Abstract
BACKGROUND
Common blood pressure (BP) trajectories are not well established in elderly persons, and their association with clinical outcomes is uncertain.
METHODS
We used hierarchical cluster analysis to identify discrete BP trajectories among 4,067 participants in the Cardiovascular Health Study using repeated BP measures from years 0 to 7. We then evaluated associations of each BP trajectory cluster with all-cause mortality, incident cardiovascular disease (CVD, defined as stroke or myocardial infarction) (N = 2,837), and incident congestive heart failure (HF) (N = 3,633) using Cox proportional hazard models.
RESULTS
Median age was 77 years at year 7. Over a median 9.3 years of follow-up, there were 2,475 deaths, 659 CVD events, and 1,049 HF events. The cluster analysis identified 3 distinct trajectory groups. Participants in cluster 1 (N = 1,838) had increases in both systolic (SBP) and diastolic (DBP) BPs, whereas persons in cluster 2 (N = 1,109) had little change in SBP but declines in DBP. Persons in cluster 3 (N = 1,120) experienced declines in both SBP and DBP. After multivariable adjustment, clusters 2 and 3 were associated with increased mortality risk relative to cluster 1 (hazard ratio = 1.21, 95% confidence interval: 1.06–1.37 and hazard ratio = 1.20, 95% confidence interval: 1.05–1.36, respectively). Compared to cluster 1, cluster 3 had higher rates of incident CVD but associations were not statistically significant in demographic-adjusted models (hazard ratio = 1.16, 95% confidence interval: 0.96–1.39). Findings were similar when stratified by use of antihypertensive therapy.
CONCLUSIONS
Among community-dwelling elders, distinct BP trajectories were identified by integrating both SBP and DBP. These clusters were found to have differential associations with outcomes.
Keywords: blood pressure, blood pressure trajectory, cardiovascular disease, elderly, hypertension, mortality.
The clinical significance of high blood pressure (BP) in elderly persons (age >75 years) remains an area of active investigation. Studies show that high BP is a risk factor for cardiovascular events and death among elders,1,2 and BP lowering to <120 mm Hg appears beneficial to lower cardiovascular disease (CVD) events in selected individuals >75 years of age.3–5, Conversely, other studies have shown that a higher BP may be associated with lower rates of mortality among octogenarians6 and elderly persons with poor functional status.5,7
Much less is known about the importance of BP change over time (trajectories) in elderly persons. Some studies have suggested that a decline in BP is common8–10 and is associated with higher rates of cardiovascular events, death, and decreased psychomotor speed.11–15 However, these studies were limited by self-reporting of events,15 lack of adjudicated outcomes,11,12,14,15 limited BP recordings,11,13,14 or inconsistent data on antihypertensive treatment.11,13–15
Hypertension is a heterogeneous condition in elderly persons, and data support the importance of both systolic (SBP) and diastolic (DBP) components.5 We designed our analysis using an unsupervised cluster approach to group elderly, community-dwelling participants on the basis of multiple BP measurements over time into distinct BP clusters based upon SBP and DBP level and slope. We then evaluated associations of these clusters with death, CVD (defined as fatal and nonfatal myocardial infarctions or cerebrovascular accidents), and congestive heart failure (HF) using a standard multivariable regression approach. An improved knowledge of BP trajectories over several years is critical in understanding the role of BP as a risk factor for adverse outcomes.
METHODS
Participants
For this study, we included all 4,067 participants enrolled in the Cardiovascular Health Study (CHS) who were alive at the year 7 visit and had at least 4 recorded BP measurements between year 0 and 7. Seated BP was measured annually from years 0 to 5 and at year 7 in the CHS. The design and rationale of the CHS has been previously published.16 Briefly, the CHS recruited 5,888 community-dwelling Black and White individuals aged ≥65 years in 2 waves from 1989 to 1993 from Medicare eligibility lists in 4 communities (Forsyth County, NC; Sacramento County, CA; Washington County, MD; and Pittsburgh, PA). CHS participants were seen in the clinic annually and contacted by phone at 6-month intervals. Major exam components were measured at each annual visit. For these analyses, we excluded 1,821 individuals. Of those excluded, 1,394 were no longer alive at the year 7 clinic visit, 346 had <4 BP measurements, and 81 were either missing, lost to follow-up, or dropped out of the study. The study was approved by the institutional review boards of University of Washington and the affiliated clinical centers.
Determination of BP trajectory
We used BP measurements that were collected during each annual CHS visit from years 0 to 5 and at year 7 to model trajectories for this study. Trained personnel obtained 3 seated BP readings using standardized procedures, and the average of the last 2 readings was recorded.
Longitudinal changes in SBP and DBP were estimated using subject-specific slopes from linear mixed models. We then identified patterns of changes in BP using median hierarchical cluster analysis to partition subjects into distinct groups. We decided a priori that the cluster construction would be informed by the SBP and DBP slopes and by levels of SBP and DBP at years 0 and 7, as these have been previously reported to be associated with clinical outcomes.5 We used the SAS ACECLUS procedure to transform the data and compute canonical variables for cluster analysis. We then used the CLUSTER and TREE procedures using Gower’s median method to determine the number of clusters and to assign each participant to a unique, distinct cluster. We determined the number of clusters by choosing the value that maximized the cubic clustering criterion and performing pseudo F statistic.
Outcomes
The primary outcome in the study was all-cause mortality. The outcome was ascertained with follow up beginning after the year 7 visit in the CHS. Deaths were identified by a review of obituaries, medical records, death certificates, the Centers for Medicare and Medicaid Services health care-utilization database for hospitalizations, and from household contacts. All outcomes (death, CVD events, HF events) were reviewed and adjudicated by the CHS outcome-assessment committee.16
Secondary outcomes analyzed separately were incident CVD and incident congestive HF. We evaluated these outcomes separately as each has different pathophysiology and mechanisms of disease. Incident CVD was defined as fatal and nonfatal cerobrascular accidents and myocardial infarctions. As with death, we considered outcomes after year 7. For analysis of CVD (N = 1,230), we excluded individuals with a prior history of CVD; for HF, we excluded individuals with a prior diagnosis of HF (N = 434). Cases of CVD events were ascertained from hospital records and included clinical histories, cardiac enzyme levels, electrocardiographic changes, and brain imaging studies.16 Incident HF was defined as having at least one of the following: (i) cardiomegaly and pulmonary edema on chest X-ray, (ii) dilated ventricle and wall motion abnormalities by echocardiography or contrast ventriculography, (iii) congestive failure as defined by a physician, plus receiving medical treatment (diuretic plus either digitalis, vasodilator, or angiotensin-converting enzyme inhibitor).
Covariates
Covariates were ascertained during the year 7 visit of the CHS, except as noted below. These included age, gender, race, body mass index, smoking status (current, former, or never), SBP and DBP, estimated glomerular filtration rate, statin therapy, antihypertensive therapy, and functional status. Estimated glomerular filtration rate was estimated from cystatin C using the CKD-EPI equation. For total cholesterol and high-density lipoprotein, data were obtained at the year 5 visit as these variables were not measured at year 7. Chronic medical diseases such as history of coronary heart disease, cerebrovascular accident, HF, and diabetes were included. Use of statin or antihypertensive medication was ascertained by a medication inventory interview and was defined as having any documented therapy between years 0 and 7.
The following were ascertained by questionnaire: age, sex, race, and smoking history. Height and weight were measured with participants wearing light clothing and no shoes. Body mass index was calculated as weight in kilograms divided by height in meters squared. Fasting blood was collected and stored at −70 °F until needed for appropriate assays, including high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, total cholesterol, and glucose. Cystatin C was measured by means of a particle-enhanced immunonephelometric assay (N Latex Cystatin C, Dade Behring) with a nephelometer (Behring Nephelometer II Analyzer, Dade Behring) and calibrated to international standard. Diabetes mellitus was determined by self-report, the use of insulin or oral hypoglycemic agents, or a fasting glucose of ≥126 mg/dl.
Functional status measures included self-report of limitation in activities of daily living, grip strength, and time to walk 15-feet. Limitation in activities of daily living was ascertained by asking whether the participant had difficulty with eating, transferring from bed to chair, mobility inside the home, dressing, bathing, and using the toilet. We categorized participants into those without limitation and those with limitation of ≥1 activity of daily living. Grip strength was measured by a hand-held Jama A dynamometer and force was measured in kilograms of 3 maximal attempts with the subject’s dominant and nondominant hands. The variable chosen for analysis was the best of 3 attempts in the dominant hand. Walking speed was determined as the time (to 0.1 seconds) required for a participant to walk a 15-foot course at his or her usual pace.
Statistical analysis
First, we compared year 7 clinical and demographic characteristics across BP trajectory clusters using chi-square and Kruskal–Wallis tests for categorical and continuous variables. We then plotted the cumulative incidence of mortality, CVD, and HF over time, with separate lines for each BP trajectory cluster. We computed incidence rates per 1,000 person-years, and then we used Cox proportional hazards regression to model associations of each BP trajectory cluster with risk of death, CVD, and HF. To account for potential bias due to the competing risk of death before onset of CVD and HF, we used Fine-Gray competing-risks analysis17 to estimate risk associations for HF and CVD.
We estimated the associations of cluster membership with each clinical outcome using sequential models. Model 1 adjusted for demographic characteristics (age, race, sex) only. Model 2 adjusted for demographics and year 7 SBP. Model 3 included demographics, year 7 SBP, cardiovascular risk factors (smoking, body mass index, total cholesterol, high-density lipoprotein, estimated glomerular filtration rate by cystatin C, statin use, antihypertensive therapy, year 7 DBP), history of chronic medical conditions (CVD, cerebrovascular event, HF, diabetes), and functional status measures (grip strength, walking speed, activity of daily living measurements). Because age and year 7 SBP have previously been shown to be important predictors of CVD and HF in the CHS,5 we performed sequential modeling to further understand the role of these variables on the relationship between BP trajectories and clinical outcomes.
Sensitivity analysis
In a sensitivity analysis, we used an empiric approach to define categories of SBP and DBP change. We first examined the distributions of the SBP and DBP slopes separately using kernel density estimates to construct smoothed density curves. We then used the distributions of change to define the following categories for SBP and DBP separately: BP decline (lower 20th percentile of change over 7 years), increase (upper 20th percentile), and stable (middle three quintiles) (Supplementary Figure 1). In a separate analysis, we used the top and bottom fifth percentile of the distribution to define categories. We then performed multivariate analysis to explore separately the association of categories of changes in SBP and DBP with mortality.
RESULTS
Participant characteristics
Among 4,067 elderly participants who completed at the 7 year visit of the CHS, the median age was 77 years (interquartile range: 77, 82), 38% were male, and 17% were Black.
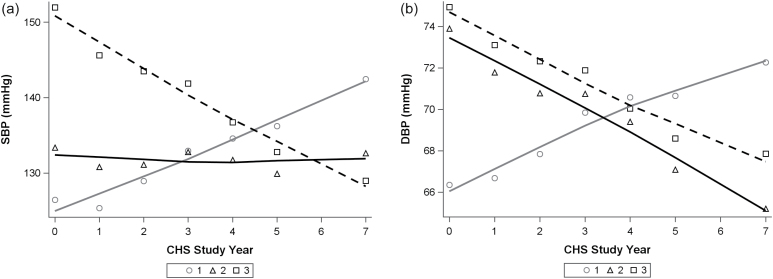
Our cluster analysis identified 3 distinct trajectories of BP change, depicted in Figure 1. At year 0 of the CHS, levels of both SBP and DBP were on average lowest in cluster 1, intermediate in cluster 2, and highest in cluster 3. Participants in cluster 1 were characterized by increases in both SBP and DBP, whereas persons in cluster 2 had little change in average in SBP but had a decline in DBP. Persons in cluster 3 had the highest initial SBP levels and experienced declines in both SBP and DBP (Table 1, Figure 1).
Figure 1.
Trajectories of (a) SBP and (b) DBP by cluster. Figures depict grouped mean values of SBP and DBP at each study visit, with smooth spline fit overlaid for each cluster. Abbreviations: CHS, Cardiovascular Health Study; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Table 1.
Demographic and clinical characteristics of CHS participants by BP trajectory cluster
| Parameter | Overall (n = 4,067) | Cluster 1 (n = 1,838) | Cluster 2 (n = 1,109) | Cluster 3 (n = 1,120) | P value |
|---|---|---|---|---|---|
| Age | 77 (75, 82) | 77 (75, 82) | 77 (74, 81) | 78 (75, 83) | <0.0001 |
| Male | 1,577 (39%) | 672 (37%) | 456 (41%) | 449 (40%) | 0.028 |
| Black | 566 (14%) | 171 (9%) | 219 (20%) | 176 (16%) | <0.0001 |
| Diabetes | 556 (16%) | 202 (13%) | 153 (16%) | 201 (22%) | <0.0001 |
| Hypertension | 1,900 (52%) | 799 (48%) | 482 (48%) | 619 (61%) | <0.0001 |
| SBP (mm Hg) year 7 | 135 (123, 149) | 140 (128, 155) | 132 (121, 145) | 130 (118, 142) | <0.0001 |
| DBP (mm Hg) year 7 | 70 (63, 77) | 72 (66, 79) | 67 (61, 73) | 69 (61, 76) | <0.0001 |
| SBP (mm Hg) year 0 | 133 (120, 147) | 125 (114, 137) | 131 (121, 142) | 150 (136, 162) | <0.001 |
| DBP (mm Hg) year 0 | 70 (63, 78) | 66 (59, 73) | 73 (66, 80) | 75 (67, 82) | <0.001 |
| Smoking | |||||
| Current | 280 (7%) | 127 (7%) | 83 (8%) | 70 (7%) | 0.55 |
| Former | 1,716 (45%) | 768 (44%) | 482 (46%) | 466 (45%) | |
| Alcohol use | 1,594 (41%) | 788 (45%) | 420 (40%) | 386 (37%) | <0.0001 |
| Antihypertensive use | 2,275 (58%) | 877 (50%) | 623 (58%) | 775 (72%) | <0.0001 |
| Statin use | 359 (9%) | 143 (8%) | 103 (10%) | 113 (10%) | 0.081 |
| LDL (mg/dl)a | 127 (106, 149) | 128 (107, 150) | 125 (106, 147) | 125 (106, 147) | 0.30 |
| BMI (kg/m2) | 26 (24, 29) | 26 (23, 29) | 27 (24, 30) | 27 (24, 30) | 0.0043 |
| eGFR-cysC (ml/min/1.73 m2) | 71 (58, 83) | 72 (60, 84) | 72 (60, 83) | 68 (53, 80) | <0.0001 |
| 15-feet walk speed (sec) | 5.0 (4.0, 6.0) | 5.0 (4.0, 6.0) | 5.0 (4.0, 6.0) | 5.0 (5.0, 7.0) | <0.0001 |
| Activities of daily living score of 1 or more | 818 (22%) | 370 (22%) | 188 (18%) | 260 (25%) | 0.0005 |
| CADb | 1,068 (26%) | 441 (24%) | 291 (26%) | 336 (30%) | 0.0015 |
| HFc | 434 (11%) | 149 (8%) | 111 (10%) | 174 (16%) | <0.0001 |
| Stroke | 308 (8%) | 125 (7%) | 69 (6%) | 114 (10%) | 0.0005 |
Data are obtained at year 7 visit at CHS unless noted and presented as median (interquartile range) or numbers (percent). Abbreviations: BP, blood pressure; BMI, body mass index; CAD, coronary artery disease; CHS, Cardiovascular Health Study; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HF, heart failure; LDL, low-density lipoprotein; SBP, systolic blood pressure.
aObtained at year 5 visit.
bPrevalent coronary artery disease.
cPrevalent congestive heart failure.
The number of BP recordings per individual was similar among clusters with an average of 6.5, 6.2, and 6.3 in clusters 1, 2, and 3, respectively. The median number of BP recordings per individual was 7 in all 3 clusters (interquartile range: 6, 7). Consistent with CHS recruitment, Black participants in our analysis had fewer BP measures on average when compared with non-Blacks (mean 4.7 vs. 6.6).
Year 7 demographic and clinical characteristics by cluster are shown in Table 1. In general, cluster 3 had higher prevalence of comorbidities and worse functional status when compared with clusters 1 and 2. Relative to cluster 1, persons in cluster 3 were on average older, had higher prevalence of diabetes mellitus, hypertension, use of antihypertensive therapy, higher body mass index, lower estimated glomerular filtration rate, and prior history of CAD, HF, and stroke.
Association of BP trajectories with all-cause mortality
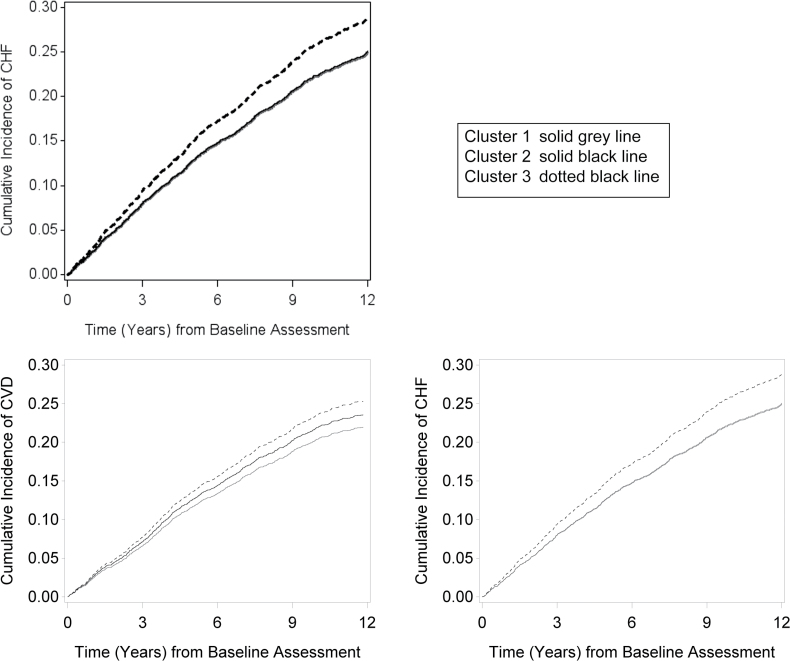
Over a median follow-up time of 9.3 years, a total of 2,475 deaths occurred. Mortality rates were lowest in cluster 1, slightly higher in cluster 2, and highest in cluster 3. Compared with cluster 1, persons in cluster 3 had a 30% higher risk for death in demographic-adjusted models, and this association was only mildly attenuated after full adjustment (Table 2). Figure 2, which shows unadjusted cumulative incidence of mortality over time, shows consistently higher rates of death in cluster 3 as compared to cluster 1 and 2 (Figure 2).
Table 2.
Association of BP trajectory cluster with outcomes
| Follow-up time | Number of events | Event rate per 1,000 PY | Demographicsa | Demographics + SBP | Multivariateb | |
|---|---|---|---|---|---|---|
| Median, IQR | HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| Mortality | ||||||
| Cluster 1 (n = 1,838) | 9.9 (5.3, 14.8) | 1,060 (58%) | 60.6 | 1 (reference) | 1 (reference) | 1 (reference) |
| Cluster 2 (n = 1,109) | 10.0 (5.2, 14.7) | 650 (59%) | 62.2 | 1.09 (0.99, 1.20) | 1.12 (1.00, 1.24) | 1.21 (1.06, 1.37) |
| Cluster 3 (n = 1,120) | 7.5 (3.9, 13.0) | 765 (68%) | 83.9 | 1.30 (1.19, 1.43) | 1.36 (1.22, 1.51) | 1.20 (1.05, 1.36) |
| Incident CVD | ||||||
| Cluster 1 (n = 1,332) | 9.2 (4.8, 14.8) | 294 (22%) | 24.0 | 1 (reference) | 1 (reference) | 1 (reference) |
| Cluster 2 (n = 779) | 9.8 (4.7, 14.7) | 183 (23%) | 25.2 | 1.10 (0.91, 1.33) | 1.26 (1.03, 1.53) | 1.18 (0.94, 1.48) |
| Cluster 3 (n = 726) | 7.7 (3.7, 13.4) | 182 (25%) | 30.6 | 1.16 (0.96, 1.39) | 1.34 (1.10, 1.64) | 1.32 (1.05, 1.66) |
| Incident HF | ||||||
| Cluster 1 (n = 1,689) | 8.9 (4.4, 13.7) | 471 (28%) | 32.3 | 1 (reference) | 1 (reference) | 1 (reference) |
| Cluster 2 (n = 998) | 8.9 (4.6, 13.7) | 280 (28%) | 32.6 | 1.02 (0.87, 1.18) | 1.13 (0.96, 1.33) | 1.02 (0.85, 1.22) |
| Cluster 3 (n = 946) | 6.9 (3.3, 12.1) | 298 (32%) | 42.2 | 1.15 (0.99, 1.34) | 1.31 (1.11, 1.54) | 1.18 (0.98, 1.43) |
Abbreviations: ADL, activity of daily living; BP, blood pressure; CI, confidence interval; CHD, coronary heart disease; CVA, cerebrovascular accident; CVD, disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; HF, heart failure; HR, hazard ratio; IQR, interquartile range; PY, person year; SBP, systolic blood pressure.
aDemographics = age, sex, race; SBP = year 7 SBP.
bMultivariate = demographics + BP (year 7 SBP, DBP) + cardiovascular risk factors (smoking, body mass index, total cholesterol, HDL, eGFR by cystatin C, statin use, antihypertensive therapy) + history of chronic medical conditions (CHD, CVA, HF, diabetes) + functional status measures (grip strength, walking speed, ADL measurements).
Figure 2.
Association of clusters with cumulative incidence of all-cause mortality, cardiovascular disease and congestive heart failure. *Baseline corresponds to the seventh year visit in the CHS. Abbreviations: BP, blood pressure; CHF, congestive heart failure; CHS, Cardiovascular Health Study; CVD, cardiovascular disease; HF, heart failure.
Compared with cluster 1, persons in cluster 2 had a 9% higher risk of death in demographic-adjusted models. This finding was largely attenuated with full adjustment (Table 2). Adjustment for year 7 SBP moderately strengthened the associations of membership in cluster 2 or 3 with death when compared with cluster 1 (Table 2). Results were similar when we stratified by use of antihypertensive therapy (Supplementary Table 1).
Association of BP trajectories with incident CVD and HF
Over a median follow-up time of 9.0 years, a total of 659 CVD and 1,049 HF events occurred. Rates of CVD and HF were lowest in cluster 1, slightly higher in cluster 2, and highest in cluster 3. In demographic-adjusted analysis, cluster 3 had a 15–16% increased risk of CVD and HF relative to cluster 1, although associations were not statistically significant (Table 2). After additional adjustment for year 7 SBP, the association of cluster 2 with CVD and HF strengthened (hazard ratio = 1.34 and 1.31, respectively).
Sensitivity analyses
In a sensitivity analysis, we used the empiric distributions of changes in SBP and DBP to define BP trajectories (Supplementary Figure 1). Though not as strong as the association observed in cluster analysis, we similarly found that a declining SBP was associated with the highest risk for death, whereas those with a stable or increasing SBP had lower mortality rates (Supplementary Table 2). In contrast to our cluster analysis, we found that an increasing DBP was associated with the highest risk for death when compared to both a stable and a decreasing DBP. The risk of death was lowest in those with a stable DBP trajectory and intermediate in those with a decreasing DBP.
DISCUSSION
In this cohort of community-dwelling elderly persons with a median age of 77, we identified 3 distinct BP trajectory groups using 7 years of BP measurements. By using cluster analysis, we found that initial levels and changes in both SBP and DBP were strong determinants of group membership. The group with the strongest association with important clinical events had the highest initial BP levels and concordantly decreasing SBP and DBP.
The present study provides unique insight into patterns of BP changes in elders. Prior studies have described that on average SBP increases steadily with advancing age, whereas DBP peaks in the fifth decade and then progressively decreases.10,18 By integrating both SBP and DBP in the same model, we found unique clusters of BP change in this cohort of community-dwelling elders. Membership within these clusters was associated with future risk of death. Specifically, we found that a BP trajectory characterized by higher year 7 BP levels and concordantly decreasing SBP and DBP was associated with a higher risk for death relative to other clusters. This finding remained robust after controlling for year 7 SBP, cardiovascular risk factors, and functional status.
Our findings highlight the utility of BP trajectories as a way to identify elderly persons at higher risk for death. Previous studies in elderly persons have not consistently shown BP change over time to be an independent predictor of death and cardiovascular events.11–13 However, these studies did not consider SBP and DBP jointly and were limited by fewer BP points and lack of adjudicated outcomes. Although we found an increasing DBP was associated with the highest risk of death in our sensitivity analysis, this finding suggests that in order to understand the relationship of BP trajectories with death, it is important to include both SBP and DBP in the analysis.
Distinct trajectories had weaker associations with risks of both CVD and HF events. Although CVD and HF rates were highest in those with a declining BP (cluster 3) and lowest in those with a rising BP (cluster 1), associations became significant only after adjustment for year 7 SBP. This suggests that the higher observed crude risk of CVD and HF in cluster 3 compared to cluster 1 is largely driven by baseline SBP. The importance of SBP as a predictor of incident CVD and CHF is consistent with prior studies that have shown that a single SBP estimate is predictive for incident CVD in an elderly cohort.5
Reasons why a declining BP may be a predictor of higher mortality risk in elders unclear. Some have suggested that a declining BP may be an indicator of poor health status.14 However, declining BP remained associated with increased mortality after full adjustment for functional status and other confounders. It is also possible that aggressive attempts to lower BP in an elderly individual may increase risk for adverse outcomes.19 However, our findings were similar when we stratified our results by use of antihypertensive therapy. It is also possible that those with a declining BP trajectory are at risk for hypotension in the community or are more likely to have greater BP variability, a known independent risk factor for future events.20,21 Future studies should investigate these possibilities.
Our study represents a large, well-characterized, diverse cohort of community-dwelling elders with outcomes adjudicated by an expert panel. Due to the observational nature of the study, we are limited in our ability to infer causality of associations and are unable to completely account for unmeasured or residual confounding that could modify the associations observed in our study. We used Fine-Gray models to account for the competing risk of death in this elderly cohort.17 In CHS, Black individuals were recruited in a second wave of enrollment. To account for potential bias, we adjusted for race and required that all individuals within the analysis have a minimum of 4 BP measures. Although we were not able to examine cause-specific mortality, future studies are needed to examine whether BP trajectory is an important determinant of non-CVD causes of death, and if the observed relationship is an effect of medication intensification or modifiable risk factors with physiologic underpinnings. Clusters identified by unsupervised learning need to be validated in other cohort studies of community-dwelling elders.
In conclusions, among community-dwelling elders, 3 distinct patterns of BP change were identified when incorporating both SBP and DBP. A concordant decreasing BP trajectory was independently associated with higher risks for death but not CVD or HF. These findings were similar when stratified by use of antihypertensive therapy. Future studies are needed to understand the importance of BP lowering in adults.
SUPPLEMENTARY MATERIAL
Supplemental data are available at American Journal of Hypertension online.
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by contracts HHSN26820120036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114 from the National Heart, Lung and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal Cardiovascular Health Study (CHS) investigators and institutions can be found at https://CHS-NHLBI.org. C.A.P. and M.C.O. are supported by the National Institutes of Health by a co-principal investigator grant (1R01AG046206). The content is solely our responsibility and does not necessarily represent the official views of the National Institute of Health.
REFERENCES
- 1. Psaty BM, Furberg CD, Kuller LH, Cushman M, Savage PJ, Levine D, O’Leary DH, Bryan RN, Anderson M, Lumley T. Association between blood pressure level and the risk of myocardial infarction, stroke, and total mortality: the cardiovascular health study. Arch Intern Med 2001; 161:1183–1192. [DOI] [PubMed] [Google Scholar]
- 2. Butler J, Kalogeropoulos AP, Georgiopoulou VV, Bibbins-Domingo K, Najjar SS, Sutton-Tyrrell KC, Harris TB, Kritchevsky SB, Lloyd-Jones DM, Newman AB, Psaty BM. Systolic blood pressure and incident heart failure in the elderly. The Cardiovascular Health Study and the Health, Ageing and Body Composition Study. Heart 2011; 97:1304–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT; SPRINT Research Group . A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Williamson JD, Supiano MA, Applegate WB, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH, Kitzman DW, Kostis JB, Krousel-Wood MA, Launer LJ, Oparil S, Rodriguez CJ, Roumie CL, Shorr RI, Sink KM, Wadley VG, Whelton PK, Whittle J, Woolard NF, Wright JT Jr, Pajewski NM; SPRINT Research Group . Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA 2016; 315:2673–2682. < 10.1001/jama.2016.7050>. [DOI] [PMC free article] [PubMed]
- 5. Peralta CA, Katz R, Newman AB, Psaty BM, Odden MC. Systolic and diastolic blood pressure, incident cardiovascular events, and death in elderly persons: the role of functional limitation in the Cardiovascular Health Study. Hypertension 2014; 64:472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mattila K, Haavisto M, Rajala S, Heikinheimo R. Blood pressure and five year survival in the very old. Br Med J Clin Res Ed 1988; 296:887–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 2012; 172:1162–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gofin J, Kark JD, Abramson JH, Epstein L. Trends in blood pressure levels over time in middle-aged and elderly Jerusalem residents. Eur Heart J 1995; 16:1988–1994. [DOI] [PubMed] [Google Scholar]
- 9. Landahl S, Bengtsson C, Sigurdsson JA, Svanborg A, Svärdsudd K. Age-related changes in blood pressure. Hypertension 1986; 8:1044–1049. [DOI] [PubMed] [Google Scholar]
- 10. Cheng S, Xanthakis V, Sullivan LM, Vasan RS. Blood pressure tracking over the adult life course: patterns and correlates in the Framingham heart study. Hypertension 2012; 60:1393–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hakala SM, Tilvis RS. Determinants and significance of declining blood pressure in old age. A prospective birth cohort study. Eur Heart J 1998; 19:1872–1878. [DOI] [PubMed] [Google Scholar]
- 12. Rogers MA, Ward K, Gure TR, Choe HM, Lee PG, Bernstein SJ, Blaum CS. Blood pressure trajectories prior to death in patients with diabetes. Diabetes Care 2011; 34:1534–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tervahauta M, Pekkanen J, Enlund H, Nissinen A. Change in blood pressure and 5-year risk of coronary heart disease among elderly men: the Finnish cohorts of the Seven Countries Study. J Hypertens 1994; 12:1183–1189. [PubMed] [Google Scholar]
- 14. Satish S, Zhang DD, Goodwin JS. Clinical significance of falling blood pressure among older adults. J Clin Epidemiol 2001; 54:961–967. [DOI] [PubMed] [Google Scholar]
- 15. Swan GE, Carmelli D, Larue A. Systolic blood pressure tracking over 25 to 30 years and cognitive performance in older adults. Stroke 1998; 29:2334–2340. [DOI] [PubMed] [Google Scholar]
- 16. Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991; 1:263–276. [DOI] [PubMed] [Google Scholar]
- 17. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94:496–509. [Google Scholar]
- 18. Pearson JD, Morrell CH, Brant LJ, Landis PK, Fleg JL. Age-associated changes in blood pressure in a longitudinal study of healthy men and women. J Gerontol A Biol Sci Med Sci 1997; 52:M177–M183. [DOI] [PubMed] [Google Scholar]
- 19. Charlesworth CJ, Peralta CA, Odden MC. Functional status and antihypertensive therapy in older adults: a new perspective on old data. Am J Hypertens 2016; 29:690–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muntner P, Whittle J, Lynch AI, Colantonio LD, Simpson LM, Einhorn PT, Levitan EB, Whelton PK, Cushman WC, Louis GT, Davis BR, Oparil S. Visit-to-visit variability of blood pressure and coronary heart disease, stroke, heart failure, and mortality: a cohort study. Ann Intern Med 2015; 163:329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suchy-Dicey AM, Wallace ER, Mitchell SV, Aguilar M, Gottesman RF, Rice K, Kronmal R, Psaty BM, Longstreth WT Jr. Blood pressure variability and the risk of all-cause mortality, incident myocardial infarction, and incident stroke in the cardiovascular health study. Am J Hypertens 2013; 26:1210–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.