Abstract
BACKGROUND
Retinal microvascular traits predict adverse health outcomes. The Singapore I Vessel Assessment (SIVA) software improved automated postprocessing of retinal photographs. In addition to microvessel caliber, it generates measures of arteriolar and venular geometry. Few studies addressed the reproducibility of SIVA measurements across a wide age range.
METHODS
In the current study, 2 blinded graders read images obtained by nonmydriatic retinal photography twice in 20 11-year-old children, born prematurely (n = 10) or at term (n = 10) and in 60 adults (age range, 18.9–86.1 years).
RESULTS
Former preterm compared with term children had lower microvessel diameter and disorganized vessel geometry with no differences in intraobserver and interobserver variability. Among adults, microvessel caliber decreased with age and blood pressure and arteriolar geometry was inversely correlated with female sex and age. Intraobserver differences estimated by the Bland–Altman method did not reach significance for any measurement. Across measurements, median reproducibility (RM) expressed as percent of the average trait value was 8.8% in children (median intraclass correlation coefficient [ICC], 0.94) and 8.0% (0.97) in adults. Likewise, interobserver differences did not reach significance with RM (ICC) of 10.6% (0.85) in children and 10.4% (0.93) in adults. Reproducibility was best for microvessel caliber (intraobserver/interobserver RM, 4.7%/6.0%; ICC, 0.98/0.96), worst for venular geometry (17.0%/18.8%; 0.93/0.84), and intermediate for arteriolar geometry (10.9%/14.9%; 0.95/0.86).
CONCLUSIONS
SIVA produces repeatable measures of the retinal microvasculature in former preterm and term children and in adults, thereby proving its usability from childhood to old age.
Keywords: blood pressure, hypertension, microcirculation, population research, reproducibility, retina, vascular geometry
Nonmydriatic retinal photography is a completely noninvasive technique that generates clinically relevant information.1–3 The retinal microvasculature undergoes extensive perinatal maturation,4,5 so that prematurely born children have smaller retinal arterioles than children delivered at term.6,7 The diameters of the retinal microvessels carry important prognostic information.8–10 Smaller arteriolar diameter,9,10 wider venular caliber,10 and lower arteriolar-to-venular diameter ratio8 predict cardiovascular mortality,9 coronary heart disease,8 and lacunar stroke.10
Retinal photographs are usually postprocessed by software.11 The Integrative Vessel Analysis (IVAN)12,13 package allows the semiautomated detection of arterioles and venules. The Singapore I Vessel Assessment (SIVA)14–16 improved automation by detection of the optic disc center and edge and automatic identification of arterioles and venules. SIVA also provides a more global representation of the retinal microvascular network with measurements extending over up to 2 disc diameters and provides additional geometric information, such as fractal dimension, tortuosity, and branching angles.14–16 While the reproducibility of measuring the diameter of the retinal microvessels is relatively well established in adults,17,18 few studies addressed this issue in children or focused on the observer-induced variability associated with the larger number of SIVA measurements.14–16 In this article, we studied intraobserver and interobserver reproducibility of the wider range of SIVA measurements14–16 in 11-year-old children born with a birth weight of less than 1,000 g or delivered at term as well as in adults, respectively, enrolled in Prematurity as Predictor of Children’s Cardiovascular-Renal Health (PREMATCH)19,20 and Flemish Study on Environment, Genes and Health Outcomes (FLEMENGHO).21,22
METHODS
Study population
The PREMATCH study (registration number at clinicaltrials.org: NCT02147457)19,20 enrolled 93 children prematurely born with a birth weight of less than 1,000 g (participation rate, 66.4%) and 87 controls born at term, who all underwent retinal photography at 11 years of age. FLEMENGHO is a family-based population study, whose participants are representative for a geographically defined area in northern Belgium.21,22 The participants remained in follow-up since their initial recruitment from 1985 until 2004. The participation rate was 78.0% at recruitment and over 75% at the follow-up examinations. In 1,065 participants, follow-up included retinal imaging performed from January 2008 until March 2015. PREMATCH19,20 and FLEMENGHO21,22 comply with the Helsinki declaration for studies in human subjects.23 The Ethics Committee of the University Hospitals Leuven approved both studies. In PREMATCH, parents or custodians provided written informed consent and the children informed assent. Likewise, FLEMENGHO participants gave or renewed written informed consent at each contact. For the current study, we randomly selected retinal photographs of the left or right eye from 10 cases and 10 controls enrolled in PREMATCH and from 60 FLEMENGHO participants.
Retinal photography and postprocessing
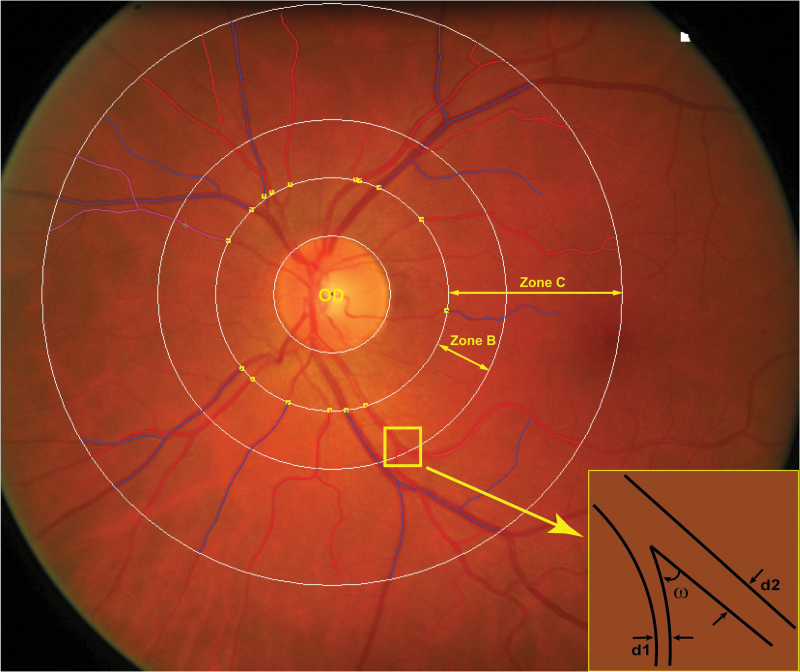
Participants were asked to refrain from heavy exercise, smoking, drinking alcohol, or caffeine-containing beverages for at least 3 hours before retinal imaging. Patients on blood pressure lowering drugs continued their medication on examination days. We applied a nonmydriatic approach in a dimly lit room to obtain retinal photographs, 1 image per eye in each participant, with the Canon CR-DGi retinal visualization system combined with the Canon D-50 digital camera (Canon Inc, Medical Equipment Group, Utsunomiya, Japan). We sorted the available images by ascending identification number and then used a random function to select images according to their rank number in the sorted list. The number of low-quality retinal photographs replaced by a higher quality image was 2 among PREMATCH children and 3 among FLEMENGHO participants. Trained observers (Q.-F.H., F.-F.W., Z.-Y.Z.,) applied the SIVA software, version 3.6 (Singapore Eye Research Institute, Singapore) to measure a range of retinal traits from 0.5 to 2.0 disc diameters away from the optic disc margin (Figure 1). The retinal microvascular diameters are expressed as central retinal arteriolar equivalent (CRAE), central retinal venular equivalent (CRVE), and the arteriole-to-venule diameter ratio (AVR) based on formulae published by Parr24,25 and Hubbard.26 The retinal vascular fractal dimension quantifies the complexity of the branching pattern of the retinal vascular tree and is computed using a box-counting method, larger values indicating a more complex branching pattern. It is derived from the gradient between the logarithm of the number of boxes (log n) plotted against the box size (log ε).27 Simple retinal tortuosity is the difference between the actual path length of the vessel segment measured by tracking and the straight line length of the segment divided by the straight line length. Curvature tortuosity is the integral of the curvature squared along the path of the vessel, normalized by the total path length, and therefore takes into account the bowing and points of inflection. The retinal vascular branching angle is expressed as the number of degrees between 2 daughter vessels at the first bifurcation. The branching asymmetry factor is the ratio of the squares of the two branching vessel widths (d12/d22). Assessment of the branching pattern of the retinal microvessels comprises the retinal vascular branching angle (ω) and the retinal vascular branching asymmetry factor (Figure 1).
Figure 1.
Retinal photograph centered on the optic disk (OD) and highlighting zone B and C, which are respectively 0.5 to 2.0 disc diameters away from the optic disc margin and in which the measurements are made. The branching asymmetry factor is the ratio of the squares of the 2 branching vessel widths (d12/d22). ω indicates the branching angle.
Graders were blinded to the identity of the study participants. Q.-F.H. read all images (n = 80) and Z.-Y.Z. (n = 20) and F.-F.W. (n = 60), respectively, postprocessed the PREMATCH and FLEMENGHO images allowing to assess interobserver variability. To quantify intraobserver variability, each observer read the photographs assigned to her twice. The total number of read-outs was therefore 80 in PREMATCH children and 240 in FLEMENGHO adults.
Other measurements
Blood pressure was the average of consecutive readings (3 in PREMATCH and 5 in FLEMENGHO) obtained after participants had rested for 5 minutes in sitting position by means of a standard mercury sphygmomanometer (Riester GmbH, Jungingen, Germany). Body mass index was weight in kilograms divided by height in meters squared. We converted anthropometric measurements in children to Z-scores based on Flemish growth charts.28 Validated questionnaires were used to obtain information on each participant’s medical history, smoking and drinking habits, and intake of medications. With participants fasting for at least 6 hours, venous blood samples were drawn for measurement of plasma glucose and serum cholesterol.
Statistical analysis
For database management and statistical analysis, we used SAS software, version 9.4 (SAS Institute, Cary, NC). For comparison of means and proportions, we applied Student’s t-test for paired or unpaired observations, as appropriate, and the χ2-statistic, respectively. To compare the retinal traits between PREMATCH cases and controls, while combining observations made by 2 graders, we applied mixed models, which accounted for clustering of the observations within children. We assessed the agreement between paired measurements by Bland and Altman’s method.29 Reproducibility was twice the SD of the pairwise differences between repeat measurements, expressed as a percentage of the average of all first and repeat measurements. To enable comparison with the literature,30–32 we also computed the intraclass correlation coefficient (ICC) between repeat measurements and the interclass correlation between observers. Based on previous experience,17 we evaluated sex, age, mean arterial pressure, smoking, serum total cholesterol, and plasma glucose as possible covariables of the retinal traits in adults. In this analysis, we used the readings of 2 observers as dependent variable and we accounted for clustering within individuals by applying general estimating equations as implemented in the PROC GENMOD procedure of the SAS package. In all analyses, statistical significance was an α-level of 0.05 or less on 2-sided tests.
RESULTS
Characteristics of participants
In PREMATCH, the 10 cases and 10 controls had similar sex distributions (55.0% girls), age, and anthropometric characteristics (P ≥ 0.32). Mean values (±SD) were 11.2 ± 1.3 years for age, 146.3 ± 8.6 cm, and 36.9 ± 7.1 kg for body height and weight and 17.1 ± 2.1 kg/m2 for body mass index. Gestational age was 27.7 weeks in cases (Supplementary Table S1). Birth weight averaged 0.76 kg in cases and 3.09 kg in controls (Supplementary Table S1). Cases compared with controls had higher systolic (113.8 ± 10.9 vs. 106.0 ± 9.0 mm Hg; P = 0.10) and diastolic blood pressures (64.9 ± 6.3 vs. 60.7 ± 5.4 mm Hg; P = 0.12) and higher mean arterial pressure (81.2 ± 6.6 vs. 75.8 ± 5.0 mm Hg; P = 0.053, but in this randomly selected subset of the PREMATCH children20 formal significance for the blood pressure differences was not reached. The characteristics of the PREMATCH children included (n = 20) and not included (n = 160) in the current reproducibility study were similar (P ≥ 0.060) with the exception of a 0.34 kg difference in the birth weight of controls (Supplementary Table S2).
Among the FLEMENGHO participants (53.3% women), mean age was 50.9 ± 16.5 years, ranging from 18.9 to 86.1 years. Of the 60 participants, 11 (18.3%) were smokers, 46 (76.7%) reported alcohol intake and 16 (26.7%) had hypertension, of whom 5 were on antihypertensive drug treatment. Mean values were 26.8 ± 5.1 kg/m2 for body mass index, 127.4 ± 17.5 mm Hg and 81.0 ± 10.8 mm Hg for systolic and diastolic blood pressure, and 5.02 ± 1.07 mmol/l and 4.61 ± 0.50 mmol/l for serum total cholesterol and plasma glucose. More details on the clinical and biochemical characteristics of the FLEMENGHO participants appear in Supplementary Table S3. The characteristics of the FLEMENGHO participants included (n = 60) and not included (n = 1005) in the current reproducibility study were all similar (P ≥ 0.21) with the exception of the prevalence of hypertension (26.7 vs. 44.0%; P = 0.009), treated hypertension (8.3 vs. 24.4%; P = 0.004), and plasma glucose (4.61 vs. 4.88 mmol/l; P = 0.0002), which were higher in not included participants (Supplementary Table S4). The proportion of hypertensive patients treated and treated and controlled was 31.3% and 18.8% among those included and 55.4% and 25.8% among those not included.
Retinal traits in PREMATCH cases and controls
As measured by Q.-F.H. and Z.-Y.Z., cases compared with controls had lower mean values for CRAE (155.2 ± 9.0 vs. 170.4 ± 9.8 µm; P < 0.0001), CRVE (224.3 ± 6.4 vs. 232.4 ± 12.9 µm; P = 0.022), and AVR (0.69 ± 0.05 vs. 0.73 ± 0.03; P = 0.003), but higher simple arteriolar tortuosity (1.129 ± 0.018 vs. 1.107 ± 0.014; P = 0.001), curvature arteriolar tortuosity (E−5 6.50 ± 0.94 vs. E−5 5.23 ± 0.63; P = 0.0001), arteriolar branching angle (91.3 ± 6.2 vs. 82.6 ± 5.9°; P = 0.0003), arteriolar asymmetry factor (0.79 ± 0.04 vs. 0.76 ± 0.03; P = 0.008), simple venular tortuosity (1.103 ± 0.010 vs. 1.095 ± 0.009; P = 0.016), and curvature venular tortuosity (E−5 5.61 ± 0.76 vs. E−5 4.92 ± 0.54; P = 0.003). Cases and controls had similar mean values of fractal dimension (1.44 ± 0.02 vs. 1.43 ± 0.02; P = 0.41), venular branching angle (80.6 ± 8.5 vs. 78.3 ± 5.3°; P = 0.32), and venular asymmetry factor (0.65 ± 0.06 vs. 0.68 ± 0.06; P = 0.090).
Correlates of retinal traits in FLEMENGHO adults
As covariables of the retinal traits in adults, we considered sex, age, mean arterial pressure, smoking, serum total cholesterol, and plasma glucose. In all adults (n = 60; 240 read-outs), while accounting for clustering within individuals, older age was significantly and independently associated with lower CRAE and CRVE and higher mean arterial pressure with smaller CRAE and AVR (Table 1). Fractal dimension decreased with age. Women compared with men had lower simple and curvature arteriolar tortuosity and lower arteriolar branching angle. Simple and curvature arteriolar tortuosity decreased with higher age. The arteriolar asymmetry factor increased with higher mean arterial pressure (Table 1). Venular simple and curvature tortuosity, branching angle, and asymmetry factor were not associated with any covariable. Serum total cholesterol and plasma glucose were not significantly associated with any retinal microvascular trait.
Table 1.
Mutually adjusted regression coefficients of retinal traits in FLEMENGHO adults
| Trait | Female sex | Age (+10 years) | MAP (+10 mm Hg) | Smoking |
|---|---|---|---|---|
| Diameters | ||||
| CRAE (µm) | 1.71 (−3.60 to 7.01) | −3.30 (−5.26 to −1.33)‡ | −2.96 (−5.25 to −0.68)† | −0.03 (−7.32 to 7.26) |
| CRVE (µm) | −1.78 (−8.85 to 5.28) | −5.73 (−8.45 to −3.01)‡ | 3.02 (−0.56 to 6.60) | −8.49 (−19.5 to 2.57) |
| AVR | 0.014 (−0.007 to 0.035) | 0.003 (−0.005 to 0.011) | −0.022 (−0.032 to −0.012)‡ | 0.025 (−0.001 to 0.051) |
| Fractal dimension | −0.002 (−0.022 to 0.017) | −0.015 (−0.021 to −0.008)‡ | 0.002 (−0.007 to 0.011) | −0.016 (−0.043 to 0.011) |
| Arterioles | ||||
| Simple tortuosity | −0.021 (−0.037 to −0.005)† | −0.006 (−0.011 to −0.001)* | 0.002 (−0.004 to 0.008) | 0.005 (−0.009 to 0.019) |
| Curvature tortuosity E−5 | −1.05 (−1.86 to −0.25)† | −0.35 (−0.61 to −0.10)† | 0.12 (−0.20 to 0.44) | 0.18 (−0.61 to 0.98) |
| Branching angle (°) | −0.26 (−8.28 to −0.24)* | 0.02 (−1.38 to 1.42) | −0.94 (−3.40 to 1.52) | −0.86 (−4.90 to 3.18) |
| Asymmetry factor | 0.023 (−0.012 to 0.058) | 0.004 (−0.008 to 0.015) | 0.022 (0.008 to 0.037)† | −0.007 (−0.048 to 0.033) |
The covariables considered included sex, age, mean arterial pressure, current smoking, serum total cholesterol, and plasma glucose. Venular simple and curvature tortuosity, branching angle, and asymmetry factor were not associated with any covariable. Serum total cholesterol and plasma glucose were not significantly associated with any retinal microvascular trait. Abbreviations: CRAE, central retinal arteriolar equivalent; CRVE, central retinal venular equivalent; MAP, mean arterial pressure (diastolic pressure plus one third of the difference between systolic and diastolic pressure). Significance of the associations: *P ≤ 0.05; †P < 0.01; ‡P ≤ 0.001.
Reproducibility of microvascular diameters
For the 2 observers involved in PREMATCH (Q.-F.H. and Z.-Y.Z.) and FLEMENGHO (Q.-F.H. and F.-F.W.), intraobserver reproducibility was similar for all retinal traits (P ≥ 0.10). There were also no differences in intraobserver (P ≥ 0.078) and interobserver (P ≥ 0.072) variability between PREMATCH cases and controls. In subsequent analyses of PREMATCH, we, therefore, pooled cases and controls and for intraobserver variability, in PREMATCH and FLEMENGHO, we pooled the readings of the 2 graders.
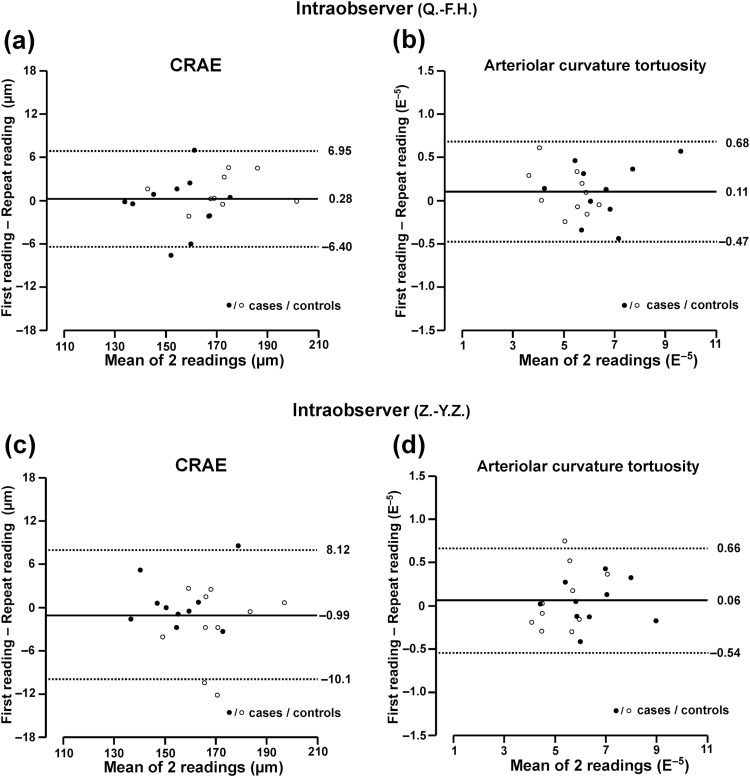
In PREMATCH (Table 2), the differences (first minus repeat) between 2 photograph readings by the same observer were −0.36 µm (P = 0.58) for CRAE, −1.35 µm (P = 0.22) for CRVE, and 0.003 units (P = 0.50) for AVR. Expressed as percent of the average trait value, reproducibility was 5.0%, 6.0%, and 7.4%, higher values indicating worse reproducibility (Table 2). The ICCs were 0.98, 0.95, and 0.95, respectively. For interobserver variability, the differences were 0.22 µm (P = 0.77) for CRAE, 0.71 µm (P = 0.59) for CRVE, and −0.003 units (P = 0.52) for AVR. The corresponding estimates of reproducibility expressed as percent of the average trait value were 5.9%, 7.3%, and 8.2% (Table 2) and the ICCs were 0.97, 0.91, and 0.93, respectively. Figure 2a and c showed the intraobserver variability of each observer.
Table 2.
Intraobserver and interobserver reproducibility of retinal microvascular diameters
| Cohort reproducibility trait | First measurement | Repeat measurement | P | Difference | Reproducibility | |||
|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | RC | %M | %MV | ||||
| PREMATCH | ||||||||
| Intraobserver | ||||||||
| CRAE (µm) | 162.6 ± 15.6 | 163.0 ± 15.3 | 0.58 | −0.36 ± 4.07 | −1.66 to 0.95 | 8.1 | 5.0 | 13.2 |
| CRVE (µm) | 227.6 ± 15.0 | 229.0 ± 14.9 | 0.22 | −1.35 ± 6.82 | −3.53 to 0.83 | 13.6 | 6.0 | 22.9 |
| AVR | 0.72 ± 0.06 | 0.71 ± 0.06 | 0.50 | 0.003 ± 0.026 | −0.006 to 0.011 | 0.05 | 7.4 | 22.6 |
| Interobserver | ||||||||
| CRAE (µm) | 162.9 ± 16.2 | 162.7 ± 14.6 | 0.77 | 0.22 ± 4.78 | −1.31 to 1.75 | 9.6 | 5.9 | 15.6 |
| CRVE (µm) | 228.7 ± 15.4 | 227.9 ± 14.6 | 0.59 | 0.71 ± 8.28 | −1.94 to 3.36 | 16.6 | 7.3 | 27.8 |
| AVR | 0.71 ± 0.05 | 0.72 ± 0.07 | 0.52 | −0.003 ± 0.029 | −0.012 to 0.006 | 0.06 | 8.2 | 25.3 |
| FLEMENGHO | ||||||||
| Intraobserver | ||||||||
| CRAE (µm) | 156.1 ± 14.0 | 156.6 ± 13.8 | 0.08 | −0.56 ± 3.42 | −1.18 to 0.06 | 6.8 | 4.4 | 12.4 |
| CRVE (µm) | 228.3 ± 19.1 | 228.6 ± 19.2 | 0.34 | −0.30 ± 3.46 | −0.93 to 0.32 | 6.9 | 3.0 | 9.1 |
| AVR | 0.69 ± 0.05 | 0.69 ± 0.05 | 0.25 | −0.001 ± 0.015 | −0.004 to 0.001 | 0.03 | 4.3 | 14.2 |
| Interobserver | ||||||||
| CRAE (µm) | 156.2 ± 13.9 | 156.4 ± 13.9 | 0.56 | −0.23 ± 4.25 | −1.00 to 0.54 | 8.5 | 5.4 | 15.4 |
| CRVE (µm) | 228.1 ± 18.9 | 228.7 ± 19.4 | 0.23 | −0.58 ± 5.31 | −1.54 to 0.38 | 10.6 | 4.6 | 13.9 |
| AVR | 0.69 ± 0.05 | 0.69 ± 0.05 | 0.72 | 0.001 ± 0.010 | −0.003 to 0.004 | 0.04 | 6.0 | 19.7 |
First and repeat measurements and differences (first minus repeat measurement) are means ± SD. The reproducibility coefficient (RC) is twice the SD of the signed differences between repeat measurements and are also expressed as percentage of the mean (%M) or 4 times the SD (%MV) of the averaged repeat measurements. Greater values indicate worse reproducibility. P is the significance of the difference between repeat measurements. Abbreviations: AVR, arteriole-to-venule ratio; CRAE, central retinal arteriolar equivalent; CRVE, central retinal venular equivalent; 95% CI, 95% confidence interval.
Figure 2.
Bland–Altman plots for intraobserver variability of 2 exemplary retinal traits in PREMATCH children: central retinal arteriolar equivalent (a, c) and curvature tortuosity (b, d). Plots include 10 cases (closed symbols) and 10 controls (open symbols), each read twice by observer 1 (Q.-F.H.) and observer 2 (Z.-Y.Z.).
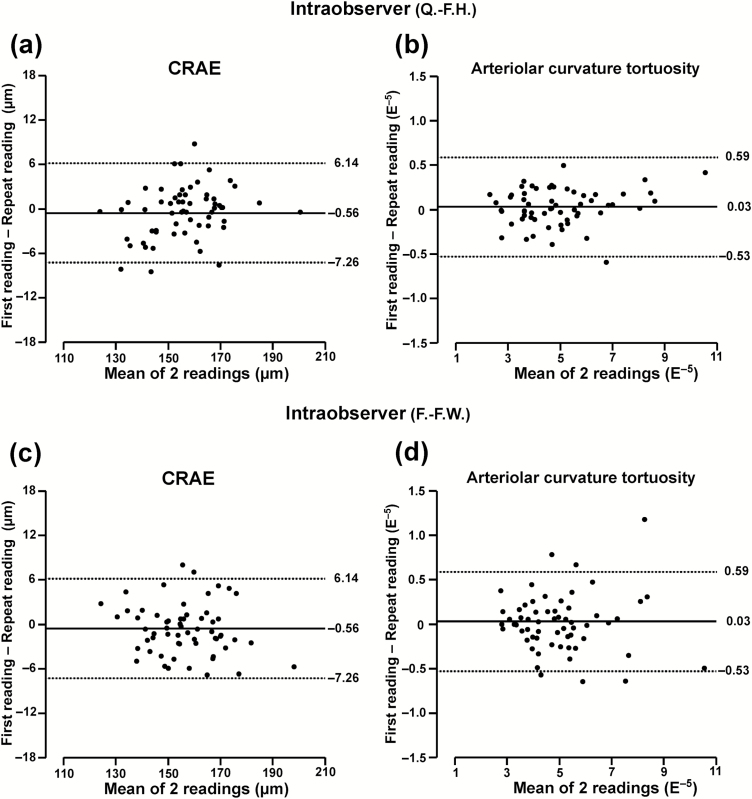
In FLEMENGHO adults (Table 2), the intraobserver differences (first minus repeat) were −0.56 µm (P = 0.08) for CRAE, −0.30 µm for CRVE (P = 0.34), and −0.001 units (P = 0.25) for AVR. The corresponding estimates of reproducibility in percent of the average trait value were 4.4%, 3.0%, and 4.3% (Table 2), and the ICCs 0.98, 0.99, and 0.98, respectively. The interobserver differences were −0.23 µm (P = 0.56) for CRAE, −0.58 µm (P = 0.23) for CRVE, and 0.001 units (P = 0.72) for AVR and reproducibility expressed in percent of the average trait value 5.4%, 4.6%, and 6.0% (Table 2). The ICCs were 0.97, 0.97, and 0.94, respectively. Figure 3a and c showed the intraobserver variability of each observer.
Figure 3.
Bland–Altman plots for intraobserver variability of 2 exemplary retinal traits in FLEMENGHO adults: central retinal arteriolar equivalent (a, c) and curvature tortuosity (b, d). Plots include 60 individuals each read twice by observer 1 (Q.-F.H.) and observer 2 (F.-F.W.).
Reproducibility of retinal microvascular patterns
For fractal dimension, the intraobserver reproducibility (Table 3), variability expressed as percent of the average trait value, and the ICC were 0.002 (P = 0.55), 2.3% and 0.95 in PREMATCH and −0.0007 (0.46), 1.4% and 0.99 in FLEMENGHO. The corresponding interobserver estimates (Table 4) were 0.004 (P = 0.36), 3.4% and 0.79 in PREMATCH and 0.001 (P = 0.38), 2.3% and 0.95 in FLEMENGHO.
Table 3.
Intraobserver reproducibility of retinal microvascular patterns
| Cohort trait | First measurement | Repeat measurement | P | Difference | Reproducibility | |||
|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | RC | %M | %MV | ||||
| PREMATCH | ||||||||
| Fractal dimension | 1.44 ± 0.03 | 1.44 ± 0.03 | 0.55 | 0.002 ± 0.016 | −0.004 to 0.007 | 0.03 | 2.3 | 24.5 |
| Arterioles | ||||||||
| Simple tortuosity | 1.12 ± 0.03 | 1.12 ± 0.03 | 0.71 | 0.0004 ± 0.007 | −0.002 to 0.003 | 0.01 | 1.3 | 14.6 |
| Curvature tortuosity | 5.91 ± 1.32 | 5.83 ± 1.30 | 0.09 | 0.083 ± 0.296 | −0.012 to 0.177 | 0.59 | 10.1 | 11.4 |
| Branching angle (°) | 86.8 ± 9.8 | 87.2 ± 9.6 | 0.76 | −0.32 ± 6.56 | −2.42 to 1.78 | 13.1 | 15.1 | 34.1 |
| Asymmetry factor | 0.77 ± 0.05 | 0.77 ± 0.06 | 0.95 | −0.0004 ± 0.039 | −0.013 to 0.012 | 0.08 | 10.2 | 35.7 |
| Venules | ||||||||
| Simple tortuosity | 1.10 ± 0.01 | 1.10 ± 0.01 | 0.41 | 0.001 ± 0.007 | −0.001 to 0.003 | 0.01 | 1.3 | 25.8 |
| Curvature tortuosity | 5.28 ± 0.95 | 5.26 ± 0.99 | 0.83 | 0.017 ± 0.490 | −0.140 to 0.173 | 0.98 | 18.6 | 25.7 |
| Branching angle (°) | 79.7 ± 10.7 | 79.2 ± 9.4 | 0.71 | 0.52 ± 8.94 | −2.34 to 3.38 | 17.9 | 22.5 | 44.7 |
| Asymmetry factor | 0.66 ± 0.08 | 0.67 ± 0.08 | 0.72 | −0.004 ± 0.061 | −0.023 to 0.016 | 0.12 | 18.5 | 38.0 |
| FLEMENGHO | ||||||||
| Fractal dimension | 1.40 ± 0.05 | 1.40 ± 0.05 | 0.46 | −0.0007 ± 0.01 | −0.002 to 0.001 | 0.02 | 1.4 | 9.9 |
| Arterioles | ||||||||
| Simple tortuosity | 1.11 ± 0.03 | 1.11 ± 0.03 | 0.28 | −0.001 ± 0.008 | −0.002 to 0.001 | 0.02 | 1.4 | 12.3 |
| Curvature tortuosity | 4.99 ± 1.64 | 4.96 ± 1.61 | 0.27 | 0.029 ± 0.285 | −0.023 to 0.080 | 0.57 | 11.5 | 8.8 |
| Branching angle (°) | 82.5 ± 9.7 | 82.3 ± 10.1 | 0.73 | 0.21 ± 6.64 | −0.99 to 1.41 | 13.3 | 16.1 | 33.7 |
| Asymmetry factor | 0.79 ± 0.08 | 0.80 ± 0.08 | 0.16 | −0.006 ± 0.046 | −0.014 to 0.002 | 0.09 | 11.7 | 27.5 |
| Venules | ||||||||
| Simple tortuosity | 1.09 ± 0.02 | 1.09 ± 0.02 | 0.62 | −0.0003 ± 0.007 | −0.002 to 0.001 | 0.01 | 1.3 | 22.3 |
| Curvature tortuosity | 4.93 ± 0.90 | 4.92 ± 0.94 | 0.69 | −0.013 ± 0.373 | −0.054 to 0.081 | 0.75 | 15.2 | 20.3 |
| Branching angle (°) | 81.0 ± 11.1 | 81.5 ± 12.0 | 0.49 | −0.53 ± 8.24 | −2.02 to 0.97 | 16.5 | 20.3 | 35.6 |
| Asymmetry factor | 0.69 ± 0.10 | 0.69 ± 0.11 | 0.71 | −0.002 ± 0.053 | −0.011 to 0.008 | 0.11 | 15.4 | 24.8 |
First and repeat measurements and differences (first minus repeat measurement) are means ± SD. For intraobserver variability, mixed models were used to combine data from 2 graders, while accounting for clustering within participants. Estimates for curvature tortuosity are multiplied by 105. The reproducibility coefficient (RC) is twice the SD of the signed differences between repeat measurements and is also expressed as percentage of the mean (%M) or 4 times the SD (%MV) of the averaged repeat measurements. Greater values indicate worse reproducibility. P is the significance of the difference between repeat measurements. Abbreviations: 95% CI, 95% confidence interval.
Table 4.
Interobserver reproducibility of retinal microvascular patterns
| Cohort trait | First measurement | Repeat measurement | P | Difference | Reproducibility | |||
|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | RC | %M | %MV | ||||
| PREMATCH | ||||||||
| Fractal dimension | 1.44 ± 0.04 | 1.44 ± 0.03 | 0.36 | 0.004 ± 0.02 | −0.004 to 0.012 | 0.05 | 3.4 | 37.1 |
| Arterioles | ||||||||
| Simple tortuosity | 1.12 ± 0.03 | 1.12 ± 0.03 | 0.15 | 0.003 ± 0.01 | −0.001 to 0.007 | 0.03 | 2.4 | 26.1 |
| Curvature tortuosity | 5.85 ± 1.37 | 5.88 ± 1.25 | 0.63 | 0.036 ± 0.464 | −0.184 to 0.113 | 0.93 | 15.8 | 17.8 |
| Branching angle (°) | 87.5 ± 10.0 | 86.5 ± 9.4 | 0.43 | 0.93 ± 7.40 | −1.44 to 3.30 | 14.8 | 17.0 | 38.5 |
| Asymmetry factor | 0.77 ± 0.05 | 0.77 ± 0.06 | 0.78 | −0.002 ± 0.5 | −0.02 to 0.01 | 0.10 | 13.0 | 45.8 |
| Venules | ||||||||
| Simple tortuosity | 1.10 ± 0.01 | 1.10 ± 0.01 | 0.05 | 0.003 ± 0.010 | −0.0001 to 0.006 | 0.02 | 1.8 | 35.0 |
| Curvature tortuosity | 5.20 ± 0.96 | 5.34 ± 0.98 | 0.19 | −0.137 ± 0.644 | −0.343 to 0.069 | 1.3 | 24.4 | 33.4 |
| Branching angle (°) | 78.5 ± 9.5 | 80.5 ± 10.5 | 0.20 | −1.94 ± 9.34 | −4.93 to 1.04 | 18.7 | 23.5 | 46.7 |
| Asymmetry factor | 0.66 ± 0.08 | 0.67 ± 0.08 | 0.18 | −0.013 ± 0.058 | −0.031 to 0.006 | 0.12 | 17.5 | 35.9 |
| FLEMENGHO | ||||||||
| Fractal dimension | 1.40 ± 0.05 | 1.40 ± 0.05 | 0.38 | 0.001 ± 0.016 | −0.002 to 0.004 | 0.03 | 2.3 | 16.6 |
| Arterioles | ||||||||
| Simple tortuosity | 1.11 ± 0.03 | 1.11 ± 0.03 | 0.48 | 0.0005 ± 0.008 | −0.001 to 0.002 | 0.02 | 1.5 | 12.9 |
| Curvature tortuosity | 4.94 ± 1.68 | 5.00 ± 1.56 | 0.06 | −0.064 ± 0.371 | −0.131 to 0.003 | 0.74 | 14.9 | 11.4 |
| Branching angle (°) | 82.0 ± 9.90 | 82.9 ± 9.84 | 0.19 | −0.86 ± 7.06 | −2.13 to 0.42 | 14.1 | 17.1 | 35.8 |
| Asymmetry factor | 0.80 ± 0.08 | 0.79 ± 0.08 | 0.36 | 0.005 ± 0.059 | −0.006 to 0.016 | 0.12 | 14.8 | 34.9 |
| Venules | ||||||||
| Simple tortuosity | 1.09 ± 0.02 | 1.09 ± 0.02 | 0.42 | −0.0007 ± 0.010 | −0.003 to 0.001 | 0.02 | 1.8 | 30.8 |
| Curvature tortuosity | 4.93 ± 0.96 | 4.92 ± 0.87 | 0.76 | 0.013 ± 0.449 | −0.069 to 0.094 | 0.90 | 18.2 | 24.5 |
| Branching angle (°) | 81.0 ± 10.6 | 81.5 ± 12.5 | 0.64 | −0.44 ± 10.4 | −2.32 to 1.43 | 20.7 | 25.5 | 44.8 |
| Asymmetry factor | 0.69 ± 0.11 | 0.70 ± 0.11 | 0.08 | −0.011 ± 0.067 | −0.023 to 0.001 | 0.13 | 19.3 | 31.1 |
First and repeat measurements and differences (first minus repeat measurement) are means ± SD. Estimates for curvature tortuosity are multiplied by 105. The reproducibility coefficient (RC) is twice the SD of the signed differences between repeat measurements and is also expressed as percentage of the mean (%M) or 4 times the SD (%MV) of the averaged repeat measurements. Greater values indicate worse reproducibility. P is the significance of the difference between repeat measurements. Abbreviations: 95% CI, 95% confidence interval.
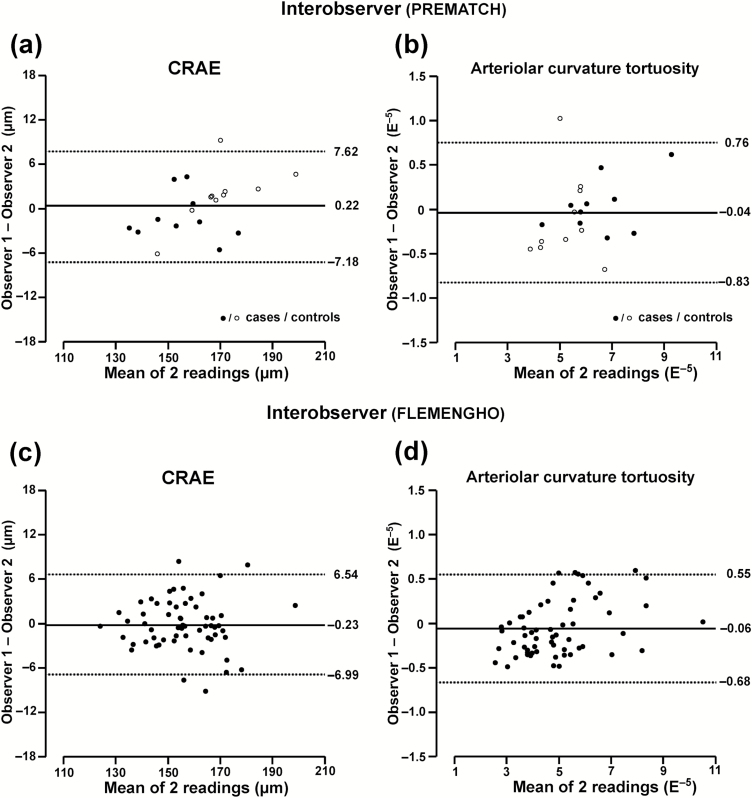
The intraobserver results for arteriolar and venular simple and curvature tortuosity, branching angle, and asymmetry factor appear in Table 3 and Figure 2b and d and Figure 3b and d. Expressed as percent of the average trait value, reproducibility ranged from 1.3% to 22.5% in PREMATCH (P ≥ 0.09) and from 1.4% to 20.3% in FLEMENGHO (P ≥ 0.16). The intraclass correlations were all higher than 0.85 except for the venular branching angle in PREMATCH (0.72) and FLEMENGHO (0.82). Table 4 and Figure 4 summarize the interobserver results for the same arteriolar and venular traits. Expressed as percent of the average trait value, reproducibility ranged from 1.8% to 24.4% in PREMATCH (P ≥ 0.05) and from 1.5% to 25.5% in FLEMENGHO (P ≥ 0.06). The intraclass correlations were all higher than 0.85 except for the arteriolar asymmetry factor (0.71) and venular branching angle (0.66) in PREMATCH and for the venular branching angle in FLEMENGHO (0.67).
Figure 4.
Bland–Altman plots for interobserver variability of 2 exemplary retinal traits in PREMATCH children and FLEMENGHO adults: central retinal arteriolar equivalent (a, c) and curvature tortuosity (b, d). The 2 readings done to determine intraobserver variability (Figures 2 and 3) were averaged to compute interobserver variability. Observers 1 and 2 were Q.-F.H. and Z.-Y.Z. in PREMATCH children (a, b) and Q.-F.H. and F.-F.W. in FLEMENGHO adults (c, d).
DISCUSSION
The retinal microcirculation is a pivotal trait in clinical and population research.1,2 Our current study builds on our previous research17,18 into the intraobserver and interobserver variability of retinal traits as postprocessed with IVAN (Vasculomatic ala Nicola, version 1.1, Department of Ophthalmology and Visual Science, University of Wisconsin-Madison, Madison, WI)33 or SIVA software. This article focused on the application of SIVA to phenotype a broader range of retinal traits in 11-year-old children born extremely prematurely or at term and in adults, thereby covering a wider age range than ever described before. The key findings can be summarized as follows: (i) preterm compared with term children had lower microvessel diameter and disorganized vessel geometry, but intraobserver and interobserver variability in cases and controls were similar; (ii) among adults, microvessel caliber decreased with age and blood pressure, while arteriolar geometry was inversely correlated with female sex and age; (iii) intraobserver and interobserver differences estimated by the Bland–Altman method did not reach significance for any measurement; (iv) and intraobserver and interobserver reproducibility were excellent for microvessel caliber, intermediate for arteriolar geometry and moderate for venular geometry. These reproducibility findings describe the performance of the software in the hands of 3 observers, but not the potential variability associated with taking the retinal photographs, because the graders read the same images.
That prematurely born children have narrower retinal arterioles than term children is well established.6,7 For instance, in the Generation R Study (Rotterdam, The Netherlands),6 retinal arteriolar calibers were read by IVAN from digitized retinal photographs in 4,122 6-year-old children. After adjustment for image grader, sex, age of the child, maternal lifestyle, and sociodemographic confounders, children born before 34 weeks of gestation and at 34–37 weeks, compared with children born at term, had narrower retinal arteriolar diameters with SD scores amounting to −0.46 (confidence interval, −0.77 to −0.15) and −0.24 (confidence interval, −0.42 to −0.05), respectively.6 Difference in age (5 vs. 11 years) and in the software (IVAN vs. SIVA) probably explain why in cases and controls combined CRAE (159.1 vs. 162.8 µm) and CRVE (219.0 vs. 228.3 µm) were slightly smaller in the Dutch6 than in our current study. In the Cardiovascular Risk in Young Finns Study,7 children aged 3–18 years were randomly selected from 5 Finnish University cities. At age 34–49 years, with adjustments applied for sex, age, employment, marital status, and smoking, premature compared with term birth was associated with narrower retinal arteriolar diameters (19.9 vs. 20.3 pixels; P = 0.034) and greater arteriolar tortuosity (0.06 vs. 0.04 × 102).7 In participants born small for gestational age, only arteriolar tortuosity was marginally higher (0.05 × 102; P = 0.074) compared with controls.
Our findings in adults are in agreement with the literature.15,30–32,34 Several investigators using SIVA reported that the use of this computer-assisted software package allows graders from paired fundus images to achieve robust and highly reproducible estimates of the retinal microvascular diameters and geometry in pregnant women32 or in participants enrolled in the Blue Mountain Eye,34 the Singapore Maley Eye,15 the Singapore Indian,31 and the Lothian Birth Cohort30 population studies. Across these studies,15,30–32,34 mean age ranged from 30.632 to 72.530 years, but was not explicitly reported for the subgroup of individuals enrolled in the reproducibility studies. However, individuals enrolled in the reproducibility studies were presumably randomly selected and were therefore representative for the whole population sample. What in our opinion sets our current study apart from most other, albeit not all,34 reports is that we assessed reproducibility using state-of-the-art Bland–Altman statistics. Other groups reported the reproducibility of retinal phenotypes as correlation coefficient30–32 or coefficient of variation.15 However, the use of a correlation coefficient for comparing repeat measurements is less accurate, as it measures the strength of a relation, but not the agreement between 2 variables.29 Furthermore, coefficients of variation represents a normalized measure of dispersion of a probability distribution. When the mean value is close to zero, the coefficient of variation will approach infinity and is therefore sensitive to small changes in the mean. To allow comparison with the literature, we also reported ICCs, which were all higher than 0.85 except for the arteriolar asymmetry factor (0.71) and venular branching angle (0.66) in PREMATCH and for the venular branching angle in FLEMENGHO (0.67). Intraobserver and interobserver reproducibility were best for microvessel caliber but less precise for vascular geometry. Our observations are consistent with results reported by Sasongko and coworkers.34 In their experience, the ICC for the arterial branching angle among 30 paired readings was 0.76, but after excluding poor quality images increased to 0.85.
Among adults, microvessel caliber decreased with age and blood pressure. That CRAE decreases with advancing age and high blood pressure is well established.2,3,15,17,35 Fewer studies reported on the correlates of retinal microvascular geometry. We found arteriolar tortuosity decreased with higher age and arteriolar asymmetry factor increased with mean blood pressure, which is consistent with previous studies.15,16 In 1,913 participants enrolled in the Singapore Malay Eye Study, with adjustments applied for sex, age, body mass index, use of antihypertensive medication and other risk factors, fractal dimension and arteriolar tortuosity decreased and venular tortuosity increased with mean arterial pressure.15 Other studies reported multivariable-adjusted inverse associations between fractal dimension and mean arterial pressure31,32 or between arteriolar tortuosity and age.16 We found lower arteriolar tortuosity in women than men, a finding not reported previously.15,16,31,32
Strong points of our current study are the large number of retinal images read (n = 320) and coverage of the whole age range. PREMATCH children (Supplementary Table S2) and FLEMENGHO participants (Supplementary Table S4) randomly selected for the current reproducibility study had similar characteristics with the exception of the prevalence of hypertension, treated hypertension, and plasma glucose among the adults (Supplementary Table S4). However, our current study must also be interpreted within the context of some potential limitations. First, PREMATCH19 and FLEMENGHO18 were carried out using different protocols. However, all PREMATCH and FLEMENGHO participants were examined at the same field center in the catchment area of the FLEMENGHO study by the same team of study nurses. Both PREMATCH and FLEMENGHO participants had their blood pressure measured by auscultation using a standard mercury sphygmomanometry. We limited the number of readings in children to 3 to minimize the discomfort caused by repeated cuff inflations, whereas in FLEMENGHO participants we averaged 5 readings to obtain the in-office blood pressure. Second, for the correlation analyses, our sample size was small. We addressed this issue by modeling individual participants as a random effect in regression analysis, allowing us to keep all images read in the analysis. Third, our findings are representative for White individuals recruited in Northern Belgium, but the literature suggests that our findings are representative for cohorts with a different ethnic composition.15,16,31,32
In population-based research, the retinal microvasculature is an established key trait, because of its association with hypertension1,17,36,37 and its role as predictor of adverse cardiovascular outcomes.8–10 Moreover, the retina shares similar embryological origin, anatomical features, and physiological properties with the brain and hence offers a unique and accessible window to study the correlates and consequences of subclinical brain pathology.38,39 While this concept has been introduced into clinical research in adults in the fields of stroke and dementia,38,40 our study suggest that it is also applicable in children. Other studies observed association of retinal microvascular diameters with renal microcirculatory traits as exemplified by the glomerular filtration rate3 or the urinary albumin excretion rate.2 These findings suggest that the retinal microcirculation in the eye might also reflect its counterpart in more distant organ systems, such as the kidney.2,3
In conclusion, SIVA produces repeatable measures of the retinal microvasculature in former preterm and term children and in adults, thereby proving that this software is a useful instrument for clinical research into the microcirculation over most of the human life span. As reported earlier,18 both IVAN and SIVA allow a reproducible assessment of retinal microvascular diameters, but SIVA generates slightly but significantly larger estimates of CRAE (3–5 µm) and CRVE (4–7 µm), with no difference in AVR. The implication is that it is only possible to combine historical AVR readings analyzed by IVAN with more recent readings analyzed by SIVA, but this approach cannot be recommended for CRAE or CRVE. Compared with IVAN, SIVA generates information over and beyond the diameter of the retinal microvasculature, including fractal dimensions, branching pattern, and tortuosity. Retinal vascular branching and tortuosity might be early indicators of microvascular damage in target organs.41 The perspective on the future is the development and validation of fully automated postprocessing software applying machine-learning algorithms, in a way similar as those already currently operational for the recognition of physiognomic traits. The expectation is that such fully automated approach will substantially reduce postprocessing time and minimize observer bias, thereby increasing the reproducibility of the assessment of microvascular retinal traits.
SUPPLEMENTARY MATERIAL
Supplementary data are available at American Journal of Hypertension online.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
The European Union (HEALTH-F7-305507 HOMAGE), the European Research Council (Advanced Researcher Grant 2011-294713-EPLORE and Proof-of-Concept Grant 713601-uPROPHET), the European Research Area Net for Cardiovascular Diseases (JTC2017-046-PROACT), and the Fonds voor Wetenschappelijk Onderzoek Vlaanderen, Ministry of the Flemish Community, Brussels, Belgium (G.0881.13) currently support the Studies Coordinating Centre in Leuven. The PREMATCH study has been facilitated by the Agency for Innovation, Science and Technology in Flanders (IWT) through the SAFEPEDRUG project (IWT/SBO 120033). The authors also acknowledge the support of the National Natural Science Foundation of China (81400312), the Shanghai Municipal Commission of Health and Family Planning (20144Y0213), and the Shanghai Jiaotong University School of Medicine (14XJ10071). The authors gratefully acknowledge the contribution of the nurses working at the examination centre (Linda Custers, Marie-Jeanne Jehoul, Daisy Thijs, and Hanne Truyens) and the clerical staff at the Studies Coordinating Centre (Annick De Soete and Renilde Wolfs).
REFERENCES
- 1. Cheung CY, Ikram MK, Sabanayagam C, Wong TY. Retinal microvasculature as a model to study the manifestations of hypertension. Hypertension 2012; 60:1094–1103. [DOI] [PubMed] [Google Scholar]
- 2. Daien V, Granados L, Kawasaki R, Villain M, Ribstein J, Cailar GD, Mimran A, Fesler P. Retinal vascular caliber associated with cardiac and renal target organ damage in never-treated hypertensive patients. Microcirculation 2017; 24:se12344. [DOI] [PubMed] [Google Scholar]
- 3. Gu YM, Petit T, Wei FF, Thijs L, Jacobs L, Zhang ZY, Yang WY, Cauwenberghs N, Knez J, Struijker-Boudier HA, Kuznetsova T, Verhamme P, Staessen JA. Renal glomerular dysfunction in relation to retinal arteriolar narrowing and high pulse pressure in seniors. Hypertens Res 2016; 39:138–143. [DOI] [PubMed] [Google Scholar]
- 4. Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other?Am J Hypertens 1988; 1:335–347. [DOI] [PubMed] [Google Scholar]
- 5. Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ 1989; 298:564–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gishti O, Jaddoe VW, Duijts L, Steegers E, Reiss I, Hofman A, Wong TY, Ikram MK, Gaillard R. Impact of birth parameters and early life growth patterns on retinal microvascular structure in children: The Generation R Study. J Hypertens 2015; 33:1429–1437. [DOI] [PubMed] [Google Scholar]
- 7. Hussain SM, Kähönen M, Raitakari OT, Skilton MR, Witt N, Chaturvedi N, Hutri-Kähönen N, Lehtimäki T, Vaahtoranta-Lehtonen H, Juonala M, Wijetunge S, Hughes AD, McG Thom SA, Metha A, Tapp RJ. Impact of fetal growth and preterm birth on the retinal microvasculature in mid-adulthood. Microcirculation 2015; 22:285–293. [DOI] [PubMed] [Google Scholar]
- 8. Wong TY, Klein R, Sharrett AR, Duncan BB, Couper DJ, Tielsch JM, Klein BE, Hubbard LD. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA 2002; 287:1153–1159. [DOI] [PubMed] [Google Scholar]
- 9. Wong TY, Klein R, Nieto FJ, Klein BE, Sharrett AR, Meuer SM, Hubbard LD, Tielsch JM. Retinal microvascular abnormalities and 10-year cardiovascular mortality: a population-based case-control study. Ophthalmology 2003; 110:933–940. [DOI] [PubMed] [Google Scholar]
- 10. Yatsuya H, Folsom AR, Wong TY, Klein R, Klein BE, Sharrett AR; ARIC Study Investigators Retinal microvascular abnormalities and risk of lacunar stroke: Atherosclerosis Risk in Communities Study. Stroke 2010; 41:1349–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yip W, Tham YC, Hsu W, Lee ML, Klein R, Klein B, Ikram MK, Wong TY, Cheung CY. Comparison of common retinal vessel caliber measurement software and a conversion algorithm. Transl Vis Sci Technol 2016; 5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wong TY, Islam FM, Klein R, Klein BE, Cotch MF, Castro C, Sharrett AR, Shahar E. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the multi-ethnic study of atherosclerosis (MESA). Invest Ophthalmol Vis Sci 2006; 47:2341–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klein R, Myers CE, Knudtson MD, Lee KE, Gangnon R, Wong TY, Klein BE. Relationship of blood pressure and other factors to serial retinal arteriolar diameter measurements over time: the beaver dam eye study. Arch Ophthalmol 2012; 130:1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheung CY, Hsu W, Lee ML, Wang JJ, Mitchell P, Lau QP, Hamzah H, Ho M, Wong TY. A new method to measure peripheral retinal vascular caliber over an extended area. Microcirculation 2010; 17:495–503. [DOI] [PubMed] [Google Scholar]
- 15. Cheung CY, Tay WT, Mitchell P, Wang JJ, Hsu W, Lee ML, Lau QP, Zhu AL, Klein R, Saw SM, Wong TY. Quantitative and qualitative retinal microvascular characteristics and blood pressure. J Hypertens 2011; 29:1380–1391. [DOI] [PubMed] [Google Scholar]
- 16. Cheung CY, Zheng Y, Hsu W, Lee ML, Lau QP, Mitchell P, Wang JJ, Klein R, Wong TY. Retinal vascular tortuosity, blood pressure, and cardiovascular risk factors. Ophthalmology 2011; 118:812–818. [DOI] [PubMed] [Google Scholar]
- 17. Liu YP, Richart T, Jin Y, Struijker-Boudier HA, Staessen JA. Retinal arteriolar and venular phenotypes in a Flemish population: reproducibility and correlates. Artery Res 2011; 5:72–79. [Google Scholar]
- 18. Wei FF, Zhang ZY, Petit T, Cauwenberghs N, Gu YM, Thijs L, Raaijmakers A, Jacobs L, Yang WY, Allegaert K, Kuznetsova T, Verhamme P, Struijker-Boudier HA, Li Y, Asayama K, Staessen JA. Retinal microvascular diameter, a hypertension-related trait, in ECG-gated vs. non-gated images analyzed by IVAN and SIVA. Hypertens Res 2016; 39:886–892. [DOI] [PubMed] [Google Scholar]
- 19. Raaijmakers A, Petit T, Gu Y, Zhang Z, Wei F, Cools B, Jacobs L, Thijs L, Thewissen L, Levtchenko E, Staessen JA, Allegaert K. Design and feasibility of “PREMATurity as predictor of children’s Cardiovascular-renal Health” (PREMATCH): A pilot study. Blood Press 2015; 24:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Raaijmakers A, Zhang ZY, Claessens J, Cauwenberghs N, van Tienoven TP, Wei FF, Jacobs L, Levtchenko E, Pauwels S, Kuznetsova T, Allegaert K, Staessen JA. Does extremely low birth weight pedispose to low-renin hypertension?Hypertension 2017; 69:443–449. [DOI] [PubMed] [Google Scholar]
- 21. Liu YP, Kuznetsova T, Jin Y, Thijs L, Asayama K, Gu YM, Bochud M, Verhamme P, Struijker-Boudier HA, Staessen JA. Heritability of the retinal microcirculation in Flemish families. Am J Hypertens 2013; 26:392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gu YM, Liu YP, Thijs L, Kuznetsova T, Wei FF, Struijker-Boudier HAJ, Verhamme P, Staessen JA. Central vs. peripheral blood pressure components as determinants of retinal microvessel diameters. Artery Res 2014; 8:35–43. [Google Scholar]
- 23. World Medical Association. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. J Am Med Ass 2013; 310:2191–2194. [DOI] [PubMed] [Google Scholar]
- 24. Parr JC, Spears GF. Mathematic relationships between the width of a retinal artery and the widths of its branches. Am J Ophthalmol 1974; 77:478–483. [DOI] [PubMed] [Google Scholar]
- 25. Parr JC, Spears GF. General caliber of the retinal arteries expressed as the equivalent width of the central retinal artery. Am J Ophthalmol 1974; 77:472–477. [DOI] [PubMed] [Google Scholar]
- 26. Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R, Cooper LS, Sharrett AR, Davis MD, Cai J. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology 1999; 106:2269–2280. [DOI] [PubMed] [Google Scholar]
- 27. Huang F, Dashtbozorg B, Zhang J, Bekkers E, Abbasi-Sureshjani S, Berendschot TT, Ter Haar Romeny BM. Reliability of using retinal vascular fractal dimension as a biomarker in the diabetic retinopathy detection. J Ophthalmol 2016; 2016:6259047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roelants M, Hauspie R, Hoppenbrouwers K. References for growth and pubertal development from birth to 21 years in Flanders, Belgium. Ann Hum Biol 2009; 36:680–694. [DOI] [PubMed] [Google Scholar]
- 29. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1:307–310. [PubMed] [Google Scholar]
- 30. McGrory S, Taylor AM, Kirin M, Corley J, Pattie A, Cox SR, Dhillon B, Wardlaw JM, Doubal FN, Starr JM, Trucco E, MacGillivray TJ, Deary IJ. Retinal microvascular network geometry and cognitive abilities in community-dwelling older people: The Lothian Birth Cohort 1936 study. Br J Ophthalmol 2017; 101:993–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thomas GN, Ong SY, Tham YC, Hsu W, Lee ML, Lau QP, Tay W, Alessi-Calandro J, Hodgson L, Kawasaki R, Wong TY, Cheung CY. Measurement of macular fractal dimension using a computer-assisted program. Invest Ophthalmol Vis Sci 2014; 55:2237–2243. [DOI] [PubMed] [Google Scholar]
- 32. Li LJ, Cheung CY, Ikram MK, Gluckman P, Meaney MJ, Chong YS, Kwek K, Wong TY, Saw SM. Blood pressure and retinal microvascular characteristics during pregnancy: Growing Up in Singapore Towards Healthy Outcomes (GUSTO) Study. Hypertension 2012; 60:223–230. [DOI] [PubMed] [Google Scholar]
- 33. Sherry LM, Wang JJ, Rochtchina E, Wong T, Klein R, Hubbard L, Mitchell P. Reliability of computer-assisted retinal vessel measurementin a population. Clin Exp Ophthalmol 2002; 30:179–182. [DOI] [PubMed] [Google Scholar]
- 34. Sasongko MB, Hodgson LA, Wong TY, Kawasaki R, Cheung CY, Hsu W, Lee ML, Lau PQ, Mitchell P, Wang JJ. Correlation and reproducibility of retinal vascular geometric measurements for stereoscopic retinal images of the same eyes. Ophthalmic Epidemiol 2012; 19:322–327. [DOI] [PubMed] [Google Scholar]
- 35. Wong TY, Klein R, Klein BE, Meuer SM, Hubbard LD. Retinal vessel diameters and their associations with age and blood pressure. Invest Ophthalmol Vis Sci 2003; 44:4644–4650. [DOI] [PubMed] [Google Scholar]
- 36. Wong TY, Shankar A, Klein R, Klein BE, Hubbard LD. Prospective cohort study of retinal vessel diameters and risk of hypertension. BMJ 2004; 329:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wong TY, Klein R, Sharrett AR, Duncan BB, Couper DJ, Klein BE, Hubbard LD, Nieto FJ; Atherosclerosis Risk in Communities Study Retinal arteriolar diameter and risk for hypertension. Ann Intern Med 2004; 140:248–255. [DOI] [PubMed] [Google Scholar]
- 38. Cheung CY, Ikram MK, Chen C, Wong TY. Imaging retina to study dementia and stroke. Prog Retin Eye Res 2017; 57:89–107. [DOI] [PubMed] [Google Scholar]
- 39. Msall ME. The retina as a window to the brain in vulnerable neonates. Pediatrics 2006; 117:2287–2289. [DOI] [PubMed] [Google Scholar]
- 40. Cheung CY, Chen C, Wong TY. Ocular fundus photography as a tool to study stroke and dementia. Semin Neurol 2015; 35:481–490. [DOI] [PubMed] [Google Scholar]
- 41. Sasongko MB, Wong TY, Donaghue KC, Cheung N, Jenkins AJ, Benitez-Aguirre P, Wang JJ. Retinal arteriolar tortuosity is associated with retinopathy and early kidney dysfunction in type 1 diabetes. Am J Ophthalmol 2012; 153:176–83.e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.