Abstract
BACKGROUND
Macrophage migration inhibitory factor (MIF) is an intracellular inhibitory regulator of the actions of angiotensin II in the central nervous system. Renovascular hypertensive 2-kidney, 1-clip (2K1C) rats have an increased activity of the renin–angiotensin system and a decrease in baroreflex function compared to normotensive (NT) rats. In the present study, we tested the effects of MIF overexpression within the nucleus of the solitary tract (NTS), a key brainstem region for cardiovascular regulation, on the development of hypertension, on baroreflex function, and on water and food intake in 2K1C rats.
METHODS
Holtzman NT rats received a silver clip around the left renal artery to induce 2K1C hypertension. Three weeks later, rats were microinjected in the NTS with AAV2-CBA-MIF, to increase the expression of MIF, or with the control vector AAV2-CBA-enhanced green fluorescent protein. Mean arterial pressure (MAP) and heart rate were recorded by telemetry. Baroreflex function was tested, and water and food intake were also measured.
RESULTS
Increasing MIF expression in the NTS of 2K1C rats attenuated the development of hypertension, reversed the impairment of baroreflex function, and reduced the increase in water intake. In contrast to 2K1C rats, similar increases in MIF expression in the NTS of NT rats produced no changes in baseline MAP, baroreflex function, or water intake.
CONCLUSIONS
These results indicate that an increased expression of MIF within the NTS attenuates the development of hypertension and restores the baroreflex function in 2K1C rats.
Keywords: angiotensin II, AT1 receptor, baroreflex, blood pressure, brainstem, hypertension, 2K1C.
The renal 2-kidneys, 1-clip (2K1C) rat model of hypertension, developed approximately 80 years ago by Goldblatt1 shows similarities with renovascular hypertension in humans, which accounts for approximately to 5–6% of high blood pressure cases in the elderly.2 This hypertension is characterized by increased renin–angiotensin system activity and sympathetic nerve activity.3–5 In addition, it is well established that the baroreflex is attenuated in 2K1C rats,6,7 which may contribute to the increase in sympathetic outflow and high blood pressure.8–10 Central mechanisms that are important for the control of baroreflex function and sympathetic nerve activity are also involved in the development of this type of hypertension.3,7,11 The nucleus of the solitary tract (NTS), the first synaptic relay station in the central nervous system for cardiovascular afferent fibers12 and one of the important central areas involved in cardiovascular regulation, particularly for the control of baroreflexes, is also suggested to participate in the maintenance of hypertension.7,13,14
Macrophage migration inhibitory factor (MIF) is a 12.5 kDa protein expressed in immune tissues and also found in the central nervous system.15,16 Within the central nervous system, MIF is found mainly in neurons in different brain areas, including the NTS of normotensive (NT) rats.14,15 MIF is an intracellular inhibitory regulator of the central actions of angiotensin II (ANG II),17,18 and its expression in neurons in culture attenuates the increased firing rate induced by ANG II acting at ANG II type 1 (AT1) receptors.17,18 The NTS is rich in AT1 receptors7 and the activation of these receptors impairs baroreflex function.19 Recently, we demonstrated that MIF overexpression in the NTS reduced blood pressure in spontaneously hypertensive rats (SHR),14 a hypertensive model that exhibits increased activity of renin–angiotensin system in the brain.20 Considering the importance of the NTS for arterial blood pressure control and its role in hypertension,7,13,14 the aim of the present study was to investigate the effects of increased expression of MIF in the NTS on the development of renovascular hypertension as well as on baroreflex function. Water and food intake were also measured.
METHODS
Animals
Male Holtzman rats (150–180 g) were used for these experiments. Animals were housed in individual stainless steel (baroreflex, fluid, and food experiments) or polypropylene cages (telemetry experiments), with ad libitum access to water and standard lab rat chow (Biobase, Águas Frias, SC, Brazil), which contain mineral and vitamin mix, 220 g of protein; 50 g of crude fat (ether extract), 70 g of fibers; and 2.7 g of sodium chloride per kg of chow. Rats were kept on a 12-hour light/dark cycle in climate-controlled rooms. The Ethics Committee for Animal Care and Use (CEUA) of the School of Dentistry of Araraquara, São Paulo State University approved all experimental protocols used (CEUA 17/2014).
Anesthesia and euthanasia
Rats were anesthetized with ketamine [80 mg/kg of body weight (b. wt.)] combined with xylazine (7 mg/kg of b. wt.) for the surgeries. During the surgeries/procedures, the level of anesthesia was monitored by checking the eye blink reflex and a reaction to paw pinch, and was adjusted if necessary. Following the surgeries animals received a prophylactic dose of penicillin (50,000 IU, intramuscularly) and a dose of the anti-inflammatory ketoprofen (1 mg/kg of b. wt., subcutaneously). At the end of the experiments, rats were euthanized by placing them under deep anesthesia with sodium thiopental (70 mg/kg of b. wt, i.p.), the kidneys were removed and weighed (wet weight) to confirm atrophy in the clipped kidney and hypertrophy in the contralateral kidney (Table 1). Thereafter, animals were decapitated or transcardially perfused with chilled 0.9% saline followed by 4% paraformaldehyde, depending on the protocol (see below), and the brainstem was removed.
Table 1.
Left kidney/right kidney weight ratio (LK/RK) in 2-kidney, 1-clip (2K1C) rats, and normotensive (NT) rats that received intra-NTS injections of AAV2-CBA-eGFP or AAV2-CBA-MIF vectors
| Group | n | LK/RK |
|---|---|---|
| NT-GFP | 20 | 0.98 ± 0.01 |
| NT-MIF | 20 | 0.99 ± 0.01 |
| 2K1C-GFP | 18 | 0.61 ± 0.04* |
| 2K1C-MIF | 12 | 0.68 ± 0.08* |
The kidney weigh was measured 7 weeks after 2K1C or sham surgery (NT group) and 4 weeks after GFP or MIF transduction in the NTS. Data are expressed as mean ± SEM; *P < 0.05 vs. NT groups; 1-way ANOVA, followed by Student–Newman–Keuls test; n = number of animals. Abbreviations: ANOVA, analysis of variance; MIF, macrophage migration inhibitory factor; NT, normotensive; 2K1C, 2-kidney, 1-clip.
Renovascular hypertension
Rats were anesthetized as above, and the left renal artery was partially obstructed using a silver clip of 0.2 mm width. NT animals were subjected to the same surgical procedure without partial renal artery occlusion (sham surgery). We will refer to Holtzman rats with sham surgery as NT rats.
Telemetry recording of arterial blood pressure
Rats were anesthetized as above, and telemetry transmitters were implanted (TA11PAC40, Data Sciences International, St. Paul, MN) into the abdominal aorta to record pulsatile arterial pressure and activity. Dataquest 4.31 software (Data Sciences International) was used to process pulsatile arterial pressure recordings and to calculate mean arterial pressure (MAP) and heart rate (HR).
In vivo gene transfer in the NTS
Rats were anesthetized as above, and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA). A partial craniotomy of the occipital bone was performed, and the dorsal surface of the brainstem was exposed. AAV2-CBA-enhanced green fluorescent protein (eGFP) or AAV2-CBA-MIF vectors were microinjected at 5 different sites along the NTS. Each microinjection consisted of 150 nl vector/site (GFP, 1.8 × 1012 genome copies (gc) and MIF, 1.2 × 1012 gc/ml) as described previously.7,14 AAV2-CBA-eGFP or AAV2-CBA-MIF were constructed as described previously and these vectors elicit gene transduction primarily in neurons, with few transductions in astrocytes.14,21
Baroreflex tests
Rats were anesthetized as above, and a catheter was inserted into the abdominal aorta through the femoral artery for pulsatile arterial pressure, MAP, and HR recording; a second polyethylene tubing was inserted into the femoral vein for drug administration, as described previously.7,14 Briefly, the arterial catheter was connected to a pressure transducer (P23 DB, Statham, Costa Mesa, CA) coupled to a signal amplifier (ETH-200 Bridge Bio Amplifier, Chicago, IL) and sampled using an analog-to-digital interface (Sampling rate of 2 kHz; ML866, ADInstruments, Bella Vista, Australia) 24 hours after catheter implantation. In conscious rats, after 30 minutes of recording at baseline conditions, the bradycardic and tachycardic reflex responses were assessed in the same animals following i.v. injection of phenylephrine (5 µg/kg, b. wt.) or sodium nitroprusside (30 µg/kg, b. wt.), respectively, with a 10-minute interval and the baroreflex sensitivity (BRS) was analyzed as described previously.7,22
Spontaneous BRS was assessed in the time-domain by means of the sequence method using a custom computer software (CardioSeries v2.4, http://www.danielpenteado.com) and it was described in details elsewhere.23,24 A spontaneous baroreflex sequence was considered only when the correlation coefficient (r) between systolic arterial pressure and pulse interval values was greater than or equal to 0.8. The BRS was determined from the slope of the linear regression lines between systolic arterial pressure and pulse interval of each baroreflex sequence and is shown as UP gain (BRS calculated from UP sequences), DOWN gain (BRS calculated from DOWN sequences), and Total gain (BRS calculated from all sequences, UP and DOWN combined).23,24
MIF detection in the NTS
To evaluate the mRNA levels of MIF (Rn00821234_g1) in the NTS, the animals were deeply anesthetized as above and decapitated. The brainstem was quickly removed and with the aid of a surgical microscope (DF Vasconcelos M900, Valença, Rio de Janeiro, Brazil) the NTS was removed by micropunch using the area postrema and calamus scriptorius as reference sites. Total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA) and isolated using a PureLink RNA mini kit (Life technologies, Grand Island, NY). The isolated RNA was converted in cDNA using a high-capacity cDNA reverse transcription kit (Life technologies, Grand Island, NY) and samples were run in duplicate using a StepOne Real-time PCR system, Taqman Universal Gene Expression Master Mix and validated Taqman probes (Applied Biosystems, Foster City, CA). Expression patterns of genes of interest were normalized to constitutively expressed glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Rn99999916_s1) and relative expression was quantified using the 2−ΔΔCt method.25
The localization of MIF and GFP within the NTS was determined by immunohistochemistry as described previously.14 Rabbit anti-MIF primary antibody was 1:300 for intact animals (animals that received no injections into the NTS) or 1:500 for MIF-overexpressed animals (Torrey Pines Biolabs, Inc., Houston, TX) were used. MIF immunofluorescence and green fluorescence (from GFP) were detected using a Leica DM5500 B Fluorescence microscope (Leica, Wetzlar, Hessen, Germany) in matched, representative sections of the tissue from different groups. The area of MIF staining was used to measured endogenous MIF immunoreactivity. Bilateral images of iNTS (area postrema level) and 1 image of cNTS (14.7 caudal to bregma) were converted into grayscale and binary formats and thresholds for black and white balance were adjusted to the same level in matched representative sections of the NTS. The area of MIF staining was indicated as gray values/section.
Water and food intake and urine collection
Water intake of each animal was recorded using 100 ml capacity-polypropylene bottles with 1-ml divisions. For food intake, a preweighed amount of regular chow pellets was given to the animals, and at the end, the ingested food was subtracted from the preweighed amount. Urine samples were collected in 0.1-ml graduated polypropylene tubes.
Statistical analysis
All data are expressed as means ± SEM. One- or 2-way analysis of variance followed by Student Newman–Keuls post hoc test or Student t test were used for comparisons. In all tests, differences were considered significant at P <0.05.
EXPERIMENTAL PROTOCOLS
Analysis of MIF overexpression in the NTS
After euthanasia, animals were either perfused or decapitated as described in Methods. Nonperfused brains were processed for qRT-PCR analyses of MIF mRNA in the NTS. Perfused brains, were processed for GFP fluorescence or MIF immunofluorescence in the NTS. To evaluate endogenous MIF, immunostaining of MIF in the NTS was done in another group of intact NT and 2K1C animals. In these groups, at the 7th week, MAP and HR were assessed in conscious rats on the day before perfusion.
Effect of increased expression of MIF in the NTS on the development of renovascular hypertension
A group of rats underwent 2K1C or sham surgery, immediately followed by implantation with telemetry transmitters into the abdominal aorta. Three weeks later animals received microinjections of either AAV2-CBA-eGFP or AAV2-CBA-MIF vectors in the NTS. Arterial pressure and activity were recorded continuously (sampling rate of 1 kHz; 1 minute every 5 minutes) for a 24-h period, once a week, starting 1 week after 2K1C surgery/telemetry transmitter implantation until the 7th week (4 weeks post-vector microinjection).
Effect of increased expression of MIF in the NTS on the baroreflex function in 2K1C rats
Another group of rats underwent sham or 2K1C surgery, followed, 3 weeks later, by microinjections of either AAV2-CBA-eGFP or AAV2-CBA-MIF vectors into the NTS. In 2K1C rats, systolic arterial pressure was monitored once a week, during the first 3 weeks by tail cuff, starting 1 week after the surgery. Only animals with an increase in systolic arterial pressure after 3 weeks of the 2K1C surgery underwent virus microinjection in the NTS. Four weeks after the viral transduction, rats were implanted with a catheter in the femoral artery and vein for MAP and HR recordings and drug administration, respectively, in conscious freely moving rats. BRS was assessed as described above.
Effect of increased expression of MIF in the NTS on water and food intake and urinary volume
Another group of rats underwent sham or 2K1C surgery, followed, 3 weeks later, by microinjections of either AAV2-CBA-eGFP or AAV2-CBA-MIF vectors into the NTS. Twenty-four hour data collection was performed once at the 1st, 3rd, 5th, and 7th week after renal clipping. On the day of data collection, rats were transferred to a metabolic cage for water and food intake and urinary volume measurement. Baseline water intake was measured in their home cages a week before the surgery. MAP and HR were recorded in conscious rats 2 days after the last measurement.
RESULTS
MIF overexpression in the NTS
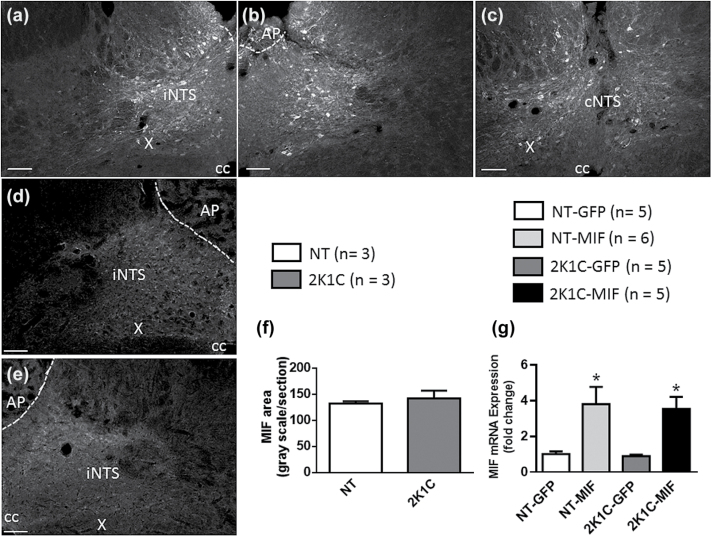
Figure 1a–c shows MIF expression along the NTS of a NT rat 4 weeks after the AAV2-CBA-MIF injection in this site. Figure 1d and e shows endogenous MIF expression in the NTS of intact NT and 2K1C rats, respectively, demonstrating that the area of endogenous MIF staining in the NTS was comparable between them (Figure 1f). In the intact animals, MAP was higher in 2K1C rats as compared to NT rats (191 ± 15, vs. 102 ± 3 mm Hg; P < 0.05). The 2K1C-MIF and NT-MIF groups showed a higher level of mRNA for MIF in the NTS compared to animals that were injected with AAV2-CBA-eGFP (P < 0.05) and the levels of MIF mRNA in GFP transduced rats was comparable (Figure 1g).
Figure 1.
Viral-mediated transduction of GFP and MIF into the NTS. Photomicrographs from coronal sections of rat brainstem (−14.0 to −14.7 mm from bregma). (a–c) Representative sequential images of the same animal showing MIF immunoreactivity in the NTS of a normotensive rat that received microinjections of AAV2-CBA-MIF 4 weeks before. (d and e) Endogenous MIF immunoreactivity from an intact normotensive (NT) and 2K1C hypertensive rats, respectively. Anti-MIF primary antibody was used at 1:300 and 1:500 dilutions for endogenous MIF expression and AAV2-CBA-MIF treated animals, respectively. Scale bar: 100 µm. (f) The area of endogenous MIF staining in the NTS of an intact NT and 2K1C hypertensive rats. (g) MIF mRNA levels in the NTS in NT or 2K1C hypertensive rats that received microinjections of AAV2-CBA-eGFP or AAV2-CBA-MIF. Data in f and g are means ± SEM; *P < 0.05 vs. corresponding GFP group; Student t test and 1-way ANOVA, followed by Student–Newman–Keuls, were used to analyze the data in f and g, respectively; n = number of animals. Abbreviations: ANOVA, analysis of variance; AP, area postrema; cc, central canal; cNTS, commissural nucleus of the solitary tract; iNTS, intermediate nucleus of the solitary tract; MIF, macrophage migration inhibitory factor; NTS, nucleus of the solitary tract; X, dorsal motor nucleus of vagus.
Increased expression of MIF in the NTS attenuates 2K1C hypertension
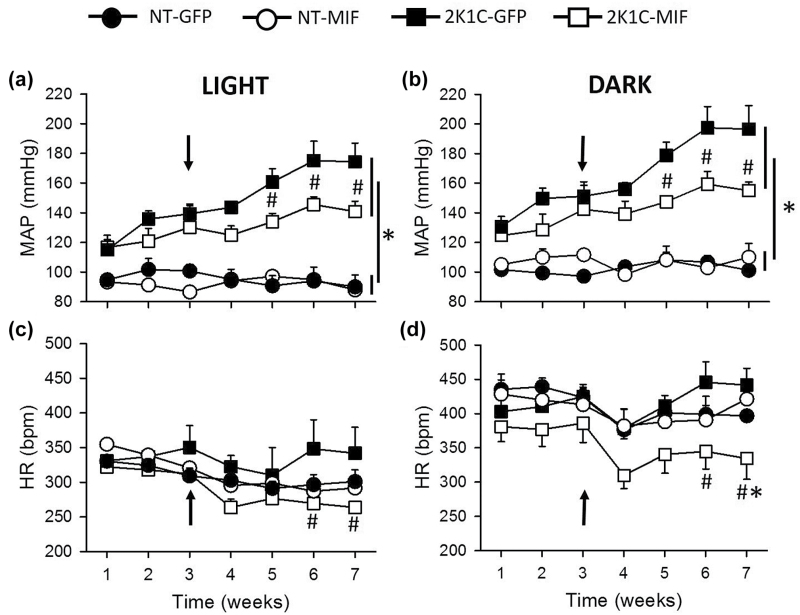
Two weeks after renal clipping, MAP was significantly different between the 2K1C (GFP or MIF) and NT (GFP or MIF) groups (P < 0.001) during the dark or light periods (Figure 2a and b). The development of hypertension was strongly attenuated in the 2K1C-MIF rats compared with the 2K1C-GFP rats (Figure 2a and b). On the 7th week, MAP was reduced in the 2K1C-MIF rats during the light period (141 ± 7, vs. 2K1C-GFP: 174 ± 12 mm Hg; P < 0.001) and the dark period (156 ± 6, vs. 2K1C-GFP: 197 ± 16 mm Hg; P < 0.05) (Figure 2a and b). In addition, HR was reduced in 2K1C-MIF rats during the light (264 ± 8, vs. 2K1C-GFP: 342 ± 37 bpm at the 7th week; P < 0.05) and dark (334 ± 30, vs. 2K1C-GFP: 442 ± 26 bpm; P < 0.05) periods (Figures 2c and d). MAP and HR in the NT-GFP and NT-MIF rats were similar during the course of the experiment (P > 0.05), Figure 2a–d.
Figure 2.
Increased MIF expression in the NTS impairs the development of hypertension and reduces HR in 2K1C rats. (a and b) Mean arterial pressure (MAP; mm Hg) and (c and d) heart rate (HR; bpm) values during the light (a, c) and dark periods (b and d) from 2K1C or normotensive (NT) rats before (1–3 weeks) and after (3–7 weeks) microinjections of AAV2-CBA-MIF or AAV2-CBA-eGFP vector into the NTS. Vector injections were made at week 3 (indicated by the arrows). The results are presented as means ± SEM. NT-GFP, n = 4; NT-MIF, n = 7; 2K1C-GFP, n = 5; 2K1C-MIF, n = 5; *P < 0.05 vs. NT groups; #P < 0.05 vs. 2K1C-GFP group; 2-way ANOVA, followed by Student–Newman–Keuls test. Abbreviations: ANOVA, analysis of variance; MIF, macrophage migration inhibitory factor; NTS, nucleus of the solitary tract.
Baroreflex function was restored in 2K1C rats with increased MIF expression in the NTS
Seven weeks after renal clipping, baseline MAP was reduced in 2K1C rats with overexpression of MIF in the NTS compared to 2K1C-GFP rats, whereas baseline HR were not different between groups (Table 2).
Table 2.
Baseline mean arterial pressure (MAP) and heart rate (HR) in 2-kidney, 1-clip (2K1C) rats, and normotensive (NT) rats that received intra-NTS injections of AAV2-CBA-eGFP or AAV2-CBA-MIF vectors
| Group | n | MAP (mm Hg) | HR (bpm) |
|---|---|---|---|
| NT-GFP | 12 | 100 ± 2 | 350 ± 12 |
| NT-MIF | 9 | 102 ± 2 | 355 ± 19 |
| 2K1C-GFP | 12 | 189 ± 7* | 392 ± 13 |
| 2K1C-MIF | 6 | 153 ± 6*# | 367 ± 13 |
MAP and HR recordings were performed 7 weeks after 2K1C or sham surgery (NT group) and 4 weeks after GFP or MIF transduction in the NTS. Data are expressed as mean ± SEM; *P < 0.05 vs. NT groups; #P < 0.05 vs. 2K1C-GFP; 1-way ANOVA, followed by Student–Newman–Keuls test; n = number of animals used in the baroreflex data (pharmacologically induced baroreflex and spontaneous baroreflex). Abbreviations: ANOVA, analysis of variance; MIF, macrophage migration inhibitory factor; NTS, nucleus of the solitary tract.
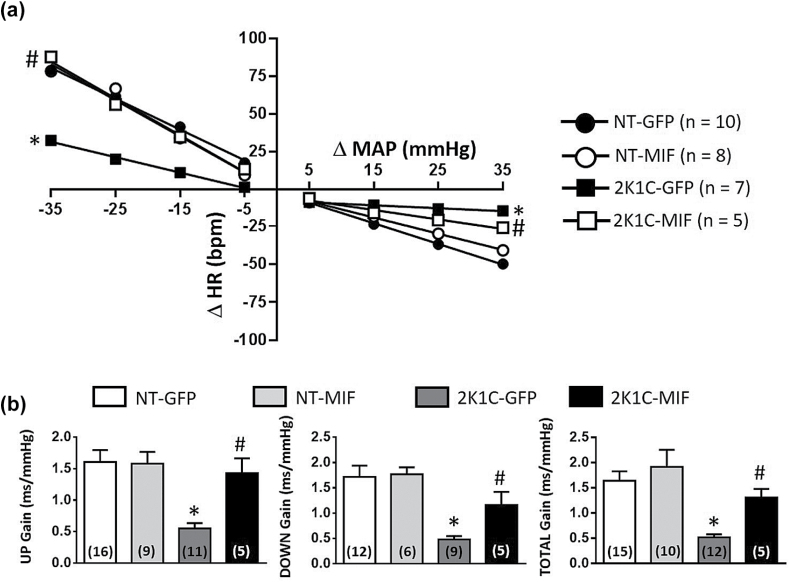
The impaired bradycardic response in 2K1C-GFP rats (slope of −0.41 ± 0.1, vs. NT-GFP slope: −1.9 ± 0.2 bpm/mm Hg; P < 0.001) was restored in the 2K1C-MIF group (slope of −1.4 ± 0.3 bpm/mm Hg, P < 0.05) reaching values comparable to the NT group (Figure 3a). The 2K1C-GFP group had also an impaired reflex tachycardia (slope of −1.7 ± 0.3, vs. NT-GFP slope: −3.3 ± 0.4 bpm/mm Hg; P < 0.001), which was also restored in the 2K1C-MIF group (slope of −4.7 ± 0.6 bpm/mm Hg; P < 0.05), reaching values comparable to those in NT rats (Figure 3a).
Figure 3.
Increased MIF expression in the NTS restores baroreflex function in 2K1C rats. Baroreflex function was analyzed in normotensive (NT) or 2K1C rats that received intra-NTS injections of AAV2-CBA-eGFP or AAV2-CBA-MIF vectors. (a) Grouped heart rate (HR) baroreflex slope in response to each 10 mm Hg change in mean arterial pressure (MAP) elicited by phenylephrine (pressor) or sodium nitroprusside (depressor); (b) Spontaneous baroreflex analysis, as follows; UP gain, DOWN gain, and Total gain. Data are presented as mean ± SEM; n = number of rats. In panel b the numbers of rats/group are inside the bars; *P < 0.05 vs. NT groups, #P < 0.05 vs. 2K1C-GFP; 1-way ANOVA, followed by Student–Newman–Keuls test. Abbreviations: ANOVA, analysis of variance; MIF, macrophage migration inhibitory factor; NTS, nucleus of the solitary tract.
The assessment of BRS by means of the sequence method (UP gain, DOWN gain, and Total gain) also revealed an improvement in baroreflex function (P < 0.001) in 2K1C-MIF rats compared to 2K1C-GFP rats (Figure 3b).
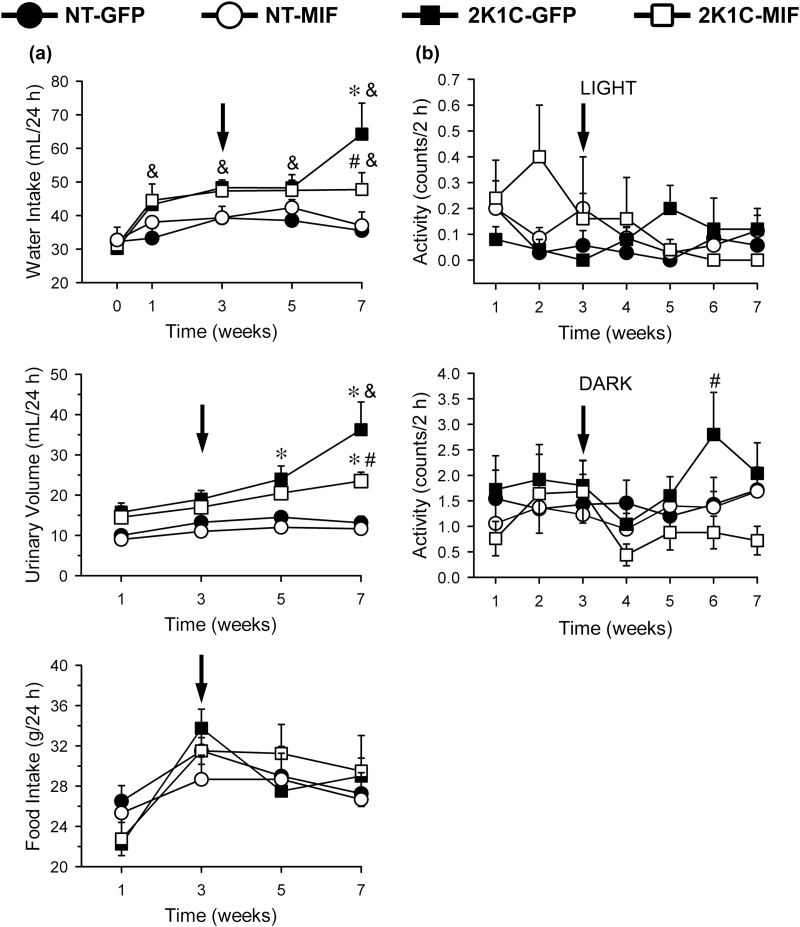
MIF overexpression in the NTS attenuated water intake in 2K1C rats
All groups of rats ingested similar daily amounts of water before the surgical procedure (Figure 4a). From 1 week after renal clipping daily water intake increased in both groups of 2K1C rats compared to their baseline levels (P < 0.05), whereas water intake in both groups of NT rats was not altered during the entire experiment (Figure 4a). During the last week of test, daily water intake increased even more in 2K1C-GFP rats, a response that was attenuated in 2K1C-MIF rats [F(3, 55) = 8.98; P < 0.05] (Figure 4a). Daily urinary volume also increased in the 2K1C-GFP rats compared to the NT rats, a response that was also attenuated in the 2K1C-MIF rats [F(3, 44) = 19.00; P < 0.05] (Figure 4a). Daily food intake was similar in all groups throughout the duration of the experiment [F(3, 44) = 0.39; P > 0.05] (Figure 4a). MAP was higher in the 2K1C-GFP rats [F(3,11) = 85.26; P < 0.05)] compared to all other groups (200 ± 7, vs. NT-GFP = 105 ± 4; NT-MIF = 106 ± 2; 2K1C-MIF = 156 ± 4 mm Hg). MAP in 2K1C-MIF rats was also different from NT groups (P < 0.05). HR was similar in all the groups [F(3, 11) = 2.45; P > 0.05)]. There were no major changes in the activity in all groups of rats during the light and dark periods (Figure 4b).
Figure 4.
(a) Water intake, urinary volume, and food intake, and (b) activity in normotensive (NT) or 2K1C rats that received intra-NTS injections of AAV2-CBA-eGFP or AAV2-CBA-MIF vectors 3 weeks after renal clipping (arrows). Activity mean was generated from a period of 2 hours during the light and the dark periods, once a week, for 7 weeks. The results are presented as means ± SEM. In panel a the number of animals/group are: NT-GFP, n = 4; NT-MIF, n = 3; 2K1C-GFP, n = 4; 2K1C-MIF, n = 4. In panel b, the number of animals are the same as in Figure 2; *P < 0.05 vs. NT groups; #P < 0.05 vs. 2K1C-GFP group; &P < 0.05 vs. baseline (0); 2-way ANOVA, followed by Student–Newman–Keuls test. Abbreviations: ANOVA, analysis of variance; MIF, macrophage migration inhibitory factor; NTS, nucleus of the solitary tract.
DISCUSSION
This study shows that MIF overexpression within the NTS attenuates hypertension, improves baroreflex function, and reduces basal HR in 2K1C rats, without changing blood pressure, HR, and baroreflex in NT rats.
These results extend those of a previous study that showed the antihypertensive effects of MIF overexpression in the NTS in SHR.14 In the previous study,14 the period of reduction of MAP in SHR observed was 1 week. In the present study, the reduction in arterial pressure produced by overexpression of MIF in the NTS in 2K1C rats was observed during a more chronic period (for at least 3 weeks). In addition, the antihypertensive effect of MIF overexpression in the NTS in 2K1C rats was stronger than that observed in SHR (reduction of 30–40 mm Hg in 2K1C rats compared to 10–20 mm Hg reduction in SHR). Following AAV2-CBA-MIF injection into the NTS, which was performed 3 weeks after clipping the renal artery, MAP stopped increasing in 2K1C-MIF rats, whereas in 2K1C-GFP rats, MAP continued to increase. Therefore, the present and previous results14 clearly suggest that overexpression of MIF in the NTS exerts a strong antihypertensive effects, without affecting MAP in NT animals. These results also reinforce the importance of the pressor mechanisms of the NTS for the development of hypertension, which was also previously suggested by other studies.7,13,14
2K1C rats have an overactivity of the renin–angiotensin system and circulating ANG II and reduced baroreflex function.5–9,26 In the 2K1C rats the impairment of baroreflex function is dependent, at least in part, on high ANG II levels,8 which may act via its AT1 receptors in the NTS to reduce baroreflex function.26 The present study shows that MIF overexpression in the NTS restores the baroreflex function in the 2K1C rats. MIF acts as an intracellular inhibitor of the actions of ANG II on AT1 receptors in the brain cardiovascular control centers.27,28 Therefore, it is possible that MIF overexpression in the NTS restores baroreflex function in 2K1C rats as a consequence of the blockade of ANG II effects on baroreflex function.
In humans, renovascular hypertension increases cardiac sympathetic activity (i.e., total body and cardiac noradrenaline spillovers), which may increase the risk for ventricular arrhythmias and sudden death.29 In 2K1C rats, basal HR increases as a consequence of decreased parasympathetic modulation of the HR.6 In the current study, although baseline HR did not increase in 2K1C rats, MIF overexpression in the NTS reduced basal HR, especially in the dark period, the period of high animal activity. The baroreceptor reflex pathways provide a major excitatory drive to the cardiac vagal activity,30 thus it is possible that the decrease in baseline HR in 2K1C-MIF rats was secondary to the improvement of the baroreflex.
An impairment of the baroreflex seems critical for the development and maintenance of 2K1C hypertension.6,9 The reduction in baroreflex gain might contribute to the increase in sympathetic outflow and high blood pressure observed in 2K1C rats.10 In the present study, MIF overexpression within the NTS in 2K1C rats produced a full recovery of the baroreflex, however, did not bring arterial pressure back to NT levels. One possible explanation for this is the action of ANG II on AT1 receptors in other brain areas such as the paraventricular nucleus of the hypothalamus and the rostral ventrolateral medulla, increasing the sympathetic drive.3,31 It is also noteworthy that high blood pressure can damage the blood brain barrier, and therefore make these regions susceptible to direct effects of circulating ANG II.32 Moreover, ANG II acting at AT1 receptors in the circumventricular organs may send facilitatory signals to the paraventricular nucleus of the hypothalamus and rostral ventrolateral medulla.33 Finally, in renovascular hypertension the increase in peripheral renin and ANG II levels5 may also contribute to the hypertension as suggested by studies showing that oral treatment with the AT1 receptor antagonist, losartan, prevented the full development of hypertension in 2K1C rats.34 Thus, it is possible that in MIF-treated 2K1C rats which display normal baroreflex function, the residual increase in arterial pressure is due to the action of mechanisms that are not influenced by MIF overexpression within the NTS.
Similarly to previous studies,35 we have demonstrated that daily water intake increased in 2K1C-GFP rats, probably as a consequence of the increase in renin activity that occurs after the induction of renal hypertension.5 However, overexpression of MIF in the NTS attenuated water intake in the 2K1C-MIF rats (Figure 4). One possibility is that the increase in MIF expression in the NTS of 2K1C rats reduces renin secretion and thereby, decreases water intake. If this is the case, this mechanism might also account for the reduced MAP in 2K1C-MIF rats. Alternately, 2K1C-GFP rats had also an increase in urinary volume, which might drive rats to drink more water. However, at this point, it is not possible to conclude if the changes in urinary volume are the cause or a consequence of the changes in water intake. In addition, daily food intake was not affected by MIF overexpression in the NTS, which reduces the possibility of nonspecific effect of this treatment on ingestive responses. The absence of effects on food intake and the changes in water intake and urinary volume in the same direction reduces the possibility that changes in ingestive responses and/or on fluid and electrolyte balance are the causes of the antihypertensive effects of MIF overexpression in the NTS in 2K1C rats.
Oxidative stress in the brain is suggested to be an important mechanism to maintain the high blood pressure in 2K1C animals.3,11,31,36 ANG II acting on AT1 receptors increases reactive oxygen species (ROS), which activates intracellular pathways involved in ANG II actions in the brain.37 A recent study demonstrated that ROS levels in the NTS and MAP in SHR are reduced following treatment with losartan or tempol (superoxide dismutase mimetic) in the drinking water, due to diminished activation of AT1 receptors and/or decreased ROS production.38 MIF is known to be an effective ROS scavenger,39 an effect mediated through its thiol-protein oxidoreductase activity. Furthermore, studies have demonstrated that overexpression of MIF in NT rat neurons in culture prevents ANG II-induced accumulation of ROS,17 and the blood pressure dampening action of MIF is due to its intrinsic thiol-protein oxidoreductase moiety.27,28 Although we did not measure ROS in the NTS of eGFP- or MIF transduced 2K1C rats, our preliminary data from SHR demonstrate that MIF over expression at the NTS lowers ROS levels at this nucleus, as assessed by electron paramagnetic resonance spectroscopy (unpublished data; Freiria-Oliveira AH; Blanch GT, Sumners C; Cardounel AJ). Collectively, these findings suggest that the mechanism of action of MIF in lowering blood pressure reside in its thiol-protein oxidoreductase antioxidant activity.
The present study demonstrated that MIF protein and mRNA levels are similar in NT and 2K1C hypertensive rats that received no treatment in the NTS. Thus, a dysregulation of endogenous levels of MIF is probably not a mechanism involved in the development of 2K1C hypertension. In addition, there were no significant change in the activity among the groups.
In conclusion, the results of this study indicate that MIF overexpression within the NTS of 2K1C rats prevents the full development of hypertension and improves baroreflex function. Furthermore, MIF overexpression within the NTS of 2K1C rats reduces the increase in daily water intake and the urinary volume apparently at the same extension, without changes on daily food intake. Although not possible to completely exclude the involvement of changes in fluid and electrolyte balance for the antihypertensive effects of MIF overexpression in the NTS, the changes in water intake and urinary volume in the same direction and not so intense reduces this possibility. The mechanisms still need to be further addressed. However, considering that an impairment of baroreflex function is suggested to be a predictor of cardiac mortality,40 the present data demonstrated a beneficial effect of MIF transduction in the NTS.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
The authors thank Reginaldo C. Queiroz, Silas P. Barbosa. Silvia Fóglia for technical assistance, Silvana A. D. Malavolta e Carla Molina for secretarial assistance, Ana L. V. de Oliveira for animal care. This research was supported by CNPq (473108/2011–9 and 304918/2011–3), FAPESP (2011/50770–1 and 2015/23467-7), and NIH (HL-076803).
REFERENCES
- 1. Goldblatt H, Lynch J, Hanzal RF, Summerville WW. Studies on experimental hypertension: I. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med 1934; 59:347–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hansen KJ, Edwards MS, Craven TE, Cherr GS, Jackson SA, Appel RG, Burke GL, Dean RH. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg 2002; 36:443–451. [DOI] [PubMed] [Google Scholar]
- 3. Oliveira-Sales EB, Nishi EE, Carillo BA, Boim MA, Dolnikoff MS, Bergamaschi CT, Campos RR. Oxidative stress in the sympathetic premotor neurons contributes to sympathetic activation in renovascular hypertension. Am J Hypertens 2009; 22:484–492. [DOI] [PubMed] [Google Scholar]
- 4. Johansson M, Elam M, Rundqvist B, Eisenhofer G, Herlitz H, Lambert G, Friberg P. Increased sympathetic nerve activity in renovascular hypertension. Circulation 1999; 99:2537–2542. [DOI] [PubMed] [Google Scholar]
- 5. Morton JJ, Wallace EC. The importance of the renin-angiotensin system in the development and maintenance of hypertension in the two-kidney one-clip hypertensive rat. Clin Sci (Lond) 1983; 64:359–370. [DOI] [PubMed] [Google Scholar]
- 6. Oliveira-Sales EB, Toward MA, Campos RR, Paton JF. Revealing the role of the autonomic nervous system in the development and maintenance of Goldblatt hypertension in rats. Auton Neurosci 2014; 183:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blanch GT, Freiria-Oliveira AH, Speretta GF, Carrera EJ, Li H, Speth RC, Colombari E, Sumners C, Colombari DS. Increased expression of angiotensin II type 2 receptors in the solitary-vagal complex blunts renovascular hypertension. Hypertension 2014; 64:777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berenguer LM, Garcia-Estañ J, Ubeda M, Ortiz AJ, Quesada T. Role of renin-angiotensin system in the impairment of baroreflex control of heart rate in renal hypertension. J Hypertens 1991; 9:1127–1133. [PubMed] [Google Scholar]
- 9. Tsyrlin VA, Galagudza MM, Kuzmenko NV, Pliss MG, Rubanova NS, Shcherbin YI. Arterial baroreceptor reflex counteracts long-term blood pressure increase in the rat model of renovascular hypertension. PLoS One 2013; 8:e64788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lohmeier TE, Irwin ED, Rossing MA, Serdar DJ, Kieval RS. Prolonged activation of the baroreflex produces sustained hypotension. Hypertension 2004; 43:306–311. [DOI] [PubMed] [Google Scholar]
- 11. Oliveira-Sales EB, Colombari DS, Davisson RL, Kasparov S, Hirata AE, Campos RR, Paton JF. Kidney-induced hypertension depends on superoxide signaling in the rostral ventrolateral medulla. Hypertension 2010; 56:290–296. [DOI] [PubMed] [Google Scholar]
- 12. Ciriello J, Hochstenbach SL, Roder S. Central projections of baroreceptor and chemoreceptor afferents fibers in the rat. In Barraco IRA (ed), Nucleus of the Solitary Tract. CRC Press: Boca Raton, Florida, 1994, pp. 35–50. [Google Scholar]
- 13. Sato MA, Menani JV, Lopes OU, Colombari E. Lesions of the commissural nucleus of the solitary tract reduce arterial pressure in spontaneously hypertensive rats. Hypertension 2001; 38:560–564. [DOI] [PubMed] [Google Scholar]
- 14. Freiria-Oliveira AH, Blanch GT, Li H, Colombari E, Colombari DS, Sumners C. Macrophage migration inhibitory factor in the nucleus of solitary tract decreases blood pressure in SHRs. Cardiovasc Res 2013; 97:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bacher M, Meinhardt A, Lan HY, Dhabhar FS, Mu W, Metz CN, Chesney JA, Gemsa D, Donnelly T, Atkins RC, Bucala R. MIF expression in the rat brain: implications for neuronal function. Mol Med 1998; 4:217–230. [PMC free article] [PubMed] [Google Scholar]
- 16. Baugh JA, Bucala R. Macrophage migration inhibitory factor. Crit Care Med 2002; 30:S27–S35. [PubMed] [Google Scholar]
- 17. Sun C, Li H, Leng L, Raizada MK, Bucala R, Sumners C. Macrophage migration inhibitory factor: an intracellular inhibitor of angiotensin II-induced increases in neuronal activity. J Neurosci 2004; 24:9944–9952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Busche S, Gallinat S, Fleegal MA, Raizada MK, Sumners C. Novel role of macrophage migration inhibitory factor in angiotensin II regulation of neuromodulation in rat brain. Endocrinology 2001; 142:4623–4630. [DOI] [PubMed] [Google Scholar]
- 19. Matsumura K, Averill DB, Ferrario CM. Angiotensin II acts at AT1 receptors in the nucleus of the solitary tract to attenuate the baroreceptor reflex. American Journal of Physiology 1998; 275:R1611–R1619. [DOI] [PubMed] [Google Scholar]
- 20. Veerasingham SJ, Raizada MK. Brain renin-angiotensin system dysfunction in hypertension: recent advances and perspectives. Br J Pharmacol 2003; 139:191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li H, Gao Y, Qi Y, Katovich MJ, Jiang N, Braseth LN, Scheuer DA, Shi P, Sumners C. Macrophage migration inhibitory factor in hypothalamic paraventricular nucleus neurons decreases blood pressure in spontaneously hypertensive rats. FASEB J 2008; 22:3175–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Speretta GF, Silva AA, Vendramini RC, Zanesco A, Delbin MA, Menani JV, Bassi M, Colombari E, Colombari DS. Resistance training prevents the cardiovascular changes caused by high-fat diet. Life Sci 2016; 146:154–162. [DOI] [PubMed] [Google Scholar]
- 23. Silva AS, Ariza D, Dias DP, Crestani CC, Martins-Pinge MC. Cardiovascular and autonomic alterations in rats with Parkinsonism induced by 6-OHDA and treated with L-DOPA. Life Sci 2015; 127:82–89. [DOI] [PubMed] [Google Scholar]
- 24. Bertinieri G, Di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G. Evaluation of baroreceptor reflex by blood pressure monitoring in unanesthetized cats. Am J Physiol 1988; 254:H377–H383. [DOI] [PubMed] [Google Scholar]
- 25. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402–408. [DOI] [PubMed] [Google Scholar]
- 26. Tan PS, Killinger S, Horiuchi J, Dampney RA. Baroreceptor reflex modulation by circulating angiotensin II is mediated by AT1 receptors in the nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol 2007; 293:R2267–R2278. [DOI] [PubMed] [Google Scholar]
- 27. Colombari E, Colombari DS, Li H, Shi P, Dong Y, Jiang N, Raizada MK, Sumners C, Murphy D, Paton JF. Macrophage migration inhibitory factor in the paraventricular nucleus plays a major role in the sympathoexcitatory response to salt. Hypertension 2010; 56:956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li H, Gao Y, Freire CD, Raizada MK, Toney GM, Sumners C. Macrophage migration inhibitory factor in the PVN attenuates the central pressor and dipsogenic actions of angiotensin II. FASEB J 2006; 20:1748–1750. [DOI] [PubMed] [Google Scholar]
- 29. Petersson MJ, Rundqvist B, Johansson M, Eisenhofer G, Lambert G, Herlitz H, Jensen G, Friberg P. Increased cardiac sympathetic drive in renovascular hypertension. J Hypertens 2002; 20:1181–1187. [DOI] [PubMed] [Google Scholar]
- 30. Kirchheim HR. Systemic arterial baroreceptor reflexes. Physiol Rev 1976; 56:100–177. [DOI] [PubMed] [Google Scholar]
- 31. Nishi EE, Bergamaschi CT, Oliveira-Sales EB, Simon KA, Campos RR. Losartan reduces oxidative stress within the rostral ventrolateral medulla of rats with renovascular hypertension. Am J Hypertens 2013; 26:858–865. [DOI] [PubMed] [Google Scholar]
- 32. Biancardi VC, Son SJ, Ahmadi S, Filosa JA, Stern JE. Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood-brain barrier. Hypertension 2014; 63:572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johnson AK, Loewy AD. Circumventricular organs and their role in visceral functions. In Loewy AD, Spyer KM (eds), Central Regulation of Autonomic Functions. Oxford University Press: New York, 1990, pp. 247–267. [Google Scholar]
- 34. Martins-Oliveira A, Castro MM, Oliveira DM, Rizzi E, Ceron CS, Guimaraes D, Reis RI, Costa-Neto CM, Casarini DE, Ribeiro AA, Gerlach RF, Tanus-Santos JE. Contrasting effects of aliskiren versus losartan on hypertensive vascular remodeling. Int J Cardiol 2013; 167:1199–1205. [DOI] [PubMed] [Google Scholar]
- 35. Möhring J, Möhring B, Näumann H-J, Philippi A, Homsy E, Orth H, Dauda G, Kazda S. Salt and water balance and renin activity in renal hypertension of rats. Am J Physiol 1975; 228:1847–1855. [DOI] [PubMed] [Google Scholar]
- 36. Burmeister MA, Young CN, Braga VA, Butler SD, Sharma RV, Davisson RL. In vivo bioluminescence imaging reveals redox-regulated activator protein-1 activation in paraventricular nucleus of mice with renovascular hypertension. Hypertension 2011; 57:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zimmerman MC, Davisson RL. Redox signaling in central neural regulation of cardiovascular function. Prog Biophys Mol Biol 2004; 84:125–149. [DOI] [PubMed] [Google Scholar]
- 38. Cheng WH, Lu PJ, Ho WY, Tung CS, Cheng PW, Hsiao M, Tseng CJ. Angiotensin II inhibits neuronal nitric oxide synthase activation through the ERK1/2-RSK signaling pathway to modulate central control of blood pressure. Circ Res 2010; 106:788–795. [DOI] [PubMed] [Google Scholar]
- 39. Kudrin A, Ray D. Cunning factor: macrophage migration inhibitory factor as a redox-regulated target. Immunol Cell Biol 2008; 86:232–238. [DOI] [PubMed] [Google Scholar]
- 40. La Rovere MT, Pinna GD, Maestri R, Sleight P. Clinical value of baroreflex sensitivity. Neth Heart J 2013; 21:61–63. [DOI] [PMC free article] [PubMed] [Google Scholar]