinal assessment of blood pressures in Black and White children. Hypertension 1993; 22:84–89. [DOI] [PubMed] [Google Scholar]
- 4. Theodore RF, Broadbent J, Nagin D, Ambler A, Hogan S, Ramrakha S, Cutfield W, Williams MJ, Harrington H, Moffitt TE, Caspi A, Milne B, Poulton R. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension 2015; 66:1108–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet 1993; 341:938–941. [DOI] [PubMed] [Google Scholar]
- 6. Relton CL, Pearce MS, Parker L. The influence of erythrocyte folate and serum vitamin B12 status on birth weight. Br J Nutr 2005; 93:593–599. [DOI] [PubMed] [Google Scholar]
- 7. Stroes ES, van Faassen EE, Yo M, Martasek P, Boer P, Govers R, Rabelink TJ. Folic acid reverts dysfunction of endothelial nitric oxide synthase. Circ Res 2000; 86:1129–1134. [DOI] [PubMed] [Google Scholar]
- 8. Verhoef P, Stampfer MJ, Buring JE, Gaziano JM, Allen RH, Stabler SP, Reynolds RD, Kok FJ, Hennekens CH, Willett WC. Homocysteine metabolism and risk of myocardial infarction: relation with vitamins B6, B12, and folate. Am J Epidemiol 1996; 143:845–859. [DOI] [PubMed] [Google Scholar]
- 9. Xun P, Liu K, Loria CM, Bujnowski D, Shikany JM, Schreiner PJ, Sidney S, He K. Folate intake and incidence of hypertension among American young adults: a 20-y follow-up study. Am J Clin Nutr 2012; 95:1023–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sinclair KD, Allegrucci C, Singh R, Gardner DS, Sebastian S, Bispham J, Thurston A, Huntley JF, Rees WD, Maloney CA, Lea RG, Craigon J, McEvoy TG, Young LE. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc Natl Acad Sci USA 2007; 104:19351–19356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Devakumar D, Chaube SS, Wells JC, Saville NM, Ayres JG, Manandhar DS, Costello A, Osrin D. Effect of antenatal multiple micronutrient supplementation on anthropometry and blood pressure in mid-childhood in Nepal: follow-up of a double-blind randomised controlled trial. Lancet Glob Health 2014; 2:e654–e663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van den Hil LC, Rob Taal H, de Jonge LL, Heppe DH, Steegers EA, Hofman A, van der Heijden AJ, Jaddoe VW. Maternal first-trimester dietary intake and childhood blood pressure: the Generation R Study. Br J Nutr 2013; 110:1454–1464. [DOI] [PubMed] [Google Scholar]
- 13. Aceti A, Santhakumaran S, Logan KM, Philipps LH, Prior E, Gale C, Hyde MJ, Modi N. The diabetic pregnancy and offspring blood pressure in childhood: a systematic review and meta-analysis. Diabetologia 2012; 55:3114–3127. [DOI] [PubMed] [Google Scholar]
- 14. Gaillard R, Steegers EA, Duijts L, Felix JF, Hofman A, Franco OH, Jaddoe VW. Childhood cardiometabolic outcomes of maternal obesity during pregnancy: the Generation R Study. Hypertension 2014; 63:683–691. [DOI] [PubMed] [Google Scholar]
- 15. Staley JR, Bradley J, Silverwood RJ, Howe LD, Tilling K, Lawlor DA, Macdonald-Wallis C. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: findings from a prospective study. J Am Heart Assoc 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. da Silva VR, Hausman DB, Kauwell GP, Sokolow A, Tackett RL, Rathbun SL, Bailey LB. Obesity affects short-term folate pharmacokinetics in women of childbearing age. Int J Obes (Lond) 2013; 37:1608–1610. [DOI] [PubMed] [Google Scholar]
- 17. Wang G, Divall S, Radovick S, Paige D, Ning Y, Chen Z, Ji Y, Hong X, Walker SO, Caruso D, Pearson C, Wang MC, Zuckerman B, Cheng TL, Wang X. Preterm birth and random plasma insulin levels at birth and in early childhood. JAMA 2014; 311:587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, Niu T, Wise PH, Bauchner H, Xu X. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA 2002; 287:195–202. [DOI] [PubMed] [Google Scholar]
- 19. Yu Y, Zhang S, Wang G, Hong X, Mallow EB, Walker SO, Pearson C, Heffner L, Zuckerman B, Wang X. The combined association of psychosocial stress and chronic hypertension with preeclampsia. Am J Obstet Gynecol 2013; 209:438.e1–438.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hong X, Wang G, Liu X, Kumar R, Tsai HJ, Arguelles L, Hao K, Pearson C, Ortiz K, Bonzagni A, Apollon S, Fu L, Caruso D, Pongracic JA, Schleimer R, Holt PG, Bauchner H, Wang X. Gene polymorphisms, breast-feeding, and development of food sensitization in early childhood. J Allergy Clin Immunol 2011; 128:374–81.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Report of the national high blood pressure education program working group on high blood pressure in pregnancy. Am J Obstet Gynecol 2000; 183:S1–S22. [PubMed] [Google Scholar]
- 22. Wang L, Wang X, Laird N, Zuckerman B, Stubblefield P, Xu X. Polymorphism in maternal LRP8 gene is associated with fetal growth. Am J Hum Genet 2006; 78:770–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Center for Health Statistics. Cdc Growth Charts, United States. <http://www.Cdc.Gov/growthcharts/> 2000. Accessed 18 April 2016
- 24. Sundström J, Neovius M, Tynelius P, Rasmussen F. Association of blood pressure in late adolescence with subsequent mortality: cohort study of Swedish male conscripts. BMJ 2011; 342:d643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li Z, Snieder H, Harshfield GA, Treiber FA, Wang X. A 15-year longitudinal study on ambulatory blood pressure tracking from childhood to early adulthood. Hypertens Res 2009; 32:404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004; 114:555–576. [PubMed] [Google Scholar]
- 27. Steinberger J, Daniels SR, Eckel RH, Hayman L, Lustig RH, McCrindle B, Mietus-Snyder ML; American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation 2009; 119:628–647. [DOI] [PubMed] [Google Scholar]
- 28. Huo Y, Li J, Qin X, Huang Y, Wang X, Gottesman RF, Tang G, Wang B, Chen D, He M, Fu J, Cai Y, Shi X, Zhang Y, Cui Y, Sun N, Li X, Cheng X, Wang J, Yang X, Yang T, Xiao C, Zhao G, Dong Q, Zhu D, Wang X, Ge J, Zhao L, Hu D, Liu L, Hou FF; CSPPT Investigators. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA 2015; 313:1325–1335. [DOI] [PubMed] [Google Scholar]
- 29. Selhub J, Jacques PF, Bostom AG, D’Agostino RB, Wilson PW, Belanger AJ, O’Leary DH, Wolf PA, Rush D, Schaefer EJ, Rosenberg IH. Relationship between plasma homocysteine, vitamin status and extracranial carotid-artery stenosis in the Framingham Study population. J Nutr 1996; 126:1258S–1265S. [DOI] [PubMed] [Google Scholar]
- 30. Krikke GG, Grooten IJ, Vrijkotte TG, van Eijsden M, Roseboom TJ, Painter RC. Vitamin B12 and folate status in early pregnancy and cardiometabolic risk factors in the offspring at age 5-6 years: findings from the ABCD multi-ethnic birth cohort. BJOG 2016; 123:384–392. [DOI] [PubMed] [Google Scholar]
- 31. McNulty B, McNulty H, Marshall B, Ward M, Molloy AM, Scott JM, Dornan J, Pentieva K. Impact of continuing folic acid after the first trimester of pregnancy: findings of a randomized trial of Folic Acid Supplementation in the Second and Third Trimesters. Am J Clin Nutr 2013; 98:92–98. [DOI] [PubMed] [Google Scholar]
- 32. Centers for Disease C, Prevention. Folate status in women of childbearing age, by race/ethnicity—United States, 1999–2000, 2001–2002, and 2003–2004. MMWR Morb Mortal Wkly Rep 2007; 55:1377–1380. [PubMed] [Google Scholar]
- 33. Szostak-Wegierek D. Intrauterine nutrition: long-term consequences for vascular health. Int J Womens Health 2014; 6:647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Magnussen EB, Vatten LJ, Lund-Nilsen TI, Salvesen KA, Davey Smith G, Romundstad PR. Prepregnancy cardiovascular risk factors as predictors of pre-eclampsia: population based cohort study. BMJ 2007; 335:978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pravenec M, Kozich V, Krijt J, Sokolová J, Zídek V, Landa V, Simáková M, Mlejnek P, Silhavy J, Oliyarnyk O, Kazdová L, Kurtz TW. Folate deficiency is associated with oxidative stress, increased blood pressure, and insulin resistance in spontaneously hypertensive rats. Am J Hypertens 2013; 26:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang RF, Yaong HC, Chen SC, Lu YF. In vitro folate supplementation alleviates oxidative stress, mitochondria-associated death signalling and apoptosis induced by 7-ketocholesterol. Br J Nutr 2004; 92:887–894. [DOI] [PubMed] [Google Scholar]
- 37. Jacquemyn Y, Ajaji M, Karepouan N, Jacquemyn N, Van Sande H. Vitamin B12 and folic acid status of term pregnant women and newborns in the Antwerp region, Belgium. Clin Exp Obstet Gynecol 2014; 41:141–143. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.