Abstract
Inflammatory cytokines cause hypertension when introduced into animals. Additional evidence indicates that cytokines induce the production of autoantibodies that activate the AT1 angiotensin receptor (AT1R). Extensive evidence shows that these autoantibodies, termed AT1-AA, contribute to hypertension. We review here recent studies showing that cytokine-induced hypertension and AT1-AA production require the ubiquitous enzyme, tissue transglutaminase (TG2). We consider 3 mechanisms by which TG2 may contribute to hypertension. (i) One involves the posttranslational modification (PTM) of AT1Rs at a glutamine residue that is present in the epitope sequence (AFHYESQ) recognized by AT1-AA. (ii) Another mechanism by which TG2 may contribute to hypertension is by PTM of AT1Rs at glutamine 315. Modification at this glutamine prevents ubiquitination-dependent proteasome degradation and allows AT1Rs to accumulate. Increased AT1R abundance is likely to account for increased sensitivity to Ang II activation and in this way contribute to hypertension. (iii) The increased TG2 produced as a result of elevated inflammatory cytokines is likely to contribute to vascular stiffness by modification of intracellular contractile proteins or by crosslinking vascular proteins in the extracellular matrix. This process, termed inward remodeling, results in reduced vascular lumen, vascular stiffness, and increased blood pressure. Based on the literature reviewed here, we hypothesize that TG2 is an essential participant in cytokine-induced hypertension. From this perspective, selective TG2 inhibitors have the potential to be pharmacologic weapons in the fight against hypertension.
Keywords: angiotensin II type I receptor, autoimmunity, blood pressure, GPCR, hypertension, inflammatory cytokines, rennin–angiotensin–aldosterone system, tissue transglutaminase, vascular stiffness.
Hypertension is a “strong, continuous, graded, and etiologically significant” risk factor1 for cardiovascular diseases affecting millions of people worldwide.2,3 The pathogenesis of essential hypertension is multifactorial, with different mechanisms contributing to hypertension in different individuals. Because of this underlying heterogeneity, a broad spectrum of antihypertensive medications is needed to meet the personalized medical needs of different individuals. Classical areas of hypertension research include the rennin–angiotensin–aldosterone system, the sympathetic nervous system, the endothelin system, the vascular system, and renal hemodynamics.4 However, mounting evidence suggests that hypertension is intrinsically associated with inflammation and autoimmunity.
INFLAMMATION CAUSES HYPERTENSION
Studies investigating the inflammatory response accompanying activation of the innate and adaptive arms of the immune system have determined a prominent role of proinflammatory cytokines in the development of hypertensive disorders.5–10 The innate immune system is mainly composed of cells of the myeloid lineage, including monocytes, macrophages, granulocytes, and dendritic cells. These cells contain pattern recognition receptors that are rapidly activated by pathogen-associated molecular patterns and damage-associated molecular patterns. In the acute immune response produced by innate immunity, the effector cells produce potent cytokines that in turn activate the adaptive immune system for its follow-up immune response including antibody production. These cytokines are also produced by activated T cells of the adaptive arm of the immune system. A particularly important family of cytokines called IL-17 are produced by a subset of T cells referred to as TH17 cells. The IL-17 family of cytokines have been implicated in autoimmune disease.11 Research has shown that elevated levels of interleukin-1β,12 tumor necrosis factor,13 interleukin-6,14 interleukin-17,15 and C-reactive protein,16–18 among others10,19,20 are consistently associated with hypertension. The inflammatory conditions induced by these cytokines can cause posttranslational modifications (PTMs) of proteins that serve as neoantigens21 that in some cases stimulate the production of autoantibodies contributing to hypertension.22 Experimental support for a causative role of inflammation in hypertension comes from animal studies showing that the introduction of these inflammatory cytokines into pregnant or nonpregnant rodents results in hypertension.23–28 Furthermore, research has shown that angiotensin II (Ang II) induces the synthesis of key proinflammatory cytokines in various cell types.29–31 Reciprocally, Ang II-induced hypertension is attenuated in animals lacking these cytokines.32–34 Overall, a critical role of inflammatory cytokines in hypertension has been well established, though the mechanisms by which these inflammatory cytokines cause hypertension is not well understood.
In an effort to understand the molecular mechanisms underlying cytokine-induced hypertension, a cytokine-induced model of experimental hypertension was developed based on the use of TNF superfamily member 14, LIGHT (Lymphotoxin, exhibits Inducible expression, and competes with HSV Glycoprotein D for HVEM, a receptor expressed by T lymphocytes).28 LIGHT is also known as TNFSF14. Considerable evidence supports a role for LIGHT in inflammation initiation, autoimmune response, and cardiovascular disorders. Circulating LIGHT is mainly secreted by cells of the innate and adaptive immune system including granulocytes, monocytes, macrophages, dendritic cells, and T cells.35,36 LIGHT activates 2 widely distributed receptors, the herpes virus entry mediator (HVEM)37 and the lymphotoxin β38,39 receptor, that activate the NFkB pathway.40,41 Both receptors are present at elevated levels in trophoblasts, endothelial cells, and cardiomyocytes in human health complications related with hypertension.28,42 LIGHT is significantly higher in the circulation of women with preeclampsia, a serious hypertensive condition of pregnancy, and is able to induce hypertension when introduced into pregnant or nonpregnant mice.28,43
AUTOIMMUNE HYPERTENSION
Recent years have witnessed increased evidence revealing the contribution of autoimmunity to hypertension.5,6,9,44–46 Autoimmunity is a common medical condition affecting approximately 5% of the US population and known to be a major factor causing well-known health problems including type 1 diabetes, multiple sclerosis, rheumatoid arthritis, and celiac disease. The autoimmune basis for these conditions was not initially recognized and only became evident after years of research. This history is now repeating itself for hypertension. Considerable evidence22,47 suggests that many forms of hypertension result from the presence of agonistic autoantibodies that activate major G protein coupled receptors (GPCRs) associated with the regulation of blood pressure. Notable examples include: (i) cardiac β1-adrenergic receptor agonistic autoantibodies in dilated cardiomyopathy,48 (ii) α1-adrenergic receptor agonistic autoantibodies in refractory hypertension,49–51 (iii) angiotensin receptor type 1 (AT1) agonistic autoantibodies (AT1-AA) in preeclampsia,52–55 malignant/refractory hypertension,56–59 and primary aldosteronism,60,61 and (iv) endothelin receptor type a agonistic autoantibodies in systemic sclerosis (SS)62 and systemic lupus erythematosus63 associated with pulmonary hypertension. Adoptive transfer experiments in laboratory animals provide convincing evidence that these receptor activating autoantibodies are active contributors to hypertension,54 and blockade of these autoantibodies with stable D-amino acid epitope peptide prevents hypertension in rabbits.64 The crucial role of agonistic autoantibodies in hypertension that has been extensively reviewed22,47,65 is further supported by the findings that the induced blood pressure increase and vascular remodeling is attenuated in mice lacking mature B cells due to B-cell-activating factor receptor-deficiency or pharmacological depletion with anti-CD20 antibody.66,67 We suggest the term “autoimmune hypertension” to describe these conditions.22,47,65,68,69
In order to understand the pathogenesis of autoimmune hypertension, it is necessary to have an experimental system in which antibody production can be induced. This has been achieved for animal models of cytokine-induced hypertension in pregnant and nonpregnant rodents.23–28 A series of reports70–73 show that cytokine-induced hypertension is associated with production of AT1-AA. Initial efforts focused on preeclampsia, a condition known to be associated with elevated inflammatory cytokines including TNF-α, IL-6, IL-17, and LIGHT/TNFSF14.70–73 Blockade of the inflammatory cytokine receptors ameliorates hypertensive features in preeclamptic rodents.74,75 A rat model of PE based on placental ischemia (the RUPP model) is characterized by elevated TNFα and the presence of AT1-AA.76 TNFα blockade with etanercept (also called Enbrel, a soluble form of the TNFα receptor) blocks AT1-AA production and prevents hypertension.74,75 Similar results were obtained with rituximab (anti-CD20, inhibits B-lymphocytes) showing a significant reduction in the number of B cells and in AT1-AA titer.67 Both Enbrel77 and rituximab78 are used to treat autoimmune diseases. Subsequent experiments showed that IL-6 is required for LIGHT/TNFSF14-induced hypertension and AT1-AA production in nonpregnant mice.43 These inflammatory cytokines establish a causal link between inflammation and autoimmunity in the pathogenesis of hypertensive disorders and provide a convenient experimental animal model to determine the mechanism of cytokine-induced autoantibody production and the contribution of these autoantibodies to hypertension.
TRANSGLUTAMINASE AND AUTOIMMUNITY
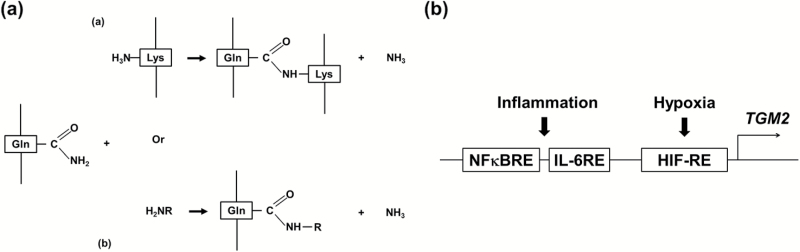
A well-recognized cause for an autoimmune response is PTM of proteins, a process that sometimes creates autoantigens recognized as foreign by the immune system.79–84 One of the best studied examples is celiac disease, an autoimmune complication affecting approximately 1% of people in developed countries.85 Celiac disease is a chronic autoimmune disorder of the small intestine caused by an abnormal immune response to a post-translationally modified dietary protein called gliadin, a component of wheat. The enzyme causing the pathogenic PTM of the glutamine-rich gliadin in celiac disease is tissue transglutaminase (TG2),86–88 the most ubiquitous and prominent member of a family of crosslinking enzymes that catalyze the PTM of glutamine residues on proteins (Figure 1a).89 In addition to the modified gliadin peptides, celiac autoantibodies also recognize TG2.90 Recent evidence indicates that TG2 modifies and stabilizes AT1 receptors in placentas of women with preeclampsia.91 More importantly, the epitope sequence of AT1-AA on the second extracellular loop of the receptor (AFHYESQ) can be crosslinked to TG2 via glutamine residue, Q187.73 The potential importance of TG2 in cytokine-induced autoantibody production in preeclampsia is enhanced by the recognition that the TGM2 gene is transcriptionally activated by hypoxia and inflammatory cytokines (Figure 1b),92–94 conditions associated with preeclampsia. Taken together, the autoimmune and inflammatory threads in the pathogenesis of hypertension converge at tissue transglutaminase.
Figure 1.
Illustration of the crosslinking function (a) and gene regulation (b) of tissue transglutaminase in cardiovascular disorders. (a) In cardiovascular diseases tissue transglutaminase is known to crosslink the glutamine residue in a peptide to either a peptide-bound lysine residue (a) or a primary amine such as serotonin (b). R in panel b could be an alkyl or aryl group. (b) The expression of tissue transglutaminase is usually induced in cardiovascular diseases due to the presence of major inflammation and hypoxia response elements in the promoter region of the gene encoding tissue transglutaminase (TGM2).
Transglutaminases are a group of structurally related enzymes that modify glutamine residues on proteins. The PTM results in the covalent isopeptide bond formation in a calcium-dependent manner between the γ-carboxamide group of a peptide-bound glutamine residue and a free amine or a peptide-bound lysine89 (Figure 1a). As the first discovered transglutaminase,95 TG2 is especially enriched in endothelial and smooth muscle cells96 and is the most ubiquitous member of the 8 enzyme family whose conserved catalytic core sequence (GQCWVFA) shares a common feature of hydrophobicity.97 Consistent with this, a general preference for the substrate glutamine residue surrounded by hydrophobic residues was identified by a combination of phage display and bioinformatics approaches.98,99 However, unlike other transglutaminases, TG2 is inhibited under normal physiological conditions where cells maintain an environment of low free calcium and high GTP and ATP that inhibits its transamidation function.100,101 In hypoxic and/or inflammatory conditions usually associated with cardiovascular disorders, TG2 is activated to perform PTMs of protein substrates due to GTP and ATP depletion and calcium influx.100,101 TG2 is involved in numerous cardiovascular disorders including preeclampsia,91 hypertension,43 cardiac hypertrophy,102,103 and atherosclerosis,104–107 although its precise role in disease pathogenesis is unclear.
TRANSGLUTAMINASE IS A CRITICAL LINK AMONG INFLAMMATION, AUTOIMMUNITY, AND HYPERTENSION
Circulating transglutaminase levels show a strong positive correlation with blood pressure, proteinuria, and AT1-AA titers in women with preeclampsia.73 These findings suggest a possible role for TG2 in the production of AT1-AA in hypertensive disorders like preeclampsia. This possibility is further supported by the presence of a Q residue in the epitope sequence (AFHYESQ) on the second extracellular loop of the AT1R, a sequence recognized by TG2 and crosslinked to the enzyme in vitro.73
In an effort to understand the role of TG2 in hypertension, renal impairment and AT1-AA production experimental models of hypertension were developed based the infusion of the inflammatory cytokine LIGHT/TNFSF14 into pregnant and nonpregnant mice. In addition to the increased blood pressure and renal impairment, elevated levels of plasma transglutaminase activity were observed in both pregnant73 and nonpregnant43 mice injected with LIGHT/TNFSF14. Cytokine injected mice also produced AT1-AA. To test the role of TG2 in cytokine-induced hypertension, renal impairment and AT1-AA production, pregnant, and nonpregnant mice were injected with LIGHT/TNFSF14 in the presence or absence of the transglutaminase inhibitor cystamine.73 The results show that cytokine-induced hypertension, renal impairment, and AT1-AA production were prevented by the presence of cystamine. Additional experiments showed that IL-6 and endothelial HIF-1α are required for LIGHT-induced hypertension, renal impairment, AT1-AA production, and increased transglutaminase.43 The requirement for endothelial HIF-1α suggests that endothelial TG2 is critical for LIGHT-induced hypertension, renal impairment, and AT1-AA production. These findings are consistent with earlier reports showing that transcription of the TG2 gene is activated by inflammatory cytokines and HIF (Figure 1). Thus, although the requirement for TG2 in cytokine-induced hypertension has only been shown for LIGHT/TNFSF14,43,73 this is likely to be the case for other cytokines that induce hypertension (e.g., TNF, IL-6, and IL-17). A related study showed that TG2 is required for AT1-AA induced hypertension in a mouse model of preeclampsia.91 Because AT1-AA active the AT1R, an in this way mimick AngII, the latter results suggest that Ang II-induced hypertension may require TG2. This possibility is supported by the fact that Ang II induces the production of inflammatory cytokines29–31 and requires these cytokines to induce hypertension.
MODIFICATION OF AT1RS BY TRANSGLUTAMINASES
The first evidence of AT1R modification by transglutaminases was presented by AbdAlla et al. who showed that AT1Rs are covalently crosslinked into homodimers by FXIIIa transglutaminase in monocytes resulting in enhanced receptor signaling.108 These receptor homodimers in monocytes show increased sensitivity to Ang II activation. As a member of the transglutaminase family, FXIIIa transglutaminase is predominantly expressed in macrophages, monocytes, and platelets,109 and is also the major plasma transglutaminase crucial in the blood clotting cascade.110,111 However, due to the limited expression pattern of FXIIIa transglutaminase, the overall molecular nature and functional consequences of AT1 receptor modification in most tissues and organs were not addressed in these early studies. Recent studies have examined AT1R modification in preeclampsia, a condition characterized by hypoxia and elevated inflammatory cytokines, conditions expected to stimulate production and activation of TG2 (Figure 1). The results show that AT1Rs112,113 and TG2114,115 are co-localized91 in the syncytiotrophoblasts at the maternal–fetal interface of the human placenta. Due to highly elevated inflammatory cytokines116 and hypoxia117 associated with preeclampsia, a significantly increased level of TG2 and isopeptide protein modification were found in the syncytiotrophoblast layer of preeclamptic placentas.91 AT1Rs were among the TG2 modified proteins and TG2-mediated AT1 receptor modification was associated with increased AT1 receptor abundance.91 Unlike the AT1 receptor dimerization observed in hypertensive monocytes108 where the FXIIIa transglutaminase is predominantly expressed, the AT1 receptor modified by TG2 in placentas shows no significant change in molecular weight. Thus, the molecular nature of TG2-mediated AT1 receptor modification in preeclamptic placentas is either intramolecular118 or a posttranslational incorporation of a small primary amine (e.g., serotonin, histamine, norepinephrine, and dopamine) as originally observed in other examples of TG2 PTM.95,119,120
To investigate whether the TG2-mediated isopeptide modification is responsible for the increased AT1 receptor abundance observed in preeclamptic placentas an experimental model of preeclampsia in pregnant mice was used based on injection of autoantibodies from women with preeclampsia. The results showed that placental TG2 was elevated in this model and that this was accompanied with elevated AT1Rs that were modified by TG2. To determine the role of TG2 in this experimental model of preeclampsia the autoantibody-injected pregnant mice were treated with cystamine, a well-established transglutaminase inhibitor121 or nanoparticle-embedded TG2 siRNA. These treatments prevented placental AT1 receptor accumulation and the transglutaminase modification together with the increase in blood pressure and urinary protein in this preeclampsia model.91 These results indicate that TG2-mediated AT1 receptor modification results in increased AT1R abundance in preeclampsia.
These were the first results to show modification of a GPCR by TG2 with the functional consequence of increased receptor abundance. The increased abundance of AT1Rs on the membrane likely contributes to the hypersensitivity to Ang II that is associated with preeclampsia122 and other hypertensive disorders. Increased abundance of TG2-modified AT1Rs may also serve as neoantigens that promote AT1-AA production. Thus, increased AT1R abundance could contribute to hypertension in multiple ways. Given the ubiquitous expression pattern of TG289 and its known association with a wide range of GPCRs,69,78–82 a previously unrecognized and general role for TG2 is the PTM of GPCRs to enhance and amplify GPCR signaling by increasing receptor abundance under stress or pathogenic conditions associated with hypoxia and inflammation.91
TISSUE TRANSGLUTAMINASE STABILIZES PLACENTAL AT1RS BY PREVENTING UBIQUITIN-DEPENDENT PROTEOSOMAL DEGRADATION
To elucidate the molecular mechanism by which TG2 modification results in an increase in AT1R abundance initial efforts focused on a glutamine residue (Q315) in the cytoplasmic tail of AT1 receptors that was shown previously to be the site for FXIIIa transglutaminase-mediated receptor crosslinking.108 Indeed, Q315 is embedded in a hydrophobic motif (FLQ315LL) evolutionarily conserved among all vertebrates higher than fishes (Figure 2) and therefore is an ideal modification site for TG2. To evaluate the importance of TG2-mediated modification on Q315 in the AT1R the glutamine was replaced with an alanine. When co-expressed with human TG2 in a stable Chinese hamster ovary cell line, the Q315A mutant AT1 receptor was no longer modified by TG2 and showed a lower cellular abundance compared with the wild-type receptor.91 These results indicate that the cellular accumulation is a direct functional consequence of TG2-mediated AT1 receptor modification at Q315. Additional experiments showed that TG2-mediated modification on Q315 of AT1 receptor prevents ubiquitin-dependent degradation123 allowing for increased receptor abundance.91 Functionally, the increased concentration of AT1Rs on the plasma membrane is likely to contribute to the heightened Ang II sensitivity characteristic of preeclampsia.122,124 These studies are the first to reveal a role for TG2-mediated PTM in regulating AT1R’s ubiquitin-dependent degradation with consequences on receptor abundance and signaling. These findings regarding TG2 modification of AT1Rs will likely apply to other GPCRs.
Figure 2.
The hydrophobic motif (in box) with the glutamine residue (in bold) for transglutaminase modification at the cytoplasmic tail of AT1 receptor is evolutionarily conserved among all the representative vertebrates higher than fish.
TISSUE TRANSGLUTAMINASE AND VASCULAR STIFFNESS
As a ubiquitous protein, TG2 is widely distributed in the vasculature, including endothelial cells, smooth muscle cells, and fibroblasts.89,96,125,126 Within arterial vascular smooth muscle cells TG2 modifies proteins that function in contraction by the addition of serotonin and norepinephrine.119,127 Excess TG2 modification of these contractile proteins could have adverse effects on vascular properties. TG2 is also found abundantly in the extracellular matrix (ECM).128–131 In the ECM, the enzyme’s transamidation function is activated by high extracellular concentrations of calcium,100,132 resulting in the crosslinking and stabilization of ECM proteins133 such as fibrinogen,134 fibronectin,135 and collagen.136 TG2-mediated vascular ECM protein crosslinking leads to thickening of the vascular wall, reduced vascular lumen, increased vasoconstriction, and vascular calcification/stiffening,137,138 all of which contribute to the development of vascular remodeling, a well-known feature of essential hypertension.139,140 Therefore, one of possible explanations for the beneficial effects of transglutaminase inhibitor cystamine on blood pressure141 is by preventing the effect of extracellular TG2 on vascular remodeling and stiffness.137,142,143 In addition to calcium concentration, the activity of TG2 in the vascular ECM is also known to be controlled by several other factors including nitrosylation,143,144 redox state,145 and mechanical force.125 Moreover, inflammation is also considered as one of the key causative factors of vascular remodeling in hypertension.140,146 Recent studies43 suggest the elevated proinflammatory cytokines in hypertension may also contribute to the detrimental vascular remodeling process by up-regulating the transcription of TGM2 in the vasculature. The results show that LIGHT/TNFSF14-induced hypertension and transglutaminase elevation are prevented in mice with an endothelial specific deletion of HIF-1α, a key transcriptional regulator of Tgm2 gene expression,92,147,148 suggesting that inflammation-induced endothelial TG2 may contribute to hypertension, in part, by triggering inward remodeling and stiffening of small arteries.
CONCLUSIONS AND FUTURE DIRECTIONS
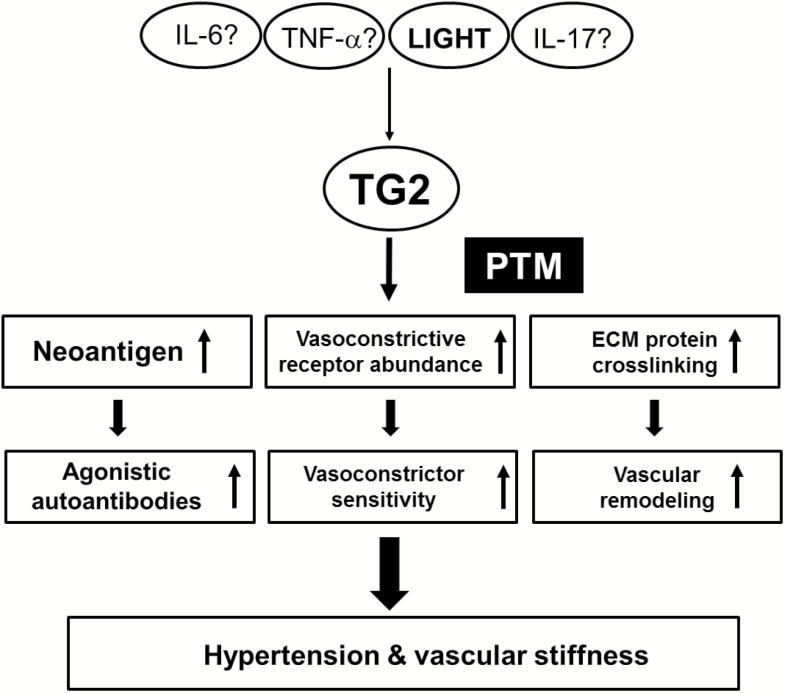
Inflammatory cytokines (TNF, IL-6, IL-17, LIGHT) cause hypertension when introduced into experimental animals.23–28 However, the mechanism by which they cause hypertension is not understood. Here, we reviewed recent literature that has begun to bridge this gap in our understanding and to reveal mechanisms by which cytokines cause hypertension. Specifically, recent publications43,73 have shown that cytokine-induced hypertension requires the widely distributed enzyme TG2. The TG2 gene (Tgm2) is transcriptionally activated by cytokines and hypoxia due to the presence of the relevant gene regulatory elements in the promoter region of the gene. Three potential mechanisms by which elevated TG2 may contribute to cytokine-induced hypertension are considered below (Figure 3).
Figure 3.
A proposed role of tissue transglutaminase (TG2) in the inflammatory cytokine-induced hypertension. The induction of TG2 in hypertension by other inflammatory cytokines than LIGHT/TNFSF14 needs to be confirmed. For more details see text (Conclusions and Future Directions).
(i) One mechanism involves the PTM of the AT1R at glutamine 18773 that is present at the end of the epitope sequence (AFHYESQ) recognized by AT1-AA.52 It has been clearly established that these autoantibodies contribute to hypertension by activation of AT1Rs based on interaction with this epitope sequence.53–55 Thus, a very likely mechanism by which TG2 contributes to hypertension is by PTM of Q187 on AT1Rs resulting in the creation of a neo-epitope that stimulates AT1-AA production by the adaptive immune system. This process requires autoimmune activation of the adaptive immune system, and for this reason is expected to require sufficient time for autoantibody production to become significant.
(ii) Another, potentially more rapid, mechanism by which TG2 may contribute to hypertension is by PTM of AT1R at glutamine 315.91 Modification at this glutamine by TG2 prevents ubiquitin-dependent proteosomal degradation and allows AT1Rs to accumulate in various cells. Increased AT1R abundance is likely to account for increased sensitivity to Ang II activation and in this way contribute to hypertension. Reduced AT1R turnover, and increased receptor abundance, caused by PTM of Q315 occurs more quickly than the time required to activate the adaptive immune system and produce AT1-AA. Chronic receptor accumulation in inflammatory and hypoxic conditions will finally result in increased sensitivity to Ang II-induced hypertension. Additionally, increased abundance of TG2-modified receptor may also serve as a neoantigen and promote AT1-AA production.
(iii) The increased TG2 produced as a result of elevated inflammatory cytokines is likely to contribute to vascular stiffness137,138 by crosslinking vascular proteins134–136 in the ECM. This process, termed inward remodeling, results in reduced vascular lumen, vascular stiffness, and increased blood pressure. Vascular remodeling as a result of increased TG2 in the ECM will commence quickly as a result of inflammation/hypoxia-mediated induction of TG2 gene expression and enzyme activation. Elevated TG2 secreted from endothelial and other cell types in the ECM will cause inward remodeling, vascular stiffness, and reduced vascular lumen by crosslinking important ECM proteins. Excess TG2 modification of contractile proteins within vascular smooth muscle cells could also have adverse effects on vascular properties.119,127 Although these vascular remodeling processes are expected to commence soon after cytokine-mediated activation of the TG2 gene and enzyme, the kinetics of the process is yet to be investigated.
The relative contributions of each of these processes to cytokine-induced hypertension can be readily examined in an experimental model of hypertension in mice. For example, the temporal relationship between the onset of hypertension and AT1-AA production can be determined. Furthermore, the role of AT1-AA in cytokine-induced hypertension can also be determined in a number of ways, most directly by stable epitope peptides that neutralize these pathogenic autoantibodies and prevent their ability to activate AT1Rs.64 Additionally, cytokine infusions could be performed in mice treated with rituximab, a monoclonal antibody that targets the B-cell antigen CD20, and prevents antibody production. The roles of AT1R receptor stabilization or vascular remodeling in cytokine-induced hypertension can be examined in the absence of autoantibody production using rituximab treated or immune-deficient mice that cannot make antibodies.66,67 Time course experiments could determine how quickly AT1R levels increase relative to how quickly hypertension occurs. Cytokine-treated animals could also be examined for increased pressor sensitivity to Ang II infusion.
Based on the literature reviewed here, we hypothesize that TG2 is an essential contributor to cytokine-induced hypertension. From this perspective, selective TG2 inhibitors are promising candidates as pharmacologic weapons to be used in the fight against hypertension. A variety of additional TG2 inhibitors are being produced and evaluated.149 With regard to drug safety, it is reassuring that TG2-deficient mice are viable with no overt pathological phenotype resulting from their enzyme deficiency. Thus, TG2-specific inhibitors may be well tolerated for the treatment of hypertension.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
This work is supported by grants to Y.X. from National Institutes of Health (1R01HL136969, 1R01HL113574, P01HL114457-01) and UTHealth Pulmonary Center of Excellence.
REFERENCES
- 1. Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Arch Intern Med 1993; 153:598–615. [DOI] [PubMed] [Google Scholar]
- 2. Vasan RS, Larson MG, Leip EP, Evans JC, O’Donnell CJ, Kannel WB, Levy D. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med 2001; 345:1291–1297. [DOI] [PubMed] [Google Scholar]
- 3. Wang W, Lee ET, Fabsitz RR, Devereux R, Best L, Welty TK, Howard BV. A longitudinal study of hypertension risk factors and their relation to cardiovascular disease: the Strong Heart Study. Hypertension 2006; 47:403–409. [DOI] [PubMed] [Google Scholar]
- 4. Hall JE, Granger JP, do Carmo JM, da Silva AA, Dubinion J, George E, Hamza S, Speed J, Hall ME. Hypertension: physiology and pathophysiology. Compr Physiol 2012; 2:2393–2442. [DOI] [PubMed] [Google Scholar]
- 5. Caillon A, Schiffrin EL. Role of inflammation and immunity in hypertension: recent epidemiological, laboratory, and clinical evidence. Curr Hypertens Rep 2016; 18:21. [DOI] [PubMed] [Google Scholar]
- 6. McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res 2015; 116:1022–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schiffrin EL. The immune system: role in hypertension. Can J Cardiol 2013; 29:543–548. [DOI] [PubMed] [Google Scholar]
- 8. Schiffrin EL. Immune mechanisms in hypertension and vascular injury. Clin Sci (Lond) 2014; 126:267–274. [DOI] [PubMed] [Google Scholar]
- 9. Harrison DG. The immune system in hypertension. Trans Am Clin Climatol Assoc 2014; 125:130–138; discussion 138. [PMC free article] [PubMed] [Google Scholar]
- 10. Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension 2011; 57:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhu S, Qian Y. IL-17/IL-17 receptor system in autoimmune disease: mechanisms and therapeutic potential. Clin Sci (Lond) 2012; 122:487–511. [DOI] [PubMed] [Google Scholar]
- 12. Dalekos GN, Elisaf MS, Papagalanis N, Tzallas C, Siamopoulos KC. Elevated interleukin-1 beta in the circulation of patients with essential hypertension before any drug therapy: a pilot study. Eur J Clin Invest 1996; 26:936–939. [DOI] [PubMed] [Google Scholar]
- 13. Xu W, Yang Q, Chen H. [Tumor necrosis factor in pregnancies associated with pregnancy induced hypertension]. Zhonghua Fu Chan Ke Za Zhi 1997; 32:9–11. [PubMed] [Google Scholar]
- 14. Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension 2001; 38:399–403. [DOI] [PubMed] [Google Scholar]
- 15. Yao W, Sun Y, Wang X, Niu K. Elevated serum level of interleukin 17 in a population with prehypertension. J Clin Hypertens (Greenwich) 2015; 17:770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dodson PM, Shine B. Retinal vein occlusion: c-reactive protein and arterial hypertension. Acta Ophthalmol (Copenh) 1984; 62:123–130. [DOI] [PubMed] [Google Scholar]
- 17. Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J Hum Hypertens 2005; 19:149–154. [DOI] [PubMed] [Google Scholar]
- 18. Parchim NF, Wang W, Iriyama T, Ashimi OA, Siddiqui AH, Blackwell S, Sibai B, Kellems RE, Xia Y. Neurokinin 3 receptor and phosphocholine transferase: missing factors for pathogenesis of C-reactive protein in preeclampsia. Hypertension 2015; 65:430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dinh QN, Drummond GR, Sobey CG, Chrissobolis S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed Res Int 2014; 2014:406960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Miguel C, Rudemiller NP, Abais JM, Mattson DL. Inflammation and hypertension: new understandings and potential therapeutic targets. Curr Hypertens Rep 2015; 17:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Doyle HA, Yang ML, Raycroft MT, Gee RJ, Mamula MJ. Autoantigens: novel forms and presentation to the immune system. Autoimmunity 2014; 47:220–233. [DOI] [PubMed] [Google Scholar]
- 22. Xia Y, Kellems RE. Angiotensin receptor agonistic autoantibodies and hypertension: preeclampsia and beyond. Circ Res 2013; 113:78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takahashi H, Nishimura M, Sakamoto M, Ikegaki I, Nakanishi T, Yoshimura M. Effects of interleukin-1 beta on blood pressure, sympathetic nerve activity, and pituitary endocrine functions in anesthetized rats. Am J Hypertens 1992; 5:224–229. [DOI] [PubMed] [Google Scholar]
- 24. Alexander BT, Cockrell KL, Massey MB, Bennett WA, Granger JP. Tumor necrosis factor-alpha-induced hypertension in pregnant rats results in decreased renal neuronal nitric oxide synthase expression. Am J Hypertens 2002; 15:170–175. [DOI] [PubMed] [Google Scholar]
- 25. Orshal JM, Khalil RA. Reduced endothelial NO-cGMP-mediated vascular relaxation and hypertension in IL-6-infused pregnant rats. Hypertension 2004; 43:434–444. [DOI] [PubMed] [Google Scholar]
- 26. LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension 2005; 46:1022–1025. [DOI] [PubMed] [Google Scholar]
- 27. Nguyen H, Chiasson VL, Chatterjee P, Kopriva SE, Young KJ, Mitchell BM. Interleukin-17 causes Rho-kinase-mediated endothelial dysfunction and hypertension. Cardiovasc Res 2013; 97:696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang W, Parchim NF, Iriyama T, Luo R, Zhao C, Liu C, Irani RA, Zhang W, Ning C, Zhang Y, Blackwell SC, Chen L, Tao L, Hicks MJ, Kellems RE, Xia Y. Excess LIGHT contributes to placental impairment, increased secretion of vasoactive factors, hypertension, and proteinuria in preeclampsia. Hypertension 2014; 63:595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Funakoshi Y, Ichiki T, Ito K, Takeshita A. Induction of interleukin-6 expression by angiotensin II in rat vascular smooth muscle cells. Hypertension 1999; 34:118–125. [DOI] [PubMed] [Google Scholar]
- 30. Han Y, Runge MS, Brasier AR. Angiotensin II induces interleukin-6 transcription in vascular smooth muscle cells through pleiotropic activation of nuclear factor-kappa B transcription factors. Circ Res 1999; 84:695–703. [DOI] [PubMed] [Google Scholar]
- 31. Ruiz-Ortega M, Ruperez M, Lorenzo O, Esteban V, Blanco J, Mezzano S, Egido J. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int Suppl 2002; 82:S12–S22. [DOI] [PubMed] [Google Scholar]
- 32. Lee DL, Sturgis LC, Labazi H, Osborne JB, Jr, Fleming C, Pollock JS, Manhiani M, Imig JD, Brands MW. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am J Physiol Heart Circ Physiol 2006; 290:H935–H940. [DOI] [PubMed] [Google Scholar]
- 33. Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 2010; 55:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang W, Wang W, Yu H, Zhang Y, Dai Y, Ning C, Tao L, Sun H, Kellems RE, Blackburn MR, Xia Y. Interleukin 6 underlies angiotensin II-induced hypertension and chronic renal damage. Hypertension 2012; 59:136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mauri DN, Ebner R, Montgomery RI, Kochel KD, Cheung TC, Yu GL, Ruben S, Murphy M, Eisenberg RJ, Cohen GH, Spear PG, Ware CF. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity 1998; 8:21–30. [DOI] [PubMed] [Google Scholar]
- 36. Tamada K, Shimozaki K, Chapoval AI, Zhai Y, Su J, Chen SF, Hsieh SL, Nagata S, Ni J, Chen L. LIGHT, a TNF-like molecule, costimulates T cell proliferation and is required for dendritic cell-mediated allogeneic T cell response. J Immunol 2000; 164:4105–4110. [DOI] [PubMed] [Google Scholar]
- 37. Kwon BS, Tan KB, Ni J, Oh KO, Lee ZH, Kim KK, Kim YJ, Wang S, Gentz R, Yu GL, Harrop J, Lyn SD, Silverman C, Porter TG, Truneh A, Young PR. A newly identified member of the tumor necrosis factor receptor superfamily with a wide tissue distribution and involvement in lymphocyte activation. J Biol Chem 1997; 272:14272–14276. [DOI] [PubMed] [Google Scholar]
- 38. Browning JL, Ngam-ek A, Lawton P, DeMarinis J, Tizard R, Chow EP, Hession C, O’Brine-Greco B, Foley SF, Ware CF. Lymphotoxin beta, a novel member of the TNF family that forms a heteromeric complex with lymphotoxin on the cell surface. Cell 1993; 72:847–856. [DOI] [PubMed] [Google Scholar]
- 39. Crowe PD, VanArsdale TL, Walter BN, Ware CF, Hession C, Ehrenfels B, Browning JL, Din WS, Goodwin RG, Smith CA. A lymphotoxin-beta-specific receptor. Science 1994; 264:707–710. [DOI] [PubMed] [Google Scholar]
- 40. Mackay F, Majeau GR, Hochman PS, Browning JL. Lymphotoxin beta receptor triggering induces activation of the nuclear factor kappaB transcription factor in some cell types. J Biol Chem 1996; 271:24934–24938. [DOI] [PubMed] [Google Scholar]
- 41. Marsters SA, Ayres TM, Skubatch M, Gray CL, Rothe M, Ashkenazi A. Herpesvirus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-kappaB and AP-1. J Biol Chem 1997; 272:14029–14032. [DOI] [PubMed] [Google Scholar]
- 42. Dahl CP, Gullestad L, Fevang B, Holm AM, Landrø L, Vinge LE, Fiane AE, Sandberg WJ, Otterdal K, Frøland SS, Damås JK, Halvorsen B, Aukrust P, Øie E, Yndestad A. Increased expression of LIGHT/TNFSF14 and its receptors in experimental and clinical heart failure. Eur J Heart Fail 2008; 10:352–359. [DOI] [PubMed] [Google Scholar]
- 43. Luo R, Liu C, Elliott SE, Wang W, Parchim N, Iriyama T, Daugherty PS, Tao L, Eltzschig HK, Blackwell SC, Sibai BM, Kellems RE, Xia Y. Transglutaminase is a critical link between inflammation and hypertension. J Am Heart Assoc 2016; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rodríguez-Iturbe B, Pons H, Quiroz Y, Lanaspa MA, Johnson RJ. Autoimmunity in the pathogenesis of hypertension. Nat Rev Nephrol 2014; 10:56–62. [DOI] [PubMed] [Google Scholar]
- 45. Rodríguez-Iturbe B, Pons H, Quiroz Y, Johnson RJ. The immunological basis of hypertension. Am J Hypertens 2014; 27:1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Trott DW, Harrison DG. The immune system in hypertension. Adv Physiol Educ 2014; 38:20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xia Y, Kellems RE. Receptor-activating autoantibodies and disease: preeclampsia and beyond. Expert Rev Clin Immunol 2011; 7:659–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jahns R, Boivin V, Lohse MJ. beta(1)-Adrenergic receptor function, autoimmunity, and pathogenesis of dilated cardiomyopathy. Trends Cardiovasc Med 2006; 16:20–24. [DOI] [PubMed] [Google Scholar]
- 49. Fu ML, Herlitz H, Wallukat G, Hilme E, Hedner T, Hoebeke J, Hjalmarson A. Functional autoimmune epitope on alpha 1-adrenergic receptors in patients with malignant hypertension. Lancet 1994; 344:1660–1663. [DOI] [PubMed] [Google Scholar]
- 50. Luther HP, Homuth V, Wallukat G. Alpha 1-adrenergic receptor antibodies in patients with primary hypertension. Hypertension 1997; 29:678–682. [DOI] [PubMed] [Google Scholar]
- 51. Wenzel K, Haase H, Wallukat G, Derer W, Bartel S, Homuth V, Herse F, Hubner N, Schulz H, Janczikowski M, Lindschau C, Schroeder C, Verlohren S, Morano I, Muller DN, Luft FC, Dietz R, Dechend R, Karczewski P. Potential relevance of alpha(1)-adrenergic receptor autoantibodies in refractory hypertension. PLoS One 2008; 3:e3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jüpner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest 1999; 103:945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xia Y, Wen H, Bobst S, Day MC, Kellems RE. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human trophoblast cells. J Soc Gynecol Investig 2003; 10:82–93. [DOI] [PubMed] [Google Scholar]
- 54. Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med 2008; 14:855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Siddiqui AH, Irani RA, Blackwell SC, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibody is highly prevalent in preeclampsia: correlation with disease severity. Hypertension 2010; 55:386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fu ML, Herlitz H, Schulze W, Wallukat G, Micke P, Eftekhari P, Sjögren KG, Hjalmarson A, Müller-Esterl W, Hoebeke J. Autoantibodies against the angiotensin receptor (AT1) in patients with hypertension. J Hypertens 2000; 18:945–953. [DOI] [PubMed] [Google Scholar]
- 57. Liao YH, Wei YM, Wang M, Wang ZH, Yuan HT, Cheng LX. Autoantibodies against AT1-receptor and alpha1-adrenergic receptor in patients with hypertension. Hypertens Res 2002; 25:641–646. [DOI] [PubMed] [Google Scholar]
- 58. Wei F, Jia XJ, Yu SQ, Gu Y, Wang L, Guo XM, Wang M, Zhu F, Cheng X, Wei YM, Zhou ZH, Fu M, Liao YH; SOT-AT1 Study Group Candesartan versus imidapril in hypertension: a randomised study to assess effects of anti-AT1 receptor autoantibodies. Heart 2011; 97:479–484. [DOI] [PubMed] [Google Scholar]
- 59. Dragun D, Müller DN, Bräsen JH, Fritsche L, Nieminen-Kelhä M, Dechend R, Kintscher U, Rudolph B, Hoebeke J, Eckert D, Mazak I, Plehm R, Schönemann C, Unger T, Budde K, Neumayer HH, Luft FC, Wallukat G. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med 2005; 352:558–569. [DOI] [PubMed] [Google Scholar]
- 60. Rossitto G, Regolisti G, Rossi E, Negro A, Nicoli D, Casali B, Toniato A, Caroccia B, Seccia TM, Walther T, Rossi GP. Elevation of angiotensin-II type-1-receptor autoantibodies titer in primary aldosteronism as a result of aldosterone-producing adenoma. Hypertension 2013; 61:526–533. [DOI] [PubMed] [Google Scholar]
- 61. Kem DC, Li H, Velarde-Miranda C, Liles C, Vanderlinde-Wood M, Galloway A, Khan M, Zillner C, Benbrook A, Rao V, Gomez-Sanchez CE, Cunningham MW, Yu X. Autoimmune mechanisms activating the angiotensin AT1 receptor in ‘primary’ aldosteronism. J Clin Endocrinol Metab 2014; 99:1790–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Becker MO, Kill A, Kutsche M, Guenther J, Rose A, Tabeling C, Witzenrath M, Kühl AA, Heidecke H, Ghofrani HA, Tiede H, Schermuly RT, Nickel N, Hoeper MM, Lukitsch I, Gollasch M, Kuebler WM, Bock S, Burmester GR, Dragun D, Riemekasten G. Vascular receptor autoantibodies in pulmonary arterial hypertension associated with systemic sclerosis. Am J Respir Crit Care Med 2014; 190:808–817. [DOI] [PubMed] [Google Scholar]
- 63. Guo L, Li M, Chen Y, Wang Q, Tian Z, Pan S, Zeng X, Ye S. Anti-endothelin receptor type A autoantibodies in systemic lupus erythematosus-associated pulmonary arterial hypertension. Arthritis Rheumatol 2015; 67:2394–2402. [DOI] [PubMed] [Google Scholar]
- 64. Li H, Kem DC, Zhang L, Huang B, Liles C, Benbrook A, Gali H, Veitla V, Scherlag BJ, Cunningham MW, Yu X. Novel retro-inverso peptide inhibitor reverses angiotensin receptor autoantibody-induced hypertension in the rabbit. Hypertension 2015; 65:793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Xia Y, Kellems RE. Is preeclampsia an autoimmune disease? Clin Immunol 2009; 133:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chan CT, Sobey CG, Lieu M, Ferens D, Kett MM, Diep H, Kim HA, Krishnan SM, Lewis CV, Salimova E, Tipping P, Vinh A, Samuel CS, Peter K, Guzik TJ, Kyaw TS, Toh BH, Bobik A, Drummond GR. Obligatory role for B cells in the development of angiotensin II-dependent hypertension. Hypertension 2015; 66:1023–1033. [DOI] [PubMed] [Google Scholar]
- 67. LaMarca B, Wallace K, Herse F, Wallukat G, Martin JN, Jr, Weimer A, Dechend R. Hypertension in response to placental ischemia during pregnancy: role of B lymphocytes. Hypertension 2011; 57:865–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Xia Y, Zhou CC, Ramin SM, Kellems RE. Angiotensin receptors, autoimmunity, and preeclampsia. J Immunol 2007; 179:3391–3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xia Y, Ramin SM, Kellems RE. Potential roles of angiotensin receptor-activating autoantibody in the pathophysiology of preeclampsia. Hypertension 2007; 50:269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. LaMarca B, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension 2008; 52:1168–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dhillion P, Wallace K, Herse F, Scott J, Wallukat G, Heath J, Mosely J, Martin JN, Jr, Dechend R, LaMarca B. IL-17-mediated oxidative stress is an important stimulator of AT1-AA and hypertension during pregnancy. Am J Physiol Regul Integr Comp Physiol 2012; 303:R353–R358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lamarca B, Speed J, Ray LF, Cockrell K, Wallukat G, Dechend R, Granger J. Hypertension in response to IL-6 during pregnancy: role of AT1-receptor activation. Int J Interferon Cytokine Mediat Res 2011; 2011:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Liu C, Luo R, Elliott SE, Wang W, Parchim NF, Iriyama T, Daugherty PS, Blackwell SC, Sibai BM, Kellems RE, Xia Y. Elevated transglutaminase activity triggers angiotensin receptor activating autoantibody production and pathophysiology of preeclampsia. J Am Heart Assoc 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. LaMarca B, Speed J, Fournier L, Babcock SA, Berry H, Cockrell K, Granger JP. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-alpha blockade. Hypertension 2008; 52:1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Irani RA, Zhang Y, Zhou CC, Blackwell SC, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Autoantibody-mediated angiotensin receptor activation contributes to preeclampsia through tumor necrosis factor-alpha signaling. Hypertension 2010; 55:1246–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Li J, LaMarca B, Reckelhoff JF. A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. Am J Physiol Heart Circ Physiol 2012; 303:H1–H8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Burness CB, Duggan ST. Etanercept (SB4): a review in autoimmune inflammatory diseases. BioDrugs 2016; 30:371–378. [DOI] [PubMed] [Google Scholar]
- 78. Randall KL. Rituximab in autoimmune diseases. Aust Prescr 2016; 39:131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Doyle HA, Mamula MJ. Post-translational protein modifications in antigen recognition and autoimmunity. Trends Immunol 2001; 22:443–449. [DOI] [PubMed] [Google Scholar]
- 80. Doyle HA, Mamula MJ. Posttranslational protein modifications: new flavors in the menu of autoantigens. Curr Opin Rheumatol 2002; 14:244–249. [DOI] [PubMed] [Google Scholar]
- 81. Anderton SM. Post-translational modifications of self antigens: implications for autoimmunity. Curr Opin Immunol 2004; 16:753–758. [DOI] [PubMed] [Google Scholar]
- 82. Cloos PA, Christgau S. Post-translational modifications of proteins: implications for aging, antigen recognition, and autoimmunity. Biogerontology 2004; 5:139–158. [DOI] [PubMed] [Google Scholar]
- 83. Doyle HA, Mamula MJ. Posttranslational modifications of self-antigens. Ann N Y Acad Sci 2005; 1050:1–9. [DOI] [PubMed] [Google Scholar]
- 84. Doyle HA, Mamula MJ. Autoantigenesis: the evolution of protein modifications in autoimmune disease. Curr Opin Immunol 2012; 24:112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Fasano A, Catassi C. Celiac disease. N Engl J Med 2012; 367:2419–2426. [DOI] [PubMed] [Google Scholar]
- 86. Molberg O, Mcadam SN, Körner R, Quarsten H, Kristiansen C, Madsen L, Fugger L, Scott H, Norén O, Roepstorff P, Lundin KE, Sjöström H, Sollid LM. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat Med 1998; 4:713–717. [DOI] [PubMed] [Google Scholar]
- 87. Sollid LM. Coeliac disease: dissecting a complex inflammatory disorder. Nat Rev Immunol 2002; 2:647–655. [DOI] [PubMed] [Google Scholar]
- 88. Sollid LM, Jabri B. Celiac disease and transglutaminase 2: a model for posttranslational modification of antigens and HLA association in the pathogenesis of autoimmune disorders. Curr Opin Immunol 2011; 23:732–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol 2003; 4:140–156. [DOI] [PubMed] [Google Scholar]
- 90. Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, Schuppan D. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med 1997; 3:797–801. [DOI] [PubMed] [Google Scholar]
- 91. Liu C, Wang W, Parchim N, Irani RA, Blackwell SC, Sibai B, Jin J, Kellems RE, Xia Y. Tissue transglutaminase contributes to the pathogenesis of preeclampsia and stabilizes placental angiotensin receptor type 1 by ubiquitination-preventing isopeptide modification. Hypertension 2014; 63:353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gundemir S, Colak G, Tucholski J, Johnson GV. Transglutaminase 2: a molecular Swiss army knife. Biochim Biophys Acta 2012; 1823:406–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kuncio GS, Tsyganskaya M, Zhu J, Liu SL, Nagy L, Thomazy V, Davies PJ, Zern MA. TNF-alpha modulates expression of the tissue transglutaminase gene in liver cells. Am J Physiol 1998; 274:G240–G245. [DOI] [PubMed] [Google Scholar]
- 94. Suto N, Ikura K, Sasaki R. Expression induced by interleukin-6 of tissue-type transglutaminase in human hepatoblastoma HepG2 cells. J Biol Chem 1993; 268:7469–7473. [PubMed] [Google Scholar]
- 95. Sarkar NK, Clarke DD, Waelsch H. An enzymically catalyzed incorporation of amines into proteins. Biochim Biophys Acta 1957; 25:451–452. [DOI] [PubMed] [Google Scholar]
- 96. Thomázy V, Fésüs L. Differential expression of tissue transglutaminase in human cells. An immunohistochemical study. Cell Tissue Res 1989; 255:215–224. [DOI] [PubMed] [Google Scholar]
- 97. Eckert RL, Kaartinen MT, Nurminskaya M, Belkin AM, Colak G, Johnson GV, Mehta K. Transglutaminase regulation of cell function. Physiol Rev 2014; 94:383–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Keresztessy Z, Csosz E, Hársfalvi J, Csomós K, Gray J, Lightowlers RN, Lakey JH, Balajthy Z, Fésüs L. Phage display selection of efficient glutamine-donor substrate peptides for transglutaminase 2. Protein Sci 2006; 15:2466–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sugimura Y, Hosono M, Wada F, Yoshimura T, Maki M, Hitomi K. Screening for the preferred substrate sequence of transglutaminase using a phage-displayed peptide library: identification of peptide substrates for TGASE 2 and Factor XIIIA. J Biol Chem 2006; 281:17699–17706. [DOI] [PubMed] [Google Scholar]
- 100. Király R, Demény M, Fésüs L. Protein transamidation by transglutaminase 2 in cells: a disputed Ca2+-dependent action of a multifunctional protein. FEBS J 2011; 278:4717–4739. [DOI] [PubMed] [Google Scholar]
- 101. Nurminskaya MV, Belkin AM. Cellular functions of tissue transglutaminase. Int Rev Cell Mol Biol 2012; 294:1–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Small K, Feng JF, Lorenz J, Donnelly ET, Yu A, Im MJ, Dorn GW, 2nd, Liggett SB. Cardiac specific overexpression of transglutaminase II (G(h)) results in a unique hypertrophy phenotype independent of phospholipase C activation. J Biol Chem 1999; 274:21291–21296. [DOI] [PubMed] [Google Scholar]
- 103. Li X, Wei XL, Meng LL, Chi MG, Yan JQ, Ma XY, Jia YS, Liang L, Yan HT, Zheng JQ. Involvement of tissue transglutaminase in endothelin 1-induced hypertrophy in cultured neonatal rat cardiomyocytes. Hypertension 2009; 54:839–844. [DOI] [PubMed] [Google Scholar]
- 104. Bowness JM, Venditti M, Tarr AH, Taylor JR. Increase in epsilon(gamma-glutamyl)lysine crosslinks in atherosclerotic aortas. Atherosclerosis 1994; 111:247–253. [DOI] [PubMed] [Google Scholar]
- 105. Haroon ZA, Wannenburg T, Gupta M, Greenberg CS, Wallin R, Sane DC. Localization of tissue transglutaminase in human carotid and coronary artery atherosclerosis: implications for plaque stability and progression. Lab Invest 2001; 81:83–93. [DOI] [PubMed] [Google Scholar]
- 106. Sumi Y, Inoue N, Azumi H, Seno T, Okuda M, Hirata K, Kawashima S, Hayashi Y, Itoh H, Yokoyama M. Expression of tissue transglutaminase and elafin in human coronary artery: implication for plaque instability. Atherosclerosis 2002; 160:31–39. [DOI] [PubMed] [Google Scholar]
- 107. Cho BR, Kim MK, Suh DH, Hahn JH, Lee BG, Choi YC, Kwon TJ, Kim SY, Kim DJ. Increased tissue transglutaminase expression in human atherosclerotic coronary arteries. Coron Artery Dis 2008; 19:459–468. [DOI] [PubMed] [Google Scholar]
- 108. AbdAlla S, Lother H, Langer A, el Faramawy Y, Quitterer U. Factor XIIIA transglutaminase crosslinks AT1 receptor dimers of monocytes at the onset of atherosclerosis. Cell 2004; 119:343–354. [DOI] [PubMed] [Google Scholar]
- 109. Greenberg CS, Birckbichler PJ, Rice RH. Transglutaminases: multifunctional cross-linking enzymes that stabilize tissues. FASEB J 1991; 5:3071–3077. [DOI] [PubMed] [Google Scholar]
- 110. Lorand L, Jeong JM, Radek JT, Wilson J. Human plasma factor XIII: subunit interactions and activation of zymogen. Methods Enzymol 1993; 222:22–35. [DOI] [PubMed] [Google Scholar]
- 111. Lorand L. Factor XIII: structure, activation, and interactions with fibrinogen and fibrin. Ann N Y Acad Sci 2001; 936:291–311. [DOI] [PubMed] [Google Scholar]
- 112. Li X, Shams M, Zhu J, Khalig A, Wilkes M, Whittle M, Barnes N, Ahmed A. Cellular localization of AT1 receptor mRNA and protein in normal placenta and its reduced expression in intrauterine growth restriction. Angiotensin II stimulates the release of vasorelaxants. J Clin Invest 1998; 101:442–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Tower CL, Lui S, Charlesworth NR, Smith SD, Aplin JD, Jones RL. Differential expression of angiotensin II type 1 and type 2 receptors at the maternal-fetal interface: potential roles in early placental development. Reproduction 2010; 140:931–942. [DOI] [PubMed] [Google Scholar]
- 114. Hager H, Gliemann J, Hamilton-Dutoit S, Ebbesen P, Koppelhus U, Jensen PH. Developmental regulation of tissue transglutaminase during human placentation and expression in neoplastic trophoblast. J Pathol 1997; 181:106–110. [DOI] [PubMed] [Google Scholar]
- 115. Robinson NJ, Glazier JD, Greenwood SL, Baker PN, Aplin JD. Tissue transglutaminase expression and activity in placenta. Placenta 2006; 27:148–157. [DOI] [PubMed] [Google Scholar]
- 116. LaMarca BD, Ryan MJ, Gilbert JS, Murphy SR, Granger JP. Inflammatory cytokines in the pathophysiology of hypertension during preeclampsia. Curr Hypertens Rep 2007; 9:480–485. [DOI] [PubMed] [Google Scholar]
- 117. Tal R. The role of hypoxia and hypoxia-inducible factor-1alpha in preeclampsia pathogenesis. Biol Reprod 2012; 87:134. [DOI] [PubMed] [Google Scholar]
- 118. Nemes Z, Petrovski G, Aerts M, Sergeant K, Devreese B, Fésüs L. Transglutaminase-mediated intramolecular cross-linking of membrane-bound alpha-synuclein promotes amyloid formation in Lewy bodies. J Biol Chem 2009; 284:27252–27264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Watts SW, Priestley JR, Thompson JM. Serotonylation of vascular proteins important to contraction. PLoS One 2009; 4:e5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Walther DJ, Stahlberg S, Vowinckel J. Novel roles for biogenic monoamines: from monoamines in transglutaminase-mediated post-translational protein modification to monoaminylation deregulation diseases. FEBS J 2011; 278:4740–4755. [DOI] [PubMed] [Google Scholar]
- 121. Siegel M, Khosla C. Transglutaminase 2 inhibitors and their therapeutic role in disease states. Pharmacol Ther 2007; 115:232–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Gant NF, Daley GL, Chand S, Whalley PJ, MacDonald PC. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest 1973; 52:2682–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Li H, Armando I, Yu P, Escano C, Mueller SC, Asico L, Pascua A, Lu Q, Wang X, Villar VA, Jones JE, Wang Z, Periasamy A, Lau YS, Soares-da-Silva P, Creswell K, Guillemette G, Sibley DR, Eisner G, Gildea JJ, Felder RA, Jose PA. Dopamine 5 receptor mediates Ang II type 1 receptor degradation via a ubiquitin-proteasome pathway in mice and human cells. J Clin Invest 2008; 118:2180–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Brown MA, Wang J, Whitworth JA. The renin-angiotensin-aldosterone system in pre-eclampsia. Clin Exp Hypertens 1997; 19:713–726. [DOI] [PubMed] [Google Scholar]
- 125. Huelsz-Prince G, Belkin AM, VanBavel E, Bakker EN. Activation of extracellular transglutaminase 2 by mechanical force in the arterial wall. J Vasc Res 2013; 50:383–395. [DOI] [PubMed] [Google Scholar]
- 126. Steppan J, Sikka G, Jandu S, Barodka V, Halushka MK, Flavahan NA, Belkin AM, Nyhan D, Butlin M, Avolio A, Berkowitz DE, Santhanam L. Exercise, vascular stiffness, and tissue transglutaminase. J Am Heart Assoc 2014; 3:e000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Johnson KB, Thompson JM, Watts SW. Modification of proteins by norepinephrine is important for vascular contraction. Front Physiol 2010; 1:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Upchurch HF, Conway E, Patterson MK, Jr, Maxwell MD. Localization of cellular transglutaminase on the extracellular matrix after wounding: characteristics of the matrix bound enzyme. J Cell Physiol 1991; 149:375–382. [DOI] [PubMed] [Google Scholar]
- 129. Aeschlimann D, Thomazy V. Protein crosslinking in assembly and remodelling of extracellular matrices: the role of transglutaminases. Connect Tissue Res 2000; 41:1–27. [DOI] [PubMed] [Google Scholar]
- 130. Zemskov EA, Janiak A, Hang J, Waghray A, Belkin AM. The role of tissue transglutaminase in cell-matrix interactions. Front Biosci 2006; 11:1057–1076. [DOI] [PubMed] [Google Scholar]
- 131. Belkin AM. Extracellular TG2: emerging functions and regulation. FEBS J 2011; 278:4704–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Smethurst PA, Griffin M. Measurement of tissue transglutaminase activity in a permeabilized cell system: its regulation by Ca2+ and nucleotides. Biochem J 1996; 313:803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Verderio EA, Johnson T, Griffin M. Tissue transglutaminase in normal and abnormal wound healing: review article. Amino Acids 2004; 26:387–404. [DOI] [PubMed] [Google Scholar]
- 134. Murthy SN, Wilson J, Guy SL, Lorand L. Intramolecular crosslinking of monomeric fibrinogen by tissue transglutaminase. Proc Nat Acad Sci USA 1991; 88:10601–10604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Akimov SS, Belkin AM. Cell-surface transglutaminase promotes fibronectin assembly via interaction with the gelatin-binding domain of fibronectin: a role in TGFbeta-dependent matrix deposition. J Cell Sci 2001; 114:2989–3000. [DOI] [PubMed] [Google Scholar]
- 136. Kleman JP, Aeschlimann D, Paulsson M, van der Rest M. Transglutaminase-catalyzed cross-linking of fibrils of collagen V/XI in A204 rhabdomyosarcoma cells. Biochemistry 1995; 34:13768–13775. [DOI] [PubMed] [Google Scholar]
- 137. Bakker EN, Buus CL, Spaan JA, Perree J, Ganga A, Rolf TM, Sorop O, Bramsen LH, Mulvany MJ, Vanbavel E. Small artery remodeling depends on tissue-type transglutaminase. Circ Res 2005; 96:119–126. [DOI] [PubMed] [Google Scholar]
- 138. Johnson KA, Polewski M, Terkeltaub RA. Transglutaminase 2 is central to induction of the arterial calcification program by smooth muscle cells. Circ Res 2008; 102:529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Heagerty AM, Aalkjaer C, Bund SJ, Korsgaard N, Mulvany MJ. Small artery structure in hypertension. Dual processes of remodeling and growth. Hypertension 1993; 21:391–397. [DOI] [PubMed] [Google Scholar]
- 140. Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension 2001; 38:581–587. [DOI] [PubMed] [Google Scholar]
- 141. Engholm M, Eftekhari A, Chwatko G, Bald E, Mulvany MJ. Effect of cystamine on blood pressure and vascular characteristics in spontaneously hypertensive rats. J Vasc Res 2011; 48:476–484. [DOI] [PubMed] [Google Scholar]
- 142. Eftekhari A, Rahman A, Schaebel LH, Chen H, Rasmussen CV, Aalkjaer C, Buus CL, Mulvany MJ. Chronic cystamine treatment inhibits small artery remodelling in rats. J Vasc Res 2007; 44:471–482. [DOI] [PubMed] [Google Scholar]
- 143. Santhanam L, Tuday EC, Webb AK, Dowzicky P, Kim JH, Oh YJ, Sikka G, Kuo M, Halushka MK, Macgregor AM, Dunn J, Gutbrod S, Yin D, Shoukas A, Nyhan D, Flavahan NA, Belkin AM, Berkowitz DE. Decreased S-nitrosylation of tissue transglutaminase contributes to age-related increases in vascular stiffness. Circ Res 2010; 107:117–125. [DOI] [PubMed] [Google Scholar]
- 144. Lai TS, Hausladen A, Slaughter TF, Eu JP, Stamler JS, Greenberg CS. Calcium regulates S-nitrosylation, denitrosylation, and activity of tissue transglutaminase. Biochemistry 2001; 40:4904–4910. [DOI] [PubMed] [Google Scholar]
- 145. van den Akker J, VanBavel E, van Geel R, Matlung HL, Guvenc Tuna B, Janssen GM, van Veelen PA, Boelens WC, De Mey JG, Bakker EN. The redox state of transglutaminase 2 controls arterial remodeling. PLoS One 2011; 6:e23067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Schiffrin EL. Mechanisms of remodelling of small arteries, antihypertensive therapy and the immune system in hypertension. Clin Invest Med 2015; 38:E394–E402. [DOI] [PubMed] [Google Scholar]
- 147. Jang GY, Jeon JH, Cho SY, Shin DM, Kim CW, Jeong EM, Bae HC, Kim TW, Lee SH, Choi Y, Lee DS, Park SC, Kim IG. Transglutaminase 2 suppresses apoptosis by modulating caspase 3 and NF-kappaB activity in hypoxic tumor cells. Oncogene 2010; 29:356–367. [DOI] [PubMed] [Google Scholar]
- 148. Penumatsa KC, Toksoz D, Warburton RR, Hilmer AJ, Liu T, Khosla C, Comhair SA, Fanburg BL. Role of hypoxia-induced transglutaminase 2 in pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol 2014; 307:L576–L585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Keillor JW, Apperley KY, Akbar A. Inhibitors of tissue transglutaminase. Trends Pharmacol Sci 2015; 36:32–40. [DOI] [PubMed] [Google Scholar]