Abstract
BACKGROUND
While obesity is a leading risk factor for preeclampsia, the mechanisms whereby obese women are more susceptible to pregnancy-induced hypertension are unclear. As high-fat diet (HFD) is an important contributor to the development of obesity, we tested the hypothesis that pregnant rats on HFD have hypertension and endothelial dysfunction due to reduced nitric oxide synthase (NOS).
METHODS
Twelve-week-old Sprague-Dawley female rats were fed normal diet (ND, 13% fat kcal) or HFD (40% fat kcal) for 9 weeks. Timed-pregnant rats were then generated and the effect of HFD on mean arterial blood pressure (MAP) and vascular function was assessed on gestational day (GD) 19.
RESULTS
MAP was not different between HFD and ND pregnant rats. Intriguingly, sensitivity to acetylcholine-induced endothelium-dependent vasorelaxation was enhanced in small mesenteric arteries of HFD dams compared to ND controls (logEC50 −7.9 ± 0.3 vs. −6.7 ± 0.3 M; P < 0.05). Additionally, HFD dams exhibited higher mesenteric artery expression of NOS3 and plasma levels of NO metabolites than ND controls (1738.0 ± 316.4 vs. 1094.0 ± 82.5 pg/mg and 72.5 ± 8.7 vs. 39.7 ± 4.5 µM, respectively; both P < 0.05). Further, to determine the role of NOS in modulating blood pressure in HFD pregnant rats, animals were treated with the nonselective inhibitor Nω-Nitro-l-arginine methyl ester hydrochloride (100 mg/l, drinking water) from GD 14 to 19. It was found that NOS inhibition increased MAP equally in HFD and ND groups.
CONCLUSIONS
Contrary to our initial hypothesis, HFD dams were normotensive and presented increased endothelial function and NO/NOS3 levels. This enhanced NOS-mediated vascular function does not appear to have a major impact on blood pressure regulation of HFD-fed pregnant rats.
Keywords: blood pressure, endothelial function, high-fat diet, hypertension, nitric oxide, pregnancy.
Preeclampsia is a syndrome unique to pregnancy that contributes enormously to maternal and neonatal morbidity and mortality.1 In addition, women who had experienced preeclampsia and their offspring are at increased risk to develop cardiovascular diseases later in life.2,3 The American College of Obstetricians and Gynecologist’s Task Force on Hypertension in Pregnancy recently changed the guidelines for diagnosis of hypertension during pregnancy. Although preeclampsia is still characterized by new-onset hypertension and proteinuria after 20 weeks of gestation, proteinuria can be now substituted by any of the following: thrombocytopenia, impaired liver function, renal insufficiency, pulmonary edema, or cerebral/visual symptoms.4 It is estimated that preeclampsia affects 3–5% of pregnancies globally,5 but it is likely that the rates will increase after widespread implementation of this new diagnostic criteria. Nonetheless, epidemiological studies performed in the United States have found that, due to the higher prevalence of risk factors, the incidence of preeclampsia has risen in the last decade.6–8 Among the well-established risk factors, obesity has been associated with a 3-fold increase in the risk of developing preeclampsia.9 It is expected that the overall impact of obesity on preeclampsia will increase even further in the future as obesity rates continue to rise worldwide. Hence, it is extremely important and urgent to understand the role that obesity plays in the pathogenesis of preeclampsia.
Genetic predisposition plus increased food intake, decreased metabolic rate, and sedentary lifestyle are major players in the development of obesity.10–12 Notably, recent studies in experimental models of pregnancy have suggested a causative role of dietary fats in preeclampsia. For instance, mice13 and rats14,15 fed a high-fat diet (HFD) prior and during pregnancy exhibited increased blood pressure by the end of gestation. However, these studies have employed plethysmography to measure blood pressure; thus, additional studies using more direct techniques are needed to confirm whether HFD promotes hypertension during pregnancy. Furthermore, maternal systemic endothelial dysfunction is a hallmark in the pathophysiology of preeclampsia.16 Interestingly, small mesenteric arteries of lard-fed pregnant rats showed decreased endothelium-dependent relaxation.17,18
While the studies above imply a detrimental effect of HFD on blood pressure and endothelial function, the mechanisms linking obesity and preeclampsia are unclear. Previous studies have demonstrated that blood pressure regulation during pregnancy relies greatly on the vasodilatory gas nitric oxide (NO),19 which is produced by the nitric oxide synthase (NOS) system of enzymes. Indeed, circulating levels of nitrite, a NO metabolite used as a surrogate measurement of NO, are increased in normal pregnant women compared to both healthy nonpregnant20 and preeclamptic women.21 Therefore, we tested the hypothesis that pregnant rats on HFD have hypertension and endothelial dysfunction due to reduced NOS.
METHODS
All protocols were approved by the University of Mississippi Medical Center’s Institutional Animal Care and Use Committee and followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals. During the entire experimental period, animals were maintained on 12:12 hour light:dark cycle at 23 °C and food and water were provided ad libitum. Details of our experimental protocols are provided as supplementary information.
Animal protocol 1: to determine the effect of HFD on blood pressure and vascular function
Twelve-week-old female Sprague-Dawley rats were fed a normal diet (ND, 13% fat kcal; n = 14) or a HFD (40% fat kcal; n = 23). Selection of diets was based on a previous study evaluating the mechanisms whereby HFD-induced obesity enhances blood pressure and appetite in male Sprague-Dawley rats.22 After 9 weeks of diet treatment, rats were bred and timed-pregnancies were generated. Rats were maintained on respective ND or HFD during the breeding period and throughout pregnancy. Initial and final body weights were recorded.
Blood pressure measurement.
Indwelling carotid catheters were implanted in anesthetized dams on gestational day (GD) 18. Mean arterial pressure (MAP) was recorded consciously on GD 19.
Tissue harvest.
On GD 19, under anesthesia, blood was drawn from the abdominal aorta of dams and centrifuged to obtain plasma samples. The mesenteric vascular arcade was collected and either placed in ice-cold physiological saline solution for functional studies or flash frozen in liquid nitrogen for densitometric studies.
Vascular reactivity measurement.
Vasorelaxation studies were performed as previously described.23 Briefly, vascular rings consisting of third-order mesenteric arteries were mounted on a wire myograph. After testing for proper endothelial function, vascular rings were constricted with phenylephrine and cumulative concentration–response curves to acetylcholine (ACh) were generated. Curves in response to ACh were produced in the absence and presence of a nonselective NOS inhibitor, Nω-Nitro-l-arginine methyl ester hydrochloride (l-NAME). Next, vascular rings were constricted with phenylephrine again and concentration–response curves to the exogenous NO-donor sodium nitroprusside were generated.
Mesenteric artery NOS3 measurements.
Whole mesenteric artery beds were homogenized in radioimmunoprecipitation assay (RIPA) lysis buffer as per manufacturer’s instructions. Total protein content in supernatant of homogenates was quantified using the bicinchoninic acid method. Mesenteric artery NOS3 expression was semi-quantitated by western blotting as previously described.24 Fluorescence was detected on an Odyssey Infrared Imaging System and densitometry was analyzed with ImageJ 1.50 software. NOS3 densities were normalized to corresponding β-actin densities. NOS3 protein levels were quantitated by enzyme-linked immunosorbent assay (ELISA), and these values were normalized to respective total protein concentrations.
Measurement of circulating NO metabolites.
Plasma levels of NO metabolites (nitrate and nitrite) were quantified by a colorimetric assay based on the Griess chemical reaction.
Animal protocol 2: to determine the effect of HFD on NOS-meditated blood pressure regulation
Twelve-week-old female Sprague-Dawley rats were fed ND (n = 13) or HFD (n = 35). After 9 weeks of diet treatment, rats were bred and timed-pregnancies were generated. Rats were maintained on respective ND or HFD during the breeding period and throughout pregnancy. From GD 14 to 19, l-NAME (100 mg/l) was added to the drinking water in a subset of ND and HFD pregnant rats, resulting in 4 experimental groups: ND + water (n = 6); ND + l-NAME (n = 7); HFD + water (n = 19); and HFD + l-NAME (n = 16). Initial and final body weights were recorded.
Body composition analysis.
On GD 18, total body lean, fat, and water contents were measured consciously in individual dams by nuclear magnetic resonance (EchoMRI).
Blood pressure measurement.
MAP was assessed as described above.
Tissue harvest.
On GD 19, under anesthesia, blood was drawn from the abdominal aorta and centrifuged to obtain serum and plasma samples. Visceral white adipose tissue was weighed. The number of viable and reabsorbed fetuses and individual fetus and placenta weights in each animal were recorded. Representative placentas were flash frozen in liquid nitrogen for further analysis.
Circulating measurements.
Plasma NO metabolites were determined as described above. Quantification of serum leptin and plasma tumor necrosis factor-alpha (TNF-α) were by done ELISA; plasma total cholesterol by fluorimetry; and plasma levels of glucose, triglycerides, and free fatty acids by colorimetry.
Placental measurements.
Whole placentas were homogenized in RIPA lysis buffer as described above. Total protein content in supernatant of homogenates was quantified using the bicinchoninic acid method. Placental TNF-α levels were quantified by ELISA, and these values were normalized to respective total protein concentrations.
Statistical analysis
Graphs and statistical analyses were prepared and performed, respectively, with GraphPad Prism 6.0 software. Comparisons between ND and HFD groups were performed using 2-tailed Student’s t-test. Comparisons among ND + water, ND + l-NAME, HFD + water, and HFD + l-NAME groups were done applying 1-way analysis of variance or Kruskal–Wallis test followed by Tukey’s or Dunn’s multiple comparison test, respectively, as appropriate. The sensitivity (logEC50) data generated in cumulative concentration-response curves were analyzed using 2-way analysis of variance followed by Bonferroni’s multiple comparison test. In addition, the only goodness-of-fit criterion was used for the logarithm of half maximal effective concentration. Values are shown as mean ± SEM. A value of P < 0.05 was considered statistically significant.
RESULTS
Effects of HFD on maternal blood pressure and vascular function
In the first protocol, initial body weight was similar between rats designated for the HFD and ND groups (223.4 ± 1.7 vs. 221.3 ± 2.7 g, respectively; P > 0.05). After 9 weeks of diet treatment, just before implementing the breeding protocol, HFD rats were heavier than ND controls (290.5 ± 3.1 vs. 277.2 ± 3.9 g; P < 0.05). However, on GD 18, body weight of HFD and ND pregnant groups did not differ (387.9 ± 4.9 vs. 382.7 ± 6.1 g; P > 0.05).
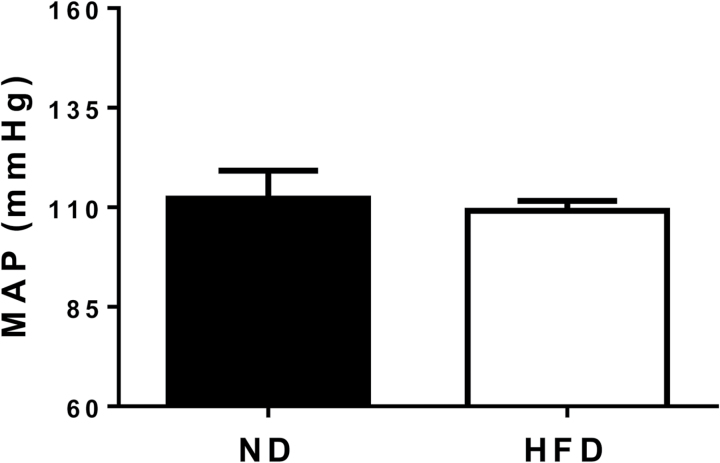
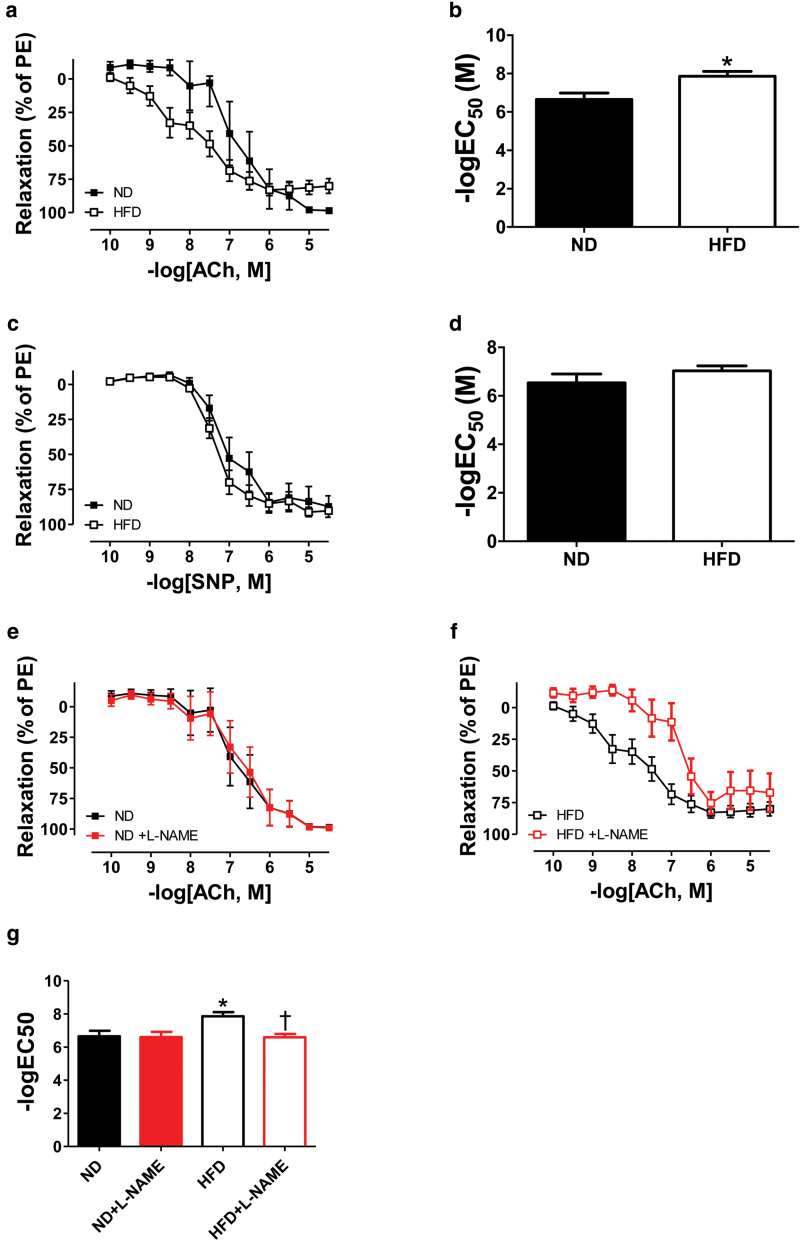
Contrary to our hypothesis, mean arterial pressure (MAP) was similar in HFD and ND dams as measured on GD 19 (Figure 1; P > 0.05). Intriguingly, myography experiments performed on GD 19 revealed that sensitivity to ACh-mediated endothelium-dependent vasorelaxation was greater, but maximum relaxation to ACh was reduced in small mesenteric arteries of HFD pregnant rats compared with ND controls (Figure 2, panel a; P < 0.05). Nonetheless, a statistically significant increase in logEC50 to ACh was observed in response to HFD feeding (Figure 2, panel b: −log 7.9 ± 0.3 vs. 6.7 ± 0.3 M; P < 0.05). Moreover, endothelium-independent vasorelaxation and logEC50 to sodium nitroprusside were comparable in HFD and ND dams (Figure 2, panels c and d, respectively; both P > 0.05). In order to investigate the mechanism responsible for the improved vascular reactivity of HFD pregnant rats, small mesenteric arteries were incubated with l-NAME, a nonselective NOS inhibitor. Curiously, while vasorelaxation of ND arteries was unaltered (Figure 2, panels e and g; both P > 0.05), l-NAME abolished the enhanced vasorelaxation and sensitivity to ACh of HFD arteries (Figure 2, panels f and g; both P < 0.05).
Figure 1.
Effects of high-fat diet (HFD) on mean arterial pressure (MAP) in pregnant rats at gestational day 19. Normal diet pregnant group (ND, n = 14); HFD pregnant group (HFD, n = 23).
Figure 2.
Effects of high-fat diet (HFD) on vascular reactivity of small mesenteric arteries isolated from pregnant rats at gestational day 19. Cumulative concentration-response curves (a) and logEC50 (b) to acetylcholine (ACh). Cumulative concentration-response curves (c) and logEC50 (d) to sodium nitroprusside (SNP). For panels a, b, c, and d, normal diet pregnant group (ND, n = 6); HFD pregnant group (HFD, n = 13). Cumulative concentration-response curves (e and f) and logEC50 (g) to ACh in the absence and presence of Nω-Nitro-l-arginine methyl ester hydrochloride (l-NAME). For panels e, f, and g, ND pregnant group (ND, n = 6); ND pregnant group + l-NAME (ND + l-NAME, n = 6); HFD pregnant group (HFD, n = 13); HFD pregnant group + l-NAME (HFD + l-NAME, n = 9). *P < 0.05 vs. ND. †P < 0.05 vs. HFD.
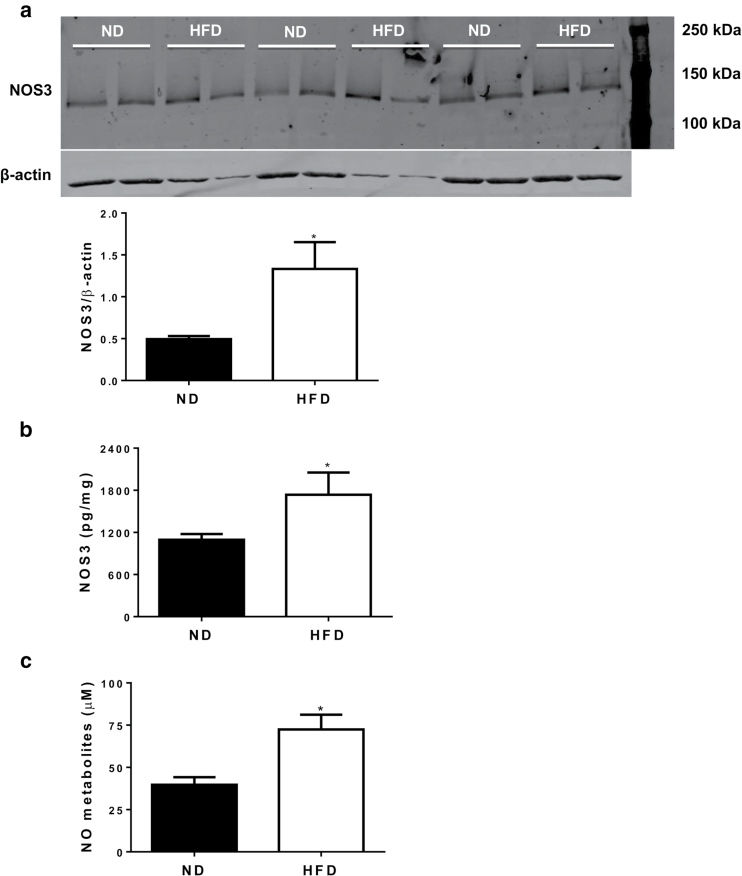
Mesenteric artery NOS3 expression determined by western blotting was increased in HFD pregnant rats compared with ND controls on GD 19 (Figure 3, panel a; P < 0.05). ELISA confirmed that NOS3 levels in HFD arteries were higher than in ND arteries (Figure 3, panel b; P < 0.05). In support of vascular function and NOS expression being enhanced in HFD dams, plasma concentration of NO metabolites was increased in HFD pregnant rats compared with ND controls in both experimental protocols (Figure 3, panel c; P < 0.05).
Figure 3.
Effects of high-fat diet (HFD) on small mesenteric artery expression of endothelial nitric oxide synthase-3 (NOS3) and circulating nitric oxide (NO) metabolites in pregnant rats at gestational day 19. Western blotting for NOS3 expression in small mesenteric arteries (a, top part: image of membrane and bottom part: quantification of bands in arbitrary units). For panel a, normal diet pregnant vessels (ND, n = 6); HFD pregnant vessels (HFD, n = 6). ELISA for NOS3 expression in small mesenteric arteries (b). For panel b, ND pregnant vessels (ND, n = 11); HFD pregnant vessels (HFD, n = 9). Plasma concentration of NO metabolites (c). For panel c, ND pregnant plasma (ND, n = 13); HFD pregnant plasma (HFD, n = 23). *P < 0.05 vs. ND.
Effect of HFD on NOS-mediated blood pressure regulation
In the second protocol, initial body weight was similar between HFD and ND groups (239.3 ± 1.8 vs. 239.7 ± 2.7 g, respectively; P > 0.05). After 9 weeks of diet treatment, HFD and ND virgin rats exhibited similar body weights (265.8 ± 6.0 vs. 260.3 ± 5.4 g; P > 0.05). On GD 18, body weight of HFD + water, HFD + l-NAME, ND + water, and ND + l-NAME pregnant rats did not differ (Table 1; P > 0.05). EchoMRI was used to determine the impact of HFD on body composition during late gestation. We observed that lean mass and total fat mass were comparable in HFD + water, HFD + l-NAME, ND + water, and ND + l-NAME dams (Table 1; both P > 0.05). In addition, there were no differences in total water content and free water content among these groups (Table 1; both P > 0.05). Notably, free water content was greatly correlated with litter size (Pearson r = 0.9398 and P < 0.0001). Thereby, body weight was normalized by free water content for each animal on GD 18. Yet, there was no difference in normalized body weight among HFD + water, HFD + l-NAME, ND + water, and ND + l-NAME pregnant rats (Table 1; P > 0.05). Upon harvesting animals on GD 19, it was observed that visceral fat weight was similar in all groups (Table 1; P > 0.05). Furthermore, serum leptin and plasma glucose concentrations were comparable in HFD + water, HFD + l-NAME, ND + water, and ND + l-NAME pregnant rats (Table 1; both P > 0.05). There was a trend for plasma levels of total cholesterol, triglycerides, and free fatty acids to be increased in HFD + water and HFD + l-NAME dams compared with both ND + water and ND + l-NAME counterparts (Table 1; all P > 0.05). Plasma TNF-α concentration did not differ statistically among these groups (Table 1; P > 0.05).
Table 1.
Effects of HFD on anthropometric and circulating parameters at gestational day 18 or 19 of pregnant rats with or without l-NAME treatment from gestational day 14 to 19
| ND + water | ND + l-NAME | HFD + water | HFD + l-NAME | |
|---|---|---|---|---|
| Body weight (g) | 471.80 ± 4.23 | 471.30 ± 9.13 | 463.50 ± 11.72 | 462.40 ± 8.93 |
| Normalized body weight (g) | 45.98 ± 3.45 | 44.13 ± 3.33 | 55.50 ± 9.40 | 49.63 ± 4.66 |
| Lean mass (g) | 333.20 ± 2.41 | 335.80 ± 9.01 | 320.00 ± 5.31 | 326.90 ± 6.20 |
| Total fat mass (g) | 96.92 ± 2.01 | 96.07 ± 11.77 | 103.40 ± 6.92 | 98.36 ± 5.81 |
| Free water content (g) | 10.59 ± 0.87 | 11.08 ± 0.89 | 10.23 ± 0.76 | 10.16 ± 0.67 |
| Total water content (g) | 266.30 ± 2.54 | 267.60 ± 7.86 | 255.80 ± 4.96 | 261.30 ± 5.45 |
| Visceral fat weight (g) | 30.47 ± 1.14 | 28.83 ± 3.04 | 32.95 ± 1.94 | 30.08 ± 1.55 |
| Serum leptin concentration (ng/ml) | 6.27 ± 0.45 | 7.34 ± 0.98 | 7.79 ± 0.89 | 7.75 ± 0.57 |
| Plasma glucose concentration (mg/dl) | 122.80 ± 5.51 | 122.30 ± 12.92 | 146.70 ± 13.82 | 132.70 ± 10.19 |
| Plasma cholesterol concentration (mg/dl) | 136.20 ± 17.30 | 136.00 ± 6.10 | 162.70 ± 5.31 | 160.70 ± 9.93 |
| Plasma triglyceride concentration (mg/dl) | 235.10 ± 44.79 | 289.70 ± 41.98 | 446.20 ± 53.51 | 403.40 ± 51.12 |
| Plasma FFA concentration (mg/dl) | 76.05 ± 15.47 | 68.59 ± 8.52 | 133.80 ± 21.43 | 158.20 ± 35.92 |
| Plasma TNF-α concentration (pg/ml) | 10.29 ± 4.66 | 5.92 ± 2.13 | 13.31 ± 3.62 | 19.67 ± 7.46 |
Pregnant rats treated with normal diet and water (ND + water, n = 6), normal diet supplemented with Nω-Nitro-l-arginine methyl ester hydrochloride (l-NAME) in drinking water (ND + l-NAME, n = 6–7), high-fat diet and water (HFD + water, n = 18–19), or high-fat diet supplemented with l-NAME in drinking water (HFD + l-NAME, n = 14–16). Abbreviations: FFA, free fatty acids; TNF-α, tumor necrosis factor-alpha. Values are mean ± SEM.
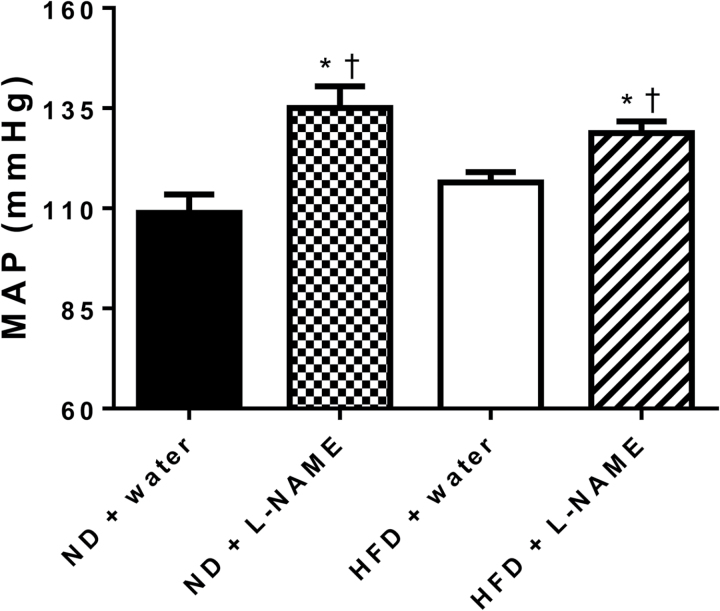
As expected, MAP was significantly increased in ND + l-NAME and HFD + l-NAME dams compared with both ND + water and HFD + water counterparts as measured on GD 19 (Figure 4; P < 0.05); however, in contrast to our hypothesis, the blood pressure rise in response to l-NAME was not blunted in the HFD group to suggest reduced NOS control of blood pressure. Nor was the blood pressure rise due to l-NAME treatment greater in the HFD group, even though vascular NOS-mediated relaxation and NOS3 expression during gestation were increased by HFD. Thus, the hypertensive response to NOS inhibition was similar between both pregnant diet groups (Figure 4; P > 0.05).
Figure 4.
Effects of Nω-Nitro-l-arginine methyl ester hydrochloride (l-NAME) on mean arterial pressure (MAP) in high-fat diet (HFD) pregnant rats at gestational day 19. Normal diet and water pregnant group (ND + water, n = 6); ND supplemented with l-NAME in drinking water pregnant group (ND + l-NAME, n = 7); HFD and water pregnant group (HFD + water, n = 19); HFD supplemented with l-NAME in drinking water (HFD + l-NAME, n = 16).
On GD 19, the number of viable (litter size) or reabsorptions (fetal demise) fetuses was not different among HFD + water, HFD + l-NAME, ND + water, and ND + l-NAME pregnant rats (Table 2; both P > 0.05). Fetal weight was reduced in HFD + water and HFD + l-NAME dams compared with ND + water controls (Table 2; P < 0.05). While ND + l-NAME placentas were heavier than ND + water controls, HFD + l-NAME placentas were lighter than ND + l-NAME counterparts (Table 2; P < 0.05). In addition, placental efficiency of ND + l-NAME pregnant rats was decreased compared with ND + water controls (Table 2; P < 0.05). Curiously, placental TNF-α levels of HFD + water and HFD + l-NAME dams were significantly reduced compared with ND + l-NAME counterparts (Table 2; P < 0.05).
Table 2.
Effects of HFD on fetal and placental parameters at gestational day 19 of pregnant rats with or without l-NAME treatment from gestational day 14 to 19
| ND + water | ND + l-NAME | HFD + water | HFD + l-NAME | |
|---|---|---|---|---|
| Litter size | 12.50 ± 0.85 | 12.57 ± 0.92 | 11.47 ± 1.11 | 12.06 ± 0.82 |
| Fetal demise | 1.00 ± 0.37 | 1.43 ± 0.48 | 1.53 ± 0.31 | 1.56 ± 0.27 |
| Fetal weight (g) | 2.23 ± 0.04 | 2.15 ± 0.05 | 2.08 ± 0.04* | 2.00 ± 0.02* |
| Placental weight (g) | 0.45 ± 0.01 | 0.52 ± 0.01* | 0.50 ± 0.03 | 0.46 ± 0.02# |
| Placental efficiency | 5.08 ± 0.18 | 4.16 ± 0.14* | 4.50 ± 0.17 | 4.50 ± 0.12 |
| Placental TNF-α levels (pg/mg) | 0.90 ± 0.12 | 1.04 ± 0.25 | 0.57 ± 0.04# | 0.61 ± 0.06# |
Pregnant rats treated with normal diet and water (ND + water, n = 6), normal diet supplemented with Nω-Nitro-l-arginine methyl ester hydrochloride (l-NAME) in drinking water (ND + l-NAME, n = 6–7), high-fat diet and water (HFD + water, n = 18–19), or high-fat diet supplemented with l-NAME in drinking water (HFD + l-NAME, n = 14–16). Abbreviations: TNF-α, tumor necrosis factor-alpha. Values are mean ± SEM. *P < 0.05 vs. ND + water; #P < 0.05 vs. ND + l-NAME.
DISCUSSION
Our main findings are that MAP was similar in HFD and ND pregnant rats, as measured on GD 19. Intriguingly, HFD feeding led to enhanced endothelium-dependent vasorelaxation most likely via activation of the NOS system, because inhibition of NOS abolished this response. In addition, mesenteric artery NOS3 expression and plasma concentration of NO metabolites were increased in HFD dams compared to ND controls. Based on these observations, we expected that blood pressure regulation in HFD dams would be dependent on NOS and thereby, l-NAME treatment would increase MAP of HFD pregnant rats over the levels of ND counterparts; however, MAP was equally elevated in HFD + l-NAME and ND + l-NAME dams. Moreover, there was only a trend for circulating levels of leptin, total cholesterol, triglycerides, free fatty acids, and TNF-α to be increased by HFD during gestation. Furthermore, HFD did not alter litter size, fetal demise, or placental weight, but reduced fetal weight. As body weight and fat content of pregnant rats were not altered by HFD, we attribute the results reported in this study as an effect of dietary fats instead of obesity.
Although high-fat feeding is a major contributor for the establishment of obesity,11,12 the role of HFD per se in promoting pregnancy-induced hypertension and the underlying mechanisms are unclear. Attempts to address this issue were initially carried out in animal models whose blood pressure was measured by tail cuff. For instance, female rats fed a HFD (45% fat kcal) from 3- to 16-week old prior to mating and during pregnancy exhibited increased systolic blood pressure compared with ND (16% fat kcal) controls on GD 15.14 Additionally, 8-week-old female mice maintained on a HFD (62% fat kcal) for 4 weeks before being mated and during pregnancy had higher systolic blood pressure and urinary protein level than ND (12% fat kcal) controls on GD 18.5.13 Yet, 8-week-old female rats treated with a HFD (62% fat kcal) only during pregnancy presented elevated systolic blood pressure and proteinuria vs. ND (12% fat kcal) controls after GD 12 and 15, respectively.15 However, we observed no differences in MAP between HFD and ND pregnant rats, as measured by carotid catheter. Thus, contrasting blood pressure results might be partially explained the distinct methods employed to assess blood pressure.
The physiological response of animals to HFD varies remarkably, depending on factors such as age, sex, genetic background, early nutritional experience, and diet composition.25 Therefore, contrasting blood pressure results might also be explained by the different diet regimens applied to feed animals (while we used a moderate-fat diet in 12-week-old rats for 12 weeks, Hayes et al. used a moderate-fat diet in 3-week-old rats for 19 weeks14 and Masuyama and Hiramatsu13 and Ge et al.15 used a very rich-fat diet in 8-week-old animals for 7 and 3 weeks, respectively). Indeed, we found that body weight was not different between HFD and ND pregnant rats. Similarly, previous animal studies have described no effect of HFD on body weight during late gestation17,18,26; however, by feeding pregnant mice a diet with 62% fat kcal, Masuyama and Hiramatsu were able to significanly increase body weight on GD 18.5.13 Moreover, our HFD and ND dams exhibited comparable total fat mass and visceral fat weight.
Furthermore, different metabolic profiles have been observed in HFD pregnant models depending on the diet composition and feeding duration. Some studies described hyperleptinemia13 and hyperlipidemia13,15 in HFD pregnant animals compared with ND controls, whereas others reported no changes in leptin,17,27 glucose,13,26,27 and lipids17 on late gestation. Likewise, we failed to demonstrate any significant effect of HFD on circulating metabolic and inflammatory factors in pregnant rats. Interestingly, visceral adipocyte estrogen receptor-alpha seems to mediate improvements in visceral fat hypertrophy, inflammation, and glucose intolerance in HFD pregnant animals by the end of gestation.27 Nevertheless, we have previously shown that chronic hyperleptinemia can elicit high blood pressure in pregnant rats.28 In that study, chronic leptin infusion caused an approximately 23-fold increase in circulating levels of leptin. Taking together, these data suggest that excessive metabolic/inflammatory disturbances are necessary to induce hypertension during pregnancy.
In order to determine the mechanism whereby HFD dams were protected against hypertension, we firstly examined the effect of HFD on vascular function. While HFD altered ACh-mediated vasorelaxation in small mesenteric arteries, sodium nitroprusside-induced vasorelaxation was unchanged. Specifically in response to ACh, sensitivity was greater, but maximum relaxation was reduced in HFD pregnant rats compared with ND controls. In addition, L-NAME completely abolished the enhanced sensitivity to ACh of HFD arteries. Earlier studies have also found that maximum relaxation, but not sensitivity, in response to ACh was decreased in small mesenteric arteries of lard-fed pregnant rats compared with controls on GD 20.17,18 Again, opposite findings among studies can be at least partially explained by the diet composition and duration of pregestational feeding. However, as impaired maximal response was noted at very high doses of ACh, the enhanced sensitivity might be more physiologically relevant than the reduced maximal response. Furthermore, we showed that mesenteric artery expression of NOS3 and plasma concentration of NO metabolites were increased in HFD pregnant rats compared with ND controls. Collectively, these data indicate that endothelium-dependent vasorelaxation is enhanced by HFD through activation of the NOS system. Because HFD may affect the circulation of other organs important for blood pressure regulation during pregnancy, future studies will evaluate whether altered endothelium-dependent vasorelaxation is restricted to small mesenteric arteries or also present in renal and uterine vessels of our HFD pregnant rats.
It is worth noting that this study was conducted under normal pregnancy conditions. We have previously demonstrated that NOS function is increased in pregnant rats, which was defined by an exaggerated blood pressure and vascular reactivity response to chronic treatment with l-NAME compared with female virgin rats.29,30 Here, after the first set of experiments indicating that blood pressure was not elevated in HFD pregnant rats due to enhanced vasorelaxation via a NOS pathway, we designed a second animal protocol to address the hypothesis that l-NAME-induced hypertension during pregnancy is exacerbated by HFD. However, MAP was similarly increased in HFD + l-NAME and ND + l-NAME pregnant rats compared with HFD + water and ND + water counterparts. Thus, we propose that mechanisms other than NOS may be in place to protect HFD dams against hypertension. Earlier studies have shown that certain components of the prostanoid, kinin, renin–angiotensin–aldosterone, and vascular endothelial growth factor systems also have vasodilatory properties relevant for blood pressure regulation during pregnancy.19 Additional studies will examine these different pathways in our HFD pregnant rats.
Intriguingly, while preeclamptic pregnancies regularly result in babies with intrauterine growth restriction, obese pregnant women has often macrosomic babies.31 However, different reports have found that high body mass index is also associated with low birth weight and small for gestational age.32–34 Contradictory findings have also been reported in experimental pregnant models. Some of the studies have noticed that HFD-fed animals exhibit increased fetal weight,13,18 whereas others observed decreased fetal weight.14,17 Yet, high-fat feeding may cause fetal demise.14,35 We showed that fetal weight was reduced in HFD pregnant rats compared with ND controls. Interestingly, HFD monkeys exhibited decreased uterine and placental volume blood flows compared with ND controls by the end of gestation.35 In addition, some of these animals presented large areas of placental infarction and calcification. Signs of HFD-induced poor placentation, depicted by trophoblast loss and increased muscularity surrounding spiral arteries plus pronounced endothelial necrosis and hypoxia in the labyrinth, have also been described in pregnant rodents.14,26,36 Moreover, Frias et al. reported that HFD led to increased placental gene expression of inflammatory cytokines in pregnant monkeys35; however, we observed a trend for placental TNF-α levels to be decreased in HFD dams compared with ND controls. Hence, further studies are required to determine the mechanisms underlying adverse fetal outcomes in our HFD pregnant rats.
In conclusion, HFD rats did not present high blood pressure on GD 19, but endothelium-dependent vasorelaxation was enhanced through activation of the NOS system. This was in contrast to our initial hypothesis. Importantly, no single study to date has examined the impact of high-fat feeding on blood pressure and vascular function in the same group of pregnant animals. Here, in order to confirm a greater role for NOS in blood pressure regulation of HFD pregnant rat, we treated animals during late gestation with l-NAME; however, we found that this nonselective NOS inhibitor increased blood pressure equally in HFD and ND dams. Thus, although HFD pregnant rats seemed to be protected against hypertension by improving NOS-mediated vascular function, other pathways are probably more relevant for blood pressure regulation in these animals. These data might explain why not all obese pregnant women develop preeclampsia and go on to have healthy pregnancies.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
Research reported in this publication was supported by the American Heart Association under the award number 14POST18970005 and by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award numbers P01HL051971, 1T32HL105324, and P20GM104357. Furthermore, the authors would like to thank Marietta Arany, Kathy Cockrell, Alex Dent, Grant Ross, and Haiyan Zhang for their technical expertise.
REFERENCES
- 1. World Health Organization. The World Health Report 2005: Make Every Mother and Child Count. WHO Press: Geneva, Switzerland, 2005, pp 252. [Google Scholar]
- 2. Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, Adwani S, Wilkinson AR, McCormick K, Sargent I, Redman C, Leeson P. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics 2012; 129:e1552–e1561. [DOI] [PubMed] [Google Scholar]
- 3. McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J 2008; 156:918–930. [DOI] [PubMed] [Google Scholar]
- 4. American College of Obstetrics and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013; 122:1122–1131. [DOI] [PubMed] [Google Scholar]
- 5. Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol 2011; 25:391–403. [DOI] [PubMed] [Google Scholar]
- 6. Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980–2010: age-period-cohort analysis. BMJ 2013; 347:f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol 2009; 113:1299–1306. [DOI] [PubMed] [Google Scholar]
- 8. Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987-2004. Am J Hypertens 2008; 21:521–526. [DOI] [PubMed] [Google Scholar]
- 9. Roberts JM, Bodnar LM, Patrick TE, Powers RW. The role of obesity in preeclampsia. Pregnancy Hypertens 2011; 1:6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chaput JP, Pérusse L, Després JP, Tremblay A, Bouchard C. Findings from the Quebec Family Study on the etiology of obesity: genetics and environmental highlights. Curr Obes Rep 2014; 3:54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Golay A, Bobbioni E. The role of dietary fat in obesity. Int J Obes Relat Metab Disord 1997; 21(suppl 3):S2–S11. [PubMed] [Google Scholar]
- 12. Hill JO, Melanson EL, Wyatt HT. Dietary fat intake and regulation of energy balance: implications for obesity. J Nutr 2000; 130(suppl 2S):284S–288S. [PubMed] [Google Scholar]
- 13. Masuyama H, Hiramatsu Y. Treatment with a constitutive androstane receptor ligand ameliorates the signs of preeclampsia in high-fat diet-induced obese pregnant mice. Mol Cell Endocrinol 2012; 348:120–127. [DOI] [PubMed] [Google Scholar]
- 14. Hayes EK, Lechowicz A, Petrik JJ, Storozhuk Y, Paez-Parent S, Dai Q, Samjoo IA, Mansell M, Gruslin A, Holloway AC, Raha S. Adverse fetal and neonatal outcomes associated with a life-long high fat diet: role of altered development of the placental vasculature. PLoS One 2012; 7:e33370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ge J, Wang J, Xue D, Zhu Z, Chen Z, Li X, Su D, Du J. Why does a high-fat diet induce preeclampsia-like symptoms in pregnant rats. Neural Regener Res 2013; 8:1872–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol 2008; 294:H541–H550. [DOI] [PubMed] [Google Scholar]
- 17. Taylor PD, Khan IY, Lakasing L, Dekou V, O’Brien-Coker I, Mallet AI, Hanson MA, Poston L. Uterine artery function in pregnant rats fed a diet supplemented with animal lard. Exp Physiol 2003; 88:389–398. [DOI] [PubMed] [Google Scholar]
- 18. Gerber RT, Holemans K, O’Brien-Coker I, Mallet AI, van Bree R, Van Assche FA, Poston L. Cholesterol-independent endothelial dysfunction in virgin and pregnant rats fed a diet high in saturated fat. J Physiol 1999; 517:607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Valdes G, Kaufmann P, Corthorn J, Erices R, Brosnihan KB, Joyner-Grantham J. Vasodilator factors in the systemic and local adaptations to pregnancy. Reprod Biol Endocrinol 2009; 7:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Okutomi T, Nomoto K, Nakamura K, Goto F. Nitric oxide metabolite in pregnant women before and after delivery. Acta Obstet Gynecol Scand 1997; 76:222–226. [PubMed] [Google Scholar]
- 21. Sandrim VC, Palei AC, Metzger IF, Gomes VA, Cavalli RC, Tanus-Santos JE. Nitric oxide formation is inversely related to serum levels of antiangiogenic factors soluble fms-like tyrosine kinase-1 and soluble endogline in preeclampsia. Hypertension 2008; 52:402–407. [DOI] [PubMed] [Google Scholar]
- 22. Dubinion JH, da Silva AA, Hall JE. Enhanced blood pressure and appetite responses to chronic central melanocortin-3/4 receptor blockade in dietary-induced obesity. J Hypertens 2010; 28:1466–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Spradley FT, Palei AC, Granger JP. Obese melanocortin-4 receptor-deficient rats exhibit augmented angiogenic balance and vasorelaxation during pregnancy. Physiol Rep 2013; 1:e00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sullivan JC, Pollock DM, Pollock JS. Altered nitric oxide synthase 3 distribution in mesenteric arteries of hypertensive rats. Hypertension 2002; 39:597–602. [DOI] [PubMed] [Google Scholar]
- 25. Rothwell NJ, Stock MJ. The development of obesity in animals: the role of dietary factors. Clin Endocrinol Metab 1984; 13:437–449. [DOI] [PubMed] [Google Scholar]
- 26. Liang C, DeCourcy K, Prater MR. High-saturated-fat diet induces gestational diabetes and placental vasculopathy in C57BL/6 mice. Metabolism 2010; 59:943–950. [DOI] [PubMed] [Google Scholar]
- 27. Pedroni SM, Turban S, Kipari T, Dunbar DR, McInnes K, Saunders PT, Morton NM, Norman JE. Pregnancy in obese mice protects selectively against visceral adiposity and is associated with increased adipocyte estrogen signalling. PLoS One 2014; 9:e94680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Palei AC, Spradley FT, Granger JP. Chronic hyperleptinemia results in the development of hypertension in pregnant rats. Am J Physiol Regul Integr Comp Physiol 2015; 308:R855–R861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kassab S, Miller MT, Hester R, Novak J, Granger JP. Systemic hemodynamics and regional blood flow during chronic nitric oxide synthesis inhibition in pregnant rats. Hypertension 1998; 31:315–320. [DOI] [PubMed] [Google Scholar]
- 30. Khalil RA, Crews JK, Novak J, Kassab S, Granger JP. Enhanced vascular reactivity during inhibition of nitric oxide synthesis in pregnant rats. Hypertension 1998; 31:1065–1069. [DOI] [PubMed] [Google Scholar]
- 31. Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One 2013; 8:e61627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Radulescu L, Munteanu O, Popa F, Cirstoiu M. The implications and consequences of maternal obesity on fetal intrauterine growth restriction. J Med Life 2013; 6:292–298. [PMC free article] [PubMed] [Google Scholar]
- 33. Rajasingam D, Seed PT, Briley AL, Shennan AH, Poston L. A prospective study of pregnancy outcome and biomarkers of oxidative stress in nulliparous obese women. Am J Obstet Gynecol 2009; 200:395.e1–395.e9. [DOI] [PubMed] [Google Scholar]
- 34. Rode L, Nilas L, Wøjdemann K, Tabor A. Obesity-related complications in Danish single cephalic term pregnancies. Obstet Gynecol 2005; 105:537–542. [DOI] [PubMed] [Google Scholar]
- 35. Frias AE, Morgan TK, Evans AE, Rasanen J, Oh KY, Thornburg KL, Grove KL. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology 2011; 152:2456–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hayes EK, Tessier DR, Percival ME, Holloway AC, Petrik JJ, Gruslin A, Raha S. Trophoblast invasion and blood vessel remodeling are altered in a rat model of lifelong maternal obesity. Reprod Sci 2014; 21:648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]