Abstract
BACKGROUND
Besides environmental risk factors, genetic factors play a crucial role in the pathogenesis of primary hypertension. The current study is to unravel whether hypertensive phenotypes vary in mice with different genetic background.
METHODS
Hypertension was induced in C57BL/6J (B6), DBA/2J (D2), and 25 BXD strains by administrating angiotensin (Ang)II (2.5 mg/kg/day infused by osmotic minipump) for 4 weeks. Systolic blood pressure was monitored before (baseline) and after 4 weeks of AngII treatment by tail cuff. Cardiac and renal fibrosis was evaluated by picrosirius red staining and collagen volume fraction (CVF) was quantitated using imaging analyzing system; cardiac transforming growth factor (TGF)-β gene expression was monitored by RT-PCR, and inflammatory response was detected by immunohistochemical ED-1 staining.
RESULTS
AngII infusion caused hypertension in all strains. However, blood pressure elevation was more evident in the D2 strain than the B6 group, while it was widely variable among BXD strains. Furthermore, chronic AngII treatment lead to development of hypertensive cardiac and renal diseases. Cardiac and renal CVF levels in the D2 strain was significantly higher than the B6 cohort, whereas these varied vastly across BXD strains. Moreover, cardiac TGF-β mRNA levels were markedly diverse among various mouse strains.
CONCLUSION
Our study unequivocally demonstrates that in response to AngII, BXDs with different genetic background expressed hypertension phenotypes with varied degree in severity. It implicates that genomics contribute to pathogenesis of primary hypertension. Building upon the genotype and hypertensive phenotypes, the BXD cohort can be further exploited experimentally to identify genes that influence blood pressure.
Keywords: blood pressure, BXD mouse strains, Genetic factors, hypertension, hypertensive phenotypes
Hypertension affects over 1 billion people worldwide. Chronic high blood pressure becomes a major risk factor for coronary artery disease, stroke, heart failure, peripheral vascular disease, vision loss, and chronic kidney disease.1,2 High blood pressure has been classified as either primary or secondary hypertension.3
Primary hypertension results from complex interactions of genes and environmental factors. Environmental factors include excess salt and body weight, smoking, and alcohol consumption.4 In addition to environment risk factors, genetic factors also play a major role in the pathogenesis of hypertension.5,6 Numerous common genetic variants with effects on blood pressure have been identified by genome-wide association studies (GWAS),7,8 a comprehensive examination of many common genetic variants in different individuals to ascertain if any specific variant(s) is associated with a trait. Human GWAS have successfully identified many genetic variants underlying susceptibility to complex diseases.9–12 The use of GWAS, which examine hundreds of thousands of single-nucleotide polymorphisms in large cohorts, has improved our understanding of blood pressure genomics and has demonstrated the presence of clearly reproducible blood pressure loci. However, these loci have so far been only effective in explaining a small proportion of the total blood pressure heritability. Hence, much of the genetic contribution to BP variability is still remained to be explored.
Even though large GWAS studies are feasible, but it is generally not possible to tightly control environmental variables.13 Large murine genetic reference populations provide a new solution to this challenge. The BXD family, currently the largest and best characterized mouse genetic reference population, is composed of 160 highly diverse lines of mice that descend from B6 and D2 parental strains.14 The BXDs have been bred specifically for systems genetics studies using both classic forward genetic methods, as well as reverse genetic studies that unravel candidate genes and their modulating effects on phenotype at the full genome level. As a group, the BXD cohort is therefore a unique and powerful model to investigate the genetic basis of diseases. The BXD family has been used to study the genetics of multiple diseases.15–18
Ang II, the central product of the renin–angiotensin system, plays a key role not only in the etiology of hypertension but also in the pathophysiology of cardiovascular and renal diseases in humans.19 Chronic AngII infusion to mice results in hypertension, leading to hypertensive heart and renal diseases and is widely used as an experimental hypertension model.20,21 Herein by using AngII infusion mouse model, we examined whether the B6, D2, and BXD strains with varied genetic background is associated with differential susceptibility of hypertensive phenotypes and are suitable for the future study to identify candidate genes that affect blood pressure. In the current study, we identified clinical and diagnostic hypertensive phenotype (high blood pressure), structural, cellular and extracellular phenotype (cardiac and renal damage and fibrosis), and molecular phenotype (biomarker of fibrosis).
MATERIAL AND METHOD
Animal model
Four- and five-month-old male B6, D2, and 25 BXD strains (n = 4–5/strain) were used in this study. Mice were treated with AngII (2.5 mg/kg/day infused by osmotic minipump) for 4 weeks.22 Systolic blood pressure was monitored before and after 4 weeks of AngII treatment by tail-cuff technique method.23 Untreated B6 and D2 cohorts served as controls. After 4 weeks of AngII treatment, the heart and kidney were removed, frozen in isopentane with dry ice, and kept at −80 °C for the following studies. This study was approved by the University of Tennessee Health Science Center Animal Care and Use Committee.
Morphology
Cryostat sections (6 µm) of frozen heart and kidney were prepared to determine the fibrillar collagen accumulation (fibrosis) by collagen-specific picrosirius red staining and examined by light microscopy as previously reported.23 Collagen volume fraction (CVF) was quantitated using a computer image analysis system (NIH image, 1.60) and was calculated as the sum of connective tissue areas, divided by the sum of connective tissue area and nonconnective tissue area in all fields of the heart or kidney section (3 sections/heart or kidney).23
Immunohistochemistry
Inflammatory response in the heart were detected by immunohistochemical ED-1 staining, a marker of macrophages. Cryostat heart sections (6 µm) were air-dried, fixed in 10% buffered formalin for 5 minutes, and washed in PBS for 10 minutes. Sections were then incubated with the primary antibody against ED-1 (Sigma, St Louis, MO) for 1 hour at room temperature. Sections were then incubated with IgG peroxidase-conjugated secondary antibody (Sigma) for 1 hour at room temperature, washed in PBS for 10 minutes, and incubated with 0.5 mg/ml diaminobenzidine tetrahydrochloride 2-hydrate + 0.05% H2O2 for 2 minutes. Negative control sections were incubated with secondary antibody alone. All sections were counterstained with hematoxylin, dehydrated, mounted, and examined by light microscopy.24
RT-PCR
Trizol Reagent (Invitrogen, Carlsbad, CA) was used to extract total RNA from cardiac tissue reserved. To prevent contamination of genome DNA, the RNA was treated with DNase by using TURBO DNA-free kit (Ambion, Austin, TX), and then purified with RNeasy Mini Kit (Qiagen, Valencia, CA). The purification and concentration of the RNA were examined with NanoDrop spectrophotometer (Thermo Scientific, Wilmington, DE), and the integrity was verified by Agilent Bioanalyzer (Agilent Technologies, Foster City, CA). cDNA was prepared from 500 ng total RNA using a high capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). The gene-specific primer sets for transforming growth factor (TGF)-β1 and endogenous quantity control TBP (TATA box-binding protein) were deduced using Universal ProbeLibrary Assay Design software (https://www.roche-applied-science.com), and corresponding probe was selected accordingly. TGF-β1 and TBP mRNA levels were detected and analyzed on an LightCycler 480 System (Roche, Indianapolis, IN) under the following cycling conditions: 1 cycle at 95 °C for 5 minutes and then 45 cycles at 95 °C for 10 seconds, 60 °C for 30 seconds, and 72 °C for 10 seconds. The PCR mix contained 0.2 µl of 10 µM primers, 0.1 µl of 10 µM Universal library probe, 5 µl of LC 480 master mix (2X), 2 µl of template cDNA and RNase-free water to 10 µl. B6, D2, and randomly selected 8 BXD strains were used for the RNA isolation and profiling.25
Statistical analysis
Statistical analysis of systolic blood pressure, CVF, and TGF-β gene expression data between controls and B6 or D2 strains and between B6 and D2 strains treated by AngII was performed using student t test. Values are expressed as mean ± SEM with P <0.05 considered significant.
RESULTS
Blood pressure
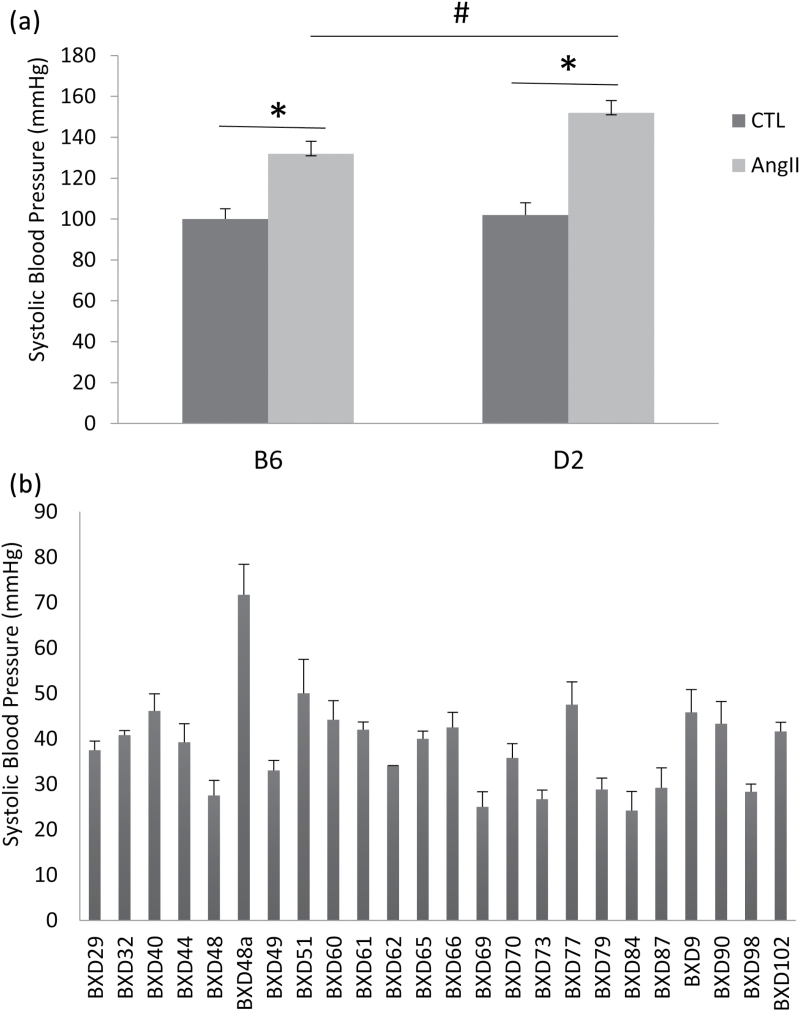
Compared to untreated B6 and D2 mice, systolic blood pressure was significantly increased in B6 and D2 strains in response to AngII treatment. However, the elevation of systolic blood pressure was significantly greater in the D2 strain than in the B6 cohort (Figure 1a).
Figure 1.
Systolic blood pressure in B6, D2, and BXD strains in response to chronic AngII treatment. AngII infusion lead to increased blood pressure in all strains. However, systolic blood pressure elevation was significantly higher in the D2 strain than in the B6 strain (a). Elevation in blood pressure was widely variable across the AngII-treated BXD strains, compared to the respective baseline values (b). *P < 0.05: untreated vs. AngII-treated B6 or D2 strain; #P < 0.05: AngII-treated B6 vs. AngII-treated D2 strain. Abbreviation: CTL, untreated controls.
Systolic blood pressure in 25 BXD strains was measured before and after 4 weeks of AngII treatment. The elevation of systolic blood pressure in response to AngII infusion in each strain was adjusted to its relative baseline. Our data revealed that AngII treatment lead to elevated blood pressure in all BXD strains. However, blood pressure elevation was widely variable among BXD strains (Figure 1b).
Hypertensive heart disease
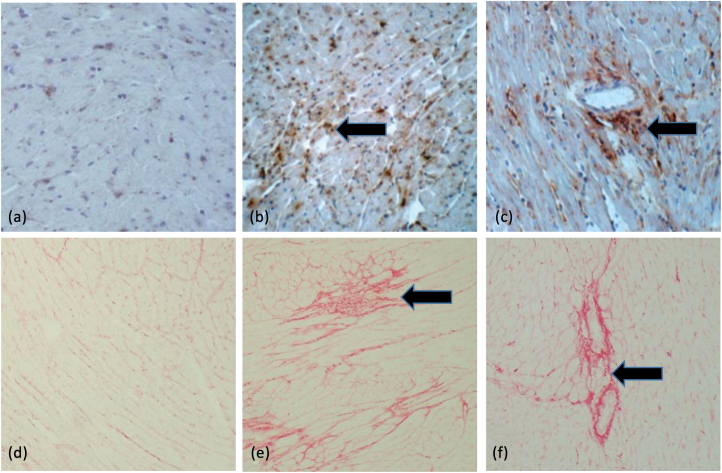
Hypertensive heart disease is a major hypertension phenotype. Cardiac and vascular injury manifested with fibrosis is a major feature of hypertensive heart disease.26,27 Our study revealed that chronic AngII infusion resulted in hypertensive heart disease in B6, D2, and BXD strains. Compared to the normal myocardium, macrophage infiltration became evident in the interstitial and perivascular space after 4 weeks of AngII treatment (Figure 2b and c). Furthermore, collagen was accumulated within the adversely remodeled myocardium and perivascular space in response to AngII infusion (Figure 2e and f). Myocardial fibrosis was coincident with the inflammatory response.
Figure 2.
Cardiac inflammatory and fibrotic responses in B6 mice in response to 4 weeks of AngII infusion. ED-1 positive macrophages (arrow) were accumulated in the damaged myocardium (b) and perivascular space (c), which were accompanied by interstitial (e) and perivascular fibrosis (arrow) (f). (a) and (d) cardiac ED1 and PSR staining in untreated control B6 mouse, respectively. ×200.
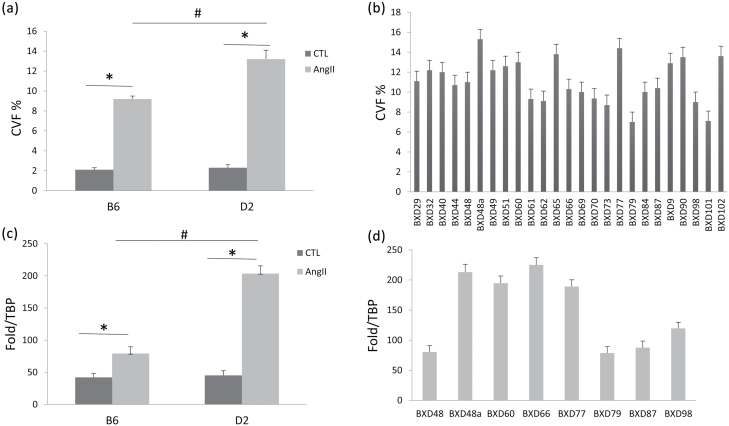
Quantitative CVF showed that cardiac collagen was increased in B6 and D2 strains, while the increase in cardiac CVF was significantly greater in the D2 strain than in the B6 strain (Figure 3a). Cardiac collagen volume, however, was remarkably varied across BXD strains (Figure 3b).
Figure 3.
Quantitative changes in cardiac CVF and TGF-β gene expression in B6, D2, and BXD strains in response to AngII infusion. Cardiac CVF occupied approximately 2% of total myocardial volume in the normal heart. Following AngII infusion, cardiac CVF was more evident in the D2 strain compared to the B6 strain (a), while cardiac CVF levels were greatly varied cross BXD strains (b). Cardiac TGF-β mRNA levels were significantly increased in both B6 and D2 strains in response to AngII infusion (c), but the increase in TGF-β mRNA was more evident in D2 than B6. Cardiac TGF-β mRNA levels were greatly different across the BXD strains (d). *P < 0.05: untreated (CTL) vs. AngII-treated B6 or D2 strain; #P < 0.05: AngII-treated B6 vs. AngII-treated D2 strain. Abbreviations: CVF, collagen volume fraction; TBP, TATA box-binding protein; TGF, transforming growth factor.
TGF-β is a key modulator of tissue fibrosis. Our data revealed that compared to controls, gene expression of cardiac TGF-β1 was significantly increased in both B6 and D2 strains, while it was more intense in D2 mice (Figure 3c). Our data further demonstrated that cardiac TGF-β1 mRNA levels were widely different among AngII-infused BXD strains (Figure 3d).
Hypertensive kidney disease
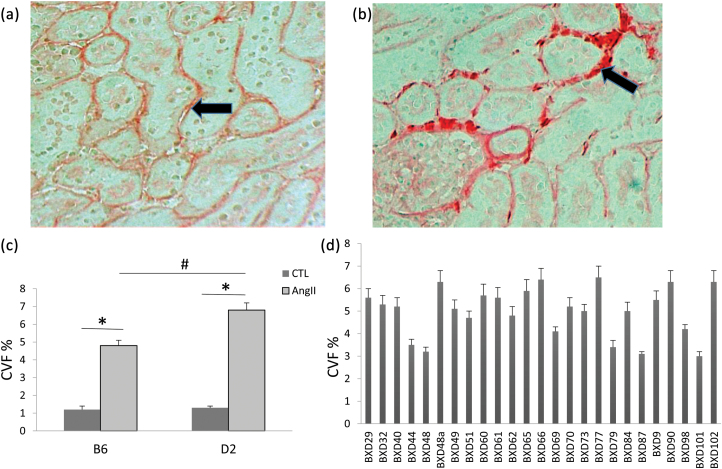
Hypertensive kidney disease is another important relevant phenotype of chronic hypertension, characterized as glomerular alterations (from mild to severe sclerosis of glomeruli), as well as periglomerular and interstitial fibrosis.2 As illustrated in Figure 4, periglomerular/interstitial fibrosis (panel B) was evident in mice receiving AngII for 4 weeks as detected by picrosirius staining.
Figure 4.
Induction of renal fibrosis by chronic AngII infusion. Compared to untreated controls, which contained a small amount of collagen in the interstitial space (a, arrow), chronic AngII treatment lead to renal fibrosis in B6 mice (b, arrow). Quantitative analysis of renal CVF revealed that the renal collagen accumulation was more evident in the D2 strain than the B6 strain (c), whereas the increase in renal CVF considerably vary across the BXD strains (d). (a)–(b): ×200. *P < 0.05: untreated (CTL) vs. AngII-treated B6 or D2 strain; #P < 0.05: AngII-treated B6 vs. AngII-treated D2 strain. Abbreviation: CVF, collagen volume fraction.
Quantitative data further revealed that renal CVF was significantly increased in both B6 and D2 strains, whereas renal fibrosis was more evident in the D2 strain compared to B6 strain (Figure 4c). Renal CVF was, however, widely differed among BXD strains (Figure 4d).
DISCUSSION
Primary hypertension reveals a broad spectrum of clinical manifestations, which differ from individual to individual. Moreover, the disorder also displays different severity of phenotypes among diverse racial/ethnic groups. African Americans have the excess prevalence and severity of hypertension than Caucasians.28 These racial and ethnic differences have been identified in the renin–angiotensin system, prevalence of salt sensitivity, ion-transport mechanisms, and calcium homeostasis.29 Genetic factors are primarily responsible for the differences in the prevalence of primary hypertension. It is known that the genetic origins of primary hypertension involve a large number of genetic variants. However, only a small fraction of the blood heritability has so far been elucidated and further experiments are needed to capture additional trait variability. Discovering of the genetic basis of differential vulnerability to hypertension is critical in predicting and developing personalized care for patients and will provide the underlying genetic determinants of the hypertensive phenotypes.
A number of genetic determinants that explain a small proportion of the genetic variance have been identified by human hypertension GWAS. Even though human GWAS are feasible, the power of these studies has been modest and difficult to tightly control the environmental variables, such as age, sex, body weight, life style, medications, severity and timeline of hypertension, etc. Animal study can overcome many of these limitations of human research and create opportunities to investigate both the mechanisms of diseases and the potential therapies.
Recombinant inbred strains are an important resource for mapping complex traits. The combined BXD strain set is the largest mouse recombinant inbred mapping panel. It is composed of over 100 lines that descend from crosses between B6 and D2 parents and inbreeding progeny for 20 or more generations.14 B6 and D2 strains are 2 of the most commonly used inbred mice in medical research and both strains have been fully sequenced. The characteristics of the D2 strain are often contrasted with those of the B6 strain. Studies have shown that the D2 strain has a greater susceptibility to certain diseases, such as the age-related hearing loss, glaucoma, audiogenic seizures, and calcified lesions of the testes, tongue, skeletal muscle, etc.30–32 The BXD strains are now fully inbred, which provides accurate data on the genotypes of all strains and, therefore, a powerful tool for collaborative analysis of quantitative traits and gene function.
Using a well-established experimental hypertension mouse model, we examined variability of hypertensive phenotypes among B6, D2, and BXD strains with different genetic background. Firstly, we evaluated the systolic blood pressure across B6, D2, and BXD strains in response to AngII infusion. AngII treatment used in the study was a presser dose, which lead to blood pressure elevation in all of these strains. However, our data further revealed that blood pressure elevation in the D2 strain was significantly greater relative to the B6 strain, whereas blood pressure levels markedly varied among BXD strains. These observations implicate that different genetic background in BXD strains results in the variable degree of elevation of blood pressure in response to AngII treatment.
Uncontrolled and prolonged elevation of blood pressure can lead to a variety of changes in the myocardial structure, coronary vasculature, and conduction system of the heart. These changes, in turn, lead to the development of left ventricular hypertrophy, coronary artery disease, cardiac injury/fibrosis, various conduction system diseases, and systolic and diastolic dysfunction.33 Our study revealed that AngII treatment resulted in varied degree of severity of cardiac interstitial and perivascular fibrosis in B6, D2, and BXD strains. Furthermore, the gene expression of cardiac TGF-β, a marker of tissue fibrosis, also differed greatly across these strains, which was also coincident with the severity of cardiac fibrosis. Thus, the severity of hypertensive heart disease is distinct among the BXD strains.
In addition to hypertensive cardiac disease, renal fibrosis was evaluated in B6, D2, and BXD in response to chronic AngII infusion. Hypertensive kidney disease is another major hypertensive phenotype, expressed as renal injury with fibrosis in the small blood vessels, glomeruli, renal tubules, and interstitial tissues.2 As observed in the heart, different severity of renal fibrosis was developed among BXD strains. Taken together, the different genetic background in BXD strains is associated with the variability of blood pressure, hypertensive heart, and kidney diseases. The variability in cardiac and renal fibrosis might be associated to different levels of hypertension in response to AngII and/or genetic modulation on cardiac and renal fibrosis.
The heterogeneity of the hypertensive phenotypes in BXD strains indicates that the BXD cohort is suitable to map loci and gene variants that affect complex hypertensive phenotypes. However, the main historical problem to identify loci and genes using BXD mice is too few strains (26 to 32 strains in most prior work) to obtain sufficient statistical power. Large panel of BXDs offers significantly enhanced mapping precision and extraordinary statistical power in determining gene loci and genes that control diseases. Our findings in 25 BXD strains have laid a solid foundation for the purpose and more BXD strains are needed to determine hypertensive phenotypes using AngII model for identification of candidate genes that impact hypertension in the future study.
In summary, the study examined hypertensive phenotypes in BXD strains in response to AngII infusion. Our data demonstrate that BXDs with different genetic background developed varied severity of hypertension phenotypes. It implicates that genomics contribute to pathogenesis of primary hypertension. The current study also indicates that building upon the genotypes and hypertensive phenotypes in BXD strains, the mouse cohort can be exploited experimentally to identify genes that influence blood pressure for the future study.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENT
This work was supported by NIH Heart, Blood, and Lung Institute (1R01HL128350, Y.S., L.L.).
REFERENCES
- 1. Georgiopoulou VV, Kalogeropoulos AP, Raggi P, Butler J. Prevention, diagnosis, and treatment of hypertensive heart disease. Cardiol Clin 2010; 28:675–691. [DOI] [PubMed] [Google Scholar]
- 2. Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage: implications for therapy. Hypertension 2004; 44:595–601. [DOI] [PubMed] [Google Scholar]
- 3. Carretero OA, Oparil S. Essential hypertension. Part I: definition and etiology. Circulation 2000; 101:329–335. [DOI] [PubMed] [Google Scholar]
- 4. Brook RD, Weder AB, Rajagopalan S. “Environmental hypertensionology” the effects of environmental factors on blood pressure in clinical practice and research. J Clin Hypertens (Greenwich) 2011; 13:836–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Padmanabhan S, Newton-Cheh C, Dominiczak AF. Genetic basis of blood pressure and hypertension. Trends Genet 2012; 28:397–408. [DOI] [PubMed] [Google Scholar]
- 6. Dominiczak AF, Negrin DC, Clark JS, Brosnan MJ, McBride MW, Alexander MY. Genes and hypertension: from gene mapping in experimental models to vascular gene transfer strategies. Hypertension 2000; 35:164–172. [DOI] [PubMed] [Google Scholar]
- 7. Ehret GB. Genome-wide association studies: contribution of genomics to understanding blood pressure and essential hypertension. Curr Hypertens Rep 2010; 12:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Padmanabhan S, Melander O, Hastie C, Menni C, Delles C, Connell JM, Dominiczak AF. Hypertension and genome-wide association studies: combining high fidelity phenotyping and hypercontrols. J Hypertens 2008; 26:1275–1281. [DOI] [PubMed] [Google Scholar]
- 9. Chen CY, Chang IS, Hsiung CA, Wasserman WW. On the identification of potential regulatory variants within genome wide association candidate SNP sets. BMC Med Genomics 2014; 7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DiStefano JK, Kingsley C, Craig Wood G, Chu X, Argyropoulos G, Still CD, Doné SC, Legendre C, Tembe W, Gerhard GS. Genome-wide analysis of hepatic lipid content in extreme obesity. Acta Diabetol 2015; 52:373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tang W, Kowgier M, Loth DW, Soler Artigas M, Joubert BR, Hodge E, Gharib SA, Smith AV, Ruczinski I, Gudnason V, Mathias RA, Harris TB, Hansel NN, Launer LJ, Barnes KC, Hansen JG, Albrecht E, Aldrich MC, Allerhand M, Barr RG, Brusselle GG, Couper DJ, Curjuric I, Davies G, Deary IJ, Dupuis J, Fall T, Foy M, Franceschini N, Gao W, Gläser S, Gu X, Hancock DB, Heinrich J, Hofman A, Imboden M, Ingelsson E, James A, Karrasch S, Koch B, Kritchevsky SB, Kumar A, Lahousse L, Li G, Lind L, Lindgren C, Liu Y, Lohman K, Lumley T, McArdle WL, Meibohm B, Morris AP, Morrison AC, Musk B, North KE, Palmer LJ, Probst-Hensch NM, Psaty BM, Rivadeneira F, Rotter JI, Schulz H, Smith LJ, Sood A, Starr JM, Strachan DP, Teumer A, Uitterlinden AG, Völzke H, Voorman A, Wain LV, Wells MT, Wilk JB, Williams OD, Heckbert SR, Stricker BH, London SJ, Fornage M, Tobin MD, O’Connor GT, Hall IP, Cassano PA. Large-scale genome-wide association studies and meta-analyses of longitudinal change in adult lung function. PLoS One 2014; 9:e100776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yatagai Y, Sakamoto T, Yamada H, Masuko H, Kaneko Y, Iijima H, Naito T, Noguchi E, Hirota T, Tamari M, Konno S, Nishimura M, Hizawa N. Genomewide association study identifies HAS2 as a novel susceptibility gene for adult asthma in a Japanese population. Clin Exp Allergy 2014; 44:1327–1334. [DOI] [PubMed] [Google Scholar]
- 13. Pearson TA, Manolio TA. How to interpret a genome-wide association study. JAMA 2008; 299:1335–1344. [DOI] [PubMed] [Google Scholar]
- 14. Peirce JL, Lu L, Gu J, Silver LM, Williams RW. A new set of BXD recombinant inbred lines from advanced intercross populations in mice. BMC Genet 2004; 5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ashbrook DG, Williams RW, Lu L, Stein JL, Hibar DP, Nichols TE, Medland SE, Thompson PM, Hager R. Joint genetic analysis of hippocampal size in mouse and human identifies a novel gene linked to neurodegenerative disease. BMC Genomics 2014; 15:850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Walters DM, White KM, Patel U, Davis MJ, Veluci-Marlow RM, Bhupanapadu Sunkesula SR, Bonner JC, Martin JR, Gladwell W, Kleeberger SR. Genetic susceptibility to interstitial pulmonary fibrosis in mice induced by vanadium pentoxide (V2O5). FASEB J 2014; 28:1098–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miyairi I, Tatireddigari VR, Mahdi OS, Rose LA, Belland RJ, Lu L, Williams RW, Byrne GI. The p47 GTPases Iigp2 and Irgb10 regulate innate immunity and inflammation to murine Chlamydia psittaci infection. J Immunol 2007; 179:1814–1824. [DOI] [PubMed] [Google Scholar]
- 18. Chesler EJ, Lu L, Shou S, Qu Y, Gu J, Wang J, Hsu HC, Mountz JD, Baldwin NE, Langston MA, Threadgill DW, Manly KF, Williams RW. Complex trait analysis of gene expression uncovers polygenic and pleiotropic networks that modulate nervous system function. Nat Genet 2005; 37:233–242. [DOI] [PubMed] [Google Scholar]
- 19. Kim S, Iwao H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol Rev 2000; 52:11–34. [PubMed] [Google Scholar]
- 20. Brand S, Amann K, Schupp N. Angiotensin II-induced hypertension dose-dependently leads to oxidative stress and DNA damage in mouse kidneys and hearts. J Hypertens 2013; 31:333–344. [DOI] [PubMed] [Google Scholar]
- 21. Gletsu N, Doan TN, Cole J, Sutliff RL, Bernstein KE. Angiotensin II-induced hypertension in mice caused an increase in insulin secretion. Vascul Pharmacol 2005; 42:83–92. [DOI] [PubMed] [Google Scholar]
- 22. Liu X, Chen Y, McCoy CW, Zhao T, Quarles DL, Pi M, Bhattacharya SK, King G, Sun Y. Differential regulatory role of soluble klothos on cardiac fibrogenesis in hypertension. Am J Hypertens 2016; 29:1140–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the rat heart: role of oxidative stress. Am J Pathol 2002; 161:1773–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhao W, Zhao T, Chen Y, Ahokas RA, Sun Y. Reactive oxygen species promote angiogenesis in the infarcted rat heart. Int J Exp Pathol 2009; 90:621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhao W, Zhao T, Chen Y, Qu Y, Gerling IC, Sun Y. Modification of oxidative stress on gene expression profiling in the rat infarcted heart. Mol Cell Biochem 2013; 379:243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sun Y, Ramires FJ, Weber KT. Fibrosis of atria and great vessels in response to angiotensin II or aldosterone infusion. Cardiovasc Res 1997; 35:138–147. [DOI] [PubMed] [Google Scholar]
- 27. Kai H, Kudo H, Takayama N, Yasuoka S, Kajimoto H, Imaizumi T. Large blood pressure variability and hypertensive cardiac remodeling–role of cardiac inflammation. Circ J 2009; 73:2198–2203. [DOI] [PubMed] [Google Scholar]
- 28. Lackland DT. Racial differences in hypertension: implications for high blood pressure management. Am J Med Sci 2014; 348:135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Minor DS, Wofford MR, Jones DW. Racial and ethnic differences in hypertension. Curr Atheroscler Rep 2008; 10:121–127. [DOI] [PubMed] [Google Scholar]
- 30. Libby RT, Anderson MG, Pang IH, Robinson ZH, Savinova OV, Cosma IM, Snow A, Wilson LA, Smith RS, Clark AF, John SW. Inherited glaucoma in DBA/2J mice: pertinent disease features for studying the neurodegeneration. Vis Neurosci 2005; 22:637–648. [DOI] [PubMed] [Google Scholar]
- 31. Neumann PE, Collins RL. Genetic dissection of susceptibility to audiogenic seizures in inbred mice. Proc Natl Acad Sci U S A 1991; 88:5408–5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matsushima Y, Imai T, Watanabe O, Kawahara H, Ohne M, Takai H. Spontaneous calcified tongue lesions in DBA mice. Jikken Dobutsu 1984; 33:539–542. [DOI] [PubMed] [Google Scholar]
- 33. Drazner MH. The progression of hypertensive heart disease. Circulation 2011; 123:327–334. [DOI] [PubMed] [Google Scholar]