Abstract
BACKGROUND
Recent evidence suggests that the mineralocorticoid receptor antagonist spironolactone should be the preferred fourth-line antihypertensive treatment in resistant hypertension (RHTN). Whether spironolactone improves blood pressure (BP) control in heart failure with preserved ejection fraction (HFpEF) and RHTN is unknown.
METHODS
We identified patients with RHTN, defined as baseline systolic blood pressure (SBP) between 140 and 160 mm Hg on 3 or more medications, in the Americas cohort of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial, in which patients with HFpEF were randomized to spironolactone vs. placebo. We evaluated the effects of spironolactone vs. placebo on BP reduction in this group and related this to the primary composite outcome of death from cardiovascular causes, aborted cardiac arrest, or hospitalization for heart failure.
RESULTS
We identified 403 participants in the Americas with RHTN. Compared to people without RHTN, those with RHTN were more frequently women, non-White, diabetics, with a higher left ventricular ejection fraction and body mass index, and a lower hemoglobin concentration. In the RHTN group, spironolactone resulted in a decrease of SBP: −6.1 (−8.9, −3.3); P < 0.001 and diastolic BP: −2.9 (−4.6, −1.2); P = 0.001 mm Hg during the first 8 months. BP became controlled after 4 weeks in 63% of patients receiving spironolactone vs. 46% receiving placebo (P = 0.003), with similar responses at 8 weeks, 4 and 8 months. Patients with RHTN derived similar overall benefit from spironolactone on the primary outcomes as those without.
CONCLUSIONS
In HFpEF patients with RHTN, spironolactone lowered BP substantially and was associated with similar benefit as those without RHTN.
CLINICAL TRIALS REGISTRATION
Trial Number NCT00094302 (ClinicalTrials.gov identifier)
Keywords: blood pressure, heart failure with preserved ejection fraction, hypertension, randomized trial, resistant hypertension, spironolactone
Arterial hypertension is one of the common comorbidities associated with heart failure with preserved ejection fraction (HFpEF), and both European and US guidelines recommend treating hypertension in HFpEF.1,2 In particular, patients with resistant hypertension (RHTN), defined as a seated office systolic blood pressure (SBP) ≥140 mm Hg or diastolic blood pressure (DBP) ≥90 mm Hg on maximally tolerated doses of 3 or more antihypertensive agents, one of which must be a diuretic appropriate for the level of kidney function, are at high risk for cardiovascular (CV) morbidity and mortality3 and are characterized by an increased prevalence of target organ damages and comorbidities, including a 3-fold increase in HF.4 As most epidemiological studies lack key elements used to define true RHTN, apparent treatment-RHTN is defined by an uncontrolled BP on 3 or more antihypertensive medication classes or use of four or more medications regardless of BP level.3 Such definition fits actually with the current and pragmatic definition of RHTN endorsed by the American Heart Association,5 stating that “RHTN is defined as BP that remains above goal in spite of the concurrent use of 3 antihypertensive agents of different classes. Ideally, one of the 3 agents should be a diuretic and all agents should be prescribed at optimal dose amounts”.
Current guidelines suggest that after optimization of the ongoing treatment, the next step is to consider the addition of other antihypertensive drugs, with the current suggested fourth-line therapy being a mineralocorticoid receptor antagonist.3,6,7 It is however unknown whether this may be applicable to an HFpEF population.
The Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial tested spironolactone vs. placebo in patients with HFpEF. Since the eligibility requirement for TOPCAT required patients to have either a controlled SBP, defined as a target SBP <140 mm Hg or be between 140 and 160 mm Hg on 3 or more antihypertensive agents, some patients with RHTN were included in TOPCAT. We therefore aimed to evaluate in this prespecified RHTN subgroup, the effect of spironolactone on BP and CV outcomes.
METHODS
The TOPCAT trial tested spironolactone vs. placebo in patients with HFpEF. TOPCAT was a phase III, multicentre, international, randomized, double-blind, placebo-controlled trial.8 Patients were eligible if they were ≥50 years of age, exhibited at least one sign and one symptom of HF, and had a left ventricular ejection fraction of ≥45%. In addition, eligible patients had either a history of hospitalization for worsening HF within the previous 12 months or an elevated natriuretic peptide level within 60 days before randomization [brain natriuretic peptide (BNP) ≥ 100 pg/ml or NT-proBNP ≥ 360 pg/ml]. A total of 3,445 patients were randomized in 6 countries [USA, Argentina, Brazil, and Canada (“the Americas”) as well as Georgia and Russia (“Eastern Europe”)]. Overall, the TOPCAT trial did not achieve a significant reduction in the primary composite outcome of CV death, aborted cardiac arrest, or hospitalization for management of HF.8 Several post-hoc analyses of the TOPCAT trial have been performed. For example, the interactions for location of randomization and left ventricular ejection fraction spectrum with spironolactone treatment effect have been published and commented upon.9,10 Briefly, TOPCAT subanalyses identified a large variation in treatment effect on CV mortality between different geographical areas [hazard ratio 0.74, 95% confidence interval (CI) 0.57–0.97 in “the Americas” vs. hazard ratio 1.31, 95% CI 0.91–1.90 in “Eastern Europe”, P for interaction = 0.012].9–11 Concerns related to the study conducted in Russia and Georgia were raised with regard to the inclusion criteria10 and to the adherence to the study medications.12
TOPCAT included patients with either a controlled SBP, defined as a target SBP <140 mm Hg, or those with SBP between 140 and 160 mm Hg if on 3 or more medications to control BP. Patients who fell into this category were considered as having RHTN patients in this prespecified subgroup analysis. Patients with SBP greater than 160 mm Hg were excluded. At each office visit, the following were obtained by short interview: current signs/symptoms consistent with HF and with administration of study drug, and current medications (subjects were asked to bring these to each visit for accurate inventory). BP was taken and recorded. Every effort was made to control BP throughout the course of follow-up.
Because of the previously reported significant regional differences between the Americas and Russia/Georgia,11 with very few events in Russia/Georgia, we restricted our analyses on the patients from the Americas.
The TOPCAT trial was conducted in accordance with the Declaration of Helsinki, with independent Institutional Review Board or ethics committee approval at all participating centers, and with written informed consent from all patients. The design and methods of the TOPCAT trial have been described previously. In addition, the trial protocol is available at NEJM.org.8 Study drugs were purchased as 15-mg tablets of spironolactone or matching placebo (United Research Laboratories and Mutual Pharmaceutical). Study drugs were initially administered at a dose of 15 mg once daily, which was increased to a maximum of 45 mg daily during the first 4 months after randomization. Subsequent dose adjustments were made as required. Measurement of potassium and creatinine levels was required within 1 week after a change in the study-drug dose and at each scheduled study visit. Study patients continued to receive other treatments for heart failure and coexisting illnesses throughout the trial.
Statistics
Baseline characteristics were summarized using median [interquartile range] and frequency (percentages) for continuous and categorical variables, respectively, and comparisons were made between RHTN and non-RHTN patients as well as between RHTN patients assigned to spironolactone and placebo using Wilcoxon rank–sum tests and Pearson’s chi-squared test. Analyses of clinical outcomes were conducted using unadjusted and adjusted Cox proportional hazards models. Changes in SBP and DBP measures from baseline at 4 weeks, 8 weeks, 4 months, and 8 months (follow-up value minus baseline value) were analyzed as outcomes using linear regression models that adjusted for baseline values of the corresponding measure. In additional, “BP control” was defined at each visit as SBP <140 and DBP <90 and was similarly considered as an outcome using logistic regression models (with baseline SBP and DBP as covariates). Where indicated, Cox models were stratified using the TOPCAT enrollment strata (recent HF hospitalization or elevated natriuretic peptide levels). Treatment-by-RHTN status interaction terms were used to assess for differential treatment effects in the RHTN patients. Comparisons of mean reported doses and frequencies of adverse events were conducted via t-tests and chi-squared tests, respectively. All analyses were conducted using STATA 14 (College Station, TX). P values less than 0.05 were considered statistically significant. No adjustments were made for multiple testing.
RESULTS
Study patients and follow-up
From 10 August 2006 to 31 January 2012, a total of 403 RHTN patients were enrolled at 119 sites in 4 countries (249 participants in the United States, 78 in Canada, 55 in Brazil, and 21 in Argentina). They were randomly assigned to receive spironolactone (191 participants) or placebo (212 participants). Their baseline features are presented in the Table 1. Compared to people without RHTN, RHTN participants were more frequently women, non-White, diabetics, displayed a higher left ventricular ejection fraction and body mass index, and a lower hemoglobin concentration. Up to 94% of patients with RHTN were treated with a diuretic. Owing to an imbalance in age, angiotensin-converting enzyme inhibitor/angiotensin receptor blockers use, and diabetes in RHTN participants (not encountered in participants without RHTN—Supplementary Table 1) assigned spironolactone vs. placebo, these 3 parameters were considered in the adjusted models.
Table 1.
Baseline characteristics in America between patients w/wo resistant HTN
| America: all patients | America: RHTN only | |||||
|---|---|---|---|---|---|---|
| No resistant HTN | Resistant HTN | P | Resistant HTN: placebo | Resistant HTN: spiro | P | |
| n = 1,361 | n = 403 | |||||
| n = 212 | n = 191 | |||||
| Age ≥ 75 | 559 (41.1%) | 160 (39.7%) | 0.62 | 75 (35.4%) | 85 (44.5%) | 0.06 |
| Female, % | 638 (46.9%) | 244 (60.5%) | 0.001 | 134 (63.2%) | 110 (57.6%) | 0.25 |
| Race_White, % | 1,099 (80.7%) | 282 (70.0%) | 0.001 | 147 (69.3%) | 135 (70.7%) | 0.77 |
| NYHA_class | 0.60 | 0.49 | ||||
| 1 | 71 (5.2%) | 28 (6.9%) | 13 (6.1%) | 15 (7.9%) | ||
| 2 | 810 (59.6%) | 233 (57.8%) | 121 (57.1%) | 112 (58.6%) | ||
| 3 | 469 (34.5%) | 140 (34.7%) | 76 (35.8%) | 64 (33.5%) | ||
| 4 | 8 (0.6%) | 2 (0.5%) | 2 (0.9%) | 0 (0.0%) | ||
| Eligibility strata | ||||||
| Hospitalization | 738 (54.2%) | 237 (58.8%) | 0.10 | 130 (61.3%) | 107 (56.0%) | 0.28 |
| Elevated BNP | 623 (45.8%) | 166 (41.2%) | 82 (38.7%) | 84 (44.0%) | ||
| Current smoker | 90 (6.6%) | 27 (6.7%) | 0.95 | 16 (7.5%) | 11 (5.8%) | 0.47 |
| Diabetes | 577 (42.4%) | 211 (52.4%) | 0.001 | 124 (58.5%) | 87 (45.5%) | 0.009 |
| Medications | ||||||
| Diuretics | 1,193 (87.7%) | 378 (93.8%) | 0.001 | 199 (93.9%) | 179 (93.7%) | 0.95 |
| ACE or ARB | 1,033 (76.0%) | 361 (89.6%) | 0.001 | 198 (93.4%) | 163 (85.3%) | 0.008 |
| CCB | 462 (34.0%) | 219 (54.3%) | 0.001 | 118 (55.7%) | 101 (52.9%) | 0.58 |
| BB | 1,069 (78.6%) | 318 (78.9%) | 0.90 | 166 (78.3%) | 152 (79.6%) | 0.75 |
| Age, years | 73 [64, 79] | 72 [64, 80] | 0.51 | 71 [63, 78] | 74 [65, 80] | 0.043 |
| Ejection fraction, % | 58 [52, 63] | 60.0 [55, 65] | 0.028 | 60 [53, 65] | 60 [55, 65] | 0.23 |
| SBP, mm Hg | 122 [114, 131] | 148 [142, 152] | 0.001 | 148 [142, 154] | 146 [141, 151] | 0.12 |
| DBP, mm Hg | 70 [60, 78] | 80.0 [70, 86] | 0.001 | 80 [70, 86] | 80 [71, 86] | 0.9 |
| HR, bpm | 68 [62, 76] | 67.0 [60, 75] | 0.005 | 68 [60, 75] | 66 [60, 74] | 0.38 |
| BMI, kg/m2 | 32.5 [27.7, 38.1] | 34.1 [29.3, 39.5] | 0.001 | 34.9 [29.7, 40.9] | 33.0 [28.9, 38.3] | 0.05 |
| K, mmol/l | 4.2 [3.9, 4.5] | 4.2 [3.9, 4.5] | 0.20 | 4.2 [3.9, 4.4] | 4.2 [3.8, 4.5] | 0.6 |
| Serum creatinine, mg/dl | 1.1 [0.9, 1.4] | 1.1 [0.9, 1.4] | 0.045 | 1.1 [0.9, 1.3] | 1.1 [0.8, 1.4] | 0.36 |
| eGFR, ml/min/1.73 m2 | 61 [49, 76] | 63 [50, 77] | 0.28 | 61 [49, 76] | 64 [50, 79] | 0.23 |
| Hb, g/dl | 12.9 [11.8, 14.0] | 12.6 [11.5, 13.9] | 0.007 | 12.6 [11.5, 13.9] | 12.6 [11.5, 13.9] | 0.74 |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BB, beta blocker; BMI, body mass index; BNP, brain natriuretic peptide; CCB, calcium channel blocker; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HR, heart rate; SBP, systolic blood pressure; w/wo, with or without.
The mean follow-up interval was 2.92 years, with 2.88 years in placebo group vs. 2.98 years in spironolactone group. A total of 25 participants—12 in the spironolactone group (6.3%) and 13 in the placebo group (6.1%)—discontinued study participation before the last expected study visit for reasons other than death. Vital status as of the last expected study visit was unknown for 2 participants in the spironolactone group (1.0%) and 3 participants in the placebo group (1.4%).
Study drug administration in the RHTN population
The mean dose at 8 months was 23.0 mg per day in the spironolactone group and 26.4 mg per day in the placebo group. There were 98 participants in the spironolactone group (51.3%) and 92 in the placebo group (43.4%) who permanently discontinued the study drug while continuing to be followed for study outcomes.
Spironolactone blood pressure effects
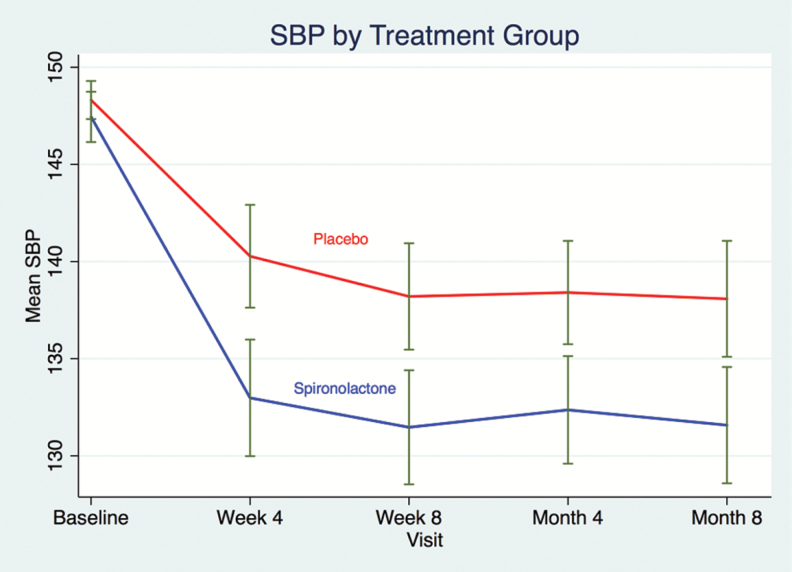
In the RHTN group, spironolactone-induced consistent and significant greater BP decreases throughout the first 8 months (SBP: −6.1 (−8.9, −3.3); P < 0.001; DBP: −2.9 (−4.6, −1.2); P = 0.001, Figure 1 presenting SBP patterns during the first 8 months) and improved BP control after 4 weeks, 8 weeks, 4 and 8 months (OR 1.89 (1.23–2.89), P = 0.003; OR: 2.07 (1.33–3.19), P = 0.001; OR: 1.77(1.16–2.69), P = 0.008; OR: 1.61(1.03–2.52); P = 0.036, respectively) (Table 2). BP decreases over the first 8 months were similar in the non-RHTN population (SBP: −3.8 (−5.1, −2.4); P < 0.001; DBP: −2.1 (−2.9, −1.3); P < 0.001; interaction P values = 0.36 and 0.10, respectively). Improvement in BP control with spironolactone was significantly weaker at 4 weeks in the non-RHTN group (OR = 1.09, interaction P = 0.033), with numerically smaller effect sizes at the other time points as well (OR = 1.33, 1.45, and 1.60 at week 8, month 4, and month 8, respectively).
Figure 1.
Kinetics of SBP in resistant hypertension during the first 8 months according to the study groups in America. Abbreviation: SBP, systolic blood pressure.
Table 2.
Between-treatment analysis of change in BP at 4 weeks, 8 weeks, 4 months, and 8 months) from the baselinea
| Americas: RHTN only | Americas: all patients | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Spiro | Placebo | Spiro | |||||||||
| n | LSM (SE) | n | LSM (SE) | Diff | P | n | LSM (SE) | n | LSM (SE) | Diff | P | |
| Week 4 | ||||||||||||
| SBP | 194 | −7.78 (1.4) | 180 | −14.08 (1.4) | −6.29 (1.96) | 0.001 | 824 | 0.72 (0.6) | 843 | −2.64 (0.6) | −3.36 (0.82) | <0.001 |
| DBP | 194 | −3.09 (0.8) | 180 | −5.84 (0.8) | −2.75 (1.15) | 0.02 | 824 | −0.27 (0.4) | 843 | −1.89 (0.4) | −1.62 (0.51) | 0.001 |
| BP controlb | 194 | 90 (46.4%) | 180 | 114 (63.3%) | OR: 1.89 (1.23–2.89) | 0.003 | 824 | 597 (72.5%) | 843 | 655 (77.6%) | OR: 1.33 (1.05–1.68) | 0.02 |
| Week 8 | ||||||||||||
| SBP | 193 | −5.92 (2.00) | 173 | −15.62 (1.45) | −5.92 (2.00) | 0.003 | 820 | 0.54 (0.6) | 825 | −4.18 (0.6) | −4.72 (0.82) | <0.001 |
| DBP | 193 | −3.86 (0.83) | 173 | −7.07 (0.88) | −3.21 (1.21) | 0.008 | 820 | −0.18 (0.4) | 825 | −3.21 (0.4) | −3.03 (0.50) | <0.001 |
| BP controlb | 193 | 93 (48.2%) | 173 | 116 (67.1%) | OR: 2.07 (1.33–3.19) | 0.001 | 820 | 595 (72.6%) | 825 | 663 (80.3%) | OR: 1.56 (1.23–1.99) | <0.001 |
| Month 4 | ||||||||||||
| SBP | 193 | −9.54 (1.33) | 173 | −15.07 (1.41) | −5.53 (1.95) | 0.005 | 809 | 0.94 (0.6) | 820 | −2.75 (0.6) | −3.69 (0.86) | <0.001 |
| DBP | 193 | −4.33 (0.84) | 173 | −6.54 (0.88) | −2.21 (1.22) | 0.07 | 809 | −0.39 (0.4) | 820 | 1.95 (0.4) | −1.56 (0.53) | 0.003 |
| BP controlb | 193 | 93 (48.2%) | 173 | 109 (63.0%) | OR: 1.77 (1.16–2.69) | 0.008 | 809 | 570 (70.5%) | 820 | 645 (78.6%) | OR: 1.55 (1.23–1.96) | <0.001 |
| Month 8 | ||||||||||||
| SBP | 174 | −9.82 (1.48) | 164 | −15.65 (1.52) | −5.83 (2.12) | 0.006 | 758 | −2.23 (0.6) | 779 | −2.88 (0.6) | −5.11 (0.89) | <0.001 |
| DBP | 174 | −4.33 (0.91) | 164 | −7.47 (0.93) | −3.14 (1.30) | 0.016 | 758 | 0.57 (0.4) | 779 | −2.12 (0.4) | −2.69 (0.55) | <0.001 |
| BP controlb | 174 | 95 (54.6%) | 164 | 110 (67.1%) | OR: 1.61 (1.03–2.52) | 0.036 | 758 | 524 (69.1%) | 779 | 609 (78.1%) | OR: 1.61 (1.27–2.04) | <0.001 |
Abbreviations: BP, blood pressure; DBP, diastolic BP; LSM, least square mean; OR, odds ratio; SBP, systolic BP.
aAdjusted for baseline SBP and treatment.
bBP control defined by SBP <140 mm Hg and DBP <90 mm Hg.
The better BP control achieved with spironolactone vs. placebo in the RHTN group throughout the first 8 months did not significantly differ across the subgroups we defined post-hoc on a pathophysiological or epidemiological basis (presence/absence of low potassium, presence/absence of diabetes, estimated glomerular filtration rate (eGFR) higher or lower than 60 ml/min/1.73 m2, low vs. high natriuretic peptides at baseline, White vs. non-White), as assessed by the corresponding nonsignificant P values for interaction (Supplementary Tables 2.1 to 2.4).
Primary outcome and component events
As previously reported,8 in the whole America’s population, patients treated with spironolactone vs. placebo displayed better outcomes. We observed no interaction between baseline RHTN and spironolactone with respect to outcomes; patients with RHTN benefited similarly to those without RHTN (P value for interaction = 0.53 for the primary outcome) (Table 3).
Table 3.
Outcomes in Americas according to the presence/absence of RHTN
| RHTN | No RHTN | P for interaction between RHTN and no RHTN and treatmenta | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number and % of participants with events, and incidence rate per 100 person-year | Unadjusted model HR, 95% CI, P value | Strata modelb HR, 95% CI, P value | Adjusted modelc HR, 95% CI, P value | Number and % of participants with events, and incidence rate per 100 person-year | Unadjusted model HR, 95% CI, P value | Strata modelb HR, 95% CI, P value | Adjusted modelc HR, 95% CI, P value | ||||
| Primary | 70 (33.0%), 13.8 per 100 pyr | 48 (25.3%), 9.6 per 100 pyr | 70 (33.0%), 13.8 per 100 pyr | 48 (25.3%), 9.6 per 100 pyr | 0.70 (0.49– 1.01), 0.06 | 210 (31.5%), 12.3 per 100 pyr | 194 (28.0%), 10.6 per 100 pyr | 0.86 (0.71–1.05), 0.14 | 0.84 (0.69– 1.02), 0.08 | 0.83 (0.68– 1.01), 0.057 | 0.53 |
| CVD | 29 (16.7%), 4.8 per 100 pyr | 18 (9.5%), 3.2 per 100 pyr | 0.67 (0.37–1.21), 0.19 | 0.71 (0.39– 1.28), 0.26 | 0.68 (0.37– 1.23), 0.20 | 98 (14.7%), 4.9 per 100 pyr | 78 (11.2%), 3.8 per 100 pyr | 0.76 (0.56–1.02), 0.07 | 0.75 (0.56– 1.01), 0.057 | 0.74 (0.55– 0.99), 0.045 | 0.71 |
| HF hospitalization | 57 (26.9%), 11.2 per 100 pyr | 40 (21.1%), 8.0 per 100 pyr | 0.72 (0.48–1.07), 0.11 | 0.74 (0.49– 1.11), 0.15 | 0.75 (0.50– 1.13), 0.16 | 159 (23.8%), 9.3 per 100 pyr | 144 (20.8%), 7.9 per 100 pyr | 0.85 (0.68–1.07), 0.16 | 0.83 (0.66– 1.04), 0.10 | 0.81 (0.65– 1.02), 0.075 | 0.45 |
| Recurrent HF | 19.4 per 100 pyr | 14.1 per 100 pyr | 0.57 (0.35–0.95), 0.029 | 0.57 (0.34– 0.93), 0.026 | 0.63 (0.38– 1.05), 0.08 | 16.2 per 100 pyr | 13.6 per 100 pyr | 0.82 (0.61–1.10), 0.18 | 0.76 (0.57– 1.01), 0.059 | 0.74 (0.56– 0.98), 0.037 | 0.22 |
| All cause mortality | 41 (19.3%), 6.4 per 100 pyr | 30 (15.8%), 5.0 per 100 pyr | 0.78 (0.48–1.24), 0.29 | 0.80 (0.50– 1.28), 0.34 | 0.72 (0.44– 1.16), 0.18 | 166 (24.9%), 8.1 per 100 pyr | 147 (21.2%), 6.8 per 100 pyr | 0.83 (0.67–1.04), 0.11 | 0.83 (0.66– 1.03), 0.09 | 0.80 (0.64– 1.01), 0.056 | 0.80 |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CI, confidence interval; CVD, cardiovascular death; HF: heart failure; HR, hazard ratio; RHTN, resistant hypertension.
aFrom unadjusted model.
bStratified by eligibility strata.
cFurther adjusted for age, ACE/ARB, diabetes.
Adverse events in the RHTN population
The rates of any serious adverse events were similar between patients with and without RHTN (63% vs. 65%). There were 364 serious adverse events in the spironolactone group and 476 in the placebo group (64.0 per 100 person-years and 78.3 per 100 person-years, respectively, in the RHTN subgroup). The prevention of serious adverse events in a time-to-first analysis observed in the RHTN subgroup (hazard ratio = 0.74, 95% CI 0.58–0.95) was not significantly different from the corresponding relationship observed in patients without RHTN (hazard ratio = 0.98, 95% CI 0.86–1.11, interaction P = 0.053).The spironolactone group had a higher rate of hyperkalemia (26.7% vs. 8.0% in the placebo group, P < 0.001) and a lower rate of hypokalemia (44.5% vs. 61.8%, P < 0.001). The spironolactone group was numerically, but not statistically significantly more likely to have a doubling of the serum creatinine level to a value above the upper limit of the normal range (22.5% vs. 17.0%, Pp = 0.16). Similarly, there were no significant between-group differences in the proportion of patients with a serum creatinine level of 3.0 mg per deciliter or higher or who required dialysis (11.5% vs. 10.8%, P = 0.83).
DISCUSSION
In patients with HFpEF and RHTN, the use of spironolactone was associated with a greater BP decrease and a better BP control. Patients with RHTN derived similar benefit from spironolactone as those without RHTN.
These results strengthen the role of low-dose spironolactone as a fourth-line therapeutic for RHTN in a HFpEF population. The efficacy of low-dose spironolactone was initially suggested in a US population (76 African American and White subjects with RHTN with and without primary aldosteronism).13
Furthermore, a post-hoc analysis of the Anglo-Scandinavian Cardiac Outcomes trial-blood pressure lowering arm (ASCOT-BPLA) trial evaluated the effect of spironolactone as a fourth-line antihypertensive agent for uncontrolled BP among 1,411 participants. The median duration of spironolactone treatment was 1.3 years (interquartile range: 0.6 to 2.6 years) and the median dose of spironolactone was 25 mg (interquartile range: 25 to 50 mg) at both the start and end of the observation period. In the ASCOT-BPLA study, spironolactone effectively lowered BP in patients with uncontrolled hypertension under a mean of approximately 3 other drugs by SBP/DBP 21.9/9.5 mm Hg (95% CI: 20.8 to 23.0/9.0 to 10.1 mm Hg; P < 0.001). Several studies confirmed this effect (see the article of Dudenbostel and Calhoun7 for review), the superiority of spironolactone to treat-RHTN compared to other classes of agents being clearly demonstrated by the PATHWAY-2 trial, as described below.6
Our analyses demonstrate a BP-lowering effect, which occurred early during the titration phase, and persisted during the 8-month exposure period we considered. Although post-hoc, our analysis provides valuable randomized evidence, and extends the results of the PATHWAY-2 trial.6 This latter study included patients with RHTN on a triple combination therapy (i.e., an angiotensin-converting enzyme inhibitor, a calcium channel blocker, and a diuretic), and plasma potassium concentrations within normal range. PATHWAY-2 showed that 12 weeks of treatment with spironolactone 25–50 mg was the most effective add-on, fourth-line drug as compared with bisoprolol (5–10 mg) and doxazosin modified release (4–8 mg) or placebo. Two hundred and thirty patients completed all treatment cycles. Importantly, exclusion criteria were an eGFR lower than 45 ml/min, recent (<6 months) CV event requiring hospitalization, and concurrent chronic illness, anticipated change of medical status during the trial or other reasons likely to preclude 40-week participation in the study. PATHWAY-2 did not target specifically HFpEF patients, therefore it is unknown whether its results may be applicable to an HFpEF population.
Moreover, the PATHWAY-2 trial enrolled patients with a mean eGFR of 91 ml/min, whereas patients with a median eGFR of 62.8 ml/min/1.73 m2 were included herein, with a good safety profile of spironolactone. Of note, the better BP control achieved with spironolactone vs. placebo in the RHTN group throughout the first 8 months did not significantly differ among patients with an eGFR higher or lower than 60 ml/min/1.73 m2 at baseline. The magnitude of the BP decrease observed in TOPCAT was somewhat lower from that observed in PATHWAY-2 after 3 months (SBP—8.70 (−9·72 to −7·69), P < 0.0001, vs. −5.53 (1.95), P = 0.005 in TOPCAT after 4 months). Importantly, the PATHWAY-2 trial lasted only 3 months, while TOPCAT performed a much longer follow-up (8-month BP effects being described in the present analysis).
Finally, we did not observe any significant interaction between the presence or absence of RHTN and the treatment group with regard to the outcomes, while the latter were improved by spironolactone in this America’s population.
Limitations
This analysis has several limitations. First, we did not consider antihypertensive drug doses, or ambulatory BP measurements to define RHTN, and 6% of the patients were not treated with diuretics. Secondary hypertension was not ruled out. However, the definition used fits with current US guidelines.5 Second, patients with SBP greater than 160 mm Hg, a history of hyperkalemia (serum potassium ≥ 5.5 mmol/l) in the past 6 months or serum potassium ≥5.0 mmol/l within the past 2 weeks, those with severe renal dysfunction, defined as an eGFR <30 ml/min (per the Modification of Diet in Renal Disease 4-component study equation), or with serum creatinine ≥2.5 mg/dl even if their GFR is ≥30 ml/min were also excluded. Therefore, the external validity and potential generalizability to “real-world” HFpEF patients is uncertain. Our analyses, restricted to the America’s population are post-hoc, although a subgroup of “RHTN” analysis was prespecified in the TOPCAT protocol. Our results are derived from a large randomized controlled trial, with adjudicated events, allowing for reliably assessing the association between spironolactone use, outcome, and BP changes.
In conclusion, in HFpEF patients with RHTN from the Americas, spironolactone enabled a better BP control. The effect of spironolactone on outcomes in patients with RHTN was similar to those without RHTN.
Perspectives
Despite the trial limitations mainly related to the reported significant regional differences between the Americas and Russia/Georgia,11 and in the absence of stronger data, TOPCAT results may be informative to physicians currently faced with clinical decisions for patients with HFpEF with RHTN with anticipated risks similar to those in patients enrolled from the Americas.
SUPPLEMENTARY MATERIAL
Supplementary data are available at American Journal of Hypertension online.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENT
The TOPCAT trial was funded by the National Heart, Lung, and Blood Institute.
REFERENCES
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 2. Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW; American College of Cardiology Foundation; American Heart Association . 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults a report of the American college of cardiology foundation/american heart association task force on practice guidelines developed in collaboration with the international society for heart and lung transplantation. J Am Coll Cardiol 2009; 53:e1–e90. [DOI] [PubMed] [Google Scholar]
- 3. Rossignol P, Massy ZA, Azizi M, Bakris G, Ritz E, Covic A, Goldsmith D, Heine GH, Jager KJ, Kanbay M, Mallamaci F, Ortiz A, Vanholder R, Wiecek A, Zoccali C, London GM, Stengel B, Fouque D; ERA-EDTA EURECA-m working group; Red de Investigación Renal (REDINREN) network; Cardiovascular and Renal Clinical Trialists (F-CRIN INI-CRCT) network . The double challenge of resistant hypertension and chronic kidney disease. Lancet 2015; 386:1588–1598. [DOI] [PubMed] [Google Scholar]
- 4. Rimoldi SF, Messerli FH, Bangalore S, Scherrer U. Resistant hypertension: what the cardiologist needs to know. Eur Heart J 2015; 36:2686–2695. [DOI] [PubMed] [Google Scholar]
- 5. Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM; American Heart Association Professional Education Committee . Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American heart association professional education committee of the council for high blood pressure research. Circulation 2008; 117:e510–e526. [DOI] [PubMed] [Google Scholar]
- 6. Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G, Ford I, Cruickshank JK, Caulfield MJ, Salsbury J, Mackenzie I, Padmanabhan S, Brown MJ; British Hypertension Society’s PATHWAY Studies Group . Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet 2015; 386:2059–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dudenbostel T, Calhoun DA. Use of aldosterone antagonists for treatment of uncontrolled resistant hypertension. Am J Hypertens 2017; 30:103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O’Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370:1383–1392. [DOI] [PubMed] [Google Scholar]
- 9. Ferreira JP, Girerd N, Rossignol P, Zannad F. Geographic differences in heart failure trials. Eur J Heart Fail 2015; 17:893–905. [DOI] [PubMed] [Google Scholar]
- 10. Rossignol P, Zannad F. Regional differences in heart failure with preserved ejection fraction trials: when nephrology meets cardiology but east does not meet west. Circulation 2015; 131:7–10. [DOI] [PubMed] [Google Scholar]
- 11. Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O’Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM, Pitt B. Regional variation in patients and outcomes in the treatment of preserved cardiac function heart failure with an aldosterone antagonist (TOPCAT) trial. Circulation 2015; 131:34–42. [DOI] [PubMed] [Google Scholar]
- 12. de Denus S, O’Meara E, Desai AS, Claggett B, Lewis EF, Leclair G, Jutras M, Lavoie J, Solomon SD, Pitt B, Pfeffer MA, Rouleau JL. Spironolactone metabolites in TOPCAT - new insights into regional variation. N Engl J Med 2017; 376:1690–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens 2003; 16:925–930. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.