Abstract
BACKGROUND
Aldosterone acts to restrain the extracellular potassium (K+) concentration. Blacks have on average lower plasma aldosterone concentrations (PACs) than Whites. Whether this ethnic difference is associated with similar changes in the concentration of K+ is unclear.
METHODS
Subjects were Blacks and Whites from an observational study of blood pressure regulation. PAC was known to be significantly lower in Blacks than Whites. We sought to test the hypothesis that the concentration of K+ remains constant despite variability in PAC. Initial enrollment took place in childhood in 1986. Some of the original enrollees were studied again in adulthood: 160 healthy Blacks and 271 healthy Whites (ages 5 to 39 years; all were studied as children and as adults).
RESULTS
Plasma renin activity [a biomarker of angiotensin II and, more proximally, extracellular fluid volume (ECFV)] and PAC were lower in Blacks (P < 0.0354 and P < 0.001, respectively, for all ages). At the same time no ethnic difference in levels of K+ was observed regardless of age. Plasma K+ concentration and PAC associated differently based on ethnicity: PAC increased in Blacks by 1.5–2.0 and in Whites by 2.3–3.0 ng/dl per mmol/l increase in K+ (P < 0.001).
CONCLUSIONS
Lower aldosterone levels in Blacks did not translate into higher K+ concentrations. We speculate that reaching the right concentration of K+ was an endpoint of aldosterone production in the presence of varying levels of ECFV and angiotensin II.
Keywords: aldosterone, angiotensin II, blood pressure, extracellular fluid volume, ethnicity, hypertension, potassium.
The concentration of extracellular potassium (K+) impacts the functions of virtually all cells.1 Deviations beyond a narrow range of K+ concentrations can have clinical consequences that include cardiac arrhythmias and sudden death.2 Recent studies also suggest that the extracellular K+ concentration could influence sodium (Na+) reabsorption in the kidney through K+-dependent phosphorylation of the Na+ chloride cotransporter3,4 as well as phosphorylation of the ligand-binding domain of the mineralocorticoid receptor.5 Regulation of the prevailing K+ concentration could have relevance to a range of biologic functions.
Aldosterone plays a pivotal role to reduce the concentration of K+. It targets the distal nephron where it increases K+ secretion. Also, K+ is a potent stimulus of aldosterone production,6 an effect enhanced by angiotensin II,7 which increases when extracellular fluid volume (ECFV) declines. Thus, the plasma aldosterone concentration (PAC) is a product of stimulation by K+ and angiotensin II with both to different degrees linked to ECFV.8 The overall response to the different signals could be a steady-state concentration of K+.
In a previous cohort study, we showed that Blacks when compared to Whites had a lower PAC.9 Because of this ethnic difference, we sought to examine in the same cohort the relation of the K+ level to the prevailing PAC. We hypothesized that a lower PAC in Blacks is not necessarily accompanied by a higher concentration of K+.
METHODS
Subjects and study design
The current report was based on data generated by a long-running prospective observational study of Blacks and Whites that started in 1986. The study protocol and subject characteristics are described in detail elsewhere.9,10 Briefly, healthy Black children and White children between 5 and 17 years of age were recruited from schools in Indianapolis, Indiana. Children with renal or cardiac disease, hypertension, diabetes mellitus, or taking medications that could affect parameters being measured including BP were excluded from participation. Subjects were followed prospectively at intervals of 6 months. Urine samples were collected overnight. Blood samples were drawn between 0800 and 1000 hours after sitting for 15 minutes.
For most subjects studied in childhood, follow-up discontinued when they left high school. In 2008, the same subjects were invited to return for further assessments as adults. Reassessments occurred every 6 months up until the year 2013. The data collection protocol was the same except for the blood sampling, which now took place at every visit. Subjects studied only in childhood were excluded from the current study. Data from 37 individuals who were receiving antihypertensive medication were excluded from the analysis. The study protocol was approved by the Indiana University Institutional Review Board. Consent was obtained from all adult subjects and from parents of children under 18 years; assent was obtained from children as appropriate.
Assay procedures
Collected blood samples were used to measure the levels of plasma renin activity (PRA) and plasma aldosterone. PRA was measured with a Clinical Assays GammaCoat radioimmunoassay kit (Baxter Healthcare, Cambridge, MA) and aldosterone by radioimmunoassay with antiserum from Diagnostic Products Corp. (Los Angeles, CA). Na+ and K+ concentrations in the plasma, and urinary excretion rates were determined using flame photometry.
Data analysis
Subjects were classified by self-identified ethnicity categories. We had previously validated the self-reported ethnicity by comparing the genetic admixture of the DNA samples of our subjects to those of 7 known ethnic groups in the HAPMAP Phase 3 data.11 We found that the self-reported ethnicity information from our study had excellent accuracy.12 We summarized the demographic and clinical characteristics of the study participants by ethnicity. Continuous variables were compared using t tests and skewed data were transformed before analysis and geometric means were reported. Categorical variables were compared using chi-square tests. All available measures were used in the calculation of the summary statistics.
We first calculated the mean values of PRA, PAC, and plasma K+ for the child cohort and the adult cohort. Considering the wide age range of the study subjects and age-related variations in PRA, PAC, and plasma K+ concentration, we compared the mean levels of these measurements in Blacks and Whites in 3 age groups: less than 20 years, 20–30 years, and greater than 30 years of age. We then used varying-coefficient regression models to examine the association between plasma K+ concentration and PAC at different ages. Varying-coefficient models allowed us to characterize the effect of K+ on PAC at specific ages.13 In the current study, regression analysis was performed using PAC as the response variable; the regression coefficient of plasma K+ was modeled as a function of age, implying that the effect of an increase in K+ on aldosterone production could vary with age; separate estimates were obtained for Blacks and Whites. The analysis also adjusted for the effects of sex, body mass index, level of PRA, and rates of urinary Na+ and K+ excretion by including them as covariates. Estimated K+ effects on PAC were displayed graphically with corresponding 95% confidence intervals. The analysis was implemented using R software (R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/). P values less than 0.05 were considered statistically significant.
RESULTS
The analysis used data generated from 1,116 samples collected from 431 subjects (160 Blacks and 271 Whites). The basic demographic and clinical characteristics of subjects are presented in Table 1. In the child cohort, the mean ages of Blacks and Whites (~15 years) were not significantly different. Systolic and diastolic blood pressures and body mass index (with or without their expression as percentiles) were significantly greater in Blacks than in Whites (P < 0.0001 to P = 0.041). Plasma K+ concentrations in children were similar in Blacks and Whites, 4.26 and 4.20 mmol/l, respectively. In children, the mean level of PRA was lower in Blacks than Whites (2.24 vs. 2.61 ng/ml/h; P = 0.035). The average PAC was also lower in Blacks than Whites (7.11 vs. 11.32 ng/dl; P < 0.0001).
Table 1.
Demographic and clinical characteristics of the study subjects (PRA uses geometric means)
| Variable | Full sample | Blacks | Whites | P value |
|---|---|---|---|---|
| Characteristics of the children at the time of measurement | ||||
| N | 302 | 98 | 204 | |
| Age (years) (range: 5–17) | 14.06 (0.10) | 14.27 (0.20) | 13.95 (0.12) | 0.1807 |
| Systolic BP (mm Hg) | 105.58 (0.37) | 106.99 (0.64) | 104.79 (0.45) | 0.0041 |
| Systolic BP % | 39.28 (0.87) | 42.72 (1.56) | 37.37 (1.03) | 0.0028 |
| Diastolic BP (mm Hg) | 62.60 (0.29) | 63.67 (0.49) | 62.02 (0.35) | 0.0052 |
| Diastolic BP % | 47.05 (0.73) | 49.33 (1.30) | 45.81 (0.87) | 0.0203 |
| BMI (kg/m2) | 21.86 (0.22) | 23.79 (0.43) | 20.73 (0.21) | <0.0001 |
| BMI % | 64.38 (1.20) | 73.12 (1.84) | 59.26 (1.49) | <0.0001 |
| Plasma K (mmol/l) | 4.22 (0.03) | 4.26 (0.08) | 4.20 (0.02) | 0.3569 |
| PAC (ng/dl) | 9.58 (0.34) | 7.11 (0.45) | 11.32 (0.44) | <0.0001 |
| PRA (ng/ml/h) | 2.47 (0.09) | 2.24 (0.14) | 2.61 (0.10) | 0.0354 |
| Urine K (mmol/mg Cr) | 0.031 (0.021) | 0.027 (0.018) | 0.032 (0.022) | <0.001 |
| Urine Na (mmol/mg Cr) | 0.122 (0.066) | 0.120 (0.064) | 0.123 (0.067) | 0.0641 |
| Characteristics of the adults at the time of measurement | ||||
| N | 233 | 93 | 140 | |
| Age (year) (range: 20–39) | 31.73 (0.25) | 30.54 (0.41) | 32.45 (0.30) | 0.0002 |
| Systolic BP (mm Hg) | 117.29 (0.71) | 118.05 (1.17) | 116.83 (0.90) | 0.3978 |
| Diastolic BP (mm Hg) | 74.68 (0.58) | 75.73 (1.02) | 74.05 (0.69) | 0.1588 |
| BMI (kg/m2) | 29.35 (0.51) | 31.74 (0.85) | 27.92 (0.61) | 0.0002 |
| Plasma K (mmol/l) | 3.81 (0.01) | 3.79 (0.02) | 3.81 (0.02) | 0.5087 |
| PAC (ng/dl) | 5.40 (0.24) | 4.25 (0.30) | 6.44 (0.34) | <0.0001 |
| PRA (ng/ml/h) | 0.94 (0.05) | 0.69 (0.06) | 1.16 (0.07) | <0.0001 |
| Urine K (mmol/mg Cr) | 0.020 (0.013) | 0.018 (0.009) | 0.021 (0.015) | <0.0001 |
| Urine Na (mmol/mg Cr) | 0.080 (0.045) | 0.078 (0.041) | 0.082 (0.047) | 0.2364 |
Abbreviations: BMI, body mass index; BP, blood pressure; PAC, plasma aldosterone concentration; PRA, plasma renin activity.
In the adult cohort, Whites were slightly older than Blacks (32.2 vs. 30.5 year; P = 0.0002). Systolic and diastolic BPs were not statistically different between Blacks and Whites, but on average Blacks had greater body mass index than Whites (31.7 vs. 27.9 kg/m2; P = 0.0003). Mean plasma K+ concentrations were nearly identical in Blacks (3.79 mmol/l) and Whites (3.81 mmol/l). Levels of PRA and of plasma aldosterone were significantly lower in Black adults than White adults: for PRA, 0.69 ng/ml/h in Blacks vs. 1.16 ng/ml/h in Whites (P < 0.0001); for PAC: 4.25 ng/dl in Blacks vs. 6.44 ng/dl in Whites (P < 0.0001).
Age-related changes in PRA, PAC, and plasma K+
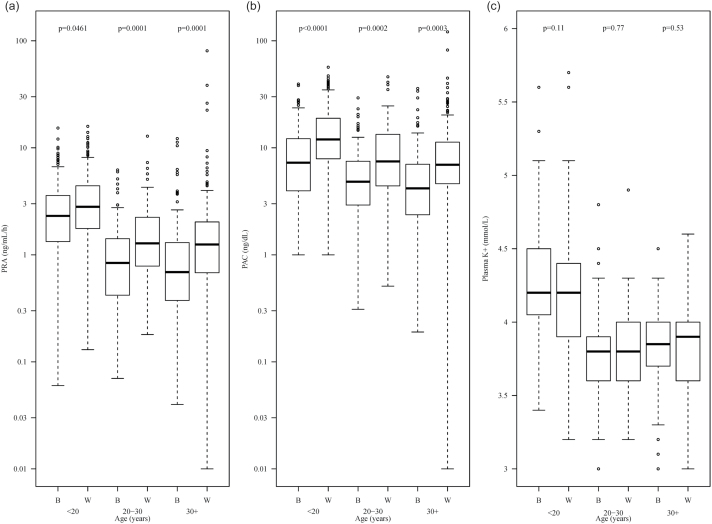
Examining the age-related variations, we calculated mean values of PRA, PAC, and plasma K+ concentration in Blacks and Whites within predefined age ranges. Comparative results are presented in Figure 1. The analysis showed that: (i) PRA, PAC, and plasma K+ concentration decreased with age regardless of ethnicity; (ii) In all age ranges, Blacks had lower PRA and PAC than Whites, but nearly identical plasma K+; P values are presented in Figure 1.
Figure 1.
Plasma renin activity (PRA), the plasma aldosterone concentration (PAC), and the plasma K+ concentration in Blacks and in Whites. (a) Distributions of PRA in Blacks and Whites by age. PRA was lower in Blacks than in Whites regardless of age (P < 0.05 for all age categories). (b) Distributions of PAC in Blacks and Whites by age. PAC was lower in Blacks than in Whites in all age categories (P < 0.003). (c) Distributions of plasma K concentration in Blacks and Whites by age. No ethnicity differences were detected.
Regression analysis showed that PAC decreased with age in both ethnic groups at the rate of approximately 0.1 ng/dl per year in Blacks and 0.3 ng/dl per year in Whites. At any given age, Whites had significantly higher PAC than Blacks (P < 0.01). A similar analysis showed that the plasma concentration of K+ decreased with age in both Blacks and Whites at approximately the same rate of 0.2–0.3 mmol/l per decade. At any given age, the mean K+ level in each ethnic group was virtually identical (P = 0.60 per linear regression model).
Urinary Na+ and K+
In children, urinary Na+ excretion rates were marginally different between Blacks and Whites (Blacks 0.120 vs. Whites 0.123 mmol/mg creatinine; P = 0.064). Black children had lower urinary K+ excretion than White children (0.027 vs. 0.032 mmol/mg creatinine; P < 0.0001). In adults, Na+ excretion rates were similar between the 2 ethnic groups (Blacks 0.078 vs. Whites 0.082 mmol/mg creatinine; P = 0.236). Black adults had a significantly lower level of urinary K+ excretion than White adults (0.018 vs. 0.021 mmol/mg creatinine; P < 0.0001).
A comparison between ethnic groups of the association of plasma K+ concentration with PAC
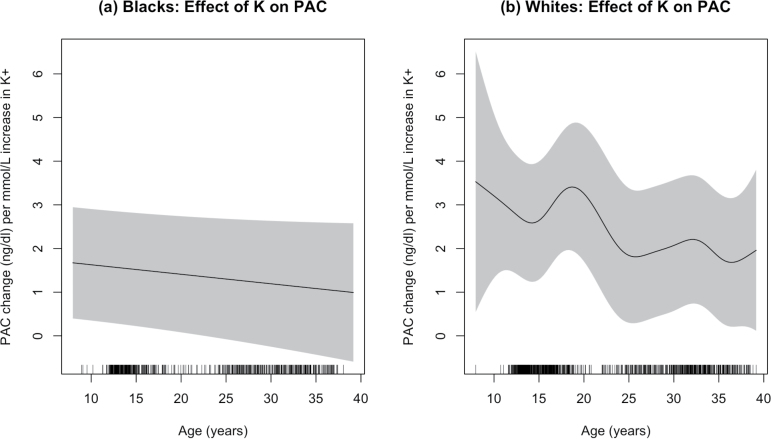
Finally, we assessed the relations between plasma K+ concentration to PAC at different ages in Blacks and in Whites. Results were adjusted for sex, body mass index, urinary Na+ and K+, and PRA. The estimated effects of plasma K+ on PAC are presented in Figure 2. The analysis showed first of all that the plasma K+ concentration was positively associated with PAC in both ethnic groups (all P < 0.0001) as evidenced by the positive values of the regression coefficients at all ages. Secondly, at any given age the plasma K+ concentration had a greater effect on PAC in Whites than in Blacks (P < 0.001). The ability of plasma K+ concentration to influence aldosterone production decreased with age in both ethnic groups. Analysis also showed that female sex, higher levels of PRA, and lower levels of urinary Na+ excretion were associated with higher PAC (P < 0.0001). Greater K+ excretion was marginally associated with increased PAC (P = 0.063).
Figure 2.
The estimated effects of an increase in the K+ concentration on PAC at different ages in Blacks (a) and Whites (b). At any age, the estimated effect of K+ concentration on PAC was much less in Blacks. For example at age 15, 1 mmol/l increase in K+ was associated with 1.6 and 2.7 ng/dl increase of PAC, respectively, in Blacks and Whites. Abbreviations: PAC, plasma aldosterone concentration.
DISCUSSION
In the present study, we found that regardless of the differences in PAC, with lower levels in Blacks, the K+ concentration was on average the same in Blacks and Whites. Blacks may depend more on an expanded ECFV to reach the correct K+ level. Expansion of ECFV leads to disposal of K+ by first reducing reabsorption of Na+ proximally in the nephron14 followed by delivery of Na+ downstream where it promotes K+ secretion.15 We also found that the association of the plasma K+ concentration with PAC was attenuated in Blacks in comparison to Whites (Figure 2); Blacks reached the same K+ concentration but with a lower PAC. The levels of angiotensin II would be expected to be lower in Blacks, thereby reducing its synergistic influence on K+ as a stimulus.7
Although findings from the present study are consistent with ECFV being decidedly influential in establishing the plasma concentration of K+, we did not have direct estimates of ECFV, which we recognize as a limitation. However, previous studies16 found that on average ECFV was greater in Blacks than Whites. There is also a large body of evidence that stands in support of Blacks having a more expanded volume including very early studies showing lower PRA levels and greater salt sensitivity of blood pressure in Blacks than in Whites.17–19
Our subjects were studied when they were children and again as young adults, allowing us opportunity to look at relationships to age. The plasma concentrations of both K+ and aldosterone declined over time, possibly from an age-related expansion of the ECFV. The ethnic differences in PAC persisted as age increased whereas K+ levels in Blacks and Whites were always the same. Had comparisons not been made within limited age ranges ( as children and as young adults), the similarities in K+ concentrations between ethnic groups could easily have been missed.
Finally, an earlier study of the current cohort showed that BP in Blacks but not in Whites was significantly related to PAC, referred to as “aldosterone sensitivity.12” As PRA decreased, aldosterone’s influence on BP became stronger, consistent with sensitivity from an expanded ECFV. In the present study, Blacks were more sensitive than Whites to a given PAC: less aldosterone was required by Blacks to maintain the same concentration of K+ observed in Whites. This could be but still another example of greater sensitivity to aldosterone that we speculate resulted from an underlying expansion of ECFV.
In conclusion, studies of 2 ethnic groups with different average PAC but similar K+ concentrations lead us to speculate that maintaining a more or less fixed extracellular concentration of K+ places an added consideration to the regulation of aldosterone production.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
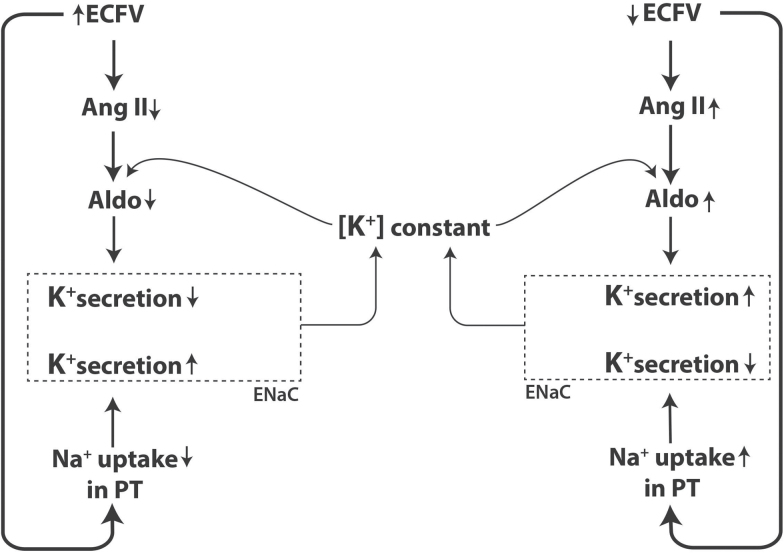
The authors are grateful to Mary Anne Wagner for invaluable laboratory work, to Linda M. Pratt for coordinating the adult study including re-recruitment of subjects and making the clinical measurements, and to Thomas Weinzerl from Medical Illustrations, IU School of Medicine, for preparing Figure 3. Funded by the National Institutes of Health (RO1-HL095086 and RO-HL 35795) and by a Department of Veterans Affairs Merit Review Award.
Figure 3.
Speculative mechanism whereby the extracellular potassium (K+) concentration remains constant despite differences in extracellular fluid volume (ECFV). An expanded ECFV results in 2 opposing influences on the plasma K+ concentration. As depicted on the left side of the figure, as volume expands, there is a reduction in sodium (Na+) uptake in proximal tubule with subsequent delivery of additional Na to cortical collecting duct where it increases activity of the epithelial Na+ channel (ENaC, depicted as rectangle with broken lines). An increase in ECFV also suppresses angiotensin II (Ang II) generation, which reduces its synergism with K+ in the adrenal’s production of aldosterone. This then decreases aldosterone-mediated activation of ENaC. Secretion of K+ is coupled to the different directions taken by ENaC activity resulting in no net change in K+ concentration. The opposite occurs when there is a deficit in ECFV (right side of figure).
REFERENCES
- 1. Hodgkin AL, Horowicz P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol 1959; 148:127–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Franse LV, Pahor M, Di Bari M, Somes GW, Cushman WC, Applegate WB. Hypokalemia associated with diuretic use and cardiovascular events in the Systolic Hypertension in the Elderly Program. Hypertension 2000; 35:1025–1030. [DOI] [PubMed] [Google Scholar]
- 3. Rengarajan S, Lee DH, Oh YT, Delpire E, Youn JH, McDonough AA. Increasing plasma [K+] by intravenous potassium infusion reduces NCC phosphorylation and drives kaliuresis and natriuresis. Am J Physiol Renal Physiol 2014; 306:F1059–F1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, Siler DA, Park HJ, Fu Y, Cohen DM, Weinstein AM, Wang WH, Yang CL, Ellison DH. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 2015; 21:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shibata S, Rinehart J, Zhang J, Moeckel G, Castañeda-Bueno M, Stiegler AL, Boggon TJ, Gamba G, Lifton RP. Mineralocorticoid receptor phosphorylation regulates ligand binding and renal response to volume depletion and hyperkalemia. Cell Metab 2013; 18:660–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dluhy RG, Axelrod L, Underwood RH, Williams GH. Studies of the control of plasma aldosterone concentration in normal man. II. Effect of dietary potassium and acute potassium infusion. J Clin Invest 1972; 51:1950–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pratt JH. Role of angiotensin II in potassium-mediated stimulation of aldosterone secretion in the dog. J Clin Invest 1982; 70:667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spät A, Hunyady L. Control of aldosterone secretion: a model for convergence in cellular signaling pathways. Physiol Rev 2004; 84:489–539. [DOI] [PubMed] [Google Scholar]
- 9. Pratt JH, Jones JJ, Miller JZ, Wagner MA, Fineberg NS. Racial differences in aldosterone excretion and plasma aldosterone concentrations in children. N Engl J Med 1989; 321:1152–1157. [DOI] [PubMed] [Google Scholar]
- 10. Manatunga AK, Jones JJ, Pratt JH. Longitudinal assessment of blood pressures in Black and White children. Hypertension 1993; 22:84–89. [DOI] [PubMed] [Google Scholar]
- 11. Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 2006; 38:904–909. [DOI] [PubMed] [Google Scholar]
- 12. Tu W, Eckert GJ, Hannon TS, Liu H, Pratt LM, Wagner MA, Dimeglio LA, Jung J, Pratt JH. Racial differences in sensitivity of blood pressure to aldosterone. Hypertension 2014; 63:1212–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hastie T.J., Tibshirani R.J. Smoothing. In Generalized Additive Models. Chapman & Hall: London, 1990, pp 29–31. [Google Scholar]
- 14. McDonough AA. Mechanisms of proximal tubule sodium transport regulation that link extracellular fluid volume and blood pressure. Am J Physiol Regul Integr Comp Physiol 2010; 298:R851–R861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stokes JB. Potassium secretion by cortical collecting tubule: relation to sodium absorption, luminal sodium concentration, and transepithelial voltage. Am J Physiol 1981; 241:F395–F402. [DOI] [PubMed] [Google Scholar]
- 16. Chrysant SG, Danisa K, Kem DC, Dillard BL, Smith WJ, Frohlich ED. Racial differences in pressure, volume and renin interrelationships in essential hypertension. Hypertension 1979; 1:136–141. [DOI] [PubMed] [Google Scholar]
- 17. Luft FC, Grim CE, Fineberg N, Weinberger MC. Effects of volume expansion and contraction in normotensive Whites, Blacks, and subjects of different ages. Circulation 1979; 59:643–650. [DOI] [PubMed] [Google Scholar]
- 18. Luft FC, Miller JZ, Grim CE, Fineberg NS, Christian JC, Daugherty SA, Weinberger MH. Salt sensitivity and resistance of blood pressure. Age and race as factors in physiological responses. Hypertension 1991; 17:I102–I108. [DOI] [PubMed] [Google Scholar]
- 19. Grim CE, Luft FC, Miller JZ, Meneely GR, Battarbee HD, Hames CG, Dahl LK. Racial differences in blood pressure in Evans County, Georgia: relationship to sodium and potassium intake and plasma renin activity. J Chronic Dis 1980; 33:87–94. [DOI] [PubMed] [Google Scholar]