Abstract
BACKGROUND
Menthol is a selective transient receptor potential melastatin 8 (TRPM8) channel agonist that induces cutaneous vasodilation in young, normotensive men and women through nitric oxide synthase (NOS)-, endothelium-derived hyperpolarizing factor (EDHF)-, and sensory nerve-mediated mechanisms. Microvascular dysfunction is present in essential hypertension and whether menthol induces vasodilation is men and women with essential hypertension is equivocal.
METHODS
Four intradermal microdialysis fibers were placed in the forearm of 9 essential hypertensive and 10 age-matched normotensive control subjects. Sites were pretreated with lactated Ringer’s (control), l-NAME (NOS inhibited), TEA (EDHF inhibited), and lidocaine (sensory nerve inhibited). The microdialysis fibers were then perfused with 7 increasing doses of menthol (0.1–500 mM). Red cell flux in response to menthol was measured with laser Doppler flowmetry. Data were normalized to mean arterial pressure and presented as a percentage of site-specific maximum vasodilation (%CVCmax).
RESULTS
At the control site, menthol caused vasodilation in both the normotensive and hypertensive groups (menthol doses 100, 250, and 500 mM; all P < 0.05 compared to baseline). There were no differences between groups (P = 0.58, main effect). There was no effect of either NOS or sensory nerve inhibition on menthol-induced vasodilation in the normotensive group; however, menthol-induced vasodilation was attenuated with NOS and sensory nerve inhibition in the hypertensive group. EDHF inhibition attenuated menthol-induced vasodilation in both groups.
CONCLUSIONS
Menthol-induced vasodilation has NO, EDHF, and sensory nerve components. Menthol-induced cutaneous vasodilation is preserved in hypertensive subjects. However, the hypertensive subjects exhibited a loss of redundant vasodilator systems.
Keywords: blood pressure, cutaneous, hypertension, menthol, microcirculation, TRPM8
Menthol, a common additive to foods, medicines, and topical analgesics, elicits a pleasant cool/mint sensation. We recently demonstrated that menthol induces cutaneous vasodilation through nitric oxide (NO), endothelium-derived hyperpolarizing factor (EDHF), and sensory nerve-dependent mechanisms.1,2 Menthol is a selective transient receptor potential melastatin 8 (TRPM8) channel agonist.3 TRPM8 channels are nonselective cation channels that open in response to both cold/cool temperatures (8–28 °C)4 and menthol,3 and are present in many cell types, including vascular smooth muscle and endothelial cells.5 The ability of menthol to induce vasodilation is in part dependent on TRPM8 channel activation. It is thought that opening of vascular TRPM8 channels enables entry of calcium into the endothelium6 which can increase endothelial NO production7 and hyperpolarize vascular smooth muscle cells by activating calcium activated potassium channels.8
Hypertension is a highly prevalent chronic disease that affects nearly one third of adult Americans9 and is a primary risk factor for the development of cardiovascular disease and stroke.10 Hypertension is characterized by increased vasoconstrictor tone11,12 and a loss of endothelium-dependent vasodilation.13,14 Multiple classes of medications are available to treat hypertension; however, antihypertensive pharmacotherapy does not effectively control blood pressure in all patients. Approximately 40% of medicated hypertensive men and women have uncontrolled blood pressure.15 Therefore, it is important to identify additional treatments to combat hypertension. Given menthol’s low cost, safety, and ability to induce vasodilation, menthol may be an appropriate nutraceutical addition to traditional antihypertensive pharmacotherapy.
In animal models, intravenous administration of menthol can lower blood pressure,16 while supplementation with orally administered menthol has been efficacious in reducing blood pressure in prehypertensive men and women.17 However, TRPM8 expression is downregulated in animal models of hypertension,18,19 and TRPM8 gene polymorphism may increase the risk for developing hypertension.20 Furthermore, the putative downstream mechanisms of menthol-mediated dilation (NO and EDHF) are downregulated in men and women with hypertension.21,22 Potential alterations in TRPM8 expression, along with attenuation of downstream mechanisms, with hypertension make the antihypertensive potential of menthol, at this point, unknown.
Our previous research on menthol utilized the skin as a clinically relevant tissue given the common use of topical menthol agents.1,2 Moreover, the cutaneous microvasculature is an easily accessible vascular bed that is representative of whole-body microvascular function23 and has been utilized to examine changes in microvasculature function in men and women with essential hypertension.24–26 Therefore, the aim of this study was to examine the magnitude and mechanisms of menthol-induced vasodilation in the cutaneous microvasculature of hypertensive men and women as a first step in elucidating the antihypertensive potential of menthol. We hypothesized that in healthy middle-aged adults, menthol would induce vasodilation through NO-, EDHF-, and sensory nerve-dependent mechanisms. We also hypothesized that menthol-induced vasodilation would be attenuated in hypertensive men and women through a loss of NO- and EDHF-dependent vasodilation.
METHODS
Subjects
The experimental protocol was approved by the institutional review board of The Pennsylvania State University and conformed to the guidelines laid out in the Declaration of Helsinki. Written and verbal informed consent was obtained from all subjects prior to participation in the study. Nine essential hypertensive subjects and 10 age-matched normotensive subjects participated in the study. Hypertensive status was determined in accordance with The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, with each participant presenting with a seated systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg on at least 2 separate occasions.27 Blood pressure status was confirmed through use of a 24-hour ambulatory blood pressure monitor (Ambulo 2400, Mortara Instruments, Milwaukee, WI) that measured blood pressure every 30 minutes during the waking hours and every hour during sleep. Due to the inclusion of sleeping hours, blood pressure from 24-hour monitors is less than what is observed during seated blood pressure measurement. Hypertensive status was confirmed from ambulatory monitor data if average 24-hour systolic blood pressure was ≥130 mm Hg and/or diastolic blood pressure was ≥80 mm Hg.28
All subjects were normally active, nonsmokers, free from chronic disease other than hypertension, and not taking any medications that could affect the cardiovascular system. All women were postmenopausal and not taking hormone replacement therapy. Prior to the study, all subjects participated in a health screening that included measurements of height, weight, waist circumference, blood pressure, a health history form, and blood chemistry and lipid analysis (Quest diagnostic, Pittsburgh, PA).
Instrumentation
All protocols took place in a thermoneutral laboratory with subjects in a semisupine position. Subjects arrived at the lab having abstained from caffeine, alcohol, and vigorous exercise for 12 hours. Four intradermal microdialysis fibers (55 kDa cutoff, CMA, Toshamnsgatan, Sweden) were place in the ventral side of the same forearm, as previously described.25 Each microdialysis fiber was randomly prescribed (i) lactated Ringer’s (control, Baxter Laboratories, Boronia, AU), (ii) 20 mM NG-Nitro-l-arginine methyl ester (l-NAME; nonspecific nitric oxide synthase (NOS) antagonist, Calbiochem, San Diego, CA), (iii) 50 mM tetraethylammonium (TEA; EDHF antagonist, Abcam, Cambridge, MA), or (iv) topical lidocaine (4% LMX4 cream; sensory nerve antagonist, Ferndale Laboratories, Ferndale, MI). l-NAME and TEA were mixed just before use in lactated Ringer’s, sterilized with syringe microfilters (Acrodisc, 0.2 µm membrane, Pall Corporation, Port Washington, NY), and wrapped in foil to prevent degradation due to light exposure. Lactated Ringer’s (control and lidocaine sites), l-NAME, and TEA were perfused through the microdialysis fibers at a rate of 2 µl min−1 (Bee Hive controller and Baby Bee syringe drive, BASi, West Lafayette, IN) for at least 60 minutes. Topical lidocaine was applied over this same time period. Sensory nerve blockade with lidocaine was confirmed through lack of sensation to a needle prick.29
After microdialysis fiber insertion, 60–90 minutes were given for hyperemia due to needle insertion trauma to fully subside. After resolution of insertion hyperemia, local heating units (Moor Instruments, Axminster, UK) were placed over each microdialysis fiber and set to 33 °C to maintain a constant skin temperature. A laser Doppler flowmeter probe (Moor Instruments) was placed within each heater to measure red cell flux, a relative measure of skin blood flow. Brachial artery blood pressure was measured via brachial auscultation on the contralateral arm every 5 minutes throughout the study (Cardiocap5, General Electric, Fairfield, CT).
Experimental protocol
After resolution of insertion trauma, baseline skin blood flow was measured for a minimum of 15 minutes. After baseline, 7 increasing doses of menthol (0.1, 1, 10, 50, 100, 250, and 500 mM; Sigma-Aldrich, St. Louis, MO), mixed with either lactated Ringer’s (control and sensory nerve-inhibited sites) or the appropriate pharmacological inhibitor, were perfused through the microdialysis fibers. Menthol of 500 mM was chosen as the top dose as it was the highest concentration of menthol that could be dissolved in lactated Ringer’s. The other menthol doses were determined through pilot experiments in young subjects. Just prior to use, menthol crystals were submerged in the appropriate inhibitor-Ringer’s solution, heated, and stirred until menthol was fully dissolved to create a 500 mM menthol solution. This solution was then diluted to the appropriate doses. Pilot data demonstrated that 4 minutes was an adequate amount of time to produce a plateau in menthol-induced vasodilation; therefore, menthol doses were perfused in 4-minute increments.
Once the menthol dose–response protocol was completed, 28 mM sodium nitroprusside (SNP: NO donor, USP, Rockville, MD) was perfused through each fiber at a rate of 4 µl min−1 and the local heating units were raised to 43 °C at a rate of 0.5 °C • 5 s−1 to obtain maximum skin blood flow.24,25
Data acquisition and analysis
Red cell flux was acquired at 40 Hz with WinDaq data acquisition software (DataQ Instruments, Akron, OH) and stored offline for later analysis. Red cell flux was divided by mean arterial pressure to obtain cutaneous vascular conductance (CVC). There was no difference in maximum CVC between groups (normotensive: 1.27 ± 0.1, hypertensive: 1.29 ± 0.1 flux mm Hg−1; P = 0.83), so data were normalized to a percentage of maximum CVC obtained during SNP perfusion/local heating (%CVCmax). Data were obtained for all microdialysis sites during baseline, menthol dose-response, and during perfusion of SNP/43 °C local heat.
Unpaired t-tests were used to determine differences in subject characteristics between groups. A 3-way repeated-measured mix-model analysis of variance was used to determine group, menthol dose, and microdialysis site differences (SAS 9.3). Specific planned comparisons were made with Bonferroni corrections where appropriate. The level of significance was set a priori at α = 0.05. All values are presented as mean ± SE.
RESULTS
Subject characteristics are presented in Table 1. The hypertensive group had significantly higher seated measures of systolic blood pressure, diastolic blood pressure, and mean arterial pressure relative to the normotensive group. There were also significant differences between groups in total cholesterol (P = 0.03). However, total cholesterol in the hypertensive group was still below clinical levels.30 Ambulatory blood pressure data are presented in Table 2. One hypertensive and 3 normotensive subjects did not complete the nighttime portion of the ambulatory monitor due to difficulty sleeping. Daytime (800–2200 hours), nighttime (2200–800 hours), and 24-hour pressures were all significantly higher in the hypertensive group relative to the normotensive group.
Table 1.
Subject characteristics
| Normotensive | Hypertensive | |
|---|---|---|
| Sex (M, F) | 3, 7 | 5, 4 |
| Age (year) | 50 ± 1 | 53 ± 2 |
| Height (m) | 1.70 ± 0.02 | 1.69 ± 0.04 |
| Weight (kg) | 77.1 ± 3.7 | 73.5 ± 4.7 |
| BMI (kg m−2) | 26.8 ± 1.3 | 25.6 ± 1.2 |
| Total cholesterol (mg dl−1) | 158 ± 18 | 205 ± 6* |
| LDL cholesterol (mg dl−1) | 105 ± 8 | 117 ± 7 |
| HDL cholesterol (mg dl−1) | 54 ± 6 | 64 ± 6 |
| HbA1C | 5.5 ± 0.1 | 5.3 ± 0.1 |
| SBP (mm Hg) | 117 ± 2 | 143 ± 2* |
| DBP (mm Hg) | 74 ± 2 | 94 ± 1* |
| MAP (mm Hg) | 88 ± 2 | 110 ± 1* |
Group mean ± SE. *P < 0.05 compared to the normotensive group. Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; F, female; HDL, high-density lipoprotein; LDL, low-density lipoprotein; M, male; MAP, mean arterial pressure; SBP, systolic blood pressure.
Table 2.
Ambulatory blood pressure data
| Normotensive | Hypertensive | |
|---|---|---|
| 24-hour | ||
| SBP (mm Hg) | 112 ± 3 | 138 ± 2* |
| DBP (mm Hg) | 71 ± 1 | 88 ± 1* |
| MAP (mm Hg) | 85 ± 2 | 104 ± 1* |
| Daytime | ||
| SBP (mm Hg) | 114 ± 3 | 139 ± 2* |
| DBP (mm Hg) | 72 ± 1 | 89 ± 1* |
| MAP (mm Hg) | 86 ± 2 | 105 ± 1* |
| Nighttime | ||
| SBP (mm Hg) | 96 ± 2 | 118 ± 6* |
| DBP (mm Hg) | 63 ± 2 | 75 ± 4* |
| MAP (mm Hg) | 74 ± 2 | 87 ± 5* |
Group mean ± SE. *P < 0.05 compared to the normotensive group. Abbreviations: DBP, diastolic blood pressure; MAP, mean arterial pressure; SBP, systolic blood pressure.
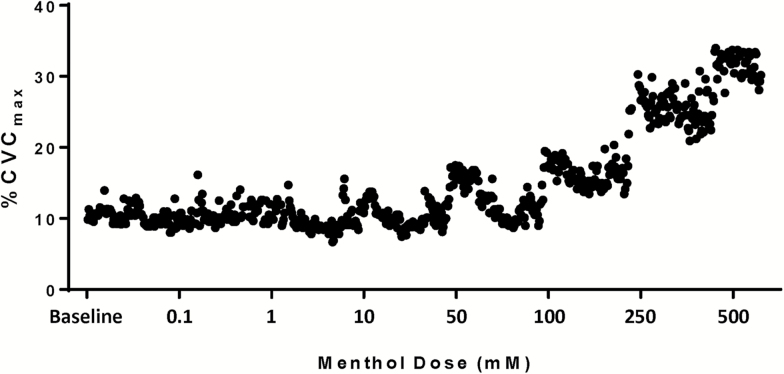
Figure 1 is an original record from the control site of a normotensive subject that illustrates the dose–response relation of menthol-induced vasodilation.
Figure 1.
Original record from the control site of a normotensive subject. Menthol elicits dose-dependent vasodilation. Abbreviation: CVC, cutaneous vascular conductance.
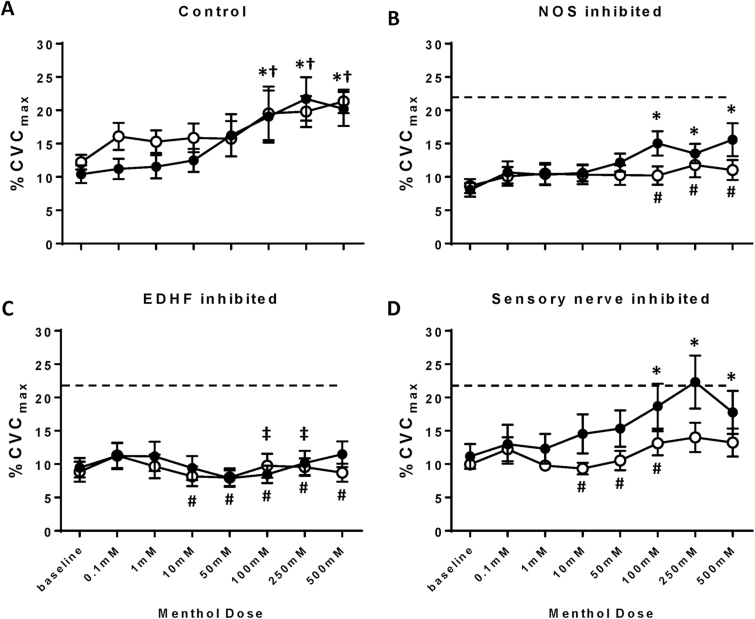
Menthol dose–response data at the control site are presented in Figure 2a. Relative to baseline, menthol induced significant vasodilation in the normotensive group at all menthol doses ≥100 mM (all P < 0.0.05). Similarly, menthol-induced significant vasodilation in the essential hypertensive group at all menthol doses ≥100 mM (all P < 0.05). There were no differences in menthol-induced vasodilation between groups at any menthol dose (all P > 0.05).
Figure 2.
Cutaneous vasodilation expressed as %CVCmax in response to increasing doses of menthol in normotensive (black symbols) and essential hypertensive (white symbols) subjects at (a) control, (b) NOS-inhibited, (c) EDHF-inhibited, and (d) sensory nerve-inhibited sites. The dashed line represents peak dilation in the control site of the normotensive subjects. *P < 0.05 between menthol dose and site-specific baseline in the normotensive group. †P < 0.05 between menthol dose and site-specific baseline in the essential hypertensive group. ‡P < 0.05 between menthol dose at pharmacologically inhibited site and corresponding dose in the control site for normotensive subjects. #P < 0.05 between menthol dose at pharmacologically inhibited site and corresponding dose in the control site for essential hypertensive subject. Abbreviations: CVC, cutaneous vascular conductance; EDHF, endothelium-derived hyperpolarizing factor; NOS, nitric oxide synthase.
Data for the NOS-inhibited sites are depicted in Figure 2b. In the normotensive group, with NOS inhibition, menthol induced cutaneous vasodilation at all menthol doses ≥100 mM (all P < 0.05). Within the normotensive group, there were no differences between the control and l-NAME sites at any menthol dose (all P > 0.05). In the essential hypertensive group, menthol did not induce vasodilation compared to baseline (all P > 0.04) and vasodilation was inhibited compared to the control site at all doses ≥100 mM (all P < 0.05). There were no between-group differences in menthol-induced vasodilation at any dose in the NOS-inhibited site (all P > 0.05).
Data for the EDHF-inhibited sites are shown in Figure 2c. With EDHFs inhibited, menthol did not induced vasodilation in either the normotensive or essential hypertensive groups (all P > 0.05). There were no differences in cutaneous vasodilation between the normotensive and essential hypertensive groups with any menthol dose (all P > 0.05). Within the normotensive group, vasodilation was significantly attenuated in the EDHF-inhibited site relative to the control site at 100 mM (P = 0.01) and 250 mM (P = 0.006) menthol. Within the essential hypertensive group, vasodilation was attenuated in the EDHF-inhibited site relative to the control site at all menthol doses ≥10 mM (all P < 0.05).
Data for the sensory nerve blockade site are depicted in Figure 2d. In the lidocaine site, menthol induced significant cutaneous vasodilation in the normotensive group with all menthol doses ≥100 mM (all P < 0.05). Within the normotensive group, there were no differences in vasodilation between the lidocaine and control sites with any menthol dose (all P > 0.05). In the essential hypertensive group, menthol did not elicit cutaneous vasodilation at any dose (all P > 0.05). Within the essential hypertensive group, vasodilation was attenuated at the lidocaine site compared to the control site at all menthol doses 10–100 mM (all P < 0.05); the attenuation approached significance at 250 and 500 mM (P = 0.06 for both). There were no differences in menthol-induced cutaneous vasodilation between the normotensive and essential hypertensive groups at any dose of menthol (all P > 0.05).
DISCUSSION
The main findings from this study were that (i) menthol induced cutaneous vasodilation to a similar degree in normotensive and essential hypertensive men and women, (ii) in normotensive subjects, only EDHF inhibition attenuated menthol-induced vasodilation, and (iii) inhibition of NO, EDHFs, and sensory nerves abolished vasodilation in the essential hypertensive subjects. These results are consistent with our previous findings, demonstrating that menthol-induced cutaneous vasodilation is EDHF-dependent.1,2 Further, these data corroborate that there are differences in vasodilator mechanisms between normotensive and hypertensive men and women.31,32
The main goal of this study was to characterize menthol-induced vasoreactivity in a group of essential hypertensive patients as a first step to evaluate the potential for menthol to act as a nutraceutical additive therapy in the treatment of hypertension. Despite evidence of altered TRPM8 expression with essential hypertension,18,20,33 we found that menthol-induced cutaneous vasodilation was preserved with no difference in the absolute magnitude of vasodilation between normotensive and essential hypertensive subjects. These data, coupled with prior data in prehypertensive subjects,17 support further investigation of menthol as a novel vasodilator in hypertensive men and women.
Similar to our previous findings, EDHFs significantly contribute to menthol-induced vasodilation. Vasodilation was abolished in both the normotensive and essential hypertensive groups when EDHFs were inhibited. In contrast, there were differential contributions of both NO and sensory nerves between groups. When NO and sensory nerves were inhibited, vasodilation was apparent in the normotensive group, whereas the response was abolished in the essential hypertensive group. These data suggest that menthol mediates vasodilation through multiple mechanisms. It is likely that healthy, middle-aged men and women can upregulate redundant vasodilator pathways when either NO or sensory nerves are inhibited to maintain vasodilation. However, there is likely attenuation of multiple redundant vasodilator pathways in hypertensive patients. Others have observed that NO-24–26 and EDHF-mediated22,31 vasodilation is attenuated with essential hypertension, supporting this theory. In the cutaneous microvasculature, sensory nerves are known to contribute to reactive hyperemia,34 reflex vasodilation,29 and menthol-induced vasodilation.1,2 Alterations in sensory nerve contributions to cutaneous vasodilatory responses in men and women with essential hypertension have not been previously evaluated. Collectively the data in the current study suggest alterations in menthol-induced cutaneous vasodilation with sensory nerve inhibition in men and women with essential hypertension.
That neither NO nor sensory nerve inhibition attenuated menthol-induced vasodilation in middle-aged subjects is counter to our previous data in young participants where inhibition of NO, EDHF, or sensory nerves abolished menthol-induced vasodilation.2 We have previously observed mechanistic differences in the control of skin blood flow between young and middle-aged subjects, despite no apparent microvascular dysfunction in the middle-aged subjects.35 It is likely that the different response to menthol between young and middle-aged subjects is due to aging-associated alterations in microvascular function. More research is required to determine if menthol induces vasodilation through other mechanisms, such as cyclooxygenase, in middle-aged subjects.
While these data suggest that menthol might be efficacious in men and women with essential hypertension, research utilizing chronic supplementation with orally administered menthol, both alone and co-prescribed with traditional antihypertensive pharmacotherapy, is required before the antihypertensive potential of menthol can be determined. Previous work in prehypertensive men and women demonstrated that menthol can be orally administered without serious side effects. Sun et al. provided participants with 144 mg of menthol per day for 8 weeks; this dose decreased systolic blood pressure by ~6 mm Hg and was free of side effects.18 Menthol administered other ways (i.e., peppermint oil) has been safely used in short-term interventions with minimal side effects (heartburn).36 However, data from long-term interventions are required to fully elucidate the safety of systemic menthol supplementation.
The postulated mechanism for menthol-induced vasodilation involves a TRPM8 channel-mediated increase in calcium flux into the vascular endothelium, resulting in upregulation of NO/EDHF. However, TRPM8 is also localized on the plasma membrane of vascular smooth muscle cells5 and it is expected that increased intracellular calcium concentrations in smooth muscle cells would induce vasoconstriction, introducing an apparent paradox in our mechanistic explanation. In endothelium-denuded vessels menthol has been shown to induce vasoconstriction; however, in pre-constricted vessels menthol can induce vasodilation,5 indicating that the actions of menthol are dependent on current vascular tone. At thermoneutral temperatures, the cutaneous microvasculature is in a relatively constricted state (i.e., has a robust capacity to vasodilate),37 suggesting that menthol is likely to induce vasodilation in the cutaneous microvasculature. Investigation of systemic menthol administration will determine if the vasodilatory effect of menthol is apparent in other tissues, or if menthol possess vasoconstrictor properties in vivo.
Limitations
While menthol is largely specific for TRPM8 channels, there is crosstalk with TRP vanilloid 3 (TRPV3) channels,38 which are capable of inducing vasodilation.39 Therefore, it is unknown whether these findings are influenced by TRPV3 channel activation. However, the main objective of this study was to determine the efficacy of menthol to induce vasodilation in essential hypertensive subjects, which was successfully demonstrated. Therefore, this limitation does not diminish the significance of these findings.
Inhibition of either NO or sensory nerves inhibited vasodilation in the hypertensive, but not normotensive, subjects. Our hypothesis is that normotensive subjects have redundant vasodilatory systems capable of inducing dilation and that these redundant systems are attenuated with hypertension. However, it is also possible that neither NO nor sensory nerves play a role in menthol-induced vasodilation in middle-aged normotensive subjects.
Menthol is highly soluble in alcohol-based solutions; however, the solubility in aqueous solutions is limited. The top dose of menthol utilized in this study (500 mM) represents the limit of menthol that can be dissolved in lactated Ringer’s solution. The inability to administer greater doses of menthol limited our ability to measure maximum menthol-mediated vasodilation. Greater dilation can be achieved with topical menthol1; it is unknown if this would differ in men and women with essential hypertension. The use of a partial ethanol solution could have increased the maximum dose of deliverable menthol; however, ethanol deactivates TRPM8 channels,40 so this would have influenced the results. Furthermore, we may have been underpowered to observe between-group differences due to the limited magnitude of menthol-induced vasodilation; this would explain why between-group differences were not observed. Despite these limitations, a significant degree of menthol-induced vasodilation was detected within a limited vasodilatory window.
While the hypertensive subjects were not hypercholesterolemic, there were differences in cholesterol between groups. Therefore, we cannot rule out that cholesterol contributed to the observed differences in menthol-mediated dilation. Finally, the essential hypertensive subjects in this study represent a unique group that present with elevated blood pressure but no other accompanying cardiovascular disease risk factors. While this is advantageous for isolating the role of blood pressure, the results are less translatable to the general public. It is possible that in a more real-world subject population with multiple other cardiovascular disease risk factors or chronic diseases accompanying hypertension, menthol-induced vasodilation may be attenuated to a greater degree due to the presence of more severe endothelial dysfunction.
In conclusion, menthol induced an equal magnitude of cutaneous vasodilation in normotensive and essential hypertensive men and women. It was also confirmed that menthol-induced cutaneous vasodilation involved NO, EDHF, and sensory nerve pathways. These data support further research into the use of menthol as a nutraceutical addition to traditional antihypertensive pharmacotherapy due to menthol’s ability to act as a vasodilator in men and women with essential hypertension.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENT
This research was funded by NIH R01 HL093238.
REFERENCES
- 1. Craighead DH, Alexander LM. Topical menthol increases cutaneous blood flow. Microvasc Res 2016; 107:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Craighead DH, McCartney NB, Tumlinson JH, Alexander LM. Mechanisms and time course of menthol-induced cutaneous vasodilation. Microvasc Res 2017; 110:43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell 2002; 108:705–715. [DOI] [PubMed] [Google Scholar]
- 4. McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002; 416:52–58. [DOI] [PubMed] [Google Scholar]
- 5. Johnson CD, Melanaphy D, Purse A, Stokesberry SA, Dickson P, Zholos AV. Transient receptor potential melastatin 8 channel involvement in the regulation of vascular tone. Am J Physiol Heart Circ Physiol 2009; 296:H1868–H1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zholos A, Johnson C, Burdyga T, Melanaphy D. TRPM channels in the vasculature. Adv Exp Med Biol 2011; 704:707–729. [DOI] [PubMed] [Google Scholar]
- 7. Cohen RA, Vanhoutte PM. Endothelium-dependent hyperpolarization. Beyond nitric oxide and cyclic GMP. Circulation 1995; 92:3337–3349. [DOI] [PubMed] [Google Scholar]
- 8. Félétou M, Vanhoutte PM. EDHF: an update. Clin Sci 2009; 117:139–155. [DOI] [PubMed] [Google Scholar]
- 9. Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011–2012. NCHS data brief 2013; 1–8. [PubMed] [Google Scholar]
- 10. Stokes J 3rd, Kannel WB, Wolf PA, D’Agostino RB, Cupples LA. Blood pressure as a risk factor for cardiovascular disease. The Framingham Study–30 years of follow-up. Hypertension 1989; 13:I13–I18. [DOI] [PubMed] [Google Scholar]
- 11. Yannoutsos A, Levy BI, Safar ME, Slama G, Blacher J. Pathophysiology of hypertension: interactions between macro and microvascular alterations through endothelial dysfunction. J Hypertens 2014; 32:216–224. [DOI] [PubMed] [Google Scholar]
- 12. Feihl F, Liaudet L, Waeber B, Levy BI. Hypertension: a disease of the microcirculation? Hypertension 2006; 48:1012–1017. [DOI] [PubMed] [Google Scholar]
- 13. Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 2000; 87:840–844. [DOI] [PubMed] [Google Scholar]
- 14. Iiyama K, Nagano M, Yo Y, Nagano N, Kamide K, Higaki J, Mikami H, Ogihara T. Impaired endothelial function with essential hypertension assessed by ultrasonography. Am Heart J 1996; 132:779–782. [DOI] [PubMed] [Google Scholar]
- 15. Gu Q, Burt VL, Dillon CF, Yoon S. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: the National Health and Nutrition Examination Survey, 2001 to 2010. Circulation 2012; 126:2105–2114. [DOI] [PubMed] [Google Scholar]
- 16. Rakieten N, Rakieten ML. The effect of 1-menthol on the systemic blood pressure. J Am Pharm Assoc Am Pharm Assoc 1957; 46:82–84. [DOI] [PubMed] [Google Scholar]
- 17. Sun J, Yang T, Wang P, Ma S, Zhu Z, Pu Y, Li L, Zhao Y, Xiong S, Liu D, Zhu Z. Activation of cold-sensing transient receptor potential melastatin subtype 8 antagonizes vasoconstriction and hypertension through attenuating RhoA/Rho kinase pathway. Hypertension 2014; 63:1354–1363. [DOI] [PubMed] [Google Scholar]
- 18. Liu XR, Liu Q, Chen GY, Hu Y, Sham JS, Lin MJ. Down-regulation of TRPM8 in pulmonary arteries of pulmonary hypertensive rats. Cell Physiol Biochem 2013; 31:892–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang F, Ni M, Zhang JM, Li DJ, Shen FM. TRPM8 downregulation by angiotensin II in vascular smooth muscle cells is involved in hypertension. Mol Med Rep 2017; 15:1900–1908. [DOI] [PubMed] [Google Scholar]
- 20. He J, Kelly TN, Zhao Q, Li H, Huang J, Wang L, Jaquish CE, Sung YJ, Shimmin LC, Lu F, Mu J, Hu D, Ji X, Shen C, Guo D, Ma J, Wang R, Shen J, Li S, Chen J, Mei H, Chen CS, Chen S, Chen J, Li J, Cao J, Lu X, Wu X, Rice TK, Gu CC, Schwander K, Hamm LL, Liu D, Rao DC, Hixson JE, Gu D. Genome-wide association study identifies 8 novel loci associated with blood pressure responses to interventions in Han Chinese. Circ Cardiovasc Genet 2013; 6:598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vanhoutte PM, Feletou M, Taddei S. Endothelium-dependent contractions in hypertension. Br J Pharmacol 2005; 144:449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Michel FS, Man GS, Man RY, Vanhoutte PM. Hypertension and the absence of EDHF-mediated responses favour endothelium-dependent contractions in renal arteries of the rat. Br J Pharmacol 2008; 155:217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol (1985) 2008; 105:370–372. [DOI] [PubMed] [Google Scholar]
- 24. Smith CJ, Santhanam L, Bruning RS, Stanhewicz A, Berkowitz DE, Holowatz LA. Upregulation of inducible nitric oxide synthase contributes to attenuated cutaneous vasodilation in essential hypertensive humans. Hypertension 2011; 58:935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holowatz LA, Kenney WL. Local ascorbate administration augments NO- and non-NO-dependent reflex cutaneous vasodilation in hypertensive humans. Am J Physiol Heart Circ Physiol 2007; 293:H1090–H1096. [DOI] [PubMed] [Google Scholar]
- 26. Holowatz LA, Kenney WL. Up-regulation of arginase activity contributes to attenuated reflex cutaneous vasodilatation in hypertensive humans. J Physiol 2007; 581:863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 28. Mancia GF, Fagard R, Narkiewicz K, Redán J, Zanchetti A, Bohm M, Christiaes T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens 2013; 31:1925–1938. [DOI] [PubMed] [Google Scholar]
- 29. Wong BJ. Sensory nerves and nitric oxide contribute to reflex cutaneous vasodilation in humans. Am J Physiol Regul Integr Comp Physiol 2013; 304:R651–R656. [DOI] [PubMed] [Google Scholar]
- 30. HHS. High Blood Cholesterol: What You Need To Know. NIH Publication No. 05-3290. Bethesda, MD: National Institutes of Health, National Heart, Lung, and Blood Institute, 2005. [Google Scholar]
- 31. Li J, Zhou Z, Jiang DJ, Li D, Tan B, Liu H, Li YJ. Reduction of NO- and EDHF-mediated vasodilatation in hypertension: role of asymmetric dimethylarginine. Clin Exp Hypertens 2007; 29:489–501. [DOI] [PubMed] [Google Scholar]
- 32. Giles TD, Sander GE, Nossaman BD, Kadowitz PJ. Impaired vasodilation in the pathogenesis of hypertension: focus on nitric oxide, endothelial-derived hyperpolarizing factors, and prostaglandins. J Clin Hypertens 2012; 14:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tabur S, Oztuzcu S, Duzen IV, Eraydin A, Eroglu S, Ozkaya M, Demiryürek AT. Role of the transient receptor potential (TRP) channel gene expressions and TRP melastatin (TRPM) channel gene polymorphisms in obesity-related metabolic syndrome. Eur Rev Med Pharmacol Sci 2015; 19:1388–1397. [PubMed] [Google Scholar]
- 34. Lorenzo S, Minson CT. Human cutaneous reactive hyperaemia: role of BKCa channels and sensory nerves. J Physiol 2007; 585:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bruning RS, Santhanam L, Stanhewicz AE, Smith CJ, Berkowitz DE, Kenney WL, Holowatz LA. Endothelial nitric oxide synthase mediates cutaneous vasodilation during local heating and is attenuated in middle-aged human skin. J Appl Physiol (1985) 2012; 112:2019–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Meamarbashi A, Rajabi A. The effects of peppermint on exercise performance. J Int Soc Sports Nutr 2013; 10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev 1974; 54:75–159. [DOI] [PubMed] [Google Scholar]
- 38. Macpherson LJ, Hwang SW, Miyamoto T, Dubin AE, Patapoutian A, Story GM. More than cool: promiscuous relationships of menthol and other sensory compounds. Mol Cell Neurosci 2006; 32:335–343. [DOI] [PubMed] [Google Scholar]
- 39. Pires PW, Sullivan MN, Pritchard HA, Robinson JJ, Earley S. Unitary TRPV3 channel Ca2+ influx events elicit endothelium-dependent dilation of cerebral parenchymal arterioles. Am J Physiol Heart Circ Physiol 2015; 309:H2031–H2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Benedikt J, Teisinger J, Vyklicky L, Vlachova V. Ethanol inhibits cold-menthol receptor TRPM8 by modulating its interaction with membrane phosphatidylinositol 4,5-bisphosphate. J Neurochem 2007; 100:211–224. [DOI] [PubMed] [Google Scholar]