Abstract
BACKGROUND
We investigated the influence of salt overconsumption on the functionality of the RhoA/Rho-associated kinase (ROCK) pathway and calcium regulation in arteries.
METHODS
The aorta and small mesenteric arteries from rats fed a chow containing 2%, 4%, or 8% NaCl were evaluated in organ baths for the activity of the RhoA/ROCK pathway and intracellular calcium mobilization. Components of these pathways and intracellular calcium levels were also assessed in samples from 4% NaCl group.
RESULTS
In arteries from animals fed regular chow, the ROCK inhibitor Y-27632 reduced the responses to phenylephrine, even when the smallest concentrations (1 and 3 μM) were tested. However, only higher concentrations of Y-27632 (10 and 50 μM) reduced phenylephrine-induced contraction in vessels from high-salt groups. Immunoblotting revealed augmented phosphorylation of the myosin phosphatase targeting subunit 1 and increased amounts of RhoA in the membrane fraction of aorta homogenates from the 4% NaCl group. Under calcium-free solution, vessels from NaCl groups presented reduced contractile responses to phenylephrine and caffeine, compared with the regular chow group. Moreover, decreased intracellular calcium at rest and after stimulation with ATP were found in aortic smooth muscle cells from 4% NaCl-fed rats, which also showed diminished levels of SERCA2 and SERCA3, but not of IP3 and ryanodine receptors, or STIM1 and Orai1 proteins.
CONCLUSIONS
Arteries from rats subjected to high-salt intake are unable to properly regulate intracellular calcium levels and present augmented activity of the calcium sensitization pathway RhoA/ROCK. These changes may precede the development of vascular diseases induced by high-salt intake.
Keywords: blood pressure, calcium sensitization, calcium signaling, high-salt, hypertension, vascular dysfunction, vascular smooth muscle cell.
Systemic arterial hypertension is often associated with both genetic and environmental events,1 and it is well recognized that excessive salt intake is a major risk factor for hypertension.2–5 In spite of the wide number of studies in this field, salt-associated cardiovascular changes and their contribution to the development of primary vascular disorders remains poorly understood.
Since a Rho-associated kinase (ROCK) was described and identified as a regulator of vascular smooth muscle contraction,6,7 the role of the RhoA/ROCK pathway in the cardiovascular system has been extensively investigated. The 2 known ROCK isoforms (ROCK I and II) are protein serine/threonine kinases with a Rho-binding regulatory domain, and binding of the small GTPase-protein RhoA to this regulatory site increases the inhibitory activity of ROCK on myosin phosphatase, resulting in physiological calcium sensitization and regulation of the vascular tone.6,8 Nevertheless, inhibition of ROCK reduces blood pressure in hypertensive rats,9 and components of the RhoA/ROCK pathway are highly expressed or more active in vessels from angiotensin II-infused rodents, 2-kidney 1-clip renal hypertensive rats, deoxycorticosterone acetate-salt (DOCA-salt) animals, Dahl salt-sensitive and spontaneously hypertensive rats.9–11
Notably, in experimental studies investigating salt-induced hypertension, high blood pressure levels are reached when elevated dietary levels of NaCl are given to mineralocorticoid-treated rodents (the DOCA-salt model), chronically angiotensin II-infused rats, or Dahl salt-sensitive rats,12–14 since overconsumption of NaCl alone is not enough to induce hypertension in healthy animals.15 At least to our knowledge, the involvement of the RhoA/ROCK pathway in the cardiovascular changes induced by continuous high-salt intake by healthy animals, not exposed to any additional condition that could contribute to increase blood pressure, remains to be investigated. The main hypothesis of this study was that overconsumption of NaCl can increase the activity of components of the RhoA/Rho-kinase pathway, accounting for changes in the vascular functionality that may precede the salt-associated hypertensive condition.
METHODS
Animals and experimental groups
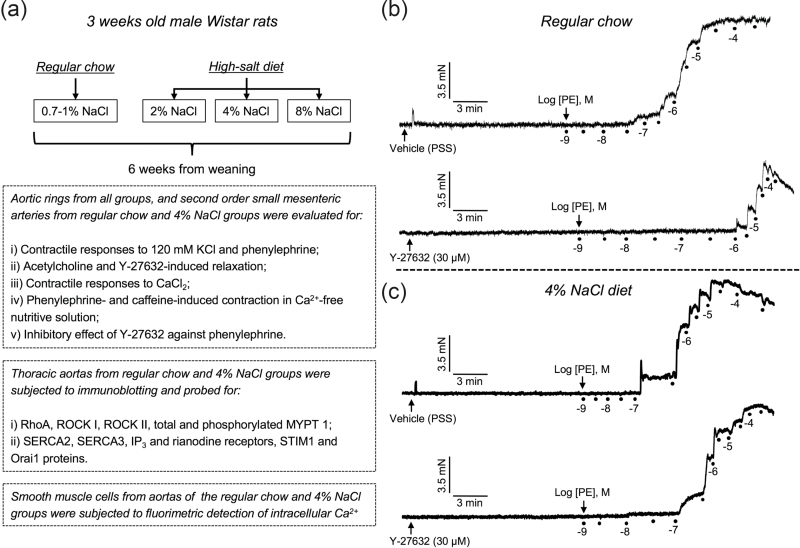
All procedures adopted in this study were approved by the Institutional Ethics Committee for Animal Use of Universidade Federal do Paraná (authorization number 345), and by the Institutional Animal Care and Use Committee from Georgia Health Sciences University (authorization number 2011-0353). Male Wistar rats (21 days, weighing 50–60 g) provided by either Universidade Federal do Paraná (Curitiba, PR, Brazil) or Harlan Laboratories (Indianapolis, IN) were used in this study. The animals were kept under a constant 12/12 hour light/dark cycle and controlled temperature (22 ± 2 °C), with ad libitum access to food and water. The experiments were conduct in a control group that received standard rat chow containing 0.7–1% NaCl, and 3 experimental groups, which were fed chow containing 2%, 4%, or 8% NaCl (purchased from Quimtia, Colombo, PR, Brazil; or Envigo, Madison, WI). The high-NaCl or regular diet was started at weaning and maintained for 6 weeks, when the animals (9 weeks old, weighing 250–300 g) were euthanized by anesthetic overdose (ketamine/xylazine, 200 and 40 mg/kg, i.p., respectively) and the thoracic aorta or second order small mesenteric arteries (external diameter: ~250 μm) were mounted in organ baths for evaluation of vascular reactivity, processed to western blotting analyses or used as source of smooth muscle cells that were subjected for analysis of intracellular calcium levels. The experiments using aortic rings were performed at Universidade Federal do Paraná (Curitiba, PR, Brazil), and all additional studies were conducted at Augusta University (Augusta, GA). The detailed methods, experimental protocols, and composition of the diets are available as Supplementary Material. A summary of the experiments performed, including typical tracings obtained in organ baths is presented in Figure 1.
Figure 1.
Summary of experimental design (a) and trace recordings in second order mesenteric arteries from rats subjected to regular chow (b) and 4% NaCl diet (c), showing the contractile responses induced by cumulative concentrations of phenylephrine (PE) in vessels previously incubated during 15 minutes with either vehicle (physiological nutritive solution, PSS), or the ROCK inhibitor Y-27632 (30 μM).
Statistical analysis
The results show the mean ± SEM of 5–6 experiments using samples from different animals from each group, unless otherwise specified, such as in the western blotting and intracellular calcium analyses. Statistical significance was determined through 1- or 2-way analysis of variance, both followed by Bonferroni correction. The Student’s t-test was used when applicable. A value of P <0.05 was accepted as statistically significant.
RESULTS
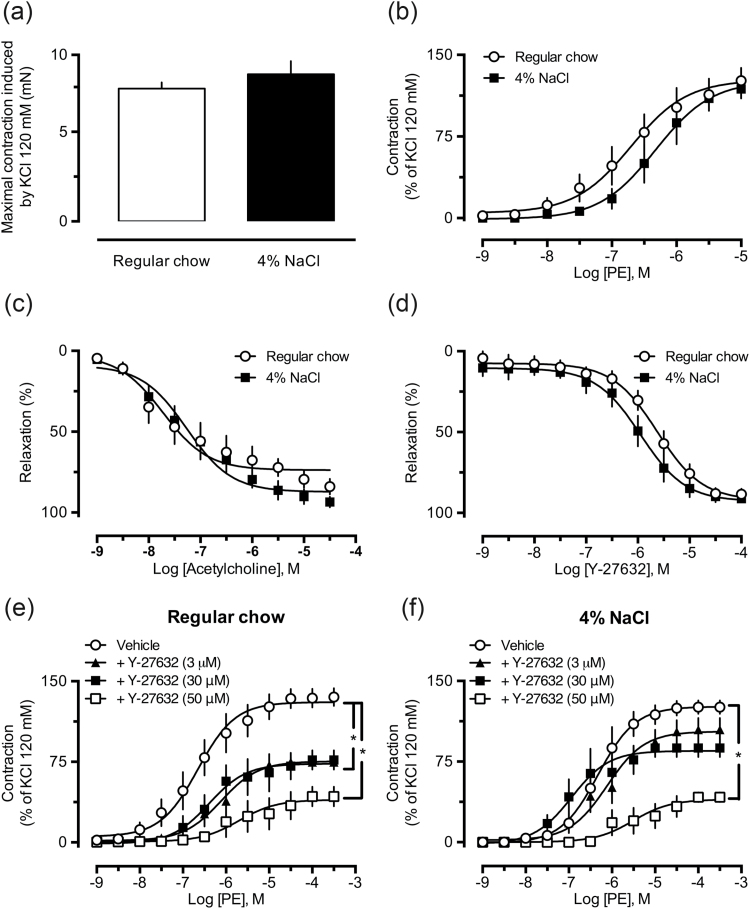
Lack of changes in vascular responsiveness to vasoconstrictors and vasodilators in vessels from high-salt diet-fed rats
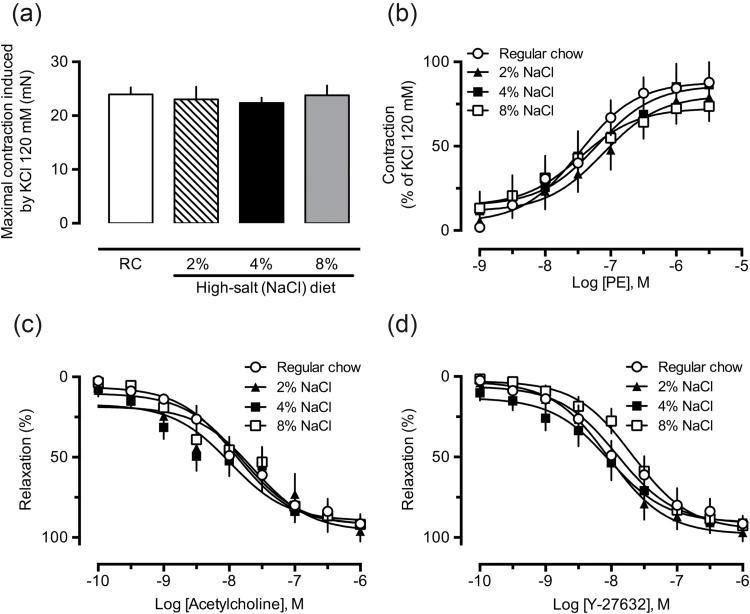
The experiments performed with endothelium-denuded aortic rings obtained from rats fed a high-salt diet did not reveal any significant change in the contractile responses induced by 120 mM KCl solution (modified PSS; Figure 2a), or cumulative concentrations of PE (Figure 2b), a selective α1-adrenergic receptor agonist. The effects of KCl (data not shown) and PE also remained unchanged in endothelium-intact aortic rings from the 2%, 4%, or 8% NaCl groups (Supplementary Material, Figure S1A–D, open circles). The relaxation induced by the muscarinic receptor agonist acetylcholine or the selective ROCK inhibitor Y-27632 in aortic rings from the high-salt groups presented the same potency and maximal effect observed in preparations from the control groups (Figure 2c and d, respectively).
Figure 2.
Aortas from rats fed a chow containing high NaCl show unaltered reactivity to vasoactive agents. The vascular reactivity to 120 mM KCl (a), cumulative concentrations of phenylephrine (PE, b), acetylcholine (c), or Y-27632 (d) were recorded in aortic rings from rats subjected to regular chow (also indicated as “RC”), 2%, 4%, or 8% NaCl diets. The results show the mean ± SEM of 5–6 preparations from different animals per group. No statistical differences were found.
Reduced inhibitory effect of Y-27632 on PE-induced vasoconstriction in aortas from high-salt diet-fed rats
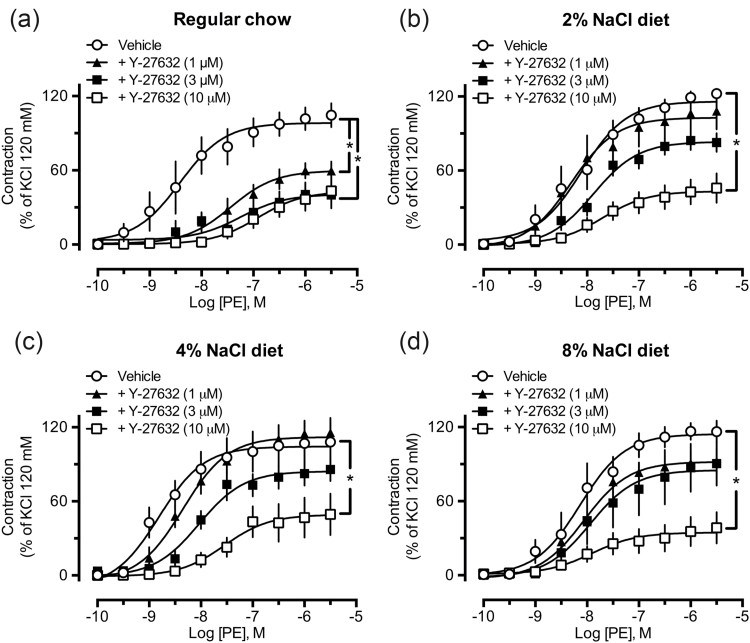
Incubation of endothelium-denuded aortic rings with the ROCK inhibitor Y-27632 for 15 minutes significantly reduced responses to PE in preparations from control rats, even when the smallest concentration adopted in this study (1 μM) was tested (Figure 3a). On the other hand, only the highest concentration of Y-27632 (10 μM) was able to significantly reduce the effects of PE in aortas obtained from high-salt diet-fed groups (2%, 4%, and 8% NaCl, Figure 3b–d, respectively). Similar results were found in endothelium-intact aortic rings (Supplementary Material, Figure S1A–D).
Figure 3.
Aortas from rats fed a chow containing high NaCl are less sensitive to the inhibitory effect of Y-27632 on phenylephrine-induced contraction. The inhibitory effect of the ROCK inhibitor Y-27632 (1, 3, or 10 μM) on PE-induced contraction is shown in aortic rings from animals subjected to regular chow (a), 2% (b), 4% (c), and 8% (d) NaCl diets. The results show the mean ± SEM of 5–6 preparations from different animals per group. *P < 0.05 compared with control (vehicle).
High-salt diet increases activation of the RhoA/ROCK pathway
The data obtained from 2%, 4%, and 8% NaCl groups did not disclose any difference among the groups, regarding the reduced sensitivity of aortic rings to the inhibitory effect of Y-27632 on PE-induced tension generation. For this, the experiments to explore the protein levels of components of the RhoA/ROCK pathway were performed only in vessels from rats fed regular (control group) or 4% NaCl chow.
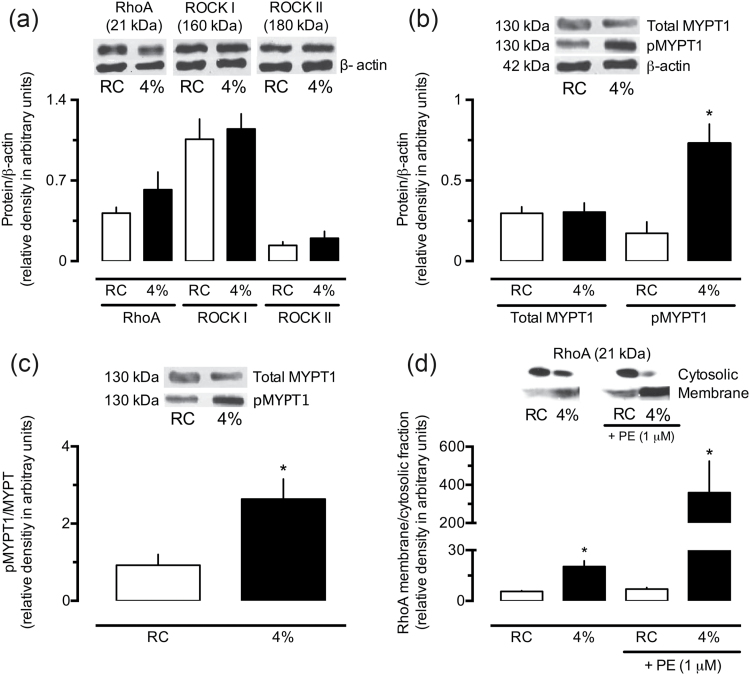
Western blot analyses revealed no differences between the levels of total RhoA, ROCK I, or ROCK II in the aortas of the control and 4% NaCl groups (Figure 4a). However, despite the total MYPT1 remained unchanged (Figure 4b), the amounts of phosphorylated-MYPT1 were significantly increased in aortas from 4% NaCl group (Figure 4b and c). Moreover, aortic homogenates from the 4% NaCl group presented an increased proportion of RhoA in the membrane, both at rest and under stimulation by PE (Figure 4d).
Figure 4.
Increased phosphorylation of MYPT1 and translocation of RhoA to the membrane in the aorta of rats subjected to a high-salt diet. The levels of RhoA, ROCK I and ROCK II (a), total and phosphorylated-MYPT1 (b and c), and membrane and cytosolic fractions of RhoA (d) were measured in aorta homogenates from rats fed regular chow (RC) or 4% NaCl chow. In panel d, the results also show the quantification of RhoA in the membrane and cytosol of aortas stimulated by phenylephrine (PE, 1 μM). The results show the mean ± SEM of densitometric analyses of 3–5 samples per group, all obtained from different animals. *P < 0.05 compared with the respective control column (RC group).
High-salt diet reduces calcium-dependent contraction
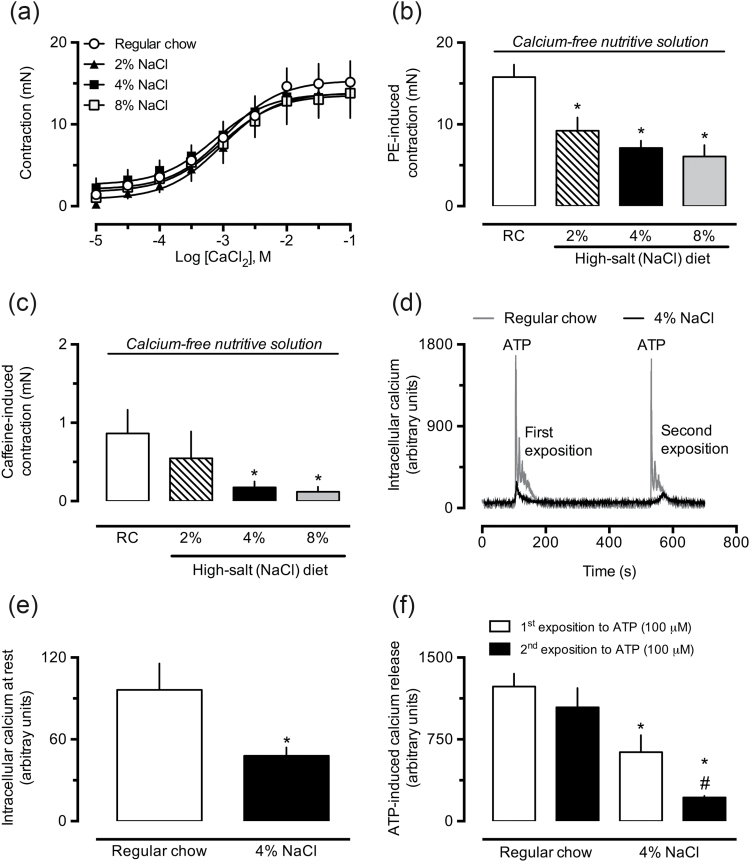
When maintained in calcium-free and depolarizing PSS, aortic rings from 2%, 4%, and 8% NaCl-supplemented groups did not demonstrate altered contractile responses to cumulative amounts of CaCl2 (Figure 5a). However, when a calcium-free nondepolarizing PSS was used, the aorta obtained from high NaCl groups presented reduced reactivity to PE (Figure 5b) and caffeine (Figure 5c), compared with control preparations.
Figure 5.
Aortas from rats fed a chow containing high NaCl maintained in calcium-free solution show reduced contractile responses and decreased intracellular calcium levels. The contractile responses to cumulative concentrations of CaCl2 (a), phenylephrine (PE, 1 μM; b), and caffeine (1 mM; c) were evaluated in aortic rings from rats fed regular chow (also indicated as “RC”) or high-NaCl chow, maintained in calcium-free nutritive solution. The fluorometric determination of calcium was conducted in smooth muscle cells freshly isolated from thoracic aorta of rats treated with regular or 4% NaCl chow, perfused with low-calcium nutritive solution, and loaded with Fura-2 (2 µg/ml). The analyses were performed before (e) and during the stimulation by ATP (100 μM; f), as illustrated in trace recordings from typical experiments (d). The results show the mean ± SEM of 3–6 samples from different animals per group. *P < 0.05 compared with control (regular chow group, RC). #P < 0.05 compared with the first exposure to ATP in the 4% NaCl group.
High-salt diet reduces intracellular calcium levels
Smooth muscle cells freshly isolated from aortas of the 4% NaCl group presented diminished amounts of cytosolic calcium at rest (Figure 5e), as well as a 50% and 80% reduction in their ability to release calcium from intracellular stores in response to ATP, after the first and second stimulation, respectively (Figure 5f), compared with cells obtained from rats receiving regular chow. Typical recordings of these experiments are shown in Figure 5d.
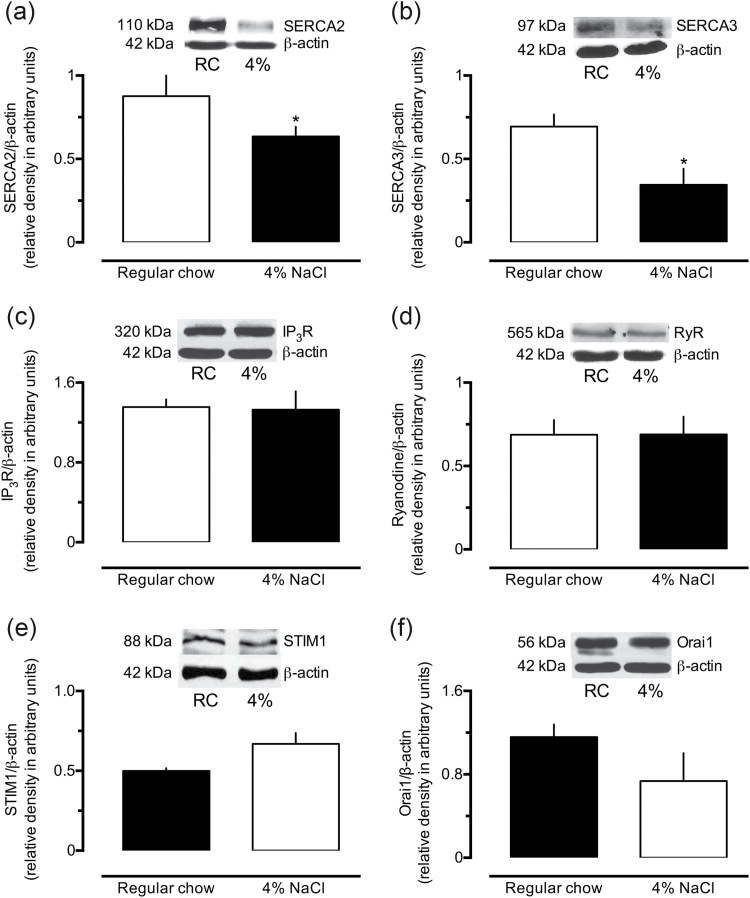
High-salt diet decreases the expression of SERCA
Aortas of the 4% NaCl group presented reduced amounts of both SERCA2 (~28%) and SERCA3 (~50%), compared with samples from control animals (Figure 6a and b, respectively). Nonetheless, compared with controls, the expression levels of IP3R (Figure 6c), RyR (Figure 6d), STIM1 (Figure 6e), and Orai1 (Figure 6f) remained unchanged in the aorta of rats exposed to the high-salt diet.
Figure 6.
Reduced levels of SERCA in aorta homogenates from rats subjected to a high-sodium diet. The levels of SERCA 2 (a), SERCA 3 (b), IP3 receptors (IP3R, c), ryanodine receptors (RyR, d), STIM 1 (e), and Orai1 (f) were measured in aorta homogenates from rats fed with regular or 4% NaCl chow. Results show the mean ± SEM of densitometric analyses of 3–5 samples per group, all obtained from different animals. *P < 0.05 compared with the respective control column.
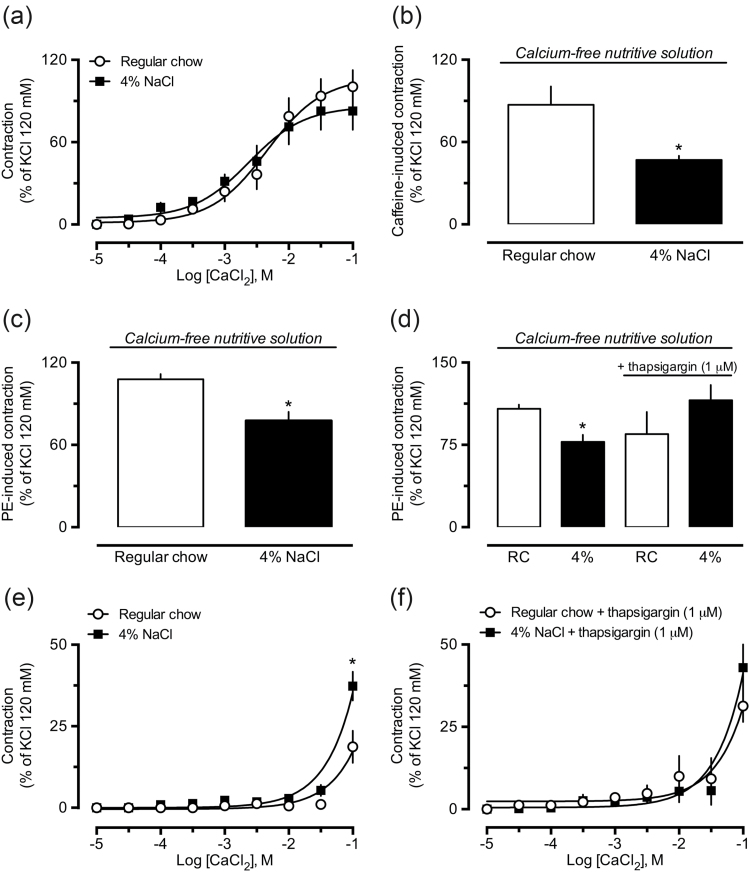
High-salt diet increases the activity of ROCK and reduces calcium-dependent contraction in small mesenteric arteries
To evaluate if our findings were not restricted to large conductance vessels such as aorta, we also measured the vascular reactivity of small mesenteric arteries from both control and 4% NaCl groups. Our experiments revealed no differences in the effects of 120 mM KCl solution (Figure 7a), PE (Figure 7b), acetylcholine (Figure 7c), and Y-27632 (Figure 7d) in small mesenteric arteries isolated from animals fed the 4% NaCl diet, compared with vessels from animals that received the control diet. Moreover, the reduced ability of Y-27632 to inhibit PE-induced contraction was fully reproducible in small mesenteric arteries from the 4% NaCl group (Figure 7e and f), as illustrated in the trace recordings presented in Figure 1b and c.
Figure 7.
Functional evidence of augmented activity of the RhoA/ROCK pathway in resistance arteries from rats subjected to a high-salt diet. The vascular reactivity of small mesenteric arteries to 120 mM KCl (a), phenylephrine (PE, b), acetylcholine (c), and Y-27632 (d) were measured in arteries obtained from regular chow and 4% NaCl diets. The inhibitory effect of the ROCK inhibitor Y-27632 (3, 30, or 50 μM) on PE-induced contraction was evaluated in small mesenteric arteries obtained from control (regular chow diet, e) and 4% NaCl group (f). The results presented are the mean ± SEM of 5–6 preparations per group. *P < 0.05 compared with the regular chow group.
As found in experiments using aortic rings under depolarizing conditions, there were no differences between the reactivity of small arteries from control and 4% NaCl groups in response to CaCl2 (Figure 8a). In addition to reduced reactivity to caffeine (Figure 8b), small mesenteric arteries from the 4% NaCl group maintained in nondepolarizing calcium-free PSS also showed decreased responses to PE (Figure 8c), which was normalized by incubation with the inhibitor of sarcoplasmic reticulum Ca2+-ATPase (SERCA), thapsigargin (Figure 8d). We also found that, compared with vessels from control animals, small mesenteric arteries from the 4% NaCl group, maintained in regular PSS, presented enhanced tone when the medium was overloaded with 100 mM CaCl2 (Figure 8e). Interestingly, addition of thapsigargin increased the sensitivity of preparations obtained from control animals to CaCl2, but failed to further increase the effects of high CaCl2 in vessels from the 4% NaCl group (Figure 8f).
Figure 8.
Functional evidence of decreased mobilization of intracellular calcium in resistance arteries from rats exposed to a high-salt diet. Compared with responses obtained in vessels from the regular chow (also indicated as “RC”), the small mesenteric arteries from 4% NaCl group maintained in calcium-free nutritive solution presented unaltered responses to CaCl2 (a), but were less reactive to caffeine (b), and phenylephrine (PE, c), which was prevented by thapsigargin (d). Panels e and f show the increase in the vascular tone evoked by CaCl2 overload in small mesenteric arteries from regular chow and 4% NaCl groups before (e) and after (f) incubation with thapsigargin. The results are the mean ± SEM of 5–6 preparations from different animals per group. *P < 0.05 compared with the respective control (regular chow group, RC).
DISCUSSION
Using vessels obtained from Wistar rats subjected to a high-salt diet for 6 weeks, our experiments showed that overconsumption of NaCl did not change the in vitro vascular reactivity to high KCl solution, phenylephrine, and acetylcholine (Figure 2a–c, respectively), classical tools used to explore contractility and endothelial function. Notably, high-salt intake is expected to result in impaired vascular reactivity to vasoactive agents and increased blood pressure in rats, but the majority of the studies showing such findings have been conducted with DOCA-salt or Dahl salt-sensitive rats,12,16,17 instead of animals under high-salt intake only. Our study used healthy, normotensive rats that did not develop hypertension (Supplementary Material, Figure S2), even after a period of exposure of 6 weeks to high-salt levels.
In spite of the lack of changes in vascular reactivity, the high-salt diet rendered aortic rings less sensitive to the inhibitory effects of Y-27632 on phenylephrine-induced contraction, in both endothelium-denuded (Figure 3) and endothelium-intact arteries (Supplementary Material, Figure S1). Given that RhoA is physiologically activated by several G-protein coupled receptors (GPCRs), including the α1A-adrenergic receptor,18 (leading to activation of ROCK), and that Y-27632 is a highly selective inhibitor of both ROCK I and II isoforms,19 the reduced potency of Y-27632 to inhibit PE-induced contraction in aortas from the NaCl-supplemented groups was taken to indicate that, in spite of the normal reactivity to vasoconstrictors and vasodilators, the functionality of the RhoA/ROCK pathway was enhanced by high-salt intake. Notably, this finding was seen in aortic rings from animals subjected to chow containing 2%, 4%, and 8% NaCl, showing that even a slight (2%), or moderate (4%) increase in salt intake may influence the vascular biology, even in the absence of detectable changes in vascular reactivity or blood pressure levels. For this reason, most of the subsequent experiments were performed using vessels obtained from the 4% NaCl group only.
Previous studies have associated the increased activity of the RhoA/ROCK pathway with augmented protein levels of RhoA and ROCK in the vasculature.20 We did not find any change in the levels of total RhoA, ROCK I, or ROCK II, but our experiments revealed that aortas from animals fed chow containing 4% NaCl present higher levels of membrane-bound RhoA than aortas from control animals, under both basal and phenylephrine-stimulated conditions (Figure 4d). Activation of RhoA by GPCRs involves translocation of RhoA from the cytosol to the plasma membrane, allowing its interaction with downstream effectors, such as ROCK.21,22 Once stimulated by RhoA, ROCK phosphorylates the myosin phosphatase target subunit 1 (MYPT1), a regulatory subunit of myosin phosphatase, inhibiting the activity of this enzyme, and favoring smooth muscle contraction mediated through Ca2+-calmodulin-dependent myosin light chain kinase.23 Indeed, the analysis of MYPT1 phosphorylation at Thr696 has been employed as a direct indicator of ROCK activity,24 and we detected augmented levels of phospho-MYPT1 in aortas of the 4% NaCl group (Figure 4b and c). Taking these results together with the reduced effects of the ROCK inhibitor Y-27632 against phenylephrine-induced contraction, and the augmented translocation of RhoA to the membrane, the increased degree of phosphorylation of MYPT1 indicates that the RhoA/ROCK pathway is more active in aortas from animals subjected to a high-salt diet. Importantly, the increased activity of this pathway has been related to several pathological conditions, including hypertension,25 but at least to our knowledge, this is the first study demonstrating that an augmented activation of the RhoA/ROCK pathway takes place in vessels from animals subjected to a high-salt intake, without any coexisting condition (i.e., angiotensin II infusion or deoxycorticosterone administration).
Because we found substantial evidence of enhanced activation of ROCK, but the vessels did not display any change in vascular reactivity (i.e., unaltered responses to phenylephrine), and because the RhoA/ROCK pathway mediates calcium sensitization, we investigated whether either calcium influx or calcium mobilization from intracellular stores could be impaired in vessels from high-salt diet-fed groups. Using a depolarizing calcium-free PSS, we found that aortas from rats fed chow containing 2%, 4%, or 8% NaCl did not present any change in calcium-induced contraction (Figure 5a), a finding that clearly indicated that calcium influx by voltage-activated membrane calcium channels, as well as the cellular contractile machinery, were not impaired by high-salt intake. However, these same vessels displayed reduced reactivity to phenylephrine and caffeine, when evaluated in nondepolarizing calcium-free nutritive solution (Figure 5b and c, respectively). Since phenylephrine- and caffeine-induced contraction in calcium-free medium are fully dependent on calcium release from the sarcoplasmic reticulum, through activation of inositol 1,4,5-trisphosphate receptors (IP3R) and ryanodine receptors (RyR), respectively, these results suggested that aortas from the high NaCl groups present disrupted control of intracellular calcium release or storage. Indeed, using the fluorescence dye FURA2, we were able to show that fresh smooth muscle cells isolated from aortas of 4% NaCl-supplemented rats display reduced free intracellular calcium at rest, as well as after stimulation by ATP (Figure 5e and f, respectively). Notably, these cells presented even lower calcium release after a second stimulation by ATP, suggesting an inability to properly reload its intracellular stores.
It is well known that the sarcoplasmic reticulum is the major intracellular structure involved in calcium storage in smooth muscle cells, providing a quick and strictly controlled regulation of cytoplasmic free calcium concentration. The release of calcium from the sarcoplasmic reticulum is primarily mediated by IP3R and RyR,26 while calcium uptake and reticulum reload occurs mainly by SERCA, and the interaction between the sensor stromal interacting molecule 1 (STIM1) and the Orai calcium release-activated calcium modulator 1 (Orai1) present in calcium release-activated calcium channels.27 Importantly, western blot analyses revealed that aortas from the 4% NaCl group did not show any difference in protein levels of IP3R, RyR, STIM1, or Orai1, but presented diminished amounts of both SERCA2 and SERCA3 subtypes (Figure 6). Thus, taking into account the reduced responsiveness to PE and caffeine presented by aortas maintained in calcium-free PSS, the reduced levels of SERCA proteins found by western blot analysis, and the reduced ability of fresh isolated smooth muscle cells to increase their intracellular concentration of free calcium, mainly after a second round of stimulation by ATP, it is reasonable to conclude that high-salt intake compromises the ability of vascular smooth muscle cells from rats to regulate intracellular calcium concentration, which appears to be, at least in part, associated with reduced SERCA pump expression leading to reduced filling of the sarcoplasmic reticulum.
Although widely used and accepted in experimental studies, the aorta is a large conductance vessel with minor influence in the systemic arterial pressure. To circumvent this limitation of our study, most of the functional protocols evaluated in the aortic rings were also investigated in small mesenteric arteries, which are resistance vessels closely related with maintenance of blood pressure and tissue perfusion. The small mesenteric arteries also did not present impaired reactivity to high KCl, phenylephrine, or acetylcholine and, despite the lack of data regarding the levels of components of the RhoA/ROCK pathway, these resistance vessels also showed reduced sensitivity to the inhibitory effect of Y-27632 against phenylephrine-induced force generation (Figure 7), as well decreased reactivity to caffeine and phenylephrine when maintained in calcium-free solution (Figure 8b and c, respectively). Interestingly, the blockage of SERCA by thapsigargin normalized the reactivity to phenylephrine in small mesenteric arteries from the 4% NaCl group, compared with preparations from control animals (Figure 8d). In addition, thapsigargin increased the contraction induced by calcium overload in small arteries from the control group, but had no effect on the already enhanced responses to calcium obtained in preparations from the 4% NaCl group (Figure 8e and f, respectively), suggesting that overconsumption of salt also disrupted the functionality or expression of SERCA in resistance arteries from the mesenteric vascular bed. Importantly, the involvement and role of such changes in other vessels potentially involved in the control of blood pressure, such as renal microcirculation, deserves further investigation.
In conclusion, the results presented here reveal that, in spite of the lack of hypertension and the absence of measurable changes in vascular reactivity, vessels from rats subjected to a 6-week period of high-salt intake in their chow display augmented activity of the RhoA/Rho-kinase pathway. This was accompanied by reduced intracellular calcium levels, which appear to be associated with the inability of the sarcoplasmic reticulum to be reloaded with calcium. Although our data do not allow us to state whether such changes result from a counterbalance to maintain vascular reactivity, neither of which is the first to be initiated, this study clearly demonstrates a significant change in vascular biology, even before the development of changes in vascular reactivity. In addition to calcium sensitization and vascular constriction, the RhoA/ROCK pathway has been implicated in several biological events in the vascular system,28,29 and calcium plays a number of roles in healthy and diseased cells. Although we have not further explored the consequences of our findings for other functions of vascular smooth muscle cells, and additional studies using in vivo approaches remain to be carried out, the knowledge regarding the effects of high-salt intake on both the RhoA/Rho-kinase pathway and calcium homeostasis may contribute to the development of innovative strategies in the management of vascular diseases associated with NaCl overconsumption.
SUPPLEMENTARY MATERIAL
Supplementary data are available at American Journal of Hypertension online.
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
S.C. received a fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil). This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil, 482214/2007-4), National Heart, Lung, and Blood Institute (USA, R01HL071138), and American Heart Association, Grant-in-Aid (15GRMT25700451).
REFERENCES
- 1. Iwamoto T, Kita S, Katsuragi T. Salt-sensitive hypertension, Na+/Ca2+ exchanger, and vascular smooth muscle. Trends Cardiovasc Med 2005; 15:273–277. [DOI] [PubMed] [Google Scholar]
- 2. Elliott P. Observational studies of salt and blood pressure. Hypertension 1991; 17:I3–I8. [DOI] [PubMed] [Google Scholar]
- 3. Brown IJ, Tzoulaki I, Candeias V, Elliott P. Salt intakes around the world: implications for public health. Int J Epidemiol 2009; 38:791–813. [DOI] [PubMed] [Google Scholar]
- 4. U.S. Department of Agricultured ARS. Nutrient Intakes from Food: Mean Amounts Consumed per Individual, by Gender and Age, What We Eat in America, NHANES 2007–2008. http://www.ars.usda.gov/ba/bhnrc/fsrg. 2010. Accessed 12 September 2013.
- 5. Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ 2007; 334:885–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J 1996; 15:2208–2216. [PMC free article] [PubMed] [Google Scholar]
- 7. Kureishi Y, Kobayashi S, Amano M, Kimura K, Kanaide H, Nakano T, Kaibuchi K, Ito M. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem 1997; 272:12257–12260. [DOI] [PubMed] [Google Scholar]
- 8. Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 1997; 389:990–994. [DOI] [PubMed] [Google Scholar]
- 9. Seko T, Ito M, Kureishi Y, Okamoto R, Moriki N, Onishi K, Isaka N, Hartshorne DJ, Nakano T. Activation of RhoA and inhibition of myosin phosphatase as important components in hypertension in vascular smooth muscle. Circ Res 2003; 92:411–418. [DOI] [PubMed] [Google Scholar]
- 10. Nishikimi T, Akimoto K, Wang X, Mori Y, Tadokoro K, Ishikawa Y, Shimokawa H, Ono H, Matsuoka H. Fasudil, a Rho-kinase inhibitor, attenuates glomerulosclerosis in Dahl salt-sensitive rats. J Hypertens 2004; 22:1787–1796. [DOI] [PubMed] [Google Scholar]
- 11. Katholi RE, Naftilan AJ, Oparil S. Importance of renal sympathetic tone in the development of DOCA-salt hypertension in the rat. Hypertension 1980; 2:266–273. [DOI] [PubMed] [Google Scholar]
- 12. Lara LS, McCormack M, Semprum-Prieto LC, Shenouda S, Majid DS, Kobori H, Navar LG, Prieto MC. AT1 receptor-mediated augmentation of angiotensinogen, oxidative stress, and inflammation in ANG II-salt hypertension. Am J Physiol Renal Physiol 2012; 302:F85–F94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rapp JP, Wang SM, Dene H. A genetic polymorphism in the renin gene of Dahl rats cosegregates with blood pressure. Science 1989; 243:542–544. [DOI] [PubMed] [Google Scholar]
- 14. Crestani S, Gasparotto Júnior A, Marques MC, Sullivan JC, Webb RC, da Silva-Santos JE. Enhanced angiotensin-converting enzyme activity and systemic reactivity to angiotensin II in normotensive rats exposed to a high-sodium diet. Vascul Pharmacol 2014; 60:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schupp N, Kolkhof P, Queisser N, Gärtner S, Schmid U, Kretschmer A, Hartmann E, Oli RG, Schäfer S, Stopper H. Mineralocorticoid receptor-mediated DNA damage in kidneys of DOCA-salt hypertensive rats. FASEB J 2011; 25:968–978. [DOI] [PubMed] [Google Scholar]
- 16. Bouhnik J, Richoux JP, Huang H, Savoie F, Baussant T, Alhenc-Gelas F, Corvol P. Hypertension in Dahl salt-sensitive rats: biochemical and immunohistochemical studies. Clin Sci (Lond) 1992; 83:13–22. [DOI] [PubMed] [Google Scholar]
- 17. Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 2000; 351:95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol 2003; 4:446–456. [DOI] [PubMed] [Google Scholar]
- 19. Sakurada S, Takuwa N, Sugimoto N, Wang Y, Seto M, Sasaki Y, Takuwa Y. Ca2+-dependent activation of Rho and Rho kinase in membrane depolarization-induced and receptor stimulation-induced vascular smooth muscle contraction. Circ Res 2003; 93:548–556. [DOI] [PubMed] [Google Scholar]
- 20. Seasholtz TM, Zhang T, Morissette MR, Howes AL, Yang AH, Brown JH. Increased expression and activity of RhoA are associated with increased DNA synthesis and reduced p27(Kip1) expression in the vasculature of hypertensive rats. Circ Res 2001; 89:488–495. [DOI] [PubMed] [Google Scholar]
- 21. Bokoch GM, Bohl BP, Chuang TH. Guanine nucleotide exchange regulates membrane translocation of Rac/Rho GTP-binding proteins. J Biol Chem 1994; 269:31674–31679. [PubMed] [Google Scholar]
- 22. Boukharov AA, Cohen CM. Guanine nucleotide-dependent translocation of RhoA from cytosol to high affinity membrane binding sites in human erythrocytes. Biochem J 1998; 330 (Pt 3):1391–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 1996; 273:245–248. [DOI] [PubMed] [Google Scholar]
- 24. Cole WC, Welsh DG. Role of myosin light chain kinase and myosin light chain phosphatase in the resistance arterial myogenic response to intravascular pressure. Arch Biochem Biophys 2011; 510:160–173. [DOI] [PubMed] [Google Scholar]
- 25. Loirand G, Guérin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res 2006; 98:322–334. [DOI] [PubMed] [Google Scholar]
- 26. Karaki H, Ozaki H, Hori M, Mitsui-Saito M, Amano K, Harada K, Miyamoto S, Nakazawa H, Won KJ, Sato K. Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev 1997; 49:157–230. [PubMed] [Google Scholar]
- 27. Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol 2006; 174:815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hall A. Rho GTPases and the actin cytoskeleton. Science 1998; 279:509–514. [DOI] [PubMed] [Google Scholar]
- 29. Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol 1996; 133:1403–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.