Abstract
Fine particulate matter <2.5 µm (PM2.5) air pollution is a leading cause of global morbidity and mortality. The largest portion of deaths is now known to be due to cardiovascular disorders. Several air pollutants can trigger acute events (e.g., myocardial infarctions, strokes, heart failure). However, mounting evidence additionally supports that longer-term exposures pose a greater magnified risk to cardiovascular health. One explanation may be that PM2.5 has proven capable of promoting the development of chronic cardiometabolic conditions including atherosclerosis, hypertension, and diabetes mellitus. Here, we provide an updated overview of recent major studies regarding the impact of PM2.5 on cardiometabolic health and outline key remaining scientific questions. We discuss the relevance of emerging trials evaluating personal-level strategies (e.g., facemasks) to prevent the harmful effects of PM2.5, and close with a call for large-scale outcome trials to allow for the promulgation of formal evidence-base recommendations regarding their appropriate usage in the global battle against air pollution.
Keywords: blood pressure, cardiovascular, diabetes mellitus, hypertension, morbidity, pollutants, prevention
Humanity faces many harmful environmental factors endemic to modern civilization including polluted drinking water, excessive noise (e.g., traffic, airports), persistent organic pollutants (e.g., pesticides), household chemicals (e.g., phthalates, bisphenol A), as well as the mounting risks posed by climate change (e.g., extreme temperatures).1 However, the largest threat to public health comes from air pollution which ranks among the leading risk factors for global morbidity and mortality.2–4 Over 90% of the global population is exposed to levels exceeding World Health Organization (WHO) Air Quality Guidelines (AQG).3 This pervasive, persistent, and involuntary nature of exposure explains why air pollution ranks among the leading risk factors for morbidity and mortality worldwide.2–4
AIR POLLUTION
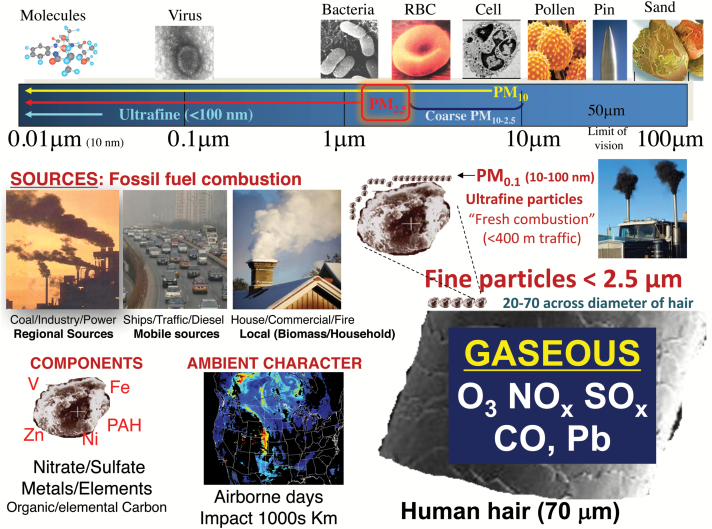
While several anthropogenic (e.g., cooking) and natural (forest fires) sources have been present for millennia, the most concerning air pollutants impacting contemporary society are derived from fossil fuel combustion (e.g., coal, diesel). This pollution is a complex mixture of gases (e.g., ozone, oxides of nitrogen and sulfur), volatile organic compounds, and particulate matter (PM) (Figure 1). This latter component represents a variety of solid particles ranging in size from 10–100 nanometers (ultrafine particles) to large coarse PM between 2.5–10 µm in diameter with concentrations measured in mass (µg) per volume of air (m3). A growing body of studies supports the toxicity of ultrafine particles5 as well as possibly coarse PM. However, the overwhelming burden of evidence impugns fine PM <2.5 µm in diameter (PM2.5) as the principal air pollutant posing the greatest threat to global public health and as such it has remained the focus of most scientific and regulatory attention.2–11 PM2.5 itself is a complex amalgam of compounds (elements, carbon species) derived from numerous sources that are small enough to deposit deep within airways and thereby elicit a host of adverse biological responses.11
Figure 1.
The complex mixture of air pollution. The combustion of from fossil fuels (coal, oil, gas, diesel) from a variety of sources (industry, traffic, power-generation, shipping) produces gaseous and particulate pollutants. Ultrafine particles (UFP) smaller than 100 nm are generally short-lived species (airborne hours) directly derived from and at highest concentrations nearby (<100–400 m) fresh combustion sources (traffic, diesel). Fine particles <2.5 µm in diameter (PM2.5) are larger yet still many times smaller than the diameter of a human hair. They are a heterogenous amalgam of numerous compounds formed from combustion (metals, carbon species) or secondarily involving reactions in the atmosphere (nitrates, sulfates). PM2.5 may be longer-lived (airborne days) and transported hundreds of miles impacting entire regions, all of which is influenced by geography and meteorological conditions. PM concentration is measured in µg/m3, with WHO AQG set at <10 µg/m3 for an annual average. For typical days in US cities, the levels range within 24-hour standards (5–35 µg/m3). In China, peak levels exceed 250–500 µg/m3 which is on par with that of secondhand smoke in indoor venues. However, the exposure dose from the active smoking of a pack of cigarettes is at least 100 times greater. Abbreviations: CO, carbon monoxide; NOx, nitrogen oxides; O3, ozone; PAH, polycyclic aromatic hydrocarbon; SOx, oxides of sulfur.
Global public health effects
The most recent calculations estimate that 3.15 million deaths per year are attributable to PM2.5, which places it among the leading 10 risk factors for global mortality.2–4 This represents 3.1% of all disability-adjusted life years lost, an alarming figure projected to double by 2050. While promoting lung diseases and cancers, more than half of the health burden is in fact due to cardiovascular diseases. Though PM2.5 impacts nearly everyone worldwide, the ecological-economic shifts during the past century have changed who is most vulnerable. PM2.5 now disproportionately concentrates among developing nations, particularly China (1.35 million deaths/year) and India. In addition to the enormous population, 99% of individuals across East Asia face average annual PM2.5 levels (~45.2 µg/m3) exceeding WHO AQG (<10 µg/m3). This is compared to <20% of people in the United States who reside in locations exceeding current AQG. In fact, average levels are now much lower across the United States (9.6 µg/m3) than past decades due in large part from National Ambient Air Quality Standards (NAAQS) beginning in the 1970s.2,12 In addition to widespread and chronic poor air quality, daily peaks of PM2.5 commonly exceed 100 µg/m3 in megacities such as Beijing and Delhi.12–14 During extreme air pollution episodes, PM2.5 can even reach extraordinary concentrations above 500–1000 µg/m3. To place this in perspective, these levels are on par with or exceed indoor secondhand smoke.15 Fortunately, such disastrous episodes have not typically threatened North America and Western Europe since the mid-20th century (e.g., 1952 London “killer smog”).6,7
While high levels of PM2.5 are well-established to prompt cardiovascular events, it is critical to note that the totality of evidence demonstrates that there is no lower concentration threshold below which exposures can be considered safe at the population-level.7,15 Even low PM2.5 levels within annual AQG targets (<10 µg/m3) faced by hundreds of millions of people living in nations with improved air quality pose significant threats to public health.2,11,15 Moreover, other air pollutants (e.g., ozone) can present their own independent risks.2,4 Many comprehensive reviews regarding the health effects of air pollution have been published.7–11 As such, our goals are to provide an updated overview of the most impactful recent studies with an emphasis on the cardiometabolic effects of PM2.5 in humans and to outline key issues we believe important to address during the next decade.
REVIEW OF THE CARDIOMETABOLIC EFFECTS OF AIR POLLUTION
Hard cardiovascular events
A wealth of evidence links air pollution to heightened cardiovascular morbidity and mortality.6–11 Several recent meta-analyses assessing the impact of short-term exposures to PM2.5 (per 10 µg/m3 increase during the prior few hours-to-days) have been published. In 34 studies, PM2.5 exposure significantly increased the risk for acute myocardial infarction by 2.5%.16 Hospitalization or death from heart failure (2.1%; 35 studies),17 stroke (1.1%; 94 studies),18 and arrhythmia (1.5%; 23 studies)19 have also been shown to be increased. Similar risks were also reported for short-term exposures to several gaseous pollutants (NO2, CO, CO2) with less consistent evidence for ozone.16–19 While these relative risks are modest, short-term exposures to PM2.5 account for up to 5% (population-attributable fraction) of myocardial infarctions worldwide because hundreds of millions of people are continuously impacted.20
Longer-term exposures over several years appear to pose amplified risks over and above the acute risks.6 A recent meta-analysis demonstrated that living within an area facing a chronic elevation in PM2.5 leads to a 10.6% increase (per 10 µg/m3) in cardiovascular mortality—roughly 5- to 10-fold the risks following acute exposures.21 This has led to the hypothesis that repetitive exposures may promote chronic disease states (e.g., hypertension, diabetes) and/or enhance the progression or vulnerability of atherosclerotic lesions.7,8,22–24 These responses could magnify health risks by heightening the underlying susceptibility for future events.
Recent epidemiological studies.
Some of the most impactful recently published data derive from the ESCAPE project—a pooling of multiple cohorts across Europe. These results largely confirm the harmful impacts of living in more polluted regions. In 11 studies (n = 100,166), a 5 µg/m3 elevation in long-term exposure to PM2.5 was associated with a 13% increase in nonfatal acute coronary events.25 However, cardiovascular mortality (hazard ratio = 1.21, 95% confidence interval = 0.87–1.69; n = 367,383; 22 cohorts),26 and strokes (hazard ratio = 1.19 95% confidence interval = 0.88–1.62; n = 99,446; 11 cohorts) showed nonsignificant upward trends.27
There has been a growth of important evidence that individuals with underlying coronary heart disease may be at particularly high risk. In the CATHGEN cohort of 5,679 patients who had a coronary angiogram for clinical reasons at Duke University, a 1 µg/m3 increase in annual average PM2.5 was associated with an 11.1% increased risk of having coronary atherosclerosis and a 14.2% increased risk of having a myocardial infarction during the prior year.28 In the Intermountain Healthcare hospital system in Utah (n = 16,314 patients), concurrent-day PM2.5 was associated with a significant increase for acute coronary syndromes.29 However, the excess risk was observed only among individuals with angiographic coronary artery disease and ST-segment elevation myocardial infarctions were preferentially triggered. This suggests that preexisting vulnerable atherosclerotic plaques are required in order to be susceptible to the acute effects of PM2.5 exposure. Accruing evidence also supports that long-term survival following an acute coronary syndrome is also reduced by chronic PM2.5 exposure.30,31 Among 8,873 patients with a myocardial infarction living in a region with good air quality (Ontario), a 10 µg/m3 increase in PM2.5 exposures over the ensuing few months-to-years increased cardiovascular mortality (hazard ratio = 1.35, 95% confidence interval = 1.09–1.67) and death from recurrent myocardial infarction (hazard ratio = 1.64, 95% confidence interval = 1.13–2.40).31
There has been a notable increase in studies evaluating the health effects of extreme high as well as low PM2.5 concentrations. Two Canadian studies have demonstrated that long-term exposures remain capable of promoting cardiovascular events even at very low exposure levels (within annual WHO AQG < 10 µg/m3).32,33 In 2.1 million Canadians,33 ischemic heart disease deaths significantly increased by 30% (per 10 µg/m3) despite average PM2.5 being 8.7 µg/m3. Increased risks were also shown in a study with even lower mean levels (6.3 µg/m3). No lower population threshold of risk was observed.32 Similar findings have been reported in the United States including in rural Iowa and North Carolina,34 as well as in the NIH-AARP35 cohort (n = 517,043) in which long-term exposure increased cardiovascular mortality by 10% (per 10 µg/m3) despite PM2.5 levels averaging 10–13 µg/m3. At the other end of the spectrum, the adverse health effects due to short-term PM2.5 exposures have recently been shown to persist even at very high concentrations.36,37 In a meta-analysis of 59 Chinese studies,36 cardiovascular mortality significantly increased by 0.36% (per 10 µg/m3) even though PM2.5 ranged from 39 to 177 µg/m3. In a time-series study in Beijing (PM2.5 ranged from 3.9 to 494 µg/m3), ischemic heart disease mortality increased by 0.25% (per 10 µg/m3).37 The shape of the dose–response was shown to be supra-linear, which accords with prior analyses.15,38 There was a steeper increase in risk at lower concentrations up to 75–100 µg/m3, with a shallower slope of the dose–response for higher exposures.37 Finally, there have been far fewer cohort studies regarding the long-term health impacts of air pollution at very high levels.39,40 In a recent analysis of 66,820 elderly residents of Hong Kong (annual PM2.5 ~35 µg/m3), mortality due to cardiovascular causes and ischemic heart disease significantly increased by 22 and 42%, respectively (per 10 µg/m3).39 These findings suggest that the adverse health effects due to chronic exposures persist at extreme PM2.5 concentrations. Further cohort studies in this regard are warranted.
Remaining challenges.
Despite substantial increases in knowledge gained during the past few years, several important questions remain. More studies are required to better elucidate the shape of the full dose–response curve because of the tremendous impact it has on the estimated global public health burden. The evidence thus far supports a supra-linear association with no (or a very low) lower threshold and an attenuation of the magnitude of increase in risk at higher levels of exposure.38 Elderly individuals are consistently at greater risk, whereas some studies suggest the same for coronary disease patients, women, those at lower socioeconomic status, and diabetics.7–11,29,41 A better understanding of both susceptible and vulnerable populations is needed, particularly in order to promulgate AQG that optimally protect the public health.41
Numerous short-42,43 and long-term44,45 studies have also attempted to identify the most toxic components (e.g., elements, carbon species) and sources (e.g., coal, traffic) of pollution responsible for eliciting adverse health effects. This is enormously difficult for methodological (e.g., variable measurement availabilities and accuracies), statistical (e.g., high intercorrelations and clustering), and study design reasons (e.g., variations in risks in regards to different health outcomes evaluated). The European ESCAPE project recently provided evidence that particle sulfur (principally derived from coal) may be of particular concern in relation to long-term exposures.44 Results from the US American Cancer Society study (n = 445,860) also support coal as being a harmful source for ischemic heart disease, but they also implicate diesel traffic.45 A growing body of evidence has implicated traffic-related air pollution as one of the most commonly encountered as well as harmful exposures.9,46 However, it remains uncertain if any specific component or source can be consistently incriminated with a high degree of confidence. Given that many pollutants likely pose differing health risks, it remains to be established if a targeted approach to regulatory policies translates into superior outcomes compared to using particle mass alone. In the meantime, the latter continues to serve as a widely available global metric of harmful exposure.
Air pollutants rarely occur in isolation to each other or other environmental exposures (e.g., noise, temperature).1,9 The totality of the ill effects elicited by mixtures of pollutants including particles of various sizes combined with gases (ozone, NO2) has only recently begun to be evaluated.9 As a prominent example, near-roadway environments lead to exposures to noise, particulate, and gaseous traffic-related air pollution, as well as psychological stressors.9,46 The independent and potentially additive (or synergistic) cardiovascular risks posed by these multiple exposures impacting most of the world’s population on a daily basis (e.g., commutes) has yet to be fully understood. Regardless, it is well-known that recent traffic exposure ranks as the single largest triggering event for myocardial infarctions worldwide.20 Many epidemiological studies have shown that residence proximity to major roadways is strongly linked to adverse cardiometabolic health effects.8–11 More work on identifying the most harmful aspects of traffic are warranted.
Chronic cardiometabolic conditions
A growing body of evidence has linked several air pollutants with the chronic development of cardiometabolic disorders.22,23,47–63 PM2.5 inhalation can trigger acute elevations in BP over hours-to-days. However, longer-term exposures can promote the development of hypertension per se.48–53 Living in a region (Ontario) with even low levels of PM2.5 (mean 10.7 µg/m3) led to a 13% increased risk for new onset hypertension (per 10 µg/m3).48 At the other end of the spectrum, extreme exposures near 100 µg/m3 in China can also trigger acute and chronic BP elevations.51,54 Several studies suggest that traffic-related air pollution may pose a particularly high risk.55,56 Not all published observations have been significant; nonetheless, overall positive associations are supported by at least 2 recent meta-analyses and comprehensive reviews of the literature.22,52,53
PM2.5 and other air pollutants can worsen insulin resistance and promote the development of DM.54,57–61 This adverse metabolic effect has been observed in regions with extremely poor as well as overall good air quality.57,58 Not all studies have reported positive findings. However, in-depth reviews62 and recent meta-analyses61 support an increase in risk (by approximately 8–13% per 10 µg/m3). The manifold mechanisms whereby PM2.5 can promote high BP and DM have been reviewed elsewhere. These include a constellation of responses such as activation of the sympathetic nervous system, endothelial dysfunction, systemic and tissue (e.g., adipocyte) inflammation and oxidative stress, altered adipocytokine expression, hypothalamic activation, impaired renal function, obesity and weight gain, and perhaps by direct actions of inhaled nanoparticulates.22.23,62–64
Remaining challenges.
It has been speculated that acute air pollution-mediated increase in BP may play a mechanistic role in the triggering of cardiovascular events (e.g., strokes, heart failure).22 Indeed, excess hypertension and DM-related mortality have been shown to occur among individuals living in more polluted locations.65,66 However, the extent to which the development of chronic cardiometabolic conditions explain the magnified risks for cardiovascular events posed by long-term exposure remains to be fully elucidated. The degree to which air pollution and environmental exposures (e.g. traffic) are contributing to the global epidemic of the cardiometabolic syndrome also awaits further clarification. Finally, other chronic illnesses related to the cardiometabolic syndrome including chronic kidney disease,63 obesity,64 sleep-related breathing disorders,67 pregnancy-related hypertension68 and diabetes,69 and neurological diseases (e.g., dementia, depression)70,71 may also be linked to air pollution and require more investigation.
Intermediate endpoints
The demonstration that air pollutants can adversely impact surrogate markers of cardiovascular risk informs on putative mechanistic pathways and provides corroboratory evidence supporting the plausibility of the epidemiological observations.7 PM2.5 has been linked to biomarkers of atherosclerosis including carotid intima-media thickness, carotid plaques, coronary artery calcium, and aortic calcification.7–11,24 Other studies show adverse vascular changes including increased arterial stiffness, impaired conduit artery flow-mediated dilatation, resistance arteriolar dysfunction, and retinal artery changes.7–11 Metabolic derangements including impaired insulin sensitivity have also been demonstrated.54,59,62
Recent studies.
There have been several publications within the past few years linking long-term air pollution and traffic exposure with an increase in biomarkers of atherosclerosis.72–75 Perhaps the largest study (n = 6,795 across 6 US regions) with the longest follow-up (mean 10 years) comes from the MESA-Air cohort.72 Each 5 µg/m3 increase in long-term PM2.5 exposure was associated with a greater progression of coronary artery calcium (4.1 Agatston units/year). PM2.5 was not associated with intima-media thickness progression in this study.72 However, a recent meta-analysis of 8 cross-sectional (n = 18,349) and 3 prospective (n = 7,268) studies showed significant associations with greater intima-media thickness.75 The overall evidence, therefore, supports that PM2.5 likely promotes an increase in intima-media thickness and coronary artery calcium, validated markers of atherosclerosis and heightened cardiovascular risk.
Earlier studies linked short-term air pollution exposures with acute endothelial dysfunction.7–11 More recently, both MESA-Air and the Framingham cohort have further demonstrated that long-term exposures to ambient levels of PM2.5 are linked to chronic reductions in brachial flow-mediated dilatation. This supports that air pollution causes vascular endothelial dysfunction, the hallmark physiological change underlying the initiation and progression of atherosclerosis and an established surrogate marker of cardiovascular risk.76,77 Traffic-related air pollution and PM2.5 have both also been linked to left and right ventricular structural changes in MESA-Air and the Jackson Heart Study.78–81 Finally, a few studies have begun to show associations between adverse cerebral changes linked to dementia (e.g., white matter disease, reduced volume, covert infarcts).82–85
Remaining challenges.
While several air pollutants have been associated with adverse changes in intermediate markers of cardiovascular damage/risk, the extent to which these alterations explain the “hard” epidemiological findings remains unknown. It is not clear to what extent these endpoints represent true “mediating” pathways of disease versus simple markers of heightened risk. It is also critical to identify the optimal endpoints to investigate that represent valid “surrogate outcomes” in interventional trials of personal-level exposure reduction.
Mechanistic evidence
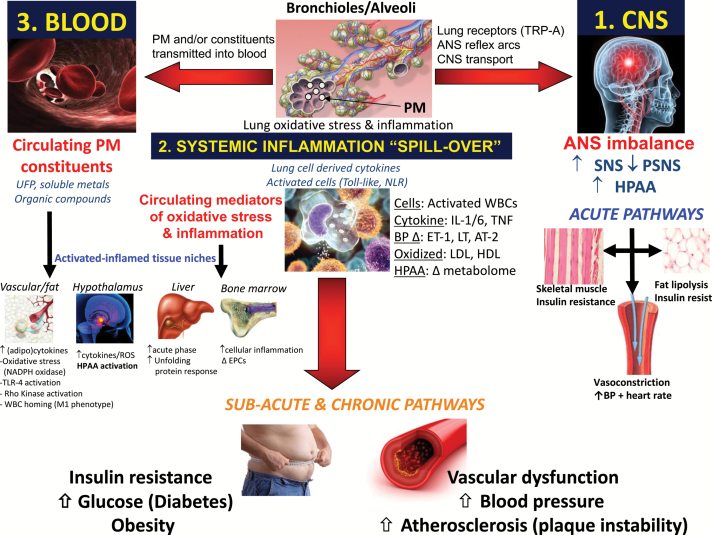
PM2.5 inhalation can trigger acute events and promote chronic cardiometabolic conditions by 3 broad mediating pathways (Figure 2). Depending upon the dose and time course of exposure, as well as the individual level of susceptibility, each pathway alone or altogether can elicit a host of adverse responses. These include vascular dysfunction (e.g., vasoconstriction, impaired vasodilatation); altered hemodynamics (e.g., increased BP and arterial stiffness); augmented thrombosis (e.g., activated platelets, adhesion molecule, and coagulation factor expression); heightened arrhythmia potential (e.g., autonomic imbalance, cardiac repolarization abnormalities); as well as numerous proatherosclerotic changes (e.g., oxidized lipids, plaque progression, and inflammation/instability). A full update on the wealth of recent basic science, toxicological, and animal experiments is beyond the scope of this review. Details in this regard can be found elsewhere.10,22–24,62,86–88 However, noteworthy human studies providing important novel mechanistic insights include the demonstration that PM2.5 can induce high-density lipoprotein dysfunction,89 trigger several epigenetic changes,90 and adversely alter circulating endothelial-platelet microparticles, growth factors (e.g., VEGF), and endothelial progenitor cell levels.91,92 Finally, a recent experiment using gold nanoparticles has provided some of the most compelling evidence supporting the potential biological relevance of mediating pathway 3 (Figure 2). The investigators demonstrated that a fraction of particles <30 nm in size were capable of reaching the systemic circulation and vascular issues following inhalation.93 Further studies in this regard are warranted.
Figure 2.
Biological pathways whereby PM2.5 promotes cardiovascular events. Inhaled PM2.5 deposit deep within pulmonary tissues (i.e., alveoli) and interact/activate local cells (e.g., resident macrophages, dendritic cells, alveolar/endothelial cells) and modify endogenous structures (e.g., cell membranes, surfactant lipids, antioxidants). Mediators of oxidative stress (e.g., free radicals) directly generated by particulate compounds (metals, organic species) or secondarily produced (e.g., modified phospholipids) by activated cellular enzyme systems (e.g., NADPH oxidase) can instigate a local inflammatory response. Several innate immune pathways may be important in coordinating this response such as via activated toll-like receptors (TLR). In pathway 1, the inhaled particles and/or oxidative stress impact a variety of afferent nerves (e.g., transient receptor potential receptors [TRP]) and rapidly alter central nervous system (CNS) autonomic nervous system (ANS) balance typically favoring sympathetic (SNS) activity over parasympathetic (PSNS) nervous system activity. In pathway 2, numerous mediators of inflammation and oxidative stress generated in the lungs (e.g., cytokines, activated immune cells, oxidized lipids) “spill-over” into the systemic circulation and thereafter carry this danger signal to remote cardiovascular tissues. In hypothetical pathway 3, nanoparticles (10–30 nm) or particulate components penetrate lung barriers and are carried within immune cells or lipoproteins or directly reach the systemic circulation. Harmful chemicals (organic carbon species, metals) deposit into cardiovascular tissues and elicit inflammatory-oxidative stress responses without the need for the signal to be indirectly transmitted via pathway 2. Abbreviations: BP, blood pressure; HPAA, hypothalamic pituitary adrenal axis; PM, particulate matter; UFP, ultrafine particles.
PERSONAL-LEVEL PREVENTIVE STRATEGIES
Overall air quality has dramatically improved across the United States during the past few decades due in large part from clean air regulations. Most counties are now in compliance with the latest NAAQS. The reduction in long-term levels of PM2.5 has translated into clear benefits to public health and significantly contributed to the increase in overall life expectancy since the 1980s.13 Unfortunately, air quality has dramatically worsened throughout much of the developing world during this same period.2,12,14 Hundreds of millions of individuals living across East and South Asia (e.g., China, India) face extraordinarily high air pollution levels far exceeding WHO AQG on a daily basis.2 It appears likely that substantial improvements in air quality remain decades off in the future in these regions due to population growth, increasing energy and transportation demands, and numerous geopolitical-economic factors.14 In the meantime, unless some actions are taken to protect the health of millions of at-risk individuals, the burden of air pollution-related cardiometabolic diseases (already a leading risk factor for morbidity and mortality across Asia)2 will only further increase.
Several “personal-level” strategies to reduce exposures and prevent the harmful effects of air pollution have been proposed (Table 1). We recently reviewed the effectiveness of these approaches from the growing number of small trials.13 A few important studies in the most germane location, heavily polluted China, have thereafter been published. The use of air purifiers with HEPA filters reduced indoor PM2.5 by 57% (96 to 41 µg/m3) among 35 college students in Shanghai. This led to significant improvements in several biomarkers of cardiometabolic health (MCP-1, IL-1, MPO, CD40L) and BP within 48 hours.94 Wearing N95 respirators facemasks in the same cohort was also protective, as BP and heart rate variability were improved within 48 hours.95 We and others are undertaking similar trials in lower polluted cities in the United States. Preliminary results from our recently completed RAPIDS trial in Detroit Michigan (ambient PM2.5 levels ranging from 10–20 µg/m3) showed that air purifiers with HEPA filters can reduce time-averaged personal-level exposures by 30–50%. Decreases in BP were observed within 1–3 days among elderly residents living in a senior residence facility (unpublished data).
Table 1.
Personal and local-level interventions to reduce exposures or susceptibility to air pollution
| Intervention type | Examples |
|---|---|
| Air purifiers | Portable indoor HEPA filter systems in living spaces and bedrooms HEPA designated filters remove PM at 0.3 microns in diameter by 99.97% |
| Facemasks | N95 respirators, surgical facemasks worn in heavily polluted regions N95 designated respirators remove at least 95% of PM at 0.3 microns |
| Reduce in-traffic exposures | In cabin HEPA filters, closing car windows, recirculating in-cabin air |
| Reduce traffic emissions | Diesel particle traps, catalytic converters, alternative fuels (natural gas, electric cars) |
| Reduce in-home penetrance | Closing windows, central air conditioning filtration, improved home efficiency |
| Cleaner cook-stoves | Cleaner-burning liquid fuel stoves, improved stove emission ventilation |
| Lifestyle changes | Avoiding point sources (roadways), exercising indoors during peak exposure times (rush hour). Awareness of health risks of travel to heavily polluted regions |
| Medications and diet | Fish oil, antioxidants, maintaining healthy diet (vegetables), evidence-based medications (statins) |
| Municipality and city changes | Green-spaces, natural (trees) barriers from roadways, improved urban design (bike paths >400 m from roadways) |
A call for clinical outcome trials
Both the American Heart Association and the European Society of Cardiology formally recognized PM2.5 as an independent risk factor for cardiovascular diseases.7,8 While prudent precautionary recommendations were provided on how to reduce exposures, it was explicitly acknowledged that no “evidence-based” guidance could be officially promulgated. PM2.5 poses an equal or greater risk to global cardiometabolic public health than most traditional risk factors (high cholesterol or blood sugar).4 Nevertheless, there has not been a single randomized outcome trial that has tested the efficacy of any preventive strategy to reduce air pollution-induced cardiovascular events. We recently reviewed the importance as well as the putative design and plausibility of clinical trials in this regard.96 Given the evidence supporting improvements in biomarkers of cardiovascular health in studies of facemasks and air purifiers,13 and the high risk for recurrent cardiovascular events among individuals with established heart disease due to long-term PM2.5 exposures, we proposed that clinical outcome trials in this population of patients are both important and feasible.96 Intervention trials would be of greatest relevance and effectiveness in heavily polluted countries where a 50% reduction in inhaled PM2.5 afforded by N95 respirators and indoor air purifiers would likely translate in substantial decreases in time-averaged exposures over months-to-years (e.g., 30–50 µg/m3). Despite the complete lack of evidence supporting or refuting their benefits on hard outcomes, many low-efficiency facemasks are already widely used across China. Here, we make another emphatic call to the scientific-medical community to design and launch large-scale randomized clinical outcome trials to test the health benefits of validated (e.g., N95 respirators, air purifiers with HEPA filters) personal-level preventive strategies among high-risk patients (e.g., acute coronary syndrome survivors). We can think of no other research effort to be undertaken during the next decade that could provide more important data. Positive results would provide a critical piece of much-needed scientific evidence that further supports a “causal role” for PM2.5 in the etiology of cardiovascular disease. Finally, the findings would be of immediate real-world relevance. They could be rapidly translated into action by widespread dissemination and evidence-based usage of simple inexpensive approaches to reduce PM2.5 exposures and thereby positively impact the health and welfare of millions of individuals worldwide.96
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGEMENTS
Author support from grants from the National Institutes of Health (R01-ES015146) (RDB, SR) and the University of Michigan-Peking University Joint Institute for Translational and Clinical Research (RDB).
References
- 1. Brook RD, Weder AB, Rajagopalan S. “Environmental hypertensionology” the effects of environmental factors on blood pressure in clinical practice and research. J Clin Hypertens (Greenwich) 2011; 13:836–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lelieveld J, Evans JS, Fnais M, Giannadaki D, Pozzer A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015; 525:367–371. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization, Health Topics: Air Pollution. <http://www.who.int/topics/air_pollution/en/>. Accessed 20 February 17.
- 4. GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388:1659–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baldauf RW, Devlin RB, Gehr P, Giannelli R, Hassett-Sipple B, Jung H, Martini G, McDonald J, Sacks JD, Walker K. Ultrafine particle metrics and research considerations: review of the 2015 UFP Workshop. Int J Environ Res Public Health 2016; 13:E1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC Jr, Tager I; Expert Panel on Population and Prevention Science of the American Heart Association . Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 2004; 109:2655–2671. [DOI] [PubMed] [Google Scholar]
- 7. Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC Jr, Whitsel L, Kaufman JD; American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism . Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 2010; 121:2331–2378. [DOI] [PubMed] [Google Scholar]
- 8. Newby DE, Mannucci PM, Tell GS, Baccarelli AA, Brook RD, Donaldson K, Forastiere F, Franchini M, Franco OH, Graham I, Hoek G, Hoffmann B, Hoylaerts MF, Künzli N, Mills N, Pekkanen J, Peters A, Piepoli MF, Rajagopalan S, Storey RF; ESC Working Group on Thrombosis, European Association for Cardiovascular Prevention and Rehabilitation; ESC Heart Failure Association . Expert position paper on air pollution and cardiovascular disease. Eur Heart J 2015; 36:83–93b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Münzel T, Sørensen M, Gori T, Schmidt F, Rao X, Brook J, Chen LC, Brook RD, Rajagopalan S. Environmental stressors and cardio-metabolic disease: part 1: epidemiologic evidence supporting a role for noise and air pollution and effects of mitigation strategies. Eur Heart J 2017; 38:550–556. [DOI] [PubMed] [Google Scholar]
- 10. Münzel T, Sørensen M, Gori T, Schmidt F, Rao X, Brook J, Chen LC, Brook RD, Rajagopalan S. Environmental stressors and cardio-metabolic disease: part II- mechanistic insights. Eur Heart J 2017; 38:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Franklin BA, Brook R, Arden Pope C 3rd. Air pollution and cardiovascular disease. Curr Probl Cardiol 2015; 40:207–238. [DOI] [PubMed] [Google Scholar]
- 12. van Donkelaar A, Martin RV, Brauer M, Boys BL. Use of satellite observations for long-term exposure assessment of global concentrations of fine particulate matter. Environ Health Perspect 2015; 123:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morishita M, Thompson KC, Brook RD. Understanding air pollution and cardiovascular diseases: is it preventable? Curr Cardiovasc Risk Reports 2015; 9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang JJ, Samet JM. Chinese haze versus Western smog: lessons learned. J Thorac Dis 2015; 7:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pope CA III, Brook RD, Burnett RT, Dockery DW. How is cardiovascular disease mortality risk affected by duration and intensity of fine particulate matter exposure? An integration of the epidemiologic evidence. Air Qual Atmos Health 2011; 4:5–14. [Google Scholar]
- 16. Mustafic H, Jabre P, Caussin C, Murad MH, Escolano S, Tafflet M, Périer MC, Marijon E, Vernerey D, Empana JP, Jouven X. Main air pollutants and myocardial infarction: a systematic review and meta-analysis. JAMA 2012; 307:713–721. [DOI] [PubMed] [Google Scholar]
- 17. Shah AS, Langrish JP, Nair H, McAllister DA, Hunter AL, Donaldson K, Newby DE, Mills NL. Global association of air pollution and heart failure: a systematic review and meta-analysis. Lancet 2013; 382:1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shah AS, Lee KK, McAllister DA, Hunter A, Nair H, Whiteley W, Langrish JP, Newby DE, Mills NL. Short term exposure to air pollution and stroke: systematic review and meta-analysis. BMJ 2015; 350:h1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Song X, Liu Y, Hu Y, Zhao X, Tian J, Ding G, Wang S. Short-term exposure to air pollution and cardiac arrhythmia: a meta-analysis and systematic review. Int J Environ Res Public Health 2016; 13:642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nawrot TS, Perez L, Künzli N, Munters E, Nemery B. Public health importance of triggers of myocardial infarction: a comparative risk assessment. Lancet 2011; 377:732–740. [DOI] [PubMed] [Google Scholar]
- 21. Hoek G, Krishnan RM, Beelen R, Peters A, Ostro B, Brunekreef B, Kaufman JD. Long-term air pollution exposure and cardio- respiratory mortality: a review. Environ Health 2013; 12:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giorgini P, Di Giosia P, Grassi D, Rubenfire M, Brook RD, Ferri C. Air pollution exposure and blood pressure: an updated review of the literature. Curr Pharm Design 2015: 22; 28–51. [DOI] [PubMed] [Google Scholar]
- 23. Rajagopalan S, Brook RD. Air pollution and type 2 diabetes: mechanistic insights. Diabetes 2012; 61:3037–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brook RD, Rajagopalan S. Particulate matter air pollution and atherosclerosis. Curr Atheroscler Rep 2010; 12:291–300. [DOI] [PubMed] [Google Scholar]
- 25. Cesaroni G, Forastiere F, Stafoggia M, Andersen ZJ, Badaloni C, Beelen R, Caracciolo B, de Faire U, Erbel R, Eriksen KT, Fratiglioni L, Galassi C, Hampel R, Heier M, Hennig F, Hilding A, Hoffmann B, Houthuijs D, Jöckel KH, Korek M, Lanki T, Leander K, Magnusson PK, Migliore E, Ostenson CG, Overvad K, Pedersen NL, J JP, Penell J, Pershagen G, Pyko A, Raaschou-Nielsen O, Ranzi A, Ricceri F, Sacerdote C, Salomaa V, Swart W, Turunen AW, Vineis P, Weinmayr G, Wolf K, de Hoogh K, Hoek G, Brunekreef B, Peters A. Long term exposure to ambient air pollution and incidence of acute coronary events: prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project. BMJ 2014; 348:f7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beelen R, Stafoggia M, Raaschou-Nielsen O, Andersen ZJ, Xun WW, Katsouyanni K, Dimakopoulou K, Brunekreef B, Weinmayr G, Hoffmann B, Wolf K, Samoli E, Houthuijs D, Nieuwenhuijsen M, Oudin A, Forsberg B, Olsson D, Salomaa V, Lanki T, Yli-Tuomi T, Oftedal B, Aamodt G, Nafstad P, De Faire U, Pedersen NL, Östenson CG, Fratiglioni L, Penell J, Korek M, Pyko A, Eriksen KT, Tjønneland A, Becker T, Eeftens M, Bots M, Meliefste K, Wang M, Bueno-de-Mesquita B, Sugiri D, Krämer U, Heinrich J, de Hoogh K, Key T, Peters A, Cyrys J, Concin H, Nagel G, Ineichen A, Schaffner E, Probst-Hensch N, Dratva J, Ducret-Stich R, Vilier A, Clavel-Chapelon F, Stempfelet M, Grioni S, Krogh V, Tsai MY, Marcon A, Ricceri F, Sacerdote C, Galassi C, Migliore E, Ranzi A, Cesaroni G, Badaloni C, Forastiere F, Tamayo I, Amiano P, Dorronsoro M, Katsoulis M, Trichopoulou A, Vineis P, Hoek G. Long-term exposure to air pollution and cardiovascular mortality: an analysis of 22 European cohorts. Epidemiology 2014; 25:368–378. [DOI] [PubMed] [Google Scholar]
- 27. Stafoggia M, Cesaroni G, Peters A, Andersen ZJ, Badaloni C, Beelen R, Caracciolo B, Cyrys J, de Faire U, de Hoogh K, Eriksen KT, Fratiglioni L, Galassi C, Gigante B, Havulinna AS, Hennig F, Hilding A, Hoek G, Hoffmann B, Houthuijs D, Korek M, Lanki T, Leander K, Magnusson PK, Meisinger C, Migliore E, Overvad K, Ostenson CG, Pedersen NL, Pekkanen J, Penell J, Pershagen G, Pundt N, Pyko A, Raaschou-Nielsen O, Ranzi A, Ricceri F, Sacerdote C, Swart WJ, Turunen AW, Vineis P, Weimar C, Weinmayr G, Wolf K, Brunekreef B, Forastiere F. Long-term exposure to ambient air pollution and incidence of cerebrovascular events: results from 11 European cohorts within the ESCAPE project. Environ Health Perspect 2014; 122:919–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McGuinn LA, Ward-Caviness CK, Neas LM, Schneider A, Diaz-Sanchez D, Cascio WE, Kraus WE, Hauser E, Dowdy E, Haynes C, Chudnovsky A, Koutrakis P, Devlin RB. Association between satellite-based estimates of long-term PM2.5 exposure and coronary artery disease. Environ Res 2016; 145:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pope CA III, Mulestein JB, Anderson JL, Cannon JB, Hales NM, Meredith KG, Le V, Horne BD. Short-term exposure to fine particulate matter air pollution is preferentially associated with the risk for ST-segment elevation acute coronary events. J Am Heart Assoc 2015; 5: e002506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tonne C, Wilkinson P. Long-term exposure to air pollution is associated with survival following acute coronary syndrome. Eur Heart J 2013; 34:1306–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen H, Burnett RT, Copes R, Kwong JC, Villeneuve PJ, Goldberg MS, Brook RD, van Donkelaar A, Jerrett M, Martin RV, Brook JR, Kopp A, Tu JV. Ambient fine particulate matter and mortality among survivors of myocardial infarction: population-based cohort study. Environ Health Perspect 2016; 124:1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pinault L, Tjepkema M, Crouse DL, Weichenthal S, van Donkelaar A, Martin RV, Brauer M, Chen H, Burnett RT. Risk estimates of mortality attributed to low concentrations of ambient fine particulate matter in the Canadian community health survey cohort. Environ Health 2016; 15:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Crouse DL, Peters PA, van Donkelaar A, Goldberg MS, Villeneuve PJ, Brion O, Khan S, Atari DO, Jerrett M, Pope CA, Brauer M, Brook JR, Martin RV, Stieb D, Burnett RT. Risk of nonaccidental and cardiovascular mortality in relation to long-term exposure to low concentrations of fine particulate matter: a Canadian national-level cohort study. Environ Health Perspect 2012; 120:708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weichenthal S, Villeneuve PJ, Burnett RT, van Donkelaar A, Martin RV, Jones RR, DellaValle CT, Sandler DP, Ward MH, Hoppin JA. Long-term exposure to fine particulate matter: association with nonaccidental and cardiovascular mortality in the agricultural health study cohort. Environ Health Perspect 2014; 122:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thurston GD, Ahn J, Cromar KR, Shao Y, Reynolds HR, Jerrett M, Lim CC, Shanley R, Park Y, Hayes RB. Ambient particulate matter air pollution exposure and mortality in the NIH-AARP Diet and Health Cohort. Environ Health Perspect 2016; 124:484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lu F, Xu D, Cheng Y, Dong S, Guo C, Jiang X, Zheng X. Systematic review and meta-analysis of the adverse health effects of ambient PM2.5 and PM10 pollution in the Chinese population. Environ Res 2015; 136:196–204. [DOI] [PubMed] [Google Scholar]
- 37. Xie W, Li G, Zhao D, Xie X, Wei Z, Wang W, Wang M, Li G, Liu W, Sun J, Jia Z, Zhang Q, Liu J. Relationship between fine particulate air pollution and ischaemic heart disease morbidity and mortality. Heart 2015; 101:257–263. [DOI] [PubMed] [Google Scholar]
- 38. Burnett RT, Pope CA 3rd, Ezzati M, Olives C, Lim SS, Mehta S, Shin HH, Singh G, Hubbell B, Brauer M, Anderson HR, Smith KR, Balmes JR, Bruce NG, Kan H, Laden F, Prüss-Ustün A, Turner MC, Gapstur SM, Diver WR, Cohen A. An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure. Environ Health Perspect 2014; 122:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wong CM, Lai HK, Tsang H, Thach TQ, Thomas GN, Lam KB, Chan KP, Yang L, Lau AK, Ayres JG, Lee SY, Chan WM, Hedley AJ, Lam TH. Satellite-based estimates of long-term exposure to fine particles and association with mortality in elderly Hong Kong residents. Environ Health Perspect 2015; 123:1167–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang LW, Chen X, Xue XD, Sun M, Han B, Li CP, Ma J, Yu H, Sun ZR, Zhao LJ, Zhao BX, Liu YM, Chen J, Wang PP, Bai ZP, Tang NJ. Long-term exposure to high particulate matter pollution and cardiovascular mortality: a 12-year cohort study in four cities in northern China. Environ Int 2014; 62:41–47. [DOI] [PubMed] [Google Scholar]
- 41. Bell ML, Zanobetti A, Dominici F. Evidence on vulnerability and susceptibility to health risks associated with short-term exposure to particulate matter: a systematic review and meta-analysis. Am J Epidemiol 2013; 178:865–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bell ML, Ebisu K, Leaderer BP, Gent JF, Lee HJ, Koutrakis P, Wang Y, Dominici F, Peng RD. Associations of PM₂.₅ constituents and sources with hospital admissions: analysis of four counties in Connecticut and Massachusetts (USA) for persons ≥ 65 years of age. Environ Health Perspect 2014; 122:138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dai L, Zanobetti A, Koutrakis P, Schwartz JD. Associations of fine particulate matter species with mortality in the United States: a multicity time-series analysis. Environ Health Perspect 2014; 122:837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Beelen R, Hoek G, Raaschou-Nielsen O, Stafoggia M, Andersen ZJ, Weinmayr G, Hoffmann B, Wolf K, Samoli E, Fischer PH, Nieuwenhuijsen MJ, Xun WW, Katsouyanni K, Dimakopoulou K, Marcon A, Vartiainen E, Lanki T, Yli-Tuomi T, Oftedal B, Schwarze PE, Nafstad P, De Faire U, Pedersen NL, Östenson CG, Fratiglioni L, Penell J, Korek M, Pershagen G, Eriksen KT, Overvad K, Sørensen M, Eeftens M, Peeters PH, Meliefste K, Wang M, Bueno-de-Mesquita HB, Sugiri D, Krämer U, Heinrich J, de Hoogh K, Key T, Peters A, Hampel R, Concin H, Nagel G, Jaensch A, Ineichen A, Tsai MY, Schaffner E, Probst-Hensch NM, Schindler C, Ragettli MS, Vilier A, Clavel-Chapelon F, Declercq C, Ricceri F, Sacerdote C, Galassi C, Migliore E, Ranzi A, Cesaroni G, Badaloni C, Forastiere F, Katsoulis M, Trichopoulou A, Keuken M, Jedynska A, Kooter IM, Kukkonen J, Sokhi RS, Vineis P, Brunekreef B. Natural-cause mortality and long-term exposure to particle components: an analysis of 19 European cohorts within the multi-center ESCAPE project. Environ Health Perspect 2015; 123:525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thurston GD, Burnett RT, Turner MC, Shi Y, Krewski D, Lall R, Ito K, Jerrett M, Gapstur SM, Diver WR, Pope CA. Ischemic heart disease mortality and long-term exposure to source-related components of U.S. fine particle air pollution. Environ Health Perspect 2016; 124:785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. HEI Panel on the Health Effects of Traffic-Related Air Pollution. Traffic-related air pollution: a critical review of the literature on emissions, exposure, and health effects. HEI Special Report 17. Boston, MA: Health Effects Institute; 2010. [Google Scholar]
- 47. Eze IC, Schaffner E, Foraster M, Imboden M, von Eckardstein A, Gerbase MW, Rothe T, Rochat T, Künzli N, Schindler C, Probst-Hensch N. Long-term exposure to ambient air pollution and metabolic syndrome in adults. PLoS One 2015; 10:e0130337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen H, Burnett RT, Kwong JC, Villeneuve PJ, Goldberg MS, Brook RD, van Donkelaar A, Jerrett M, Martin RV, Kopp A, Brook JR, Copes R. Spatial association between ambient fine particulate matter and incident hypertension. Circulation 2013; 129:562–569. [DOI] [PubMed] [Google Scholar]
- 49. Zhang Z, Laden F, Forman JP, Hart JE. Long-term exposure to particulate matter and self-reported hypertension: a prospective analysis in the Nurses’ Health Study. Environ Health Perspect 2016; 124:1414–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chan SH, Van Hee VC, Bergen S, Szpiro AA, DeRoo LA, London SJ, Marshall JD, Kaufman JD, Sandler DP. Long-term air pollution exposure and blood pressure in the Sister Study. Environ Health Perspect 2015; 123:951–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dong GH, Qian ZM, Xaverius PK, Trevathan E, Maalouf S, Parker J, Yang L, Liu MM, Wang D, Ren WH, Ma W, Wang J, Zelicoff A, Fu Q, Simckes M. Association between long-term air pollution and increased blood pressure and hypertension in China. Hypertension 2013; 61:578–584. [DOI] [PubMed] [Google Scholar]
- 52. Liang R, Zhang B, Zhao X, Ruan Y, Lian H, Fan Z. Effect of exposure to PM2.5 on blood pressure: a systematic review and meta-analysis. J Hypertens 2014; 32:2130–2141. [DOI] [PubMed] [Google Scholar]
- 53. Cai Y, Zhang B, Ke W, Feng B, Lin H, Xiao J, Zeng W, Li X, Tao J, Yang Z, Ma W, Liu T. Associations of short-term and long-term exposure to ambient air pollutants with hypertension: a systematic review and meta-analysis. Hypertension 2016; 68:62–70. [DOI] [PubMed] [Google Scholar]
- 54. Brook RD, Sun Z, Brook JR, Zhao X, Ruan Y, Yan J, Mukherjee B, Rao X, Duan F, Sun L, Liang R, Lian H, Zhang S, Fang Q, Gu D, Sun Q, Fan Z, Rajagopalan S. Extreme air pollution conditions adversely affect blood pressure and insulin resistance: the Air Pollution and Cardiometabolic Disease Study. Hypertension 2016; 67:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fuks KB, Weinmayr G, Foraster M, Dratva J, Hampel R, Houthuijs D, Oftedal B, Oudin A, Panasevich S, Penell J, Sommar JN, Sørensen M, Tiittanen P, Wolf K, Xun WW, Aguilera I, Basagaña X, Beelen R, Bots ML, Brunekreef B, Bueno-de-Mesquita HB, Caracciolo B, Cirach M, de Faire U, de Nazelle A, Eeftens M, Elosua R, Erbel R, Forsberg B, Fratiglioni L, Gaspoz JM, Hilding A, Jula A, Korek M, Krämer U, Künzli N, Lanki T, Leander K, Magnusson PK, Marrugat J, Nieuwenhuijsen MJ, Ostenson CG, Pedersen NL, Pershagen G, Phuleria HC, Probst-Hensch NM, Raaschou-Nielsen O, Schaffner E, Schikowski T, Schindler C, Schwarze PE, Søgaard AJ, Sugiri D, Swart WJ, Tsai MY, Turunen AW, Vineis P, Peters A, Hoffmann B. Arterial blood pressure and long-term exposure to traffic-related air pollution: an analysis in the European Study of Cohorts for Air Pollution Effects (ESCAPE). Environ Health Perspect 2014; 122:896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kingsley SL, Eliot MN, Whitsel EA, Wang Y, Coull BA, Hou L, Margolis HG, Margolis KL, Mu L, Wu WC, Johnson KC, Allison MA, Manson JE, Eaton CB, Wellenius GA. Residential proximity to major roadways and incident hypertension in post-menopausal women. Environ Res 2015; 142:522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen H, Burnett RT, Kwong JC, Villeneuve PJ, Goldberg MS, Brook RD, van Donkelaar A, Jerrett M, Martin RV, Brook JR, Copes R. Risk of incident diabetes in relation to long-term exposure to fine particulate matter in Ontario, Canada. Environ Health Perspect 2013; 121:804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu C, Yang C, Zhao Y, Ma Z, Bi J, Liu Y, Meng X, Wang Y, Cai J, Kan H, Chen R. Associations between long-term exposure to ambient particulate air pollution and type 2 diabetes prevalence, blood glucose and glycosylated hemoglobin levels in China. Environ Int 2016; 92-93:416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen Z, Salam MT, Toledo-Corral C, Watanabe RM, Xiang AH, Buchanan TA, Habre R, Bastain TM, Lurmann F, Wilson JP, Trigo E, Gilliland FD. Ambient air pollutants have adverse effects on insulin and glucose homeostasis in Mexican Americans. Diabetes Care 2016; 39:547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hansen AB, Ravnskjær L, Loft S, Andersen KK, Bräuner EV, Baastrup R, Yao C, Ketzel M, Becker T, Brandt J, Hertel O, Andersen ZJ. Long-term exposure to fine particulate matter and incidence of diabetes in the Danish Nurse Cohort. Environ Int 2016; 91:243–250. [DOI] [PubMed] [Google Scholar]
- 61. Eze IC, Hemkens LG, Bucher HC, Hoffmann B, Schindler C, Künzli N, Schikowski T, Probst-Hensch NM. Association between ambient air pollution and diabetes mellitus in Europe and North America: systematic review and meta-analysis. Environ Health Perspect 2015; 123:381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rao X, Montresor-Lopez J, Puett R, Rajagopalan S, Brook RD. Ambient air pollution: an emerging risk factor for diabetes mellitus. Curr Diab Rep 2015; 15:603. [DOI] [PubMed] [Google Scholar]
- 63. Mehta AJ, Zanobetti A, Bind MA, Kloog I, Koutrakis P, Sparrow D, Vokonas PS, Schwartz JD. Long-term exposure to ambient fine particulate matter and renal function in older men: the Veterans Administration Normative Aging Study. Environ Health Perspect 2016; 124:1353–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Li W, Dorans KS, Wilker EH, Rice MB, Schwartz J, Coull BA, Koutrakis P, Gold DR, Fox CS, Mittleman MA. Residential proximity to major roadways, fine particulate matter, and adiposity: the Framingham Heart Study. Obesity (Silver Spring) 2016; 24:2593–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Brook RD, Cakmak S, Turner MC, Brook JR, Crouse DL, Peters PA, van Donkelaar A, Villeneuve PJ, Brion O, Jerrett M, Martin RV, Rajagopalan S, Goldberg MS, Pope CA 3rd, Burnett RT. Long-term fine particulate matter exposure and mortality from diabetes in Canada. Diabetes Care 2013; 36:3313–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pope CA 3rd, Turner MC, Burnett RT, Jerrett M, Gapstur SM, Diver WR, Krewski D, Brook RD. Relationships between fine particulate matter air pollution, cardiometabolic disorders, and cardiovascular mortality. Circ Res 2015; 116:108–115. [DOI] [PubMed] [Google Scholar]
- 67. Zanobetti A, Redline S, Schwartz J, Rosen D, Patel S, O’Connor GT, Lebowitz M, Coull BA, Gold DR. Associations of PM10 with sleep and sleep-disordered breathing in adults from seven U.S. urban areas. Am J Respir Crit Care Med 2010; 182:819–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pedersen M, Stayner L, Slama R, Sørensen M, Figueras F, Nieuwenhuijsen MJ, Raaschou-Nielsen O, Dadvand P. Ambient air pollution and pregnancy-induced hypertensive disorders: a systematic review and meta-analysis. Hypertension 2014; 64:494–500. [DOI] [PubMed] [Google Scholar]
- 69. Hu H, Ha S, Henderson BH, Warner TD, Roth J, Kan H, Xu X. Association of atmospheric particulate matter and ozone with gestational diabetes mellitus. Environ Health Perspect 2015; 123:853–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chen H, Kwong JC, Copes R, Tu K, Villeneuve PJ, van Donkelaar A, Hystad P, Martin RV, Murray BJ, Jessiman B, Wilton AS, Kopp A, Burnett RT. Living near major roads and the incidence of dementia, Parkinson’s disease, and multiple sclerosis: a population-based cohort study. Lancet 2017; 389:718–726. [DOI] [PubMed] [Google Scholar]
- 71. Kim KN, Lim YH, Bae HJ, Kim M, Jung K, Hong YC. Long-term fine particulate matter exposure and major depressive disorder in a community-based urban cohort. Environ Health Perspect 2016; 124:1547–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kaufman JD, Adar SD, Barr RG, Budoff M, Burke GL, Curl CL, Daviglus ML, Diez Roux AV, Gassett AJ, Jacobs DR Jr, Kronmal R, Larson TV, Navas-Acien A, Olives C, Sampson PD, Sheppard L, Siscovick DS, Stein JH, Szpiro AA, Watson KE. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the Multi-Ethnic Study of Atherosclerosis and Air Pollution): a longitudinal cohort study. Lancet 2016; 388:696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dorans KS, Wilker EH, Li W, Rice MB, Ljungman PL, Schwartz J, Coull BA, Kloog I, Koutrakis P, D’Agostino RB Sr, Massaro JM, Hoffmann U, O’Donnell CJ, Mittleman MA. Residential proximity to major roads, exposure to fine particulate matter, and coronary artery calcium: the Framingham Heart Study. Arterioscler Thromb Vasc Biol 2016; 36:1679–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Akintoye E, Shi L, Obaitan I, Olusunmade M, Wang Y, Newman JD, Dodson JA. Association between fine particulate matter exposure and subclinical atherosclerosis: A meta-analysis. Eur J Prev Cardiol 2016; 23:602–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Provost EB, Madhloum N, Int Panis L, De Boever P, Nawrot TS. Carotid intima-media thickness, a marker of subclinical atherosclerosis, and particulate air pollution exposure: the meta-analytical evidence. PLoS One 2015; 10:e0127014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Krishnan RM, Adar SD, Szpiro AA, Jorgensen NW, Van Hee VC, Barr RG, O’Neill MS, Herrington DM, Polak JF, Kaufman JD. Vascular responses to long- and short-term exposure to fine particulate matter: MESA Air (Multi-Ethnic Study of Atherosclerosis and Air Pollution). J Am Coll Cardiol 2012; 60:2158–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wilker EH, Ljungman PL, Rice MB, Kloog I, Schwartz J, Gold DR, Koutrakis P, Vita JA, Mitchell GF, Vasan RS, Benjamin EJ, Hamburg NM, Mittleman MA. Relation of long-term exposure to air pollution to brachial artery flow-mediated dilation and reactive hyperemia. Am J Cardiol 2014; 113:2057–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Weaver AM, Wellenius GA, Wu WC, Hickson DA, Kamalesh M, Wang Y. Residential distance to major roadways and cardiac structure in African Americans: cross-sectional results from the Jackson Heart Study. Environ Health 2017; 16:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Aaron CP, Chervona Y, Kawut SM, Diez Roux AV, Shen M, Bluemke DA, Van Hee VC, Kaufman JD, Barr RG. Particulate matter exposure and cardiopulmonary differences in the Multi-Ethnic Study of Atherosclerosis. Environ Health Perspect 2016; 124: 1166–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Van Hee VC, Adar SD, Szpiro AA, Barr RG, Bluemke DA, Diez Roux AV, Gill EA, Sheppard L, Kaufman JD. Exposure to traffic and left ventricular mass and function: the Multi-Ethnic Study of Atherosclerosis. Am J Respir Crit Care Med 2009; 179: 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Leary PJ, Kaufman JD, Barr RG, Bluemke DA, Curl CL, Hough CL, Lima JA, Szpiro AA, Van Hee VC, Kawut SM. Traffic-related air pollution and the right ventricle. The multi-ethnic study of atherosclerosis. Am J Respir Crit Care Med 2014; 189:1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wilker EH, Preis SR, Beiser AS, Wolf PA, Au R, Kloog I, Li W, Schwartz J, Koutrakis P, DeCarli C, Seshadri S, Mittleman MA. Long-term exposure to fine particulate matter, residential proximity to major roads and measures of brain structure. Stroke 2015; 46:1161–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wilker EH, Martinez-Ramirez S, Kloog I, Schwartz J, Mostofsky E, Koutrakis P, Mittleman MA, Viswanathan A. Fine particulate matter, residential proximity to major roads, and markers of small vessel disease in a memory study population. J Alzheimers Dis 2016; 53:1315–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chen JC, Wang X, Wellenius GA, Serre ML, Driscoll I, Casanova R, McArdle JJ, Manson JE, Chui HC, Espeland MA. Ambient air pollution and neurotoxicity on brain structure: evidence from women’s health initiative memory study. Ann Neurol 2015; 78:466–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Casanova R, Wang X, Reyes J, Akita Y, Serre ML, Vizuete W, Chui HC, Driscoll I, Resnick SM, Espeland MA, Chen JC. A voxel-based morphometry study reveals local brain structural alterations associated with ambient fine particles in older women. Front Hum Neurosci 2016; 10:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lawal AO. Air particulate matter induced oxidative stress and inflammation in cardiovascular disease and atherosclerosis: the role of Nrf2 and AhR-mediated pathways. Toxicol Lett 2017; 270:88–95. [DOI] [PubMed] [Google Scholar]
- 87. Perez CM, Hazari MS, Farraj AK. Role of autonomic reflex arcs in cardiovascular responses to air pollution exposure. Cardiovasc Toxicol 2015; 15:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bauer RN, Diaz-Sanchez D, Jaspers I. Effects of air pollutants on innate immunity: the role of Toll-like receptors and nucleotide-binding oligomerization domain-like receptors. J Allergy Clin Immunol 2012; 129:14–24; quiz 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ramanathan G, Yin F, Speck M, Tseng CH, Brook JR, Silverman F, Urch B, Brook RD, Araujo JA. Effects of urban fine particulate matter and ozone on HDL functionality. Part Fibre Toxicol 2016; 13:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Brenton CV, Marutani AN. Air pollution and epigenetics: recent findings. Curr Environ Health Rep 2014; 1: 35–45. [Google Scholar]
- 91. Pope CA 3rd, Bhatnagar A, McCracken JP, Abplanalp W, Conklin DJ, O’Toole T. Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ Res 2016; 119:1204–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. O’Toole TE, Hellmann J, Wheat L, Haberzettl P, Lee J, Conklin DJ, Bhatnagar A, Pope CA 3rd. Episodic exposure to fine particulate air pollution decreases circulating levels of endothelial progenitor cells. Circ Res 2010; 107:200–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Miller MR, Raftis JB, Langrish JP, McLean SG, Samutrtai P, Connel SPet al. Inhaled nanoparticles accumulate at sites of vascular disease. ACSnano 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chen R, Zhao A, Chen H, Zhao Z, Cai J, Wang C, Yang C, Li H, Xu X, Ha S, Li T, Kan H. Cardiopulmonary benefits of reducing indoor particles of outdoor origin: a randomized, double-blind crossover trial of air purifiers. J Am Coll Cardiol 2015; 65:2279–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Shi J, Lin Z, Chen R, Wang C, Yang C, Cai J, Lin J, Xu X, Ross JA, Zhao Z, Kan H. Cardiovascular benefits of wearing particulate-filtering respirators: a randomized crossover trial. Environ Health Perspect 2017; 125:175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Brook RD, Newby DE, Rajagopalan S. The global threat of outdoor ambient air pollution to cardiovascular health. Time for intervention. JAMA Cardiol 2017; 2:353–354. [DOI] [PubMed] [Google Scholar]