Abstract
BACKGROUND
Dietary sodium and potassium affect the fluctuation in blood pressure (BP) and renal function. Corin, with its enzymatic activity to convert pro-atrial natriuretic peptide (pro-ANP) to biologically active ANP, regulates BP, cardiac, and renal functions. We investigated whether corin expression responds to a high-salt (HS) diet to regulate salt and water balance.
METHODS
Forty-two volunteers followed 3 sequential diets for 7 days each: a low-salt (LS) diet (3.0 g/day NaCl), a HS diet (18.0 g/day NaCl), followed by an HS diet with K+ supplementation (HS + K+) (18.0 g/day NaCl and 4.5 g/day KCl).
RESULTS
Corin level was higher with the HS diet than the LS and HS + K+ diets and was positively correlated with systolic BP (SBP) and 24-hour urinary Na+ and microalbumin (U-mALB) excretion. In rodents, serum and renal levels of corin were transiently increased with the HS diet and were decreased if the HS diet was continued for up to 7 days. HS loading increased SBP, 24-hour urinary Na+, U-mALB excretion, and the expression of proprotein convertase subtilisin/kexin-6 (PCSK6), a corin activator. Knockdown of PCSK6 or corin in high salt-treated M1-cortical collecting duct (M1-CCD) cells increased the expression of aquaporin 2 (AQP2) and β-epithelial Na+ channel (β-ENaC).
CONCLUSIONS
Short-term HS may induce the PCSK6–corin–ANP–AQP2/β-ENaC pathway in the kidney. Enhanced serum corin level in humans and rodents is positively correlated with HS-induced SBP and 24-hour urinary Na+ and U-mALB excretion, which suggests that corin is involved in the salt-water balance in response to HS intake.
CLINICAL TRIALS REGISTRATION
Public Trials Registry Number NCT02915315
Keywords: blood pressure, corin, high-salt diet, hypertension, potassium, PCSK6
Chronic high salt (HS) intake exacerbates hypertension and renal damage.1 However, short-term salt stress can activate several protective mechanisms in the kidney to protect against the stress. These mechanisms include increased expression of "a" Na+ sensor in the medulla and dephosphorylation of sodium chloride cotransporter and oxidative stress response kinase-1/STE20/SPS1-related proline alanine-rich kinase (OSR1/SPAK) to regulate aldosterone action in the kidney.2,3
Corin is a type II transmembrane serine protease that converts pro-atrial natriuretic peptide (pro-ANP) to biologically active atrial natriuretic peptide (ANP).4 Given that ANP inhibits aquaporin 2 (AQP2) and β-epithelial Na+ channel (β-ENaC) to regulate blood pressure (BP),5 corin regulation of ANP production would be renal-protective. Indeed, patients with chronic kidney disease show reduced urinary level of corin.6 Additionally, corin has been found to improve cardiac function and ameliorate heart failure by regulating the salt–water balance and maintaining normal BP via ANP homeostasis.7 Corin variants and mutations are linked to hypertension, pre-eclampsia, and cardiac hypertrophy.8–12 Also, accumulating evidence indicates that serum corin level is elevated in people with hypertension, obesity, hyperglycemia, pre-eclampsia, atrial fibrillation, and dyslipidemia but decreased in patients with heart failure and acute myocardial infarction.13–20 In mouse models of corin deficiency, ANP metabolism is impaired, which leads to salt-sensitive hypertension.21
At the upstream, corin is cleaved and activated by proprotein convertase subtilisin/kexin-6 (PCSK6, also known as PACE4).22 Functioning as a proteinase, PCSK6 is involved in the proteolytic cleavage of various precursor proteins and thus important in the regulation of protein maturation. As a corin activator, PCSK6 is pivotal in Na+ homeostasis and BP regulation.
We used in vitro, in vivo, and clinical experiments to determine whether corin is induced by short-term HS intake. A HS diet increased corin level in the circulation and corin expression in the kidney, which were mitigated by K+ supplementation in humans and rodents. Moreover, corin level was positively correlated with systolic BP (SBP) and 24-hour urinary Na+ and microalbumin (U-mALB) excretion but inversely with urinary K+ excretion.
MATERIALS AND METHODS
Study participants
We enrolled participants living in a rural community of Shaanxi Province, China who had similar dietary habits. A questionnaire was used to collect data on demographic characteristics (age, sex, education, ethnicity, occupation, physical activity, cardiovascular disease, and physical examination findings). We excluded participants with stage 2 hypertension, secondary hypertension, use of antihypertensive medication, history of clinical cardiovascular disease, chronic kidney disease, diabetes, pregnancy, high alcohol intake, and concomitant low-salt (LS) diet. The research was approved by the institutional ethics committee of Xi’an Jiaotong University Medical School. The study protocol was carried out in accordance with the Declaration of Helsinki (2008). All participants were specifically informed about the purpose and methods of this study and voluntarily provided written informed consent to participate.
Baseline surveys and dietary intervention
The salt-intake and K+ supplementation intervention protocol was performed as described.23,24 Briefly, during the 3-day baseline period, trained staff collected data, including height, weight, waist circumference, and BP measurements by questionnaire and physical examination. Then, participants received a 7-day LS diet (3.0 g NaCl or 51.3 mmol Na+ per day) followed by a 7-day HS diet (18 g NaCl or 307.8 mmol Na+ per day), then the HS diet and an additional 4.5 g KCl or 60 mmol K+ per day for 7 days. To ensure compliance with the intervention, participants were required to consume their daily meals in the study kitchen under supervision of study staff for the entire period. All study foods were cooked without salt, and prepackaged salt was added to meals by the on-site study staff. Each participant was also instructed to avoid eating food not cooked in the study kitchen for the next 21 days.
Animals
Eight-week-old male Sprague-Dawley (SD) rats were purchased from the Experimental Animal Center of Xi’an Jiaotong University. All protocols were approved by the Institutional Animal Ethics Committee of Xi’an Jiaotong University (XJTU1AF2014LSL-023). Four rats receiving a normal diet (0.3% NaCl) were set as controls, and 8 rats received an HS diet (8% NaCl) for 3 days, then a normal diet for 7 days. This intermittent intervention was conducted twice sequentially.
With the HS and/or HS + K+ diet, each group consisted of 9 rats. Day 1, 3, and 7 groups each included 3 rats with an HS diet (8% NaCl) and 3 with an HS diet with K+ supplementation (8% KCl). Six rats with normal diet (0.3% NaCl) were designated as controls. All rats were housed in metabolic cages (3700M022, TECNIPLAST, Italy). Kidney tissues were dissected and flushed with cold phosphate buffered saline (PBS), kept in 4% paraformaldehyde or quickly frozen in liquid nitrogen, and stored at −80 °C.
Cell culture and transfection
M1-cortical collecting duct (M1-CCD) cells were cultured in Dulbecco’s modified Eagle medium and maintained in a humidified 95% air, 5% CO2 incubator at 37 °C, transfected with corin or PCSK6 siRNA (Genepharma, Shanghai) with use of Lipofectamine 2000 RNAi Max (Invitrogen), then with NaCl (20 mmol/l) for 24 hour. Target mRNA expression was assessed by real-time quantitative polymerase chain reaction (qPCR).
qPCR analysis
RNA was isolated from cultured cells or tissue by using TRIzol reagent (Invitrogen). Total RNA was reverse-transcribed by using the iScript cDNA synthesis kit (Invitrogen), then analyzed by qPCR with SYBR Green (Promega) in a 7500 real-time PCR system (Applied Biosystems). Relative mRNA levels were calculated by the ΔΔCt method with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal control.
Western blot analysis
Protein extracts from tissue were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membrane, which were incubated with antibodies for corin (Abcam, MA), ANP and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA). Protein bands were visualized by enhanced chemiluminescence detection.
Statistical analyses
Data are presented as mean ± SEM. Comparisons between 2 groups involved Student t test. Comparisons between 3 or more groups involved 1-way analysis of variance followed by Bonferroni post-hoc test for equal sample sizes or Turkey–Kramer test for unequal sample sizes. Correlations were determined by Spearman correlation analysis after determining the (non)normal distribution of data. Statistical analyses involved use of SPSS for Windows, v18.0 (SPSS; Chicago, IL) and GraphPad Prism v5.01 (GraphPad Software, San Diego, CA). Two-tailed P <0.05 was considered statistically significant.
RESULTS
HS diet increases circulatory level of corin
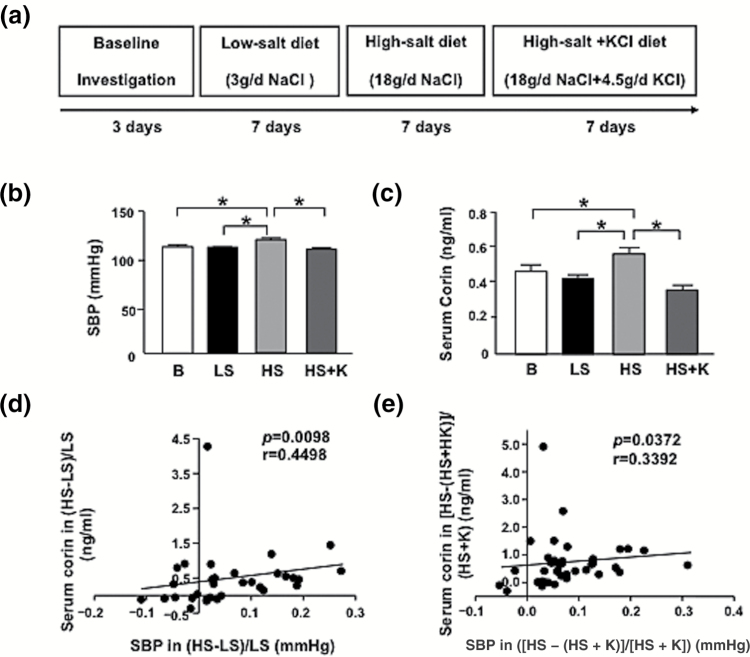
We included 42 participants in the study; the mean age was 50.9 ± 1.3 years (Table 1). Four participants presented hypertension, but none were taking medications at the time of the study. With the LS, HS, and HS + K diet, the mean SBP was 108.7 ± 1.8, 117.3 ± 2.7, and 107.5 ± 1.9 mm Hg, respectively, at the end of the 7-day periods (Figure 1a, b). The corresponding serum corin level was 0.416 ± 0.021, 0.553 ± 0.05, and 0.356 ± 0.02 ng/ml, respectively (Figure 1c). Changes in corin level were positively correlated with changes in SBP from the HS to LS diet as well as HS to HS + K diet (r = 0.4498, P = 0.0098; Figure 1d; r = 0.3392, P = 0.0372; Figure 1e).
Table 1.
Baseline characteristics of the study population
| Parameters | Values |
|---|---|
| Age (years) | 50.9 ± 8.42 |
| Sex (n, %) | |
| Male | 21 (50%) |
| Female | 21 (50%) |
| Body mass index (kg/m2) | 23.5 ± 2.6 |
| Smoking (n, %) | 17 (40.5) |
| Alcohol (n, %) | 3 (7.1) |
| Hypertension | 4 (9.5) |
| Systolic BP (mm Hg) | 110.7 ± 14.26 |
| Diastolic BP (mm Hg) | 72.6 ± 8.42 |
| Mean arterial pressure (mm Hg) | 85.3 ± 9.72 |
| eGFR (ml/min/1.73 m2) | 121.5 ± 15.55 |
| Serum creatinine (µmol/l) | 57.3 ± 9.07 |
| LDL-cholesterol (mmol/l) | 2.35 ± 0.71 |
| HDL-cholesterol (mmol/l) | 1.21 ± 0.26 |
| Triglyceides (mmol/l) | 1.32 ± 0.71 |
| Total cholesterol (mmol/l) | 4.18 ± 0.91 |
| Glucose (mmol/l) | 3.91 ± 0.71 |
Data are mean ± SD or no. (%). Hypertension was defined as the mean systolic BP ≥140 mm Hg and/or mean diastolic BP ≥90 mm Hg. Alcohol drinking was defined as having 12 or more drinks in the past 12 months. Cigarette smoking was defined as having smoked ≥100 cigarettes in the lifetime and smoking at the time of the intervention. Abbreviations: BP, blood pressure; eGFR, estimated glomerular filtration rate; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Figure 1.
Short-term HS loading augments SBP and serum corin level in humans. (a) Schematic illustration of human cohort receiving HS or HS + K diet. The 3 periods of intervention include 7 days of a low-salt (LS) diet (3 g NaCl/day), a HS diet (18 g NaCl/day), then the HS diet with potassium supplementation (18 g NaCl + 4.5 g KCl/day), n = 42. (b) SBP levels measured during intervention periods. (c) Serum corin level detected by ELISA. (d, e) Correlation of changes in SBP and serum corin level from HS to LS diet ([HS − LS]/LS) (d) and HS to HS + potassium (K) diet ([HS − (HS + K)]/[HS + K]) (e). Data are mean ± SEM. *p < 0.05; B denotes baseline. Abbreviations: HS, high-salt; LS, low-salt; SBP, systolic blood pressure.
Corin level positively correlated with urinary Na+ and U-mALB excretion
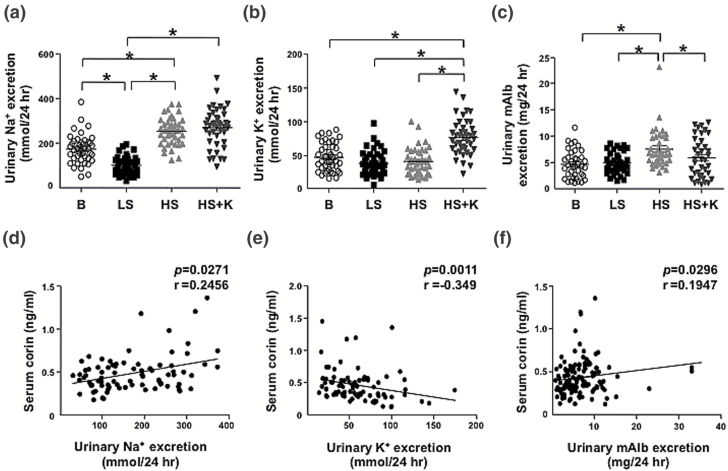
The compliance of participants with the study protocol was assessed by 24-hour urinary Na+ and K+ levels for each diet period. The urinary Na+ excretion decreased under the LS diet as compared with baseline but was reversed under the HS diet (Figure 2a). However, urinary K+ level was similar under the LS and HS diet, but significantly increased under the HS + K diet (Figure 2b), so potassium supplementation substantially increased the urinary K+ level. In addition, U-mALB excretion was elevated during the HS diet, which was mitigated by the HS + K diet (Figure 2c). Serum corin level was positively correlated with urinary excretion of Na+ and U-mALB (r = 0.2456, P = 0.0271; Figure 2d; r = 0.1947, P = 0.0296; Figure 2f) and negatively with potassium excretion (r = −0.349, P = 0.0011; Figure 2e).
Figure 2.
Serum corin level positively correlates with urinary sodium (Na+) and mALB excretion and inversely with urinary K+ excretion in humans. (a–c) 24-hour urinary Na+, K+, and mALB excretion on the last day of each diet period. (d–f) Correlations between serum corin level and exertion of 24-hour urinary Na+ (d), K+ (e), and mALB (f). Data are mean ± SEM. *P < 0.05. Abbreviations: HS, high-salt; LS, low-salt; mALB, microalbumin; SBP, systolic blood pressure.
Corin level increased in rat kidney in response to HS diet
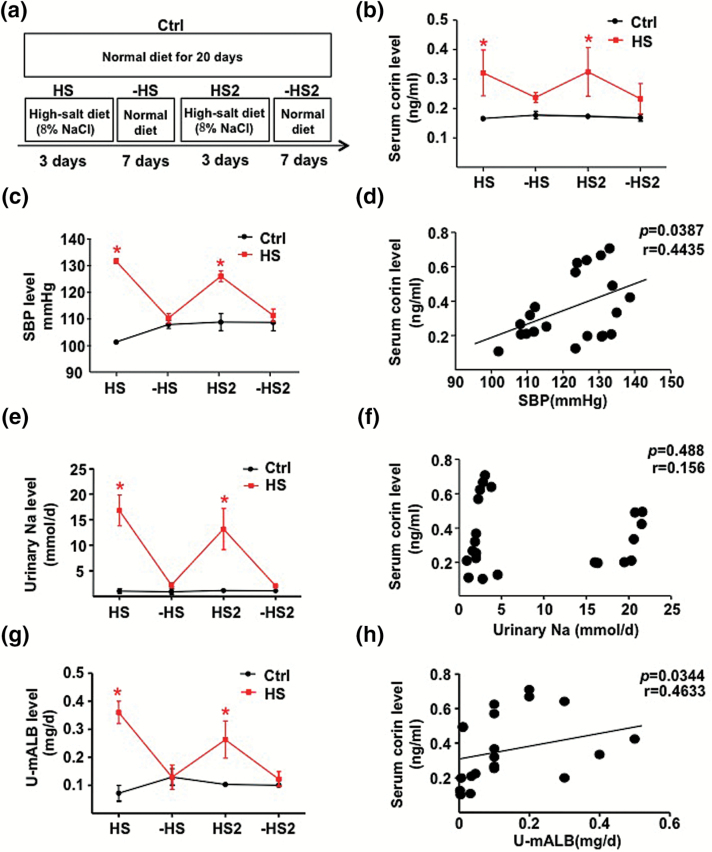
To explore the mechanism of the HS-diet–induced corin, we fed SD rats an HS diet (8% NaCl) for 3 days, then a normal diet (0.3% NaCl) for the next 7 days, with a repeat (Figure 3a). Similar to the human cohort, the serum level of corin and SBP were elevated during the 2 HS diet periods as compared with the regular diet periods or in control rats receiving a regular diet for the entire 20-day period (Figure 3b, c). In addition, the urinary 24-hour Na+ and U-mALB excretion responded positively to the HS diet (Figure 3e, g). Serum corin level was positively correlated with SBP (r = 0.4435, P = 0.0387) and U-mALB excretion (r = 0.4633, P = 0.0344) but not 24-hour urinary Na+ excretion (r = 0.488, P = 0.156) (Figure 3d, f, h). In addition, we found that serum corin level was positively correlated with 24 hour-urine volume (r = 0.6909, P = 0.0007; Supplementary Figure 1).
Figure 3.
HS loading increases corin level in rats. (a) Schematic illustration of HS loading in rats. (b) Circulating level of corin in various stages for rats with salt diets (n = 8) or controls (n = 4). (c, e, g) SBP and 24-hour urinary Na+ and U-mALB excretion. (d, f, h) Correlations of serum corin level with SBP and 24-hour urinary Na+ and U-mALB excretion. Data are mean ± SEM. Abbreviations: HS, high-salt; mALB, microalbumin; SBP, systolic blood pressure.
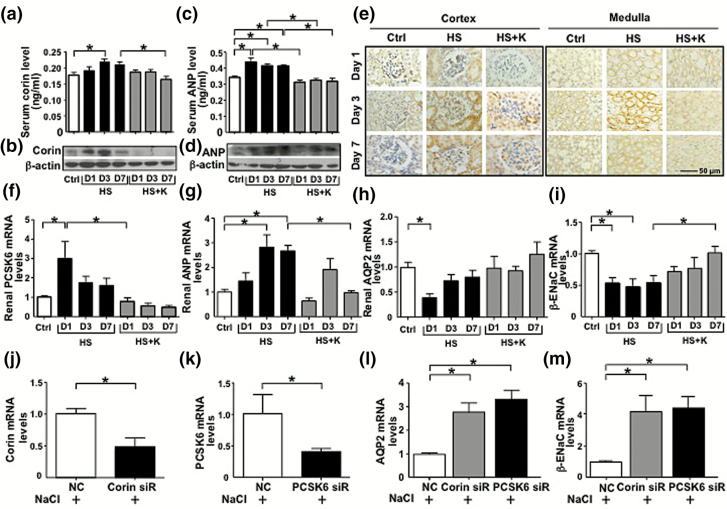
In the kidney, corin is expressed in proximal tubular epithelial cells and in the collecting ducts.25 Given the elevated serum level of corin in rats in response to a short-term HS diet, we explored whether such augmented corin level in circulation was correlated with corin expression in the kidney. We fed rats the HS or HS + K diet for 1, 3, or 7 days or a normal diet for 3 days. Corin and ANP expressions in serum and rat kidney were significantly augmented by the HS diet at day 3 and decreased at day 7 as compared with the HS + K diet (Figure 4a–d). Immunohistochemistry confirmed that corin, with expression mainly in the renal cortex and medulla, was induced 3 days after the HS diet (Figure 4e). Hence, corin level in the serum and kidney was increased by the HS diet and was diminished with longer-term HS diet.
Figure 4.
HS regulates PCSK6–corin–ANP–AQP2/β-ENaC pathway in the rat kidney and M1-CCD cells. (a–d) Rats were fed with HS diet together with or without K+ for 1, 3, and 7 days (n = 8 for each group) or a normal diet for 3 days (control group, n = 8). ELISA and Western blot and were used to measure serum and renal levels of corin and ANP in diet groups. (e) Immunohistochemistry of protein level of corin in the renal cortex and medulla. Scale bar: 50 µm. (f–m) Real-time qPCR quantification of renal PCSK6, ANP, AQP2, and β-ENaC mRNA levels in diet groups (f–i) and mRNA level of corin, PCSK6, AQP2, and β-ENaC in M1-CCD cells transfected with corin, PCSK6, or control siRNA for 24 hour, then treated with 20 mM NaCl for 24 hour (j–m). Data are mean ± SEM *P < 0.05. Abbreviations: ANP, atrial natriuretic peptide; AQP2, aquaporin 2; β-ENaC, β-epithelial Na+ channel; HS, high-salt; LS, low-salt; M1-CCD, M1-cortical collecting duct; PCSK6, proprotein convertase subtilisin/kexin-6.
Short-term HS intake regulates the PCSK6–corin axis in the kidney
Because PCSK6 proteolytically cleaves and activates corin,22 we next examined the effect of short-term HS intake on the renal level of PCSK6. Rats receiving the HS diet for 1 day showed elevated PCSK6 mRNA level in kidneys, which was not seen at 3 and 7 days after the HS diet (Figure 4f). Importantly, animals receiving the HS + K diet did not show this short-term HS-diet–induced PCSK6 level. Corin negatively regulates Na+ and water retention via enhanced activation of ANP; then activated ANP inhibits AQP2 and β-ENaC activity.5 Consistently, HS intake, while increasing ANP mRNA expression, inhibited the mRNA levels of AQP2 and β-ENaC (Figure 4g–i). As potassium might affect other physiological effects, such as extracellular electric potential and intracellular pH, we have repeated a similar experiment with the use of amiloride instead of potassium. As shown in Supplementary Figure 2, HS diet-elevated SBP and corin mRNA in the kidney was attenuated by amiloride supplementation. Amiloride administration contributed to the decreased SBP, which might result in lower levels of corin. On the other hand, HS diet-reduced β-ENaC expression was increased by amiloride supplementation. Thus, a short-term HS intake regulated the PCSK6–corin–ANP–AQP2/β-ENaC pathway in the rat kidney.
To investigate whether high concentrations of extracelluar NaCl upregulate this PCSK6–corin–ANP–AQP2/β-ENaC pathway at the cellular level, we treated M1-CCD cells with 20 mM NaCl. The mRNA level of corin was decreased and levels of AQP2 and β-ENaC were increased with corin and PCSK6 siRNA knockdown (Figure 4j–m).
DISCUSSION
Dietary Na+ and K+ affect the fluctuation in BP and renal function, and corin regulates BP as well as cardiac and renal functions. We investigated whether corin expression responds to a HS diet to regulate salt and water balance at organ, tissue, and cellular levels. Short-term HS intake increased corin level in the blood circulation of humans and SD rats, which was attenuated with K+ supplementation. In addition, corin level was positively correlated with changes in SBP and 24-hour urinary Na+ and U-mALB excretion. Such HS induction of corin was accompanied by increased expression of corin in the kidney of SD rats, which was diminished with more than 3 days of the HS diet. PCSK6 activation of corin attenuated the salt–water retention by inhibiting AQP2 and β-ENaC. Short-term HS may induce the PCSK6–corin–ANP–AQP2/β-ENaC pathway in the kidney for salt–water balance. Noticeably, in addition to the mechanism studied herein, there may be multiple mechanisms involved in renal responses to HS intake in relation to BP elevation in human.
As mentioned previously, corin increased in some cardiovascular diseases, such as hypertension and dyslipidemia but decreased in patients with heart failure and acute myocardial infarction.13,18–20 The mechanisms underlying such variations in corin level with various diseases are still elusive. One explanation might be the temporal compensation of corin associated with the onset and severity of various cardiovascular diseases. To this end, corin might be induced by an acute insult in the cardiovascular system as part of defensive or protective compensatory responses. However, a prolonged pathophysiological milieu may inactivate corin. If so, the increased level of circulatory corin and expression of corin in the kidney would be a compensatory response to a short-term HS diet. Indeed, in our rat experiments (Figure 3), corin level followed the same trend of HS intake. However, in animals receiving more than 3 days of the HS diet, corin level was decreased in both serum and kidney (Figure 4).
According to the published work by Polzin et al., corin is co-localized with ANP in the kidney.26 Furthermore, Dong et al. showed that corin and ANP both are mainly expressed in the tubular epithelial cells and collecting ducts.25 Therefore, the corin-mediated pro-ANP cleavage would occur in the kidney. Accumulating epidemiological, clinical, and mechanistic studies have shown that dietary potassium positively affects BP. One mechanism is that a high potassium intake inhibits ENaC activity and Na+ absorption.27 Consistent with those results, our data indicated that potassium supplementation to the HS diet decreased SBP and abrogated serum and renal corin level in both humans and SD rats. Moreover, serum corin level was negatively correlated with urinary K+ level (Figure 2e). Given that corin inhibits ENaC, the positive role of potassium supplementation in regulating BP and salt–water retention might be mediated in part through corin. Because the HS + K+ diet rectified the HS-diet–increased corin level, K+ may act as a competitive inhibitor of Na+ during the induction of corin in the renal cortex and medulla.28
Corin plays an important role in salt-sensitive hypertension by regulating the salt–water balance.29 We found a correlation between changes in BP and urinary Na+ level and changes serum corin level in humans (Figures 1d, e, and 2d) as well as BP and serum corin level in rats (Figure 3d). However, we found no significant correlation between serum corin level and urinary Na+ level in rats (Figure 3f), which might be due to the limited number of rats tested. The rationale for the lower SBP, urinary sodium excretion, and U-mAlb level in response to the second interval of high sodium intake might be that renal tissues had been desensitized by the pre-exposure of HS loading (Figure 3).
High dietary Na+ affects renal function, as indicated by increased 24-hour U-mALB excretion, a clinical sign of early renal damage.30Figure 2c, f indicated that 7-day HS diet induced an increase in 24-hour urinary albumin excretion in humans that was positively correlated with corin serum levels. However, urine albumin excretion was less than that of the clinical manifestation of early renal damage (30 mg/24 hour). Therefore, we suggest that corin may protect from HS-diet–induced urinary albumin prior to renal dysfunction.
Figure 4a, b show that the levels of renal and serum corin had a similar trend in response to HS intake. Thus, the increased renal expression of corin should contribute, at least in part, to the elevated serum corin in response to a short period of HS intake. Corin is highly expressed in the heart in addition to the kidney.31 As mentioned previously, serum levels of corin are significantly reduced in patients with acute myocardial infarction and inversely associated with infarction incidents.20 Furthermore, cardiac overexpression of corin in mice improves dilated cardiomyopathy.7 Thus, serum level of corin might be an indicator of heart disease, and increased corin level in the heart might have a beneficial role in cardiac function. Whether the short-term HS loading would increase corin level in the heart and its related effect on cardiac function needs further study.
PCSK6 is known to cleave and then activate corin, and Pcsk6 ablation in mice causes salt-sensitive hypertension.22 We found PCSK6 induced in the rat kidney in response to HS loading (Figure 4f). PCSK6 expression is increased in carotid atherosclerotic lesions, which is positively correlated with inflammation and apoptosis markers including interleukin 1β, tumor necrosis factor α, and nuclear factor κB.32 Although the exact mechanism by which the atherosclerotic milieu induces PCSK6 is unknown, cardiovascular insults including short-term HS loading in the kidney may be common denominators for PCSK6 activation.
We recognize several limitations to our human cohort study. The study population was relatively small and restricted to northern Chinese people. Because of the relatively small human cohort in this study, participants were not randomized to the order of the diet. Furthermore, there was no washout period between diets or control group, which may introduce the possibility of carry-over effects. Moreover, a comparison between corin and other biomarkers (e.g., angiotensin-converting enzyme 2 and β2 microglobulin) may help address the usefulness of corin as an additional prognostic marker in evaluating cardiac and renal functions.33,34 Furthermore, the long-term physiologic response to sodium and potassium with respect to corin activity and ANP levels warrants future investigation.
In conclusion, this study, encompassing humans, rodents, and cell culture, showed that short-term HS loading induced the PCSK6–corin–ANP–AQP2/β-ENaC pathway in the kidney, which may result in elevated level of corin in circulation. This finding suggests a mechanistic basis of volume regulation by HS diet. This knowledge may help in developing a therapeutic strategy involving corin.
SUPPLEMENTARY MATERIAL
Supplementary data are available at American Journal of Hypertension online.
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Drs. Yifei Miao, Juan Zhou and Fuqiang Liu, and Mr. Baochang Lai their consultation; and Ms. Jianjie Dong, Xinxing Shi for their technical assistance. We are grateful for financial support by the National Natural Science Fund of China (grant Nos. 81400230 to M.H., 91339116 and 91639301 to Z.Y.Y., 81270349 and 81670452 to J.S., and 81570381 and 81370357 to J.J.M.); NIH research grants (R01HL105318 and R01HL125643 to J.S.); and the Taiwan Ministry of Science and Technology Academic Excellence Program (grant No: MOST 105-2633-B-009-003).
REFERENCES
- 1. Susic D, Fares H, Frohlich ED. Salt, arterial pressure, and cardiovascular and renal damage. Ochsner J 2009; 9:197–203. [PMC free article] [PubMed] [Google Scholar]
- 2. Lara LS, Satou R, Bourgeois CR, Gonzalez AA, Zsombok A, Prieto MC, Navar LG. The sodium-activated sodium channel is expressed in the rat kidney thick ascending limb and collecting duct cells and is upregulated during high salt intake. Am J Physiol Renal Physiol 2012; 303:F105–F109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chiga M, Rai T, Yang SS, Ohta A, Takizawa T, Sasaki S, Uchida S. Dietary salt regulates the phosphorylation of OSR1/SPAK kinases and the sodium chloride cotransporter through aldosterone. Kidney Int 2008; 74:1403–1409. [DOI] [PubMed] [Google Scholar]
- 4. Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci USA 2000; 97:8525–8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klein JD. Corin: an ANP protease that may regulate sodium reabsorption in nephrotic syndrome. Kidney Int 2010; 78:635–637. [DOI] [PubMed] [Google Scholar]
- 6. Fang C, Shen L, Dong L, Liu M, Shi S, Dong N, Wu Q. Reduced urinary corin levels in patients with chronic kidney disease. Clin Sci (Lond) 2013; 124:709–717. [DOI] [PubMed] [Google Scholar]
- 7. Gladysheva IP, Wang D, McNamee RA, Houng AK, Mohamad AA, Fan TM, Reed GL. Corin overexpression improves cardiac function, heart failure, and survival in mice with dilated cardiomyopathy. Hypertension 2013; 61:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dong N, Fang C, Jiang Y, Zhou T, Liu M, Zhou J, Shen J, Fukuda K, Qin J, Wu Q. Corin mutation R539C from hypertensive patients impairs zymogen activation and generates an inactive alternative ectodomain fragment. J Biol Chem 2013; 288:7867–7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cui Y, Wang W, Dong N, Lou J, Srinivasan DK, Cheng W, Huang X, Liu M, Fang C, Peng J, Chen S, Wu S, Liu Z, Dong L, Zhou Y, Wu Q. Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature 2012; 484:246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dries DL, Victor RG, Rame JE, Cooper RS, Wu X, Zhu X, Leonard D, Ho SI, Wu Q, Post W, Drazner MH. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation 2005; 112:2403–2410. [DOI] [PubMed] [Google Scholar]
- 11. Rame JE, Drazner MH, Post W, Peshock R, Lima J, Cooper RS, Dries DL. Corin I555(P568) allele is associated with enhanced cardiac hypertrophic response to increased systemic afterload. Hypertension 2007; 49:857–864. [DOI] [PubMed] [Google Scholar]
- 12. Wang W, Liao X, Fukuda K, Knappe S, Wu F, Dries DL, Qin J, Wu Q. Corin variant associated with hypertension and cardiac hypertrophy exhibits impaired zymogen activation and natriuretic peptide processing activity. Circ Res 2008; 103:502–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peng H, Zhang Q, Cai X, Liu Y, Ding J, Tian H, Chao X, Shen H, Jiang L, Jin J, Zhang Y. Association between high serum soluble corin and hypertension: a cross-sectional study in a general population of China. Am J Hypertens 2015; 28:1141–1149. [DOI] [PubMed] [Google Scholar]
- 14. Peng H, Zhang Q, Shen H, Liu Y, Chao X, Tian H, Cai X, Jin J. Association between serum soluble corin and obesity in Chinese adults: a cross-sectional study. Obesity 2015; 23:856–861. [DOI] [PubMed] [Google Scholar]
- 15. Liu Y, Peng H, Zhang Q, Zhang P, Tian Y, Chao X, Zhang Y. Association between serum soluble corin and hyperglycaemia: a cross-sectional study among Chinese adults. BMJ Open 2015; 5:e009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miyazaki J, Nishizawa H, Kambayashi A, Ito M, Noda Y, Terasawa S, Kato T, Miyamura H, Shiogama K, Sekiya T, Kurahashi H, Fujii T. Increased levels of soluble corin in pre-eclampsia and fetal growth restriction. Placenta 2016; 48:20–25. [DOI] [PubMed] [Google Scholar]
- 17. Chen F, Xia Y, Liu Y, Zhang Y, Song W, Zhong Y, Gao L, Jin Y, Li S, Jiang Y, Yang Y. Increased plasma corin levels in patients with atrial fibrillation. Clin Chim Acta 2015; 447:79–85. [DOI] [PubMed] [Google Scholar]
- 18. Wang X, Chen S, Zhang Q, Liu Y, Liu L, Li H, Peng H. Increased serum soluble corin in dyslipidemia: a cross-sectional study. Clin Chim Acta 2015; 450:310–315. [DOI] [PubMed] [Google Scholar]
- 19. Dong N, Chen S, Yang J, He L, Liu P, Zheng D, Li L, Zhou Y, Ruan C, Plow E, Wu Q. Plasma soluble corin in patients with heart failure. Circ Heart Fail 2010; 3:207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang SM, Shen JX, Li H, Zhao P, Xu G, Chen JC. Association between serum corin levels and risk of acute myocardial infarction. Clin Chim Acta 2016; 452:134–137. [DOI] [PubMed] [Google Scholar]
- 21. Wang W, Shen J, Cui Y, Jiang J, Chen S, Peng J, Wu Q. Impaired sodium excretion and salt-sensitive hypertension in corin-deficient mice. Kidney Int 2012; 82:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen S, Cao P, Dong N, Peng J, Zhang C, Wang H, Zhou T, Yang J, Zhang Y, Martelli EE, Naga Prasad SV, Miller RE, Malfait AM, Zhou Y, Wu Q. PCSK6-mediated corin activation is essential for normal blood pressure. Nat Med 2015; 21:1048–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fang Y, Mu JJ, He LC, Wang SC, Liu ZQ. Salt loading on plasma asymmetrical dimethylarginine and the protective role of potassium supplement in normotensive salt-sensitive Asians. Hypertension 2006; 48:724–729. [DOI] [PubMed] [Google Scholar]
- 24. Liu F, Mu J, Yuan Z, Zhang M, Zheng S, Lian Q, Liu E, Xu H, Ren K, Huang Q. Potassium supplement ameliorates salt-induced haemostatic abnormalities in normotensive subjects. Acta Cardiol 2011; 66:635–639. [DOI] [PubMed] [Google Scholar]
- 25. Dong L, Wang H, Dong N, Zhang C, Xue B, Wu Q. Localization of corin and atrial natriuretic peptide expression in human renal segments. Clin Sci (Lond) 2016; 130:1655–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Polzin D, Kaminski H, Kastner C, Wang W, Kramer S, Gambaryan S, Russwurm M, Peters H, Wu Q, Vandewalle A, Bachmann S, Theilig F. Decreased renal corin expression contributes to sodium retention in proteinuric kidney diseases. Kidney Int 2010; 78:650–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sun P, Antoun J, Lin DH, Yue P, Gotlinger KH, Capdevila J, Wang WH. Cyp2c44 epoxygenase is essential for preventing the renal sodium absorption during increasing dietary potassium intake. Hypertension 2012; 59:339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kanbay M, Bayram Y, Solak Y, Sanders PW. Dietary potassium: a key mediator of the cardiovascular response to dietary sodium chloride. J Am Soc Hypertens 2013; 7:395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu Q, Xu-Cai YO, Chen S, Wang W. Corin: new insights into the natriuretic peptide system. Kidney Int 2009; 75:142–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Engelen L, Soedamah-Muthu SS, Geleijnse JM, Toeller M, Chaturvedi N, Fuller JH, Schalkwijk CG, Stehouwer CD. Higher dietary salt intake is associated with microalbuminuria, but not with retinopathy in individuals with type 1 diabetes: the EURODIAB Prospective Complications Study. Diabetologia 2014; 57:2315–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ichiki T, Huntley BK, Heublein DM, Sandberg SM, McKie PM, Martin FL, Jougasaki M, Burnett JC Jr. Corin is present in the normal human heart, kidney, and blood, with pro-B-type natriuretic peptide processing in the circulation. Clin Chem 2011; 57:40–47. [DOI] [PubMed] [Google Scholar]
- 32. Perisic L, Hedin E, Razuvaev A, Lengquist M, Osterholm C, Folkersen L, Gillgren P, Paulsson-Berne G, Ponten F, Odeberg J, Hedin U. Profiling of atherosclerotic lesions by gene and tissue microarrays reveals PCSK6 as a novel protease in unstable carotid atherosclerosis. Arterioscler Thromb Vasc Biol 2013; 33:2432–2443. [DOI] [PubMed] [Google Scholar]
- 33. Mendoza-Torres E, Oyarzún A, Mondaca-Ruff D, Azocar A, Castro PF, Jalil JE, Chiong M, Lavandero S, Ocaranza MP. ACE2 and vasoactive peptides: novel players in cardiovascular/renal remodeling and hypertension. Ther Adv Cardiovasc Dis 2015; 9:217–237. [DOI] [PubMed] [Google Scholar]
- 34. Otaki Y, Watanabe T, Shishido T, Takahashi H, Funayama A, Narumi T, Kadowaki S, Hasegawa H, Honda S, Netsu S, Ishino M, Arimoto T, Miyashita T, Miyamoto T, Konta T, Kubota I. The impact of renal tubular damage, as assessed by urinary β2-microglobulin-creatinine ratio, on cardiac prognosis in patients with chronic heart failure. Circ Heart Fail 2013; 6:662–668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.